Abstract
Background
Laryngeal squamous cell carcinoma (LSCC) is the most common type in head and neck squamous cell carcinoma (HNSCC), and the development and progression of LSCC are multistep processes accompanied by changes of molecular biology.
Objective
The purpose of this study was to investigate the molecular basis of tumorigenesis and regional lymph node metastasis in LSCC, and provide a set of genes that may be useful for the development of novel diagnostic markers and/or more effective therapeutic strategies.
Methods
A total number of 10 patients who underwent surgery for primary laryngeal squamous cell carcinoma were recruited for microarray analysis. LSCC tissues compared with corresponding adjacent non-neoplastic tissues were analysed by Illumina mRNA microarrays, and LSCC tissues with regional lymph node metastasis and LSCC tissues without regional lymph node metastasis were analyzed in the same manner. The most frequently differently expressed genes screened by microarrays were also validated by qRT-PCR in another 42 patients diagnosed for LSCC.
Results
Analysed by Illumina mRNA microarrays, there were 361 genes significantly related to tumorigenesis while 246 genes significantly related to regional lymph node metastasis in LSCC. We found that the six genes (CDK1, CDK2, CDK4, MCM2, MCM3, MCM4) were most frequently differently expressed functional genes related to tumorigenesis while eIF3a and RPN2 were most frequently differently expressed functional genes related to regional lymph node metastasis in LSCC. The expressions of these genes were also validated by qRT-PCR.
Conclusions
The research revealed a gene expression signature of tumorigenesis and regional lymph node metastasis in laryngeal squamous cell carcinoma. Of the total, the deregulation of several genes (CDK1, CDK2, CDK4, MCM2, MCM3, MCM4, EIF3a and RPN2) were potentially associated with disease development and progression. The result will contribute to the understanding of the molecular basis of LSCC and help to improve diagnosis and treatment.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most frequent cancer, and laryngeal squamous cell carcinoma (LSCC) is the most common type, accounting for 1% to 2% of all malignancies worldwide [1-3]. Corresponding to ~25% of HNSCC cases, of which the long-term survival rate has remained at 50%, LSCC should be drawn attention [4]. However, cervical lymph nodes metastases and distant metastases are the factors that significantly affect the prognosis in LSCC patients [5]. An important step in the process of tumor metastases is the detachment of malignant cells from their original site [6]. In normal epithelial tissues, cell–cell adhesion is mediated by a large number of cell adhesion molecules. Defective interactions between adhesion molecules play a critical role in oncogenesis and metastasis [7]. Metastasis to a regional lymph node is the first indication of tumor metastasis competence [8].The understanding of the process involving this lymphatic progression will help to handle the treatment of these aggressive tumors. Neoplastic cells with metastasis capacity acquire distinct cellular capabilities, such as the ability to proliferate without limit, to evade apoptosis, to escape immune surveillance, and to express factors that alter the growth of blood and lymphatic vessels so as to create conduits for tumor metastasis [9].
The development and progression of LSCC are multistep processes accompanied by changes of molecular biology. Various studies have revealed numerous molecular abnormalities in LSCC, including MMP-2 [10], HER2 [11], E-cadherin [12], AEG-1 [13], EGFL7 [14], NF-kappaB [15], CXCR2 [16] and so on. However, the complete array of molecular changes that occur during oncogenesis and metastasis remains elusive.
Genome-wide analysis using microarrays has emerged as an important tool for biological studies by allowing the large-scale identification and comprehensive analysis of gene expression profiles and mutation mapping. This technology has enabled discovery of large gene-sets whose altered expression levels reflect specific disease status or given treatment response, which provides clues of related biological mechanisms. The data of this technology also could be recognized as an effective method for validation of biomarkers, discovery of gene functions, and development of new drugs targeting specific genes [17]. Large-scale studies involving microarrays have identified specific gene expression signatures associated with expression changes in HNSCC tissue samples compared to normal tissue [18]. However, the microarray gene expression studies on LSCC with regional lymph node metastasis need to be further explored.
In the present study, we focus on the gene dysregulation about tumorigenesis and regional lymph node metastasis in LSCC. A number of ten laryngeal squamous cell carcinoma tissues and corresponding adjacent non-neoplastic tissues were recruited. We constructed an mRNA microarray platform containing probes for 34601 genes. Gene dysregulation related with tumorigenesis and regional lymph node metastasis was analyzed by biological analysis software, and significant and functional gene dysregulation was validated further. Our findings contribute to the understanding of the molecular basis of tumorigenesis and regional lymph node metastasis in LSCC, and provide a set of genes that may be useful for the development of novel diagnostic markers and/or more effective therapeutic strategies.
Materials and Methods
All patients we had selected were treated in Department of Head and Neck Surgery, Beijing Tongren Hospital, and all patients provided written informed consent before their participation. The Ethics Committee of Capital Medical University approval was obtained for the use of all samples by using a protocol that conforms to the provisions of the Declaration of Helsinki.
Tissue samples and patients
A total number of 10 patients (no females) who underwent surgery for primary laryngeal squamous cell carcinoma were recruited for microarray gene expression analysis, and the TNM stage of each patient was determined as Table 1 . A second group of 42 patients (no females) who underwent surgery for primary LSCC were also recruited for qRT-PCR, and the TNM stage of each patient was determined as Table 2 . The two patient cohorts used for microarrays and qRT-PCR investigations were separated. The cancer tissues and corresponding adjacent non-neoplastic tissues were collected during surgery. Each specimen was immediately snap-frozen in liquid nitrogen and stored at -80 °C for subsequent study. The pathology of all the cancer tissues were squamous cell carcinoma, which were evaluated by pathologists.
Table 1. Clinical data of patients for microarrays.
| Tumor tissues | age | T | N | M | corresponding non-neoplastic tissues |
|---|---|---|---|---|---|
| WA | 53 | 2 | 0 | 0 | WB |
| WC | 55 | 4 | 3 | 0 | WD |
| WE | 52 | 3 | 0 | 0 | WF |
| WG | 56 | 4 | 0 | 0 | WH |
| WI | 74 | 3 | 0 | 0 | WJ |
| WK | 64 | 1 | 0 | 0 | WL |
| LA | 71 | 4 | 2a | 0 | LB |
| LC | 58 | 4 | 2b | 0 | LD |
| LE | 52 | 3 | 2b | 0 | LF |
| LG | 66 | 3 | 2c | 0 | LH |
Table 2. Clinical data of patients for qRT-PCR.
| TNM | number | average age |
|---|---|---|
| T1N0M0 | 1 | 51 |
| T2N0M0 | 6 | 47 |
| T3N0M0 | 8 | 56 |
| T4N0M0 | 8 | 60 |
| T3NxM0(X≠0) | 9 | 62 |
| T4NxM0(X≠0) | 10 | 59 |
RNA extraction and quality assessment
Total RNA was extracted from tissue samples using Trizol Reagent (Invitrogen). Then the RNA quantity was determined using denaturing gel electrophoresis which produced at least 2 distinct bands representing the 28S and 18S ribosomal RNA, confirming that the total RNA was not contaminated with DNA and the RNA was not degraded.
cRNA amplification
Reverse transcription to synthesize first strand cDNA was primed with the T7 Oligo(dT) Primer to synthesize cDNA containing a T7 promoter sequence.Second Strand cDNA Synthesis converted the single-stranded cDNA into a double-stranded DNA (dsDNA) template for transcription.The reaction employed DNA Polymerase and RNase H to simultaneously degrade the RNA and synthesize second strand cDNA. Then, cDNA purification removed RNA, primers, enzymes, and salts that would inhibit in vitro transcription.After that, in vitro transcription to synthesize cRNA generated multiple copies of biotinylated cRNA from the double-stranded cDNA templates.At last, cRNA purification removed unincorporated NTPs, salts, enzymes, and inorganic phosphate. After purification, the cRNA was ready for use with Illumina’s direct hybridization array kits.
Illumina Human HT-12 BeadChip
The RNA samples which passed the quality test were hybridized with reagents for hybridization according to protocols.Hatched in room temperature,and then processed through high-temperature wash and ethanol wash.After three room- temperature washes, image could be read by the software called Illumina Bead Chip Reader. The dataset had been submitted to Gene Expression Omnibus, and the accession number was GSE51985.
Quantitative real-time PCR
The transcriptional level of the target genes were measured by qRT-PCR detection.Trizol was applied to extract total cellular RNA. Prepared Template RNA (5μl) / primer (1μl) mixture in Microtube tube. Keep in 70 °C for 10 minutes, then rapid quenched in ice no more than 5 minutes. After that, centrifuged for a few seconds so that the template RNA / primer solution of denatured aggregation gathered at the bottom of the tube Microtube. Then added 5 × M-MLV Buffer, RNase Inhibitor and dNTP Mixture preparation called reverse transcription reaction solution in the Microtube tube, 4μl totally. This solution had to keep in 42 °C for 1 hour. Cooled by ice after hold in 95 °C for 15 minutes, then we got the cDNA solution. Mixed this 1μl cDNA solution, Taq DNA Polymerase, 2XSYBR to 20μl the mixed system. Hold it in 95 °C for 5 mins for denaturating, then followed by 45 cycles totally which were keeping in 95 °C for 30s, keeping in 65 °C for 30s, and keeping in 72 °C for 5 mins. The gene expression levels were determined based on Livak method [19].The results that 2-ΔΔCT values of all samples were analysed automatically by computer control with β-actin gene as an internal reference. The primer sequences as Table 3 :
Table 3. Primer sequences.
| Gene | Primer Sequence |
|---|---|
| β-actin | F:GTGAAGGTGACAGCAGTCGGTT |
| R:AGTGGGGTGGCTTTTAGGA | |
| CDK1 | F:CTTTTATGTCTTGCTTAAGT |
| R:CATAATTCTAAATAAAACTG | |
| CDK2 | F:CTCCTACCCCATAGGAGTTAG |
| R:GTCCAATATAGGTAATCATC | |
| CDK4 | F:CTTTCCTGCAAAACCTTAAAG |
| R:GGACTCCAGTCCTCAAGCTCTG | |
| MCM2 | F:CCTCTGTGCTTTATGGACAC |
| R:GGAGGCTCACGAAACAGAGG | |
| MCM3 | F:GGTGATGAAGCTGAGTTCAGG |
| R:CTGAAGACTCATGAAAACCC | |
| MCM4 | F:GTATTTTTTGGTAGAGACGGCTTC |
| R:GTGACGTGGGTCGGAAAC | |
| EIF3a | F:GTAAACATTACAAACATTGG |
| R:GCGTTCACACTTAGGTTTGTC | |
| RPN2 | F:GGACTCAGCTCAACATGTTCCAG |
| R:GATGCTTGTCGTGTAATCAAGG |
Microarray analysis of laryngeal squamous cell carcinoma tissues VS corresponding adjacent non-neoplastic tissues
Illumina Genomestudio-Gene Expression software was used for background correction and missing value difference processing,and then the data was normalized by quantitle method. Illumina Custom software was used for analyzing different gene expression.To reduce the false positive rate, SAM method was chosen for analyzing the different gene expression further by Mev software (FDR<0.05 was chosen). Paired t tests were used for analysis, and differences were considered statistically significant at P-value <0.05.
Microarray analysis of LSCC tissues with regional lymph node metastasis VS cancer tissues without regional lymph node metastasis
Independent sample t tests were used for analysis, and other studies were conducted in the same way as the above.
Pathway analysis
Recently, pathway-based methods have been developed to be adopted in the development of a disease or some other physiological process. Pathway-based methods are powerful tools that can give new insights into various biological phenomena from the system or functional levels [20]. The GO database provides a controlled vocabulary of terms to define biological descriptors (GO categories) and to support biologically meaningful annotation of gene products [21], while the KEGG pathways database has been widely used for the systematic analysis of gene functions that involve networks of molecular interactions in cells [22].
In this study, GO categories and KEGG pathways were used to identify pathways with considerable enrichment of the genes by on-line analysis called “Web-Based Gene Set Analysis Toolkit”. P-value was calculated using hypergeometric distribution and the cut-off was set at < 0.05.
Statistical analysis for qRT-PCR
All data were imported to SPSS 20.0.The data which did not meet normality would be conversed into normality. Independent sample t tests or paired sample t tests were used for analysis involving two samples. Differences were considered statistically significant at P-value <0.05.
Results
Gene expression analysis of tumorigenesis in LSCC
Expression analysis using the mRNA microarrays was initially performed on 10 laryngeal squamous cell carcinoma tissues and their corresponding adjacent non-neoplastic tissues. Of the 34601 genes analyzed, 361 genes showed statistically significant differences in the expression between LSCC tissues and corresponding non-neoplastic tissues (P <0.05). Among these 361genes, 232 showed a higher expression in tumor than in non-tumor tissue, and 129 presented the contrasting pattern. Supervised hierarchical clustering analysis revealed that the expression patterns of the selected set of 361 differentially expressed genes were able to perfectly distinguish tumors from non-neoplastic tissues in the set of samples, which suggested heterogeneity between cancer cases and normal tissues. The result was shown below in Figure 1 and Figure 2.
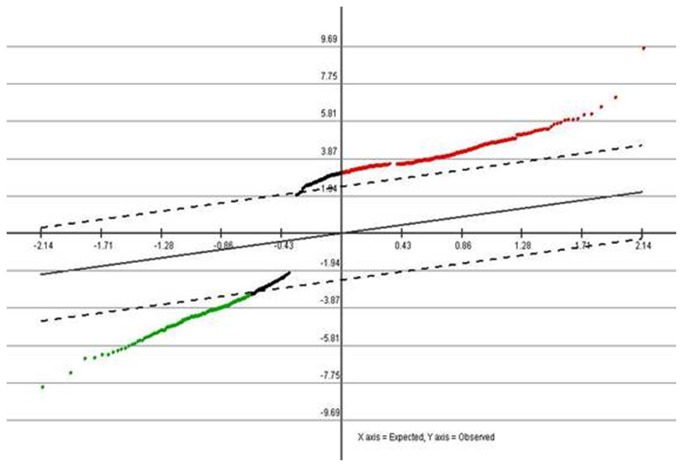
Figure 1. SAM Graph of genes related to tumorigenesis in LSCC.
Red points indicated higher expression genes(P<0.05) and green points indicated lower expression genes(P<0.05) across the 361 samples.
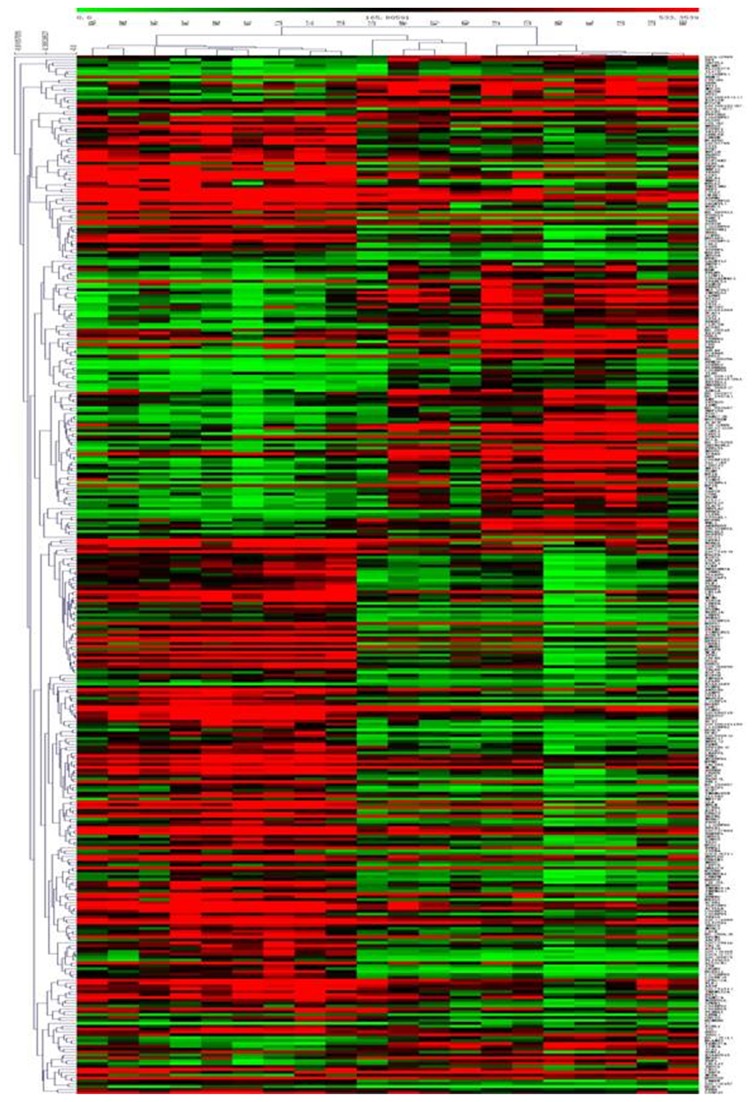
Figure 2. Hierarchical Trees: All significant genes of tumorigenesis in LSCC.
For each gene (row), red indicated a higher expression and green a lower one relative to the average level of expression of that gene across the 361 samples (columns).
To identify typical nuclear genes which might be diagnostic or therapeutic markers from the identified 361 genes, the GO database and the KEGG pathways database were used for biological process enrichment analysis. Analyzed by GO database,we observed that these genes were involved in processes such as mitosis, cell cycle phase, cell cycle process, ATP-banding, apoptosis, nuclear division and so on (P <0.05). DNA replication pathway, cell cycle pathway and p53 signaling pathway played especially important role in tumorigenesis of LSCC analyzed by KEGG pathways database(P<0.05).Of the 361 genes, we found that the six genes(CDK1, CDK2, CDK4, MCM2, MCM3, MCM4) were related with both cell cycle in GO database and DNA replication, cell cycle pathways in KEGG database. The six genes were overexpression in cancer tissues compared to adjacent non-neoplastic tissues, suggesting they might be useful target markers. The results were shown below in Table 4, Table 5 and Table 6.
Table 4. Microarray analysis of six genes between LSCC tissues and their corresponding adjacent non-neoplastic tissues.
| genes | fold change | P |
|---|---|---|
| mcm2 | 3.58 | 0.005 |
| mcm3 | 2.13 | 0.020 |
| mcm4 | 2.74 | 0.008 |
| CDK1 | 2.58 | 0.028 |
| CDK2 | 2.15 | 0.025 |
| CDK4 | 2.38 | 0.002 |
Table 5. Six genes in GO database ID.
| GO database (ID) | genes | P |
|---|---|---|
| GO:0000278 | CDK1,CDK2,CDK4,mcm2,mcm3,mcm4 | 6.68e-19 |
| GO:0022403 | CDK1,CDK2,CDK4,mcm2,mcm3,mcm4 | 1.66e-18 |
| GO:0022402 | CDK1,CDK2,CDK4,mcm2,mcm3,mcm4 | 3.75e-18 |
| GO:0007049 | CDK1,CDK2,CDK4,mcm2,mcm3,mcm4 | 2.13e-17 |
| GO:0007067 | CDK1,CDK2 | 2.71e-14 |
| GO:0000280 | CDK1,CDK2 | 2.71e-14 |
| GO:0000087 | CDK1,CDK2 | 4.75e-14 |
| GO:0000279 | CDK1,CDK2 | 3.00e-14 |
Table 6. Six genes in KEGG pathways database ID.
| KEGG pathways database (ID) | genes | P |
|---|---|---|
| Cell cycle(04110) | CDK1,CDK2,CDK4,mcm2,mcm3,mcm4 | 0.0005 |
| p53 signaling pathway(04115) | CDK1,CDK2,CDK4 | 0.00078 |
| DNA replication(03030 | mcm2,mcm3,mcm4 | 5.25e-07 |
Drug association analysis database is one of pathway-based methods in “Web-Based Gene Set Analysis Toolkit”. The analysis of the 361 genes by this database as previously introduced suggested that CDK1 was related to paclitaxel, mechlorethamine and CDK2 was also related to mechlorethamine, which indicated CDK1 and CDK2 also might be therapeutic target genes. The results of the 2 genes in the pathway analysis ID (CDK1, CDK2) were shown below in Table 7.
Table 7. CDK1,CDK2 in drug association analysis database ID.
Minichromosome maintenance proteins are essential for DNA replication in all eukaryotic cells and for restricting replication to once per cell cycle [23],and cyclin-dependent kinases (CDKs) interact at specific stages of the cell cycle to drive the cell cycle from one phase to the next [24]. To investigate whether the typical nuclear genes were able to distinguish LSCC from non-tumor larynx tissues, these 6 genes (CDK1, CDK2, CDK4, MCM2, MCM3 and MCM4) were run on qRT-PCR for a subset of 42 cancer tissues and their adjacent non-neoplastic tissues. Compared with non-tumor larynx tissues, the mRNA expression levels of the 6 genes in LSCC tissues were statistically different (P <0.05), and the results of cancer tissues were significantly up-regulated. The result was shown below in Table 8.
Table 8. QRT-PCR analysis between LSCC tissues and their corresponding adjacent non-neoplastic tissues (paired sample t tests).
| genes | relative mRNA expression levels |
||
|---|---|---|---|
| carcinoma tissues | non-neoplastic tissues | P | |
| mcm2 | 1.57 ±1.02 | 1.05±0.62 | 0.037 |
| mcm3 | 1.12±0.84 | 0.96±0.71 | 0.045 |
| mcm4 | 1.61±0.92 | 1.08±0.54 | 0.012 |
| CDK1 | 2.53±1.23 | 1.52±0.98 | 0.005 |
| CDK2 | 2.78±1.65 | 1.15±0.74 | 0.005 |
| CDK4 | 2.92±1.22 | 1.02±0.60 | 0.001 |
Gene expression analysis of regional lymph node metastasis in LSCC
The LSCC tissues with regional lymph node metastasis(5 samples) and LSCC tissues without regional lymph node metastasis (5 samples) were compared, and the methods were introduced in the previous paragraph. In this study, 246 genes showed statistically significant differences in the expression with regional lymph node metastasis (P <0.05). Among these genes, 13 genes showed a higher expression in tumors with regional lymph node metastasis, while 233 presented the contrasting pattern. Two subgroups of tumor samples were distinguishable in the training set based on the expression profile of the different genes, which suggested regional lymph node metastasis was affected by gene regulation in LSCC. The result is shown below in Figure 3 and Figure 4.
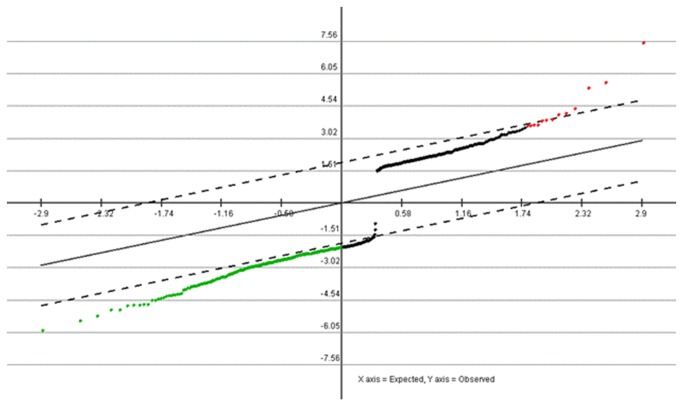
Figure 3. SAM Graph of genes related to regional lymph node metastasis in LSCC.
Red points indicated higher expression genes (P<0.05) and green points indicated lower expression genes (P<0.05) across the 246 samples.
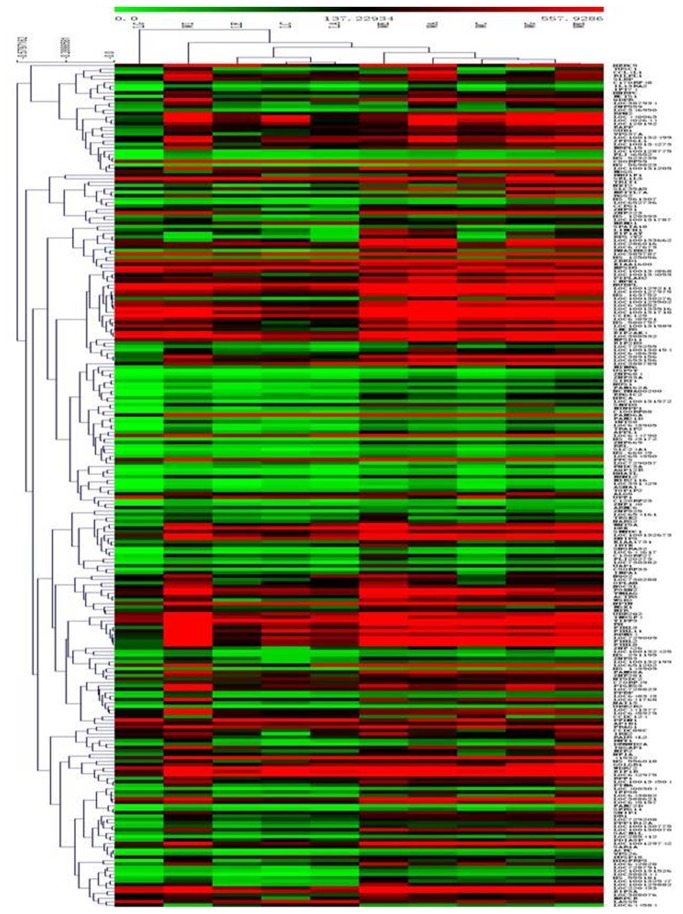
Figure 4. Hierarchical Trees:All significant genes of regional lymph node metastasis in LSCC.
For each gene (row), red indicated a higher expression and green a lower one relative to the average level of expression of that gene across the 246 samples (columns). To identify typical nuclear genes which might be therapeutic markers from the identified 246 genes, the GO database and the KEGG pathways database were also used for biological process enrichment analysis. Analyzed by GO database, we observed that these genes were involved in processes such as cellular macromolecule metabolic process, translation, organic substance biosynthetic process, biosynthetic process, cellular metabolic process, RNA binding and so on (P <0.05). RNA transport pathway and N-Glycan biosynthesis pathway played especially important role in regional lymph node metastasis of LSCC analyzed by KEGG pathways database(P<0.05). Of the 246 genes, we found that the genes such as eIF3a, eIF2b3, RPN2, UPF1, ALG3 were statistically related with regional lymph node metastasis of LSCC by the analysis of GO database and KEGG database. Among the significantly related genes, eIF3a and RPN2 were extremely representative genes in the two functional pathways analyzed by KEGG, and the expression levels in regional lymph node metastasis tissues were significantly down-regulated by mRNA microarrays analysis (The results are shown in Table 9, Table 10 and Table 11). Subsequently, qRT-PCR was used for validation.
Table 9. Microarray analysis of two genes between tissues with regional lymph node metastasis or not.
| genes | fold change | P |
|---|---|---|
| eIF3a | 0.28 | 0.028 |
| RPN2 | 0.47 | 0.035 |
Table 10. Two genes in GO database ID.
| GO database (ID) | genes | P |
|---|---|---|
| GO:0044260 | eIF3a, RPN2 | 0.0003 |
| GO:0006412 | eIF3a, RPN2 | 0.0003 |
| GO:0044267 | eIF3a, RPN2 | 0.0020 |
| GO:1901576 | eIF3a, RPN2 | 0.0016 |
| GO:0009058 | eIF3a | 0.0022 |
| GO:0044237 | eIF3a, RPN2 | 0.0017 |
| GO:0003743 | eIF3a | 0.0242 |
| GO:0004576 | RPN2 | 0.0019 |
Table 11. Two genes in KEGG pathways database ID.
| KEGG pathways database (ID) | genes | P |
|---|---|---|
| RNA transport(03013) | eIF3a | 0.0021 |
| N-Glycan biosynthesis (00510) | RPN2 | 0.0089 |
EIF3a, the largest subunit of eIF3 complex(translation initiation factor), has been shown to play a role in regulating synthesis of proteins including a-tubulin, ribonucleotide reductase M2, and p27 as well as in cell proliferation, cell cycle control and cell differentiation [25,26]. RPN2, part of an N-oligosaccharyl transferase complex, affects cell apoptosis and cell growth [27]. EIF3a and RPN2 were run on qRT-PCR for a subset of the LSCC tissues with regional lymph node metastasis (19 samples) and those without regional lymph node metastasis (23 samples). Compared with non-regional lymph node metastasis cancer tissues, the mRNA expression levels of the 2 genes in regional lymph node metastasis cancer tissues were both statistically different (P <0.05),and the results in regional lymph node metastasis cancer tissues were significantly down-regulated. The results of the 2 genes are shown in Table 12. Because both eIF3a and RPN2 reacted to docetaxel [28,29], they might be useful target markers which should be payed sufficient attention.
Table 12. QRT-PCR analysis between tissues with regional lymph node metastasis or not (independent sample t tests).
| Genes | relative mRNA expression levels |
||
|---|---|---|---|
| metastasis | no metastasis | P | |
| eIF3a | 0.85 ±0.52 | 1.17±0.74 | 0.038 |
| RPN2 | 0.76±0.50 | 0.98±0.61 | 0.042 |
Discussion
The mRNA microarray is proving to be a valuable resource for biomarker identification. In this study, we found a substantial number of new differentially expressed genes affecting tumorigenesis and regional lymph node metastasis in laryngeal squamous cell carcinoma at an FDR ≤ 0.05. Illumina Human HT-12 BeadChip was chosen, as it could detect the expression levels of 34601 genes in our clinical samples. This is the first research involving mRNA microarray analysis to determine gene expression changes during disease development and progression in LSCC.
We identified 361 genes as differentially regulated in LSCC tissues as compared to corresponding non-neoplastic tissues. Among these 361genes, 232 showed a higher expression in tumor than in non-tumor tissue, and 129 presented the contrasting pattern. The differentially expressed genes were mainly involved in processes such as mitosis, cell cycle phase, cell cycle process, ATP-banding, apoptosis, nuclear division and so on. At the molecular level, six genes (CDK1, CDK2, CDK4, MCM2, MCM3 and MCM4) were the most frequently selected genes affecting tumorigenesis in LSCC, and they were also validated by qRT-PCR. MCM2, MCM3 and MCM4 are minichromosome maintenance proteins, which are essential for DNA replication in all eukaryotic cells and for restricting replication to once per cell cycle [23]. Minichromosome maintenance protein (MCM) is a family of six highly conserved and highly homologous proteins (MCM2-7). The MCM2-7 polypeptides form a functional hexameric complex [30] that comprises an important part of the ‘prereplicative complex’ of replication proteins at replication origins during the G1 phase. The protein then irreversibly dissociates to ensure that DNA synthesis is initiated only once during each cell cycle [31] and not evident in quiescent, differentiated and senescent cells, and all of the six MCM proteins show similar and comparable expressions in a range of tissue sections [32]. In previous study, MCM2, MCM3 and MCM4 were dysregulated in malignant salivary gland tumours [33], gastric cardiac cancer [34], thyroid malignancy [35], non-small cell lung cancer [36], malignant melanoma [37], colon cancer, promyelocytic leukemia [38], cervical squamous cell carcinoma [39]. In our research, the high expression of the 3 genes consequently contributed to larynx carcinogenesis, which suggested they might be useful target markers.
It is also known that cyclin-dependent kinases (CDKs) interact at specific stages of the cell cycle to drive the cell cycle from one phase to the next in cells. For example, CDK1/Cyclin B complex plays an important role for regulation of G2/M phase [40,41]. CDK2-cyclin E complex is known to initiate both DNA replication and centrosome duplication during the G1-S transition in the cell cycle [24]. Constitutive expression of CDK4 results in hyperphosphorylation of Rb and increased E2F activity, leading to inappropriate progression through the G1/S phase of the cell cycle [40]. In previous study, the genes (CDK1, CDK2 and CDK4) were dysregulated in breast cancer [42], ovarian cancer [43], colon cancer [44], hepatocellular carcinoma [45], thyroid carcinoma [46], and lung cancer [47]. We also found that the high expression of CDK1, CDK2 and CDK4, part of cyclin-dependent kinases, were related to tumorigenesis in LSCC, and the results were validated by qRT-PCR. They were also useful target markers related to tumorigenesis as the genes (MCM2, MCM3 and MCM4) mentioned previously in the study.
Moreover, analyzed by drug association database, CDK1 was related to paclitaxel, mechlorethamine and CDK2 was related to mechlorethamine. This result indicated that CDK1 and CDK2 also might be therapeutic target genes.
We also investigated the genes related to regional lymph node metastasis besides tumorigenesis. Regional lymph node metastasis plays an important role as a prognostic factor in laryngeal squamous cell carcinoma. Research has been carried out for many years to pinpoint the factors, which facilitate spreading of the tumor into lymph nodes. However, it is still difficult to give explicit results [48]. This study used by microarrays analysis revealed that some functional molecules were crucial for malignant cells to metastasize in molecular biology. The LSCC tissues with regional lymph node metastasis those without regional lymph node metastasis were compared, and 246 genes were identified as differentially regulated. Among these genes, 13 genes showed a higher expression in tumors with regional lymph node metastasis, while 233 presented the contrasting pattern. Being different from the genes related to tumorigenesis, these genes were mainly involved in processes such as cellular macromolecule metabolic process, translation, organic substance biosynthetic process, biosynthetic process, cellular metabolic process, RNA binding and so on. The result indicated that the basis of molecular biology was different between tumorigenesis and regional lymph node metastasis in laryngeal squamous cell carcinoma, which suggested that disease development and progression of LSCC were differently progressive processes. Analysed by GO database and KEGG pathways database, eIF3a and RPN2 which were low-expression in regional lymph node metastasis tissues were the most frequently selected genes affecting regional lymph node metastasis in our study and validated by qRT-PCR. As the function of eIF3a and RPN2 had been previously discussed, they need to draw sufficient attention.
EIF3a appeared to be essential for cancer cells to maintain malignant phenotype. Suppressing endogenous eIF3a expression had been shown to reverse the malignant phenotype of human cancer cells and overexpression of eIF3a had been found in many cancers such as cancers of lung, breast, cervix, stomach, and esophagus [49]. However, it has been observed previously that cervical and esophageal cancer patients with high eIF3a level had better relapse-free and overall survival than those with low eIF3a expression [50,51]. Moreover, when human lung cancer A549 cells were treated with high concentration of docetaxel, the expression level of eIF3a mRNA tended to increase in a time-depend manner. Docetaxe could slightly increase the expression level of eIF3a mRNA [28]. EIF3a upregulation in lung cancer patients also correlated with their response to platinum-based chemotherapy and contributed to increased cisplatin (cis-dichlorodiammine platinum(II) (CDDP)) sensitivity [52]. These observations suggest that eIF3a has an important role in cancer cell response to chemotherapeutics, possibly by regulating gene expression.RPN2 may also have an significant role in cancer cell response to chemotherapeutics. Recently, Honma et al [27] reported that downregulation of ribophorin II (RPN2)) promoted docetaxel-dependent apoptosis and cell growth inhibition of MCF7-ADR human breast cancer cells that are resistant to docetaxel It also has been found that RPN2 suppression increased sensitivity to docetaxel in oesophageal squamous cell carcinoma in vitro [53]. Considering our detection, expression of RPN2 in biopsy specimens could be a useful predictive marker for response to neoadjuvant chemotherapy in LSCC.
Conclusion
In conclusion, our microarray analysis revealed a gene expression signature of tumorigenesis and regional lymph node metastasis in laryngeal squamous cell carcinoma, and several genes whose deregulation is potentially associated with disease development and progression were validated by qRT-PCR. Further studies of the genes are required to explore the specific mechanisms and evaluate the clinical application values. Our findings will contribute to the understanding of the molecular basis of laryngeal squamous cell carcinoma, thus helping to improve diagnosis and treatment.
Funding Statement
Sponsored by National Natural Science Foundation of China(NSFC) (81072204). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wu H, Liu T, Wang R, Tian S, Liu M et al. (2011) MicroRNA-16 targets zyxin and promotes cell motility in human laryngeal carcinoma cell line HEp-2. IUBMB Life 63(2): 101-108. PubMed: 21360639. [DOI] [PubMed] [Google Scholar]
- 2. Fleskens SA, van der Laak JA, Slootweg PJ, Takes RP (2010) Management of Laryngeal Premalignant Lesions in the Netherlands. Laryngoscope 120(7): 1326-1335. doi: 10.1002/lary.20888. PubMed: 20564658. [DOI] [PubMed] [Google Scholar]
- 3. Ren J, Zhu D, Liu M, Sun Y, Tian L (2010) Downregulation of miR-21 modulates Ras expression to promote apoptosis and suppress invasion of Laryngeal squamous cell carcinoma. Eur J Cancer 46(18): 3409-3416. doi: 10.1016/j.ejca.2010.07.047. PubMed: 20801640. [DOI] [PubMed] [Google Scholar]
- 4. Hunter KD, Parkinson EK, Harrison PR (2005) Profiling early head and neck cancer. Nat Rev Cancer 5: 127-135. doi: 10.1038/nrc1549. PubMed: 15685196. [DOI] [PubMed] [Google Scholar]
- 5. Cosetti M, Yu GP, Schantz SP (2008) Five-year survival rates and time trends of laryngeal cancer in the US population. Arch Otolaryngol Head Neck Surg 134: 370–379. doi: 10.1001/archotol.134.4.370. PubMed: 18427002. [DOI] [PubMed] [Google Scholar]
- 6. Geiger TR, Peeper DS (2009) Metastasis mechanisms. Biochim Biophys Acta 1796: 293–308. PubMed: 19683560. [DOI] [PubMed] [Google Scholar]
- 7. Makrilia N, Kollias A, Manolopoulos L, Syrigos K (2009) Cell adhesion molecules: role and clinical significance in cancer. Cancer Invest 27: 1023–1037. doi: 10.3109/07357900902769749. PubMed: 19909018. [DOI] [PubMed] [Google Scholar]
- 8. Thiele W, Sleeman JP (2006) Tumor-induced lymphangiogenesis: a target for cancer therapy? J Biotechnol 124(1): 224-241. doi: 10.1016/j.jbiotec.2006.01.007. PubMed: 16497404. [DOI] [PubMed] [Google Scholar]
- 9. Boonkitticharoen V, Kulapaditharom B, Leopairut J, Kraiphibul P, Larbcharoensub N et al. (2008) Vascular endothelial growth factor a and proliferation marker in prediction of lymph node metastasis in oral and pharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 134(12): 1305-1311. doi: 10.1001/archotol.134.12.1305. PubMed: 19075127. [DOI] [PubMed] [Google Scholar]
- 10. Mallis A, Teymoortash A, Mastronikolis NS, Werner JA, Papadas TA (2012) MMP-2 expression in 102 patients with glottic laryngeal cancer. Eur Arch Otorhinolaryngol 269(2): 639-642. doi: 10.1007/s00405-011-1625-8. PubMed: 21667117. [DOI] [PubMed] [Google Scholar]
- 11. Tse GM, Yu KH, Chan AW, King AD, Chen GG et al. (2009) HER2 expression predicts improved survival in patients with cervical node-positive head and neck squamous cellcarcinoma. Otolaryngol Head Neck Surg 141(4): 467-473. doi: 10.1016/j.otohns.2009.06.747. PubMed: 19786214. [DOI] [PubMed] [Google Scholar]
- 12. Li JJ, Zhang GH, Yang XM, Li SS, Liu X et al. (2012) Reduced E-cadherin expression is associated with lymph node metastases in laryngeal squamous cell carcinoma. Auris Nasus Larynx 39(2): 186-192. doi: 10.1016/j.anl.2011.04.003. PubMed: 21570223. [DOI] [PubMed] [Google Scholar]
- 13. Liu Y, Li G, Su ZW, Qiu YZ, Zhang X et al. (2013) Expression of astrocyte elevated gene-1 protein and its clinical significance in laryngealsquamous cell carcinoma. Zhonghua Bing Li Xue Za Zhi 42(2): 111-115. PubMed: 23710918. [DOI] [PubMed] [Google Scholar]
- 14. Gao S, Yang X, Li S, Tang Q (2013) The study on clinical significance of the expression of EGFL7 in laryngeal squamous cell carcinoma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 27(3):147-150. [PubMed] [Google Scholar]
- 15. Yoshida K, Sasaki R, Nishimura H, Okamoto Y, Suzuki Y et al. (2010) Nuclear factor-kappaB expression as a novel marker of radioresistance in early-stage laryngealcancer. Head Neck 32(5): 646-655. PubMed: 19885926. [DOI] [PubMed] [Google Scholar]
- 16. Han L, Jiang B, Wu H, Wang X, Tang X et al. (2012) High expression of CXCR2 is associated with tumorigenesis, progression, and prognosis oflaryngeal squamous cell carcinoma. Med Oncol 29(4): 2466-2472. doi: 10.1007/s12032-011-0152-1. PubMed: 22274915. [DOI] [PubMed] [Google Scholar]
- 17. Yim WC, Min K, Jung D, Lee BM, Kwon Y (2011) Cross experimental analysis of microarray gene expression data from volatile organic compounds treated targets. Mol Cell. Toxicol 7: 233-241. [Google Scholar]
- 18. Colombo J, Fachel AA, De Freitas Calmon M, Cury PM, Fukuyama EE et al. (2009) Gene expression profiling reveals molecular marker candidates of laryngeal squamous cell carcinoma. Oncol Rep 21(3): 649-663. PubMed: 19212623. [PubMed] [Google Scholar]
- 19. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4): 402-408. doi: 10.1006/meth.2001.1262. PubMed: 11846609. [DOI] [PubMed] [Google Scholar]
- 20. Jiang Y, Zhang R, Lv H, Li J, Wang M et al. (2013) HGPGD: The Human Gene Population Genetic Difference. Database - PLOS ONE 8(5): e64150. doi: 10.1371/journal.pone.0064150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris MA, Deegan JI, Lomax J, Ashburner M, Tweedie S et al. (2008) The Gene Ontology project in 2008. Nucleic Acids Res 36: 440–444. [Google Scholar]
- 22. Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28: 27–30. doi: 10.1093/nar/28.7.e27. PubMed: 10592173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kearsey SE, Labib K (1998) MCM proteins: evolution, properties, and role in DNA replication. Biochim Biophys Acta 1398: 113–136. doi: 10.1016/S0167-4781(98)00033-5. PubMed: 9689912. [DOI] [PubMed] [Google Scholar]
- 24. Han JY, Wang H, Xie YT, Li Y, Zheng LY et al. (2012) Association of Germline Variation in CCNE1 and CDK2 with Breast Cancer Risk, Progression and Survival among Chinese Han Women. PLOS ONE 7(11): e49296. doi: 10.1371/journal.pone.0049296. PubMed: 23185313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dong Z, Liu LH, Han B, Pincheira R, Zhang JT (2004) Role of eIF3 p170 in controlling synthesis of ribonucleotide reductase M2 and cell growth. Oncogene 23: 3790–3801. doi: 10.1038/sj.onc.1207465. PubMed: 15094776. [DOI] [PubMed] [Google Scholar]
- 26. Liu Z, Dong Z, Yang Z, Chen Q, Pan Y et al. (2007) Role of eIF3a(eIF3 p170) in intestinal cell differentiation and its association with early development. Differentiation 75: 652–661. doi: 10.1111/j.1432-0436.2007.00165.x. PubMed: 17381544. [DOI] [PubMed] [Google Scholar]
- 27. Honma K, Iwao-Koizumi K, Takeshita F, Yamamoto Y, Yoshida T et al. (2008) RPN2 gene confers docetaxel resistance in breast cancer. Nat Med 14(9): 939-948. doi: 10.1038/nm.1858. PubMed: 18724378. [DOI] [PubMed] [Google Scholar]
- 28. Gong Z, Xu X, Hou R, Guo Y, Sheng F et al. (2010) Effect of docetaxel on expression of eIF3a in human lung cancer A549 cell line. Zhong NAN da Xue Xue Bao Yi Xue Ban 35(8): 771-776. PubMed: 20818067. [DOI] [PubMed] [Google Scholar]
- 29. Kurashige J, Watanabe M, Iwatsuki M, Kinoshita K, Saito S et al. (2012) RPN2 expression predicts response to docetaxel in oesophageal squamous cell carcinoma. Br J Cancer 107(8): 1233-1238. doi: 10.1038/bjc.2012.396. PubMed: 22955852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prokhorova TA, Blow JJ (2000) Sequential MCM/P1 subcomplex assembly is required to form a heterohexamer with replication licensing activity. J Biol Chem 275: 2491-2498. doi: 10.1074/jbc.275.4.2491. PubMed: 10644704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tachibana KE, Gonzalez MA, Coleman N (2005) Cell-cycledependent regulation of DNA replication and its relevance to cancer pathology. J Pathol 205: 123-129. doi: 10.1002/path.1708. PubMed: 15643673. [DOI] [PubMed] [Google Scholar]
- 32. Stoeber K, Tlsty TD, Happerfield L, Thomas GA, Romanov S et al. (2001) DNA replication licensing and human cell proliferation. J Cell Sci 114: 2027-2041. PubMed: 11493639. [DOI] [PubMed] [Google Scholar]
- 33. Vargas PA, Cheng Y, Barrett AW, Craig GT, Speight PM (2008) Expression of Mcm-2, Ki-67 and geminin in benign and malignant salivary gland tumours. J Oral Pathol Med 37(5): 309-318. doi: 10.1111/j.1600-0714.2007.00631.x. PubMed: 18248354. [DOI] [PubMed] [Google Scholar]
- 34. Liu M, Li JS, Tian DP, Huang B, Rosqvist S et al. (2013) MCM2 expression levels predict diagnosis and prognosis in gastric cardiac cancer. Histol Histopathol 28(4): 481-492. PubMed: 23329420. [DOI] [PubMed] [Google Scholar]
- 35. Mehrotra P, Gonzalez MA, Johnson SJ, Coleman N, Wilson JA et al. (2006) Mcm-2 and Ki-67 Have Limited Potential in Preoperative Diagnosis of Thyroid Malignancy. Laryngoscope 116(8): 1434-1438. doi: 10.1097/01.mlg.0000225931.59644.93. PubMed: 16885749. [DOI] [PubMed] [Google Scholar]
- 36. Kikuchi J, Kinoshita I, Shimizu Y, Kikuchi E, Takeda K et al. (2011) Minichromosome maintenance (MCM) protein 4 as a marker for proliferation and its clinical and clinicopathological significance in non-small cell lung cancer. Lung Cancer 72(2): 229-237. doi: 10.1016/j.lungcan.2010.08.020. PubMed: 20884074. [DOI] [PubMed] [Google Scholar]
- 37. Nodin B, Fridberg M, Jonsson L, Bergman J, Uhlén M et al. (2012) High MCM3 expression is an independent biomarker of poor prognosis and correlates with reduced RBM3 expression in a prospective cohort of malignant melanoma. Diagn Pathol 7: 82. doi: 10.1186/1746-1596-7-82. PubMed: 22805320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ha SA, Shin SM, Namkoong H, Lee H, Cho GW et al. (2004) Cancer-Associated Expression of Minichromosome Maintenance 3 Gene in Several Human Cancers and Its Involvement in Tumorigenesis. Clin Cancer Res 10(24): 8386-8395. doi: 10.1158/1078-0432.CCR-04-1029. PubMed: 15623617. [DOI] [PubMed] [Google Scholar]
- 39. Gan N, Du Y, Zhang W, Zhou J (2010) Increase of Mcm3 and Mcm4 expression in cervical squamous cell carcinoma. Eur J Gynaecol Oncol 31(3): 291-294. PubMed: 21077471. [PubMed] [Google Scholar]
- 40. Chou LC, Yang JS, Huang LJ, Wu HC, Lu CC et al. (2009) The synthesized 2-(2-fluorophenyl)-6,7-methylenedioxyquinolin-4-one (CHM-1) promoted G2/M arrest through inhibition of CDK1 and induced apoptosis through the mitochondrial-dependent pathway in CT-26 murine colorectal adenocarcinoma cells. J Gastroenterol 44: 1055-1063. doi: 10.1007/s00535-009-0111-1. PubMed: 19688288. [DOI] [PubMed] [Google Scholar]
- 41. Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9: 153-166. doi: 10.1038/nrc2602. PubMed: 19238148. [DOI] [PubMed] [Google Scholar]
- 42. Han YK, Lee JH, Park GY, Chun SH, Han JY et al. (2013) A possible usage of a CDK4 inhibitor for breast cancer stem cell-targeted therapy. Biochem Biophys Res Commun 430(4): 1329-1333. doi: 10.1016/j.bbrc.2012.10.119. PubMed: 23261434. [DOI] [PubMed] [Google Scholar]
- 43. Shirali S, Aghaei M, Shabani M, Fathi M, Sohrabi M et al. (2013) Adenosine induces cell cycle arrest and apoptosis via cyclinD1/Cdk4 and Bcl-2/Bax pathways in human ovarian cancer cell line OVCAR-3. Tumour Biol 34(2): 1085-1095. doi: 10.1007/s13277-013-0650-1. PubMed: 23345014. [DOI] [PubMed] [Google Scholar]
- 44. Shi T, Mazumdar T, Devecchio J, Duan ZH, Agyeman A et al. (2010) cDNA Microarray Gene Expression Profiling of Hedgehog Signaling Pathway Inhibition in Human Colon Cancer Cells. PLOS ONE 5(10): e13054. doi: 10.1371/journal.pone.0013054. PubMed: 20957031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lu JW, Lin YM, Chang JG, Yeh KT, Chen RM et al. (2013) Clinical implications of deregulated CDK4 and Cyclin D1 expression in patients with human hepatocellular carcinoma. Med Oncol 30(1): 379. doi: 10.1007/s12032-012-0379-5. PubMed: 23292829. [DOI] [PubMed] [Google Scholar]
- 46. Lim YC, Cha YY (2011) Epigallocatechin-3-gallate Induces Growth Inhibition and Apoptosis of Human Anaplastic Thyroid Carcinoma Cells Through Suppression of EGFR/ERK Pathway and Cyclin B1/CDK1 Complex. J Surg Oncol 104(7): 776-780. doi: 10.1002/jso.21999. PubMed: 21725973. [DOI] [PubMed] [Google Scholar]
- 47. Zhang C, Elkahloun AG, Robertson M, Gills JJ, Tsurutani J et al. (2011) Loss of Cytoplasmic CDK1 Predicts Poor Survival in Human Lung Cancer and Confers Chemotherapeutic Resistance. PLOS ONE 6(8): e23849. doi: 10.1371/journal.pone.0023849. PubMed: 21887332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mielcarek-Kuchta D, Olofsson J, Golusinski W (2008) Laminin expression in advanced laryngeal squamous cell carcinoma does not correlate to neck metastases. Eur Arch Otorhinolaryngol 265(10): 1257-1261. doi: 10.1007/s00405-008-0666-0. PubMed: 18516614. [DOI] [PubMed] [Google Scholar]
- 49. Dong Z, Liu LH, Han B, Pincheira R, Zhang JT (2004) Role of eIF3 p170 in controlling synthesis of ribonucleotide reductase M2 and cell growth. Oncogene 23: 3790–3801. doi: 10.1038/sj.onc.1207465. PubMed: 15094776. [DOI] [PubMed] [Google Scholar]
- 50. Dellas A, Torhorst J, Bachmann F, Bänziger R, Schultheiss E et al. (1998) Expression of p150 in cervical neoplasia and its potential value in predicting survival. Cancer 83: 1376–1383. doi: 10.1002/(SICI)1097-0142(19981001)83:7. PubMed: 9762939. [DOI] [PubMed] [Google Scholar]
- 51. Chen G, Burger MM (1999) p150 expression and its prognostic value in squamous-cell carcinoma of the esophagus. Int J Cancer 84: 95–100. doi: 10.1002/(SICI)1097-0215(19990420)84:2. PubMed: 10096238. [DOI] [PubMed] [Google Scholar]
- 52. Yin JY, Shen J, Dong ZZ, Huang Q, Zhong MZ et al. (2011) Effect of eIF3a on response of lung cancer patients to platinum-based chemotherapy by regulating. DNA Repair - Clinical Cancer Res 17(13): 4600-4609. doi: 10.1158/1078-0432.CCR-10-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kurashige J, Watanabe M, Iwatsuki M, Kinoshita K, Saito S et al. (2012) RPN2 expression predicts response to docetaxel in oesophageal squamous cell carcinoma. Br J Cancer 107(8): 1233-1238. doi: 10.1038/bjc.2012.396. PubMed: 22955852. [DOI] [PMC free article] [PubMed] [Google Scholar]