Abstract
Background
For patients with chronic liver disease, different optimal liver stiffness cut-off values correspond to different stages of fibrosis, which are specific for the underlying liver disease and population.
Aims
To establish the normal ranges of liver stiffness in the healthy Chinese population without underlying liver disease.
Methods
This is a prospective cross sectional study of 2,528 healthy volunteers recruited from the general population and the Red Cross Transfusion Center in Hong Kong. All participants underwent a comprehensive questionnaire survey, measurement of weight, height, and blood pressure. Fasting liver function tests, glucose and cholesterol was performed. Abdominal ultrasound and transient elastography were performed on all participants.
Results
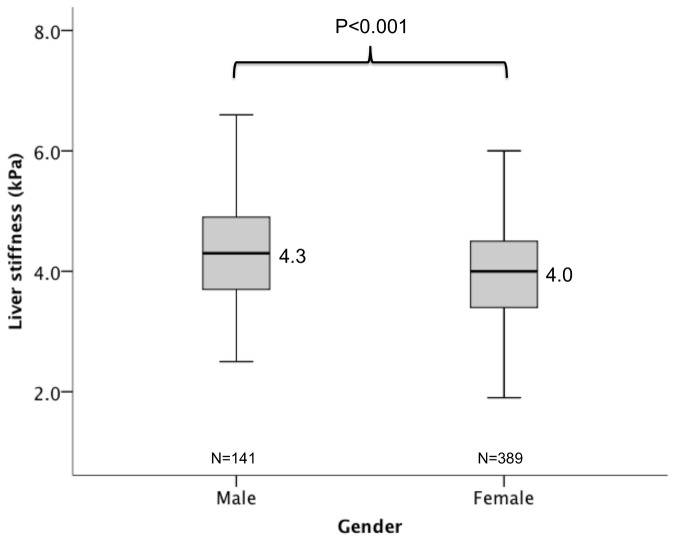
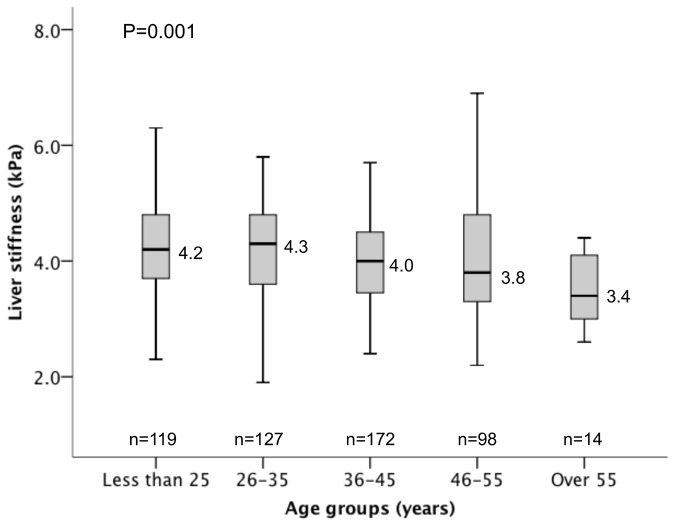
Of the 2,528 subjects, 1,998 were excluded with either abnormal liver parenchyma on ultrasound, chronic medical condition, abnormal blood tests including liver enzymes, fasting glucose, fasting cholesterol, high body mass index, high blood pressure, or invalid liver stiffness scan. The reference range for the 530 subjects without known liver disease was 2.3 to 5.9 kPa (mean 4.1, SD 0.89). The median liver stiffness was higher in males compared with females (4.3 vs 4.0 kPa respectively, p<0.001). There was also a decline in median Lliver stiffness in the older age group, from 4.2 kPa in those <25 years to 3.4 kPa for those >55 years (p=0.001).
Conclusions
The healthy reference range for liver stiffness in the Chinese population is 2.3 to 5.9 kPa. Female gender and older age group was associated with a lower median liver stiffness.
Introduction
For people with chronic liver diseases, assessment of liver fibrosis is important for several reasons. Firstly, the degree of fibrosis is an indication of the severity of the underlying liver disease. Secondly, it may have prognostic significance. In addition, treatment decisions may be dependent on the presence of significant fibrosis. Although liver biopsy remains the gold standard for assessing liver fibrosis, patients may be unwillingly to undergo a biopsy procedure and clinicians may be reluctant to advocate it because of the potential adverse effects associated with this invasive procedure [1,2]. Furthermore, there are also disadvantages associated with liver biopsy. Its accuracy is dependent on the quality of tissue sample obtained, including the number of portal tracts, length of specimen, and degree of fragmentation. As biopsies only sample tiny portions of the liver, they are subjected to sampling errors. Its interpretation is further subjected to intra- and inter- observer variability [3]. For these reasons, non-invasive methods to assess liver fibrosis have been developed as an alternative to liver biopsy.
In the past decade, liver stiffness measurement (LSM) using transient elastography has become one of the most viable alternative non-invasive methods to biopsy in assessing liver fibrosis. Previous studies have documented the diagnostic accuracy of LSM in grading fibrosis for a variety of chronic liver diseases [4-8]. In addition, LSM has been shown to have prognostic significance in predicting long-term outcome, and in the assessment of treatment response [9,10]. One of the earliest key concepts of LSM to evolve is that different optimal liver stiffness cut-off values correspond to different stages of fibrosis, and these cut-off values are disease-specific [4-7]. Although cut-off values for advanced fibrosis and cirrhosis have been well established for different diseases, the normal reference range of LSM in specific population groups have not been well defined, especially from large population studies. Histology from normal liver is rarely available, therefore normal ranges of liver stiffness is much harder to establish. An earlier small-scaled study using normal livers from subjects undergoing donor hepatectomy have identified a normal cut-off liver stiffness of <7.2 kPa [11].
The Hong Kong Liver Health Census was established in 2010 by a group of hepatologists from the University of Hong Kong in collaboration with the Hong Kong Liver Foundation and Hong Kong Red Cross Blood Transfusion Service. The aim of the current study was to define the normal ranges of liver stiffness in the healthy Chinese population without underlying liver disease.
Subjects and Methods
The Liver Health Census recruited participants from blood donors of the Hong Kong Red Cross Blood Transfusion Services and volunteers from the general population. All participants included in the census were screened negative for hepatitis B surface antigen (HBsAg), antibody to hepatitis C virus (anti-HCV), and human immunodeficiency virus (HIV). Ultrasound of the abdomen and transient elastography was performed in all participants. Laboratory blood testing was performed on the same day. All participants underwent weight, height, and completed a detailed questionnaire.
Ethics statement
Written informed consent was obtained from all participants. The study was approved by the Institutional Review Board of the University of Hong Kong and Hospital Authority Western Cluster (No. UW-10-325).
Liver stiffness measurement
LSM was performed using transient elastography (Fibroscan, Echosens, Paris) by an experienced operator. The procedure has been described previously [12]. Briefly, LSM was performed with the subject lying in the supine position. Using time-motion ultrasound image, measurements were obtained once a segment of the liver was located with a thickness of over 6cm and free of large vascular structures. Results were included in the final analysis only if the following three criteria were met: at least 10 valid measurements, success rate >60% and the interquartile range (IQR)-to-liver stiffness ration were ≤0.30. The median liver stiffness value of each participant was representative of the liver stiffness, and expressed in units of kilopascals (kPa).
Abdominal ultrasound
All participants underwent abdominal ultrasonography by two experienced radiologists, using the Envisor ultrasound system (Philips Ultrasound, Philips Medical System, The Netherlands). Fatty liver was diagnosed by the presence of increased liver echogenicity.
Detailed questionnaire
All participants completed a detailed questionnaire including their past and current medical history, and their current intake of medications, herbal remedies, over-the-counter remedies, and alcohol.
Laboratory test
Participants were excluded from the census if prior testing indicated they were infected with hepatitis B, hepatitis C, or HIV. Fasting blood tests were performed on the same day of the ultrasound and transient elastography. Liver biochemistry including alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT) and bilirubin levels were measured, along with cholesterol and glucose.
Statistical analysis
All statistical analysis was performed using SPSS version 16.0 (Chicago, IL). Continuous variables with skewed distribution were analyzed using the Mann-Whitney test. Continuous variables with more than two categories were analyzed using the Kruskal Wallis test. The correlation co-efficient between LSM and other parameters were calculated using the Pearson test. The reference range was calculated using the mean ±2 standard deviations. A p-value of <0.05 was considered statistically significant.
Results
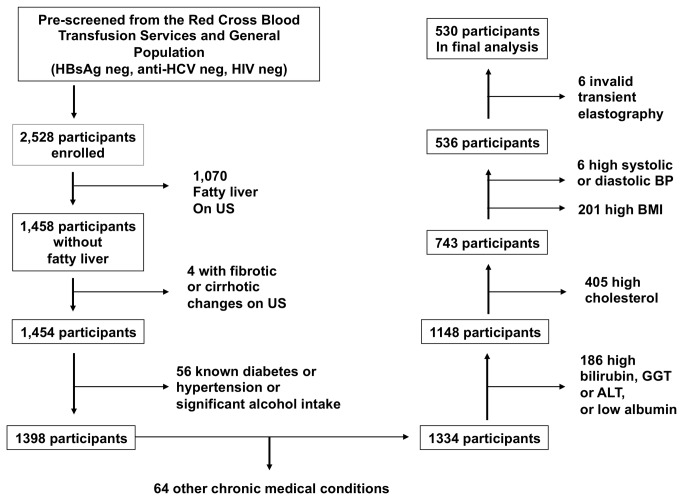
A total of 2,528 subjects were enrolled into the liver health census. As mentioned previously, these subjects were already screened negative for HBV, HCV, and HIV infection. To define a normal liver stiffness range in healthy individuals without known liver disease, we excluded also those with sonographical evidence of fatty liver disease (n=1070) and fibrosis/cirrhosis (n=4). Next, those with pre-existing diabetes, hypertension, other chronic medical conditions, and significant alcohol intake (as defined by >20g/day) were excluded (n=120). Those with elevated bilirubin (>17.1 umol/L), ALT (>45 U/L), GGT >84 U/L), fasting glucose (>6.0 mmol/L)[13], fasting cholesterol (>5.0 mmol/L)[14], and low albumin (<35 g/L) were then excluded (n=591). A further 207 patients were excluded with higher BMI (>23 kg/m2) or elevated blood pressure (systolic >140 mmHg or diastolic >90 mmHg)[15]. In the remaining 536, 6 (1%) subjects had invalid transient elastography measurements (as defined by the criteria set above), and were excluded. This is consistent with the predicted failure rate of <5%[16].
The remaining 530 participants had valid LSM and were included in the final analysis. The flow of participants is outlined in Figure 1. Basic demographics and characteristics are summarized in Table 1. All patients in the final analysis therefore had normal liver parenchyma on ultrasonography, normal liver enzymes, absence of chronic medical condition, no significant alcohol intake, and normal fasting glucose and cholesterol and normal BMI. The laboratory and liver stiffness results are summarized in Table 2.
Figure 1. Flow diagram of health participants from the Hong Kong Liver Health Census.
Table 1. Basic demographics and characteristics of healthy participants.
| Parameters | Values |
|---|---|
| Number of participants (N) | 530 |
| Age (years) | 37 (18-63) |
| Male sex [n(%)] | 141 (26.6%) |
| Body mass index (kg/m2) | 20.6 (16.0 - 23.0) |
| Systolic blood pressure (mmHg) | 108 (80 - 139) |
| Diastolic blood pressure (mmHg) | 66 (49 - 89) |
Continuous variables expressed as median (range)
Table 2. Laboratory results and liver stiffness results of participants.
| Parameters | Values |
|---|---|
| Blood tests | |
| Bilirubin (umol/L) | 8.9 (2.1-16.9) |
| Alanine aminotransferase (U/L) | 30 (16 - 45) |
| Gamma glutaryltransferase (U/L) | 23 (9 - 73) |
| Albumin (g/L) | 41 (36-50) |
| Fasting glucose (mmol/L) | 4.8 (3.9 - 6.0) |
| Fasting cholesterol (mmol/L) | 4.8 (2.6 - 5.0) |
| Liver stiffness measurement | |
| Liver stiffness (kPa) | 4.1 (1.9 - 7.8) |
| Interquartile range | 0.5 (0.0 - 1.6) |
| Success rate | 100% (71 - 100) |
| Interquartile range to liver stiffness ratio | 0.13 (0.00 - 0.30) |
Continuous variables expressed as median (range).
Correlation of LSM with patient parameters
There was a negative correlation between age and LSM (r=-0.168, p<0.001). The LSM for those with age ≤25, 26-35, 36-45, 46-55, and >55 years were 4.2, 4.3, 4.0, 3.8, and 3.4 kPa respectively (p=0.001), as shown in Figure 2. The median LSM in males was higher than females (4.3 vs 4.0 kPa respectively, p<0.001)(Figure 3). There was no correlation observed between LSM and BMI (r=-0.72, p=0.097), systolic blood pressure (r=0.061, p=0.162), and diastolic blood pressure (r=0.014, p=0.743).
Figure 2. Liver stiffness according to gender.
Figure 3. Liver stiffness according to different age groups.
Correlation of LSM with laboratory parameters
There was no significant correlation between LSM and fasting cholesterol (r=-0.61, p=0.163), fasting glucose (-0.78, p=0.071), ALT (r=0.072, p=0.100), and GGT (r=0.072, p=0.100). There was a weak correlation between LSM and bilirubin (r=0.096. p=0.028).
Normal reference range
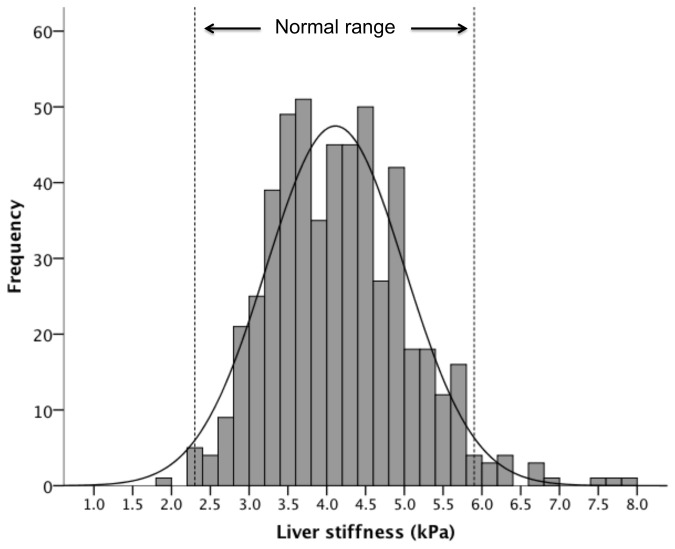
The reference range for liver stiffness was determined for the current healthy population without known liver disease. The mean was determined, and the range of normal values was established by calculating ±2 standard deviations from the mean. The reference range for the 530 subjects without known liver disease was 2.3 to 5.9 kPa (mean 4.11, SD 0.89), with the distribution shown in Figure 4. The reference range was similar between males and females (2.6 to 6.1 kPa and 2.3 to 5.8 kPa respectively).
Figure 4. The distribution and normal range of liver stiffness in normal subjects.
Discussion
In recent years, transient elastography has become more widely available for the non-invasive assessment of liver fibrosis. There are well-established optimal cut-off values for different stages of fibrosis, which appear to be specific for individual diseases and populations. Normal ranges for liver stiffness have been less well defined due to the lack of large population studies on healthy subjects. Histological specimens from normal livers in subjects without liver disease are expectedly rare. However, a large population study of normal healthy subjects is essential to derive a normal reference range of liver stiffness, which provides a solid and firm reference for populations with liver disease
The current study included participants from The Hong Kong Liver Health Census, selecting those without ultrasonographic evidence of fatty liver, fibrosis, and cirrhosis. In addition, none of these subjects were hepatitis B carriers, a disease that continues to be endemic with a prevalence rate of 8% in our general population. The study identified a healthy liver stiffness range of 2.3 to 5.9 kPa in the Chinese population.
A recent study on 437 South Asian subjects showed a reference range to be 3.2 and 8.5 kPa by calculating the 5th and 95th percentile respectively [17]. A higher LSM was identified in lean and obese subjects. As the current study excluded subjects with high BMI (>23 kg/m2), no correlation between liver stiffness and BMI was observed among those with normal BMI, Another study of 445 South Asian subjects without known liver disease and normal liver enzymes showed a mean LSM of 5.1 kPa, with both ALT and BMI influencing the LSM significantly [18]. These differences highlight the important fact that liver stiffness values are not universal, and reference ranges should be derived for different populations.
Although there was correlation shown between LSM and bilirubin, the correlation was very weak only. A previous large population study of 1,268 chronic hepatitis B Chinese patients demonstrated that LSM correlated with higher age and also increasing levels of ALT [12]. It has long been realized that ALT was a significant independent factor in which markedly elevated ALT levels can increase the LSM spuriously [19,20]. Another study also showed that even small increments in ALT were associated with higher LSM in CHB patients [21]. There was no correlation between liver stiffness and ALT in the current study. This is likely due to the fact that only those with normal ALT were included.
In a previous study of 428 patients with normal liver enzymes and no known liver disease, the liver stiffness was significantly higher in males compared with females [22]. This is also consistent with our current study showing a higher median stiffness in males compared with females (4.3 vs 4.0 kPa respectively, p<0.001). However, this difference is minimal, and therefore having separate normal ranges is unlikely to be useful.
One limitation of the current study is the unavailability of liver biopsies to confirm the absence of fibrosis in the study population. However, the use of liver biopsies in a large population of healthy individuals is unlikely to be feasible. By using stringent criteria to define a healthy population, the margin of error is likely to be minimized. Despite this, undiagnosed liver diseases may still occur. Although viral serology for HBV, HCV, and HIV were performed, neither hepatitis B core antibody (anti-HBc) nor autoimmune antibodies were screened to exclude past HBV infection and autoimmune liver diseases respectively.
In conclusion, the normal range for liver stiffness in the Chinese population is 2.3 to 5.9 kPa. Female gender and older age were associated with lower liver stiffness in subjects without known liver disease.
Acknowledgments
Hong Kong Liver Health Census Investigators: James Fung, Man-Fung Yuen, Lee Cheuk-Kwong, Monica Chan, Wai-Kay Seto, Ching-Lung Lai, Doris Ho, Karen Lam, Emily Lau, Iris Lee, Lu Lei, Chadwick Lie, Alex Liu, Jacky Ng, Joan Tsao.
Funding Statement
The Hong Kong Liver Health Census was an investigator-initiated, not-for-profit study, in conjunction with the Hong Kong Liver Foundation. Financial support was provided by unrestricted grants from Bristol-Myers-Squibbs (Hong Kong), Chong Lap (Hong Kong), GlaxoSmithKline,(Hong Kong) and Novartis Pharmaceuticals (Hong Kong). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Froehlich F, Lamy O, Fried M, Gonvers JJ (1993) Practice and complications of liver biopsy. Results of a nationwide survey in Switzerland. Dig Dis Sci 38: 1480-1484. doi: 10.1007/BF01308607. PubMed: 8344104. [DOI] [PubMed] [Google Scholar]
- 2. Perrault J, McGill DB, Ott BJ, Taylor WF (1978) Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology 74: 103-106. PubMed: 618417. [PubMed] [Google Scholar]
- 3. Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG et al. (2002) Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 97: 2614-2618. doi: 10.1111/j.1572-0241.2002.06038.x. PubMed: 12385448. [DOI] [PubMed] [Google Scholar]
- 4. Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R et al. (2009) Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int 29: 242-247. doi: 10.1111/j.1478-3231.2008.01802.x. PubMed: 18637064. [DOI] [PubMed] [Google Scholar]
- 5. Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E et al. (2005) Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 128: 343-350. doi: 10.1053/j.gastro.2004.11.018. PubMed: 15685546. [DOI] [PubMed] [Google Scholar]
- 6. Corpechot C, El Naggar A, Poujol-Robert A, Ziol M, Wendum D et al. (2006) Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology 43: 1118-1124. doi: 10.1002/hep.21151. PubMed: 16628644. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen-Khac E, Chatelain D, Tramier B, Decrombecque C, Robert B et al. (2008) Assessment of asymptomatic liver fibrosis in alcoholic patients using fibroscan: prospective comparison with seven non-invasive laboratory tests. Aliment Pharmacol Ther 28: 1188-1198. doi: 10.1111/j.1365-2036.2008.03831.x. PubMed: 18705692. [DOI] [PubMed] [Google Scholar]
- 8. Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J et al. (2008) Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 134: 960-974. doi: 10.1053/j.gastro.2008.01.034. PubMed: 18395077. [DOI] [PubMed] [Google Scholar]
- 9. Fung J, Lai CL, Seto WK, Wong DK, Yuen MF (2011) Prognostic significance of liver stiffness for hepatocellular carcinoma and mortality in HBeAg-negative chronic hepatitis B. J Viral Hepat, 18: 738–44. PubMed: 20659306. [DOI] [PubMed] [Google Scholar]
- 10. Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T et al. (2009) Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology 49: 1954-1961. doi: 10.1002/hep.22870. PubMed: 19434742. [DOI] [PubMed] [Google Scholar]
- 11. Fung J, Lai CL, Chan SC, But D, Seto WK et al. (2010) Correlation of liver stiffness and histological features in healthy persons and in patients with occult hepatitis B, chronic active hepatitis B, or hepatitis B cirrhosis. Am J Gastroenterol 105: 1116-1122. doi: 10.1038/ajg.2009.665. PubMed: 19920809. [DOI] [PubMed] [Google Scholar]
- 12. Fung J, Lai CL, Fong DY, Yuen JC, Wong DK et al. (2008) Correlation of liver biochemistry with liver stiffness in chronic hepatitis B and development of a predictive model for liver fibrosis. Liver Int 28: 1408-1416. doi: 10.1111/j.1478-3231.2008.01784.x. PubMed: 18482268. [DOI] [PubMed] [Google Scholar]
- 13. Mayfield J (1998) Diagnosis and classification of diabetes mellitus: new criteria. Am Fam Physician 58: 1355-1362, 1369-1370 [PubMed] [Google Scholar]
- 14. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106: 3143-3421 PubMed; : 12485966 [PubMed] [Google Scholar]
- 15. Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J et al. (2007) Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation 115: 2761-2788. doi: 10.1161/CIRCULATIONAHA.107.183885. PubMed: 17502569. [DOI] [PubMed] [Google Scholar]
- 16. Foucher J, Castéra L, Bernard PH, Adhoute X, Laharie D et al. (2006) Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol 18: 411-412. doi: 10.1097/00042737-200604000-00015. PubMed: 16538113. [DOI] [PubMed] [Google Scholar]
- 17. Das K, Sarkar R, Ahmed SM, Mridha AR, Mukherjee PS et al. (2012) "Normal" liver stiffness measure (LSM) values are higher in both lean and obese individuals: a population-based study from a developing country. Hepatology 55: 584-593. doi: 10.1002/hep.24694. PubMed: 21952989. [DOI] [PubMed] [Google Scholar]
- 18. Kumar M, Sharma P, Garg H, Kumar R, Bhatia V et al. (2011) Transient elastographic evaluation in adult subjects without overt liver disease: influence of alanine aminotransferase levels. J Gastroenterol Hepatol 26: 1318-1325. doi: 10.1111/j.1440-1746.2011.06736.x. PubMed: 21443658. [DOI] [PubMed] [Google Scholar]
- 19. Fung J, Lai CL, But D, Hsu A, Seto WK et al. (2010) Reduction of liver stiffness following resolution of acute flares of chronic hepatitis B. Hepatol. Int. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arena U, Vizzutti F, Corti G, Ambu S, Stasi C et al. (2008) Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 47: 380-384. PubMed: 18095306. [DOI] [PubMed] [Google Scholar]
- 21. Fung J, Lai CL, Cheng C, Wu R, Wong DK et al. (2011) Mild-to-moderate elevation of alanine aminotransferase increases liver stiffness measurement by transient elastography in patients with chronic hepatitis B. Am J Gastroenterol 106: 492-496. doi: 10.1038/ajg.2010.463. PubMed: 21157442. [DOI] [PubMed] [Google Scholar]
- 22. Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC et al. (2008) Liver stiffness values in apparently healthy subjects: Influence of gender and metabolic syndrome. J Hepatol 48: 606-613. doi: 10.1016/j.jhep.2007.11.020. PubMed: 18222014. [DOI] [PubMed] [Google Scholar]