Abstract
Inflammasomes are large macromolecular signaling complexes that control the proteolytic activation of two highly proinflammatory IL-1 family cytokines, IL-1β and IL-18. The NLRP3 inflammasome is of special interest because it can assemble in response to a diverse array of stimuli and because the inflammation it triggers has been implicated in a wide variety of disease pathologies. To avoid aberrant activation, the NLRP3 inflammasome is modulated on multiple levels, ranging from transcriptional control to post-translational protein modifications. Emerging genetic and pharmacological evidence suggests that NLRP3 inflammasome activation may also be involved in acute lung inflammation after viral infection and during progression of several chronic pulmonary diseases, including idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease, and asthma. Here, we review the most recent contributions to our understanding of the regulatory mechanisms controlling activation of the NLRP3 inflammasome and discuss the contribution of the NLRP3 inflammasome to the pathology of lung diseases.
CME Accreditation Statement: This activity (“ASIP 2014 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2014 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
The main functions of the immune system are to protect the host from microbial infections, to detect and combat cancerous cells, and to respond to and repair tissue damage. The innate immune system has evolved germline-encoded signaling receptors with which microbial molecules (pathogen-associated molecular patterns) and altered host molecules (danger-associated molecular patterns) can be detected. Activation of these signaling receptors leads to the production of a wide variety of inflammatory mediators that orchestrate a coordinated immune response toward pathogens or tissue damage, with the goal of restoring homeostasis. In tissues that are colonized by commensal microbes, such as the gut, skin, or lungs, the immune system faces the particular challenge of distinguishing commensal microbes from foreign pathogens. Tissue-specific mechanisms evolved to ensure a carefully balanced immune response that is tailored for the normal local microflora.
The lung is continuously exposed to a variety of inhaled infectious agents and exogenous particulates, as well as to host-derived danger signals, and thus, the innate immune response plays a critical role in protecting the pulmonary system from disease. The lung comprises a set of specialized cells of the innate immune system that express several families of innate immune pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), RIG-I-like receptors (RLRs), and NOD-like receptors (NLRs), which initiate signaling pathways that promote the induction of inflammatory mediators. In the lung, production of cytokines [eg, tumor necrosis factor (TNF)] and chemokines (eg, IL-8) by immune cells is critical for coordinating the acute immune response, including recruitment and activation of other immune cells (eg, neutrophils), as well as for subsequent activation of lymphocytes.1
Highly inflammatory cytokines of the IL-1 family, including IL-1β and IL-18, are central to processes mediating lung inflammation. The proteolytic activation of these cytokines is under the control of several innate immune receptors that are able to form large multiprotein signaling platforms, termed inflammasomes.2 The inflammasome formed downstream of the receptor NACHT, LRR and PYD domains-containing protein 3 (NLRP3; alias NALP3) can be activated not only by pathogens, but also in response to sterile tissue damage or metabolic stress, resulting in sustained inflammatory reactions. This observation led to the discovery that the NLRP3 inflammasome is central to the pathogenesis of a wide variety of chronic inflammatory diseases, including several common metabolic disorders (eg, atherosclerosis and type 2 diabetes).3 Emerging genetic and pharmacological evidence suggests that, although NLRP3 inflammasome activation is critical for driving acute lung inflammation aiding in the clearance of viral or bacterial infections, persistent activation of NLRP3 by irritants may be involved in the progression of several chronic pulmonary diseases, including idiopathic pulmonary fibrosis (IPF), chronic obstructive pulmonary disease (COPD), and asthma. Chronic respiratory diseases account for more than 4 million deaths annually worldwide,4 and the underlying immunological mechanisms that govern these airway pathologies remain the topic of intense investigation. Here, we will first review the most recent contributions to our understanding of the activation and regulation of the NLRP3 inflammasome and then discuss the role of NLRP3 in the pathology of lung diseases.
Production of IL-1 Family Cytokines via Inflammasomes
Proinflammatory cytokines of the IL-1 family are particularly potent inducers of inflammation.5 By virtue of the potentially destructive proinflammatory effects of uncontrolled IL-1β release, its production is tightly regulated. First, the expression of a nonactive IL-1β precursor (pro-IL-1β) must be induced in immune cells via activation (eg, by TLRs) of signaling pathways upstream of the transcription factor NFκB. Second, pro-IL-1β must be processed by caspase-1 into its bioactive form before its release from cells. Although the signaling pathways and inflammatory outcomes of IL-1β activation have long been known, the mechanisms by which immune cells produce this cytokine have come to light only in the last decade, with the discovery of inflammasomes.2 Inflammasomes consist of a receptor molecule (sensor), the adaptor molecule apoptosis-associated speck-like protein containing a CARD (ASC), and caspase-1. Their formation leads to the production of bioactive IL-1β and IL-18. To date, a number of cytosolic receptors are known to trigger formation of an inflammasome, including the NLR protein family members NLRP1, NLRP3, NLRP6, NLRP7, NLRP12, and NLRC4.6 In addition, the PYHIN protein family members AIM2 and IFI16 can also form inflammasomes.7,8
In general, the NLR proteins comprise three major domains: a C-terminal leucine-rich repeat (LRR) domain, which is thought to have regulatory functions and to be involved in ligand sensing,9 a central nucleotide binding (NACHT) domain required for ATP-dependent self oligomerization,10 and an N-terminal pyrin domain (PYD, present in NLRPs) or caspase activation and recruitment domain (CARD, present in NLRC4) enabling protein–protein interactions. The crystal structure of murine NLRC4 was resolved in 2013, revealing a closed inhibitory conformation of the protein in which the NACHT domain is guarded by the LRR.11 This suggests that NLRs exhibit a closed structure in the cytosol until ligand binding or an activation signal, whereupon they undergo a conformational change allowing subsequent NLR oligomerization and interaction with ASC.
In the case of NLRP3, some evidence exists that its conformation may be regulated by interactions with the cochaperone molecules Hsp90 and SGT1, which appear to be critical for its activation.12 The interaction of NLR receptors with ASC, which is mediated via PYD–PYD binding, results in assembly of large multimeric complexes consisting predominantly of dimers and oligomers of ASC.13 The formation of these so-called ASC specks allows for the recruitment of pro-caspase-1 via homotypic CARD–CARD interactions with ASC. The accumulation of pro-caspase-1 within these complexes induces an autocatalytic event, ultimately resulting in the formation of active caspase-1 heterotetramers that are able to process inactive cytosolic pro-IL-1β and pro-IL-18 into their functional forms.5 In addition to facilitating the cleavage and maturation of these cytokines, activation of caspase-1 also induces pyroptosis, a specific and highly proinflammatory form of programmed cell death.14 Thus, in contrast to most other PRRs that regulate the transcription of inflammatory mediators, the inflammasomes mediate post-translational activation of IL-1 family cytokines and the induction of a specialized form of cell death.
Activation of the NLRP3 Inflammasome
The NLRP3 inflammasome is of special interest because it can assemble in response to a wide variety of stimuli with diverse physical and chemical properties. These include various exogenous activators, ranging from microbial components [eg, influenza A virus (IAV)] to particulates found in the environment (eg, silica crystals or asbestos fibers). Furthermore, many endogenous molecules also induce activation of the NLRP3 inflammasome after their accumulation or alteration under conditions of tissue damage or metabolic dysfunction. For instance, under normal physiological conditions uric acid exists in a harmless soluble form. When circulating levels become severely elevated, however, it can undergo a phase transition to form monosodium urate crystals, which activate the NLRP3 inflammasome, ultimately resulting in the IL-1β–driven chronic inflammatory state seen in gout.15–17 Likewise, although normal intracellular ATP levels are innocuous, a rapid increase in extracellular ATP (eATP), such as is seen after tissue damage or cell death, acts as an endogenous danger signal activating the NLRP3 inflammasome via binding P2X purinoreceptor 7 (P2X7), which acts as a ligand-gated ion channel.18
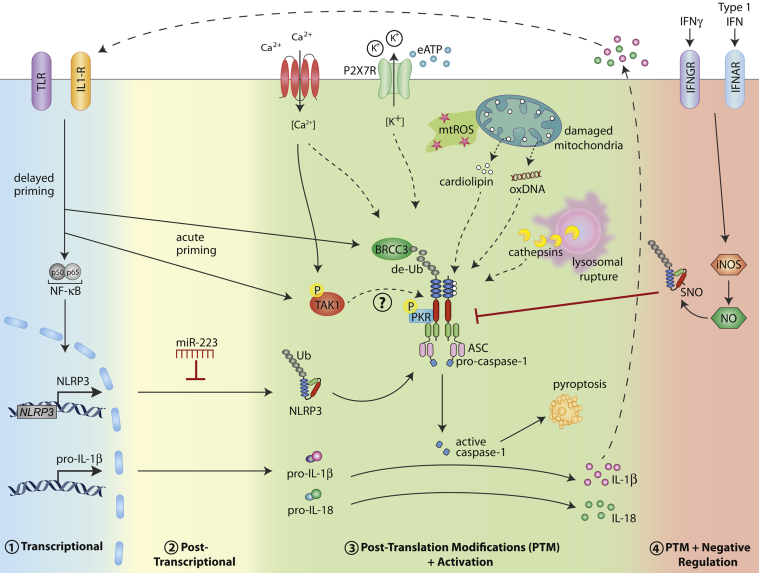
Given that NLRP3 inflammasome formation is induced by such a broad array of signals, it seems unlikely that direct binding of these stimuli to the receptor occurs. Instead, NLRP3 is thought to sense and/or bind a common upstream activation signal or signals, which to date remain to be fully described. What has been shown is that various intracellular events, often caused by cellular stress, can facilitate NLRP3 activation; these include alterations in redox potential, lysosomal stability, and ion concentrations (Figure 1).
Figure 1.
Multiple levels of NLRP3 inflammasome regulation. Various types of cellular stress, including intracellular ROS production, lysosomal leakage, and ion fluxes (K+ efflux and Ca2+ influx), can trigger activation of the NLRP3 inflammasome. The events leading to NLRP3 activation appear to involve pathways mediating mitochondrial damage and the release of mitochondrial content into the cytosol [eg, oxidized DNA (oxDNA) and cardiolipin]. In addition, high levels of eATP activate NLRP3 after eATP binding the P2X7 receptor. Activation of NLRP3 leads to maturation and release of IL-1β and IL-18 cytokines after caspase-1–dependent proteolysis. In addition, capase-1 activation results in cell death via pyroptosis. The activation status of NLRP3 is modulated on multiple levels, to avoid aberrant activation. 1) In macrophages, NLRP3 (and pro-IL-1β) protein levels are controlled by a delayed transcriptional priming step mediated via activation of PRRs and cytokine receptors upstream of the transcription factor NFκB. 2) In resting myeloid cells, NLRP3 is negatively regulated via miR-223 at the post-transcriptional level. 3) Acutely, NLRP3 can be activated by BRCC3-dependent deubiquitination (de-Ub). The kinase activity of Syk, PKR, and TAK1 all play a role in NLRP3 activation, suggesting phosphorylation of NLRP3 may also be an activation requirement. 4) Type I IFN or IFNGR signaling leads to production of NO via activation of inducible nitric oxide synthase (iNOS). NO can inhibit NLRP3 inflammasome formation via SNO modification.
Intracellular ROS
Oxidative stress in the form of reactive oxygen species (ROS) has been widely implicated in NLRP3 activation. Initially, intracellular ROS produced via the NADPH oxidase system were thought to activate NLRP3; however, both mouse and human cells defective in NADPH oxidase exhibit normal NLRP3 activation.19,20 More recently, mitochondrial ROS have been associated with NLRP3 activation.21–23 The precise role of ROS remains somewhat controversial, because ROS may be required only during the transcriptional priming step, rather than for post-translational NLRP3 activation itself.24
Lysosomal Destabilization
Another form of cell stress can be induced by the ingestion of fibrillar protein aggregates (eg, amyloid β) or crystalline structures (eg, cholesterol crystals) by immune cells, leading to NLRP3 activation through the induction of lysosomal perturbation, and the release of proteases such as cathepsins.25–27 The mechanisms by which lysosomal damage induces NLRP3 activation remain poorly understood. Of note, although proteolytic cleavage of NLRP3 is yet to be reported, such a mechanism has been shown to be critical for activation of the NLRP1 inflammasome.28,29
Ion Flux
Changes in cytosolic ion levels, such as increases in Ca2+ 30–32 or decreases in K+,13,33,34 also appear to be important for NLRP3 function. A recent study in which Muñoz-Planillo et al35 extensively tested many of the candidate upstream NLRP3 activators suggests that K+ efflux may represent a common signal required for NLRP3 activation.
The exact mechanism by which NLRP3 is activated remains a subject of vigorous research, but it is likely to involve a culmination or a convergence of the upstream events discussed above. Interestingly, there is a growing body of evidence to suggest that signals emanating from damaged mitochondria could be the common feature linking these intracellular events. First, several studies have demonstrated that NLRP3 localizes to the mitochondria after activation,21,36 potentially via interaction with mitochondrial antiviral-signaling protein (MAVS),37 and efflux of K+ directly from mitochondria is known to modulate the production of mitochondrial ROS.38 Phagolysosomal rupture has been shown to induce Ca2+ mobilization, which can subsequently induce mitochondrial damage and activation of NLRP3.30 Damage to the mitochondria by Ca2+ can result in ROS production, as well as in release of other mitochondrial-derived products that could potentially be sensed by NLRP3, such as oxidized mitochondrial DNA.30,39 In 2013, Iyer et al40 reported that NLRP3 activators can induce the release of the mitochondrial membrane lipid cardiolipin and showed that binding of cardiolipin to the LRR region of NLRP3 in parallel with K+ efflux is required for NLRP3 activation in macrophages, independent of mitochondrial ROS production. Taken together, these findings are suggestive of a central role for mitochondrial dysfunction and potentially mitochondrial cardiolipin in activation of NLRP3. Nonetheless, further investigation is required to clearly establish how activation of the NLRP3 inflammasome occurs.
Multiple Levels of NLRP3 Regulation
Although it remains unclear exactly how NLRP3 becomes activated, extensive research to identify a common activator has revealed the complex nature in which NLRP3 itself is directly regulated on various levels, ranging from transcriptional control to post-translational protein modifications (Figure 1).
Transcriptional Control
In macrophages, endogenous NLRP3 levels are not sufficient to facilitate inflammasome activation; consequently, these cells require an initial NFκB-dependent transcriptional priming step to induce NLRP3 protein to a functional level before its activation. Thus, the sensitivity of immune cells to NLRP3 stimuli is under the control of other innate immune signaling receptors (eg, TLRs) or cytokine receptors (eg, TNFR), which can induce the transcription of NLRP3.41,42 In macrophages, several hours of stimulation are required to achieve optimal levels of NLRP3 protein sufficient for inflammasome activation. Moreover, constitutive NLRP3 overexpression, which allows priming-independent caspase-1 cleavage in response to stimuli, demonstrates that the level of NLRP3 protein is rate-limiting for its activation.41,43
Post-Transcriptional Regulation
More recently, it was also demonstrated that NLRP3 expression is negatively regulated in cells of the myeloid lineage (CD11b+) on the post-transcriptional level by a specific miRNA, miR-223.44,45 Binding of miR-223 to a conserved site within the 3′-untranslated region of NLRP3 results in reduced translation of NLRP3 protein and a subsequent reduction in inflammasome activation. Interestingly, expression of miR-223 is not under the control of a specific proinflammatory signal, but rather exhibits differential expression among myeloid cells: high in neutrophils, moderate in macrophages, and low in dendritic cells (DCs). Thus, the regulatory system mediated by miR-223 has likely evolved to allow for cell-specific sensitivity to NLRP3 activators and the requirement for an additional level of transcriptional regulation in some immune cells, thus avoiding aberrant NLRP3 inflammasome activation.
Post-Translational Modifications and NLRP3 Activation
Several recent reports suggest that NLRP3 must undergo post-translational modifications before inflammasome activation that are independent of its transcriptional requirements. Indeed, acute lipopolysaccharide treatment of macrophages (for as little as 10 minutes) was shown to mediate subsequent NLRP3-dependent caspase-1 cleavage, even under conditions of protein synthesis inhibition.43 This may be explained by a rapid mitochondrial ROS-dependent deubiquitination event on NLRP3, which was shown to be required before activation.43 The deubiquitinase responsible for mediating this NLRP3 modification was subsequently identified as BRCC3.46
Aside from deubiquitination, other protein modifications are likely to govern the activation status of NLRP3. Although no reports to date have demonstrated direct phosphorylation of NLRP3, several studies suggest that kinase activity may also regulate its activation status. Indeed, the tyrosine kinase Syk has been implicated in NLRP3 activation during the antifungal response to Candida albicans. Recognition of C. albicans by immunoreceptor tyrosine-based activation motif (ITAM)-coupled receptors induces Syk activation and signaling resulting in formation of the NLRP3 inflammasome, as well as synthesis of its substrate pro-IL-1β.47 In addition, Lu et al48 recently reported that protein kinase R (PKR) directly interacts with NLRP1, NLRP3, NLRC4, and AIM2 and that genetic ablation of the kinase domain of this protein severely impairs inflammasome-induced caspase-1 cleavage and IL-1β secretion. Given that PKR appears to be required for activation of several inflammasomes, placement of this protein kinase upstream of these receptors is unlikely, because this would remove the ligand specificity of their activation. More recent findings in macrophages reported no dependence on PKR during NLRP3 inflammasome activation.49 The reason for these discrepancies remains unclear, and further studies need to be conducted to confirm a role for this protein kinase in NLRP3 activation. The kinase activity of TGF-β-activated kinase 1 (TAK1) also appears to play a role in NLRP3 activation, because treatment of macrophages with a specific TAK1 inhibitor (5Z-7-oxozeaenol) blocks NLRP3 inflammasome activation independent of its ability to inhibit TLR-induced NFκB responses.50 Interestingly, TAK1 activation after intracellular Ca2+ mobilization has also been shown to be required for NLRP3 activation under conditions of cellular perturbation induced by cell swelling.51
Taken together, the findings on Syk, PKR, and TAK1 raise the possibility that activation of an upstream protein kinase may potentially regulate the phosphorylation status of NLRP3 and its ability to form a functional inflammasome. Indeed, a phosphorylation event has been shown to be critical for the function of the NLRC4 inflammasome.11,52 A single phosphorylation site at Ser533 by protein kinase Cδ (PKCδ) was identified by affinity purification and subsequent mass spectrometry of a tagged version of NLRC4 from Salmonella typhimurium–infected macrophages.52 In a similar fashion as for NLRC4, such proteomic approaches could yield valuable insights into the post-translational regulation of NLRP3 and its activation mechanism.
Post-Translational Modifications and NLRP3 Inhibition
The inflammatory response driven by activation of the NLRP3 inflammasome can be crucial for clearance of invading microbial pathogens; however, its activation must be shut down in a timely manner, to avoid the possibly damaging effects of prolonged inflammation. One such mechanism was recently described whereby NLRP3 inflammasome activation can be subdued after post-translational modification induced by exposure to nitric oxide (NO).53,54 Production of intracellular NO downstream of type I or type II interferon (IFN) receptor signaling (via IFNAR or IFNGR, respectively) was found to lead to thiol S-nitrosylation (SNO) of NLRP3, thereby inhibiting its ability to interact with ASC and to form an inflammasome in macrophages. This mechanism is particularly important in the control of lung immunopathology during Mycobacterium tuberculosis infection. Macrophages infected by M. tuberculosis activate the NLRP3 inflammasome, resulting in secretion of IL-18, which can subsequently stimulate the production of IFN-γ from T cells or natural killer cells. In turn, IFN-γ can activate IFNGR on macrophages to stimulate NO production and the nitrosylation of NLRP3, thus preventing further NLRP3 activation.53
NLRP3 Expression in the Lung
Most studies on the regulation and function of inflammasomes have been performed on murine bone marrow–derived macrophages or DCs. As noted above, the inflammasomes likely play important roles in mediating an antimicrobial response in tissues. In addition, chronic activation of inflammasomes in tissue-resident immune cells or even stromal cells could contribute to pathology such as chronic inflammation or fibrotic responses. An examination across murine tissues found Nlrp3 mRNA to be most highly expressed in the spleen, and next highest in the lung.55 The high expression of NLRP3 in the lung was attributed to the large amount of immune cells that populate this organ. Indeed, alveolar macrophages comprise more than 90% of cells obtained from the bronchoalveolar lavage (BAL) fluid of naïve mice.56 Alveolar macrophages express high levels of Nlrp3 mRNA, as do other myeloid cells such as DCs derived from murine lungs (Immunological Genome Project Consortium57), and are the primary source of IL-1β and IL-18 produced locally. In addition to pulmonary macrophages and DCs, lung epithelial cells also express NLRP3 and produce IL-1β in response to several stimuli.58,59
Activation of the NLRP3 inflammasome is thought to contribute to a number of inflammatory conditions. In the following sections, we provide a short overview of the emerging role of NLRP3 in various lung pathologies.
NLRP3 Inflammasome in Host Defense against IAV
IAV infects millions of people worldwide during seasonal flu epidemics, placing a significant financial burden on national health care systems. In the United States alone, IAV accounts for nearly 40,000 deaths per year.60 Several studies have revealed a protective phenotype for NLRP3 in mouse models of IAV infection, as demonstrated by reduced morbidity in NLRP3-deficient and caspase-1–deficient animals.61–63 The increased mortality of Nlrp3-deficient mice correlated with significantly lower IL-1β and IL-18 cytokine levels and less cellular infiltration in the BAL fluid after intranasal challenge with IAV. Given its protective role in IAV infection, and the high yearly infection rate for IAV, it is conceivable that there is a strong evolutionary pressure on the NLRP3 gene. To date, the signal responsible for NLRP3 activation after IAV remains unclear, but it may be via direct recognition of the single-stranded RNA virus itself,62 or possibly via the function of viral encoded proteins.63,64
More broadly, IAV infection evokes an integrated innate immune response dependent on members of multiple PRR families in addition to NLRP3. Several of these signaling pathways lead to strong production of type I IFNs, including recognition of viral RNA by TLR365 and activation of RIG-I by 5′-triphosphate on genomic viral single-stranded RNA.66 Although production of type I IFN can negatively regulate NLRP3 activation via nitrosylation (as discussed above), it can also be important in regulating its activation in the context of specific infections. Rathinam et al67 recently reported that, after TLR4-mediated recognition of Gram-negative bacteria, TIR domain-containing adaptor protein inducing IFN-β (TRIF) mediates the expression and activation of caspase-11 via type I IFN signaling, which in turn synergizes with caspase-1 during NLRP3 inflammasome activation. Although this concept remains to be tested experimentally in the case of IAV infection, we are tempted to speculate that the strong IFN response evoked by PRRs during IAV may also play a role in NLRP3 inflammasome activation via the TRIF–caspase-11 axis.
The NLRP3 Inflammasome in Pulmonary Fibrosis
Occupational Pulmonary Fibrosis
Asbestos and silica are naturally occurring minerals with distinct chemical and physical properties. For many years asbestos was a common material used in industry and construction, until it was revealed that extended exposure to asbestos fibers could lead to fibrosis of lung tissue and the form of pneumoconiosis now termed asbestosis.68 Prolonged inhalation of dust containing crystalline silicon dioxide (silica crystals), such as is encountered in the various mining, construction, and manufacturing industries, triggers similar lung pathologies in the form of pneumoconiosis termed silicosis. Inhalation of asbestos fibers or silica crystals leads to their deposition within the small airways of the lung, where they are encountered by resident cells of the innate immune system, such as alveolar macrophages and DCs. Phagocytosis of these particulates by macrophages elicits the sustained inflammatory state that is a hallmark of both diseases.68 This chronic inflammation ultimately leads to pulmonary fibrosis, often progressing to pneumoconiosis and lung cancer.
The damaging inflammation responsible for driving these processes is dependent on aberrant activation of the NLRP3 inflammasome.25,69,70 In response to silica and asbestos, macrophages secrete IL-1β in a manner dependent on NLRP3 activation after lysosomal disruption and intracellular ROS production. Furthermore, after intranasal administration of asbestos, Nlrp3-deficient mice showed diminished recruitment of inflammatory cells into the lungs, which correlated with a significant decrease in IL-1β cytokine present in the BAL fluid.70 Similarly, in response to silica inhalation, Asc-deficient and Nlrp3-deficient mice exhibited significantly less infiltration of inflammatory cells into alveoli (ie, reduced granuloma formation), as well as reduced collagen deposition and pulmonary fibrosis, compared with wild-type animals.69 Taken together, these findings demonstrate that inflammation mediated through recognition of exogenous particulates (danger signals) by the NLRP3 inflammasome can lead to progression of chronic occupational lung pathologies.
IPF
In contrast to asbestosis and silicosis, most cases of pulmonary fibrosis are idiopathic, with unknown causative agents. IPF is a progressive and fatal interstitial pneumonitis characterized by recurrent episodes of acute lung injury with subsequent scarring and lung disease. There is currently no effective medical therapy.
The most widely used model of experimental IPF is that induced by instillation of bleomycin, an antitumor agent that induces DNA damage via oxidative injury and cell death of alveolar macrophages and epithelial cells.71 Inflammation, repair, and fibrosis in this model are dependent on IL-1β production and IL-1R1/MyD88 signaling.72 Of note, bleomycin-induced IL-1β production is dependent on the adapter molecule ASC. These studies were extended to investigate the upstream mechanisms leading to IL-1β release and identified a critical role for the NLRP3 inflammasome in the pathology of bleomycin-induced lung injury.73 Nlrp3-deficient mice exhibited a significant reduction in neutrophil recruitment and active matrix metalloproteinase 2 (MMP-2) in the BAL fluid, compared with wild-type mice. Interestingly, accumulation of uric acid was also observed in the BAL fluid after bleomycin induction. Inhibition of uric acid synthesis with allopurinol or administration of uricase, which converts uric acid to more soluble allantoin, significantly diminished bleomycin-induced increase in uric acid, neutrophil influx, and IL-1β production. Notably, uric acid crystals administered intranasally were engulfed by alveolar macrophages and induced a dose-dependent macrophage and neutrophil recruitment into the BAL fluid. This was dependent on the NLRP3 inflammasome, as demonstrated by a significant decrease in IL-1β production and neutrophil recruitment into BAL fluid of Nlrp3-deficient mice.73 Taken together, these findings suggest that bleomycin-induced lung injury results in local accumulation of uric acid in the lung that undergoes phase transition to form uric acid crystals, which may in turn activate the NLRP3 inflammasome and result in IL-1β production and pulmonary fibrosis.
The NLRP3 inflammasome has also been implicated in a model of lung injury induced by mechanical ventilation. Interestingly, in this model of ventilator-induced lung injury, uric acid accumulated in the BAL fluid.74 A subsequent study implicated eATP, another known activator of the NLRP3 inflammasome, as a mediator of bleomycin-induced fibrosis.75 Elevated levels of eATP were found in the BAL fluid of patients with IPF and in mice after bleomycin instillation. Strikingly, patients with exacerbated IPF exhibited a fourfold to fivefold increase in eATP levels in the BAL fluid. The mechanism proposed is that the P2X7 receptor is activated by eATP, leading to activation of the NLRP3 inflammasome and mature IL-1β production.75 Interestingly, in addition to bleomycin, the chemotherapeutic agents gemcitabine and 5-fluorouracil have recently been shown to activate NLRP3 in myeloid-derived suppressor cells.76 Taken together, these findings support a significant role for NLRP3 in IPF.
A Controversial Role for the NLRP3 Inflammasome in Allergic Asthma
An estimated 300 million people currently live with asthma worldwide, and an alarming 250,000 people die from the disease each year.4 Allergic asthma is an inflammatory airway disease exhibiting a distinct inflammatory profile through activation of the adaptive T helper 2 (Th2) pathway, which is initiated by allergen uptake and processing by antigen-presenting cells. The ensuing Th2-type response leads to airway eosinophilia, mucus hypersecretion, structural changes to the airway wall, and various types of airway obstruction.77 TLR4 activation is critical for allergic lung inflammation, and low levels of lipopolysaccharide have been shown to enhance the Th2-type response to allergens.78 A role for the NLRP3 inflammasome remains controversial, however, although some indirect evidence for NLRP3 activation in allergic airway disease exists. This evidence includes elevated eATP in the BAL fluid after allergen challenge,79 which can trigger the NLRP3 inflammasome via the P2X7 ion channel, as well as increased IL-1β cytokine levels in the serum,80 induced sputum,81 and BAL fluid82 of asthma patients.
Mouse models of allergic asthma classically involve sensitization and challenge with various protein antigens, such as ovalbumin (OVA), or encompass inhalation of aeroantigens, such as house dust mite (HDM). Notably, HDM and the widely used Th2-promoting adjuvant aluminum hydroxide (alum) are known activators of the NLRP3 inflammasome.25,70,83 However, controversy exists as to whether NLRP3 plays a role in alum-induced adjuvanticity and induction of Th2-type immunity. A crucial role for the NLRP3 inflammasome in development of allergic airway inflammation was described in both an adjuvant-dependent (alum-OVA) and an adjuvant-free (OVA) model of allergic asthma.84,85 Th2 cell priming was impaired in mice deficient in Nlrp3, Asc, or caspase-1, as demonstrated by decreased OVA-specific Ig antibody induction, airway eosinophilia, and Th2 cytokine production.84,85 Reduced Th2-type responses were also observed for mice deficient in IL-1R1, IL-1β, and IL-1α, confirming the critical role of IL-1R1 signaling in allergic inflammation.84
In contrast to these reports, Kool et al86 suggested that NLRP3 does not significantly contribute to either OVA-mediated or HDM-mediated allergic airway inflammation in mice. Their study identified uric acid as a potent Th2-cell adjuvant acting independent of the NLRP3 inflammasome–IL-1 axis. In the proposed mechanism, uric acid is both necessary and sufficient to induce Th2-mediated immune responses in mice by triggering DC activation in a Syk-dependent and PI3-kinase δ–dependent manner.86 More recently, Allen et al87 compared four different allergic models and found no difference between allergic response in Nlrp3-deficient mice, compared with controls. In fact, only the adjuvant-free OVA model showed a modest and selected role for NLRP3. Several explanations have been proposed to account for the discrepancies among studies, including variations in the preparation, type, route of administration, and concentration of the antigen used, the timing of the model, or differences in composition of the host microbiome.87,88 Although such differences may, in part, help explain the discrepancies between studies, the role of NLRP3 in allergic asthma remains unclear and requires further investigation. The contribution of NLRP3 to the allergic asthma models described above is summarized in Table 1.
Table 1.
Contribution of NLRP3 in Animal Models of Allergic Airway Disease
| Animal model | References |
|
|---|---|---|
| Role for NLRP3 | No Role for NLRP3 | |
| Adjuvant-free OVA model: sensitization and intranasal challenge with OVA | Besnard et al84 | Allen et al87 |
| Adjuvant-dependent OVA model: sensitization with OVA and adjuvant; intranasal challenge with OVA | Eisenbarth et al85 | Kool et al86; Allen et al87 |
| House dust mite (HDM)–driven model: intranasal exposure to HDM antigen | Kool et al86; Allen et al87 | |
In addition to the conventional Th2-type response to allergens, the NLRP3 inflammasome has been implicated in mixed Th2- and Th17-mediated allergic airway disease models.89 Of note, in these allergic sensitizations, serum amyloid A expression is induced in the airways, which can provoke a robust IL-1β–dependent inflammatory response. Serum amyloid A is found in human asthma patients,90,91 and it can activate several PRRs, including the NLRP3 inflammasome.89 Thus, it appears possible that, especially in severe asthma, NLRP3 inflammasome activation contributes to lung pathology. A role for the inflammasome-dependent cytokine IL-18 has also been described. Of particular interest, IL-18 is a known trigger of several Th2-like cytokines, including IL-4, IL-5, IL-9, and IL-13. In a murine model of IL-18 overexpression in the lung, anti-CD4 antibody therapy or deletion of IL-13 led to improvement of OVA-induced airway hyperresponsiveness and airway inflammation. These studies suggest that IL-18 contributes to lung pathology driven by activated T cells and T cell–derived cytokines.92
A Possible Role for the NLRP3 Inflammasome in COPD
COPD was responsible for 5% of deaths globally in 2005 [Chronic obstructive pulmonary disease (COPD), World Health Organization Fact sheet no. 315; http://www.who.int/mediacentre/factsheets/fs315/en, last accessed October 22, 2013], and it is projected to be the fourth leading cause of death by 2030.4 COPD is a multicomponent disease manifested as chronic bronchitis, chronic airway obstruction, and emphysema. This results in shortness of breath, increasing cough and sputum production, and progressive airflow obstruction that is not fully reversible.93 Development of the chronic inflammatory airway pathology in COPD is thought to be caused by inhalation of noxious particles or gas, most commonly cigarette smoke (CS). Acute symptomatic exacerbations in COPD patients, which are commonly brought about by secondary viral or bacterial infections of the lung, contribute to structural changes in the airway. Many cell types in the lung (including epithelial cells, alveolar macrophages, and DCs), as well as immune cells recruited from the periphery, respond to the noxious inhaled substances that cause COPD. Activated cells produce proinflammatory cytokines, ROS, and tissue-degrading enzymes, which mediate tissue injury and remodeling, emphysema induction, and chronic inflammation.94 CS causes alveolar epithelial cell injury,95 leading to infiltration of inflammatory cells into the mucosa, submucosa, and glandular tissue to orchestrate the innate inflammatory response.93 In addition, airway epithelial cells release transforming growth factor β (TGF-β), which contributes to the induction of fibrotic tissue remodeling.96
Although several indirect lines of evidence link inflammasome-dependent cytokines to disease pathology of COPD,97,98 a direct role for the NLRP3 inflammasome has yet to be clearly shown. Cigarette smoking leads to IL-1β release in the human lung.99 In addition, elevated levels of IL-1α and IL-1β are found in the lungs of COPD patients, and their secretion is amplified in lungs during disease exacerbations.100,101 Furthermore, mice overexpressing IL-1β in the lung present a phenotype similar to COPD, including lung inflammation, emphysema, and pulmonary fibrosis,102 and mice lacking IL-1R are profoundly protected from CS lung pathology.103–105
Another IL-1 family cytokine, IL-18, is also processed via the NLRP3 inflammasome, and it appears to be causally related to COPD. In patients with COPD, IL-18 levels are elevated in blood and lungs.106,107 IL-18 levels in sputum108 and even serum109 inversely correlate with lung function in COPD patients, suggesting a significant role in pathogenesis. Additionally, compelling data have emerged from the generation of lung-specific transgenic mice overexpressing IL-18, which undergo chronic inflammatory changes in the lungs with severe emphysematous and associated pulmonary hypertension similar to that seen in COPD.110 Furthermore, mice deficient in IL-18R are partially protected from CS-induced lung injury and inflammation.106
Inhibition of caspase-1 in a murine CS-induced emphysema model significantly decreased airway inflammation.111 Notably, increased caspase-1 activation was observed in lung samples from smokers and emphysema patients, compared with nonsmokers.112 Taken together, these findings suggest that inflammasome activation occurs in the lungs of COPD patients.
CS contains a wide variety of toxic molecules that can trigger innate immune receptors, including TLRs and inflammasomes. Additionally, the induction of cell death by CS leads to the release of endogenous danger signals. For example, the chromatin-binding high mobility group box 1 protein (HMGB1) is found in BAL fluid113 and sputum114 of COPD patients. HMGB1 is a known TLR activator,115,116 and could contribute to the inflammatory response in the lung by eliciting proinflammatory cytokines and the priming of cells for inflammasome activation. Also, various molecules that are known activators of the NLRP3 inflammasome are found at elevated levels after CS exposure or in the lungs of COPD patients. For example, eATP accumulates in the airways of both COPD patients and animal models of COPD, and a growing body of evidence highlights eATP and the P2X7 receptor in the pathogenesis of lung diseases.94 In a study by Eltom et al,112 CS exposure was found to induce neutrophilia, leading to increased caspase-1 activity and to release of IL-1β and IL-18 in the lungs of mice. Pharmacological blockade or genetic deficiency of the P2X7 receptor attenuated CS-induced caspase-1 activation, IL-1β release, and airway neutrophilia in this model.112
Interestingly, increases in uric acid levels have also been observed in the BAL fluid from COPD patients.98 As has been observed in bleomycin-induced models of IPF, the presence of elevated uric acid may result in the formation of uric acid crystals within the lungs, which could subsequently activate the NLRP3 inflammasome. Taken together, these findings suggest a possible role for NLRP3 inflammasome–mediated production of IL-1 family cytokines in COPD. We speculate that exacerbations of COPD could also involve the NLRP3 inflammasome. In 60% to 80% of cases, these episodes are triggered after viral and bacterial infections of the respiratory tract.93 Many of these pathogens are likely recognized via PRRs, including TLRs. Activation of such pathways induce the transcriptional priming of NLRP3 and in turn could enhance its subsequent activation by eATP or uric acid crystals present in COPD lungs at high concentrations.
Although there is now compelling evidence that inflammasome-dependent cytokines are found in COPD, and that triggers of the NLRP3 inflammasome are elevated during disease pathogenesis, direct evidence that the NLRP3 inflammasome is indeed driving COPD remains to be clearly established. To our knowledge, only one study has directly examined the role of NLRP3 in a model of pulmonary inflammation after CS exposure by using Nlrp3-deficient mice. In that study, Pauwels et al105 found that, although IL-1β and IL-1R were central for mediating pulmonary inflammation after CS exposure, NLRP3 and caspase-1 appeared to play no role. These findings could indicate that NLRP3 inflammasome activation may be required only at certain phases of disease pathology in this model.
Alternatively or additionally, in this model IL-1β could also be processed in an NLRP3-independent manner during the CS-induced lung inflammation. Indeed, independent of inflammasomes, IL-1β processing by inflammatory caspases other than caspase-1 has been identified,117,118 and there are other proteases that can mediate NLRP3 inflammasome-independent IL-1β processing.119 Furthermore, the importance of IL-1R signaling in COPD models may also be explained by findings showing that IL-1α is a key proinflammatory cytokine linked to CS-induced inflammation and lung injury. Neutralization of IL-1α (but not of IL-1β) reduced CS-mediated lung neutrophilia,103 as well as DC accumulation and activation in the lungs.120 In contrast to IL-1β, both the precursor and cleaved forms of IL-1α are biologically active. IL-1α can be released from dying cells,121,122 and thus could lead to IL-1–mediated inflammatory responses. Additionally, in response to eATP, for example, IL-1α release is also regulated by NLRP3,123 and thus NLRP3 activation may contribute to IL-1α–mediated responses. Therefore, as in the case of studies of asthma, further investigation is required before ruling out a possible contribution of NLRP3 activation during the pathogenesis of COPD.
Conclusions and Perspectives
PRRs constitute an integral component of the immune system of the lung and are necessary for inflammatory processes involved in defense against invading pathogens and for restoration of tissue homeostasis. However, erroneous activation of the innate defense mechanisms in the lung can have drastic consequences for the host. Although many different PRRs are undoubtedly triggered during inflammatory conditions in the lung and likely contribute to disease manifestation, several lines of evidence suggest a central role for the NLRP3 inflammasome.
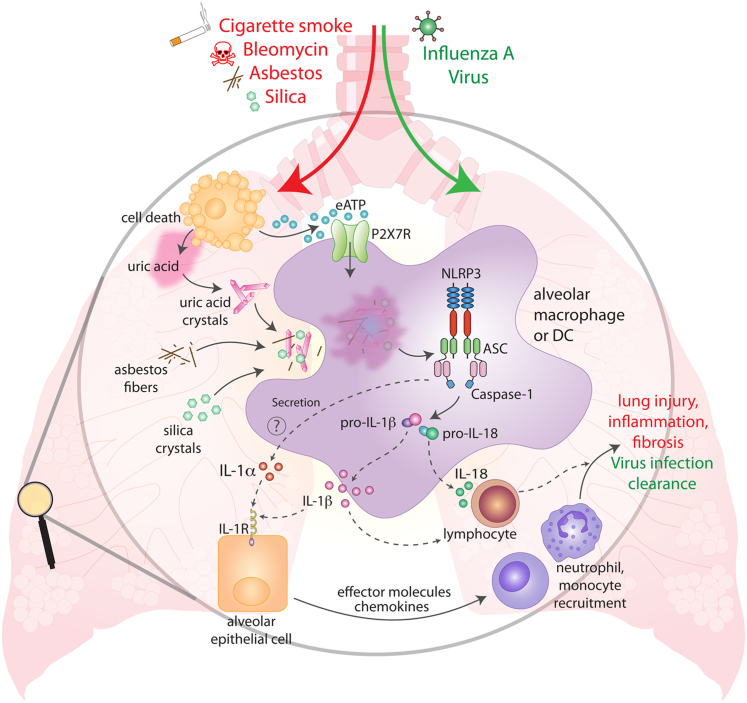
Here, we have described recent findings relevant to the understanding of NLRP3 regulation and activation. In addition, we have summarized how innate immune mechanisms, especially activation of the NLRP3 inflammasome, can contribute to lung pathology in several disease conditions (Figure 2). Acutely, activation of NLRP3 is important for the clearance of viral and bacterial lung infections. However, sustained activation of NLRP3 after inhalation of irritants can lead to more chronic and deleterious inflammatory effects in the lung. The accumulation of well-defined NLRP3 activators, eATP and uric acid crystals, as well as the presence of inflammasome-dependent cytokines in patients with chronic inflammatory lung pathologies strongly suggests an involvement of the NLRP3 inflammasome. A possible scenario leading to chronic inflammation in pulmonary tissues could involve activation of alveolar epithelial cells by IL-1β and IL-1α cytokines produced from alveolar macrophages or DCs through continual activation of the NLRP3 inflammasome by inhaled irritants or locally produced danger signals. Activation of epithelial cells could then trigger the production of chemokines and effector molecules that mediate a robust inflammatory response. In addition, NLRP3-dependent secretion of IL-1β and IL-18 from lung macrophages or DCs could result in activation and polarization of lymphocytes (Figure 2). Persistent production of such inflammatory mediators could lead to the tissue injury and fibrosis seen in such chronic lung pathologies.
Figure 2.
The NLRP3 inflammasome in lung inflammation and injury. Evidence from patients with lung diseases and experimental animal models suggests that a number of inhaled triggers can cause NLRP3 inflammasome activation in the lung, including cigarette smoke, asbestos, silica, bleomycin, and IAV. Inhaled silica crystals or asbestos fibers can induce NLRP3 activation directly via lysosomal damage and ROS production after phagocytosis by alveolar macrophages. The NLRP3 inflammasome can also be activated indirectly in the lung after the release of danger signals from dying or injured cells (eg, eATP and uric acid crystals). Activation of the NLRP3 inflammasome drives the production of IL-1β and IL-18 cytokines, causing the infiltration of additional immune cells and lymphocytes that sustain the inflammatory response potentially leading to chronic lung injury and pulmonary fibrosis.
Advances in our understanding of the detrimental effects of inhaled irritants such as asbestos and silica have assisted in minimizing exposure to these NLRP3 activators. However, another potential threat to the lung comes from the increasing use of nanoparticles (eg, titanium dioxide) in diverse products and manufacturing processes, including cosmetics, biomedicine, and electronics. Recent studies have revealed that nanoparticles can trigger the NLRP3 inflammasome.124,125 Alarmingly, inhalation of nanoparticles evokes a tissue response similar to that seen for asbestos and silica,126 raising the concern that long-term exposure to these materials could cause chronic pathologies similar to those observed after inhalation of asbestos and silica.
As we have outlined above, in some murine models of chronic lung diseases, including asthma and COPD, the role of NLRP3 remains unclear. Therefore, although much progress has been made toward the understanding of inflammatory lung pathologies, many challenges remain for full characterization of the role of NLRP3 in these contexts. For instance, future efforts should aim at better defining in which tissue-resident cells the NLRP3 inflammasome is activated in various lung disease models and which cells are activated downstream by inflammasome effector molecules. A further challenge will be to dissect the contribution of IL-1β and IL-1α to these disease pathologies. This is of particular importance, because pharmacological approaches to directly target IL-1β cytokines and their signaling receptors are currently being pursued.127 The partially redundant and complementary roles of inflammasome effector molecules in lung pathologies also warrant the search for novel therapeutics that directly target the NLRP3 inflammasome or mechanisms that control its activation.
Acknowledgment
We thank Andrea Stutz for helpful discussion and critical review of the manuscript.
Footnotes
Supported in part by grants from the NIH and the German Research Foundation–Deutsche Forschungsgesellschaft (DFG) (E.L.). E.L. is a member of the DFG Cluster of Excellence ImmunoSensation, the German Center for Infection Research (DZIF) in Bonn, Germany, and the Center of Molecular Inflammation Research at the Department of Cancer Research and Molecular Medicine at the Norwegian University of Science and Technology (NTNU) in Trondheim, Norway.
References
- 1.Toews G.B. Cytokines and the lung. Eur Respir J Suppl. 2001;34:3s–17s. doi: 10.1183/09031936.01.00266001. [DOI] [PubMed] [Google Scholar]
- 2.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 3.De Nardo D., Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011;32:373–379. doi: 10.1016/j.it.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bousquet J., Khaltaev N., editors. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. WHO Press; Geneva: 2007. pp v, 15, 21. [Google Scholar]
- 5.Dinarello C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 6.Latz E., Xiao T.S., Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D.R., Latz E., Fitzgerald K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerur N., Veettil M.V., Sharma-Walia N., Bottero V., Sadagopan S., Otageri P., Chandran B. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryant C., Fitzgerald K.A. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol. 2009;19:455–464. doi: 10.1016/j.tcb.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Duncan J.A., Bergstralh D.T., Wang Y., Willingham S.B., Ye Z., Zimmermann A.G., Ting J.P. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci USA. 2007;104:8041–8046. doi: 10.1073/pnas.0611496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Z., Yan C., Liu P., Huang Z., Ma R., Zhang C., Wang R., Zhang Y., Martinon F., Miao D., Deng H., Wang J., Chang J., Chai J. Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science. 2013;341:172–175. doi: 10.1126/science.1236381. [DOI] [PubMed] [Google Scholar]
- 12.Mayor A., Martinon F., De Smedt T., Pétrilli V., Tschopp J. A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat Immunol. 2007;8:497–503. doi: 10.1038/ni1459. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes-Alnemri T., Wu J., Yu J.W., Datta P., Miller B., Jankowski W., Rosenberg S., Zhang J., Alnemri E.S. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miao E.A., Rajan J.V., Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalbeth N., Haskard D.O. Mechanisms of inflammation in gout. Rheumatology (Oxford) 2005;44:1090–1096. doi: 10.1093/rheumatology/keh640. [DOI] [PubMed] [Google Scholar]
- 16.Martinon F. Mechanisms of uric acid crystal-mediated autoinflammation. Immunol Rev. 2010;233:218–232. doi: 10.1111/j.0105-2896.2009.00860.x. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y., Evans J.E., Rock K.L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 18.Pelegrin P., Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282:2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- 19.Latz E. NOX-free inflammasome activation. Blood. 2010;116:1393–1394. doi: 10.1182/blood-2010-06-287342. [DOI] [PubMed] [Google Scholar]
- 20.van Bruggen R., Köker M.Y., Jansen M., van Houdt M., Roos D., Kuijpers T.W., van den Berg T.K. Human NLRP3 inflammasome activation is Nox1-4 independent. Blood. 2010;115:5398–5400. doi: 10.1182/blood-2009-10-250803. [DOI] [PubMed] [Google Scholar]
- 21.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation [Erratum appeared in Nature 2011, 475:122] Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 22.Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M.T., Brickey W.J., Ting J.P. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakahira K., Haspel J.A., Rathinam V.A., Lee S.J., Dolinay T., Lam H.C., Englert J.A., Rabinovitch M., Cernadas M., Kim H.P., Fitzgerald K.A., Ryter S.W., Choi A.M. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauernfeind F., Bartok E., Rieger A., Franchi L., Núñez G., Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornung V., Bauernfeind F., Halle A., Samstad E.O., Kono H., Rock K.L., Fitzgerald K.A., Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halle A., Hornung V., Petzold G.C., Stewart C.R., Monks B.G., Reinheckel T., Fitzgerald K.A., Latz E., Moore K.J., Golenbock D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duewell P., Kono H., Rayner K.J., Sirois C.M., Vladimer G., Bauernfeind F.G., Abela G.S., Franchi L., Núñez G., Schnurr M., Espevik T., Lien E., Fitzgerald K.A., Rock K.L., Moore K.J., Wright S.D., Hornung V., Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals [Erratum appeared in Nature 2010, 466:652] Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finger J.N., Lich J.D., Dare L.C., Cook M.N., Brown K.K., Duraiswami C., Bertin J., Gough P.J. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J Biol Chem. 2012;287:25030–25037. doi: 10.1074/jbc.M112.378323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frew B.C., Joag V.R., Mogridge J. Proteolytic processing of Nlrp1b is required for inflammasome activity. PLoS Pathog. 2012;8:e1002659. doi: 10.1371/journal.ppat.1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murakami T., Ockinger J., Yu J., Byles V., McColl A., Hofer A.M., Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci USA. 2012;109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee G.S., Subramanian N., Kim A.I., Aksentijevich I., Goldbach-Mansky R., Sacks D.B., Germain R.N., Kastner D.L., Chae J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossol M., Pierer M., Raulien N., Quandt D., Meusch U., Rothe K., Schubert K., Schöneberg T., Schaefer M., Krügel U., Smajilovic S., Bräuner-Osborne H., Baerwald C., Wagner U. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun. 2012;3:1329. doi: 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pétrilli V., Papin S., Dostert C., Mayor A., Martinon F., Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 34.Perregaux D., Gabel C.A. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 35.Muñoz-Planillo R., Kuffa P., Martínez-Colón G., Smith B.L., Rajendiran T.M., Núñez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misawa T., Takahama M., Kozaki T., Lee H., Zou J., Saitoh T., Akira S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013;14:454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 37.Subramanian N., Natarajan K., Clatworthy M.R., Wang Z., Germain R.N. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. 2013;153:348–361. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malinska D., Mirandola S.R., Kunz W.S. Mitochondrial potassium channels and reactive oxygen species. FEBS Lett. 2010;584:2043–2048. doi: 10.1016/j.febslet.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Shimada K., Crother T.R., Karlin J., Dagvadorj J., Chiba N., Chen S., Ramanujan V.K., Wolf A.J., Vergnes L., Ojcius D.M., Rentsendorj A., Vargas M., Guerrero C., Wang Y., Fitzgerald K.A., Underhill D.M., Town T., Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iyer S.S., He Q., Janczy J.R., Elliott E.I., Zhong Z., Olivier A.K., Sadler J.J., Knepper-Adrian V., Han R., Qiao L., Eisenbarth S.C., Nauseef W.M., Cassel S.L., Sutterwala F.S. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39:311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bauernfeind F.G., Horvath G., Stutz A., Alnemri E.S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B.G., Fitzgerald K.A., Hornung V., Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franchi L., Eigenbrod T., Núñez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juliana C., Fernandes-Alnemri T., Kang S., Farias A., Qin F., Alnemri E.S. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauernfeind F., Rieger A., Schildberg F.A., Knolle P.A., Schmid-Burgk J.L., Hornung V. NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol. 2012;189:4175–4181. doi: 10.4049/jimmunol.1201516. [DOI] [PubMed] [Google Scholar]
- 45.Haneklaus M., Gerlic M., Kurowska-Stolarska M., Rainey A.A., Pich D., McInnes I.B., Hammerschmidt W., O’Neill L.A., Masters S.L. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1beta production. J Immunol. 2012;189:3795–3799. doi: 10.4049/jimmunol.1200312. [DOI] [PubMed] [Google Scholar]
- 46.Py B.F., Kim M.S., Vakifahmetoglu-Norberg H., Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49:331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Gross O., Poeck H., Bscheider M., Dostert C., Hannesschläger N., Endres S., Hartmann G., Tardivel A., Schweighoffer E., Tybulewicz V., Mocsai A., Tschopp J., Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 48.Lu B., Nakamura T., Inouye K., Li J., Tang Y., Lundbäck P., Valdes-Ferrer S.I., Olofsson P.S., Kalb T., Roth J., Zou Y., Erlandsson-Harris H., Yang H., Ting J.P., Wang H., Andersson U., Antoine D.J., Chavan S.S., Hotamisligil G.S., Tracey K.J. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He Y., Franchi L., Núñez G. The protein kinase PKR is critical for LPS-induced iNOS production but dispensable for inflammasome activation in macrophages. Eur J Immunol. 2013;43:1147–1152. doi: 10.1002/eji.201243187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong Y.N., Wang X., Wang J., Yang Z., Li S., Yang J., Liu L., Lei X., Shao F. Chemical probing reveals insights into the signaling mechanism of inflammasome activation. Cell Res. 2010;20:1289–1305. doi: 10.1038/cr.2010.135. [DOI] [PubMed] [Google Scholar]
- 51.Compan V., Baroja-Mazo A., López-Castejón G., Gomez A.I., Martínez C.M., Angosto D., Montero M.T., Herranz A.S., Bazán E., Reimers D., Mulero V., Pelegrín P. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 2012;37:487–500. doi: 10.1016/j.immuni.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Qu Y., Misaghi S., Izrael-Tomasevic A., Newton K., Gilmour L.L., Lamkanfi M., Louie S., Kayagaki N., Liu J., Komuves L., Cupp J.E., Arnott D., Monack D., Dixit V.M. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature. 2012;490:539–542. doi: 10.1038/nature11429. [DOI] [PubMed] [Google Scholar]
- 53.Mishra B.B., Rathinam V.A., Martens G.W., Martinot A.J., Kornfeld H., Fitzgerald K.A., Sassetti C.M. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nat Immunol. 2013;14:52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernandez-Cuellar E., Tsuchiya K., Hara H., Fang R., Sakai S., Kawamura I., Akira S., Mitsuyama M. Cutting edge: nitric oxide inhibits the NLRP3 inflammasome. J Immunol. 2012;189:5113–5117. doi: 10.4049/jimmunol.1202479. [DOI] [PubMed] [Google Scholar]
- 55.Guarda G., Zenger M., Yazdi A.S., Schroder K., Ferrero I., Menu P., Tardivel A., Mattmann C., Tschopp J. Differential expression of NLRP3 among hematopoietic cells. J Immunol. 2011;186:2529–2534. doi: 10.4049/jimmunol.1002720. [DOI] [PubMed] [Google Scholar]
- 56.Gwyer Findlay E., Hussell T. Macrophage-mediated inflammation and disease: a focus on the lung. Mediators Inflamm. 2012;2012:140937. doi: 10.1155/2012/140937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heng T.S., Painter M.W. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 58.Peeters P.M., Perkins T.N., Wouters E.F., Mossman B.T., Reynaert N.L. Silica induces NLRP3 inflammasome activation in human lung epithelial cells. Part Fibre Toxicol. 2013;10:3. doi: 10.1186/1743-8977-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tran H.B., Lewis M.D., Tan L.W., Lester S.E., Baker L.M., Ng J., Hamilton-Bruce M.A., Hill C.L., Koblar S.A., Rischmueller M., Ruffin R.E., Wormald P.J., Zalewski P.D., Lang C.J. Immunolocalization of NLRP3 inflammasome in normal murine airway epithelium and changes following induction of ovalbumin-induced airway inflammation. J Allergy (Cairo) 2012;2012:819176. doi: 10.1155/2012/819176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taubenberger J.K., Morens D.M. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allen I.C., Scull M.A., Moore C.B., Holl E.K., McElvania-TeKippe E., Taxman D.J., Guthrie E.H., Pickles R.J., Ting J.P. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas P.G., Dash P., Aldridge J.R., Jr., Ellebedy A.H., Reynolds C., Funk A.J., Martin W.J., Lamkanfi M., Webby R.J., Boyd K.L., Doherty P.C., Kanneganti T.D. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ichinohe T., Lee H.K., Ogura Y., Flavell R., Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McAuley J.L., Tate M.D., Mackenzie-Kludas C.J., Pinar A., Zeng W., Stutz A., Latz E., Brown L.E., Mansell A. Activation of the NLRP3 inflammasome by IAV virulence protein PB1-F2 contributes to severe pathophysiology and disease. PLoS Pathog. 2013;9:e1003392. doi: 10.1371/journal.ppat.1003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guillot B., Portalès P., Thanh A.D., Merlet S., Dereure O., Clot J., Corbeau P. The expression of cytotoxic mediators is altered in mononuclear cells of patients with melanoma and increased by interferon-alpha treatment. Br J Dermatol. 2005;152:690–696. doi: 10.1111/j.1365-2133.2005.06512.x. [DOI] [PubMed] [Google Scholar]
- 66.Pichlmair A., Schulz O., Tan C.P., Naslund T.I., Liljeström P., Weber F., Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 67.Rathinam V.A., Vanaja S.K., Waggoner L., Sokolovska A., Becker C., Stuart L.M., Leong J.M., Fitzgerald K.A. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by Gram-negative bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mossman B.T., Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med. 1998;157:1666–1680. doi: 10.1164/ajrccm.157.5.9707141. [DOI] [PubMed] [Google Scholar]
- 69.Cassel S.L., Eisenbarth S.C., Iyer S.S., Sadler J.J., Colegio O.R., Tephly L.A., Carter A.B., Rothman P.B., Flavell R.A., Sutterwala F.S. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dostert C., Pétrilli V., Van Bruggen R., Steele C., Mossman B.T., Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith R.E., Strieter R.M., Phan S.H., Kunkel S.L. C-C chemokines: novel mediators of the profibrotic inflammatory response to bleomycin challenge. Am J Respir Cell Mol Biol. 1996;15:693–702. doi: 10.1165/ajrcmb.15.6.8969262. [DOI] [PubMed] [Google Scholar]
- 72.Gasse P., Mary C., Guenon I., Noulin N., Charron S., Schnyder-Candrian S., Schnyder B., Akira S., Quesniaux V.F., Lagente V., Ryffel B., Couillin I. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest. 2007;117:3786–3799. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gasse P., Riteau N., Charron S., Girre S., Fick L., Pétrilli V., Tschopp J., Lagente V., Quesniaux V.F., Ryffel B., Couillin I. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- 74.Kuipers M.T., Aslami H., Janczy J.R., van der Sluijs K.F., Vlaar A.P., Wolthuis E.K., Choi G., Roelofs J.J., Flavell R.A., Sutterwala F.S., Bresser P., Leemans J.C., van der Poll T., Schultz M.J., Wieland C.W. Ventilator-induced lung injury is mediated by the NLRP3 inflammasome. Anesthesiology. 2012;116:1104–1115. doi: 10.1097/ALN.0b013e3182518bc0. [DOI] [PubMed] [Google Scholar]
- 75.Riteau N., Gasse P., Fauconnier L., Gombault A., Couegnat M., Fick L., Kanellopoulos J., Quesniaux V.F., Marchand-Adam S., Crestani B., Ryffel B., Couillin I. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010;182:774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- 76.Bruchard M., Mignot G., Derangère V., Chalmin F., Chevriaux A., Végran F., Boireau W., Simon B., Ryffel B., Connat J.L., Kanellopoulos J., Martin F., Rébé C., Apetoh L., Ghiringhelli F. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19:57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 77.Barnes P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 78.Eisenbarth S.C., Piggott D.A., Huleatt J.W., Visintin I., Herrick C.A., Bottomly K. Lipopolysaccharide-enhanced, Toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Idzko M., Hammad H., van Nimwegen M., Kool M., Willart M.A., Muskens F., Hoogsteden H.C., Luttmann W., Ferrari D., Di Virgilio F., Virchow J.C., Jr., Lambrecht B.N. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 80.Thomas S.S., Chhabra S.K. A study on the serum levels of interleukin-1beta in bronchial asthma. J Indian Med Assoc. 2003;101:282. 284, 286 passim. [PubMed] [Google Scholar]
- 81.Konno S., Gonokami Y., Kurokawa M., Kawazu K., Asano K., Okamoto K., Adachi M. Cytokine concentrations in sputum of asthmatic patients. Int Arch Allergy Immunol. 1996;109:73–78. doi: 10.1159/000237234. [DOI] [PubMed] [Google Scholar]
- 82.Broide D.H., Lotz M., Cuomo A.J., Coburn D.A., Federman E.C., Wasserman S.I. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992;89:958–967. doi: 10.1016/0091-6749(92)90218-q. [DOI] [PubMed] [Google Scholar]
- 83.Dai X., Sayama K., Tohyama M., Shirakata Y., Hanakawa Y., Tokumaru S., Yang L., Hirakawa S., Hashimoto K. Mite allergen is a danger signal for the skin via activation of inflammasome in keratinocytes. J Allergy Clin Immunol. 2011;127 doi: 10.1016/j.jaci.2010.12.006. 806–814.e1–e4. [DOI] [PubMed] [Google Scholar]
- 84.Besnard A.G., Guillou N., Tschopp J., Erard F., Couillin I., Iwakura Y., Quesniaux V., Ryffel B., Togbe D. NLRP3 inflammasome is required in murine asthma in the absence of aluminum adjuvant. Allergy. 2011;66:1047–1057. doi: 10.1111/j.1398-9995.2011.02586.x. [DOI] [PubMed] [Google Scholar]
- 85.Eisenbarth S.C., Colegio O.R., O’Connor W., Sutterwala F.S., Flavell R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kool M., Willart M.A., van Nimwegen M., Bergen I., Pouliot P., Virchow J.C., Rogers N., Osorio F., Reis e Sousa C., Hammad H., Lambrecht B.N. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 2011;34:527–540. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 87.Allen I.C., Jania C.M., Wilson J.E., Tekeppe E.M., Hua X., Brickey W.J., Kwan M., Koller B.H., Tilley S.L., Ting J.P. Analysis of NLRP3 in the development of allergic airway disease in mice. J Immunol. 2012;188:2884–2893. doi: 10.4049/jimmunol.1102488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kool M., Pétrilli V., De Smedt T., Rolaz A., Hammad H., van Nimwegen M., Bergen I.M., Castillo R., Lambrecht B.N., Tschopp J. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 89.Ather J.L., Ckless K., Martin R., Foley K.L., Suratt B.T., Boyson J.E., Fitzgerald K.A., Flavell R.A., Eisenbarth S.C., Poynter M.E. Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J Immunol. 2011;187:64–73. doi: 10.4049/jimmunol.1100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu T.L., Chang P.Y., Tsao K.C., Sun C.F., Wu L.L., Wu J.T. A panel of multiple markers associated with chronic systemic inflammation and the risk of atherogenesis is detectable in asthma and chronic obstructive pulmonary disease. J Clin Lab Anal. 2007;21:367–371. doi: 10.1002/jcla.20197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ozseker F., Buyukozturk S., Depboylu B., Yilmazbayhan D., Karayigit E., Gelincik A., Genc S., Colakoglu B., Dal M., Issever H. Serum amyloid A (SAA) in induced sputum of asthmatics: a new look to an old marker. Int Immunopharmacol. 2006;6:1569–1576. doi: 10.1016/j.intimp.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 92.Sawada M., Kawayama T., Imaoka H., Sakazaki Y., Oda H., Takenaka S., Kaku Y., Azuma K., Tajiri M., Edakuni N., Okamoto M., Kato S., Hoshino T. IL-18 induces airway hyperresponsiveness and pulmonary inflammation via CD4+ T cell and IL-13. PLoS One. 2013;8:e54623. doi: 10.1371/journal.pone.0054623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Decramer M., Janssens W., Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mortaz E., Folkerts G., Nijkamp F.P., Henricks P.A. ATP and the pathogenesis of COPD. Eur J Pharmacol. 2010;638:1–4. doi: 10.1016/j.ejphar.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 95.Kosmider B., Messier E.M., Chu H.W., Mason R.J. Human alveolar epithelial cell injury induced by cigarette smoke. PLoS One. 2011;6:e26059. doi: 10.1371/journal.pone.0026059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barnes P.J., Shapiro S.D., Pauwels R.A. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 97.Birrell M.A., Eltom S. The role of the NLRP3 inflammasome in the pathogenesis of airway disease. Pharmacol Ther. 2011;130:364–370. doi: 10.1016/j.pharmthera.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 98.Wanderer A.A. Interleukin-1beta targeted therapy in severe persistent asthma (SPA) and chronic obstructive pulmonary disease (COPD): proposed similarities between biphasic pathobiology of SPA/COPD and ischemia-reperfusion injury. Isr Med Assoc J. 2008;10:837–842. [PubMed] [Google Scholar]
- 99.Kuschner W.G., D’Alessandro A., Wong H., Blanc P.D. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. Eur Respir J. 1996;9:1989–1994. doi: 10.1183/09031936.96.09101989. [DOI] [PubMed] [Google Scholar]
- 100.Aaron S.D., Angel J.B., Lunau M., Wright K., Fex C., Le Saux N., Dales R.E. Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:349–355. doi: 10.1164/ajrccm.163.2.2003122. [DOI] [PubMed] [Google Scholar]
- 101.Chung K.F. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2001;34:50s–59s. [PubMed] [Google Scholar]
- 102.Lappalainen U., Whitsett J.A., Wert S.E., Tichelaar J.W., Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 103.Botelho F.M., Bauer C.M., Finch D., Nikota J.K., Zavitz C.C., Kelly A., Lambert K.N., Piper S., Foster M.L., Goldring J.J., Wedzicha J.A., Bassett J., Bramson J., Iwakura Y., Sleeman M., Kolbeck R., Coyle A.J., Humbles A.A., Stampfli M.R. IL-1alpha/IL-1R1 expression in chronic obstructive pulmonary disease and mechanistic relevance to smoke-induced neutrophilia in mice. PLoS One. 2011;6:e28457. doi: 10.1371/journal.pone.0028457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Doz E., Noulin N., Boichot E., Guénon I., Fick L., Le Bert M., Lagente V., Ryffel B., Schnyder B., Quesniaux V., Couillin I. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008;180:1169–1178. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- 105.Pauwels N.S., Bracke K.R., Dupont L.L., Van Pottelberge G.R., Provoost S., Vanden Berghe T., Vandenabeele P., Lambrecht B.N., Joos G.F., Brusselle G.G. Role of IL-1alpha and the Nlrp3/caspase-1/IL-1beta axis in cigarette smoke-induced pulmonary inflammation and COPD. Eur Respir J. 2011;38:1019–1028. doi: 10.1183/09031936.00158110. [DOI] [PubMed] [Google Scholar]
- 106.Kang M., Homer R.J., Gallo A., Lee C.G., Crothers K.A., Cho S.J., Rochester C., Cain H., Chupp G., Yoon H.J., Elias J.A. IL-18 is induced and IL-18 receptor alpha plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J Immunol. 2007;178:1948–1959. doi: 10.4049/jimmunol.178.3.1948. [DOI] [PubMed] [Google Scholar]
- 107.Petersen A.M., Penkowa M., Iversen M., Frydelund-Larsen L., Andersen J.L., Mortensen J., Lange P., Pedersen B.K. Elevated levels of IL-18 in plasma and skeletal muscle in chronic obstructive pulmonary disease [Erratum appeared in Lung 2011, 189:519] Lung. 2007;185:161–171. doi: 10.1007/s00408-007-9000-7. [DOI] [PubMed] [Google Scholar]
- 108.Rovina N., Dima E., Gerassimou C., Kollintza A., Gratziou C., Roussos C. Interleukin-18 in induced sputum: association with lung function in chronic obstructive pulmonary disease. Respir Med. 2009;103:1056–1062. doi: 10.1016/j.rmed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 109.Imaoka H., Hoshino T., Takei S., Kinoshita T., Okamoto M., Kawayama T., Kato S., Iwasaki H., Watanabe K., Aizawa H. Interleukin-18 production and pulmonary function in COPD. Eur Respir J. 2008;31:287–297. doi: 10.1183/09031936.00019207. [DOI] [PubMed] [Google Scholar]
- 110.Hoshino T., Kato S., Oka N., Imaoka H., Kinoshita T., Takei S., Kitasato Y., Kawayama T., Imaizumi T., Yamada K., Young H.A., Aizawa H. Pulmonary inflammation and emphysema: role of the cytokines IL-18 and IL-13. Am J Respir Crit Care Med. 2007;176:49–62. doi: 10.1164/rccm.200603-316OC. [DOI] [PubMed] [Google Scholar]
- 111.Churg A., Zhou S., Wang X., Wang R., Wright J.L. The role of interleukin-1beta in murine cigarette smoke-induced emphysema and small airway remodeling. Am J Respir Cell Mol Biol. 2009;40:482–490. doi: 10.1165/rcmb.2008-0038OC. [DOI] [PubMed] [Google Scholar]
- 112.Eltom S., Stevenson C.S., Rastrick J., Dale N., Raemdonck K., Wong S., Catley M.C., Belvisi M.G., Birrell M.A. P2X7 receptor and caspase 1 activation are central to airway inflammation observed after exposure to tobacco smoke. PLoS One. 2011;6:e24097. doi: 10.1371/journal.pone.0024097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ferhani N., Letuve S., Kozhich A., Thibaudeau O., Grandsaigne M., Maret M., Dombret M.C., Sims G.P., Kolbeck R., Coyle A.J., Aubier M., Pretolani M. Expression of high-mobility group box 1 and of receptor for advanced glycation end products in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:917–927. doi: 10.1164/rccm.200903-0340OC. [DOI] [PubMed] [Google Scholar]
- 114.Hou C., Zhao H., Liu L., Li W., Zhou X., Lv Y., Shen X., Liang Z., Cai S., Zou F. High mobility group protein B1 (HMGB1) in asthma: comparison of patients with chronic obstructive pulmonary disease and healthy controls. Mol Med. 2011;17:807–815. doi: 10.2119/molmed.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yanai H., Ban T., Wang Z., Choi M.K., Kawamura T., Negishi H., Nakasato M., Lu Y., Hangai S., Koshiba R., Savitsky D., Ronfani L., Akira S., Bianchi M.E., Honda K., Tamura T., Kodama T., Taniguchi T. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 116.Yu M., Wang H., Ding A., Golenbock D.T., Latz E., Czura C.J., Fenton M.J., Tracey K.J., Yang H. HMGB1 signals through Toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 117.Bossaller L., Chiang P.I., Schmidt-Lauber C., Ganesan S., Kaiser W.J., Rathinam V.A., Mocarski E.S., Subramanian D., Green D.R., Silverman N., Fitzgerald K.A., Marshak-Rothstein A., Latz E. Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. J Immunol. 2012;189:5508–5512. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maelfait J., Vercammen E., Janssens S., Schotte P., Haegman M., Magez S., Beyaert R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kono H., Orlowski G.M., Patel Z., Rock K.L. The IL-1-dependent sterile inflammatory response has a substantial caspase-1-independent component that requires cathepsin C. J Immunol. 2012;189:3734–3740. doi: 10.4049/jimmunol.1200136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Botelho F.M., Nikota J.K., Bauer C.M., Morissette M.C., Iwakura Y., Kolbeck R., Finch D., Humbles A.A., Stämpfli M.R. Cigarette smoke-induced accumulation of lung dendritic cells is interleukin-1alpha-dependent in mice. Respir Res. 2012;13:81. doi: 10.1186/1465-9921-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen C.J., Kono H., Golenbock D., Reed G., Akira S., Rock K.L. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 122.Eigenbrod T., Park J.H., Harder J., Iwakura Y., Núñez G. Cutting edge: critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1 alpha released from dying cells. J Immunol. 2008;181:8194–8198. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gross O., Yazdi A.S., Thomas C.J., Masin M., Heinz L.X., Guarda G., Quadroni M., Drexler S.K., Tschopp J. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 124.Demento S.L., Eisenbarth S.C., Foellmer H.G., Platt C., Caplan M.J., Saltzman W.M., Mellman I., Ledizet M., Fikrig E., Flavell R.A., Fahmy T.M. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009;27:3013–3021. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang M., Flavin K., Kopf I., Radics G., Hearnden C.H.A., McManus G.J., Moran B., Villalta-Cerdas A., Echegoyen L.A., Giordani S., Lavelle E.C. Functionalization of carbon nanoparticles modulates inflammatory cell recruitment and NLRP3 inflammasome activation. Small. 2013 doi: 10.1002/smll.201300481. doi:10.1002/smll.201300481 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 126.Yazdi A.S., Guarda G., Riteau N., Drexler S.K., Tardivel A., Couillin I., Tschopp J. Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1alpha and IL-1beta. Proc Natl Acad Sci USA. 2010;107:19449–19454. doi: 10.1073/pnas.1008155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dhimolea E. Canakinumab. MAbs. 2010;2:3–13. doi: 10.4161/mabs.2.1.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]