Abstract
Objective
To evaluate the effects of overeating (140% of energy requirements) a high-fat low-energy density diet (HF/LED, 1.05kcal/g), high-fat high-energy density diet (HF/HED, 1.60kcal/g), and high-carbohydrate (HC) LED (1.05kcal/g) for 2-days on subsequent 4-day energy intake (EI), activity levels, appetite, and mood.
Design and Methods
Using a randomized cross-over design, energy expenditure and EI were standardized during overeating.
Results
In 20 adults with a mean±SD BMI of 30.7±4.6kg/m2, EI was not suppressed until the second day after overeating and accounted for ~30% of the excess EI. Reductions in EI did not differ among the 3 diets or across days. Overeating had no effect on subsequent energy expenditure but steps/day decreased after the HC/LED and HF/HED. Sleep time was increased after the HF/HED compared to both LEDs. After overeating a HF/HED vs. HF/LED, carbohydrate cravings, hunger, prospective food consumption, and sadness increased and satisfaction, relaxation, and tranquility decreased.
Conclusions
Diet type, time, or their interaction had no impact on compensation over 4 days. No adaptive thermogenesis was observed. The HF/HED vs. HF/LED had detrimental effects on food cravings, appetite, and mood. These results suggest short-term overeating is associated with incomplete compensation.
Keywords: hyperphagia, appetite, spontaneous physical activity, energy expenditure, hunger, sleep
Introduction
Obesity is a known epidemic with over 68% of Americans being classified as overweight or obese (1). Obesity is associated with many health problems including cardiovascular disease and diabetes (2). Obesity is the result of a positive energy balance, which occurs when more energy is ingested than expended. Weight loss is achieved by reducing energy intake and/or increasing physical activity (energy expenditure). Increased body weight may be driven by hyperphagia on holidays and weekends (3, 4) and less physical activity on Sundays (4).
Physical activity and exercise are involved in energy homeostasis. As was recently reported from 5 decades of NHANES data, there has been a gradual decrease in physical activity in the workforce that may be accounting for much of the increase in body weight seen during the timeframe (5). Edholm found that physical activity was positively related to energy intake with a 48 hour delay (6). It is generally accepted that there is a loose positive coupling of energy expenditure from exercise and food intake (7). Since physical activity may be coupled with food intake and directly affect energy balance, it is critical to account for this part of the energy balance equation (energy expenditure) when performing food intake experiments.
Overeating produces a positive energy balance and has not been found to affect physical activity (8). The amount of food or energy that people ingest varies from day-to-day (9) with an average intra-individual coefficient of variation (CV) of 25% (10). de Castro originally demonstrated there was a 2–3 day lag in habitual free-living energy intake (11) but more recently Bray et al. showed 3–4 day corrective responses to habitual variations in food intake, which may be critical in energy balance homeostasis (10). Saris et al. also noted a lag in intake and expenditure in bicyclists during the Tour de France bicycle race (12). Thus a lag time appears to exist between energy intake and subsequent corrective responses.
Lowering the energy density of food decreases energy intake (13, 14). Over 4 days, obese, but not lean women decreased food intake on a low fat low energy density diet compared to a low fat high energy density matched diet (15). The authors suggested that energy intake may be regulated by weight or mass of food rather than its total energy. Thus the more energy consumed per unit weight of food, the more energy will be consumed. Fat (9 kcal/g) and water (0 kcal/g) are the main contributors to energy density as carbohydrate and protein each have 4 kcal/g. The current study tested the hypothesis that manipulating energy density and fat content might affect subsequent energy intake following overeating.
It has been demonstrated that dietary fat is positively related to energy intake (16) whereas the carbohydrate balance of humans is found to be negatively associated with energy balance (17). Thus higher carbohydrate intake and lower energy density may decrease energy intake especially during times of overeating. The purpose of this study was to test the effects of overeating a high-fat low-energy density diet (HF/LED), high-fat high-energy density diet (HF/HED), and high-carbohydrate low-energy density diet (HC/LED) for 2-days on subsequent 4-day food intake, activity levels, energy expenditure, and ratings of appetite and mood. To our knowledge, we are among the first to control activity/energy expenditure during overeating and to examine differences in energy balance after overeating high fat diets that varied in energy density.
Methods
The two studies reported herein were conducted according to the guidelines in the Declaration of Helsinki and all participants were given verbal and written explanations about the study, provided signed informed consent, and received a monetary stipend. The studies were approved by the Pennington Biomedical Research Center’s Institutional Review Board and were registered at clinical trials.gov NCT 01653886 (pilot study) and NCT 01653145 (main study).
Pilot Study
A pilot study was conducted to develop study menus that were similar in appearance, aroma and taste. The menus were created using the Standard Reference database. The pilot study occurred prior to the parent study and is a separate study with different participants. Sixteen participants (9 M and 7 F, mean ± SD, 32 ± 7 years old, 26.6 ± 1.4 kg/m2) started and completed the pilot study. All participants completed the taste tests on a single day.
The following four diets were utilized in the pilot and main study: 1) a baseline diet (15% protein, 30% fat, and 55% carbohydrate; 11 g fiber per 1000 kcal), 2) a high-fat low-energy density diet (HF/LED, 4.4 kJ/g or 1.05 kcal/g), 3) a high-fat high-energy density diet (HF/HED, 6.7 kJ/g or 1.60 kcal/g), and 4) a high-carbohydrate low-energy density diet (HC/LED, 4.4 kJ/g or 1.05 kcal/g). The high fat diets were created by adding fat with 15% protein to the baseline diet, resulting in diets that were 50% fat, 35% carbohydrate, and 15% protein. The high carbohydrate diet was created by adding carbohydrate with 15% protein to the baseline diet, resulting in a diet that was 20% fat, 65% carbohydrate, and 15% protein. The three treatment diets were low or high in energy density (low = 4.4 kJ/g or 1.05 kcal/g; high = 6.7 kJ/g or 1.60 kcal/g) which was consistent with previous studies (15). Energy density was manipulated by modifying the water content of foods such as soups. Participants overeating the HF/LED, HF/HED, and HC/LED diets consumed 7 g, 10 g, and 14 g of fiber per 1000 kcal, respectively. The diets are described in Supplemental Table 1 and the individual food items are described in Table 1.
Table 1.
Dietary menus for overeating and food intake test days
| Overeating Menu |
Food Intake Test Menus | ||||
|---|---|---|---|---|---|
| Days 1 & 2 | Day 1 | Day 2 | Day 3 | Day 4 | |
| Breakfast | |||||
| Oatmeal | Bagel | Blueberry Muffin |
Egg Substitute |
Egg Substitute |
|
| Bagel | Cream Cheese | Tropical Fruit Bowl |
Whole Wheat Bread |
Butter | |
| Grape Juice | Tropical fruit mix |
Low fat milk (1%) |
Butter | Grits | |
| Orange Juice | Low fat milk (1%) |
Low fat milk (1%) |
Peach Fruit Cup |
||
| Soy or skim milk |
Low fat milk (1%) |
||||
| Wheat Bread | |||||
| Banana | |||||
| Butter or Margarine |
|||||
| Lunch | |||||
| Wheat bread | Wheat bread | Whole wheat pita bread |
Bun | French bread pizza |
|
| Ham | Turkey | Chicken salad | Grilled chicken |
Pineapples | |
| breast | |||||
| Cheddar Cheese |
Lettuce and tomato |
Lettuce and tomato |
Mayonnaise | Strawberry yogurt |
|
| Mayonnaise | Mayonnaise | Sun chips | Mustard | Salad | |
| Pretzels or potato chips |
Mustard | Apple | Fruit salad | Italian dressing |
|
| Apple | Baked Lays | Strawberry yogurt |
|||
| Baby carrots and ranch dressing |
Pears | Salad | |||
| Broccoli and cheese soup |
String Cheese | Ranch Dressing |
|||
| Snack | |||||
| Wheat thins | Granola bar | Graham crackers |
Vanilla wafers |
Wheat thins | |
| Dried cranberries |
Orange juice | Peanut butter | Chocolate pudding |
String cheese |
|
| Peanut butter | Orange juice | Apple Juice | Grape juice | ||
| Cranberry juice | |||||
| Dinner | |||||
| Chicken alfredo | Spaghetti and meatballs |
Pork chop | Catfish almondine |
Lemon sage chicken |
|
| Mixed vegetables |
Broccoli | Mashed potatoes |
Green beans | Mixed vegetables |
|
| Fruit cocktail | Dinner roll (white) |
Carrots | Rice pilaf | Wild rice | |
| Dinner roll (wheat) |
Peaches | Dinner roll (white) |
Dinner roll (white) |
Dinner roll (white) |
|
| Soy or skim milk |
Pineapple | Peaches | Pears | ||
| Cream of mushroom or broccoli cheese soup |
|||||
Participant were not allowed salt, caffeine, or artificial sweetener but were allowed salt-free seasonings and herbs.
Energy density was manipulated by modifying the water content of foods such as soups.
Each participant completed taste tests at breakfast, lunch and dinner consisting of the baseline and the 3 treatment menu foods (HF/HED, HF/LED, HC/LED) resulting in each participant rating the palatability of the 12 meals. Every participant completed taste test ratings on a 9-point scale evaluating appearance, flavor, and taste. An ANOVA found no differences between meals at breakfast and dinner. However at lunch appearance and taste were significantly different between the baseline and HF/LED menus. The only menu item which differed was a low energy density soup. Thus the soup was modified to improve the texture and overall ratings of the meal.
Main Study
Screening
Inclusion criteria were: 1) BMI ≥ 20 and ≤ 40 kg/m2, 2) age 18–50 years for men and 18–45 years for pre-menopausal women, and 3) regular menses, with no less than 28 day cycles (women only). Women were included in the study who used monophasic birth control pills, copper IUD, or vaginal ring, or who had a complete hysterectomy. Screening and testing of premenopausal women was completed during the luteal phase of the menstrual cycle to control the effect of menstrual cycle phase on food intake.
Exclusion criteria were: 1) diseases or conditions that affected metabolism, appetite, or body weight, which included diabetes and cardiovascular disease, 2) post-menopausal or partial hysterectomy (women only), 3) use of prescription or over the counter medications that affected metabolism or body weight (e.g., sibutramine, orlistat), 4) restrained eating (>14 on the Eating Inventory restraint scale), 5) symptoms of depression (> 13 on the Beck Depression Inventory II), 6) tobacco use, and 8) heavy exercise (> 1 hour per day 5 or more days per week).
Participants completed two screening visits prior to being enrolled in the study. At Screening Visit 1 (SV1), potential participants provided written informed consent and their initial eligibility was confirmed. Clinic measurements, including height, weight, blood pressure and pulse rate were collected at this visit. Participants also completed the Beck Depression Inventory II (18) and the Eating Inventory (19) (see self – report questionnaires for psychometrics). Participants wore the SenseWear® Armband (Body Media, Inc., Pittsburgh, PA) between screening visits. At Screening Visit 2 (SV2) (approximately one week after Screening Visit 1), participants returned the Armband and were enrolled in the study if all eligibility criteria were met and they wished to enroll.
Experimental Design
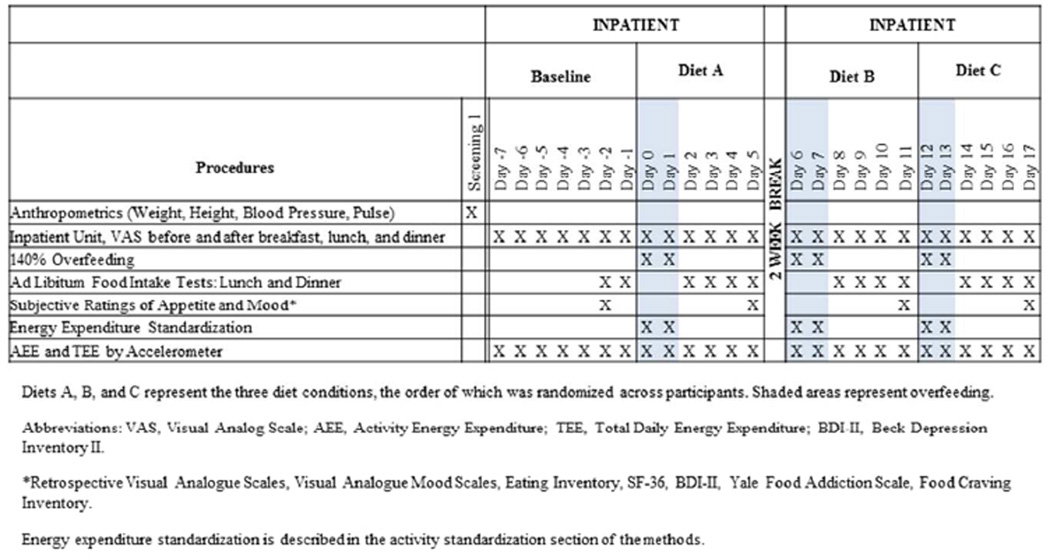
Twenty participants (15 M, 5 F; 1 normal weight, 8 overweight and 11 obese) were enrolled and completed the randomized, balanced, cross-over study. Participants resided on the PBRC inpatient unit (metabolic ward) during overeating and during outcomes assessments. All participants first completed a one-week (day -7 through day -1) baseline period before completing the three randomized overeating diets. Baseline ad libitium food intake tests were completed on days -2 and -1 (Figure 1). Participants lived at home for two weeks mid-way through the study. This was so menstruating females could be tested on the same phase of their menstrual cycle (luteal phase) during all diets.
Figure 1.
Experimental design and study schedule
The baseline diet was designed to meet energy requirements; hence, from days -7 to -3 energy intake was adjusted based on daily body weights to achieve weight maintenance. If participants were not weight stable (within ± 0.5 kg) during days -7 to -3, foods with the same energy density as the diet were added or subtracted in 100 kcal increments in an effort to achieve body weight stability (energy balance). During overeating, participants were provided with 140% of the energy necessary for energy balance/weight maintenance on the inpatient unit. The amount of energy necessary for weight maintenance was determined by calculating resting metabolic rate (RMR) with the Mifflin equation and multiplying estimated RMR by an activity factor of 1.45. This activity factor was derived from physical activity level data collected from PBRC’s inpatient unit as well as other studies (20). This activity factor was approximately 80% of their free living energy balance requirements. Thus, since participants were in energy balance, 140% of the energy necessary for energy balance/weight maintenance on the inpatient unit is about 12% more energy intake than in free living conditions.
Anthropometric Measures
Body weight, height, blood pressure, and pulse were measured in the clinic at screening. Metabolic body weight (subject weight minus the gown weight to the nearest 0.1 kg) was measured on all days participants resided in the Inpatient Unit (Day -7 to Day 17) on a scale (A&D, UC-32THW). Participants were weighed after the first void in the morning in a hospital gown and underwear only. All measurements were taken in duplicate.
Activity
During positive energy balance, energy expenditure was maintained at the levels recorded during baseline. On day -4 during baseline, energy expenditure was measured with indirect calorimetry (Parvomedics, Sandy, UT) while participants sat or stood and watched TV for 10–20 minutes. Energy expenditure was also measured while participants walked 1, 2, 3 mph (4–5 minutes each speed). From these measures, physical activity was tailored so energy expenditure on all overeating days would be fixed and equal to 100% of the energy requirements of living in the inpatient unit.
During the days of positive energy balance, energy expenditure was quantified with an ActivPAL accelerometer (PAL Technologies, Glascow, UK) and closely monitored every 1–2 hours. Participants were instructed to increase or decrease their energy expenditure to match their baseline levels of EE (within ± 10 kcal/hr). Walking and standing was spread throughout the day and supervised by inpatient staff. The average treadmill walking speed was 1.6 ± 0.2 mph. Aside from designated times to wash themselves, the participants were asked to sit the rest of the day.
Food Intake
During overeating days, participants were required to consume all food provided. However they were allowed to carry-over unfinished food items to the next meal but were required to finish on the assigned day.
During all ad libitum feeding days when food intake was being quantified as an outcome variable (including baseline days -2 and -1), a standardized breakfast and afternoon snack were provided consisting of 663 kcal (30% fat, 55% carbohydrate and 15% protein). Since it was standardized, the breakfast and afternoon snack are not included in the ad libitum meal results. The ad libitum meals were 30% fat, 55% carbohydrate, and 15% protein and consisted of the participants estimated RMR multiplied by an activity factor of 2.3. At lunch and dinner, food was provided and participants were asked to eat to a ‘comfortable level of fullness’. On these ad libitum feeding days, the total amount of food consumed (weighted to the nearest 0.1 g) was evaluated for 4 days in the inpatient unit following the 2 days of overeating.
Physical Activity Assessment
SenseWear armbands (Body Media, Inc.) were worn by participants throughout the baseline and the first 3 days of ad libitum feeding portions of the study to quantify changes in activity levels and energy expenditure. It was worn on the upper arm and has previously been validated (21). The armband was worn at all times by participants (i.e. greater than 95% on body time) except during periods of water submersion (i.e., showering). The arm band measures several parameters including daily energy expenditure (kcal/d), measured active energy expenditure (kcal/d), physical activity duration (min/d), daily steps (steps/d), time lying down (min/d), measured sleep (min/d), and average metabolic equivalents (METs). Measured sleep is an output on the Sensewear report determined from proprietary algorithms based on accelerometry, heat flux sensor, body temperature, and galvanic skin response (sweat). A metabolic equivalent is equal to resting VO2 which is approximately 3.5 ml · kg−1 · min−1. METs are the cost of exercise as a multiple of resting VO2.
Visual Analogue Scales (VAS)
Subjects were asked to complete subjective ratings of appetite for the following questions (22); how hungry do you feel; how full does your stomach feel; how strong is your desire to eat; how much food do you think you could eat; how satisfied do you feel? The subject rated the intensity of their feeling with a dash that corresponded with their perception at that moment. The lower anchor was ‘Not at all’ (scored 0) and the upper anchor was ‘Extremely’ (scored 100) on a computer with a line that was divided into 100 equal units. VAS were completed and scored on all days before and after breakfast, before and after lunch and before and after dinner for a total of 6×/day.
Retrospective Visual Analogue Scales (RVAS)
RVAS were used to measure hunger, fullness, desire to eat, prospective food consumption and satisfaction. When rating RVAS participants record on a computer using a line that is divided into 100 equal units. They are similar to VAS except they reflect how the participant felt ‘overall’ during the previous week (23). RVAS were administered on days -1, 5, 11, and 17 two hours after lunch.
Visual Analogue Mood Scales (VAMS)
VAMS were used to measure mood states. Specifically lower and upper anchors were as follows: happy, sad; tense, relaxed; troubled, tranquil; calm, excited; alert, drowsy; lethargic, energetic. Thus, happiness, relaxation, anxiety, depression, and alertness were measured on days -1, 5, 11, and 17 with the same computerized line which is divided into 100 equal units (24).
Self-Report Questionnaires
All self-report questionnaires were completed on days -1, 5, 11, and 17 (the last day of the 4-day follow-up period after overeating).
The Beck Depression Inventory II (BDI-II) (18) is a 21-item self-report measure that was used to measure depressive symptoms. Higher scores indicative of more severe symptoms of depression.
The Eating Inventory (EI) (19) was designed to measure different dimensions of eating behavior including both cognitive and behavioral aspects. Three factor-analyzed subscales (Cognitive Restraint, Disinhibition, and Hunger) were derived from the questionnaire. The scale involves 36 true or false questions and 15 multiple choice questions.
The Food Craving Inventory (FCI) (25) was used as a measure of specific food craving. The FCI assesses the frequency with which an individual experiences a craving for 4 types of foods: high fats, sweets, carbohydrates/starches, and fast food fats. A total craving score is also derived.
The Medical Outcomes Study Short Form Health Survey (SF-36) (26) is a questionnaire of general health and emotional well-being. It covers 8 key scales that are divided into 2 main dimensions: physical component summary and mental component summary. The physical component summary includes: physical functioning, role-physical, bodily pain, and general health. The mental component summary includes: vitality, social functioning, role-emotional, mental health.
Statistical Analysis
Power analysis
A power analysis was conducted for the primary outcome variable (energy intake). The power analysis used an alpha level of .05, power was held at 80%, and two-directional tests were utilized. Variability estimates were obtained from our laboratory. With 20 participants, we were powered to detect a difference of 355 kcal (Effect Size = .66, Standard Deviation = 537) or a 16% difference in food intake between conditions. Food intake differed by 16% between low and high energy density diets in a previous study (15); therefore, we believed that adequate statistical power was achieved.
Statistical analysis
Data were normally distributed thus did not require transformation or nonparametric statistical analyses. Observations made during days -2 and −1 of the study were considered to be baseline measurements. A mixed model repeated measures analysis of covariance (ANCOVA) was used to test if change from baseline on food intake differed significantly by condition (HF/LED, HC/LED, and HF/HED), with and without adjusting for baseline. The presence of order effects was tested with repeated-measures ANCOVA to account for the cross-over design. Day was a repeated factor (except for when data were averaged). Armband and questionnaire data were analyzed similarly. Unplanned a priori comparisons were made for sex (between subjects effect) for food intake variables. Planned a priori comparisons were made between the 3 diets conditions as well as a priori least squares means (LS Means) for change from baseline in dietary treatments.
A mixed model analysis of variance (ANOVA) for a 2×2 crossover trial with repeated day effect was performed to analyze the area under the curve (AUC) for VAS ratings of hunger, fullness, satisfaction, desire to eat, and prospective food intake. The model included factors with fixed effects, sequence effects, visit main effects, treatment main effects, in addition to the random effects of subjects within treatment sequence groups. The repeated effect was days within each visit. Area under the curve was estimated using the linear trapezoidal rule. Baseline values were entered as covariates in the determination of AUC for VAS ratings.
Means were considered to be statistically significant if p ≤ 0.05. When post hoc tests were conducted, they followed the Tukey-Kramer adjustment, with the exception of the a priori planned comparisons. All analyses were carried out using SAS Version 9.2 software package.
Results
Subject Characteristics, Anthropometrics, and Food Intake
The subject characteristics are described in Table 2. Following overeating, day and a treatment had an effect on body weight but no interaction. For all groups combined, following overeating, body weight on days 1, 2, and 3 was higher than baseline whereas day 4 was not different (Supplemental Table 2). Also, for all groups combined, body weight decreased on each subsequent day after overeating (e.g., body weight was lower the second day after overeating compared to the first day, etc.). Change in body weight from baseline to after overeating also differed by treatment. Specifically, following the HC/LED, body weight increased compared to following the HF/HED (HC/LED, 1.09 ± 0.22 kg; HF/HED, 0.58 ± 0.22 kg; p□0.01) while following the HF/LED body weight was not different from either treatment (0.76 ± 0.22 kg).
Table 2.
Characteristics of the study sample
| Total (N=20) | Minimum | Maximum | |
|---|---|---|---|
| Sex | 15 M, 5 F | ||
| Age (y) | 34 ± 9 | 20 | 49 |
| BMI (kg/m2) | 30.7 ± 4.6 | 22.0 | 37.6 |
| Body Weight (kg) | 92.8 ± 16.2 | 65.7 | 126.1 |
| Blood Pressure (mmHg) | |||
| Systolic | 119 ± 10 | 100 | 131 |
| Diastolic | 78 ± 8 | 60 | 89 |
Data are expressed as Mean ± SD.
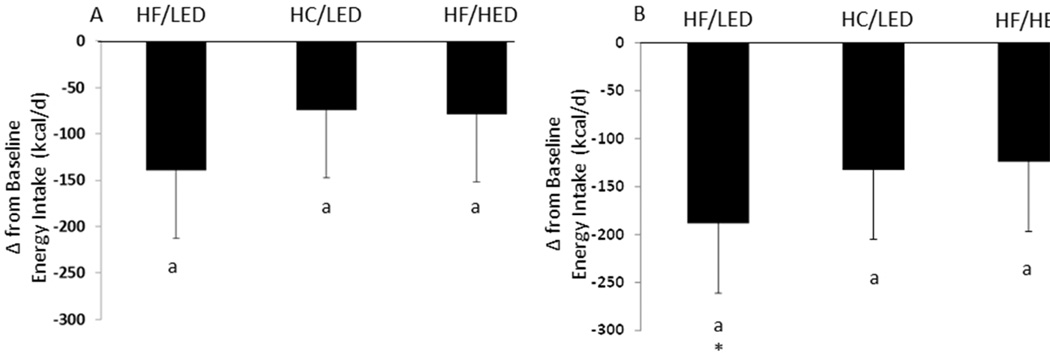
Following overeating, there were no treatment by day interactions or main treatment effects for food intake over all 4 days. On all 3 diet treatments (Table 3), subjects consumed more the day following the 2 days of overeating compared to the subsequent ad libitum test days (p<0.0001; Supplemental Table 2). When examining the data as change from baseline, days 1–4 energy intake were not statistically different from baseline (Figure 2A, but there was a trend for day 2 to be lower than baseline (−8.48 ± 4.65%; p=0.087). On days 2, 3, and 4, the HF/LED reduced energy intake by −189 ± 73 kcal compared to baseline (Figure 2B; p<0.05).
Table 3.
Energy and macronutrient intake, body weight, and physical activity over the four days following overeating a high fat/low energy dense diet, a high carbohydrate/low energy dense diet, and a high fat/high energy dense diet
| High fat/low energy dense diet | High carbohydrate/low energy dense diet | High fat/high energy dense diet | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 1 | Day 2 | Day 3 | Day 4 | Day 1 | Day 2 | Day 3 | Day 4 | |
| BW (kg) | 91.17± 2.53*a |
90.64 ± 2.53*b |
90.46 ± 2.53*c |
90.28 ± 2.53*d |
91.68 ± 2.53*a |
91.06 ± 2.53*b |
90.75 ± 2.53*c |
90.39 ± 2.53*d |
90.82 ± 2.53*a |
90.54 ± 2.53*b |
90.37 ± 2.53*bc |
90.13 ± 2.53*d |
| Δ BW (kg) | 1.33 ± 0.23*a |
0.80 ± 0.23*b |
0.62±0.23*c | 0.44 ± 0.23d |
1.84 ± 0.23*a |
1.22 ± 0.23*b |
0.91 ± 0.23*c |
0.55 ± 0.23*d |
0.98 ± 0.23*a |
0.70 ± 0.23*b |
0.52 ± 0.23*bc |
0.29 ± 0.23d |
| Food Intake | ||||||||||||
| Energy (kcal) | 1825 ± 115*a |
1577 ± 115*b |
1600 ± 115*b | 1629 ± 115*b |
1908 ± 115*a |
1601 ± 115*b |
1692 ± 115*b |
1689 ± 115*b |
1865 ± 115*a |
1631 ± 115*b |
1693 ± 115*b |
1684 ± 115*b |
| Δ Energy (kcal) | 28 ± 87a | −220 ± 86*b | −197 ± 86*b | −168 ± 86b | 111 ± 86a |
−196 ± 86*b |
−105 ± 86b |
−108 ± 86b |
67 ± 86a |
−166 ± 86b |
−104 ± 86b |
−113 ± 86b |
| Δ Energy (%) | 2.87 ± 5.51a |
−10.40 ± 5.45b |
−9.19 ± 5.45b |
−6.68 ± 5.45b |
7.98 ± 5.45a |
−8.71 ± 5.45b |
−2.77 ± 5.45b |
−4.22 ±5.45b |
4.74 ± 5.45a |
−6.33 ± 5.45b |
−3.12 ± 5.45b |
−6.15 ± 5.45b |
| Δ Carbohydrate (%) | 2.22 ± 6.48a |
−11.82 ± 6.40b |
−13.35 ± 6.40*b |
−14.41 ± 6.40*b |
7.39 ± 6.40a |
−9.21 ± 6.40b |
−4.79 ± 6.40b |
−9.53 ± 6.40b |
4.88 ± 6.40a |
−6.77 ± 4.60b |
−8.20 ± 6.40b |
−11.50 ± 6.40b |
| Δ Fat (%) | 8.16 ± 6.02a |
−9.57 ± 5.97b |
−3.19 ± 5.97b |
8.63 ± 5.97a |
12.89 ± 5.97*a |
−8.97 ± 5.97b |
2.69 ± 5.97c |
7.74 ± 5.97ac |
7.80 ± 5.97a |
−6.59 ± 5.97b |
5.50 ± 5.97a |
5.80 ± 5.97a |
| Δ Protein (%) | −9.22 ± 5.10a |
−2.12±5.00a | −1.45±5.00a | −11.50± 5.00*a |
−3.41 ± 4.99a |
−1.59 ± 4.99a |
2.20 ± 4.99a |
−9.90 ± 4.99a |
−5.31 ± 4.99ab |
1.32 ± 4.99a |
2.85 ± 4.99a |
−11.71 ± 4.99*b |
| Physical Activity Assessment |
||||||||||||
| Δ Energy Expenditure (kcal/d) |
−58 ± 40a | −31±41ab | 18 ± 40b | −33 ± 37a | −86 ± 37*a |
−69 ± 38a | −77 ± 40a |
−50 ± 40a |
−65 ± 41a |
|||
| Δ Measured Active Energy Expenditure (kcal/d) |
−9 ± 16a | −12 ± 17a | 12 ± 16a | −12 ± 15a | −21 ± 15a | −17 ± 15a | −23 ± 16a |
−1 ± 16a | −19 ± 16a |
|||
| Δ Physical Activity Duration (min/d) |
−2.3 ± 2.9a |
−1.8±3.1a | 1.8±2.9a | −2.3 ± 2.7a |
−4.3 ± 2.7a |
−3.3 ± 2.8a |
−4.5 ± 3.0a |
−0.2 ± 2.9a |
−3.2 ± 3.1a |
|||
| Δ Daily Steps (steps/d) | −456 ± 191*a |
−267 ± 200a | −93 ± 191a | −226 ± 182a |
−561 ± 182*a |
−364 ± 190a |
−373 ± 195a |
−398 ± 191*a |
−266 ± 200a |
|||
| Δ Time Lying Down (min/d) |
72.9 ± 32.5*a |
−5.0±34.2b | −14.4 ± 32.5b |
43.3 ± 30.4a |
68.4 ± 30.4*a |
18.9 ± 32.0a |
83.4 ± 33.2*ab |
104.2 ± 32.4*a |
29.9 ± 34.2b |
|||
| Δ Measured Sleep (min/d) |
62.3 ± 20.0*a |
−27.5 ± 21.2b |
−35.1 ± 20.0b |
27.5 ± 18.7a |
14.4 ± 18.7ab |
−26.4 ± 19.8b |
50.4 ± 20.5*a |
45.0 ± 20.0*a |
35.1 ± 21.2a |
|||
| Δ Ave. METs | −0.03 ± 0.02a |
−0.02 ± 0.02ab |
0.01±0.02b | −0.01 ± 0.02a |
−0.04 ± 0.02*a |
−0.03 ± 0.02a |
−0.04 ± 0.02a |
−0.02 ± 0.02a |
−0.03 ± 0.02a |
|||
Figure 2.
(A) Change in Energy Intake following a High Fat/Low Energy Density diet (HF/LED), a High Carbohydrate/Low Energy Density diet (HC/LED), and a High Fat/High Energy Density diet (HF/HED). Values are means for fifteen males and five females with standard errors represented by vertical bars. (B) Change in Energy Intake (mean energy intake during days 2, 3, and 4 minus baseline only) following a HF/LED, a HC/LED, and a HF/HED. Values are means for fifteen males and five females with standard errors represented by vertical bars. *Energy intake after the HF/LED was significantly (p<0.05) lower than baseline.
When data were examined for macronutrient differences for all 4 days following overeating, no treatment by day interactions or main effects among dietary treatment groups were seen when analyzed as percent change from baseline. The energy and macronutrient change from baseline values for all groups combined is shown in Table 3. Carbohydrate intake was higher on the day following overeating compared to subsequent days (p<0.0001). Fat decreased on day 2 of ad libitum feeding and day 3 continued the decrease in fat intake (p<0.01). On days 1 and 4, fat intake was not different. Protein intake was higher on days 1 and 4 compared to day 3 (p<0.05) and day 2 was not different than any other day.
When data were also examined in grams and the average of the 4 days of ad libitum feeding (no interaction term or day effect in the statistical model) following overeating were analyzed, no treatment differences were seen for energy (kcal), weight (grams), energy density (kcal/g), fat (g), protein (g), or carbohydrate (g). These data are not shown.
Also, although underpowered to detect differences, when data were examined with sex and sex by treatment effects in the model, energy (kcal), weight (grams), energy density (kcal/g), fat (g), protein (g), or protein (g) did not approach significance. All p-values were greater than p=0.20. These data are also not shown.
Activity
No treatment by day interactions or treatment differences were seen with total daily energy expenditure, daily steps, step rates (HF/LED 85 ± 3 steps per minute, HF/HED 85 ± 3 steps per minute, and HC/LED 85 ± 3 steps per minute), daily energy expenditure change from baseline, or daily step change from baseline assessed by accelerometer during overeating.
Physical Activity Assessment
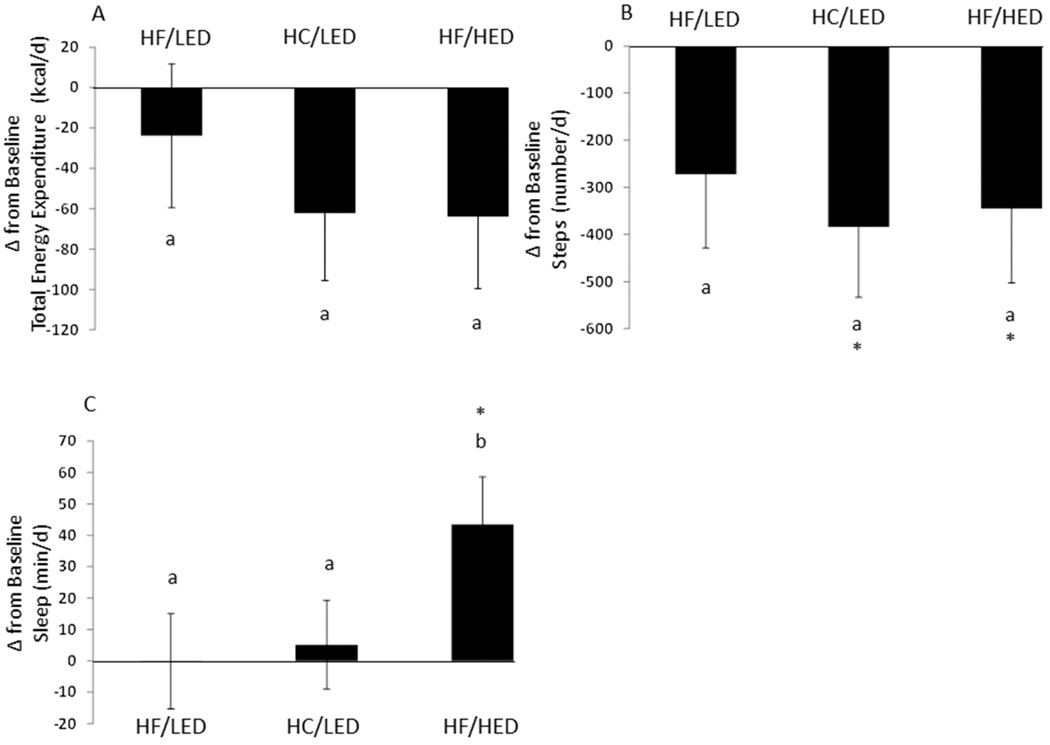
Following overeating, no treatment by day interactions, treatment differences, or day differences were seen with change from baseline energy expenditure, measured active energy expenditure, and physical activity duration. Following overeating, days 1 and 2 had reduced steps compared to baseline (Table 3). Following the two LEDs, both HF and HC, steps were reduced compared to baseline (Figure 3). A treatment by day interaction was seen with change from baseline average metabolic equivalents. A reduction in average metabolic equivalents after the HF-low energy density group was seen on day 1 compared to day 3 (−0.038 ± 0.013; p<0.05).
Figure 3.
Change in Total Energy Expenditure, Step Count and Sleep following a High Fat/Low Energy Density diet (HF/LED), High Carbohydrate/Low Energy Density diet (HC/LED), and High Fat/High Energy Density diet (HF/HED). Values are means for fifteen males and five females with standard errors represented by vertical bars.
(A) Energy expenditure was not different from baseline in any group. (B) Following overeating the HC/LED and HF/HED diets, number of steps decreased compared to baseline (p<0.05). (C) Following overeating the HF/HED diet, sleep time increased compared to baseline (p<0.05). Following overeating the HF/HED diet, sleep time increased compared to the sleep time following the HF/LED and HC/LED treatments (p<0.05).
No day by treatment interaction was observed with sleep time, but change from baseline day and treatment effects on sleep time were observed. Specifically, increased sleep time occurred on day 1 following overeating compared to baseline. Also, following overeating the HF/HED, sleep time increased compared to baseline. Following overeating the HF/HED, day 1 had increased sleep time compared to days 2 and 3. Also, sleep increased after the HF/HED compared to after the HF/LED and HC/LED diets. Minutes lying down increased following overeating on days 1 and 2 compared to baseline. Minutes lying down were reduced on day 3 following overeating compared to days 1 and 2.
Visual Analogue Scale (VAS)
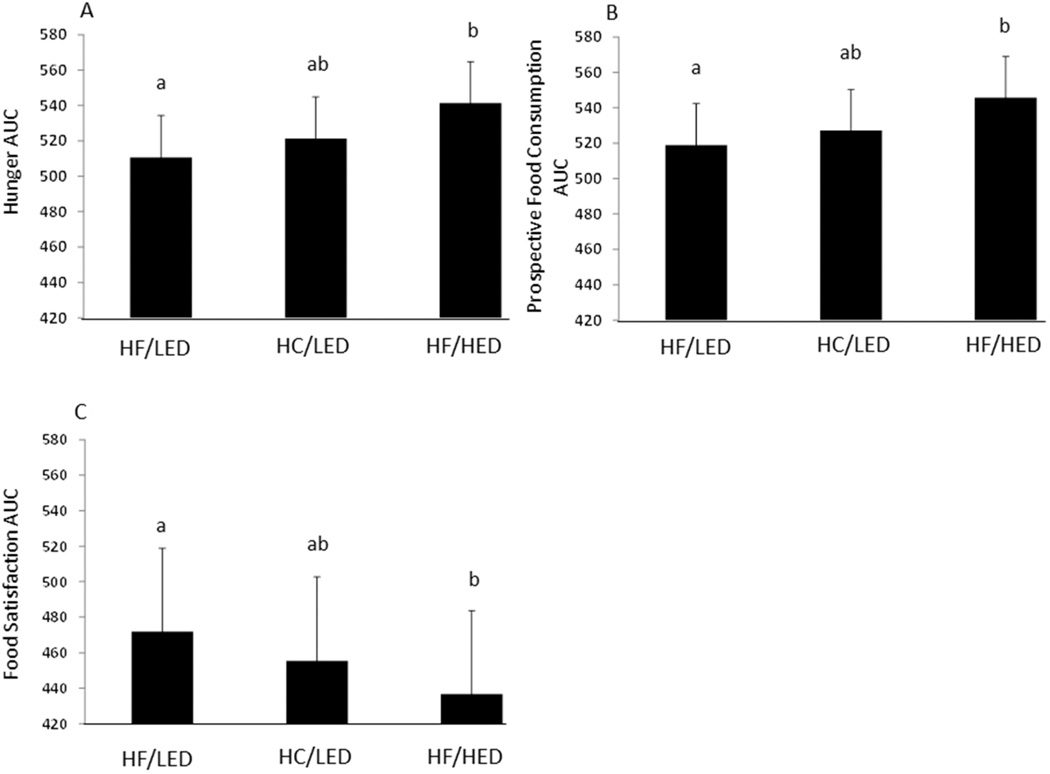
Using the area under the curve for the VAS scales, feelings of hunger decreased after the HF/LED compared to after a HF/HED dietary treatment (Figure 4; p<0.05) and no differences in hunger were seen following other dietary treatments. Also, following the HF/LED, feelings of ‘how much food do you think you could eat at this very moment?’ decreased compared to after the HED version of this dietary treatment (p<0.05) with no differences following other dietary treatments. Lastly, after the HF/LED, ratings of ‘how satisfied do you feel at this moment?’ increased compared to following the HED version of this dietary treatment (p<0.05) and no differences following other dietary treatments. After overeating, no treatment differences were seen with fullness or desire to eat.
Figure 4.
Change in Appetite Ratings from Visual Analogue Scales following High Fat/Low Energy Density diet (HF/LED), High Carbohydrate/Low Energy Density diet (HC/LED), and High Fat/High Energy Density diet (HF/HED). Values are means for fifteen males and five females with standard errors represented by vertical bars. Values with a different superscript are different at p<0.05.
(A) Following overeating a HF/HED diet, hunger increased compared to following overeating a HF/LED diet. (B) Following overeating a HF/HED diet, prospective food consumption was higher compared to following a HF/LED. (C) Following overeating a HF/HED diet, food satisfaction was lowered compared to after a HF/LED diet.
Retrospective Visual Analogue Scale (RVAS)
With change from baseline for RVAS, after the HC/LED higher fullness was reported than after the HF/HED treatment (13 ± 6; p<0.05). Also, after eating the HF/LED participants had decreased feelings of how much food do you think you could have eaten during the past week compared to after the HF/HED treatment (−14 ± 6; p<0.05); no differences were seen following other dietary treatment groups. Lastly, following the HC/LED decreased feelings of hunger were reported compared to after the HF/HED treatment (−18 ± 8; p<0.05), and no differences were seen following other dietary treatment groups. No treatment differences were seen following overeating with satisfaction or desire to eat.
Visual Analogue Mood Scales (VAMS)
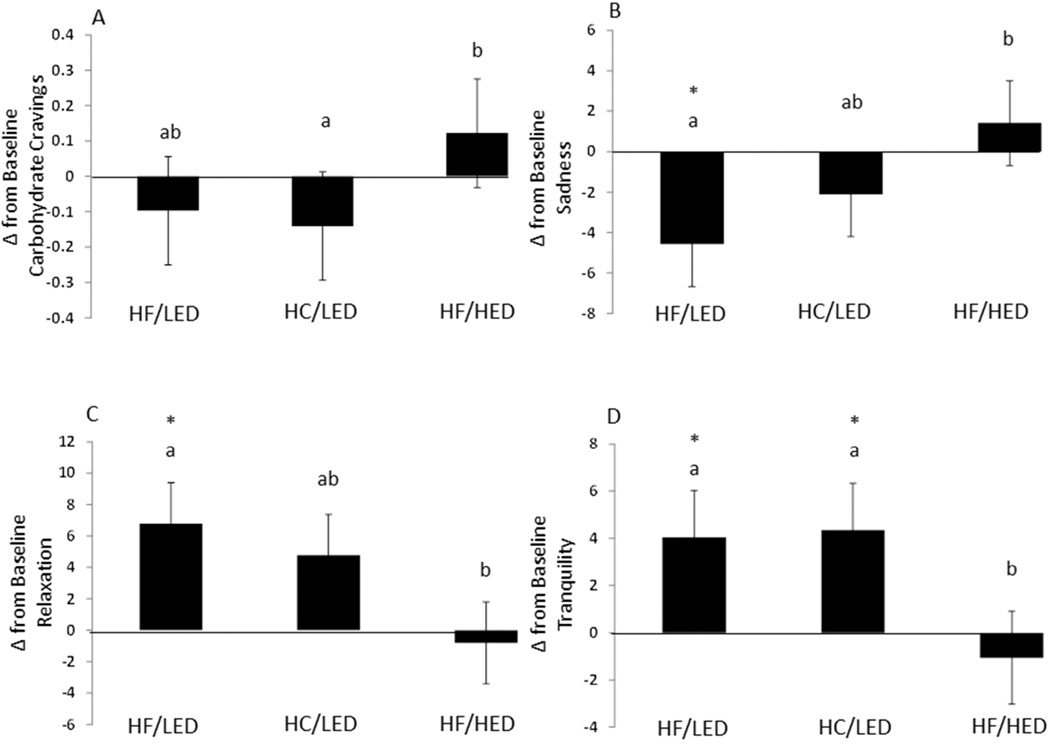
With change from baseline for VAMS, after the HF/LED treatment more happiness (less sadness) was felt than after the HF/HED treatment (Figure 5; 6 ± 3; p<0.05). Following overeating the HF/LED treatment participants felt more relaxed (less tense) than after the HF/HED treatment (−8 ± 4; p<0.05) but no differences were seen after the HF/LED and HC/LED dietary treatments. After consuming the HF/LED and HC/LED treatments, participants felt more tranquil (less troubled) than following the HF/HED dietary treatment (−5 ± 2, −5 ± 2; p<0.05). No differences were seen after the HF/LED and HC/LED dietary treatments. No other treatment differences were seen.
Figure 5.
Change in Food Cravings and Mood States following High Fat/Low Energy Density Diet (HF/LED), High Carbohydrate/Low Energy Density Diet (HC/LED), and High Fat/High Energy Density Diet (HC/LED). Values are means for fifteen males and five females with standard errors represented by vertical bars. Values with a different superscript are different at p<0.05.
(A) Following the HF/HED diet carbohydrate and starch cravings increased compared to following the HC/LED dietary treatment (p<0.05). (B) Following the HF/HED diet feelings of sadness (less happiness) increased compared to following the HF/LED dietary treatment group (p<0.05). Following overeating the HF/LED diet sadness was decreased compared to baseline (p<0.05). (C) Following overeating the HF/HED diet feelings of relaxation decreased compared to following the HF/LED dietary treatment group. Following overeating the HF/LED diet was increased compared to baseline (p<0.05). (D) Following overeating the HF/HED diet decreased tranquility compared to following the HF/LED diet (p<0.05). Following overeating HF/LED and HC/LED diet increased tranquility compared to baseline (p<0.05).
Self-Report Questionnaires
Following overeating, no change from baseline differences in restraint, disinhibition, or hunger were seen with the Eating Inventory. After overeating, no change from baseline differences were seen with the Beck Depression Inventory or SF-36. After overeating, with the Food Craving Inventory, change from baseline HC/LED carbohydrate and starch craving decreased compared to after the HF/HED dietary treatment, and no other changes were seen (Figure 5).
Discussion
Following two days of overeating a HC/LED, HF/HED, and HF/LED, the effects of the subsequent 4 days ad libitum food intake, appetite, mood, and energy expenditure were examined. de Castro and Bray et al. suggested that ad libitum corrective food intake responses may be occurring with a 2–4 day lag (10, 11). However, utilizing a 2-day overeating paradigm, a 2-day lag was shown suggesting that a partial corrective response existed with all treatments. With the overeating paradigm used in this study, there may be a faster partial corrective response (2 day vs. 2–4 days) to energy intake compared to conditions of deviations of energy intake near energy balance. During a longer term overeating study, young adults have been shown to compensate for the excess energy provided (27). Young males that were overfed 4.09 MJ/d (978 kcal/d) for a period of 21 days reduced food intake following overeating allowing body weight to return to baseline. During our short-term overeating study, a ~30% compensation of energy intake over 4 days was observed. Including this ~30% compensation, the overall net positive energy balance from beginning of overeating till 4 days after it ended was about ~1000 kcal or 250 kcal/d. This uncompensated 1000 kcal increase in energy intake is not negligible. Energy intake decreased following overeating but was not statistically lower than baseline on any day of ad libitum feeding. We could not determine if compensation continued after day 4, which would reduce this positive energy balance but body weight was not different from baseline by day 4 suggesting the 4 day ad libitum feeding was of sufficient length to examine for compensation in energy intake as supported by previous articles by Edholm (6), deCastro (11), Saris (12) and Bray et al. (10) suggesting compensation occurs within 4 days. Body weight following the HC/LED was higher than HF/HED possibly due to food weight (g) eaten with the HC/LED and the fiber content. However, during and following overeating HF/LED had similar food intake weights (g) as the HC/LED and even lower fiber content but body weight was not significantly different. Thus food weight and fiber are possibilities but do not fully explain the unexpected body weight effect. The days following overeating did not have any energy, macronutrient, or food intake weight effect differences among groups. The body weight response was an unanticipated result which the authors cannot fully explain.
The current study also analyzed ad libitum macronutrient intake. Carbohydrate intake decreased ~15% on days 2–4 of ad libitum feeding compared to day 1. These data are not consistent with the hypothesis that carbohydrate intake is negatively associated with energy intake. However, carbohydrate intake was reduced on days 2, 3, and 4 of the ad libitum diet period and thus supports another study that found carbohydrate intake and energy intake are positively associated (28). It is likely that increased glycogen stores from the high carbohydrate diet resulted in increased body weight compared to the 4 days following overeating in the HF/HED treatment. Also, it is well known that macronutrients are not independent of each other. When one macronutrient is altered at least one other macronutrient must be increased or decreased to account for the difference. In the current study, fat and carbohydrate intakes were manipulated. Following overeating, fat intake dropped on day 2 and slowly rose back up to be similar to day 1 on days 3 and 4 of ad libitum intake. This suggests the reduction in fat intake on day 2 and carbohydrate intake on days 2–4 may be a determinant for the energy compensation shown starting on the 2nd day.
Previous research suggested that energy density but not fat intake affects ad libitum energy intake in women (15, 29), leaving the applicability to males unknown. The current study included males and females but did not demonstrate a sex effect. Males and females responded similarly to all treatments. Following overeating of energy density matched foods, ad libitum fed volunteers continued overeating (compared to baseline) on day of 1 of ad libitum energy intake. The partial compensation was not an effect of energy density when analyzed over 4 days. On days 2, 3, and 4 of ad libitum feeding, the HF/LED treatment decreased energy intake compared to baseline, whereas neither the other two (HF/HED and HC/LED) reduced energy intake compared to baseline. During the ad libitum feeding period, our results show energy intake may be affected by energy density and fat intake. It is important to note that the definition of energy density is kcal divided by gram weight of food. However often volume is considered, but not included of the definition of energy density (14).
The modest differences in food intake in this study resulted in changes in appetite measured with the VAS and RVAS that were consistent with the decreases in energy intake compared to baseline following the HF/LED. Specifically, following overeating, the HF/LED decreased feelings of hunger and prospective food consumption but increased feelings of satisfaction compared to the HF/HED when assessed with VAS. Following overeating, the HF/LED decreased prospective food consumption compared to HF/HED with RVAS. The appetitive response changes are suggestive of decreased energy intake following the HF/LED. With the psychological questionnaires, only carbohydrate/starch craving was altered. Following the HF/HED dietary treatment greater cravings of carbohydrates and starches were reported compared to following the HC/LED but not the HF/LED likely due to the 30% reduction in carbohydrate intake during HF overfeeding..
Lastly changes in mood state were seen during this short-term intervention. The 4 ad libitum feeding days following the HF/LED were associated with less sadness (more happiness), relaxation, and tranquility whereas the days following the HF/HED were associated with more sadness, tension, and troubled feelings. These findings are consistent with previous studies in females provided an isocaloric low carbohydrate diet (higher fat) having higher tension, depression, anger, total mood score, and less vigor compared to moderate carbohydrate and high carbohydrate diets for one week (30). However, longer-term low carbohydrate (higher fat) diets with energy restriction lead to improvements in mood in most studies (31, 32) but not all (33). Overconsumption of a high energy density diet may have a particularly harmful effect on mood state. This suggests the previous findings on mood state are not macronutrient specific (i.e. fat) per se, but instead may be related to other attributes or characteristics of the diet which, while speculative, may include psychological factors or the energy density of the diet.
The alterations in energy density also changed step count and sleeping time. Specifically following overeating, the participants had decreased steps and increased lying and sleeping time suggesting greater sedentary behavior. Thus step counts actually went in the opposite direction of adaptive thermogenesis. Only the HF/HED dietary treatment increased sleeping time. This suggests short-term overeating as well as high energy density diets negatively affect sleep. The finding that obesity is associated with decreased wakefulness has previously been shown in mice (34). However, previously in humans increased wakefulness has been associated with obesity (35) but these data rely on self-report. With isocaloric feeding, very low carbohydrate diets (high fat diets) do increase slow wave sleep compared to a control mixed diet (36). Our data critically evaluated sleep with the validated Sensewear Armband and suggested that after short-term HF/HED overeating participants increase sleep time compared to baseline and LEDs.
Previously, Levine et al. found that overeating causes an increase in spontaneous physical activity (SPA) (37). Levine et al. overfed volunteers 1000 kcal for 8 weeks (20% protein, 40% carbohydrate and 40% fat). SPA was the main determinant of fat gain during overeating with an average increase of 336 kcal/d. However other studies have failed to reproduce this effect (38). He et al. performed a 3 day overeating study where participants consumed 150% of weight maintenance energy requirements (overeating) followed by 3 days of ad libitum feeding. No differences in SPA or energy expenditure were detected between weight maintenance and overeating. Murgatroyd et al. imposed physical activity following a 21% overeating with 35 or 60% energy consumed as dietary fat. Imposed physical activity (i.e. exercise not SPA) helped prevent positive energy balance (39). Similar to the current study, SPA was not affected by 3 day overeating (25% overeating) in obesity prone and obesity resistant humans (40). Thus the current study’s short physiologic energy imbalances are suggestive of lack of an expenditure compensation (i.e. SPA) that may be leading to increasing body weight gain in adults.
This study had numerous strengths. The patients were housed on the inpatient unit. Chances of dietary alterations or additions were thus negligible because it was a controlled feeding and physical activity study. To our knowledge, we are among the first to control physical activity (the energy expenditure side of energy balance) during overeating, resulting in a precise positive energy balance that was the same across the diet conditions. A potential but necessary weakness was that the baseline feeding was not randomized. Baseline feeding was necessary in order to estimate energy intake for overeating. However as previously described, the 3 overeating diets were randomized among participants. Also as described in the statistical methods, order was included in the mixed model and found to be non-significant for all parameters. Thus the authors do not feel an adaptation period was a study weakness. Another potential weakness was that participants were not exposed to the high carbohydrate high energy density treatment (HC/HED), though this condition was included in previous studies, whereas we are among the first to include high fat diets that were low and high in energy density. Also the pilot study assessed palatability but sweetness was not specifically measured across the diets. This may have altered the mood and cravings of volunteers. Lastly, a possible study weakness was literature from studies including children was excluded. The authors did not feel differences between children and adults on an inpatient unit lent itself well to comparisons. Hence the results from this study are not generalizable to children.
In conclusion, following 2 days of overeating, energy expenditure was not altered. However, food intake was non-significantly reduced on day 2 of ad libitum feeding which corresponded with a reduction in carbohydrate and fat intake. The reduction in carbohydrate and fat intake may be a physiological effort to correct for the previous positive energy balance. Treatment had no effect over the 4 day ad libitum feeding period but the HF/LED reduced energy intake compared to baseline on days 2, 3, and 4 suggesting that overeating may shorten the time necessary for the corrective response with energy intake. These modest food intake changes did alter appetitive responses. The HF/LED decreased carbohydrate and starch cravings, lying time, and sleep time compared to the high energy density version of the high fat diet despite identical macronutrient composition. Thus the current study’s short physiologic duration of energy imbalances like on weekends or holidays are suggestive of an incomplete energy compensation with no spontaneous energy expenditure compensation. This may be leading to increasing body weight gain in adults.
Supplementary Material
What is already known about this subject
Body weight has been found to increase during weekends and holidays, suggesting that people do not compensate after short periods of overeating.
Physical activity is thought to be loosely positively coupled with energy intake.
Lowering energy density decreases dietary energy intake. Previously, women reduced energy intake following a low fat low energy dense diet compared to a similar low fat high energy dense diet.
What this study adds
We control for energy expenditure during overeating and monitor energy expenditure before, during, and after overeating.
We control overeating with high fat and high carbohydrate diets that varied in energy density. To our knowledge, this is the first time a high-fat high energy density diet (HF/HED) has been examined.
Following overeating, a lack of adaptive thermogenesis was seen. Step count decreased after the high carbohydrate low energy density diet (HC/LED) and HF/HED. Also following overeating, sleep time increased in the HF/HED and increased on day 1 following overeating.
Acknowledgements
The authors would like to thank the participants, PBRC metabolic kitchen and PBRC inpatient unit. The authors responsibilities were as follows: GAB, MTH, CMC, and CKM designed research; TWZ conducted research; HH analyzed data; JWA and CKM wrote the paper; and JWA and CKM had primary responsibility for final content.
The study was supported by USDA 2010-34323-21052, NIH K23 DK 068052, and NIH P30 DK072476.
This study was registered at www.clinicaltrials.gov and numbered: NCT 01653886 (pilot study) and NCT 01653145 (main study).
Footnotes
Disclosure: The authors have no conflicts of interest and the authors have no competing interests.
References
- 1.Diseases NIDDK, editor. Overweight and Obesity Statistics. WIN Weight-control Information Network Bethesda. 2010. pp. 1–7. [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 3.Yanovski JA, Yanovski SZ, Sovik KN, Nguyen TT, O'Neil PM, Sebring NG. A prospective study of holiday weight gain. N Engl J Med. 2000;342:861–867. doi: 10.1056/NEJM200003233421206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Racette SB, Weiss EP, Schechtman KB, Steger-May K, Villareal DT, Obert KA, et al. Influence of weekend lifestyle patterns on body weight. Obesity (Silver Spring) 2008;16:1826–1830. doi: 10.1038/oby.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, et al. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PloS one. 2011;6:e19657. doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edholm OG, Fletcher JG, Widdowson EM, McCance RA. The energy expenditure and food intake of individual men. The British journal of nutrition. 1955;9:286–300. doi: 10.1079/bjn19550040. [DOI] [PubMed] [Google Scholar]
- 7.Blundell JE, King NA. Effects of exercise on appetite control: loose coupling between energy expenditure and energy intake. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1998;22(Suppl 2):S22–S29. [PubMed] [Google Scholar]
- 8.Westerterp KR. Physical activity, food intake, and body weight regulation: insights from doubly labeled water studies. Nutrition reviews. 2010;68:148–154. doi: 10.1111/j.1753-4887.2010.00270.x. [DOI] [PubMed] [Google Scholar]
- 9.Martin CK, Williamson DA, Geiselman PJ, Walden H, Smeets M, Morales S, et al. Consistency of food intake over four eating sessions in the laboratory. Eat Behav. 2005;6:365–372. doi: 10.1016/j.eatbeh.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Bray GA, Flatt JP, Volaufova J, Delany JP, Champagne CM. Corrective responses in human food intake identified from an analysis of 7-d food-intake records. Am J Clin Nutr. 2008;88:1504–1510. doi: 10.3945/ajcn.2008.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Castro JM. Prior day's intake has macronutrient-specific delayed negative feedback effects on the spontaneous food intake of free-living humans. J Nutr. 1998;128:61–67. doi: 10.1093/jn/128.1.61. [DOI] [PubMed] [Google Scholar]
- 12.Saris WHM. Limits of human endurance: lessons from the Tour de France. In: Kinney JM, Tucker HN, editors. Physiology, stress, and malnutrition: functional correlates, nutritional intervention. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 451–462. [Google Scholar]
- 13.Rolls BJ, Bell EA, Thorwart ML. Water incorporated into a food but not served with a food decreases energy intake in lean women. Am J Clin Nutr. 1999;70:448–455. doi: 10.1093/ajcn/70.4.448. [DOI] [PubMed] [Google Scholar]
- 14.Westerterp-Plantenga MS. Analysis of energy density of food in relation to energy intake regulation in human subjects. Br J Nutr. 2001;85:351–361. doi: 10.1079/bjn2000272. [DOI] [PubMed] [Google Scholar]
- 15.Rolls BJ, Bell EA, Castellanos VH, Chow M, Pelkman CL, Thorwart ML. Energy density but not fat content of foods affected energy intake in lean and obese women. Am J Clin Nutr. 1999;69:863–871. doi: 10.1093/ajcn/69.5.863. [DOI] [PubMed] [Google Scholar]
- 16.Tremblay A, Plourde G, Despres JP, Bouchard C. Impact of dietary fat content and fat oxidation on energy intake in humans. The American journal of clinical nutrition. 1989;49:799–805. doi: 10.1093/ajcn/49.5.799. [DOI] [PubMed] [Google Scholar]
- 17.Stubbs RJ, Harbron CG, Murgatroyd PR, Prentice AM. Covert manipulation of dietary fat and energy density: effect on substrate flux and food intake in men eating ad libitum. Am J Clin Nutr. 1995;62:316–329. doi: 10.1093/ajcn/62.2.316. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Brown GK, Steer RA. Beck Depression Inventory-II. San Antonio: Psychological Corporation; 1996. [Google Scholar]
- 19.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 20.Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr. 1996;50:72–92. [PubMed] [Google Scholar]
- 21.Fruin ML, Rankin JW. Validity of a multi-sensor armband in estimating rest and exercise energy expenditure. Med Sci Sports Exerc. 2004;36:1063–1069. doi: 10.1249/01.mss.0000128144.91337.38. [DOI] [PubMed] [Google Scholar]
- 22.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 23.Womble LG, Wadden TA, Chandler JM, Martin AR. Agreement between weekly vs. daily assessment of appetite. Appetite. 2003;40:131–135. doi: 10.1016/s0195-6663(02)00170-8. [DOI] [PubMed] [Google Scholar]
- 24.Bond A, Lader M. The use of analogue scales in rating subjective feelings. British Journal of Medical Psychology. 1974;47:211–218. [Google Scholar]
- 25.White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obes Res. 2002;10:107–114. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 27.Roberts SB, Fuss P, Heyman MB, Evans WJ, Tsay R, Rasmussen H, et al. Control of food intake in older men. JAMA : the journal of the American Medical Association. 1994;272:1601–1606. doi: 10.1001/jama.1994.03520200057036. [DOI] [PubMed] [Google Scholar]
- 28.Galgani JE, de Jonge L, Most MM, Bray GA, Smith SR. Effect of a 3-day high-fat feeding period on carbohydrate balance and ad libitum energy intake in humans. International journal of obesity. 2010;34:886–891. doi: 10.1038/ijo.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell EA, Rolls BJ. Energy density of foods affects energy intake across multiple levels of fat content in lean and obese women. Am J Clin Nutr. 2001;73:1010–1018. doi: 10.1093/ajcn/73.6.1010. [DOI] [PubMed] [Google Scholar]
- 30.Keith RE, O'Keeffe KA, Blessing DL, Wilson GD. Alterations in dietary carbohydrate, protein, and fat intake and mood state in trained female cyclists. Medicine and science in sports and exercise. 1991;23:212–216. [PubMed] [Google Scholar]
- 31.Halyburton AK, Brinkworth GD, Wilson CJ, Noakes M, Buckley JD, Keogh JB, et al. Low- and high-carbohydrate weight-loss diets have similar effects on mood but not cognitive performance. The American journal of clinical nutrition. 2007;86:580–587. doi: 10.1093/ajcn/86.3.580. [DOI] [PubMed] [Google Scholar]
- 32.McClernon FJ, Yancy WS, Jr., Eberstein JA, Atkins RC, Westman EC. The effects of a low-carbohydrate ketogenic diet and a low-fat diet on mood, hunger, and other self-reported symptoms. Obesity (Silver Spring) 2007;15:182–187. doi: 10.1038/oby.2007.516. [DOI] [PubMed] [Google Scholar]
- 33.Brinkworth GD, Buckley JD, Noakes M, Clifton PM, Wilson CJ. Long-term effects of a very low-carbohydrate diet and a low-fat diet on mood and cognitive function. Arch Intern Med. 2009;169:1873–1880. doi: 10.1001/archinternmed.2009.329. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins JB, Omori T, Guan Z, Vgontzas AN, Bixler EO, Fang J. Sleep is increased in mice with obesity induced by high-fat food. Physiology & behavior. 2006;87:255–262. doi: 10.1016/j.physbeh.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165:25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 36.Afaghi A, O'Connor H, Chow CM. Acute effects of the very low carbohydrate diet on sleep indices. Nutr Neurosci. 2008;11:146–154. doi: 10.1179/147683008X301540. [DOI] [PubMed] [Google Scholar]
- 37.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 38.He J, Votruba S, Pomeroy J, Bonfiglio S, Krakoff J. Measurement of ad libitum food intake, physical activity, and sedentary time in response to overfeeding. PLoS One. 2012;7:e36225. doi: 10.1371/journal.pone.0036225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murgatroyd PR, Goldberg GR, Leahy FE, Gilsenan MB, Prentice AM. Effects of inactivity and diet composition on human energy balance. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1999;23:1269–1275. doi: 10.1038/sj.ijo.0801062. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt SL, Harmon KA, Sharp TA, Kealey EH, Bessesen DH. The effects of overfeeding on spontaneous physical activity in obesity prone and obesity resistant humans. Obesity. 2012;20:2186–2193. doi: 10.1038/oby.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.