Abstract
Methamphetamine is the second most frequently used illicit drug in the United States. Methamphetamine abuse is associated with increased risk of HIV-1 acquisition, higher viral loads, and enhanced HIV-1 pathogenesis. Although a direct link between methamphetamine abuse and HIV-1 pathogenesis remains to be established in patients, methamphetamine has been shown to increase HIV-1 replication in macrophages, dendritic cells, and cells of HIV transgenic mice. Intriguingly, the effects of methamphetamine on HIV-1 replication in human CD4+ T cells that serve as the primary targets of infection in vivo are not clearly understood. Therefore, we examined HIV-1 replication in primary CD4+ T cells in the presence of methamphetamine in a dose-dependent manner. Our results demonstrate that methamphetamine had a minimal effect on HIV-1 replication at concentrations of 1 to 50 μmol/L. However, at concentrations >100 μmol/L, it inhibited HIV-1 replication in a dose-dependent manner. We also discovered that methamphetamine up-regulated the cellular anti–HIV-1 microRNAs (miR-125b, miR-150, and miR-28-5p) in CD4+ T cells. Knockdown experiments illustrated that up-regulation of the anti-HIV miRNAs inhibited HIV-1 replication. These results are contrary to the paradigm that methamphetamine accentuates HIV-1 pathogenesis by increasing HIV-1 replication. Therefore, our findings underline the complex interaction between drug use and HIV-1 and necessitate comprehensive understanding of the effects of methamphetamine on HIV-1 pathogenesis.
Substance use is a major barrier for combating the HIV pandemic because it is associated with increased HIV transmission, increased viral load, and poor adherence to therapy.1–4 Accumulating evidence also suggests associations between substance use and HIV disease progression and AIDS-associated clinical outcomes.5–8 Recreational methamphetamine (METH) use is one of the fastest-growing substance use problems in the United States.9 METH use enhances high-risk sexual behaviors and increases the likelihood of HIV-1 acquisition.10 METH is also associated with higher viral loads, development of antiretroviral resistance, and rapid progression to AIDS.11–14 However, direct and molecular effects of METH on HIV-1 infection and disease progression remain poorly understood. Several mechanisms have been proposed to support the effects of METH on HIV-1 pathogenesis. METH has been shown to increase HIV-1 replication in dendritic cells (DCs)15 and monocyte-derived macrophages.16 Furthermore, METH has been suggested to activate HIV-1 long-terminal repeat (LTR) promoter-mediated transcription.17 A study using the JR-CSF/hu-CycT1 mouse model demonstrated that METH could increase HIV-1 replication in CD4+ T cells.18 However, the effects of METH on HIV-1 replication in human CD4+ T cells that are primary targets of HIV-1 infection and replication in vivo19 remain largely unclear. Therefore, we evaluated effects of METH on HIV-1 replication in human primary CD4+ T cells. We purified primary CD4+ T cells from the peripheral blood mononuclear cells (PBMCs) and activated by phytohemagglutinin (PHA). The activated CD4+ T cells were then infected with HIV-1, and replication was monitored with or without METH. Intriguingly, our results demonstrate that METH inhibits HIV-1 replication in CD4+ T cells in a dose-dependent manner. Our molecular studies suggest that METH inhibits HIV-1 replication by up-regulating the cellular anti–HIV-1 miRNAs. The inhibitory effect of METH described herein is in contrast to the earlier reports describing potentiating effects of METH on HIV-1 replication. Therefore, it is critical to better understand the molecular interplay between METH abuse and HIV-1 pathogenesis.
Materials and Methods
Isolation of PBMCs and CD4+ T Cells
Blood was purchased from the New York Blood Center as per the Meharry Medical College (Nashville, TN) Institutional Review Board. PBMCs were isolated by Ficoll-PaquePremium reagent (GE Healthcare, Pittsburgh, PA), and CD4+ T cells were isolated by negative selection as per our published protocol.20 The purity of CD4+ T cells was measured as per a published method.20 Cells with >95% purity were activated by 5 μg/mL PHA for 48 hours and cultured with 20 U/mL IL-2 (Sigma, St. Louis, MO). SupT1 cells were obtained from ATCC (Manassas, VA) and maintained in complete RPMI 1640 medium that contains 10% fetal bovine serum and antibiotics.
Cytotoxicity of METH
Methamphetamine hydrochloride was obtained from Sigma. We used 1 to 1000 μmol/L METH to mimic the physiological concentrations of METH in drug abusers; these concentrations can vary from 10 to 50 μmol/L in blood and from 240 to 1144 μmol/L in spleen and brain.21,22 Cells were treated with METH for 24 to 48 hours, and cytotoxicity was measured by MTT assay as per the manufacturer’s instructions (Millipore, Billerica, MA). Apoptosis was measured by staining cells with annexin V (AV) and propidium iodide (PI) (Beckman Coulter, Brea, CA). After treatment, cells were washed, stained, and analyzed by flow cytometry using FACSCalibur (Becton Dickinson, San Jose, CA).
Measurement of HIV-1 Replication
Infectious HIV-1 and VSV-G pseudotyped HIV-1 were produced as per our published protocol.20 A total of 1 × 106 activated CD4+ T cells were infected with HIV-1 LAI (X4) and BAL (R5) virions, with or without spinoculation in the presence of 5 μg/mL polybrene (Sigma), and 4 × 105 cells/mL were cultured, with or without METH. Infection was measured by detecting the intracellular HIV-1 p24 by fluorescence-activated cell sorter (FACS), as per our published protocol.20 Extracellular p24 was measured in the supernatants of the infected cells using the HIV-1 p24 antigen-capture ELISA (Frederick, MD), as per supplier protocol. SupT1 cells were infected with pseudotyped virions [HIV-1 green fluorescent protein (GFP) or HIV-1 luciferase], and infection was measured by FACS or luciferase activity, as per our published protocol.20 In infection experiments, METH was added after infection.
miRNA Expression and Knockdown Assays
Total RNA was isolated from cells by an miRNeasy mini kit (Qiagen, Valencia, CA), and real-time RT-PCR was performed using miRNA-specific primers (Exiqon, Vedbaek, Denmark). miRNA expression was normalized to 5s-rRNA expression. For knockdown assays, inhibitors and negative controls were purchased from Dharmacon (Lafayette, CO). Inhibitors or scrambled controls (100 pmol) were transfected to SupT1 cells using a Neon Transfection System (Life Technolgies, Carlsbad, CA). Cells were recovered in prewarmed antibiotic-free RPMI 1640 medium and incubated for 3 hours at 37°C. Then, the cells were infected to determine the effects of anti–HIV-1 miRNAs on HIV-1 replication.
Transcription Assay
HIV-1 LTR-GFP reporter construct (AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD) was transfected into SupT1 cells by the Neon Transfection System and into primary CD4+ T cells by Amaxa Nucleofactor (Lonza, Walkersville, MD) as per manufacturer’s protocol. The cells were treated with METH after infection, and GFP expression was measured by FACS after 24 hours.
Statistical Analysis
Data were analyzed by one-way analysis of variance. Comparisons between two groups were conducted using Student’s t-test. Results were considered significant when P < 0.05. Data are presented as means ± SD.
Results
METH Inhibits HIV-1 Replication in CD4+ T Cells
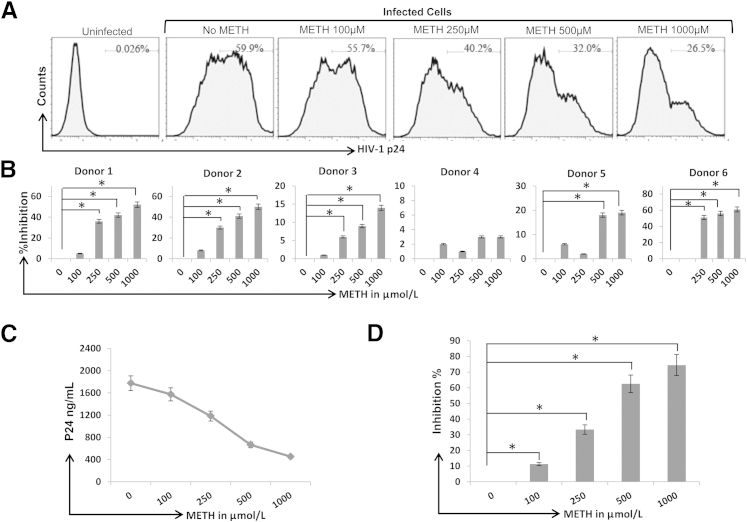
To examine the effects of METH on HIV-1 replication in CD4+ T cells, first we infected the CD4+ T-cell model SupT1 cells with pseudotyped HIV-1 GFP reporter virus and treated the cells with METH in a dose-dependent manner (1 to 1000 μmol/L). After 48 hours of infection, intracellular GFP was measured by FACS to monitor single-cycle HIV-1 replication. METH up to a 50 μmol/L concentration had no impact on GFP expression, whereas at concentrations >100 μmol/L, METH reduced GFP expression in a dose-dependent manner (Supplemental Figure S1A). The maximum inhibitory effect was observed at 1000 μmol/L of METH with approximately threefold decrease in GFP expression (Supplemental Figure S1B). Then, we infected primary CD4+ T cells with infectious HIV-1 LAI virions (X4 tropic). After 72 hours of infection, intracellular and extracellular p24 levels were measured by FACS and ELISA, respectively. Our data illustrated that METH up to 50 μmol/L had no effect on intracellular p24 expression (Supplemental Figure S1C). However, intracellular p24 expression decreased in a dose-dependent manner with 100 to 1000 μmol/L concentrations of METH (Figure 1A). Notably, a significant reduction in intracellular p24 levels was observed in cells derived from five of six donors (Figure 1B). METH also showed a dose-dependent inhibitory effect on virion release, as measured by the p24 levels in the supernatants of infected primary CD4+ T cells (Figure 1, C and D). In both intracellular and extracellular p24 assays, maximum inhibitory activity was observed with METH at 1000 μmol/L. Infection at a lower multiplicity of infection and without spinoculation also produced similar results (Supplemental Figure S2), solidifying the inhibitory effects of METH on HIV-1 replication. An earlier study by Toussi et al18 reported that METH increased replication of R5 virions in primary CD4+ T cells. Therefore, we also infected primary CD4+ T cells with HIV-1 BAL (R5 tropic) virions and measured replication with or without METH. Our data showed that METH also inhibits replication of HIV-1 BAL virions in a dose-dependent manner (Figure 2, A and B). The low level of infection of BAL virions is not surprising given that CCR5-using R5 virions have lower infectivity toward CD4+ T cells compared with C-X-C receptor (CXCR) 4 using X4 tropic virions.23 Collectively, these data strongly suggest that METH inhibits HIV-1 replication in CD4+ T cells.
Figure 1.
METH inhibits HIV-1 replication in primary CD4+ T cells. A: Primary CD4+ T cells were isolated by negative selection from human PBMCs. After isolation, the purity of CD4+ T cells was determined by FACS. CD4+ T cells with a purity >95% were activated by PHA for 48 to 72 hours, infected with HIV-1 LAI by spinoculation, and cultured in the presence or absence of METH. Productive infection was measured by detecting intracellular viral p24 protein 3 days after infection by FACS. B: Data from six different donors with percentage inhibitory activity of METH calculated from intracellular p24 expression in infected CD4+ T cells. C: Measurement of extracellular p24 levels in the supernatants of infected primary CD4+ T cells by ELISA assay. D: Percentage inhibition of p24 released from infected primary CD4+ T cells isolated from three different donors. Results are expressed as means ± SD from three independent experiments. Statistical analysis was performed by analysis of variance. ∗P < 0.05.
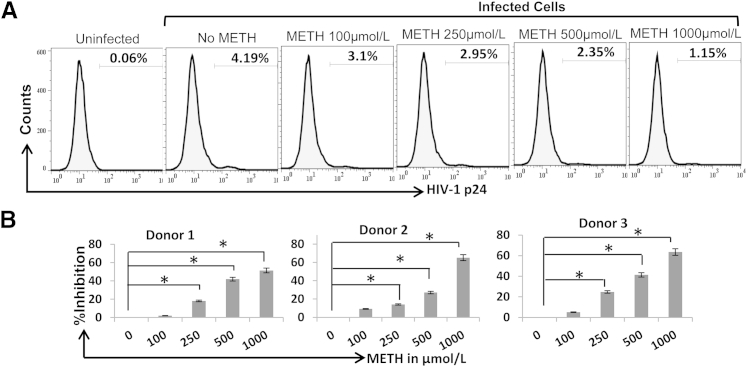
Figure 2.
METH inhibits replication of R5 tropic HIV-1 in CD4+ T cells. A: Activated primary CD4+ T cells were infected with HIV-1 BAL virions by spinoculation and cultured in the presence or absence of METH. Productive infection was measured by detecting intracellular viral p24 protein 3 to 4 days after infection by FACS. B: Data from three different donors with relative inhibition in intracellular p24 expression in the presence of METH. Results are expressed as means ± SD from three independent experiments. Statistical analysis was performed by analysis of variance. ∗P < 0.05.
The Inhibitory Effects of METH Are Not Due to Reduced Viral Transcription
Because METH has been reported to regulate HIV-1 LTR-driven transcription,17,18 we examined whether METH targeted HIV-1 transcription. We transfected the HIV-1 LTR-GFP reporter construct into SupT1 and primary CD4+ T cells and measured GFP expression by FACS after 24 to 48 hours. Notably, METH up to 1000 μmol/L concentration showed minimal/no inhibitory effect on HIV-1 LTR-driven GFP expression in either SupT1 or primary CD4+ T cells (Figure 3A). These data suggest that the inhibitory effects of METH on HIV-1 replication in CD4+ T cells are most likely not due to decreased viral transcription.
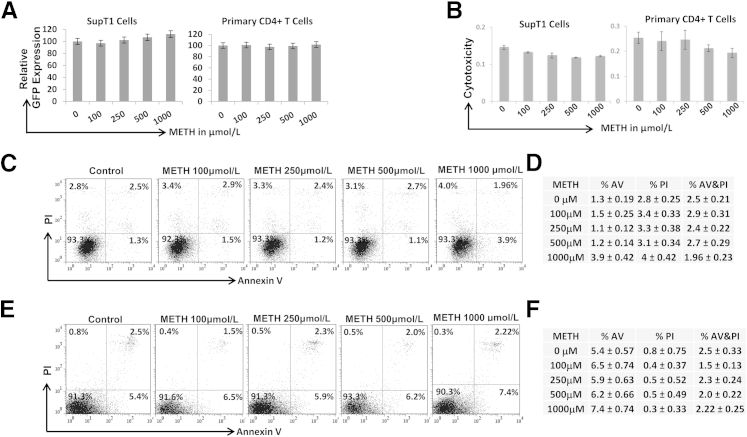
Figure 3.
Effects of METH on HIV-1 LTR-driven transcription and cytotoxicity. A: SupT1 cells and primary CD4+ T cells were transfected with HIV-1 LTR-GFP reporter construct and treated with METH for 24 hours. HIV-1 LTR-driven GFP expression was measured by FACS and expressed as relative GFP expression. B: MTT-based cytotoxicity assay using SupT1 and primary CD4+ T cells. Cells were treated with METH, and cytotoxicity was measured by MTT assay after 24 hours. Cellular apoptosis was measured by AV and PI staining using FACS in SupT1 cells (C and D) and primary CD4+ T cells (E and F) from three different donors. Results are expressed as means ± SD from three independent experiments. Statistical analysis was performed by analysis of variance.
Cytotoxicity Does Not Contribute to the Inhibitory Effects of METH on HIV-1 Replication
Given that METH has been shown to induce cytotoxicity to various cell types, we examined whether cytotoxicity contributed toward the inhibitory effects of METH. To test this, an MTT-based cytotoxicity assay was conducted. Our data indicated that METH up to 1000 μmol/L had minimal toxicity toward SupT1 and primary CD4+ T cells (Figure 3B). We also tested METH’s effect on CD4+ T-cell apoptosis by AV and PI staining to examine METH’s effect on early apoptosis (AV positive), late apoptosis (PI and AV positive), and necrosis (PI positive). Notably, METH up to 500 μmol/L minimally altered the percentage of AV (+), AV/PI (+), and PI (+) SupT1 cells (Figure 3, C and D). Similar effects were also observed with primary CD4+ T cells (Figure 3, E and F). METH at 1000 μmol/L marginally increased the AV (+) stained SupT1 cells from 1.3% to 3.9% and the primary CD4+ T cells from 5.4% to 7.4%. However, there was minimal change in AV/PI (+) and PI (+) staining. These results suggest that, at physiological concentrations, METH has minimal impact on CD4+ T-cell apoptosis.
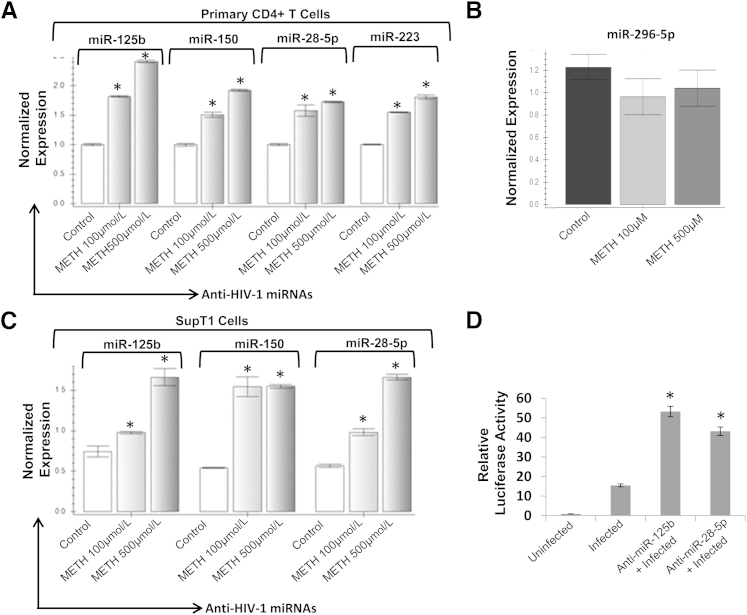
METH Up-Regulates Cellular Anti–HIV-1 miRNAs in CD4+ T Cells
Drugs of abuse, such as cocaine and opioids, have been demonstrated to target the cellular anti–HIV-1 miRNAs to regulate HIV-1 replication.20,24 Therefore, we examined the effects of METH on anti-HIV miRNAs in CD4+ T cells. We used 100 and 500 μmol/L METH for these studies based on the data on HIV-1 replication. Our real-time PCR analysis revealed that METH up-regulated expressions of miR-28-5p, miR-125b, miR-150, and miR-223 in primary CD4+ T cells (Figure 4A). Notably, the expression of the anti-viral miR-296-5p was not affected by METH in primary CD4+ T cells (Figure 4B). Our data also illustrated that, in SupT1 cells, miR-28-5p, miR-125b, and miR-150 expressions were up-regulated upon METH treatment (Figure 4C). However, we were unable to amplify miR-223 in SupT1 cells, suggesting that this miRNA may not be expressed in this CD4+ T-cell model. Notably, expressions of anti–HIV-1 miRNAs (except for miR-150) were higher in cells treated with 500 compared with 100 μmol/L, suggesting a possible correlation between levels of miRNAs and inhibition of HIV-1 replication. METH treatment also up-regulated the expressions of the anti–HIV-1 miRNAs in infected primary CD4+ T cells and SupT1 cells (Supplemental Figure S3A). To examine whether up-regulation of anti–HIV-1 miRNAs can inhibit HIV-1 replication in CD4+ T cells, we performed knockdown experiments in SupT1 cells. Because miR-125b and miR-150 have an overlapping target site on the HIV-1 genome25 (Supplemental Figure S3B), we excluded miR-150 from our experiments. Furthermore, miR-223 was also excluded because this miRNA was undetectable in SupT1 cells (Figure 4B). The miR-125b and miR-28-5p were knocked down in SupT1 cells by transfecting the respective anti-miRs and measuring knockdown efficiency by real-time PCR (data not shown). After 24 hours, the cells with the anti-miR or scrambled controls were then infected with pseudotyped HIV-1 luciferase reporter virions. Data presented in Figure 4D illustrated that both anti–miR-125b and anti–miR-28-5p transfected cells have increased luciferase activity compared with cells with the scrambled controls. Thus, our data solidified that miR-125b and miR-28-5p negatively regulate HIV-1 replication and suggested that METH-induced up-regulation of anti–HIV-1 miRNAs may inhibit HIV-1 replication in CD4+ T cells.
Figure 4.
METH up-regulates anti–HIV-1 miRNAs in CD4+ T cells. METH up-regulates anti–HIV-1 miRNA expression in primary CD4+ T cells (n = 6) (A) and CD4+ T-cell model SupT1 cells (C). Cells were treated with METH for 48 hours, and expression of cellular miRNAs was analyzed by real-time RT-PCR using RNA isolated from METH-treated cells. miRNA expression levels were determined by miRNA-specific primers and normalized to 5s-rRNA. B: Expression of miR-296-5p in primary CD4+ T cells (n = 3) as a positive control. D: Knockdown experiments were conducted in SupT1 cells using anti-miRs. Anti-miRs or scrambled control oligos were transfected into SupT1 cells using the Neon Transfection System. These cells were then infected with VSV-G–pseudotyped HIV-1 luciferase reporter virus. Infection was determined by measuring luciferase activity in the cellular lysates. Luciferase activity was normalized to total protein content of the lysate. Knockdown of miR-125b and miR-28-5p resulted in increased luciferase activity. Results are expressed as means ± SD from three independent experiments. Statistical analysis was performed by analysis of variance. ∗P < 0.05.
Discussion
METH use is a major public health concern, with approximately 35 million users worldwide.26 In the United States, METH abuse has reached epidemic levels, with an estimated 1.5 million regular users and 11 million reported to use it at least once in their lifetime.27,28 METH use is particularly prevalent among HIV-1 patients, with 10% to 15% of HIV-1–positive individuals acknowledging METH use.29 This may be due to the fact that METH is the most widely used recreational drug among men who have sex with men29–31 and is associated with doubling the risk of HIV-1 acquisition.32–36 In addition to men who have sex with men, a strong correlation between METH use and HIV infection has been observed among heterosexual men37 and female sex workers.38 Notably, METH use has also been suggested to play a role in rapid progression to AIDS and to increase virus load in the central nervous system of HIV-infected people.14,39 However, the molecular mechanisms underlying the interaction between METH abuse and HIV-1 infection/replication/disease progression are not clearly understood.
In vitro studies suggest that METH enhances HIV-1 replication in various HIV-1–permissive cells, including monocyte-derived macrophages and DCs.15,16 METH has also been shown to increase replication of the feline immunodeficiency virus in astrocytes.40 Intriguingly, the effects of METH on HIV-1 replication in human CD4+ T cells that are the primary targets of HIV-1 infection and replication in vivo remain largely unclear. Therefore, we examined the effects of METH on HIV-1 replication using primary CD4+ T cells isolated from human PBMCs. The concentration of METH used in our study mimics that of drug abusers, which can vary from 10 to 50 μmol/L in blood and from 240 to 1144 μmol/L in spleen and brain.21,22 Our data revealed that METH at concentrations of 1 to 50 μmol/L had no effect on HIV-1 replication in SupT1 and primary CD4+ T cells (Supplemental Figure S1). However, at concentrations of 100 to 1000 μmol/L, METH inhibited viral replication in a dose-dependent manner in both primary and model CD4+ T cells (Figure 1). This is in contrast to the study by Toussi et al18 that showed that METH at concentrations up to 150 μmol/L enhances HIV-1 replication in the peripheral CD4+ T cells of JR-CSF/hu-CycT1 HIV-1 transgenic mouse. This double-transgenic mouse is populated with mouse cells that express the HIV-1 JR-CSF provirus and produce human cyclin T1 gene, enabling support of Tat-mediated transactivation of HIV LTR in the mouse CD4+ T cells and myeloid-lineage cells.41 The limitations of this model are the following: i) it is not a humanized mouse model and, therefore, does not use human CD4+ T cells, ii) the provirus is integrated in every mouse cell and can be expressed by any cell type that supports HIV-1 LTR transcription, and iii) the mouse cells cannot be infected with HIV-1 and, therefore, only examine effects of METH on HIV-1 LTR-driven transcription. These authors also described that METH enhances replication of R5 tropic JR-CSF HIV-1 in human CD4+ T cells. This is in contrast to our data that showed inhibitory effects of METH on replication of X4 tropic HIV-1 LAI in CD4+ T cells. HIV-1 infection is mediated by the cellular receptor, CD4, and the coreceptors, CXCR4 and CCR5.42 X4 virions use CXCR4, whereas CCR5 is used by R5 tropic virions.42 Because primary CD4+ T cells express both the CXCR4 and CCR5 coreceptors, they can be infected by both X4 and R5 virions.42 There is evidence that CD4+ T cells infected with R5 HIV-1 produce more virions over time than X4 virion–infected CD4+ T cells.23 Therefore, we rationalized that METH’s effect on HIV-1 replication in CD4+ T cells may depend on coreceptor requirement for entry. Intriguingly, the infection experiments with R5 HIV-1 BAL virions also illustrated that METH inhibits replication of R5 virions in primary CD4+ T cells (Figure 2). We acknowledge that intracellular p24 was measured in the R5 infection experiments in contrast to extracellular p24 measurements by Toussi et al.18 However, it is unlikely that the methods used can explain the contrasting data given that extracellular p24 level is most likely dependent on intracellular p24 production. It is also likely that METH may have differential effects on the replication kinetics of BAL and JR-CSF virions in CD4+ T cells. A major difference is that we treated the cells with METH only once, whereas Toussi et al18 added METH to the cells every day. Whether this distinct pattern of METH exposure is responsible for the contrasting data remains to be elucidated. In addition, the possibility that metabolites generated under single and long-term METH exposure may have a differential effect on CD4+ T-cell function and HIV-1 replication cannot be excluded. Nevertheless, when the coreceptor requirement or viral entry was abrogated by VSV-G pseudotyping, METH also inhibited single-cycle replication of HIV-1 in CD4+ T cells (Supplemental Figure S1, A and B). Therefore, it is reasonable to conclude that METH’s effect on HIV-1 replication may not depend solely on infectivity and/or coreceptor requirement but also on postentry steps.
HIV-1 postentry steps broadly include reverse transcription, integration, transcription, translation, assembly, and release. Published data suggest that METH may regulate the entry step of the HIV-1 life cycle. For example, METH has been shown to induce dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin to facilitate virus dissemination43 and decrease the secretion of the chemokines, such as macrophage inflammatory proteins 1α and 1β and regulated on activation normal T-cell expressed and secreted, that prevent entry of virus into cells.44 METH has also been shown to increase CCR5 expression and down-regulate the expression of anti-viral cytokine, interferon-α.16,45 However, there is also precedence that METH regulates viral postentry steps, such as HIV-1 LTR-driven transcription.17,18 Therefore, we examined the effects of METH on viral transcription in CD4+ T cells using HIV-1 LTR-based reporter constructs. Interestingly, METH treatment did not alter HIV-1 LTR-driven transcription in SupT1 or primary CD4+ T cells (Figure 3A), implying that the inhibitory effect of METH on HIV-1 replication was most likely not due to decreased viral transcription. Furthermore, METH’s inhibitory effect on HIV-1 replication is most likely not due to induction of cellular apoptosis because METH did not increase the percentage of AV (+) and/or PI (+) cells (Figure 3, C–F). Therefore, we investigated the effects of METH on viral translation because our earlier report suggested that drugs of abuse, such as cocaine, regulate HIV-1 protein translation in CD4+ T cells by down-regulating the anti–HIV-1 miRNA miR-125b.20 In addition, opioids are also shown to target viral translation by regulating anti–HIV-1 miRNAs.24 The real-time PCR data revealed that METH up-regulates the expression of anti–HIV-1 miRNAs, such as miR-28-5p, miR-125b, miR-150, and miR-223 in primary CD4+ T cells (Figure 4A). Knockdown analysis solidified a role of miR-125b and miR-28-5p in regulating HIV-1 replication in CD4+ T cells (Figure 4D). Therefore, we hypothesize that METH inhibits HIV-1 replication in CD4+ T cells by up-regulating the cellular anti–HIV-miRNAs and inhibiting viral protein translation. However, further studies are required to establish a direct link between up-regulation of anti–HIV-1 miRNAs and HIV-1 replication in METH-treated cells.
Accumulating evidence suggests that METH can alter or suppress functions of immune cells, including CD4+ T cells. METH has been demonstrated to regulate T-cell proliferation, cytokine production, oxidative stress, and mitochondrial function.46,47 However, the mechanisms by which METH may regulate cellular miRNAs in CD4+ T cells are not clearly understood. We envision several pathways that can be targeted by METH to regulate anti–HIV-1 miRNAs in CD4+ T cells. For example, METH is known to regulate the catecholamine neurotransmitter, dopamine (DA).48 Notably, DA signaling is known to regulate expression of cellular miRNAs, such as miR-132 and miR-181a.49,50 Because CD4+ T cells synthesize, transport, reuptake, and express DA receptors,51 it is plausible that METH may target the DA signaling to regulate the anti–HIV-1 miRNAs. Furthermore, METH may use epigenetic mechanisms, such as DNA methylation, to regulate cellular miRNAs. This is because the promoter of miR-125b contains CpG-rich regions52 and the bioinformatics analyses illustrate that the promoter sequences of miR-150 also contain CpG islands (data not shown). Because METH has been known to regulate gene expression by altering DNA methylation in neuronal cells,53 it is also possible that METH may up-regulate these miRNAs by promoter methylation. In addition, METH’s effect on the biogenesis pathway of cellular miRNAs in CD4+ T cells cannot be excluded. However, further studies are required to elucidate the exact mechanism by which METH regulates cellular miRNAs and exerts an inhibitory effect on HIV-1 replication in CD4+ T cells.
Although epidemiological studies suggest a possible association between METH use and HIV-1 disease progression, a direct link between METH use and HIV-1 replication/disease progression in human patients remains to be established. There are also inherent difficulties associated with studying drug-abusing HIV-1–infected patients. For example, history and route of drug use, amount and formulation of drug used, single or concurrent use of other drugs, and poor nutrition can also influence outcomes of HIV-1 disease. Furthermore, use of METH and other illicit drugs is often associated with a reduction or nonadherence to antiretroviral therapy, which severely complicates a direct correlation between substance use and worsening of HIV-1 disease.54–58 Therefore, the increased pathogenesis in METH-abusing individuals has been mainly attributed to nonadherence to antiretroviral therapy. Data presented herein depict that METH at concentrations (10 to 50 μmol/L) reported in the blood of METH abusers has no/minimal effect on HIV-1 replication in CD4+ T cells, implying that METH abuse may not accentuate viral load in the periphery. This contention is supported by a study of simian immunodeficiency virus–infected macaques by Marcondes et al39 illustrating that METH administration had no effect on plasma viral loads. However, these authors reported that METH led to a significantly increased viral load in the brain.39 Therefore, it is plausible that METH may not affect viral load in the periphery but can enhance HIV-1 viral load in the brain. Given that our studies are conducted in the pure cultures of CD4+ T cells, the in vivo implications of these data may be limited. Further studies are needed to examine whether METH confers similar effects on HIV-1 replication in the mixed cultures of PBMCs. Most importantly, studies with a humanized mouse model will help us comprehensively evaluate the molecular effects of METH on HIV-1 replication and disease progression. Nevertheless, our observations emphasize the complex interaction between substance use and HIV-1 disease and highlight the critical need for molecular studies to comprehensively evaluate effects of substance use on HIV-1 infection, replication, and disease progression.
Footnotes
Supported by National Institute on Drug Abuse/NIH grants DA024558, DA30896, and DA033892, the Vanderbilt Clinical Translational Science Award grant UL1RR024975, the Meharry Translational Research Center Clinical Translational Science Award grant, National Center for Research Resources/NIH grantU54 RR026140, National Institute on Minority Health and Health Disparities/NIH grant U54 MD007593, and National Institute on Drug Abuse/NIH Diversity-Promoting Institutions Drug Abuse Research Program grant R24DA021471 (C.D.).
Supplemental Data
Dose-dependent effects of METH on HIV-1 replication in SupT1 and primary CD4+ T cells. A: SupT1 cells were infected with VSV-G–pseudotyped HIV-1–GFP reporter virions and treated with 1 to 1000 μmol/L METH. Single-cycle replication was measured after 48 hours of infection by detecting percentage of GFP-positive cells by FACS. B: Percentage inhibitory activity of METH calculated from GFP expression in infected SupT1 cells. C: Primary CD4+ T cells were isolated by negative selection from human PBMCs. After isolation, these cells were activated by PHA for 48 to 72 hours, infected with HIV-1 LAI by spinoculation, and cultured in the presence or absence of 1 to 50 μmol/L METH. Productive infection was measured by detecting intracellular viral p24 protein 3 days after infection by FACS. D: Data from three different donors with relative infection as determined from the measurement of intracellular p24 expression in the presence of METH. Results are expressed as means ± SE from three independent experiments. Statistical analysis was performed by analysis of variance. ∗P < 0.05.
METH inhibits HIV-1 replication in CD4+ T cells. A: Activated primary CD4+ T cells were infected with X4 virions at 1 multiplicity of infection by spinoculation and cultured in the presence or absence of METH. Productive infection was measured by detecting intracellular viral p24 protein 3 to 4 days after infection by FACS. B: Data from three different donors with relative inhibition in intracellular p24 expression in the presence of METH. C: Infection of primary CD4+ T cells without spinoculation. X4 virions were added to activated primary CD4+ T cells and incubated at 37°C overnight. Productive infection was measured by detecting intracellular viral p24 protein 3 to 4 days after infection by FACS. D: Data from three different donors with relative inhibition in intracellular p24 expression in the presence of METH. Results are expressed as means ± SE from three independent experiments. Statistical analysis was performed by analysis of variance. ∗P < 0.05.
METH up-regulates anti–HIV-1 miRNAs in HIV-1–infected CD4+ T cells. A: METH up-regulates anti–HIV-1 miRNA expression in infected primary CD4+ T cells and SupT1 cells. Primary CD4+ T cells were infected with HIV-1 LAI virions, whereas SupT1 cells were infected with VSV-G–pseudotyped HIV-1 GFP virions. Then, these cells were treated with 500 μmol/L METH for 48 hours, and expression of cellular miRNAs was analyzed by real-time RT-PCR using RNA isolated from METH-treated cells. miRNA expression levels were determined by miRNA-specific primers and normalized to 5s-rRNA. Results are expressed as means ± SE conducted in triplicate. B: Overlapping targets of miR-125b and miR-150 on the HIV-1 genome.
References
- 1.Kipp A.M., Desruisseau A.J., Qian H.Z. Non-injection drug use and HIV disease progression in the era of combination antiretroviral therapy. J Subst Abuse Treat. 2011;40:386–396. doi: 10.1016/j.jsat.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman H., Pross S., Klein T.W. Addictive drugs and their relationship with infectious diseases. FEMS Immunol Med Microbiol. 2006;47:330–342. doi: 10.1111/j.1574-695X.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- 3.Khalsa J.H., Royal W. Do drugs of abuse impact on HIV disease? J Neuroimmunol. 2004;147:6–8. doi: 10.1016/j.jneuroim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Cabral G.A. Drugs of abuse, immune modulation, and AIDS. J Neuroimmune Pharmacol. 2006;1:280–295. doi: 10.1007/s11481-006-9023-5. [DOI] [PubMed] [Google Scholar]
- 5.Baum M.K., Rafie C., Lai S., Sales S., Page B., Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009;50:93–99. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- 6.Cook J.A., Burke-Miller J.K., Cohen M.H., Cook R.L., Vlahov D., Wilson T.E., Golub E.T., Schwartz R.M., Howard A.A., Ponath C., Plankey M.W., Levine A., Grey D.D. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008;22:1355–1363. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cofrancesco J., Jr., Scherzer R., Tien P.C., Gibert C.L., Southwell H., Sidney S., Dobs A., Grunfeld C. Illicit drug use and HIV treatment outcomes in a US cohort. AIDS. 2008;22:357–365. doi: 10.1097/QAD.0b013e3282f3cc21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian H.Z., Stinnette S.E., Rebeiro P.F., Kipp A.M., Shepherd B.E., Samenow C.P., Jenkins C.A., No P., McGowan C.C., Hulgan T., Sterling T.R. The relationship between injection and noninjection drug use and HIV disease progression. J Subst Abuse Treat. 2011;41:14–20. doi: 10.1016/j.jsat.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freese T.E., Obert J., Dickow A., Cohen J., Lord R.H. Methamphetamine abuse: issues for special populations. J Psychoactive Drugs. 2000;32:177–182. doi: 10.1080/02791072.2000.10400226. [DOI] [PubMed] [Google Scholar]
- 10.Shoptaw S., Reback C.J., Freese T.E. Patient characteristics, HIV serostatus, and risk behaviors among gay and bisexual males seeking treatment for methamphetamine abuse and dependence in Los Angeles. J Addict Dis. 2002;21:91–105. doi: 10.1300/j069v21n01_08. [DOI] [PubMed] [Google Scholar]
- 11.Ellis R.J., Childers M.E., Cherner M., Lazzaretto D., Letendre S., Grant I. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188:1820–1826. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- 12.Smith D.M., Wong J.K., Hightower G.K., Ignacio C.C., Koelsch K.K., Petropoulos C.J., Richman D.D., Little S.J. HIV drug resistance acquired through superinfection. AIDS. 2005;19:1251–1256. doi: 10.1097/01.aids.0000180095.12276.ac. [DOI] [PubMed] [Google Scholar]
- 13.Colfax G.N., Vittinghoff E., Grant R., Lum P., Spotts G., Hecht F.M. Frequent methamphetamine use is associated with primary non-nucleoside reverse transcriptase inhibitor resistance. AIDS. 2007;21:239–241. doi: 10.1097/QAD.0b013e3280114a29. [DOI] [PubMed] [Google Scholar]
- 14.Moore R.D., Keruly J.C., Chaisson R.E. Differences in HIV disease progression by injecting drug use in HIV-infected persons in care. J Acquir Immune Defic Syndr. 2004;35:46–51. doi: 10.1097/00126334-200401010-00006. [DOI] [PubMed] [Google Scholar]
- 15.Nair M.P., Saiyed Z.M., Nair N., Gandhi N.H., Rodriguez J.W., Boukli N., Provencio-Vasquez E., Malow R.M., Miguez-Burbano M.J. Methamphetamine enhances HIV-1 infectivity in monocyte derived dendritic cells. J Neuroimmune Pharmacol. 2009;4:129–139. doi: 10.1007/s11481-008-9128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang H., Wang X., Chen H., Song L., Ye L., Wang S.H., Wang Y.J., Zhou L., Ho W.Z. Methamphetamine enhances HIV infection of macrophages. Am J Pathol. 2008;172:1617–1624. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wires E.S., Alvarez D., Dobrowolski C., Wang Y., Morales M., Karn J., Harvey B.K. Methamphetamine activates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappaB) and induces human immunodeficiency virus (HIV) transcription in human microglial cells. J Neurovirol. 2012;18:400–410. doi: 10.1007/s13365-012-0103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toussi S.S., Joseph A., Zheng J.H., Dutta M., Santambrogio L., Goldstein H. Short communication: methamphetamine treatment increases in vitro and in vivo HIV replication. AIDS Res Hum Retroviruses. 2009;25:1117–1121. doi: 10.1089/aid.2008.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane H.C. Pathogenesis of HIV infection: total CD4+ T-cell pool, immune activation, and inflammation. Top HIV Med. 2010;18:2–6. [PubMed] [Google Scholar]
- 20.Mantri C.K., Dash-Pandhare J., Mantri J., Dash C. Cocaine enhances HIV-1 replication in CD4+ T cells by down-regulating miR-125b. PLoS One. 2012;7:e51387. doi: 10.1371/journal.pone.0051387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talloczy Z., Martinez J., Joset D., Ray Y., Gacser A., Toussi S., Mizushima N., Nosanchuk J.D., Goldstein H., Loike J., Sulzer D., Santambrogio L. Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog. 2008;4:e28. doi: 10.1371/journal.ppat.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riviere G.J., Gentry W.B., Owens S.M. Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J Pharmacol Exp Ther. 2000;292:1042–1047. [PubMed] [Google Scholar]
- 23.Schweighardt B., Roy A.M., Meiklejohn D.A., Grace E.J., 2nd, Moretto W.J., Heymann J.J., Nixon D.F. R5 human immunodeficiency virus type 1 (HIV-1) replicates more efficiently in primary CD4+ T-cell cultures than X4 HIV-1. J Virol. 2004;78:9164–9173. doi: 10.1128/JVI.78.17.9164-9173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Ye L., Zhou Y., Liu M.Q., Zhou D.J., Ho W.Z. Inhibition of anti-HIV microRNA expression: a mechanism for opioid-mediated enhancement of HIV infection of monocytes. Am J Pathol. 2011;178:41–47. doi: 10.1016/j.ajpath.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J., Wang F., Argyris E., Chen K., Liang Z., Tian H., Huang W., Squires K., Verlinghieri G., Zhang H. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med. 2007;13:1241–1247. doi: 10.1038/nm1639. [DOI] [PubMed] [Google Scholar]
- 26.Reiner B.C., Keblesh J.P., Xiong H. Methamphetamine abuse, HIV infection, and neurotoxicity. Int J Physiol Pathophysiol Pharmacol. 2009;1:162–179. [PMC free article] [PubMed] [Google Scholar]
- 27.Colfax G., Shoptaw S. The methamphetamine epidemic: implications for HIV prevention and treatment. Curr HIV/AIDS Rep. 2005;2:194–199. doi: 10.1007/s11904-005-0016-4. [DOI] [PubMed] [Google Scholar]
- 28.Rawson R.A., Anglin M.D., Ling W. Will the methamphetamine problem go away? J Addict Dis. 2002;21:5–19. doi: 10.1300/j069v21n01_02. [DOI] [PubMed] [Google Scholar]
- 29.Purcell D.W., Moss S., Remien R.H., Woods W.J., Parsons J.T. Illicit substance use, sexual risk, and HIV-positive gay and bisexual men: differences by serostatus of casual partners. AIDS. 2005;19(Suppl 1):S37–S47. doi: 10.1097/01.aids.0000167350.00503.db. [DOI] [PubMed] [Google Scholar]
- 30.Halkitis P.N., Parsons J.T., Stirratt M.J. A double epidemic: crystal methamphetamine drug use in relation to HIV transmission among gay men. J Homosex. 2001;41:17–35. doi: 10.1300/J082v41n02_02. [DOI] [PubMed] [Google Scholar]
- 31.Semple S.J., Patterson T.L., Grant I. Motivations associated with methamphetamine use among HIV+ men who have sex with men. J Subst Abuse Treat. 2002;22:149–156. doi: 10.1016/s0740-5472(02)00223-4. [DOI] [PubMed] [Google Scholar]
- 32.Drumright L.N., Little S.J., Strathdee S.A., Slymen D.J., Araneta M.R., Malcarne V.L., Daar E.S., Gorbach P.M. Unprotected anal intercourse and substance use among men who have sex with men with recent HIV infection. J Acquir Immune Defic Syndr. 2006;43:344–350. doi: 10.1097/01.qai.0000230530.02212.86. [DOI] [PubMed] [Google Scholar]
- 33.Peck J.A., Reback C.J., Yang X., Rotheram-Fuller E., Shoptaw S. Sustained reductions in drug use and depression symptoms from treatment for drug abuse in methamphetamine-dependent gay and bisexual men. J Urban Health. 2005;82:i100–i108. doi: 10.1093/jurban/jti029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plankey M.W., Ostrow D.G., Stall R., Cox C., Li X., Peck J.A., Jacobson L.P. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007;45:85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoptaw S., Reback C.J. Associations between methamphetamine use and HIV among men who have sex with men: a model for guiding public policy. J Urban Health. 2006;83:1151–1157. doi: 10.1007/s11524-006-9119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoptaw S., Reback C.J. Methamphetamine use and infectious disease-related behaviors in men who have sex with men: implications for interventions. Addiction. 2007;102(Suppl 1):130–135. doi: 10.1111/j.1360-0443.2006.01775.x. [DOI] [PubMed] [Google Scholar]
- 37.Wohl A.R., Johnson D.F., Lu S., Jordan W., Beall G., Currier J., Simon P.A. HIV risk behaviors among African American men in Los Angeles County who self-identify as heterosexual. J Acquir Immune Defic Syndr. 2002;31:354–360. doi: 10.1097/00126334-200211010-00013. [DOI] [PubMed] [Google Scholar]
- 38.Patterson T.L., Semple S.J., Staines H., Lozada R., Orozovich P., Bucardo J., Philbin M.M., Pu M., Fraga M., Amaro H., Torre Ade L., Martinez G., Magis-Rodriguez C., Strathdee S.A. Prevalence and correlates of HIV infection among female sex workers in 2 Mexico-US border cities. J Infect Dis. 2008;197:728–732. doi: 10.1086/527379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcondes M.C., Flynn C., Watry D.D., Zandonatti M., Fox H.S. Methamphetamine increases brain viral load and activates natural killer cells in simian immunodeficiency virus-infected monkeys. Am J Pathol. 2010;177:355–361. doi: 10.2353/ajpath.2010.090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gavrilin M.A., Mathes L.E., Podell M. Methamphetamine enhances cell-associated feline immunodeficiency virus replication in astrocytes. J Neurovirol. 2002;8:240–249. doi: 10.1080/13550280290049660. [DOI] [PubMed] [Google Scholar]
- 41.Sun J., Soos T., Kewalramani V.N., Osiecki K., Zheng J.H., Falkin L., Santambrogio L., Littman D.R., Goldstein H. CD4-specific transgenic expression of human cyclin T1 markedly increases human immunodeficiency virus type 1 (HIV-1) production by CD4+ T lymphocytes and myeloid cells in mice transgenic for a provirus encoding a monocyte-tropic HIV-1 isolate. J Virol. 2006;80:1850–1862. doi: 10.1128/JVI.80.4.1850-1862.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grivel J.C., Shattock R.J., Margolis L.B. Selective transmission of R5 HIV-1 variants: where is the gatekeeper? J Transl Med. 2011;9(Suppl 1):S6. doi: 10.1186/1479-5876-9-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nair M.P., Mahajan S., Sykes D., Bapardekar M.V., Reynolds J.L. Methamphetamine modulates DC-SIGN expression by mature dendritic cells. J Neuroimmune Pharmacol. 2006;1:296–304. doi: 10.1007/s11481-006-9027-1. [DOI] [PubMed] [Google Scholar]
- 44.Nair M.P., Saiyed Z.M. Effect of methamphetamine on expression of HIV coreceptors and CC-chemokines by dendritic cells. Life Sci. 2011;88:987–994. doi: 10.1016/j.lfs.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahajan S.D., Hu Z., Reynolds J.L., Aalinkeel R., Schwartz S.A., Nair M.P. Methamphetamine modulates gene expression patterns in monocyte derived mature dendritic cells: implications for HIV-1 pathogenesis. Mol Diagn Ther. 2006;10:257–269. doi: 10.1007/BF03256465. [DOI] [PubMed] [Google Scholar]
- 46.Potula R., Persidsky Y. Adding fuel to the fire: methamphetamine enhances HIV infection. Am J Pathol. 2008;172:1467–1470. doi: 10.2353/ajpath.2008.080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harms R., Morsey B., Boyer C.W., Fox H.S., Sarvetnick N. Methamphetamine administration targets multiple immune subsets and induces phenotypic alterations suggestive of immunosuppression. PLoS One. 2012;7:e49897. doi: 10.1371/journal.pone.0049897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cadet J.L., Krasnova I.N., Jayanthi S., Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- 49.Saba R., Storchel P.H., Aksoy-Aksel A., Kepura F., Lippi G., Plant T.D., Schratt G.M. Dopamine-regulated microRNA MiR-181a controls GluA2 surface expression in hippocampal neurons. Mol Cell Biol. 2012;32:619–632. doi: 10.1128/MCB.05896-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang D., Li T., Wang Y., Tang Y., Cui H., Tang Y., Zhang X., Chen D., Shen N., Le W. miR-132 regulates the differentiation of dopamine neurons by directly targeting Nurr1 expression. J Cell Sci. 2012;125:1673–1682. doi: 10.1242/jcs.086421. [DOI] [PubMed] [Google Scholar]
- 51.Sarkar C., Chakroborty D., Basu S. Neurotransmitters as regulators of tumor angiogenesis and immunity: the role of catecholamines. J Neuroimmune Pharmacol. 2013;8:7–14. doi: 10.1007/s11481-012-9395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y., Yan L.X., Wu Q.N., Du Z.M., Chen J., Liao D.Z., Huang M.Y., Hou J.H., Wu Q.L., Zeng M.S., Huang W.L., Zeng Y.X., Shao J.Y. miR-125b is methylated and functions as a tumor suppressor by regulating the ETS1 proto-oncogene in human invasive breast cancer. Cancer Res. 2011;71:3552–3562. doi: 10.1158/0008-5472.CAN-10-2435. [DOI] [PubMed] [Google Scholar]
- 53.Numachi Y., Shen H., Yoshida S., Fujiyama K., Toda S., Matsuoka H., Sora I., Sato M. Methamphetamine alters expression of DNA methyltransferase 1 mRNA in rat brain. Neurosci Lett. 2007;414:213–217. doi: 10.1016/j.neulet.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 54.Arnsten J.H., Demas P.A., Grant R.W., Gourevitch M.N., Farzadegan H., Howard A.A., Schoenbaum E.E. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qian HZ, McGowan CC, Mitchell VJ, Cassell H, Perez G, Bebawy S, Stinnette SE, Hulgan T, Sterling TR: Non-injection substance abuse, depression, and adherence to antiretroviral therapy among HIV-infected patients. 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention, 17-20 July 2011, Rome, Italy, Abstract TUPE220
- 56.Parsons J.T., Kowalczyk W.J., Botsko M., Tomassilli J., Golub S.A. Aggregate versus day level association between methamphetamine use and HIV medication non-adherence among gay and bisexual men. AIDS Behav. 2013;17:1478–1487. doi: 10.1007/s10461-013-0463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marquez C., Mitchell S.J., Hare C.B., John M., Klausner J.D. Methamphetamine use, sexual activity, patient-provider communication, and medication adherence among HIV-infected patients in care, San Francisco 2004-2006. AIDS are. 2009;21:575–582. doi: 10.1080/09540120802385579. [DOI] [PubMed] [Google Scholar]
- 58.Reback C.J., Larkins S., Shoptaw S. Methamphetamine abuse as a barrier to HIV medication adherence among gay and bisexual men. AIDS care. 2003;15:775–785. doi: 10.1080/09540120310001618621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dose-dependent effects of METH on HIV-1 replication in SupT1 and primary CD4+ T cells. A: SupT1 cells were infected with VSV-G–pseudotyped HIV-1–GFP reporter virions and treated with 1 to 1000 μmol/L METH. Single-cycle replication was measured after 48 hours of infection by detecting percentage of GFP-positive cells by FACS. B: Percentage inhibitory activity of METH calculated from GFP expression in infected SupT1 cells. C: Primary CD4+ T cells were isolated by negative selection from human PBMCs. After isolation, these cells were activated by PHA for 48 to 72 hours, infected with HIV-1 LAI by spinoculation, and cultured in the presence or absence of 1 to 50 μmol/L METH. Productive infection was measured by detecting intracellular viral p24 protein 3 days after infection by FACS. D: Data from three different donors with relative infection as determined from the measurement of intracellular p24 expression in the presence of METH. Results are expressed as means ± SE from three independent experiments. Statistical analysis was performed by analysis of variance. ∗P < 0.05.
METH inhibits HIV-1 replication in CD4+ T cells. A: Activated primary CD4+ T cells were infected with X4 virions at 1 multiplicity of infection by spinoculation and cultured in the presence or absence of METH. Productive infection was measured by detecting intracellular viral p24 protein 3 to 4 days after infection by FACS. B: Data from three different donors with relative inhibition in intracellular p24 expression in the presence of METH. C: Infection of primary CD4+ T cells without spinoculation. X4 virions were added to activated primary CD4+ T cells and incubated at 37°C overnight. Productive infection was measured by detecting intracellular viral p24 protein 3 to 4 days after infection by FACS. D: Data from three different donors with relative inhibition in intracellular p24 expression in the presence of METH. Results are expressed as means ± SE from three independent experiments. Statistical analysis was performed by analysis of variance. ∗P < 0.05.
METH up-regulates anti–HIV-1 miRNAs in HIV-1–infected CD4+ T cells. A: METH up-regulates anti–HIV-1 miRNA expression in infected primary CD4+ T cells and SupT1 cells. Primary CD4+ T cells were infected with HIV-1 LAI virions, whereas SupT1 cells were infected with VSV-G–pseudotyped HIV-1 GFP virions. Then, these cells were treated with 500 μmol/L METH for 48 hours, and expression of cellular miRNAs was analyzed by real-time RT-PCR using RNA isolated from METH-treated cells. miRNA expression levels were determined by miRNA-specific primers and normalized to 5s-rRNA. Results are expressed as means ± SE conducted in triplicate. B: Overlapping targets of miR-125b and miR-150 on the HIV-1 genome.