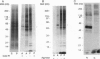Abstract
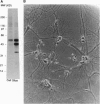
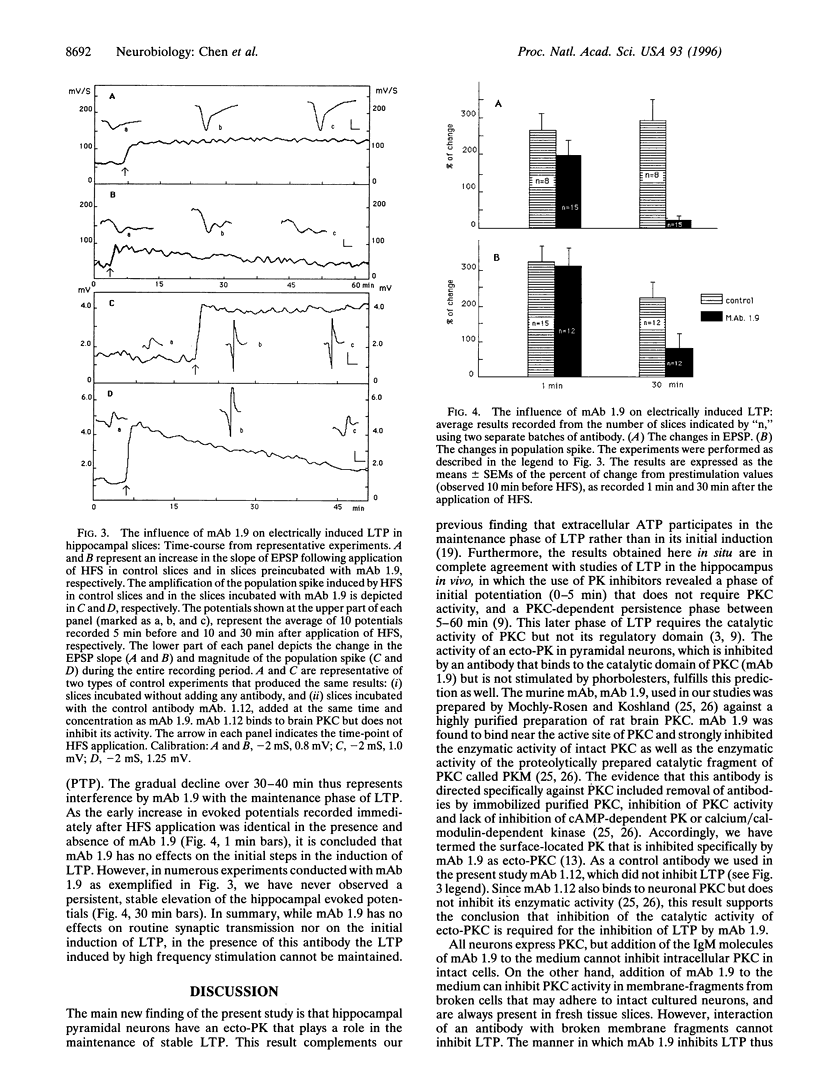
During the induction of long-term potentiation (LTP) in hippocampal slices adenosine triphosphate (ATP) is secreted into the synaptic cleft, and a 48 kDa/50 kDa protein duplex becomes phosphorylated by extracellular ATP. All the criteria required as evidence that these two proteins serve as principal substrates of ecto-protein kinase activity on the surface of hippocampal pyramidal neurons have been fulfilled. This phosphorylation activity was detected on the surface of pyramidal neurons assayed after synaptogenesis, but not in immature neurons nor in glial cells. Addition to the extracellular medium of a monoclonal antibody termed mAb 1.9, directed to the catalytic domain of protein kinase C (PKC), inhibited selectively this surface protein phosphorylation activity and blocked the stabilization of LTP induced by high frequency stimulation (HFS) in hippocampal slices. This antibody did not interfere with routine synaptic transmission nor prevent the initial enhancement of synaptic responses observed during the 1-5 min period immediately after the application of HFS (the induction phase of LTP). However, the initial increase in the slope of excitatory postsynaptic potentials, as well as the elevated amplitude of the population spike induced by HFS, both declined gradually and returned to prestimulus values within 30-40 min after HFS was applied in the presence of mAb 1.9. A control antibody that binds to PKC but does not inhibit its activity had no effect on LTP. The selective inhibitory effects observed with mAb 1.9 provide the first direct evidence of a causal role for ecto-PK in the maintenance of stable LTP, an event implicated in the process of learning and the formation of memory in the brain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Liu I., Briccetti F. M., Barnwell J. W., Kwakye-Berko F., Dokun A., Goldberger J., Pernambuco M. Analysis of CD36 binding domains: ligand specificity controlled by dephosphorylation of an ectodomain. Science. 1993 Nov 26;262(5138):1436–1440. doi: 10.1126/science.7504322. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley P. A., Sheu F. S., Routtenberg A. Inhibition of protein kinase C blocks two components of LTP persistence, leaving initial potentiation intact. J Neurosci. 1990 Oct;10(10):3353–3360. doi: 10.1523/JNEUROSCI.10-10-03353.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker L. V., De Graan P. N., Oestreicher A. B., Versteeg D. H., Gispen W. H. Inhibition of noradrenaline release by antibodies to B-50 (GAP-43). Nature. 1989 Nov 2;342(6245):74–76. doi: 10.1038/342074a0. [DOI] [PubMed] [Google Scholar]
- Ehrlich Y. H. Extracellular protein phosphorylation in neuronal responsiveness and adaptation. Adv Exp Med Biol. 1987;221:187–199. doi: 10.1007/978-1-4684-7618-7_14. [DOI] [PubMed] [Google Scholar]
- Ehrlich Y. H., Hogan M. V., Pawlowska Z., Naik U., Kornecki E. Ectoprotein kinase in the regulation of cellular responsiveness to extracellular ATP. Ann N Y Acad Sci. 1990;603:401–416. doi: 10.1111/j.1749-6632.1990.tb37689.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich Y. H., Rabjohns R. R., Routtenberg A. Experiential input alters the phosphorylation of specific proteins in brain membranes. Pharmacol Biochem Behav. 1977 Feb;6(2):169–174. doi: 10.1016/0091-3057(77)90068-5. [DOI] [PubMed] [Google Scholar]
- Ehrlich Y. H., Snider R. M., Kornecki E., Garfield M. G., Lenox R. H. Modulation of neuronal signal transduction systems by extracellular ATP. J Neurochem. 1988 Jan;50(1):295–301. doi: 10.1111/j.1471-4159.1988.tb13263.x. [DOI] [PubMed] [Google Scholar]
- Fujii S., Kato H., Furuse H., Ito K., Osada H., Hamaguchi T., Kuroda Y. The mechanism of ATP-induced long-term potentiation involves extracellular phosphorylation of membrane proteins in guinea-pig hippocampal CA1 neurons. Neurosci Lett. 1995 Mar 3;187(2):130–132. doi: 10.1016/0304-3940(95)11347-9. [DOI] [PubMed] [Google Scholar]
- Hardwick J. C., Ehrlich Y. H., Hendley E. D. Extracellular ATP stimulates norepinephrine uptake in PC12 cells. J Neurochem. 1989 Nov;53(5):1512–1518. doi: 10.1111/j.1471-4159.1989.tb08546.x. [DOI] [PubMed] [Google Scholar]
- Hogan M. V., Pawlowska Z., Yang H. A., Kornecki E., Ehrlich Y. H. Surface phosphorylation by ecto-protein kinase C in brain neurons: a target for Alzheimer's beta-amyloid peptides. J Neurochem. 1995 Nov;65(5):2022–2030. doi: 10.1046/j.1471-4159.1995.65052022.x. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kuroda Y., Ichikawa M., Muramoto K., Kobayashi K., Matsuda Y., Ogura A., Kudo Y. Block of synapse formation between cerebral cortical neurons by a protein kinase inhibitor. Neurosci Lett. 1992 Feb 3;135(2):255–258. doi: 10.1016/0304-3940(92)90449-h. [DOI] [PubMed] [Google Scholar]
- Malenka R. C. Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994 Aug 26;78(4):535–538. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D., Koshland D. E., Jr A general procedure for screening inhibitory antibodies: application for identifying anti-protein kinase C antibodies. Anal Biochem. 1988 Apr;170(1):31–37. doi: 10.1016/0003-2697(88)90085-1. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D., Koshland D. E., Jr Domain structure and phosphorylation of protein kinase C. J Biol Chem. 1987 Feb 15;262(5):2291–2297. [PubMed] [Google Scholar]
- Muramoto K., Taniguchi H., Kawahara M., Kobayashi K., Nonomura Y., Kuroda Y. A substrate of ecto-protein kinase is microtubule-associated protein 1B in cortical cell cultures undergoing synaptogenesis. Biochem Biophys Res Commun. 1994 Dec 15;205(2):1467–1473. doi: 10.1006/bbrc.1994.2830. [DOI] [PubMed] [Google Scholar]
- Neary J. T., Crow T., Alkon D. L. Change in a specific phosphoprotein band following associative learning in Hermissenda. Nature. 1981 Oct 22;293(5834):658–660. doi: 10.1038/293658a0. [DOI] [PubMed] [Google Scholar]
- Nelson T. J., Collin C., Alkon D. L. Isolation of a G protein that is modified by learning and reduces potassium currents in Hermissenda. Science. 1990 Mar 23;247(4949 Pt 1):1479–1483. doi: 10.1126/science.247.4949.1479. [DOI] [PubMed] [Google Scholar]
- Pawlowska Z., Hogan M. V., Kornecki E., Ehrlich Y. H. Ecto-protein kinase and surface protein phosphorylation in PC12 cells: interactions with nerve growth factor. J Neurochem. 1993 Feb;60(2):678–686. doi: 10.1111/j.1471-4159.1993.tb03201.x. [DOI] [PubMed] [Google Scholar]
- Ross A. H., McKinnon C. A., Daou M. C., Ratliff K., Wolf D. E. Differential biological effects of K252 kinase inhibitors are related to membrane solubility but not to permeability. J Neurochem. 1995 Dec;65(6):2748–2756. doi: 10.1046/j.1471-4159.1995.65062748.x. [DOI] [PubMed] [Google Scholar]
- Sacktor T. C., Osten P., Valsamis H., Jiang X., Naik M. U., Sublette E. Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol. 1975 May;247(1):145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler T. J., DiScenna P. Long-term potentiation. Annu Rev Neurosci. 1987;10:131–161. doi: 10.1146/annurev.ne.10.030187.001023. [DOI] [PubMed] [Google Scholar]
- Wieraszko A., Ehrlich Y. H. On the role of extracellular ATP in the induction of long-term potentiation in the hippocampus. J Neurochem. 1994 Nov;63(5):1731–1738. doi: 10.1046/j.1471-4159.1994.63051731.x. [DOI] [PubMed] [Google Scholar]
- Wieraszko A., Goldsmith G., Seyfried T. N. Stimulation-dependent release of adenosine triphosphate from hippocampal slices. Brain Res. 1989 Apr 24;485(2):244–250. doi: 10.1016/0006-8993(89)90567-2. [DOI] [PubMed] [Google Scholar]
- Wieraszko A., Seyfried T. N. ATP-induced synaptic potentiation in hippocampal slices. Brain Res. 1989 Jul 10;491(2):356–359. doi: 10.1016/0006-8993(89)90070-x. [DOI] [PubMed] [Google Scholar]