Abstract
Excessive neutrophil migration across the pulmonary endothelium into the lung and release of oxidants and proteases are key elements in pathogenesis of acute lung injury. Previously, we identified protein kinase C-delta (PKCδ) as an important regulator of proinflammatory signaling in human neutrophils and demonstrated that intratracheal instillation of a TAT-conjugated PKCδ inhibitory peptide (PKCδ-TAT) is lung protective in a rat model of sepsis-induced indirect pulmonary injury (cecal ligation and puncture). In the present study, intratracheal instillation of this PKCδ inhibitor resulted in peptide distribution throughout the lung parenchyma and pulmonary endothelium and decreased neutrophil influx, with concomitant attenuation of sepsis-induced endothelial ICAM-1 and VCAM-1 expression in this model. To further delineate the role of PKCδ in regulating neutrophil migration, we used an in vitro transmigration model with human pulmonary microvascular endothelial cells (PMVECs). Consistent with in vivo findings, inhibition of PMVEC PKCδ decreased IL-1β–mediated neutrophil transmigration. PKCδ regulation was stimulus-dependent; PKCδ was required for transmigration mediated by IL-1β and fMLP (integrin-dependent), but not IL-8 (integrin-independent). PKCδ was essential for IL-1β–mediated neutrophil adherence and NF-κB–dependent expression of ICAM-1 and VCAM-1. In PMVECs, IL-1β–mediated production of ROS and activation of redox-sensitive NF-κB were PKCδ dependent, suggesting an upstream signaling role. Thus, PKCδ has an important role in regulating neutrophil–endothelial cell interactions and recruitment to the inflamed lung.
Sepsis and sepsis-induced lung injury are among the leading causes of death in intensive care units, resulting in more than 200,000 deaths per year in the United States.1 The lung is the organ most often affected; lung injury results in pulmonary dysfunction, which can develop into acute lung injury or the more severe acute respiratory distress syndrome (ARDS).2–4 Sepsis is characterized by an intense inflammatory response leading to excessive neutrophil infiltration of the lungs, producing tissue damage.2,5–7 Although neutrophils are critical to host defense against pathogens, neutrophil dysregulation has a critical role in the early course of lung injury and development of respiratory failure, through release of proteases and oxygen radicals that damage lung tissue and result in lung edema and impaired gas exchange.5–8
ARDS can develop from direct pulmonary sepsis (eg, pneumonia) or nonpulmonary sepsis (eg, intra-abdominal sepsis). Although both lead to common pulmonary alterations associated with ARDS, the underlying pathophysiology may be distinct.9–12 During pulmonary infections, there is direct interaction with pathogens and pathogen-associated molecular patterns involving lung epithelium and alveolar macrophages that generate proinflammatory mediators and chemotactic gradients which recruit neutrophils and other immune cells to the site of pulmonary infection. Conversely, indirect pulmonary injury arises from proinflammatory mediators released from remote infectious foci, leading to a systemic inflammatory response, activation of circulating neutrophils, and increased global vascular endothelial permeability.9–12
To date, therapeutic approaches to the treatment of sepsis-induced acute lung injury or ARDS have been largely supportive, and no specific pharmacological therapies are available to protect the lung from neutrophil-mediated damage.13–15 Potential therapeutic target sites include local control of the response of the lung to systemic inflammation, as well as direct modulation of neutrophil migration and activation. The inflammatory response involves multiple overlapping and redundant mechanisms, which in turn involve numerous cell types and signaling pathways. Recent research efforts have focused on common control points in signaling that are activated by diverse signals. Several control points are appropriate for drug targeting, and protein kinase inhibitors have become a major focus for the development of anti-inflammatory drugs.16–18
Our research group identified the protein kinase C isotype delta (PKCδ) as a critical regulator of the inflammatory response and an important signal transducer of multiple signaling pathways.19–24 PKCδ is activated by proinflammatory mediators involved in the septic response (including pathogen-associated molecular patterns such as LPS and the bacterial peptide fMLP), as well as proinflammatory cytokines (including TNF-α and IL-1β).20,25 Moreover, PKCδ is activated in the lungs of a rat model of sepsis-induced indirect lung injury.24 Studies with PKCδ-deficient mice and PKCδ inhibitors have indicated a role for PKCδ in regulating immune cell trafficking to the lung in response to pulmonary inflammation triggered by asbestos exposure, LPS, stroke–reperfusion injury, or pancreatitis.26–29 Recently, our research group demonstrated that targeted inhibition of pulmonary PKCδ with a peptide inhibitor has an anti-inflammatory and lung-protective effect in a rat model of sepsis-induced lung injury.24 PKCδ is an important regulator of both neutrophil and endothelial and epithelial proinflammatory signaling.20–23,25,30,31 However, the mechanism by which PKCδ modulates neutrophil-mediated lung injury is not known.
The endothelium plays an integral role in the pathogenesis of sepsis-induced lung injury by facilitating the recruitment and activation of neutrophils through the production of chemokines and cytokines and the expression of adhesion molecules.2,32 In the present study, we investigated the in vivo role of PKCδ in neutrophil migration to the lung in a rat model of sepsis-induced indirect lung injury. In further mechanistic studies, we investigated the in vitro role of endothelial PKCδ in regulating the crosstalk between human neutrophils and pulmonary endothelium. Our studies demonstrated that PKCδ plays a key role in regulating pulmonary endothelial cell adhesion molecule expression and the influx of neutrophils in response to indirect acute lung injury. In vitro studies demonstrate that endothelial PKCδ is an important regulator of neutrophil transmigration. Furthermore, our studies demonstrated that PKCδ involvement is stimulus-dependent, acting through regulation of endothelial reactive oxygen species (ROS) production, NF-κB activation, and adhesion molecule expression.
Materials and Methods
Chemicals and Reagents
Recombinant human IL-1β and mouse monoclonal antibodies against PECAM-1 were obtained from EMD Millipore (Billerica, MA). Recombinant human IL-8 was obtained from R&D Systems (Minneapolis, MN). EGTA, fMLP, Na-orthovanadate, 4-(2-aminoethyl)-benzenesulfonyl fluoride, leupeptin, protease inhibitor cocktail, and phosphatase inhibitor cocktail were obtained from Sigma-Aldrich (St. Louis, MO). Mouse monoclonal anti–VCAM-1 and polyclonal goat anti–ICAM-1 (for IHC) were obtained from BD Bioscience (San Diego, CA). Mouse monoclonal antibody against ICAM-1 was obtained from AbD Serotec (Raleigh, NC). The Vybrant cell adhesion assay kit and 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) were obtained from Life Technologies (Carlsbad, CA). Polyclonal rabbit anti-human PKCδ, PKCβII, PKCα, and PKCζ were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal rabbit anti–VCAM-1 (for IHC), polyclonal goat anti-MPO heavy chain (L20 clone, for IHC), horseradish peroxidase (HRP)–conjugated donkey anti-goat, and goat anti-rabbit secondary antibodies, and 3-amino-9-ethyl-carbazole (AEC) substrate chromogens were also obtained from Santa Cruz Biotechnology. Pierce subcellular protein fractionation kit, SuperSignal ULTRA chemiluminescence substrate, dimethyl pimelimidate (DMP), and bicinchoninic acid (BCA) reagents were obtained from Thermo Fisher Scientific (Rockford, IL). Diphenyleneiodonium chloride (DPI) was obtained from Enzo Life Sciences (Farmingdale, NY). Apocynin was obtained from EMD Biosciences (San Diego, CA).
PKCδ Inhibitor Peptide Synthesis
PKCδ activity was selectively inhibited by a peptide antagonist that consisted of a peptide derived from the first unique region (V1) of PKCδ (SFNSYELGSL; amino acids 8 to 17) coupled via an N-terminal Cys–Cys bond to a membrane-permeant peptide sequence in the HIV TAT gene product (YGRKKRRQRRR; amino acids 47 to 57 of TAT).33 Coupling this inhibitor to a membrane-permeant TAT peptide sequence permits effective intracellular delivery into target cells.22–24,33,34 Extensive in vitro and in vivo studies have demonstrated that, when taken up by cells, the PKCδ-TAT peptide produces a unique dominant-negative phenotype that effectively inhibits activation of PKCδ, but not other PKC isotypes.22,33,34 The PKCδ-TAT peptide was also synthesized with a fluorescent tag, 5,6-carboxytetramethylrhodamine (TMR), at a noncritical site on the inhibitor portion of the peptide complex, to monitor in vivo lung distribution. This is critical, because the Cys–Cys disulfide bond between the inhibitory peptide and TAT peptide is subject to cleavage via reduction in the reducing environment of the cytoplasm. A carrier–carrier dimer (TAT-TAT) was used as a peptide control. The peptides were synthesized by Mimotopes (Melbourne, Australia) by 9-fluorenylmethoxycarbonyl solid-phase chemistry. Peptides were purified to >95% by preparative reverse-phase high-performance liquid chromatography.
Animal Protocol
Animal procedures and handling were conducted in accordance to National Institutes of Health standards and were approved by the Institutional Animal Care and Use Committee at Temple University School of Medicine. Male Sprague–Dawley rats (225 to 250 g) (Charles River Laboratories International, Wilmington, MA) were used in all experiments. Rats were housed in a climate-controlled facility and were given free access to food and water. Sepsis was induced by the cecal ligation and puncture (CLP) method, as described previously.24 For controls, sham surgery (laparotomy without cecal ligation or puncture) was performed. In the CLP animal groups, closure of the abdominal incision was followed by open tracheotomy with a 24-gauge intravenous cannula.24 Animals were randomized to receive either the PKCδ-TAT inhibitory peptide (200 μg/kg in 200 μL of PBS), the TAT-TAT control peptide (200 μg/kg in 200 μL of PBS), or a like volume of PBS (vehicle). This dose of the PKCδ inhibitor was selected based on in vitro and in vivo studies.23,24,34 After the procedure, the cannula was removed and the skin incision closed.
At 24 hours after surgery, animals were euthanized. The lungs were gravity-fixed with 10% neutral buffered formalin instillation into the airways; the trachea was then tied off, to maintain inflation during fixation. Lungs were fixed for 2 to 3 hours at room temperature, then held overnight in formalin at 4°C. After fixation, lungs were washed several times with PBS and stored in 70% ethanol (EtOH) at 4°C. Lung tissue samples were obtained from different locations (left and right lung) and depths (ventral and dorsal), to assess the uniformity of observed histological features and patterns of protein localization. Lung tissue was paraffin-embedded, cut into sections (8 to 10 μm thick), and stained with H&E or with specific antibodies for immunohistochemical detection of myeloperoxidase (MPO), ICAM-1, and VCAM-1.
Immunohistochemical Localization of MPO, ICAM-1, and VCAM-1
Rat lung tissue sections were deparaffinized according to standard protocols. Antigen retrieval was achieved by boiling the tissue slides for 4 to 20 minutes in citrate buffer pH 6.0, as needed and when applicable. In our hands, with the specific antibodies used, ICAM-1 required only brief boiling (4 minutes), MPO required a longer period of boiling (15 to 20 minutes), and VCAM-1 did not require antigen retrieval. After the antigen-retrieval steps, slides were blocked with 3% bovine serum albumin (BSA). For HRP-based detection, slides were incubated with 1.5% hydrogen peroxide for 10 minutes, to block endogenous peroxidase activity. The sections were then incubated with primary antibody (1:40 dilution for MPO, 1:200 for ICAM-1, and 1:50 for VCAM-1) for 2 to 4 hours at room temperature (ICAM-1 and MPO) or overnight at 4°C (VCAM-1). Secondary detection was performed using an Alexa Fluor 488–conjugated donkey anti-goat secondary antibody (Life Technologies) for fluorescence visualization of MPO and ICAM-1 staining. For chromogen-based visualization of ICAM-1 and VCAM-1 staining, HRP-conjugated secondary antibodies were applied and the stains were developed using the AEC chromogen. All secondary antibodies were diluted 1:1000. Negative controls were processed by omitting the primary antibody.
Representative photomicrographs were taken using a standard light and fluorescence microscope and image acquisition software SPOT version 4.5 (Diagnostic Instruments, Inc., Sterling Heights, MI). Cellular localization of ICAM-1 and VCAM-1 throughout the various levels of the pulmonary endothelium and lung parenchyma was determined based on known morphology. ImageJ software version 1.46r (NIH, Bethesda, MD) was used to count the total numbers of nuclei (DAPI stain) and of MPO+ cells in fluorescence micrographs, allowing for calculation of the percentage of total cells. Automated counts were validated with manual counts in selected micrographs. Counts were typically performed on a minimum of 10 randomly acquired fields per animal, per group, with n = 4 animals each for sham surgery, CLP+PBS, CLP+PKCδ-TAT, and CLP+TAT-TAT groups. At a minimum, 40 fields (×400) were counted per group.
Biodistribution of PKCδ-TAT Inhibitory Peptide in the Lung after IT Delivery
The uptake and distribution of the fluorescently labeled (TMR-tagged) PKCδ-TAT peptide was monitored after intratracheal (IT) delivery of the peptide (200 μg/kg). At 30 minutes after IT administration, as described above, the rats were euthanized and lungs were gravity-fixed and processed for paraffin embedding, as described above. Sections (10 μm thick) were cut, mounted on slides, deparaffinized, and rehydrated before DAPI counterstaining. Visualization of TMR fluorescence was performed on a standard fluorescence microscope, and cellular uptake (with an emphasis on the pulmonary endothelium) was assessed based on morphology and location in the tissue.
Endothelial Cell Culture
Human pulmonary microvascular endothelial cells (PMVECs) were obtained from Lonza (Walkersville, MD) and were cultured using endothelial growth medium 2 (Lonza) with added bovine brain extract, vascular endothelial growth factor, epidermal growth factor, gentamicin, and hydrocortisone according to the manufacturer’s specifications. Cells were obtained at passage 5 and used between passages 5 and 8. Cells were passed from T-25 flasks to experimental plates when 70% to 80% confluent. PMVECs were detached using trypsin–EDTA and were cultured on collagen-coated (type IV, human placental collagen; Sigma-Aldrich) Transwell inserts (Costar, 3.0-μm pore size; Corning Life Sciences, Tewksbury, MA) for transmigration experiments, or on 96-well plates for cell-surface enzyme-linked immunosorbent assays (ELISA), ROS assays, and neutrophil adherence experiments. Medium was changed on the cell monolayers every 48 hours.
PKCδ siRNA
PMVEC monolayers were washed and incubated in EGM-2 medium without antibiotics for 24 hours. Cells were washed and treated with Validated Stealth RNAi (Life Technologies) to target PKCδ (target sequence 5′-CCACUACAUCAAGAACCAUGAGUUU-3′), as described previously.22 siRNA with equivalent percentage of GC nucleotide content was used as a control. Delivery of 500 nmol/L Validated Stealth siRNA with Invitrogen Lipofectamine 2000 transfection reagent (Life Technologies) was performed in OPTI-MEM I reduced-serum medium (Life Technologies). After 4 to 6 hours of transfection, the cells were cultured in EGM-2 with antibiotics for 48 hours. PKCδ knockdown efficiency and specificity were monitored by immunoblotting for PKCδ and other PKC isotypes (PKCα, PKCβII, and PKCζ).
Preparation of Human Neutrophils
Neutrophils were isolated from 10 U/mL heparinized venous blood obtained from healthy adult donors, after informed consent, in accordance with Institutional Review Board protocols at Temple University (Philadelphia, PA). Donors were healthy adult (over the age of 18) men and women who were recruited from the Temple University community. Standard isolation techniques35 were used with Ficoll–Hypaque (GE Healthcare, Pittsburgh, PA) centrifugation, followed by dextran sedimentation and hypotonic lysis to remove residual erythrocytes. Cells were suspended in 10 mmol/L HEPES buffer (pH 7.4). Neutrophil viability was 98%, as determined by Trypan Blue exclusion.
Neutrophil Transendothelial Migration
PMVECs were seeded on collagen-coated Transwell inserts (24-well plates) and were cultured until the monolayers were confluent. PMVEC monolayer confluency was monitored by measuring resistance changes across the endothelial cell monolayer using an EndOhm epithelial voltohmmeter (World Precision Instruments, Sarasota, FL).36 The microvascular endothelial cell monolayers reached an average resistance of 18 to 20 Ω/cm2 across the monolayer within 5 to 6 days. The cells were pretreated with buffer or 2 μmol/L PKCδ inhibitor before the addition of 10 U/mL IL-1β. Neutrophils (1 × 106 cells per well) were added to the upper wells and allowed to migrate across the PMVECs for 90 minutes at 37°C in a 5% CO2–enriched atmosphere. In siRNA experiments, cells were treated with PKCδ targeted siRNA or percent-GC control RNA (as described above). After PKCδ depletion, cells were treated with 10 U/mL IL-1β. Neutrophils (1 × 106 per well) were added to the upper wells and allowed to migrate across PMVECs for 90 minutes at 37°C in a 5% CO2–enriched atmosphere. After incubation, the Transwell inserts were removed, and neutrophils in the bottom wells were counted. In some experiments, just before the addition of neutrophils to the top well, 2 nmol/L IL-8 or 1 nmol/L fMLP was added to the bottom well of PMVEC monolayers pretreated with buffer only or with 2 μmol/L PKCδ inhibitor. After 90 minutes at 37°C in a 5% CO2–enriched atmosphere, the Transwell inserts were removed and the migrated neutrophils were counted.
Neutrophil Binding to PMVECs
PMVEC monolayers were grown to confluency on 96-well plates and pretreated with buffer, 10 U/mL IL-1β, or 0.01 to 10 μmol/L IL-1β+PKCδ inhibitor for 18 hours. Isolated neutrophils were suspended in Dulbecco’s modified Eagle’s medium+BSA (0.2%) at a concentration of 2 × 106 cells/mL and incubated with the acetoxymethyl ester of calcein for 30 minutes at 37°C, according to the manufacturer’s instructions (Vybrant cell adhesion assay kit; Life Technologies). The cells were washed, and 2.5 × 105 calcein-loaded neutrophils per well were added to PMVEC monolayers and incubated for 30 minutes at 37°C. Nonadherent neutrophils were removed by careful washing. Neutrophil binding was determined fluorometrically using a FlexStation fluorescence microplate reader (Molecular Devices, Sunnyvale, CA) at an excitation wavelength of 494 nm and an emission wavelength of 515 nm. The number of bound neutrophils was calculated from a standard curve prepared from calcein-loaded neutrophils.
Surface Expression of ICAM-1, VCAM-1, and PECAM-1 on PMVECs
The surface expression of ICAM-1, VCAM-1, and PECAM-1 on PMVECs was determined by a cell-surface ELISA.37 PMVECs were grown to confluence on 96-well plates and then were pretreated with buffer or 0.01 to 5 μmol/L PKCδ inhibitor for 1 hour before the addition of 10 U/mL IL-1β. In some experiments, the cells were pretreated with 10 μmol/L ROS inhibitors DPI and 500 μmol/L apocynin or their respective controls [ie, dimethyl sulfoxide (DMSO) or EtOH] before the addition of IL-1β. The cells were incubated for 18 hours, washed twice in washing buffer (PBS with 0.5% BSA, 1 mmol/L CaCl2, and 1 mmol/L MgCl2), and then fixed for 15 minutes at room temperature in 1% paraformaldehyde. The cell monolayers were washed and blocked for 1 hour at 37°C in blocking buffer (washing buffer with 2% BSA). The cells were incubated with 0.05 μg/mL anti-human ICAM-1 (clone 6.5B5; AbD Serotec), 0.25 μg/mL VCAM-1 (clone 51-10C9; BD Pharmingen, San Diego, CA), or 0.25 μg/mL PECAM-1 (clone P2B1; EMD Millipore) for 1 hour, washed, and incubated with goat anti-mouse IgG conjugated to HRP (1:1000) for 1 hour. The cell monolayers were washed, tetramethylbenzidine liquid substrate (Sigma-Aldrich) was added, and optical density at 650 nm (OD650) was determined. Mouse IgG1 was used as an isotype control at a concentration of 0.25 μg/mL for VCAM-1 and PECAM-1 ELISA and 0.05 μg/mL for ICAM-1 ELISA. The value obtained with mouse IgG1 was subtracted from the value obtained for expression of each adhesion molecule.
Preparation of PMVEC Nuclear Fractions
PMVEC monolayers were incubated with buffer or 10 U/mL IL-1β in the absence or presence of 2 μmol/L of the PKCδ inhibitor or 10 μmol/L of the ROS inhibitor DPI for 15 minutes. Nuclear extracts were prepared using a Pierce subcellular protein fractionation kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Samples for Western blot analysis were prepared by mixing an aliquot of the nuclear extracts with 2× sample buffer and heating for 15 minutes at 65°C. Purity of nuclear fractions was routinely determined by probing fractions for cytoplasmic (lactate dehydrogenase) and nuclear (HDAC-2) markers. Nuclear extracts were run on a 4% to 12% gradient SDS-PAGE, transferred to a nitrocellulose membrane, and blocked for 1 hour at room temperature with Tris-buffered saline (pH 7.5) containing 0.1% Tween 20 and 1% BSA–3% casein.20 The membranes were incubated with a rabbit polyclonal anti-p65 NF-κB antibody, washed, and incubated with HRP-conjugated goat anti-rabbit IgG. Immunoreactive bands were visualized using Pierce SuperSignal ULTRA chemiluminescence substrate (Thermo Fisher Scientific). Translocation of the p65 NF-κB subunit to the nucleus was quantitated by densitometry analysis of Western blots with ImageJ software version 1.46r (NIH) and the values were expressed in arbitrary densitometry units.
PMVEC ROS Production
PMVEC monolayers were incubated with 10 μmol/L cell permeable fluorogenic probe 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA; Life Technologies) for 1 hour at 37°C in a 5% CO2–enriched atmosphere. Cells were washed and then incubated ± 2 μmol/L PKCδ inhibitor for 1 hour. A background reading was obtained using a FlexStation microplate reader (Molecular Devices). IL-1β (10 U/mL) was added, and ROS production was monitored for 2 hours. PMVECs treated with buffer (Dulbecco’s modified Eagle’s medium without Phenol Red) were used to correct for background fluorescence, and 100 μmol/L H2O2 was used as a continuous oxidant to confirm loading of the probe.
ROS Inhibitor Assays
VCAM-1 expression and p65 NF-κB nuclear translocation were assayed in the presence or absence of the ROS inhibitors DPI in DMSO or apocynin in EtOH. In brief, PMVECs were cultured to confluence and pretreated with 10 μmol/L DPI or 500 μmol/L apocynin for 15 minutes at 37°C in a 5% CO2–enriched atmosphere. In VCAM-1 expression assays, cells were treated with 10 U/mL IL-1β for 18 hours and expression was determined by cell-surface ELISA, as described above. In NF-κB translocation assays, PMVECs were pretreated with 10 μmol/L DPI for 15 minutes and then were stimulated with 10 U/mL IL-1β for 15 minutes at 37°C in a 5% CO2–enriched atmosphere. Nuclear fractions were prepared and the presence of nuclear p65 NF-κB was determined by immunoblotting, as described above.
Statistical Analysis
Data were analyzed by Student’s t-test for two group comparisons or analysis of variance followed for multiple comparisons. The Tukey–Kramer multiple comparisons post-test was used to evaluate the significance between experimental groups if analysis of variance indicated a significant difference. Differences were considered significant if P < 0.05. Data are expressed as means ± SEM for the number (n) of studies performed.
Results
Biodistribution of the PKCδ Inhibitor in the Lung
Previously, our research group demonstrated that IT administration of a TAT-conjugated, highly specific PKCδ peptide inhibitor decreases inflammation and exerts a lung-protective effect in a rat model of sepsis-induced lung injury.24 Coupling of the PKCδ inhibitory peptide to a protein transduction domain (TAT peptide) permits effective intracellular delivery into multiple cell types22,23,33,34 and transport across highly impermeable barriers in vivo.38 However, the biodistribution of this peptide within the lung after IT administration had not previously been examined. The endothelium is a key target, given that pulmonary endothelial cells have an active role in the recruitment of neutrophils and are important contributors to the pathogenesis of acute lung injury or ARDS.39 We therefore determined the distribution and uptake of the fluorescent TMR-tagged inhibitor within the lung, to ascertain whether the inhibitor penetrates the pulmonary epithelium and is taken up by lung parenchyma cells after IT delivery of the peptide. Qualitative assessment of TMR fluorescence in lung tissue sections revealed consistent, largely homogeneous distribution throughout the distal lung parenchyma (Figure 1A). The inhibitory peptide was observed in alveolar wall cells, as well as in the endothelial cells lining small veins and arterioles (Figure 1A). Robust TMR fluorescence was also consistently observed at all levels of the pulmonary endothelium, from the large pulmonary vessels (Figure 1B) down to the capillary endothelium of the alveoli (Figure 1, A and C). Thus, IT administration of the PKCδ-TAT peptide results in penetration of the epithelium and uptake of the inhibitor by pulmonary endothelial cells and throughout the lung parenchyma, indicating that direct effects of PKCδ inhibition in endothelial cells are likely widespread and may contribute to the observed therapeutic effects in the lung on septic challenge.
Figure 1.
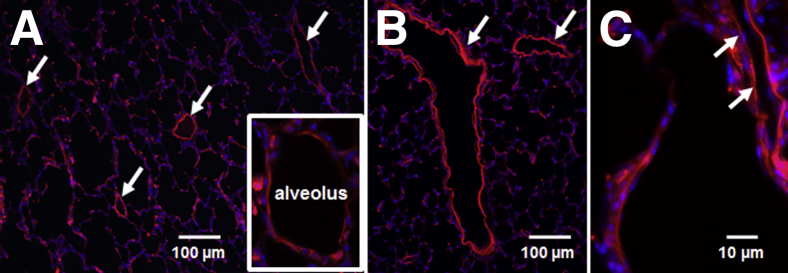
TAT-mediated transport facilitates uptake of the PKCδ inhibitory peptide throughout the pulmonary endothelium and peripheral lung parenchyma. Rat lungs were gravity-fixed 30 minutes after IT administration of a fluorescent TMR-tagged TAT-conjugated PKCδ inhibitory peptide. Representative lung tissue sections are shown (n = 3 animals). A: TMR fluorescence (red) reveals largely homogeneous distribution of the peptide throughout the peripheral lung parenchyma, in the endothelium of small veins and arterioles (arrows), and in alveolar wall cells (inset). B: Robust TMR fluorescence (red) indicates uptake of the peptide in large vessel endothelium (arrows) and surrounding parenchyma. C: Cropped view of TMR fluorescence (red) in the capillary of an alveolar septum (arrows), indicating uptake of the peptide at the level of capillary endothelium. Original magnification: ×100 (A and B); ×400 (C).
IT Administration of a PKCδ-TAT Inhibitory Peptide Attenuates Sepsis-Induced Lung Injury and Neutrophil Influx 24 Hours after CLP
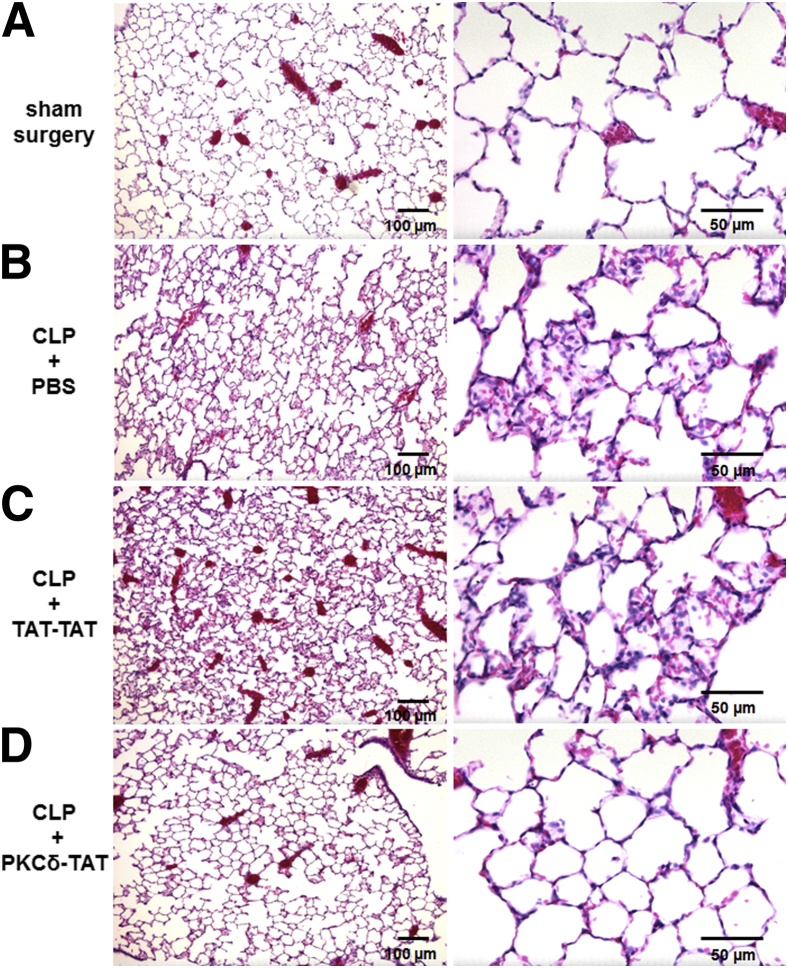
To confirm that the protection observed in our model of indirect pulmonary injury is mediated through specific effects of the PKCδ inhibitory peptide (SFNSYELGSL), rather than nonspecific effects of the TAT protein transduction domain peptide (YGRKKRRQRRR), we compared the effects of IT administration of the PKCδ-TAT peptide and the TAT-TAT control peptide after CLP. Lung sections were prepared 24 hours after sham or CLP surgery and were evaluated for alterations consistent with lung injury. In the sham-surgery rats, H&E staining demonstrated normal lung architecture, with occasional small foci of inflammatory infiltrates, but no appreciable thickening of alveolar walls and septa (Figure 2A). Lungs from CLP+PBS rats exhibited histopathological features consistent with acute inflammation and indirect pulmonary injury, including increased inflammatory cell infiltrate, thickening of alveolar walls and septa, and an overall disruption of alveolar architecture (Figure 2B), all of which are hallmark histopathological features of clinical ARDS.4,40 Lungs from CLP+TAT-TAT rats had all of the same histopathological features seen in the lungs of CLP+PBS rats (Figure 2C), indicating an absence of therapeutic effects mediated by the TAT peptide sequence. By contrast, the lungs of CLP+PKCδ-TAT rats had a histological appearance similar to that of sham-surgery controls (Figure 2D), indicating a specific therapeutic effect of the PKCδ inhibitory peptide. PKCδ inhibition preserved normal lung architecture, with marked reductions in the CLP-induced inflammatory infiltrates, without the alveolar wall thickening, hemorrhaging, and proteinaceous exudate induced by sepsis.
Figure 2.
Lung protective effects of IT administration of PKCδ-TAT in the setting of sepsis-induced indirect lung injury are specific to the PKCδ inhibitory peptide sequence. H&E staining in representative lung tissue sections from 24 hours after surgery (n = 4 animals per group). A: In the sham-surgery group, lung architecture was normal, with open alveoli and thin alveolar walls. B: In the CLP+PBS group, by 24 hours sepsis had induced indirect pulmonary injury, with widespread inflammatory infiltrate, thickening of alveolar walls and septa, as well as visible hemorrhaging and proteinaceous exudate filling some alveoli. C: In the CLP+TAT-TAT group, histopathological features were similar to those of the CLP+PBS group, indicating that the TAT peptide sequence does not exert a lung-protective effect. D: PKCδ inhibition limited the development of histological changes consistent with lung injury, with reduced inflammatory infiltrate, maintenance of alveolar wall thickness, and an absence of hemorrhaging and proteinaceous exudate induced by sepsis. Scale bars: 100 μm (left column); 50 μm (right column). Original magnification: ×100 (left column); ×400 (right column).
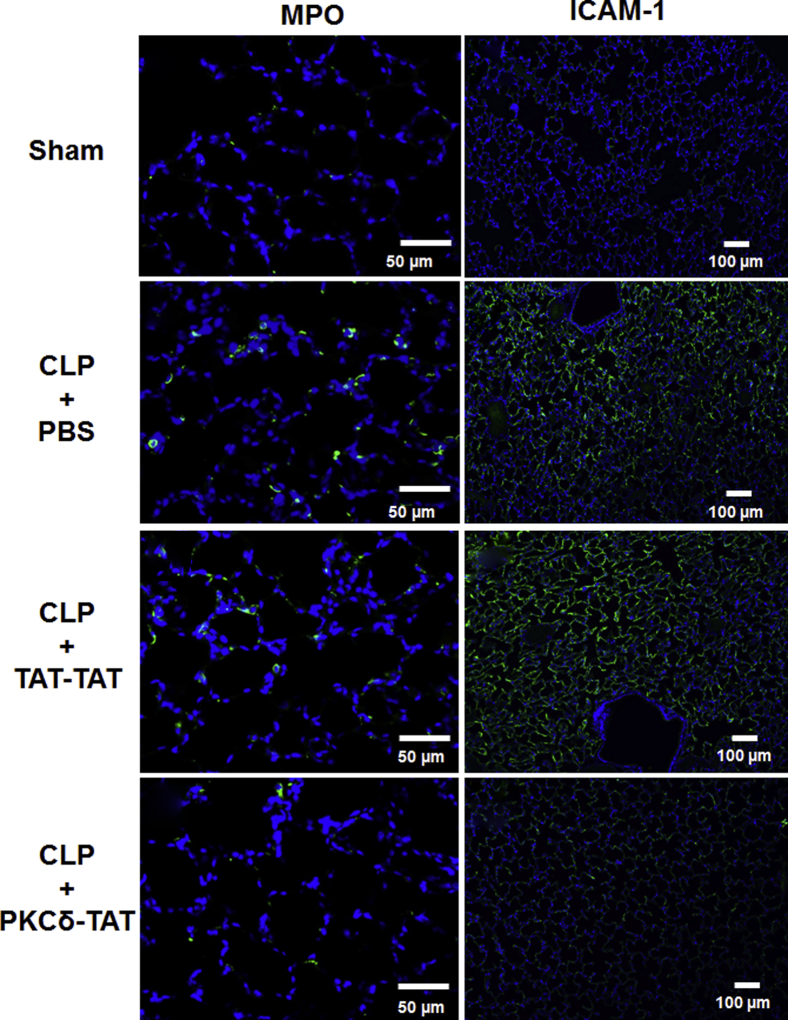
Intra-abdominal sepsis produces systemic inflammation and influx of neutrophils into the lung, a hallmark of clinical ARDS.2,5–7,41 To assess the degree to which the observed decreases in cellular infiltrates on PKCδ inhibition reflect decreased neutrophil influx, we performed immunohistochemistry to detect MPO+ cells in lung tissue sections (Figure 3). The lungs of sham-surgery rats had very low numbers of MPO+ cells (2.2 ± 1.2% of total cells) (Figure 3). By contrast, the lungs of CLP+PBS rats had significantly higher numbers of MPO+ cells (23.4 ± 6.8% of total cells; P < 0.001 versus sham surgery), indicative of acute lung inflammation (Figure 3). The lungs of CLP+TAT-TAT rats had similarly elevated levels of MPO+ cells (21.6 ± 7.8% of total cells; P < 0.001 versus sham surgery) (Figure 3). Consistent with the decreased cellular infiltrate observed with H&E staining, IT delivery of the PKCδ-TAT peptide significantly reduced the number of MPO+ cells in the lungs of CLP septic rats to near the level of sham-surgery controls (7.4 ± 3.1% of total cells; P < 0.001 versus CLP+PBS vehicle) (Figure 3).
Figure 3.
IT administration of PKCδ-TAT attenuates neutrophil migration into rat lung and attenuates ICAM-1 expression in sepsis-induced indirect lung injury. Immunohistochemical detection of MPO in representative lung tissue sections from 24 hours after surgery (n = 4 animals per group). In the sham surgery group, only a few MPO+ cells were seen in each field. In the CLP+PBS group, sepsis induced infiltration of numerous MPO+ cells throughout the lung parenchyma. Administration of the TAT-TAT control peptide had no significant effect on sepsis-induced influx of MPO+ cells into the parenchyma, compared with CLP+PBS vehicle. Administration of the inhibitory peptide led to a significant reduction of sepsis-induced MPO+ cell numbers in the lung. After primary antibody incubation, ICAM-1 was visualized using an Alexa Fluor 488–conjugated secondary antibody (green), with DAPI counterstaining (blue). Representative lung tissue sections from 24 hours after surgery are shown (n = 4 animals per group). In the sham surgery group, levels of ICAM-1 were barely detectable. In the CLP+PBS group, widespread and intense sepsis-induced ICAM-1 staining throughout the lung parenchyma was observed. In the CLP+TAT-TAT group, high levels of ICAM-1 were observed, with distribution similar to that of the CLP+PBS group. In the CLP+PKCδ-TAT group, marked reduction in sepsis-induced ICAM-1 expression was observed, with some small patches of staining seen in alveoli. Scale bars: 50 μm (left column); 100 μm (right column).
Inhibition of PKCδ Reduces ICAM-1 and VCAM-1 Expression in Lung Tissue after Septic Challenge
ICAM-1 and VCAM-1 are key adhesion molecules involved in neutrophil recruitment to the lung in sepsis, and are known to be up-regulated on activation of vascular endothelium by proinflammatory cytokines released during sepsis.6,42 To test the hypothesis that PKCδ promotes increased expression of ICAM-1 and VCAM-1 in sepsis-induced lung injury, and that decreased expression of these adhesion molecules is in part responsible for the lung-protective effect of PKCδ inhibition through decreased neutrophil influx,24 we compared expression of ICAM-1 and VCAM-1 in the lungs across experimental groups.
To examine ICAM-1 expression in septic lung tissue, we performed immunohistochemistry with fluorescence visualization to assess overall levels and distribution of ICAM-1 (Figure 3), as well as AEC chromogen–based detection to visualize ICAM-1 in the context of the overall histological appearance of the tissue and to identify cellular localization (Figure 4). At 24 hours after surgery, there was only scant ICAM-1 expression in the alveolar walls of lungs from sham-surgery rats, whereas the lungs of both CLP+PBS and CLP+TAT-TAT rats exhibited widespread ICAM-1 localization throughout the parenchyma and alveolar walls (Figure 3). ICAM-1 localization in the lungs of CLP+PKCδ-TAT rats was not nearly as widespread or robust as seen in the other two CLP groups, but was appreciably higher than in sham-surgery controls (Figure 3).
Figure 4.
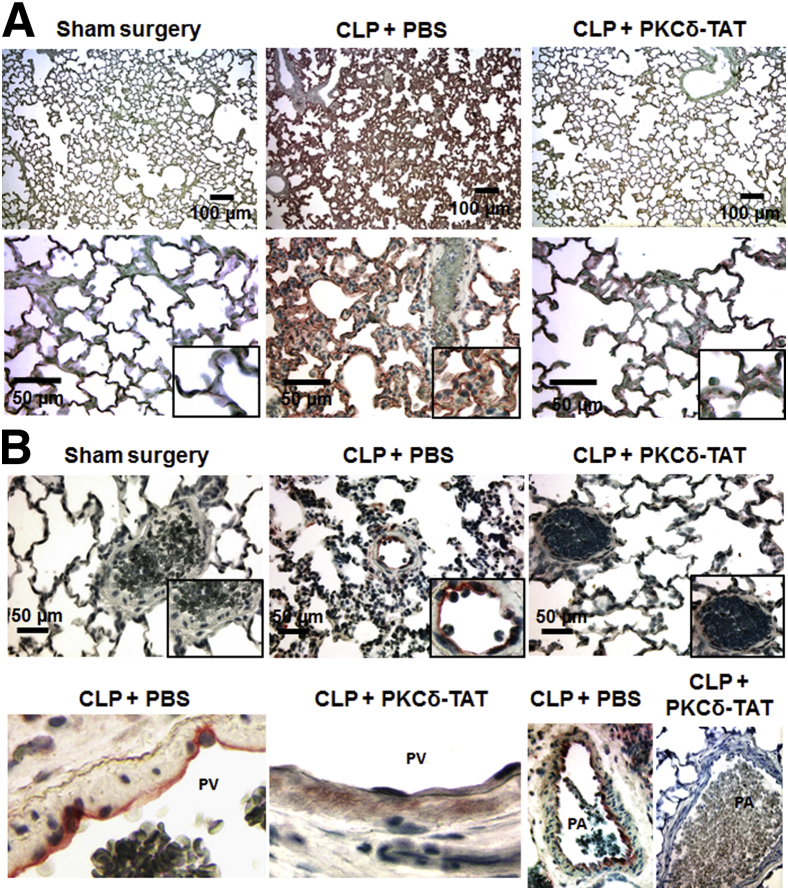
IT administration of the PKCδ inhibitor attenuates sepsis-induced expression of the adhesion molecules ICAM-1 and VCAM-1 in the pulmonary endothelium. A: Immunohistochemical detection of ICAM-1 in representative lung tissue sections from 24 hours after surgery (minimum of n = 3 animals per group). After primary antibody incubation, ICAM-1 was visualized using HRP-conjugated secondary antibodies and the peroxidase substrate chromogen AEC, which produces a red reaction product. In the sham-surgery group, ICAM-1 staining was barely detectable, and lung architecture was normal. In the CLP+PBS group, robust ICAM-1 localization was observed throughout the distal lung tissue and in the large arteries accompanying the bronchial airways. In the CLP+PKCδ-TAT group, ICAM-1 staining was reduced to near the levels with sham surgery. B: Immunohistochemical detection of VCAM-1 in representative lung tissue sections from 24 hours after surgery (minimum of n = 3 animals per group). After primary antibody incubation, VCAM-1 was visualized using HRP-conjugated secondary antibodies and the peroxidase substrate chromogen AEC (red), which produces a red reaction product. B, top row: VCAM-1 staining was not detectable in the sham surgery group. A representative VCAM-1–positive small artery situated within the injured, inflamed parenchyma is shown for the CLP+PBS group; adhered leukocytes along the VCAM-1+ endothelium are shown at higher magnification in the inset. In the CLP+PKCδ-TAT image, VCAM-1 staining is absent in the endothelium of a similarly situated small artery in the distal parenchyma. B, bottom: Cropped views of venous and arterial localization of VCAM-1. Left to right: VCAM-1 staining in the venous endothelium (CLP+PBS); absence of VCAM-1 staining in the venous endothelium (CLP+PKCδ-TAT); a large pulmonary artery with VCAM-1+ endothelium and thickened underlying interstitium (CLP+PBS); and a large pulmonary artery with absent VCAM-1 staining in the endothelium, and with normal thickness of the underlying interstitium (CLP+PKCδ-TAT). Scale bars: 100 μm (A, top); 50 μm (A, bottom, and B, top). Original magnification, ×400 (B, bottom row). PA, pulmonary artery; PV, pulmonary vein.
AEC chromogen-based visualization of ICAM-1 in the lungs of CLP+PBS rats revealed widespread and robust ICAM-1 expression throughout the pulmonary endothelium, including the pulmonary arteries and veins as well as the alveolar microcirculation throughout the parenchyma (Figure 4A). In addition, the disruption of lung architecture, interstitial thickening, and increased cellularity that are hallmarks of sepsis-induced lung injury were clearly evident in the CLP septic animals. By contrast, sham-surgery controls had normal lung architecture and cellularity, with barely detectable ICAM-1 stain in the alveolar microcirculation (Figure 4A). There was a significant reduction in the distribution and intensity of ICAM-1 staining in the lungs of CLP+PKCδ-TAT rats, and this was associated with preservation of a more histiotypic lung architecture (Figure 4A). In the CLP+PKCδ-TAT rats, some ICAM-1 staining was still evident in capillaries of the alveolar walls, compared with sham-surgery controls (Figure 4A). However, the capillary ICAM-1 staining in the lungs of CLP+PKCδ-TAT rats was less pronounced than in the CLP+PBS rats (Figure 4A).
Immunohistochemical detection of VCAM-1 demonstrated a similar pattern of staining, consistent with inflammatory activation. VCAM-1 staining was virtually absent in the lungs of sham-surgery controls, but was consistently observed in the pulmonary endothelium of CLP+PBS rats (Figure 4B). Increased leukocyte adherence to the VCAM-1+ endothelium was a common feature in septic lungs, and the interstitial regions surrounding VCAM-1+ endothelium appeared thickened and edematous, with evidence of inflammatory infiltrate, all of which are hallmarks of lung injury (Figure 4B). As was observed with ICAM-1, VCAM-1 staining in the lungs of CLP+PKCδ-TAT rats was reduced to levels similar to those of sham-surgery controls (Figure 4B). In large pulmonary veins, the endothelium consistently stained positive for VCAM-1 in CLP+PBS rats; this component of the inflammatory response was significantly attenuated on PKCδ inhibition (Figure 4B). Similarly, VCAM-1 activation in the large pulmonary arteries and the concomitant thickening of the underlying interstitium were attenuated on PKCδ inhibition (Figure 4B), further establishing the endothelial-specific anti-inflammatory and lung-protective effects of PKCδ inhibition in the setting of experimental sepsis.
Role of PKCδ in Neutrophil Transmigration
Our in vivo studies demonstrated that IT administration of the PKCδ-TAT peptide inhibitor attenuates neutrophil influx and modulates endothelial adhesion molecule expression in a model of indirect pulmonary injury. Having confirmed that the PKCδ inhibitory peptide is taken up throughout the pulmonary endothelium in vivo (Figure 1), where it may act directly to reduce adhesion molecule expression (Figures 3 and 4), we used an in vitro model of neutrophil transmigration across primary human PMVECs grown in a Transwell culture system to delineate the role of endothelial PKCδ activity in regulating neutrophil transmigration.
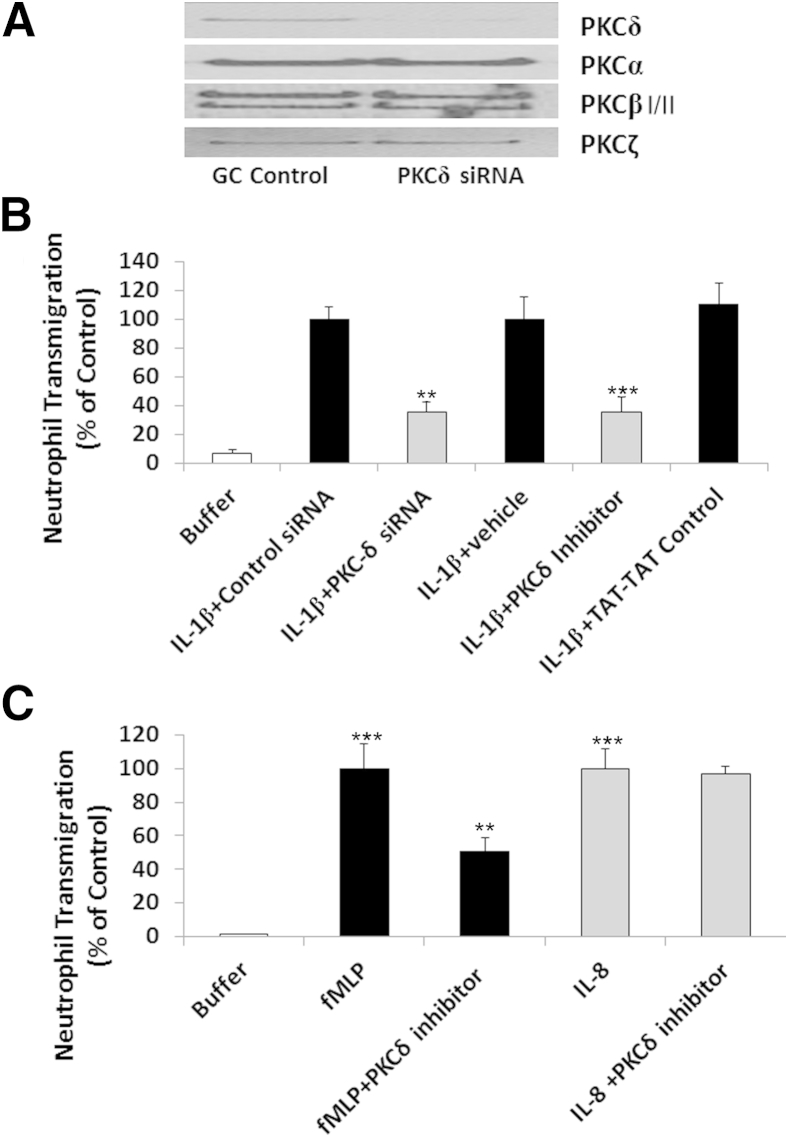
Endothelial PKCδ activity was inhibited using genetic (PKCδ siRNA) and pharmacological (PKCδ antagonist) techniques. Treatment of PMVECs with siRNA-depleted PMVEC monolayers of PKCδ, but not PKCα, PKCβI/II, or PKCζ (Figure 5A). There was minimal neutrophil migration across PMVEC monolayers in the absence of stimulus (ie, in buffer-treated PMVECs) (Figure 5B). IL-1β-mediated activation of PMVECs increased neutrophil transmigration by more than eightfold. Neutrophil migration across IL-1β–activated endothelium was significantly attenuated in PMVECs depleted of PKCδ. In contrast, PMVECs transfected with the percent-GC control siRNA showed no significant differences in neutrophil migration across IL-1β–treated PMVECs, compared with PMVECs treated with IL-1β alone. Pretreatment with PKCδ-TAT also significantly decreased neutrophil migration across IL-1β–activated PMVECs, compared with PMVECs treated with IL-1β+TAT-TAT control peptide or IL-1β+vehicle, indicating that endothelial PKCδ activity is an important component of IL-1β–induced neutrophil transmigration (Figure 5B). Because we obtained similar results with either the peptide antagonist or PKCδ siRNA, further mechanistic studies were performed using PKCδ-TAT.
Figure 5.
Role of PKCδ in neutrophil transmigration. A: Selective depletion of PKCδ by stealth PKCδ siRNA in PMVECs. Controls were PMVECs treated with siRNA with equivalent percentage of GC nucleotide content (GC control) as the stealth PKCδ siRNA. Levels of specific PKC isotypes were determined in cell lysates by immunoblotting with isotype-specific antibodies to PKCδ, PKCα, PKCβI/II, and PKCζ. Blots are representative of three separate Western blotting experiments. B: Neutrophil transmigration through IL-1β–activated PMVECs. PMVECs were grown on Transwell inserts and incubated with IL-1β or buffer after transfection with PKCδ stealth siRNA or siRNA containing equivalent percentage of GC nucleotide content (Control). In a second series of experiments, PMVECs were grown on Transwell inserts and were pretreated with buffer, 10 U/mL IL-1β+vehicle, IL-1β+TAT-TAT control peptide, or IL-1β+PKCδ inhibitor. Neutrophils (1 × 106 per well) were added to the upper well of the Transwell culture system and allowed to migrate for 1.5 hours through PMVECs. C: Role of PKCδ in neutrophil transmigration mediated by IL-8 and fMLP. Chemoattractants fMLP (1 nmol/L) or IL-8 (2 nmol/L) were added to the bottom well of a Transwell culture system. Neutrophils (2 × 106 per well) were added to the upper well and were allowed to migrate for 1.5 hours through PMVECs pretreated with buffer or with 2 μmol/L PKCδ inhibitor. Data are expressed as means ± SEM. n = 4 (B, siRNA experiments); n = 3–9 (B, PKCδ inhibitor experiments); n = 6–9 (C). ∗∗P < 0.01, ∗∗∗P < 0.001 versus respective control or controls (B). ∗∗P < 0.01 fMLP versus fMLP+PKCδ inhibitor–treated PMVECs; ∗∗∗P < 0.001 fMLP or IL-8 versus buffer (C).
To determine whether the role of PKCδ as a regulator of neutrophil transmigration across PMVEC monolayers is stimulus dependent, we used fMLP and IL-8, two diverse signaling molecules known to elicit different modes of neutrophil recruitment. In these experiments, the chemoattractants were added to the lower chamber of the Transwell culture system just before the addition of neutrophils to the upper well. Both fMLP and IL-8 triggered significant neutrophil transmigration through PMVECs, compared with buffer alone (Figure 5C). Pretreatment of the PMVEC monolayers with the PKCδ inhibitor significantly attenuated neutrophil migration in response to fMLP. By contrast, inhibition of PKCδ activity had no significant effect on IL-8–mediated neutrophil transmigration, indicating a PKCδ-independent mechanism. Thus, PKCδ activation is required for migration through IL-1β–activated endothelium or in response to the chemotactic peptide fMLP, but PKCδ activity is not required for IL-8–mediated migration.
Role of PKCδ in Neutrophil Binding and Adhesion Molecule Expression in PMVECs
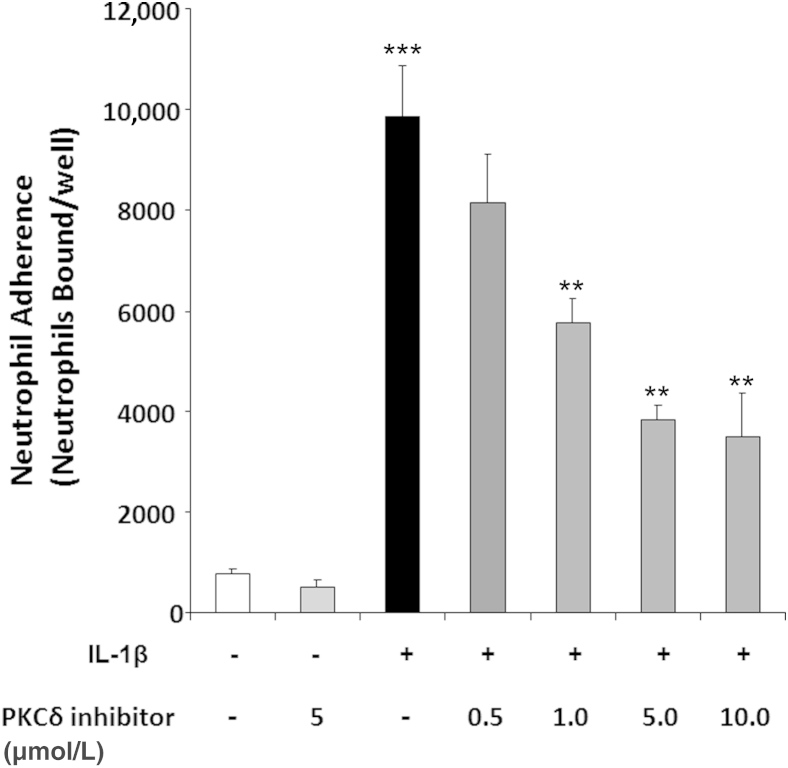
To further identify the regulatory role of PKCδ in neutrophil transmigration, we investigated whether PKCδ activity has a functional role in neutrophil–endothelial cell interaction and expression of adhesion molecules required for migration. The effect of endothelial PKCδ inhibition on IL-1β–mediated neutrophil adherence was determined using PMVEC monolayers. Treatment of PMVECs with IL-1β significantly enhanced calcein-labeled neutrophil adherence to PMVEC monolayers, compared with unstimulated PMVEC monolayers (Figure 6). Incubation with the PKCδ inhibitor decreased neutrophil adherence to IL-1β–stimulated PMVEC monolayers in a dose-dependent manner. In contrast, PKCδ inhibition had no effect on neutrophil adhesion to nonactivated PMVECs, suggesting that PKCδ involvement is limited to proinflammatory signaling pathways.
Figure 6.
Neutrophil binding to IL-1β–activated PMVECs is PKCδ dependent. PMVEC monolayers were treated overnight with buffer or 10 U/mL IL-1β ± 0.5 to 10 μmol/L PKCδ inhibitor. Calcein-loaded neutrophils (2 × 105) were incubated with PMVECs for 30 minutes at 37°C. Neutrophil binding was calculated from a standard curve prepared from calcein-loaded neutrophils. Data are expressed as means ± SEM from one representative experiment, performed in triplicate, from four different neutrophil donors. ∗∗P < 0.01 IL-1β versus IL-1β+PKCδ inhibitor (1 to 10 μmol/L); ∗∗∗P < 0.001 buffer versus IL-1β.
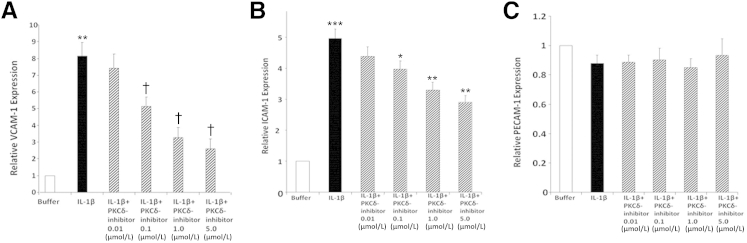
Endothelial adhesion molecules such as ICAM-1, VCAM-1, and PECAM-1 have been identified as important regulators of neutrophil adhesion and transmigration through pulmonary endothelium in response to IL-1β and fMLP, but not IL-8.2,43–45 We next investigated whether PKCδ has a role in regulating IL-1β–mediated PMVEC activation. PMVEC monolayers were activated with IL-1β in the absence or presence of the PKCδ inhibitor. There was little VCAM-1 or ICAM-1 expression on PMVEC monolayers under basal or resting conditions (Figure 7, A and B). IL-1β stimulated a significant increase in expression of both VCAM-1 and ICAM-1. IL-1β–mediated expression of VCAM-1 and ICAM-1 was significantly attenuated in a dose-dependent manner by incubation with the PKCδ inhibitor, indicating a regulatory role for PKCδ in cytokine-mediated activation of PMVECs. In contrast, PECAM-1 expression was independent of both IL-1β treatment and PKCδ inhibition (Figure 7C). Thus, PKCδ selectively regulates activation of PMVECs and IL-1β–induced cell-surface expression of ICAM-1 and VCAM-1 but not PECAM-1. Furthermore, the decreased expression of ICAM-1 and VCAM-1 after inhibition of PKCδ was associated with decreased adherence of neutrophils to activated endothelium.
Figure 7.
Role of PKCδ in PMVEC adhesion molecule expression. The expression of VCAM-1, ICAM-1, and PECAM-1 on PMVEC monolayers was determined by a cell-surface ELISA. Human PMVEC monolayers were treated overnight with buffer or IL-1β ± PKCδ inhibitor (0.01 to 5 mol/L). A: IL-1β–stimulated VCAM-1 expression. Constitutive expression of VCAM-1 (buffer) was normalized to 1, and expression in response to IL-1β treatment ± PKCδ inhibitor was compared with constitutive VCAM-1 expression. B: IL-1β–stimulated ICAM-1 expression, relative to constitutive ICAM-1 expression (as described for A). C: IL-1β–stimulated PECAM-1 expression, relative to constitutive PECAM-1 expression (as described for A). Data are expressed as means ± SEM. n = 7 (A); n = 5 (B); n = 6 (C). ∗∗P < 0.01, buffer versus IL-1β–treated PMVECs, †P < 0.01, IL-1β–treated PMVECs versus 10 U/mL IL-1β+PKCδ inhibitor (0.1–5 μmol/L) (A). ∗P < 0.05, IL-1β–treated PMVECs versus IL-1β+PKCδ inhibitor (0.1 μmol/L); ∗∗P < 0.01, IL-1β–treated PMVECs versus IL-1β+PKCδ inhibitor (1–5 μmol/L); and ∗∗∗P < 0.001, buffer versus IL-1β–treated PMVECs (B).
PKCδ Regulates IL-1β–Mediated Activation of NF-κB
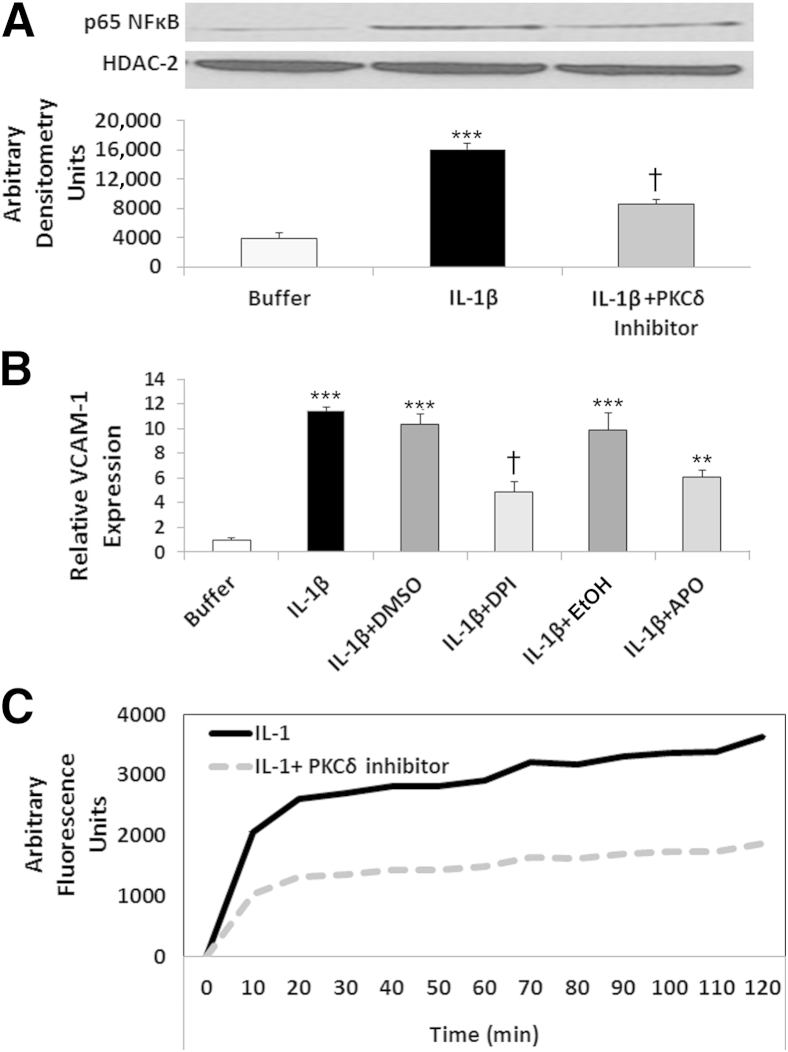
IL-1β–mediated VCAM-1 and ICAM-1 expression is dependent on activation of the transcription factor NF-κB,46 and we have previously demonstrated a role for PKCδ in NF-κB activation in TNF-treated human neutrophils.20,21 In addition, PKCδ is implicated as an important regulator of NF-κB in other types of endothelial or epithelial cells.26,30,31,47–50 To investigate the role of PKCδ in IL-1β–mediated activation of NF-κB in PMVECs, we determined the effect of PKCδ inhibition on p65 NF-κB translocation to the nucleus. In the absence of stimuli, there was little detectable p65 NF-κB present in the nuclear fraction of PMVECs (Figure 8A). The addition of IL-1β to PMVEC monolayers resulted in a fourfold increase in NF-κB translocation, which was significantly attenuated by PKCδ inhibition, indicating a role for PKCδ in regulating IL-1β–mediated activation of NF-κB in PMVECs.
Figure 8.
Role for PKCδ in IL-1β–mediated ROS production and activation of the redox-sensitive transcription factor NF-κB. A: IL-1β–mediated p65 NF-κB translocation is PKCδ dependent. PMVECs were treated with buffer or 10 U/mL IL-1β ± PKCδ inhibitor. Nuclear extracts, prepared after 15 minutes of incubation, were probed for the presence of p65 NF-κB by Western blotting. Representative blots are shown, with quantitation. HDAC-2 is a marker for nuclear fractions. B: Regulatory role for ROS in IL-1β–mediated VCAM-1 expression in PMVECs. VCAM-1 expression was measured by cell-surface ELISA. Constitutive expression of VCAM-1 (buffer) was normalized to 1, and expression in response to IL-1β treatment ± the ROS inhibitors DPI (10 μmol/L) or apocynin (APO; 500 μmol/L) or appropriate vehicle controls (DMSO and EtOH, respectively) was compared. C: PKCδ inhibition attenuates ROS production in IL-1β–treated PMVECs. CM-H2DCFDA–loaded PMVEC monolayers were treated with 10 U/mL IL-1β ± 2 μmol/L PKCδ inhibitor. Controls (Dulbecco’s modified Eagle’s medium) exhibited no change in oxidation of the probe, and H2O2 confirmed presence of the probe (data not shown). Graph in C is representative of four separate experiments run in triplicate (P < 0.03 IL-1β versus IL-1β+PKCδ inhibitor). Data are expressed as means ± SEM (A and B). n = 5 (A); n = 4 (B). ∗∗∗P < 0.001 IL-1β–treated PMVECs versus buffer-treated PMVECs; †P < 0.001 IL-1β–treated PMVECs versus IL-1β+PKCδ inhibitor–treated PMVECs (A). ∗∗∗P < 0.001 buffer versus IL-1β, IL-1β+DMSO, and IL-1β+EtOH; †P < 0.001 IL-1β+DPI versus IL-1β and IL-1β+DMSO; and ∗∗P < 0.001 IL-1β+APO versus IL-1β and IL-1β+EtOH (B).
A Regulatory Role for ROS in IL-1β–Mediated NF-κB Activation and Adhesion Molecule Expression
ROS produced by endothelial cells in response to cytokines are important regulators of the inflammatory response, and endothelial-derived redox signaling is implicated in regulating neutrophil transmigration.51,52 Of specific importance to our experimental system, IL-1β activates ROS production via NADPH oxidase (Nox2).53 To determine the role of ROS production in IL-1β–stimulated NF-κB activation and adhesion molecule expression, we pretreated PMVEC monolayers with the ROS inhibitors DPI and apocynin before adding IL-1β. ROS inhibition significantly decreased IL-1β–induced expression of VCAM-1, compared with IL-1β treatment alone or with the appropriate vehicle (Figure 8B). ROS inhibition also decreased IL-1β–mediated nuclear translocation of p65 NF-κB (P < 0.04, IL-1+vehicle versus IL-1+DPI) (n = 3), indicating a role for ROS production in NF-κB activation and adhesion molecule expression in response to IL-1β treatment of PMVECs.
PKCδ Inhibition Attenuates ROS Production in IL-1β–Treated PMVECs
ROS production requires assembly of the NOX complex, and different PKC isotypes (including PKCδ) have been implicated in the assembly and activation of these enzyme complexes.23,54,55 To investigate whether PKCδ regulates IL-1β–mediated NOX activation in PMVECs, we examined the effect of PKCδ inhibition on ROS production. In PMVECs, IL-1β elicited a rapid and sustained production of ROS in PMVEC monolayers (Figure 8C). PKCδ inhibition significantly attenuated PMVEC-derived ROS production, indicating that PKCδ is a signaling component upstream of NOX required for IL-1β–mediated ROS production in PMVECs.
Discussion
Our present results demonstrate that PKCδ is an important regulator of the acute inflammatory response in the lung, in part through the control of neutrophil infiltration and activation. IT administration of a PKCδ inhibitor protected the lung from sepsis-induced histopathological changes (Figure 2) and attenuated neutrophil migration, as evidenced by a significant decrease in the influx of MPO+ cells into the lung (Figure 3). Furthermore, PKCδ inhibition attenuated sepsis-induced expression of the endothelial adhesion molecules ICAM-1 and VCAM-1 in the lungs of CLP septic rats (Figures 3 and 4), suggesting a role for PKCδ in the regulation of neutrophil–endothelial cell interaction. Importantly for clinical translation, the present results demonstrate that IT delivery (across the lung epithelium) of the PKCδ inhibitor coupled to a protein transduction domain (TAT) facilitates distribution within the lung parenchyma (Figure 1), including pulmonary endothelial cells, thus validating the feasibility of modulating the pulmonary endothelium without the need for systemic infusion (which would likely result in more widespread distribution of the inhibitor).
The specific mechanisms involved in neutrophil recruitment to the lung during indirect pulmonary injury have yet to be clearly defined, but they likely involve unique organ-specific pathways.56 In contrast to most organs, in which neutrophil accumulation is mediated through postcapillary venules, in the lung neutrophil migration occurs principally across the capillary endothelium.2,37,45,57 Although the extensive pulmonary capillary system creates a physical trap for neutrophil sequestration, vascular inflammation is a critical aspect of indirect pulmonary injury that promotes neutrophil influx.9,12,58 Although the proinflammatory signaling role of PKCδ in endothelial cells and neutrophils has been described in a variety of experimental systems,59 the specific role of PKCδ in pulmonary endothelial cells in response to indirect pulmonary injury is not known.
In the lung, neutrophil recruitment involves both integrin-dependent and integrin-independent pathways.37,43,45,60,61 Although both pathways may function simultaneously, the relative importance of a specific pathway is stimulus-dependent.37,45 Integrin-dependent neutrophil recruitment to the lung involves neutrophil β2- and β1-integrins and their endothelial binding partners ICAM-1 and VCAM-1, respectively.7,32,62–64 Lung ICAM-1 and VCAM-1 are up-regulated in patients with ARDS and in animal models of lung injury.2,65,66 In sepsis and other diseases with systemic inflammation, VCAM-1–mediated neutrophil recruitment to the lung is increased through enhanced neutrophil expression of β1-integrins, such as α4β1.6,67 We observed a consistent increase in endothelium ICAM-1 and VCAM-1 expression in CLP septic rat lungs, and this critical component of the inflammatory response was attenuated on PKCδ inhibition throughout the arterial, venous, and capillary compartments of the pulmonary endothelium (Figures 3 and 4). Of crucial importance is the observation that PKCδ inhibition attenuates pulmonary expression of these adhesion molecules in the setting of sepsis, an event associated with decreased influx of MPO+ cells (Figure 3). This establishes a link between PKCδ and up-regulation of proinflammatory adhesion molecules in the injured lung, suggesting a potential mechanism for reduced neutrophil influx and lung protection.
To identify the critical site or sites of PKCδ regulation, we next examined human neutrophil migration across primary human PMVEC monolayers. Importantly, in our in vitro transmigration model only endothelial cell PKCδ was inhibited, thereby permitting delineation of the relative contribution of endothelial PKCδ in modulating neutrophil migration. A key observation in the present study is the finding that endothelial PKCδ involvement in neutrophil transmigration is selective and stimulus-dependent. Specifically, PKCδ is an important regulator of integrin-dependent neutrophil transmigration, a process involving interactions with ICAM-1 and VCAM-1. In vitro, neutrophil transmigration through IL-1β–activated endothelium or in response to fMLP is dependent on interactions with these adhesion molecules.37,43–45,57,68 In contrast, integrin-independent transmigration (specifically, IL-8–mediated migration) does not require interactions with ICAM-1, VCAM-1, or PECAM-143–45,60 and, as shown in the present study, it is also PKCδ independent.
In vivo, inhibition of PKCδ resulted in decreased expression of ICAM-1 and VCAM-1 in the lungs of CLP septic animals, and in vitro studies demonstrated a direct role for PKCδ in the regulation of VCAM-1 and ICAM-1 expression. Similarly, a regulatory role for PKCδ in the regulation of VCAM-1 and ICAM-1 expression has been reported in HUVECs and in epithelial cells,25,47,48,50 suggesting that PKCδ regulates common proinflammatory signaling pathways in these different cell types. In contrast to its role in VCAM-1 and ICAM-1 expression, PKCδ is not a regulator of PECAM-1 expression in PMVECs. Thus, PKCδ is not a global regulator of adhesion molecule expression in the endothelium; rather, this kinase regulates specific proinflammatory signaling pathways. Taken together, these in vitro findings support the hypothesis that decreased expression of proinflammatory adhesion molecules ICAM-1 and VCAM-1 in the pulmonary endothelium, and the consequently reduced neutrophil influx into the lung, is a potential mechanism of lung protection on PKCδ inhibition in the setting of sepsis in vivo. Although our in vitro studies identified a critical role for PKCδ in regulating adhesion molecules, these studies did not rule out other possible lung-protective effects of PKCδ inhibition in vivo (such as direct effects on neutrophils, alveolar macrophages, or epithelial and stromal cells) that may also affect neutrophil migration into the lung during sepsis.59
In endothelial cells, ROS are implicated as important regulators of NF-κB and the expression of VCAM-1 and ICAM-1.53,69–71 IL-1β activation of NF-κB in the endothelium is mediated via the IL-1R1 receptor and is dependent on receptor endocytosis and endosomal ROS production.53,69,71 Our present findings indicate that, in PMVECs, PKCδ is a key regulator of ROS production in response to IL-1β. Furthermore, we have demonstrated that ROS production in PMVECs is required for both NF-κB activation and adhesion molecule expression. Although we cannot rule out effects on other transcription factors, such as AP-1 or PKCδ-dependent post-translational modifications,50 our findings indicate that PKCδ has an important role in the up-regulation of ICAM-1 and VCAM-1 in response to proinflammatory cytokines in a defined, human cell-based in vitro system. Furthermore, pathophysiological improvements in CLP septic rat lungs corresponded to decreased ICAM-1 and VCAM-1 levels on PKCδ inhibition in vivo, suggesting an evolutionarily conserved and therefore potentially clinically relevant mechanism.
In summary, our findings implicate PKCδ as a key regulator of pulmonary endothelial adhesion molecule expression and the influx of neutrophils in response to systemic sepsis and indirect pulmonary injury. Of clinical import, the up-regulation of adhesion molecules in response to inflammatory activation can be attenuated by inhibiting PKCδ in both rodents and primary human cells, an approach that resulted in significant lung protection in vivo in the setting of sepsis. It is therefore tempting to speculate that selective delivery of PKCδ inhibitors to the lung could modulate aberrant neutrophil–endothelial cell interactions during acute inflammation, but without producing global immunosuppression (given that we observed selective and stimulus-specific roles of PKCδ during inflammatory signaling). In conclusion, the present findings suggest that targeting pulmonary PKCδ activity may offer a unique therapeutic strategy to regulate neutrophil migration and activation in sepsis-induced pulmonary dysfunction and ARDS.24,72
Footnotes
Supported in part by NIH grants RO1 HL111552 (L.E.K.), R01 DK064344 (R.S.), 5T32-HL007777 (M.J.M.) and DoD/ONR N00014-12-1-0597 (M.R.W.).
M.J.M and T.Z. contributed equally to this work.
Disclosure: L.E.K. is listed as an inventor on U.S. patent 8470766 entitled “Protein kinase C therapy for the treatment of acute lung injury” and assigned to Children’s Hospital of Philadelphia and the Trustees of the University of Pennsylvania.
Current address of T.Z., Shanghai Children’s Hospital, Shanghai, China; of D.J.K., University of Maryland School of Medicine, Baltimore, MD.
References
- 1.Angus D.C., Linde-Zwirble W.T., Lidicker J., Clermont G., Carcillo J., Pinsky M.R. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Reutershan J., Ley K. Bench-to-bedside review: acute respiratory distress syndrome–how neutrophils migrate into the lung. Crit Care. 2004;8:453–461. doi: 10.1186/cc2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 4.Matthay M.A., Ware L.B., Zimmerman G.A. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31(4 Suppl):S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 6.Guo R.F., Riedemann N.C., Laudes I.J., Sarma V.J., Kunkel R.G., Dilley K.A., Paulauskis J.D., Ward P.A. Altered neutrophil trafficking during sepsis. J Immunol. 2002;169:307–314. doi: 10.4049/jimmunol.169.1.307. [DOI] [PubMed] [Google Scholar]
- 7.Lee W.L., Downey G.P. Neutrophil activation and acute lung injury. Curr Opin Crit Care. 2001;7:1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg K.P., Milberg J.A., Martin T.R., Maunder R.J., Cockrill B.A., Hudson L.D. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;150:113–122. doi: 10.1164/ajrccm.150.1.8025736. [DOI] [PubMed] [Google Scholar]
- 9.Pelosi P., D’Onofrio D., Chiumello D., Paolo S., Chiara G., Capelozzi V.L., Barbas C.S.V., Chiaranda M., Gattinoni L. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl. 2003;22:48s–56s. doi: 10.1183/09031936.03.00420803. [DOI] [PubMed] [Google Scholar]
- 10.Sevransky J.E., Martin G.S., Mendez-Tellez P., Shanholtz C., Brower R., Pronovost P.J., Needham D.M. Pulmonary vs nonpulmonary sepsis and mortality in acute lung injury. Chest. 2008;134:534–538. doi: 10.1378/chest.08-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheu C.C., Gong M.N., Zhai R., Bajwa E.K., Chen F., Thompson B.T., Christiani D.C. The influence of infection sites on development and mortality of ARDS. Intensive Care Med. 2010;36:963–970. doi: 10.1007/s00134-010-1851-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perl M., Lomas-Neira J., Venet F., Chung C.S., Ayala A. Pathogenesis of indirect (secondary) acute lung injury. Expert Rev Respir Med. 2011;5:115–126. doi: 10.1586/ers.10.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler A.P., Bernard G.R. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg K.P., Hudson L.D., Goodman R.B., Hough C.L., Lanken P.N., Hyzy R., Thompson B.T., Ancukiewicz M., National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 15.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 16.Gaestel M., Kotlyarov A., Kracht M. Targeting innate immunity protein kinase signalling in inflammation. Nat Rev Drug Discov. 2009;8:480–499. doi: 10.1038/nrd2829. [DOI] [PubMed] [Google Scholar]
- 17.Cohen P. Targeting protein kinases for the development of anti-inflammatory drugs. Curr Opin Cell Biol. 2009;21:317–324. doi: 10.1016/j.ceb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Müller S., Knapp S. Targeting kinases for the treatment of inflammatory diseases. Expert Opin Drug Discov. 2010;5:867–881. doi: 10.1517/17460441.2010.504203. [DOI] [PubMed] [Google Scholar]
- 19.Kilpatrick L.E., Song Y.H., Rossi M.W., Korchak H.M. Serine phosphorylation of p60 tumor necrosis factor receptor by PKC-delta in TNF-alpha-activated neutrophils. Am J Physiol Cell Physiol. 2000;279:C2011–C2018. doi: 10.1152/ajpcell.2000.279.6.C2011. [DOI] [PubMed] [Google Scholar]
- 20.Kilpatrick L.E., Lee J.Y., Haines K.M., Campbell D.E., Sullivan K.E., Korchak H.M. A role for PKC-delta and PI 3-kinase in TNF-alpha-mediated antiapoptotic signaling in the human neutrophil. Am J Physiol Cell Physiol. 2002;283:C48–C57. doi: 10.1152/ajpcell.00385.2001. [DOI] [PubMed] [Google Scholar]
- 21.Kilpatrick L.E., Sun S., Korchak H.M. Selective regulation by delta-PKC and PI 3-kinase in the assembly of the antiapoptotic TNFR-1 signaling complex in neutrophils. Am J Physiol Cell Physiol. 2004;287:C633–C642. doi: 10.1152/ajpcell.00486.2003. [DOI] [PubMed] [Google Scholar]
- 22.Kilpatrick L.E., Sun S., Mackie D., Baik F., Li H., Korchak H.M. Regulation of TNF mediated antiapoptotic signaling in human neutrophils: role of delta-PKC and ERK1/2 [Erratum appeared in J Leukoc Biol 2008, 83:797] J Leukoc Biol. 2006;80:1512–1521. doi: 10.1189/jlb.0406284. [DOI] [PubMed] [Google Scholar]
- 23.Kilpatrick L.E., Sun S., Li H., Vary T.C., Korchak H.M. Regulation of TNF-induced oxygen radical production in human neutrophils: role of delta-PKC. J Leukoc Biol. 2010;87:153–164. doi: 10.1189/jlb.0408230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilpatrick L.E., Standage S.W., Li H., Raj N.R., Korchak H.M., Wolfson M.R., Deutschman C.S. Protection against sepsis-induced lung injury by selective inhibition of protein kinase C-δ (δ-PKC) J Leukoc Biol. 2011;89:3–10. doi: 10.1189/jlb.0510281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page K., Li J., Zhou L., Iasvovskaia S., Corbit K.C., Soh J.W., Weinstein I.B., Brasier A.R., Lin A., Hershenson M.B. Regulation of airway epithelial cell NF-kappa B-dependent gene expression by protein kinase C delta. J Immunol. 2003;170:5681–5689. doi: 10.4049/jimmunol.170.11.5681. [DOI] [PubMed] [Google Scholar]
- 26.Chou W.H., Choi D.S., Zhang H., Mu D., McMahon T., Kharazia V.N., Lowell C.A., Ferriero D.M., Messing R.O. Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. J Clin Invest. 2004;114:49–56. doi: 10.1172/JCI21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chichger H., Grinnell K.L., Casserly B., Chung C.S., Braza J., Lomas-Neira J., Ayala A., Rounds S., Klinger J.R., Harrington E.O. Genetic disruption of protein kinase Cdelta reduces endotoxin-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;303:L880–L888. doi: 10.1152/ajplung.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramnath R., Sun J., Bhatia M. PKC δ mediates pro-inflammatory responses in a mouse model of caerulein-induced acute pancreatitis. J Mol Med (Berl) 2010;88:1055–1063. doi: 10.1007/s00109-010-0647-9. [DOI] [PubMed] [Google Scholar]
- 29.Shukla A., Lounsbury K.M., Barrett T.F., Gell J., Rincon M., Butnor K.J., Taatjes D.J., Davis G.S., Vacek P., Nakayama K.I., Nakayama K., Steele C., Mossman B.T. Asbestos-induced peribronchiolar cell proliferation and cytokine production are attenuated in lungs of protein kinase C-delta knockout mice. Am J Pathol. 2007;170:140–151. doi: 10.2353/ajpath.2007.060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cummings R., Zhao Y., Jacoby D., Spannhake E.W., Ohba M., Garcia J.G., Watkins T., He D., Saatian B., Natarajan V. Protein kinase Cdelta mediates lysophosphatidic acid-induced NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells. J Biol Chem. 2004;279:41085–41094. doi: 10.1074/jbc.M404045200. [DOI] [PubMed] [Google Scholar]
- 31.Rahman A., Anwar K.N., Uddin S., Xu N., Ye R.D., Platanias L.C., Malik A.B. Protein kinase C-delta regulates thrombin-induced ICAM-1 gene expression in endothelial cells via activation of p38 mitogen-activated protein kinase. Mol Cell Biol. 2001;21:5554–5565. doi: 10.1128/MCB.21.16.5554-5565.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maniatis N.A., Orfanos S.E. The endothelium in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care. 2008;14:22–30. doi: 10.1097/MCC.0b013e3282f269b9. [DOI] [PubMed] [Google Scholar]
- 33.Chen L., Hahn H., Wu G., Chen C.H., Liron T., Schechtman D., Cavallaro G., Banci L., Guo Y., Bolli R., Dorn G.W., 2nd, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci USA. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Begley R., Liron T., Baryza J., Mochly-Rosen D. Biodistribution of intracellularly acting peptides conjugated reversibly to Tat. Biochem Biophys Res Commun. 2004;318:949–954. doi: 10.1016/j.bbrc.2004.04.121. [DOI] [PubMed] [Google Scholar]
- 35.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 36.O’Brien C.D., Lim P., Sun J., Albelda S.M. PECAM-1-dependent neutrophil transmigration is independent of monolayer PECAM-1 signaling or localization. Blood. 2003;101:2816–2825. doi: 10.1182/blood-2002-08-2396. [DOI] [PubMed] [Google Scholar]
- 37.Moreland J.G., Bailey G., Nauseef W.M., Weiss J.P. Organism-specific neutrophil-endothelial cell interactions in response to Escherichia coli, Streptococcus pneumoniae, and Staphylococcus aureus. J Immunol. 2004;172:426–432. doi: 10.4049/jimmunol.172.1.426. [DOI] [PubMed] [Google Scholar]
- 38.Qin Y., Zhang Q., Chen H., Yuan W., Kuai R., Xie F., Zhang L., Wang X., Zhang Z., Liu J., He Q. Comparison of four different peptides to enhance accumulation of liposomes into the brain. J Drug Target. 2012;20:235–245. doi: 10.3109/1061186X.2011.639022. [DOI] [PubMed] [Google Scholar]
- 39.Orfanos S.E., Mavrommati I., Korovesi I., Roussos C. Pulmonary endothelium in acute lung injury: from basic science to the critically ill. Intensive Care Med. 2004;30:1702–1714. doi: 10.1007/s00134-004-2370-x. [DOI] [PubMed] [Google Scholar]
- 40.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 41.Aldridge A.J. Role of the neutrophil in septic shock and the adult respiratory distress syndrome. Eur J Surg. 2002;168:204–214. doi: 10.1080/11024150260102807. [DOI] [PubMed] [Google Scholar]
- 42.Asaduzzaman M., Zhang S., Lavasani S., Wang Y., Thorlacius H. LFA-1 and MAC-1 mediate pulmonary recruitment of neutrophils and tissue damage in abdominal sepsis. Shock. 2008;30:254–259. doi: 10.1097/shk.0b013e318162c567. [DOI] [PubMed] [Google Scholar]
- 43.Mackarel A.J., Russell K.J., Brady C.S., FitzGerald M.X., O’Connor C.M. Interleukin-8 and leukotriene-B4, but not formylmethionyl leucylphenylalanine, stimulate CD18-independent migration of neutrophils across human pulmonary endothelial cells in vitro. Am J Respir Cell Mol Biol. 2000;23:154–161. doi: 10.1165/ajrcmb.23.2.3853. [DOI] [PubMed] [Google Scholar]
- 44.Mackarel A.J., Russell K.J., Ryan C.M., Hislip S.J., Rendall J.C., FitzGerald M.X., O’Connor C.M. CD18 dependency of transendothelial neutrophil migration differs during acute pulmonary inflammation. J Immunol. 2001;167:2839–2846. doi: 10.4049/jimmunol.167.5.2839. [DOI] [PubMed] [Google Scholar]
- 45.Doerschuk C.M. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation. 2001;8:71. [PubMed] [Google Scholar]
- 46.De Martin R., Hoeth M., Hofer-Warbinek R., Schmid J.A. The transcription factor NF-kappa B and the regulation of vascular cell function. Arterioscler Thromb Vasc Biol. 2000;20:E83–E88. doi: 10.1161/01.atv.20.11.e83. [DOI] [PubMed] [Google Scholar]
- 47.Minami T., Abid M.R., Zhang J., King G., Kodama T., Aird W.C. Thrombin stimulation of vascular adhesion molecule-1 in endothelial cells is mediated by protein kinase C (PKC)-delta-NF-kappa B and PKC-zeta-GATA signaling pathways. J Biol Chem. 2003;278:6976–6984. doi: 10.1074/jbc.M208974200. [DOI] [PubMed] [Google Scholar]
- 48.Bijli K.M., Fazal F., Minhajuddin M., Rahman A. Activation of Syk by protein kinase C-delta regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via tyrosine phosphorylation of RelA/p65. J Biol Chem. 2008;283:14674–14684. doi: 10.1074/jbc.M802094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minhajuddin M., Bijli K.M., Fazal F., Sassano A., Nakayama K.I., Hay N., Platanias L.C., Rahman A. Protein kinase C-delta and phosphatidylinositol 3-kinase/Akt activate mammalian target of rapamycin to modulate NF-kappaB activation and intercellular adhesion molecule-1 (ICAM-1) expression in endothelial cells. J Biol Chem. 2009;284:4052–4061. doi: 10.1074/jbc.M805032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woo C.H., Lim J.H., Kim J.H. VCAM-1 upregulation via PKCdelta-p38 kinase-linked cascade mediates the TNF-alpha-induced leukocyte adhesion and emigration in the lung airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2005;288:L307–L316. doi: 10.1152/ajplung.00105.2004. [DOI] [PubMed] [Google Scholar]
- 51.Aghajanian A., Wittchen E.S., Allingham M.J., Garrett T.A., Burridge K. Endothelial cell junctions and the regulation of vascular permeability and leukocyte transmigration. J Thromb Haemost. 2008;6:1453–1460. doi: 10.1111/j.1538-7836.2008.03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lum H., Roebuck K.A. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 53.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fontayne A., Dang P.M., Gougerot-Pocidalo M.A., El-Benna J. Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- 55.Cai W., Torreggiani M., Zhu L., Chen X., He J.C., Striker G.E., Vlassara H. AGER1 regulates endothelial cell NADPH oxidase-dependent oxidant stress via PKC-d: implications for vascular disease. Am J Physiol Cell Physiol. 2010;298:C624–C634. doi: 10.1152/ajpcell.00463.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rossaint J., Zarbock A. Tissue-specific neutrophil recruitment into the lung, liver, and kidney. J Innate Immun. 2013;5:348–357. doi: 10.1159/000345943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burns A.R., Smith C.W., Walker D.C. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 58.Hu R., Xu H., Jiang H., Zhang Y., Sun Y. The role of TLR4 in the pathogenesis of indirect acute lung injury. Front Biosc (Landmark Ed) 2013;18:1244–1255. doi: 10.2741/4176. [DOI] [PubMed] [Google Scholar]
- 59.Mondrinos M.J., Kennedy P.A., Lyons M., Deutschman C.S., Kilpatrick L.E. Protein kinase C and acute respiratory distress syndrome. Shock. 2013;39:467–479. doi: 10.1097/SHK.0b013e318294f85a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doerschuk C.M., Tasaka S., Wang Q. CD11/CD18-dependent and -independent neutrophil emigration in the lungs: how do neutrophils know which route to take? Am J Respir Cell Mol Biol. 2000;23:133–136. doi: 10.1165/ajrcmb.23.2.f193. [DOI] [PubMed] [Google Scholar]
- 61.Yan S.R., Sapru K., Issekutz A.C. The CD11/CD18 (beta2) integrins modulate neutrophil caspase activation and survival following TNF-alpha or endotoxin induced transendothelial migration. Immunol Cell Biol. 2004;82:435–446. doi: 10.1111/j.0818-9641.2004.01268.x. [DOI] [PubMed] [Google Scholar]
- 62.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 63.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 64.Kadioglu A., De Filippo K., Bangert M., Fernandes V.E., Richards L., Jones K., Andrew P.W., Hogg N. The integrins Mac-1 and alpha4beta1 perform crucial roles in neutrophil and T cell recruitment to lungs during Streptococcus pneumoniae infection. J Immunol. 2011;186:5907–5915. doi: 10.4049/jimmunol.1001533. [DOI] [PubMed] [Google Scholar]
- 65.Müller A.M., Cronen C., Müller K.M., Kirkpatrick C.J. Heterogeneous expression of cell adhesion molecules by endothelial cells in ARDS. J Pathol. 2002;198:270–275. doi: 10.1002/path.1186. [DOI] [PubMed] [Google Scholar]
- 66.Guo R.F., Ward P.A. Mediators and regulation of neutrophil accumulation in inflammatory responses in lung: insights from the IgG immune complex model. Free Radic Biol Med. 2002;33:303–310. doi: 10.1016/s0891-5849(02)00823-7. [DOI] [PubMed] [Google Scholar]
- 67.Ibbotson G.C., Doig C., Kaur J., Gill V., Ostrovsky L., Fairhead T., Kubes P. Functional alpha4-integrin: a newly identified pathway of neutrophil recruitment in critically ill septic patients. Nat Med. 2001;7:465–470. doi: 10.1038/86539. [DOI] [PubMed] [Google Scholar]
- 68.Reutershan J., Basit A., Galkina E.V., Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;289:L807–L815. doi: 10.1152/ajplung.00477.2004. [DOI] [PubMed] [Google Scholar]
- 69.Gloire G., Legrand-Poels S., Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 70.Li Q., Harraz M.M., Zhou W., Zhang L.N., Ding W., Zhang Y., Eggleston T., Yeaman C., Banfi B., Engelhardt J.F. Nox2 and Rac1 Regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rahman A., Fazal F. Hug tightly and say goodbye: role of endothelial ICAM-1 in leukocyte transmigration. Antioxid Redox Signal. 2009;11:823–839. doi: 10.1089/ars.2008.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martins J.O. Can PKCδ be a novel therapeutic target? J Leukoc Biol. 2011;89:1–2. doi: 10.1189/jlb.0810452. [DOI] [PubMed] [Google Scholar]