Abstract
Although cancer has historically been viewed as a disorder of proliferation, recent evidence has suggested that it should also be considered a metabolic disease. Growing tumors rewire their metabolic programs to meet and even exceed the bioenergetic and biosynthetic demands of continuous cell growth. The metabolic profile observed in cancer cells often includes increased consumption of glucose and glutamine, increased glycolysis, changes in the use of metabolic enzyme isoforms, and increased secretion of lactate. Oncogenes and tumor suppressors have been discovered to have roles in cancer-associated changes in metabolism as well. The metabolic profile of tumor cells has been suggested to reflect the rapid proliferative rate. Cancer-associated metabolic changes may also reveal the importance of protection against reactive oxygen species or a role for secreted lactate in the tumor microenvironment. This article reviews recent research in the field of cancer metabolism, raising the following questions: Why do cancer cells shift their metabolism in this way? Are the changes in metabolism in cancer cells a consequence of the changes in proliferation or a driver of cancer progression? Can cancer metabolism be targeted to benefit patients?
CME Accreditation Statement: This activity (“ASIP 2014 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2014 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Discoveries of Otto Warburg
Otto Warburg’s pioneering work in the 1920s established that tumor cells exhibit altered metabolism. Warburg discovered an important distinction between the relative use of different modes of energy production in normal cells and tumors. In normal tissues, most of the pyruvate formed from glycolysis enters the tricarboxylic acid (TCA) cycle and is oxidized via oxidative phosphorylation. In tumors, in contrast, the pyruvate is largely converted to lactic acid and energy is produced anaerobically.1 This finding seemed counterintuitive. Surely, a rapidly proliferating cancer cell would prefer the 36 ATPs that can be claimed by complete oxidation of a glucose molecule to the two ATPs available through glycolysis. Furthermore, this shift in metabolism in which pyruvate is converted to lactate and secreted, rather than being oxidized, occurred in tumors even when there was sufficient oxygen to support mitochondrial function. The conversion of most pyruvate to lactate through fermentation, even when oxygen is present, is called aerobic glycolysis or the Warburg effect.
Evidence that Aerobic Glycolysis Promotes Tumorigenesis
Since these early discoveries, rapid consumption of glucose and secretion of lactate have been discovered to be a characteristic of many types of tumors. By using the imaging agent 2-[18F]fluoro-2-deoxy-d-glucose, coupled with positron emission tomography (PET), primary and metastatic lesions can be identified with a specificity and sensitivity near 90%.2 Furthermore, glucose uptake assessed with PET correlates with poor prognosis in oral squamous cell carcinoma,3 gastric cancer,4 and neoplasms of other tissues.5 Tumor-produced lactate concentrations also correlate with shorter survival and increased metastases in cervical and head and neck cancer.6–8 Overall, the association between a glycolytic phenotype and poor prognosis, along with the consistency of the phenotype and its usefulness for diagnosis, supports a model in which metabolic changes are a reproducible characteristic of cancer cells and may even promote disease progression.
In this review, we consider the way in which cancer cells rewire their metabolism with a focus on a few key questions. What is the metabolic phenotype of cancer cells and how is it achieved molecularly? How do oncogenes and tumor suppressors coordinate and enforce the metabolic changes that occur with cancer? Is the metabolic phenotype of cancer cells a reflection of their rapid growth? Why do tumor cells undergo this dramatic shift (ie, what advantage would an inefficient energy production program confer)? Are metabolic changes drivers of cancer progression or do they just come along for the ride? And finally, is the cancer metabolic profile sufficiently distinct from that of normal cells that it can be targeted therapeutically?
Molecular Basis for the Cancer Cell Metabolic Phenotype
Cancer Cells Reengineer Glycolysis
Cancer cells evade the mechanisms that normally regulate glycolytic flux using multiple different strategies. The levels of many different glycolytic enzymes are induced in tumors9 (Figure 1 and Table 1). In addition, cancer cells subvert the feedback mechanisms that normally allosterically inhibit rate-controlling steps in glycolysis. For instance, phosphofructokinase (PFK) is inhibited by ATP; when the cell is energy rich, glycolysis should decrease. However, when glucose is abundant, the metabolite fructose 2,6-bisphosphate is formed from fructose 6-phosphate by 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatases (PFKFBP1-4), and fructose 2,6-bisphosphate can override ATP-mediated PFK inhibition. In tumor cells, high levels of glucose transport2,10,11 and hexokinase activity10,24,25 lead to elevated levels of fructose 2,6-bisphosphate, which allosterically activates PFK. The specific PFK isozymes overexpressed in cancer cells are less sensitive to allosteric inhibition by ATP and more strongly activated by fructose 2,6-bisphosphate.31 Cancer cells also trick themselves and generate cues that there are higher levels of blood glucose than actually exist by overexpressing PFKFBPs, increasing the levels of fructose 2,6-bisphosphate and, thus, driving glycolysis.34 As a result of these different mechanisms of activation, PFK activity is much higher in cancer cells than normal tissue.31
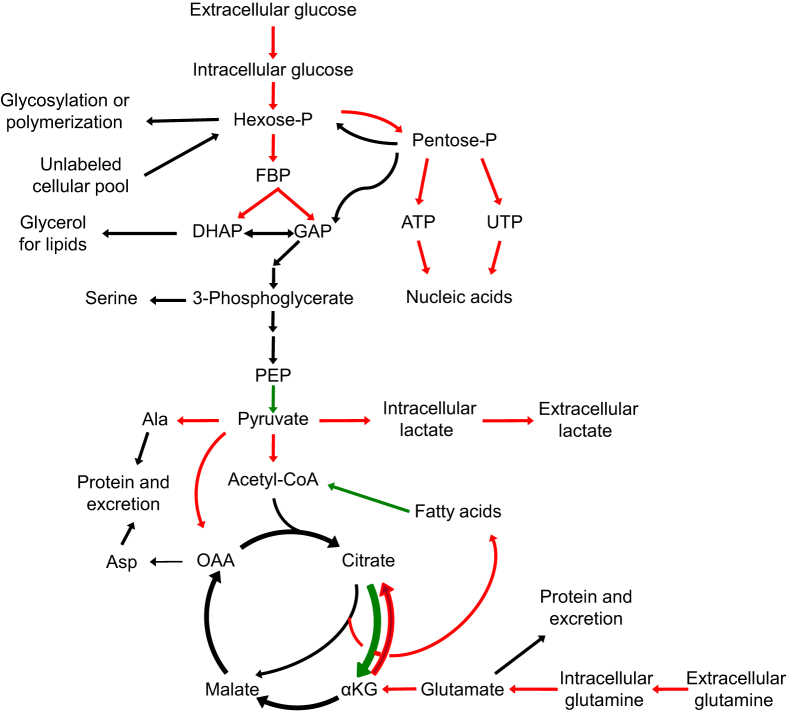
Figure 1.
Cancer metabolism. Scheme shows central carbon metabolism. Metabolic reactions that tend to be faster in tumors are identified in red, whereas reactions that tend to be slower in tumors are identified in green. DHAP, dihydroxyacetone phosphate; GAP, glyceraldehyde 3-phosphate; KG, α-ketoglutarate; OAA, oxaloacetate; PEP, phosphoenolpyruvate.
Table 1.
Metabolic Changes in Tumors and Activated Lymphocytes
| Metabolic step | Cancer cells | Primary tumors | Functional importance | Potential target | Activated lymphocytes | Potential oncogene target |
|---|---|---|---|---|---|---|
| Glucose uptake/glucose transporters | Increased10 | Increased2,11 | Yes12,13 | Yes14 | Increased15–18 | Induced by MYC,19,20 AKT,15 and HIF21 and repressed by p5322,23 |
| Hexokinase | Hexokinase II increased24,25 | Hexokinase II increased25 | Yes26 | Yes27 | Increased17,28 | Induced by MYC29 and AKT30 |
| Phosphofructokinase | Liver isozyme induced31 | Liver isozyme increased31 | Yes32 | Yes32 | Increased17 | Induced by MYC20 and AKT33 |
| 6-Phosphofructo-2-kinase | Induced34 | Increased34 | Yes35 | Yes36 | Increased37 | Induced by p5338 |
| Pyruvate kinase | Shift to PKM239 | Shift to PKM239 | Yes39–41 | Yes39–41 | Increased17,28 | |
| Pyruvate dehydrogenase kinase | Increased42 | Yes43,44 | Yes44,45 | Increased by HIF46 and repressed by p5347 | ||
| Lactate dehydrogenase | Increased48 | Yes49,50 | Yes51 | Increased28 | Increased by MYC50 | |
| Monocarboxylate transporters | Increased52 | Increased52 | Yes53 | Yes53 | Increased28 | Repressed by p5354 |
| Lactate secretion | Increased49 | Yes49,50 | Increased15 | Increased by MYC19 and repressed by p5322 | ||
| ATP citrate lyase | Increased55 | Yes56 | Yes56 | Activated by AKT57 | ||
| Glutamine consumption/glutamine transporters | Increased58 | Increased17,28,59 | Increased by MYC60 | |||
| Glutaminase | Increased61 | Yes62 | Yes19,62 | Increased17,59 | Increased by MYC61 | |
| Glutamate dehydrogenase | Yes63 | Yes63 | Increased59 | |||
| Glutamate oxaloacetate transaminase | Yes63 | Yes60,63,64 | Increased28,59 | |||
| Oxidative phosphorylation | May increase65–67 | Yes67 | Yes67 | Increased18 | Induced by MYC67 and p5322 |
Cancer cell lines and tumors also reexpress the embryonic isoform (PKM2) of pyruvate kinase (PK).39 PKM2 is distinguished from other PK isoforms because it can associate with tyrosine-phosphorylated peptides,68 an association that results in a transition to a dimeric form with low affinity for its substrate, phosphoenolpyruvate.69 The less active PKM2 allows for a diversion of glycolytic metabolites to serine and glycine biosynthetic pathways.70 Phosphorylated PKM2 can also translocate to the nucleus, phosphorylate histone H3, and act as a transcriptional co-activator that induces expression of genes involved in glycolysis.71
The shunting of pyruvate to secreted lactate in tumors is associated with elevated levels of lactate dehydrogenase (LDH)48 and monocarboxylate transporters (MCTs) that cotransport lactate and a proton out of the cell.52 Elevated LDH levels have been discovered in Burkitt’s lymphoma48 and non-small cell lung cancer,72 whereas increased MCT levels have been detected in ovarian,73 prostate,52 gastric,74 and cervical75 carcinomas. The shift of pyruvate toward lactate production and away from oxidative phosphorylation also reflects decreased activity of the pyruvate dehydrogenase complex, which can result from induction of the inhibitory pyruvate dehydrogenase kinases (PDKs).42
There is substantial evidence that elevated glucose consumption and increased lactate secretion in tumors contribute to their growth. Patients with type 2 diabetes have high levels of blood glucose and an increased risk of developing cancers of the pancreas, liver, colon, gastrointestinal tract, breast, and endometrium.76 Inhibiting expression of a glucose transporter GLUT1,12 PKM2,40 LDH,49 or PDK43 results in reduced tumorigenicity in xenograft models. Reducing the levels of 6-phosphofructo-2-kinase suppresses glycolytic flux, growth in soft agar, and tumor growth in mice.35 Knocking down the β-catalytic subunit of the mitochondrial H+-ATP synthase results in a higher glycolytic rate and a more aggressive tumor-forming phenotype.77 Taken together, these studies highlight the importance of the glycolytic phenotype for tumor progression.
Multiple approaches to reducing glycolytic flux are being considered as potential cancer therapies (Figure 2 and Table 1). In one strategy, patients eat low-carbohydrate diets, thus starving their tumors of glucose, and it was shown to be promising in a recent pilot study.14 Pharmacological approaches are also being attempted. Lonidamine, a derivative of indazole-3-carboxylic acid that inhibits hexokinase, reduces cancer cell proliferation, and sensitizes xenograft tumors to death by radiation and other compounds.27 An inhibitor of PFKFB3, 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one, decreases intracellular concentrations of fructose 2,6-bisphosphate, suppresses glucose uptake, reduces the growth of cells from multiple types of cancer in vitro, and inhibits the growth of established tumors in vivo.36 Dichloroacetate, a pyruvate mimetic that inhibits pyruvate dehydrogenase kinase, increases pyruvate dehydrogenase activity and the oxidation of glucose, reduces the proliferation of breast cancer cell lines, inhibits proliferation, and slows xenograft tumor growth.44 In a pilot study, dichloroacetate resulted in radiological regression in three of five patients with glioblastoma multiforme.45 In sum, there are substantial data to suggest that impeding glycolysis, or redirecting pyruvate toward oxidative pathways and away from its conversion to lactate, inhibits tumor growth.
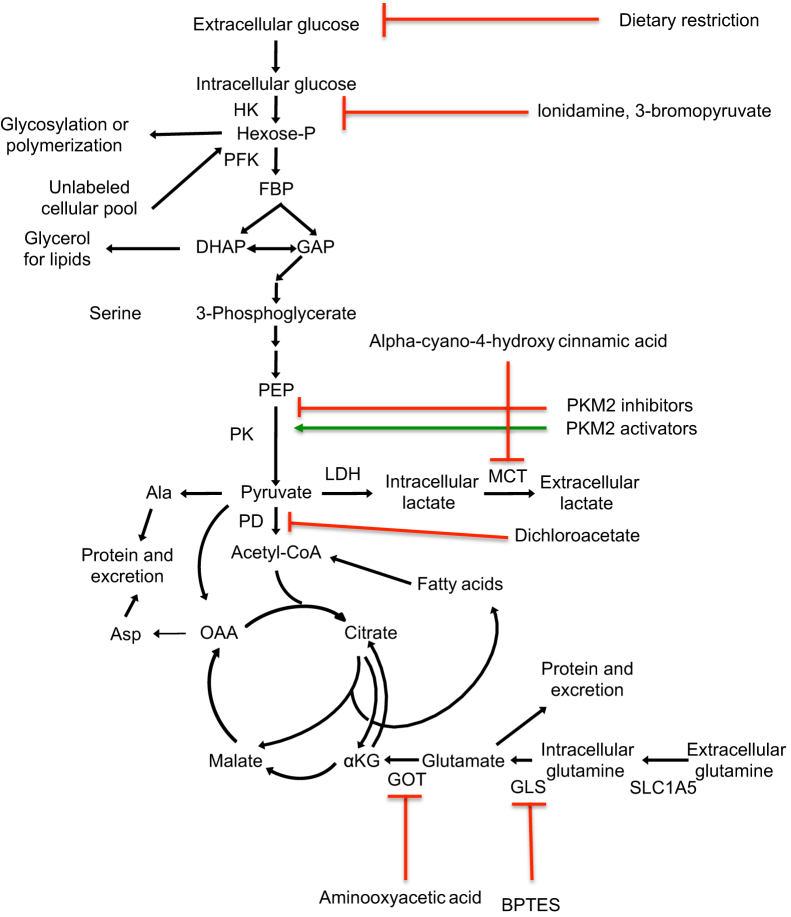
Figure 2.
Metabolic approaches to treating cancer. Scheme shows some of the compounds being explored as anticancer agents and the metabolic reactions that they target. Red lines indicate inhibition; green lines, activation. BPTES, bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide; DHAP, dihydroxyacetone phosphate; GAP, glyceraldehyde 3-phosphate; GLS, glutamine synthetase; GOT, glutamate oxaloacetate transaminase; HK, hexokinase; MCT, monocarboxylate transporters; OAA, oxaloacetate; PD, pyruvate dehydrogenase; PEP, phosphoenolpyruvate; PK, pyruvate kinase.
Glutamine Is the Major Anaplerotic Source for Cancer Cells
Some cancer cells also run the TCA cycle in a pattern that distinguishes them from most non-transformed cells. In some cancer cells, pyruvate from glycolysis enters a truncated TCA cycle that ends as citrate is shuttled from the mitochondrial matrix to the cytosol.78 Citrate is cleaved by ATP citrate lyase (ACL) to provide acetyl-CoA that can be used for fatty acid synthesis. Disruption of ACL impairs tumor growth.56 This truncated TCA cycle results in a flow of metabolites out of the TCA cycle (cataplerosis) that needs to be balanced by an influx of metabolites (anaplerosis). In many cancer cells, glutamine fulfills this role: it is converted to glutamate and then to the TCA intermediate, α-ketoglutarate.79 Although glucose is the precursor for 90% of secreted lactate in cancer cells, oxidative conversion of glutamine accounts for as much as 40% of TCA cycle intermediates79 and ≥30% of the ATP generated.61,79 To meet the glutamine requirements, some cancer cells dramatically increase glutamine consumption through induction of glutamine transporters.58 Cancer cells also induce enzymes that metabolize glutamine, such as glutaminases, that convert glutamine to glutamate (glutaminase1 and glutaminase C)61 and glutamate oxaloacetate transaminases that convert glutamate to α-ketoglutarate.80
Glutamine withdrawal results in the death of some cancer cells,60 which is surprising because glutamine is a nonessential amino acid that can be synthesized from glucose. The strict requirement of some tumors for glutamine makes glutaminolysis enzymes attractive anticancer targets. Glutaminase inhibitors, such as bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide, reduce cancer cell growth, transformation, and tumorigenesis.19,62 Transaminase inhibitors have also been suggested as anticancer agents because glutamine-derived carbons are more likely to enter the TCA cycle through transamination in cancer cells, whereas normal cells tend to rely more heavily on glutamate dehydrogenase.80 Transaminase inhibitor, aminooxyacetic acid, has a cytotoxic effect specifically on cancer cells,60,63,64 with little effect on healthy cells.64 Treatment with aminooxyacetic acid reduced the growth of breast cancer cells in a mouse xenograft model without any obvious dose-limiting toxicities.64
Reevaluation of the Warburg Effect
Warburg hypothesized that the shift from respiration to aerobic glycolysis in cancer cells reflects defective mitochondrial respiration.1 In support of this model, tumors tend to down-regulate the expression of genes involved in oxidative phosphorylation in general,81 and specifically, the β-F1 subunit of the ATP(synth)ase.82 In addition, mutations in mitochondrial DNA have been observed in multiple tumor types.83 Furthermore, experiments in which the levels of mitochondrial components are modulated have largely reinforced the importance of the glycolytic phenotype for tumor growth in vivo.77 Taken together, the findings of the functional importance of high glycolytic rates and mitochondrial abnormalities in tumors have contributed to the prevailing paradigm that tumors generate most of their ATP through glycolysis.
However, this model is being reevaluated for several reasons. First, recent studies have indicated that some tumor cell lines do perform oxidative metabolism.65,66,84 In some studies, respiration actually increases in tumor mitochondria.65,67 In one study, glycolysis contributed 50% to 70% of ATP for some cancer cell lines, consistent with Warburg’s findings, but as little as 10% of cellular ATP in other cell lines.67 Furthermore, there are studies that indicate that mitochondrial activity and oxidative phosphorylation support tumor growth.85,86 In particular, overexpression of the mitochondrial citrate transporter has been shown to increase tumor growth in xenograft models, whereas inhibition of the mitochondrial citrate transporter, which enhances glycolysis, actually reduces tumor growth.87 Further supporting such a model, some human and rodent tumors are susceptible to death induced by highly specific respiratory inhibitors.67
The Warburg effect is also being reconsidered by investigators who have argued that some of the cells within a tumor actually consume, rather than secrete, lactate. Lactic acid recycling occurs in normal physiological conditions as contracting skeletal muscle supplies lactate to the liver. The liver uses gluconeogenesis to convert lactate back to glucose that is released into the bloodstream and absorbed by muscle, thus completing the Cori cycle. In the tumor microenvironment, oxidative tumor cells (eg, those near blood vessels) have been proposed to consume lactate secreted by tumor cells that are engaging in aerobic glycolysis.53 Absorbed lactate can be converted to pyruvate and used to fuel oxidative phosphorylation in these well-oxygenated cells. The reliance of aerobic cells within a tumor on lactate as a fuel may preserve the available glucose for the hypoxic cells that strictly require it.53
Metabolism of the Tumor Stroma
It has also been proposed that cells within the host tissue, the stroma, and not the tumor cells, perform aerobic glycolysis. Stromal cells, for example, the fibroblasts, in the tumor microenvironment can actively support malignant transformation88 and metastasis.89 A hypothesis has been proposed that the tumor stroma is glycolytic and that stromal cells express MCTs that exude lactate, whereas tumor cells perform oxidative metabolism and express transporters that consume lactate.90,91 The proposed model is that tumor growth is fueled by lactate, ketones, and glutamine provided by stromal cells that are then absorbed by cancer cells and used for oxidative phosphorylation. It has been further suggested that the PET avidity observed by tumors reflects 2-deoxy-glucose uptake by nearby stromal and inflammatory cells rather than the cancer cells themselves.84 This model has been called the reverse Warburg effect because the increased glycolysis occurs in the surrounding stromal cells, rather than the tumor cells.91 From this perspective, cancer is viewed as a parasitic disease that steals energy-rich metabolites from the host organism.91–93
Summary of Molecular Mechanisms of Cancer Metabolism
In summary, although studies have recently questioned the glucose flux paradigm,87,91 the prevailing model is that there is higher flux of glucose through most metabolic pathways in tumor cells compared with normal cells. More glucose is transmitted to metabolic intermediates, lactate, citrate, and fatty acid synthase, and possibly even more to oxidative phosphorylation.78 Meeting all of these conditions would seem to require a large increase in glucose uptake in tumors. PET imaging has confirmed the increased glucose consumption in many, but not all, tumors, and glucose consumption rates exceed the amounts that can be easily explained by needs for energy or metabolites.2 Glutamine consumption follows a similar pattern of excess consumption.79 We consider now the mechanisms that enforce this metabolic shift and possible explanations for its occurrence.
Oncogenes and Tumor Suppressors Enforce the Metabolic Shift
The key to understanding the mechanism(s) affecting changes in metabolism in tumors lies in the discovery that oncogenes and tumor suppressors consistently activated or deleted in tumors are important regulators of metabolism.78,94 The oncogenic molecules AKT, MYC, and hypoxia-inducible factor-1 (HIF-1) can all contribute to the metabolic shift that occurs during carcinogenesis (Figure 3 and Table 1), whereas the tumor suppressor p53 acts to minimize the glycolytic phenotype and its loss contributes to aerobic glycolysis and the tumor metabolic phenotype. In tumors, multiple oncogenic mutations likely cooperate with each other to result in a phenotype in which cells absorb nutrients to meet or even exceed the bioenergetic demands of cell growth and proliferation.
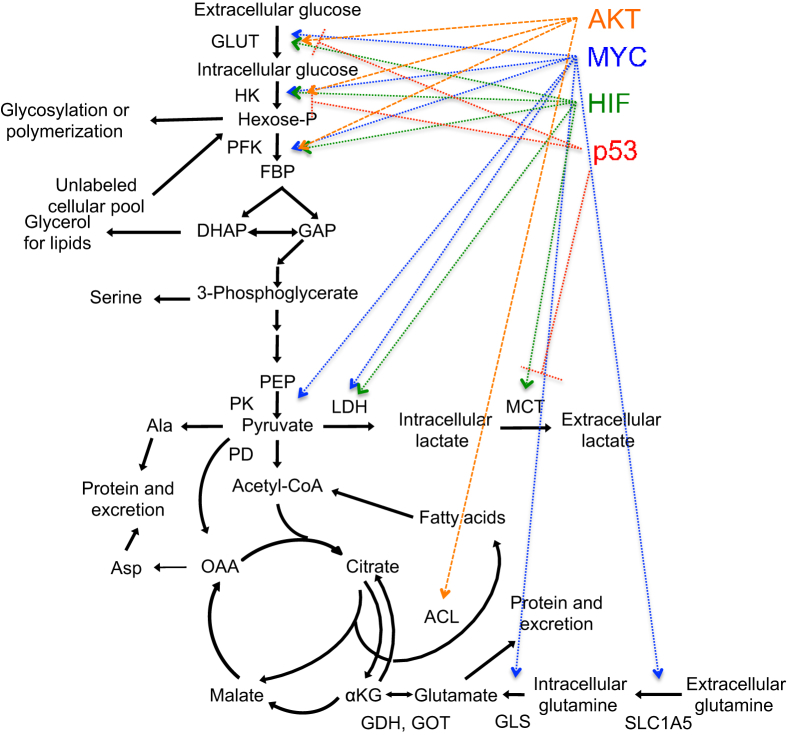
Figure 3.
Metabolic effects of oncogenes and tumor suppressors. Scheme shows the metabolic reactions in central carbon metabolism affected by AKT (orange), MYC (blue), HIF (green) and p53 (red). Arrows indicate activation; lines, repression. DHAP, dihydroxyacetone phosphate; GAP, glyceraldehyde 3-phosphate; GDH, glutamate dehydrogenase; GLS, glutamine synthetase; HK, hexokinase; KG, α-ketoglutarate; LDH, lactate dehydrogenase; OAA, oxaloacetate; PEP, phosphoenolpyruvate.
PI3K/AKT
In non-transformed cells, the phosphatidyl inositol-3-kinase (PI3K) pathway is activated in response to growth signals.15 In a sizable fraction of all cancers, the PI3K pathway is constitutively activated through mutation or amplification,95 resulting in constitutive activation of AKT kinase and a growth-promoting metabolic program. AKT activation increases the glycolytic rate, in part by increasing GLUT1 expression15 and translocation of GLUT1 to the plasma membrane.16 AKT causes the glycolytic enzyme, hexokinase, to associate with the mitochondrial outer membrane.30 AKT also performs an activating phosphorylation of PFK that releases its inhibition by ATP.33 Finally, AKT promotes the conversion of citrate to fatty acids by phosphorylating and activating ACL.57 By simultaneously reducing the expression of carnitine palmitoyltransferase 1A,96 an enzyme that initiates the esterification and breakdown of long-chain fatty acids, AKT may eliminate a potential nutrient source and contribute to the glucose addiction of some cancer cells. Thus, activation of the PI3K/AKT pathway can be a powerful mechanism for altered tumor cell metabolism.
MYC
Deregulated expression of c-MYC, an early serum response transcription factor, is one of the most common oncogenic events in cancer.97 Although MYC has well-established roles in the regulation of cell proliferation, differentiation, and apoptosis, MYC also drives the accumulation of cellular biomass by regulating nucleotide biosynthesis, ribosome and mitochondrial biogenesis, and metabolism.98 In an MYC-inducible human Burkitt’s lymphoma model, glucose consumption, lactate production, glutamine uptake, and glutamine incorporation into the TCA cycle were all induced by MYC.19,20,50 The induction of LDH by MYC has been specifically demonstrated to be functionally important for tumor growth, because MYC-dependent tumors exhibit reduced proliferative capacity and ability to grow in soft agar when LDH expression is reduced.50 MYC also promotes glutamine metabolism by inducing the expression of glutamine transporters60 and by up-regulating levels of glutaminase indirectly via repression of the miRNA miR-23.61 As a result, some MYC-transformed cells have an absolute requirement for glutamine to maintain continuous replenishment of TCA cycle intermediates.19,60,99
HIF
The oxygen-sensitive HIF-1 transcription factor is a heterodimer composed of constitutively expressed β subunits and oxygen-sensitive α subunits.100 In well-oxygenated cells, HIF-1α is hydroxylated, which facilitates its ubiquitination and degradation by the proteasome. In hypoxic conditions, HIF-1 is stabilized and activated. During tumorigenesis, localized hypoxic regions in which HIF-1 is stabilized may develop. This results in the expression of HIF-1 target genes, such as angiogenesis factors that increase oxygen delivery to hypoxic tissues.67 HIF-1 also facilitates the activation of an oxygen-independent mode of energy extraction (ie, glycolysis in oxygen-deprived cancer cells by inducing many enzymes in the glycolytic pathway).21 HIF-1α also promotes aerobic glycolysis by transcriptionally inducing PDK,46 thus reducing the oxidative stress expected to occur if the electron transport chain were active. Hypoxic tumors, which induce HIF-1 and glycolysis most strongly, tend to be more invasive and metastatic than those with normal oxygen levels.13 Furthermore, high HIF-1 is associated with higher mortality.101 Thus, hypoxia experienced by tumors promotes HIF-1 expression, which, in turn, coordinates a transition to an aerobic glycolytic phenotype.
p53
The p53 tumor suppressor is also being reconsidered from a metabolic perspective. The role of p53 in orchestrating cell cycle arrest, apoptosis, or senescence in response to DNA damage or cellular stress has been thought to explain its role as a tumor suppressor.102 More recently, p53, like MYC, has been discovered to be an important regulator of cellular metabolism. p53−/− Cells have higher rates of glycolysis, produce more lactate, and exhibit decreased mitochondrial respiration compared with wild-type cells,22 indicating that wild-type p53 suppresses an aerobic glycolysis phenotype. p53 Functions that might enforce these metabolic changes include down-regulation of glucose transporters,23 up-regulation of a fructose-bisphosphate-phosphatase that lowers levels of fructose 2,6-bisphosphate,38 repression of lactate transporters,54 repression of PDKs,47 induction of the mitochondrial oxidation regulator, synthesis of cytochrome c oxidase 2,22 and competition with HIF-1 for limiting amounts of a shared transcriptional co-activator.103
A recent article has critically tested the importance of the role of p53 in metabolism in the prevention of tumorigenesis. Cells with three p53 lysine mutations (p533KR) lack the normal functions of p53 in cell-cycle arrest, senescence, or apoptosis, but retain the ability to suppress glycolytic rates and maintain low reactive oxygen species (ROS) levels.104 Although p53-null mice rapidly develop thymic lymphomas leading to death, surprisingly, p533KR/3KR mice do not exhibit early-onset tumor formation.104 These findings suggest that less conventional functions of p53, such as inhibiting the metabolic shift to aerobic glycolysis and reducing ROS levels, are critical for the ability of p53 to suppress early-onset spontaneous tumorigenesis.
The studies previously described demonstrate that p53 can modulate metabolism. Recent studies have shown that the availability of carbohydrates can, in turn, affect p53 levels. Glucose restriction has been reported to specifically induce deacetylation and degradation of mutant, but not wild-type, p53 both in vitro and in vivo.105,106 Because wild-type p53 inhibits tumor growth and mutant forms of p53 can promote tumorigenesis,107 the findings suggest that there may be reciprocal regulation between diet and metabolism on the one hand, and p53 status on the other, that affects tumor growth.
Cancer Metabolic Phenotype
Activated Lymphocytes Share Metabolic Properties with Cancer Cells
The metabolic program of cancer cells, although different from that of most normal, differentiated cells, shares significant similarities with some proliferating cells, including activated lymphocytes. Mature, resting lymphocytes rely on oxidative metabolism of glucose and glutamine for the energetic needs.28 Recognition of their corresponding antigen results in activation of the lymphocytes and is accompanied by a dramatic shift in metabolism.108 Activated lymphocytes increase in size, divide rapidly, consume glucose and glutamine in excess of what can be easily explained by their need for biosynthesis or ATP, and secrete the extraneous material as lactate.15,17,18 Many of the molecular changes that occur when lymphocytes are activated are similar to those that occur in tumors, including increased activity of glucose transporters,15,16 glycolytic enzymes,17,28 PFKFBP3,37 lactate dehydrogenase,28 and MCTs.28 To compensate for the loss of citrate from the TCA cycle, glutamine consumption increases when lymphocytes are activated,17,59 and this is associated with higher levels of glutamine transporters17,28,59 and enzymes involved in glutaminolysis (Table 1).17,28,59 The increased glucose flux in activated lymphocytes also results in higher levels of oxidative phosphorylation.18 The similarity between the metabolic profile of tumor cells and activated lymphocytes suggests that this metabolic pattern and may be associated more generally with rapid cell division.
Not All Proliferating Cells Use Aerobic Glycolysis
In addition to lymphocytes, many fast-growing unicellular organisms, including the baker’s yeast Saccharomyces cerevisiae, rely on glucose fermentation during proliferation, even when oxygen is available.109 However, despite the similarities between tumors, activated lymphocytes, and fermenting yeast, respiration can sustain fast cell growth. Some tumor cells rely on oxidation to generate ATP,65,66 and some aerobic yeasts, such as Yarrowia lipolytica, rely on respiration for growth.110 Conversely, nondividing cells can preferentially rely on glycolysis. Hematopoietic stem cells, which are largely quiescent, have higher glycolytic activity, lower mitochondrial activity,111 and higher PDK activity,112 compared with their more proliferative descendants. In a primary human fibroblast model system, a shift between proliferation and quiescence was not found to be associated with a dramatic difference in glycolytic rate.113 Finally, recent studies report that the shift to glycolysis in lymphocytes is not necessary for proliferation or survival, but rather supports cytokine secretion.114 Thus, in some model systems, the metabolic changes observed in tumors occur with a shift to a high proliferative rate, but this transition is not always observed when proliferative rate changes; even if it does occur, it may not facilitate faster proliferation.
The Advantages of the Tumor Cell Metabolic Profile to the Tumor
Rapid ATP
Why is a less efficient catabolic pathway so strongly induced in tumor cells? One suggestion is that aerobic glycolysis is advantageous because it provides ATP more rapidly than oxidative phosphorylation.66 However, some cancer cells actually recover a significant fraction of their ATP from oxidative phosphorylation.66 Furthermore, it is not clear that ATP levels, or the speed which ATP can be extracted, is actually limiting for cellular growth.94 Even rapidly dividing mammalian cells have been found to maintain high ratios of ATP/ADP.39 And, signaling pathways exist that allow cells to increase low ATP levels by activating catabolic pathways that generate ATP.94 For these reasons, the rationale that cells shift to aerobic glycolysis to recover rapid ATP is being reconsidered, and other interpretations for the Warburg effect have been offered.
Carbon Skeletons for Growth
Although there may not be selective pressure for generating ATP, per se, one can imagine selective pressure for the rate of cellular proliferative expansion.94 Organisms in which immune cells can respond to the presence of invaders by rapidly mounting an immune response ought to be less likely to succumb to infection and, therefore, be more fit. Increased glycolysis in tumor cells provides a constant supply of metabolic intermediates that can be diverted to support cell growth.94 Furthermore, because glucose is one of the two main nutrients that the cell consumes, it is needed to provide all of the molecules necessary for cell growth.
To make a fatty acyl chain, a single glucose molecule can provide five times the ATP required, whereas seven glucose molecules are needed to generate the necessary NADPH through the pentose phosphate pathway.94 If all of the available glucose were converted efficiently and completely to ATP in mitochondria, there would not be any glucose to provide acetyl-CoA to make fatty acids. There would also be no glucose available to divert from glycolysis for the synthesis of NADPH, nonessential amino acids, or ribose needed for generating nucleotides. Furthermore, complete oxidation of each glucose molecule would result in high ATP levels that would feedback and shut down glycolysis.94 The fact that rapidly proliferating lymphocytes and yeast also rely heavily on glycolysis over oxidative phosphorylation could support the argument that the cancer metabolism phenotype is the metabolic profile that channels glucose among the available pathways in a way that facilitates rapid proliferation and growth.109
But, one might reasonably wonder, if the goal of cancer cells is to increase their biomass, then why do they secrete and waste 90% of the glucose carbons they consume?18,79,109 There are several possible explanations. One possibility is that the cell needs a high rate of flux through glycolysis to ensure that metabolic intermediates can be siphoned off to anabolic pathways without dramatically affecting the sizes of the metabolite pools.109,115 Another important consideration is that achieving a high level of glycolytic flux actually requires NAD+ to be regenerated, which is achieved by converting pyruvate into lactate.109 Furthermore, the secreted lactate is not, in fact, lost. As previously described, aerobic tumor cells might absorb the extracellular lactate released by glycolytic cells, convert it to pyruvate, and use it as a fuel for mitochondrial oxidative phosphorylation.53
Optimization of Fitness
A somewhat different perspective is to view the Warburg effect as an extension of a pattern of metabolic pathway use that exists in simpler model organisms. As growth rate, cell size, and ribosomal content increase, there is often an associated shift toward metabolic pathways with less efficient energy recovery.116 This has been interpreted as a tradeoff between two different catabolic pathways, one of which is more expensive to generate, but generates more ATP, and the other uses less enzyme, but produces less energy. At low extracellular substrate concentrations, intracellular substrate is expensive, so an efficient catabolic method is necessary. At higher substrate concentrations, however, the catabolic pathway that requires less energy to produce its components becomes more valuable. Thus, a pathway that seems wasteful in that all possible ATP is not recovered from each nutrient, may be cheap in terms of the resources needed to construct the pathway, and may actually be the more desirable pathway when cells are in a nutrient-rich environment. A logical extension of the argument to cancer cells might be to recognize that performing oxidative phosphorylation requires the generation and maintenance of entire organelles, the mitochondria, complete with their own genomes and ribosomes, and an expensive-to-maintain membrane potential. Respiration, from this perspective, is a costly catabolic path that requires a substantial investment, but is useful for efficiently extracting ATP when nutrients are scarce. When nutrients are abundant, the less resource-intensive process of glycolysis might be more desirable. Thus, if resources are not limiting, cells may benefit from engaging a cheap, but seemingly wasteful, metabolic program.
Despite these cogent arguments, there are still unanswered questions about the metabolic phenotype of cancer cells. For instance, if the cancer cell phenotype is designed to facilitate cell growth, then why do cancer cell lines have higher glucose, lactate, and glutamine fluxes per unit area of cell membrane, higher hexokinase activity, and higher pentose phosphate pathway activity than nonmalignant cells growing at the same rate?117 Are other benefits conferred on the tumor by this metabolic strategy in addition to simply a faster growth rate?
Minimizing ROS
The use of aerobic glycolysis allows cells to expend less energy in the generation and maintenance of mitochondria and protects tumor cells from ROS that would be generated by performing oxidative phosphorylation in conditions of limited oxygen. In addition, both the glucose and the glutamine consumed by cancer cells can be metabolized to generate NADPH,79 a necessary cofactor for the replenishment of the cell’s most important antioxidant, reduced glutathione. The importance of the pentose phosphate pathway and ROS detoxification in tumor cell growth was highlighted in a recent study in which hypoxia was found to induce glycosylation and inhibition of PFK, leading to redirection of glycolytic intermediates into the pentose phosphate pathway.32 Blocking PFK glycosylation reduced cancer cell proliferation in vitro and impaired tumor formation in vivo. Thus, reducing ROS levels and protecting against ROS-mediated cell death may represent an advantage conferred by a Warburg effect metabolic phenotype.
Protection against Apoptosis
In addition to controlling ROS levels, the aerobic glycolysis phenotype of cancer cells may also protect them from apoptosis by inhibiting the release of pro-apoptotic factors from the mitochondria through the mitochondrial permeability transition pore. The ease with which this pore opens depends on the mitochondrial membrane potential generated as hydrogen ions are transferred out of the inner mitochondrial membrane during oxidative phosphorylation. The low flux through the electron transport chain in cancer cells results in mitochondria with higher membrane potential45 and a higher threshold for transition pore opening, thus suppressing apoptosis. If the hyperpolarization in cancer mitochondria is reversed by forcing pyruvate into the mitochondria, glucose oxidation increases, mitochondrial membrane potential decreases, and cancer cells undergo more cell death.45 Thus, active electron transport flux may facilitate mitochondria-mediated cell death, and cancer cells may maintain viability, in part, by minimizing respiration.
High levels of glycolysis also protect against apoptosis via hexokinase. Hexokinases can be found physically associated with the outer surface of mitochondria.24 Some tumor cells have higher levels of hexokinase24,25 and a tighter association between hexokinase and the mitochondrial membrane.118 The localization of hexokinase to the mitochondria, which is facilitated by active AKT,29 inhibits the release of apoptosis-inducing factors, and suppresses apoptosis.119 Thus, aerobic glycolysis may provide a survival advantage for tumor cells that helps to explain its prevalence in human cancers.
Adaptation to the Tumor Microenvironment
Another possibility is that aerobic glycolysis is selected for in tumors because they are found in a hypoxic environment. According to this model, as a tumor grows, cells will be found further and further from the blood supply and po2 levels decline even more rapidly with distance from blood vessels than glucose levels. Lack of oxygen will reduce mitochondrial respiration and lead to a decline in mitochondrial ATP. Lower ATP levels are expected to relieve allosteric inhibition of PFK and PK and promote glycolysis. Hypoxia also induces HIF-1α stabilization and activity, which will promote glycolysis and the growth of new blood vessels. Even if new blood vessels are formed, the solid tumor microenvironment will still be characterized by disorganized microvasculature and cycles of normoxia-hypoxia.120 Aerobic glycolysis would continue to benefit cells in this environment. Thus, the tumor microenvironment, in this model, induces an aerobic glycolysis metabolic profile and then provides a selective advantage for tumor cells with high glycolytic metabolism. Aerobic glycolysis would provide a strong selective advantage during metastasis as well and, indeed, cells pretreated with hypoxia are more likely to survive during metastasis than their normoxic counterparts.121
There are a few questions surrounding this model. Some studies have questioned whether oxygen levels in the tumor microenvironment are, in fact, lower than the Km for the rate-limiting enzymes in oxidative phosphorylation.67 Others have questioned the implied timing of the model, and argued that cancer cells activate a glycolytic metabolism even before they are exposed to hypoxic conditions.94 In addition, the aerobic glycolysis metabolic profile is not limited to hypoxic tumors.94 Leukemic cells and lung tumors found in airways are highly glycolytic, even though they are exposed to oxygen.94 Furthermore, although the tumor microenvironment might select for cells with an aerobic glycolysis phenotype, tumor cells maintain the metabolic phenotypes in culture under normoxic conditions. This may reflect the stabilization of HIF-1α and the persistent effects on gene expression of the combination of HIF-1α, oncogenes, and tumor suppressors. Thus, a more inclusive model might be that, in response to a combination of microenvironmental conditions, including hypoxia, and the activity of oncogenes and tumor suppressors, cancer cells acquire a metabolic phenotype that is stable and heritable, persists even when oxygen is available, and provides a selective advantage in the tumor environment and during metastasis.
Functional Role of Secreted Lactate
A final proposed explanation for the Warburg effect is that lactate secreted from tumor cells has an important functional role in promoting tumorigenesis. In support of this explanation, much of the glucose consumed by cancer cells is converted to lactate,18,79,109 and high levels of lactate are associated with a poor tumor prognosis.7 MCTs cotransport lactate and a hydrogen ion out of the cell, resulting in an acidification of the local environment. The ensuing decrease in pH might promote cancer cell invasion and metastasis by killing normal host cells, thus generating space for the tumor and possibly releasing nutrients that the tumor can consume. A low pH might also stimulate invasion122 and metastasis123 by activating pH-sensitive metalloproteinases and/or cathepsins that degrade proteins in the extracellular matrix and basement membranes.124 Furthermore, as previously described, secreted lactate has been proposed to provide nutrients to surrounding cells.53 Lactate secreted by cancer cells has also been proposed to feed nontumor, stromal cells.125 Thus, from the perspective of lactate recycling, the cancer can be considered a microecosystem in which the different tumor components engage in complementary metabolic pathways that allow for the recycling of the waste product metabolites of aerobic glycolysis to support tumor growth.53,84,125
Finally, the secretion of lactic acid has also been proposed to play a role in suppressing the host anticancer immune response.126 The metabolism of cytotoxic T lymphocytes, like that of the tumor cells, requires lactate secretion to drive high rates of glycolysis. In an advanced tumor, the high levels of lactate in the microenvironment may impede the ability of immune cells to export the intracellular lactate because secretion depends on a concentration gradient between intracellular and extracellular lactate. The resulting lactate overload reduces the ability of the T cells to secrete cytokines,126 thus reducing the defense normally provided by the host immune response.
Conclusions
The Role of Metabolic Changes in Cancer
For many years, cancer was considered fundamentally a disease of uncontrolled cell proliferation. Although metabolic changes were acknowledged to occur in cancer cells, it was considered a secondary phenomenon. More recently, the metabolic changes that occur during cancer are being reconsidered as more central to the disease itself. So, is cancer a disease of metabolism? Are the proliferation changes primary and the metabolic changes come along for the ride, or vice versa? One possible model is that oncogenes and tumor suppressors make cancer cells hyperproliferative, and the coordinated shift in metabolism is a consequence. For instance, MYC would be expected to promote proliferation, whereas the loss of p53 may protect cells from senescence. Because these molecules also affect metabolism, metabolic changes would ensue.
A variation on this model would stress that the effects of oncogenes and tumor suppressors on proliferation are closely associated with metabolic changes that are also necessary to promote cell growth. The similarity in the changes between cancer cells and rapidly proliferating immune cells,15,17,18 and even yeast,109 supports a model in which altered metabolism provides the building blocks needed to form new cells. From this perspective, inappropriate cell proliferation would still be considered the primary driver of the tumorigenesis phenotype, and the metabolic changes are considered a coordinated and complementary program that supports the higher proliferative rate. Treating cell proliferation will, as a consequence, reverse the metabolic phenotype. A dramatic demonstration in support of this view is the ability of the tyrosine kinase inhibitor, imatinib, to normalize glucose metabolism in leukemic cells.127
An alternative model would propose that changes in metabolism are necessary to support biomass accumulation and drive the cancer phenotype. This argument is based on the premise that the aerobic glycolysis phenotype per se, and not just increased growth rate, contributes to tumorigenesis, a statement supported by the findings that glycolytic tumors are more invasive and more likely to cause the patient’s death.6 This argument might stress that the excessive lactate secreted by tumor cells indicates that glucose carbons are not required just for biomass accumulation, but rather that secreted lactate likely actively promotes tumorigenesis, possibly by suppressing the host immune response or promoting invasion or metastasis. This argument would also stress that the changes in metabolism in tumor cells are more extreme than,117 and somewhat distinct from,24 those observed in most proliferating cells, some of which do not demonstrate the aerobic glycolytic phenotype of activated lymphocytes.113 For example, the association of hexokinase with mitochondria is observed in hepatoma cells, but not in normal liver, even when it is regenerating.24 Glucose transporters are induced in pancreatic cancer, but not mass-forming pancreatitis.11 Finally, one might argue, well-established oncogenes and tumor suppressors repeatedly observed as amplified, mutated, or deleted in tumors, such as those previously reported, RAS128 and JAK2V617F,129 are being discovered to have direct effects on metabolism.
An extreme version of this model would argue that all of the more classically accepted attributes of tumors actually derive from the metabolic phenotype of tumor cells.130 Then, is an aerobic glycolytic phenotype sufficient to transform a cell in the absence of other nonmetabolic cancer attributes? It seems unlikely—many immune cells temporarily adopt an aerobic glycolysis phenotype in response to antigen exposure. When they no longer receive inflammatory signals, they revert to the resting state and rarely form tumors.108 On the other hand, a p53 mutant that can counter aerobic glycolysis and ROS production, but cannot induce apoptosis, senescence, or cell cycle arrest, retains the ability to suppress tumorigenesis.104 These recent findings with p53 support a model in which metabolic changes are critical drivers of tumorigenesis, and highlight the need for more studies to clarify this issue.
The Prospects for Targeting Cancer through Metabolism
The first anticancer agents targeted metabolic pathways required for proliferation (eg, by depleting pools of nucleotide precursors).131 Successful anticancer agents designed more recently have largely focused on a specific activated oncogene. These targeted therapies have been extremely successful in achieving a rapid remission of some tumors, but unfortunately, for many patients, the disease recurs. Metabolism-based therapeutics might have advantages over gene-based therapies. Although most genes are important drivers of only a subset of tumor types, some of the shifts in metabolism observed in tumors are common to tumors derived from many different tissues. In addition, it may be more challenging, although certainly not impossible, for a tumor to acquire mutations that confer resistance to an anti-metabolism therapy than a gene-based therapy.108 If the metabolic characteristics of tumors are essential for the tumor’s growth and survival, targeting the tumor’s metabolism could have a dramatic effect on tumor viability.
However, there are drawbacks to a metabolism-based approach to therapy as well. Metabolism-based therapies face a major hurdle of non-specific toxicity: the same metabolic pathways are required for the survival of all cells. Activated immune cells might be expected to be especially vulnerable to anticancer therapies, which is especially concerning because these are the cells that would normally target the tumor.108 Neurons consume large amounts of glucose, and peripheral neuropathy has been detected as the dose-limiting toxicity for some anti-glycolytic therapies.45
Nevertheless, there is some reason to be hopeful about the prospects of metabolic targeting. A combination of energy metabolism inhibitors with other antitumor drugs could represent a powerful new approach to treatment.78 Energetic collapse due to blocked glycolysis could make other physical and chemical anticancer agents more effective (eg, by reducing the effectiveness of efflux transporters and allowing drugs to accumulate to higher effective doses). Alternatively, forcing cancer cells to reactivate the mitochondria might strengthen the therapeutic activity of antineoplastic treatments that depend on the induction of free radicals.
There is also hope that tumor-specific metabolic programs can be exploited for therapy. Some tumors organize the TCA cycles so that they are addicted to glucose for anaplerosis and survival,99 whereas other tumors are glutamine dependent.60,99 Tumors characterized by a strict reliance on either glucose or glutamine may be targetable through this metabolic vulnerability. There may be opportunities to target cancer-specific isozymes24,119 or pathways that are relied on more heavily by cancer cells than normal cells (eg, the conversion of glutamine to glutamate through transamination).64 PKM2 is another attractive target; both allosteric activators and inhibitors of PKM2 reduce tumor growth.40,41 Further studies that elucidate the molecular basis for distinguishing cancer cell metabolism from a proliferative phenotype, and the range of metabolic profiles in different types of cancer cells, will allow for prioritization among the targets that have been identified and will likely suggest even more targets for exploration.
Footnotes
Supported by the Princeton University National Institutes of General Medical Sciences Center of Excellence grant P50 GM071508, the Cancer Institute of New Jersey, the New Jersey Commission on Cancer Research, National Cancer Institute grant 1RC1 CA147961-01, Johnson & Johnson Foundation Focused Funding grant, the PhRMA Foundation grant, and the Jonsson Comprehensive Cancer Center. H.A.C. was the Milton E. Cassel scholar of the Rita Allen Foundation.
Current addresses of H.A.C., Department of Molecular, Cell and Developmental Biology, University of California, Los Angeles, CA, and Department of Biological Chemistry, David Geffen School of Medicine Los Angeles, Los Angeles, CA.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Czernin J., Phelps M.E. Positron emission tomography scanning: current and future applications. Ann Rev Med. 2002;53:89–112. doi: 10.1146/annurev.med.53.082901.104028. [DOI] [PubMed] [Google Scholar]
- 3.Kunkel M., Reichert T.E., Benz P., Lehr H.A., Jeong J.H., Wieand S., Bartenstein P., Wagner W., Whiteside T.L. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97:1015–1024. doi: 10.1002/cncr.11159. [DOI] [PubMed] [Google Scholar]
- 4.Mochiki E., Kuwano H., Katoh H., Asao T., Oriuchi N., Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004;28:247–253. doi: 10.1007/s00268-003-7191-5. [DOI] [PubMed] [Google Scholar]
- 5.Podoloff D.A., Advani R.H., Allred C., Benson A.B., 3rd, Brown E., Burstein H.J., Carlson R.W., Coleman R.E., Czuczman M.S., Delbeke D., Edge S.B., Ettinger D.S., Grannis F.W., Jr., Hillner B.E., Hoffman J.M., Kiel K., Komaki R., Larson S.M., Mankoff D.A., Rosenzweig K.E., Skibber J.M., Yahalom J., Yu J.M., Zelenetz A.D. NCCN task force report: positron emission tomography (PET)/computed tomography (CT) scanning in cancer. J Natl Compr Canc Netw. 2007;5(Suppl 1):S1–S22. [PubMed] [Google Scholar]
- 6.Walenta S., Wetterling M., Lehrke M., Schwickert G., Sundfor K., Rofstad E.K., Mueller-Klieser W. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60:916–921. [PubMed] [Google Scholar]
- 7.Brizel D.M., Schroeder T., Scher R.L., Walenta S., Clough R.W., Dewhirst M.W., Mueller-Klieser W. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:349–353. doi: 10.1016/s0360-3016(01)01630-3. [DOI] [PubMed] [Google Scholar]
- 8.Schwickert G., Walenta S., Sundfor K., Rofstad E.K., Mueller-Klieser W. Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res. 1995;55:4757–4759. [PubMed] [Google Scholar]
- 9.Altenberg B., Greulich K.O. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–1020. doi: 10.1016/j.ygeno.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Medina R.A., Owen G.I. Glucose transporters: expression, regulation and cancer. Biol Res. 2002;35:9–26. doi: 10.4067/s0716-97602002000100004. [DOI] [PubMed] [Google Scholar]
- 11.Reske S.N., Grillenberger K.G., Glatting G., Port M., Hildebrandt M., Gansauge F., Beger H.G. Overexpression of glucose transporter 1 and increased FDG uptake in pancreatic carcinoma. J Nucl Med. 1997;38:1344–1348. [PubMed] [Google Scholar]
- 12.Rastogi S., Banerjee S., Chellappan S., Simon G.R. Glut-1 antibodies induce growth arrest and apoptosis in human cancer cell lines. Cancer Lett. 2007;257:244–251. doi: 10.1016/j.canlet.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Keunen O., Johansson M., Oudin A., Sanzey M., Rahim S.A., Fack F., Thorsen F., Taxt T., Bartos M., Jirik R., Miletic H., Wang J., Stieber D., Stuhr L., Moen I., Rygh C.B., Bjerkvig R., Niclou S.P. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A. 2011;108:3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fine E.J., Segal-Isaacson C.J., Feinman R.D., Herszkopf S., Romano M.C., Tomuta N., Bontempo A.F., Negassa A., Sparano J.A. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012;28:1028–1035. doi: 10.1016/j.nut.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Frauwirth K.A., Riley J.L., Harris M.H., Parry R.V., Rathmell J.C., Plas D.R., Elstrom R.L., June C.H., Thompson C.B. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs S.R., Herman C.E., Maciver N.J., Wofford J.A., Wieman H.L., Hammen J.J., Rathmell J.C. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brand K. Glutamine and glucose metabolism during thymocyte proliferation: pathways of glutamine and glutamate metabolism. Biochem J. 1985;228:353–361. doi: 10.1042/bj2280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hume D.A., Radik J.L., Ferber E., Weidemann M.J. Aerobic glycolysis and lymphocyte transformation. Biochem J. 1978;174:703–709. doi: 10.1042/bj1740703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le A., Lane A.N., Hamaker M., Bose S., Gouw A., Barbi J., Tsukamoto T., Rojas C.J., Slusher B.S., Zhang H., Zimmerman L.J., Liebler D.C., Slebos R.J., Lorkiewicz P.K., Higashi R.M., Fan T.W., Dang C.V. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osthus R.C., Shim H., Kim S., Li Q., Reddy R., Mukherjee M., Xu Y., Wonsey D., Lee L.A., Dang C.V. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000;275:21797–21800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- 21.Mathupala S.P., Rempel A., Pedersen P.L. Glucose catabolism in cancer cells: identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J Biol Chem. 2001;276:43407–43412. doi: 10.1074/jbc.M108181200. [DOI] [PubMed] [Google Scholar]
- 22.Matoba S., Kang J.G., Patino W.D., Wragg A., Boehm M., Gavrilova O., Hurley P.J., Bunz F., Hwang P.M. p53 Regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 23.Schwartzenberg-Bar-Yoseph F., Armoni M., Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 2004;64:2627–2633. doi: 10.1158/0008-5472.can-03-0846. [DOI] [PubMed] [Google Scholar]
- 24.Bustamante E., Pedersen P.L. High aerobic glycolysis of rat hepatoma cells in culture: role of mitochondrial hexokinase. Proc Natl Acad Sci U S A. 1977;74:3735–3739. doi: 10.1073/pnas.74.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marin-Hernandez A., Rodriguez-Enriquez S., Vital-Gonzalez P.A., Flores-Rodriguez F.L., Macias-Silva M., Sosa-Garrocho M., Moreno-Sanchez R. Determining and understanding the control of glycolysis in fast-growth tumor cells: flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J. 2006;273:1975–1988. doi: 10.1111/j.1742-4658.2006.05214.x. [DOI] [PubMed] [Google Scholar]
- 26.Wolf A., Agnihotri S., Micallef J., Mukherjee J., Sabha N., Cairns R., Hawkins C., Guha A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalia V.K., Prabhakara S., Narayanan V. Modulation of cellular radiation responses by 2-deoxy-D-glucose and other glycolytic inhibitors: implications for cancer therapy. J Cancer Res Ther. 2009;5(Suppl 1):S57–S60. doi: 10.4103/0973-1482.55145. [DOI] [PubMed] [Google Scholar]
- 28.Wang R., Dillon C.P., Shi L.Z., Milasta S., Carter R., Finkelstein D., McCormick L.L., Fitzgerald P., Chi H., Munger J., Green D.R. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottlob K., Majewski N., Kennedy S., Kandel E., Robey R.B., Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J.W., Gao P., Liu Y.C., Semenza G.L., Dang C.V. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol. 2007;27:7381–7393. doi: 10.1128/MCB.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vora S., Halper J.P., Knowles D.M. Alterations in the activity and isozymic profile of human phosphofructokinase during malignant transformation in vivo and in vitro: transformation- and progression-linked discriminants of malignancy. Cancer Res. 1985;45:2993–3001. [PubMed] [Google Scholar]
- 32.Yi W., Clark P.M., Mason D.E., Keenan M.C., Hill C., Goddard W.A., 3rd, Peters E.C., Driggers E.M., Hsieh-Wilson L.C. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deprez J., Vertommen D., Alessi D.R., Hue L., Rider M.H. Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J Biol Chem. 1997;272:17269–17275. doi: 10.1074/jbc.272.28.17269. [DOI] [PubMed] [Google Scholar]
- 34.Atsumi T., Chesney J., Metz C., Leng L., Donnelly S., Makita Z., Mitchell R., Bucala R. High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res. 2002;62:5881–5887. [PubMed] [Google Scholar]
- 35.Telang S., Yalcin A., Clem A.L., Bucala R., Lane A.N., Eaton J.W., Chesney J. Ras transformation requires metabolic control by 6-phosphofructo-2-kinase. Oncogene. 2006;25:7225–7234. doi: 10.1038/sj.onc.1209709. [DOI] [PubMed] [Google Scholar]
- 36.Clem B., Telang S., Clem A., Yalcin A., Meier J., Simmons A., Rasku M.A., Arumugam S., Dean W.L., Eaton J., Lane A., Trent J.O., Chesney J. Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther. 2008;7:110–120. doi: 10.1158/1535-7163.MCT-07-0482. [DOI] [PubMed] [Google Scholar]
- 37.Telang S., Clem B.F., Klarer A.C., Clem A.L., Trent J.O., Bucala R., Chesney J. Small molecule inhibition of 6-phosphofructo-2-kinase suppresses T cell activation. J Transl Med. 2012;10:95. doi: 10.1186/1479-5876-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bensaad K., Tsuruta A., Selak M.A., Vidal M.N., Nakano K., Bartrons R., Gottlieb E., Vousden K.H. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 39.Christofk H.R., Vander Heiden M.G., Harris M.H., Ramanathan A., Gerszten R.E., Wei R., Fleming M.D., Schreiber S.L., Cantley L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg M.S., Sharp P.A. Pyruvate kinase M2-specific siRNA induces apoptosis and tumor regression. J Exp Med. 2012;209:217–224. doi: 10.1084/jem.20111487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anastasiou D., Yu Y., Israelsen W.J., Jiang J.K., Boxer M.B., Hong B.S. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol. 2012;8:1008. doi: 10.1038/nchembio.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wigfield S.M., Winter S.C., Giatromanolaki A., Taylor J., Koukourakis M.L., Harris A.L. PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer. Br J Cancer. 2008;98:1975–1984. doi: 10.1038/sj.bjc.6604356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McFate T., Mohyeldin A., Lu H., Thakar J., Henriques J., Halim N.D., Wu H., Schell M.J., Tsang T.M., Teahan O., Zhou S., Califano J.A., Jeoung N.H., Harris R.A., Verma A. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem. 2008;283:22700–22708. doi: 10.1074/jbc.M801765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun R.C., Fadia M., Dahlstrom J.E., Parish C.R., Board P.G., Blackburn A.C. Reversal of the glycolytic phenotype by dichloroacetate inhibits metastatic breast cancer cell growth in vitro and in vivo. Breast Cancer Res Treat. 2010;120:253–260. doi: 10.1007/s10549-009-0435-9. [DOI] [PubMed] [Google Scholar]
- 45.Michelakis E.D., Sutendra G., Dromparis P., Webster L., Haromy A., Niven E., Maguire C., Gammer T.L., Mackey J.R., Fulton D., Abdulkarim B., McMurtry M.S., Petruk K.C. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000677. 31ra34. [DOI] [PubMed] [Google Scholar]
- 46.Kim J.W., Tchernyshyov I., Semenza G.L., Dang C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Contractor T., Harris C.R. p53 negatively regulates transcription of the pyruvate dehydrogenase kinase Pdk2. Cancer Res. 2012;72:560–567. doi: 10.1158/0008-5472.CAN-11-1215. [DOI] [PubMed] [Google Scholar]
- 48.Magrath I., Lee Y.J., Anderson T., Henle W., Ziegler J., Simon R., Schein P. Prognostic factors in Burkitt’s lymphoma: importance of total tumor burden. Cancer. 1980;45:1507–1515. doi: 10.1002/1097-0142(19800315)45:6<1507::aid-cncr2820450634>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 49.Fantin V.R., St-Pierre J., Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 50.Shim H., Dolde C., Lewis B.C., Wu C.S., Dang G., Jungmann R.A., Dalla-Favera R., Dang C.V. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Granchi C., Roy S., De Simone A., Salvetti I., Tuccinardi T., Martinelli A., Macchia M., Lanza M., Betti L., Giannaccini G., Lucacchini A., Giovannetti E., Sciarrillo R., Peters G.J., Minutolo F. N-hydroxyindole-based inhibitors of lactate dehydrogenase against cancer cell proliferation. Eur J Med Chem. 2011;46:5398–5407. doi: 10.1016/j.ejmech.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 52.Hao J., Chen H., Madigan M.C., Cozzi P.J., Beretov J., Xiao W., Delprado W.J., Russell P.J., Li Y. Co-expression of CD147 (EMMPRIN), CD44v3-10, MDR1 and monocarboxylate transporters is associated with prostate cancer drug resistance and progression. Br J Cancer. 2010;103:1008–1018. doi: 10.1038/sj.bjc.6605839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sonveaux P., Vegran F., Schroeder T., Wergin M.C., Verrax J., Rabbani Z.N., De Saedeleer C.J., Kennedy K.M., Diepart C., Jordan B.F., Kelley M.J., Gallez B., Wahl M.L., Feron O., Dewhirst M.W. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boidot R., Vegran F., Meulle A., Le Breton A., Dessy C., Sonveaux P., Lizard-Nacol S., Feron O. Regulation of monocarboxylate transporter MCT1 expression by p53 mediates inward and outward lactate fluxes in tumors. Cancer Res. 2012;72:939–948. doi: 10.1158/0008-5472.CAN-11-2474. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y., Wang Y., Shen L., Pang Y., Qiao Z., Liu P. Prognostic and therapeutic implications of increased ATP citrate lyase expression in human epithelial ovarian cancer. Oncol Rep. 2012;27:1156–1162. doi: 10.3892/or.2012.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hatzivassiliou G., Zhao F., Bauer D.E., Andreadis C., Shaw A.N., Dhanak D., Hingorani S.R., Tuveson D.A., Thompson C.B. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Manning B.D., Cantley L.C. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hassanein M., Hoeksema M.D., Shiota M., Qian J., Harris B.K., Chen H., Clark J.E., Alborn W.E., Eisenberg R., Massion P.P. SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin Cancer Res. 2013;19:560–570. doi: 10.1158/1078-0432.CCR-12-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carr E.L., Kelman A., Wu G.S., Gopaul R., Senkevitch E., Aghvanyan A., Turay A.M., Frauwirth K.A. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol. 2010;185:1037–1044. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wise D.R., DeBerardinis R.J., Mancuso A., Sayed N., Zhang X.Y., Pfeiffer H.K., Nissim I., Daikhin E., Yudkoff M., McMahon S.B., Thompson C.B. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao P., Tchernyshyov I., Chang T.C., Lee Y.S., Kita K., Ochi T., Zeller K.I., De Marzo A.M., Van Eyk J.E., Mendell J.T., Dang C.V. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seltzer M.J., Bennett B.D., Joshi A.D., Gao P., Thomas A.G., Ferraris D.V., Tsukamoto T., Rojas C.J., Slusher B.S., Rabinowitz J.D., Dang C.V., Riggins G.J. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70:8981–8987. doi: 10.1158/0008-5472.CAN-10-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qing G., Li B., Vu A., Skuli N., Walton Z.E., Liu X., Mayes P.A., Wise D.R., Thompson C.B., Maris J.M., Hogarty M.D., Simon M.C. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell. 2012;22:631–644. doi: 10.1016/j.ccr.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thornburg J.M., Nelson K.K., Clem B.F., Lane A.N., Arumugam S., Simmons A., Eaton J.W., Telang S., Chesney J. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. 2008;10:R84. doi: 10.1186/bcr2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez-Enriquez S., Vital-Gonzalez P.A., Flores-Rodriguez F.L., Marin-Hernandez A., Ruiz-Azuara L., Moreno-Sanchez R. Control of cellular proliferation by modulation of oxidative phosphorylation in human and rodent fast-growing tumor cells. Toxicol Appl Pharmacol. 2006;215:208–217. doi: 10.1016/j.taap.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 66.Guppy M., Leedman P., Zu X., Russell V. Contribution by different fuels and metabolic pathways to the total ATP turnover of proliferating MCF-7 breast cancer cells. Biochem J. 2002;364:309–315. doi: 10.1042/bj3640309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moreno-Sanchez R., Rodriguez-Enriquez S., Saavedra E., Marin-Hernandez A., Gallardo-Perez J.C. The bioenergetics of cancer: is glycolysis the main ATP supplier in all tumor cells? Biofactors. 2009;35:209–225. doi: 10.1002/biof.31. [DOI] [PubMed] [Google Scholar]
- 68.Christofk H.R., Vander Heiden M.G., Wu N., Asara J.M., Cantley L.C. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 69.Mazurek S., Boschek C.B., Hugo F., Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 70.Chaneton B., Hillmann P., Zheng L., Martin A.C., Maddocks O.D., Chokkathukalam A., Coyle J.E., Jankevics A., Holding F.P., Vousden K.H., Frezza C., O’Reilly M., Gottlieb E. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature. 2012;491:458–462. doi: 10.1038/nature11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo W., Hu H., Chang R., Zhong J., Knabel M., O’Meally R., Cole R.N., Pandey A., Semenza G.L. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koukourakis M.I., Giatromanolaki A., Sivridis E., Bougioukas G., Didilis V., Gatter K.C., Harris A.L. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89:877–885. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen H., Wang L., Beretov J., Hao J., Xiao W., Li Y. Co-expression of CD147/EMMPRIN with monocarboxylate transporters and multiple drug resistance proteins is associated with epithelial ovarian cancer progression. Clin Exp Metastasis. 2010;27:557–569. doi: 10.1007/s10585-010-9345-9. [DOI] [PubMed] [Google Scholar]
- 74.Pinheiro C., Longatto-Filho A., Simoes K., Jacob C.E., Bresciani C.J., Zilberstein B., Cecconello I., Alves V.A., Schmitt F., Baltazar F. The prognostic value of CD147/EMMPRIN is associated with monocarboxylate transporter 1 co-expression in gastric cancer. Eur J Cancer. 2009;45:2418–2424. doi: 10.1016/j.ejca.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 75.Pinheiro C., Longatto-Filho A., Pereira S.M., Etlinger D., Moreira M.A., Jubé L.F., Queiroz G.S., Schmitt F., Baltazar F. Monocarboxylate transporters 1 and 4 are associated with CD147 in cervical carcinoma. Dis Markers. 2009;26:97–103. doi: 10.3233/DMA-2009-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giovannucci E., Harlan D.M., Archer M.C., Bergenstal R.M., Gapstur S.M., Habel L.A., Pollak M., Regensteiner J.G., Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanchez-Arago M., Chamorro M., Cuezva J.M. Selection of cancer cells with repressed mitochondria triggers colon cancer progression. Carcinogenesis. 2010;31:567–576. doi: 10.1093/carcin/bgq012. [DOI] [PubMed] [Google Scholar]
- 78.Kroemer G., Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 79.DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moreadith R.W., Lehninger A.L. The pathways of glutamate and glutamine oxidation by tumor cell mitochondria: role of mitochondrial NAD(P)+-dependent malic enzyme. J Biol Chem. 1984;259:6215–6221. [PubMed] [Google Scholar]
- 81.Simonnet H., Alazard N., Pfeiffer K., Gallou C., Beroud C., Demont J., Bouvier R., Schagger H., Godinot C. Low mitochondrial respiratory chain content correlates with tumor aggressiveness in renal cell carcinoma. Carcinogenesis. 2002;23:759–768. doi: 10.1093/carcin/23.5.759. [DOI] [PubMed] [Google Scholar]
- 82.Lopez-Rios F., Sanchez-Arago M., Garcia-Garcia E., Ortega A.D., Berrendero J.R., Pozo-Rodriguez F., Lopez-Encuentra A., Ballestin C., Cuezva J.M. Loss of the mitochondrial bioenergetic capacity underlies the glucose avidity of carcinomas. Cancer Res. 2007;67:9013–9017. doi: 10.1158/0008-5472.CAN-07-1678. [DOI] [PubMed] [Google Scholar]
- 83.Chatterjee A., Mambo E., Sidransky D. Mitochondrial DNA mutations in human cancer. Oncogene. 2006;25:4663–4674. doi: 10.1038/sj.onc.1209604. [DOI] [PubMed] [Google Scholar]
- 84.Sotgia F., Martinez-Outschoorn U.E., Pavlides S., Howell A., Pestell R.G., Lisanti M.P. Understanding the Warburg effect and the prognostic value of stromal caveolin-1 as a marker of a lethal tumor microenvironment. Breast Cancer Res. 2011;13:213. doi: 10.1186/bcr2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fogal V., Richardson A.D., Karmali P.P., Scheffler I.E., Smith J.W., Ruoslahti E. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol Cell Biol. 2010;30:1303–1318. doi: 10.1128/MCB.01101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu M., Shi Y., Wei X., Yang Y., Zhou Y., Hao X., Zhang N., Niu R. Depletion of mitochondrial DNA by ethidium bromide treatment inhibits the proliferation and tumorigenesis of T47D human breast cancer cells. Toxicol Lett. 2007;170:83–93. doi: 10.1016/j.toxlet.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 87.Catalina-Rodriguez O., Kolukula V.K., Tomita Y., Preet A., Palmieri F., Wellstein A., Byers S., Giaccia A.J., Glasgow E., Albanese C., Avantaggiati M.L. The mitochondrial citrate transporter, CIC, is essential for mitochondrial homeostasis. Oncotarget. 2012;3:1220–1235. doi: 10.18632/oncotarget.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Orimo A., Gupta P.B., Sgroi D.C., Arenzana-Seisdedos F., Delaunay T., Naeem R., Carey V.J., Richardson A.L., Weinberg R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 89.Karnoub A.E., Dash A.B., Vo A.P., Sullivan A., Brooks M.W., Bell G.W., Richardson A.L., Polyak K., Tubo R., Weinberg R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 90.Whitaker-Menezes D., Martinez-Outschoorn U.E., Flomenberg N., Birbe R.C., Witkiewicz A.K., Howell A., Pavlides S., Tsirigos A., Ertel A., Pestell R.G., Broda P., Minetti C., Lisanti M.P., Sotgia F. Hyperactivation of oxidative mitochondrial metabolism in epithelial cancer cells in situ: visualizing the therapeutic effects of metformin in tumor tissue. Cell Cycle. 2011;10:4047–4064. doi: 10.4161/cc.10.23.18151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinez-Outschoorn U.E., Pestell R.G., Howell A., Tykocinski M.L., Nagajyothi F., Machado F.S., Tanowitz H.B., Sotgia F., Lisanti M.P. Energy transfer in “parasitic” cancer metabolism: mitochondria are the powerhouse and Achilles’ heel of tumor cells. Cell Cycle. 2011;10:4208–4216. doi: 10.4161/cc.10.24.18487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinez-Outschoorn U.E., Pavlides S., Howell A., Pestell R.G., Tanowitz H.B., Sotgia F., Lisanti M.P. Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment. Int J Biochem Cell Biol. 2011;43:1045–1051. doi: 10.1016/j.biocel.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martinez-Outschoorn U.E., Balliet R.M., Rivadeneira D.B., Chiavarina B., Pavlides S., Wang C., Whitaker-Menezes D., Daumer K.M., Lin Z., Witkiewicz A.K., Flomenberg N., Howell A., Pestell R.G., Knudsen E.S., Sotgia F., Lisanti M.P. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9:3256–3276. doi: 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shaw R.J., Cantley L.C. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 96.Deberardinis R.J., Lum J.J., Thompson C.B. Phosphatidylinositol 3-kinase-dependent modulation of carnitine palmitoyltransferase 1A expression regulates lipid metabolism during hematopoietic cell growth. J Biol Chem. 2006;281:37372–37380. doi: 10.1074/jbc.M608372200. [DOI] [PubMed] [Google Scholar]
- 97.Nesbit C.E., Tersak J.M., Prochownik E.V. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 98.Miller D.M., Thomas S.D., Islam A., Muench D., Sedoris K. c-Myc and cancer metabolism. Clin Cancer Res. 2012;18:5546–5553. doi: 10.1158/1078-0432.CCR-12-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yuneva M., Zamboni N., Oefner P., Sachidanandam R., Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaelin W.G., Jr., Ratcliffe P.J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 101.Semenza G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vousden K.H., Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 103.Schmid T., Zhou J., Kohl R., Brune B. p300 relieves p53-evoked transcriptional repression of hypoxia-inducible factor-1 (HIF-1) Biochem J. 2004;380:289–295. doi: 10.1042/BJ20031299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li T., Kon N., Jiang L., Tan M., Ludwig T., Zhao Y., Baer R., Gu W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–1283. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rodriguez O.C., Choudhury S., Kolukula V., Vietsch E.E., Catania J., Preet A., Reynoso K., Bargonetti J., Wellstein A., Albanese C., Avantaggiati M.L. Dietary downregulation of mutant p53 levels via glucose restriction: mechanisms and implications for tumor therapy. Cell Cycle. 2012;11:4436–4446. doi: 10.4161/cc.22778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moon S.H., Prives C. Mutant p53 succumbs to starvation. Cell Cycle. 2013;12:867. doi: 10.4161/cc.23911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brosh R., Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9:701–713. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- 108.Altman B.J., Dang C.V. Normal and cancer cell metabolism: lymphocytes and lymphoma. FEBS J. 2012;279:2598–2609. doi: 10.1111/j.1742-4658.2012.08651.x. [DOI] [PubMed] [Google Scholar]
- 109.Lunt S.Y., Vander Heiden M.G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 110.Christen S., Sauer U. Intracellular characterization of aerobic glucose metabolism in seven yeast species by 13C flux analysis and metabolomics. FEMS Yeast Res. 2011;11:263–272. doi: 10.1111/j.1567-1364.2010.00713.x. [DOI] [PubMed] [Google Scholar]
- 111.Simsek T., Kocabas F., Zheng J., Deberardinis R.J., Mahmoud A.I., Olson E.N., Schneider J.W., Zhang C.C., Sadek H.A. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takubo K., Nagamatsu G., Kobayashi C.I., Nakamura-Ishizu A., Kobayashi H., Ikeda E., Goda N., Rahimi Y., Johnson R.S., Soga T., Hirao A., Suematsu M., Suda T. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 2013;12:49–61. doi: 10.1016/j.stem.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lemons J.M., Feng X.J., Bennett B.D., Legesse-Miller A., Johnson E.L., Raitman I., Pollina E.A., Rabitz H.A., Rabinowitz J.D., Coller H.A. Quiescent fibroblasts exhibit high metabolic activity. PLoS Biol. 2010;8:e1000514. doi: 10.1371/journal.pbio.1000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chang C.H., Curtis J.D., Maggi L.B., Jr., Faubert B., Villarino A.V., O’Sullivan D., Huang S.C., van der Windt G.J., Blagih J., Qiu J., Weber J.D., Pearce E.J., Jones R.G., Pearce E.L. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Newsholme E.A., Crabtree B., Ardawi M.S. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci Rep. 1985;5:393–400. doi: 10.1007/BF01116556. [DOI] [PubMed] [Google Scholar]
- 116.Molenaar D., van Berlo R., de Ridder D., Teusink B. Shifts in growth strategies reflect tradeoffs in cellular economics. Mol Syst Biol. 2009;5:323. doi: 10.1038/msb.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meadows A.L., Kong B., Berdichevsky M., Roy S., Rosiva R., Blanch H.W., Clark D.S. Metabolic and morphological differences between rapidly proliferating cancerous and normal breast epithelial cells. Biotechnol Prog. 2008;24:334–341. doi: 10.1021/bp070301d. [DOI] [PubMed] [Google Scholar]
- 118.Pedersen P.L. Warburg, me and hexokinase 2: multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr. 2007;39:211–222. doi: 10.1007/s10863-007-9094-x. [DOI] [PubMed] [Google Scholar]
- 119.Pastorino J.G., Hoek J.B. Hexokinase II: the integration of energy metabolism and control of apoptosis. Curr Med Chem. 2003;10:1535–1551. doi: 10.2174/0929867033457269. [DOI] [PubMed] [Google Scholar]
- 120.Kimura H., Braun R.D., Ong E.T., Hsu R., Secomb T.W., Papahadjopoulos D., Hong K., Dewhirst M.W. Fluctuations in red cell flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Res. 1996;56:5522–5528. [PubMed] [Google Scholar]
- 121.Rofstad E.K., Danielsen T. Hypoxia-induced metastasis of human melanoma cells: involvement of vascular endothelial growth factor-mediated angiogenesis. Br J Cancer. 1999;80:1697–1707. doi: 10.1038/sj.bjc.6690586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Martínez-Zaguilán R., Seftor E.A., Seftor R.E., Chu Y.W., Gillies R.J., Hendrix M.J. Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis. 1996;14:176–186. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- 123.Schlappack O.K., Zimmermann A., Hill R.P. Glucose starvation and acidosis: effect on experimental metastatic potential: DNA content and MTX resistance of murine tumour cells. Br J Cancer. 1991;64:663–670. doi: 10.1038/bjc.1991.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rozhin J., Sameni M., Ziegler G., Sloane B.F. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. 1994;54:6517–6525. [PubMed] [Google Scholar]
- 125.Rattigan Y.I., Patel B.B., Ackerstaff E., Sukenick G., Koutcher J.A., Glod J.W., Banerjee D. Lactate is a mediator of metabolic cooperation between stromal carcinoma associated fibroblasts and glycolytic tumor cells in the tumor microenvironment. Exp Cell Res. 2012;318:326–335. doi: 10.1016/j.yexcr.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fischer K., Hoffmann P., Voelkl S., Meidenbauer N., Ammer J., Edinger M., Gottfried E., Schwarz S., Rothe G., Hoves S., Renner K., Timischl B., Mackensen A., Kunz-Schughart L., Andreesen R., Krause S.W., Kreutz M. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 127.Gottschalk S., Anderson N., Hainz C., Eckhardt S.G., Serkova N.J. Imatinib (STI571)-mediated changes in glucose metabolism in human leukemia BCR-ABL-positive cells. Clin Cancer Res. 2004;10:6661–6668. doi: 10.1158/1078-0432.CCR-04-0039. [DOI] [PubMed] [Google Scholar]
- 128.Ying H., Kimmelman A.C., Lyssiotis C.A., Hua S., Chu G.C., Fletcher-Sananikone E., Locasale J.W., Son J., Zhang H., Coloff J.L., Yan H., Wang W., Chen S., Viale A., Zheng H., Paik J.H., Lim C., Guimaraes A.R., Martin E.S., Chang J., Hezel A.F., Perry S.R., Hu J., Gan B., Xiao Y., Asara J.M., Weissleder R., Wang Y.A., Chin L., Cantley L.C., DePinho R.A. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Reddy M.M., Fernandes M.S., Deshpande A., Weisberg E., Inguilizian H.V., Abdel-Wahab O., Kung A.L., Levine R.L., Griffin J.D., Sattler M. The JAK2V617F oncogene requires expression of inducible phosphofructokinase/fructose-bisphosphatase 3 for cell growth and increased metabolic activity. Leukemia. 2012;26:481–489. doi: 10.1038/leu.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Seyfried T.N., Shelton L.M. Cancer as a metabolic disease. Nutr Metab. 2010;7:7. doi: 10.1186/1743-7075-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Farber S., Diamond L.K. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamine acid. N Engl J Med. 1948;238:787–793. doi: 10.1056/NEJM194806032382301. [DOI] [PubMed] [Google Scholar]