Abstract
Cells protect themselves from zinc toxicity by inducing proteins such as metallothionein (MT) that bind it tightly, by sequestering it in organelles, or by exporting it. In this study, the interplay between zinc binding by MT and its efflux by zinc transporter 1 (ZnT1) was examined genetically. Inactivation of the Znt1 gene in baby hamster kidney (BHK) cells that do not express their Mt genes results in a zinc-sensitive phenotype and a high level of “free” zinc. Restoration of Mt gene expression increases resistance to zinc toxicity ≈4-fold, but only slightly reduces free zinc levels. Expression of ZnT1 provides greater protection (≈7-fold) and lowers free zinc substantially. Selection for zinc resistance in BHK cells that cannot synthesize either MT or ZnT1 is ineffective. However, parental BHK cells that grow in high concentrations (>500 μM) of zinc can be selected; these cells have amplified their endogenous Znt1 genes. The Znt1 gene is also amplified in zinc-resistant mouse cells that cannot induce their Mt genes. However, if Mt genes can be expressed, then they are preferentially amplified. Thus, both ZnT1 and MT genes contribute to zinc resistance in BHK cells, whereas ZnT1 plays a larger role in regulating free zinc levels.
Zinc is an essential nutrient that can also be toxic (1). Zinc serves as a critical structural component of thousands of zinc-finger proteins with diverse functions (2). The total zinc content of a typical fibroblast-like cell grown in ordinary tissue culture medium is ≈0.25 fmol per cell or ≈200 μM (3). Cells exposed to zinc-deficient medium stop growing when total cellular zinc levels fall to ≈0.2 fmol per cell (3). At that point, all nonessential zinc has been used and critical zinc-containing proteins can no longer be synthesized. On the flip side, exposure of cells to high concentrations of zinc activates several protective mechanisms that include down-regulation of zinc uptake transporters, induction of zinc efflux transporters, sequestration of zinc in intracellular compartments, and induction of metal-binding proteins (4-6). Induction of metallothioneins (MT), which bind several heavy metals with high affinity, is a common protective mechanism (5). Most cells can induce MT synthesis in response to heavy metal challenge. This induction depends on the metal-regulatory transcription factor (MTF-1), but the mechanism by which it senses elevated metals to activate transcription of Mt genes is not established (7-9). However, some cell lines fail to induce Mt transcription in response to metals, either because their Mt genes are silenced or because they lack expression of MTF-1. Such cell lines are useful for exploring mechanisms of zinc resistance other than sequestration by MT. Mammalian cells that cannot synthesize MT can accumulate zinc up to ≈0.6 fmol per cell before it becomes toxic, but if MTs can be induced, then cells can accumulate considerably more zinc. The mechanisms of zinc toxicity are not well defined, but inhibition of key metabolic enzymes, stimulation of apoptosis, and interference with protein folding has been suggested (10-12).
Repeated mutagenesis of a baby hamster kidney (BHK) cell line that does not express its endogenous Mt genes but carries a zinc-responsive reporter gene (MRE-βGeo) that is inducible by MTF-1 produced a zinc-sensitive cell line that has high constitutive expression of the reporter gene (13). Transfection of these cells with a cDNA expression library resulted in clones with restored zinc-resistance and reduced expression of the reporter gene. Cloning, sequencing, and biochemical studies revealed that one of the cDNAs that restored zinc resistance encoded a membrane protein, zinc transporter 1 (ZnT1), that facilitates zinc efflux from cells (3). Deliberate overexpression of Znt1 in BHK cells lowers total cellular zinc levels, reduces expression of MRE-βGeo, and confers resistance to zinc toxicity (3). These are all attributes one would predict for a transporter that exports zinc out of the cell. Subsequently, it has become clear that ZnT1 is the first member of group of related molecules (the SLC30 group) that transport zinc out of the cell or into various intracellular compartments (14). Although several indirect lines of evidence suggested that the defect in the zinc-sensitive BHK cell line reflected loss of Znt1 gene function (3), the molecular basis of the defect was never established. The experiments described here provide further genetic evidence that Znt1 gene (also known as Slc30a1) expression is important for zinc homeostasis in BHK cells.
Materials and Methods
Cell Culture and Selection Techniques. Derivation of the zinc-sensitive BHK cells (line 3286, hereafter referred to as ZnS) and the zinc-resistant parental cells (line 3038, hereafter referred to as ZnR) that carry the MRE-βGeo reporter gene has been described (13). These BHK cells and the Hepa1A cells (15) and DKO7 cells lacking Mtf1 gene expression (16) were maintained at 37°C in DMEM supplemented with 10% FBS, penicillin, and streptomycin. This medium contains ≈4 μM Zn. BHK cells resistant to an even higher concentration of zinc were derived by gradually increasing the concentration of zinc sulfate. At each passage, some cells were transferred to medium containing ≈20% more zinc than the medium in which they had been growing. When they grew well at higher concentrations of zinc, the process was repeated.
DNA Sequencing. DNA from the ZnS and ZnR BHK cell lines (13) was harvested by standard techniques and subjected to PCR amplification by using primers based on the known mouse and rat Znt1 gene sequences (3). PCR products were sequenced directly to identify differences between the Znt1 gene in the two cell lines. To ascertain whether mutations were on the same allele, 55 μg of genomic DNA from ZnS cells was digested with HindIII plus NotI (which flanks the entire gene) and electrophoresed on a 0.7% agarose gel. DNA of ≈7.5 kb was purified, ligated with Bluescript plasmid vector (Stratagene) cut with the same enzymes, and then electroporated into DH10B bacteria. Screening with a rat Znt1 probe identified positive colonies; plasmid DNA was isolated and sequenced with primers near the mutations that were identified by PCR.
Transfection and Activation of Mt Genes. Two different methods were used to establish Mt gene expression in BHK cells. One method involved activation of endogenous Mt genes by treating cells with 5-azacyctine (Sigma) and then selection for cells that could grow in 3 μM Cd. Treatment with either 5 μM 5-azacytidine for 24 h or 1.5 μM 5-azacytidine for 48 h was effective in generating about three colonies per 106 treated cells; lower and higher concentrations were ineffective. Alternatively, cells were transfected with a mouse cosmid (3121B-26) carrying both Mt1 and Mt2 genes. Cosmid DNA (110 ng) was transfected along with 10 μg of carrier mouse DNA by the calcium-phosphate method, and cells resistant to 3 μM Cd were selected. Colonies were picked and expanded, and the DNA was analyzed by quantitative Southern blots with a mouse Mt1 gene probe and mouse DNA as a standard to identify clones with one or two copies.
DNA Analysis. To measure cell number, cells were washed in PBS, suspended in 0.2% SDS, sonicated, and the DNA content was determined by fluorescence of bisbenzimide H33258 (Riedelde Haën, Seelze-Hannover, Germany). For Southern blots, DNA was harvested from cultured cells by proteinase K digestion with 1% SDS, followed by phenol-chloroform extraction and ethanol precipitation. DNA concentration was determined by H33258 dye binding, and equal amounts of DNA (typically ≈6 μg) were digested with restriction enzymes indicated in the legends and then subjected to Southern blot procedure. The nylon membranes (Zeta-probe, Bio-Rad) were hybridized with either rat Znt1, rat Znt2, or mouse Mt1 gene probes. Most blots were hybridized with a probe from another gene to check for equal loading and transfer of the DNA. DNA dilutions from amplified samples were included on the gel to provide an estimate of gene copy number.
Biochemical Assays. β-galactosidase activity of BHK cells was measured by using o-nitrophenyl-β-galactopyranoside (Sigma) as substrate (13). To measure zinc efflux, cells were grown in 6-well dishes in zinc-free medium (Chelex-treated serum; see ref. 3) supplemented with 4 μM 65Zn (≈2,000 cpm/nmol) for 2 days. After washing the cells and adding fresh medium, cells and medium were collected from individual wells at various times, and the radioactivity was determined by using a γ-counter (3).
Metallothionein Protein Assay. Cells were treated with the optimal concentration of zinc (30 μM for ZnS cells, 75 μM for ZnS subclones, or 125 μM for ZnR cells and subclones) for 16 h. Then the cells were harvested, washed once with PBS, resuspended in 1 ml of 10 mM Tris·Cl, pH 7.5, and sonicated briefly. An aliquot was removed to measure DNA, and the remainder was boiled for 2 min and centrifuged. An aliquot of the supernatant (0.2 ml, equivalent ≈3 × 106 cells) was mixed with 4,000 pmol of 109Cd (≈800 cpm/pmol; Perkin-Elmer, Boston), incubated 15 min at 22°C to allow cadmium exchange for zinc in MT; then cadmium that was not bound to MT was removed by two rounds of hemoglobin extraction (17), and the radioactivity in the supernatant was measured with a scintillation counter. The background with no protein extract (≈1 pmol) was subtracted.
Results
Zinc-Sensitive BHK Cells Have Frame-Shift Mutations in Znt1. A zinc-sensitive BHK cell line (3286, ZnS) was identified after multiple rounds of mutagenesis with the frame-shift mutagen ICR-191 (13). Transfection of these cells with a rat kidney cDNA library and selection for zinc resistance led to the discovery of the Znt1 gene; however, expression of other genes (Mt1, Mt2, Znt2 or Znt4) in these cells can also confer zinc resistance (refs. 3 and 18 and unpublished observations). Because indirect lines of evidence suggested that Znt1 might be responsible, PCR primers were designed to amplify and sequence regions of the Znt1 gene from the ZnS cells and the parental, zinc-resistant (3038, ZnR) BHK cells. The Znt1 gene in ZnR cells encodes a protein with 30 amino acid differences plus a 6-aa insertion (at position 153) relative to the 503-aa mouse protein (3). However, the Znt1 gene from ZnS cells has two frame-shift mutations: a G insertion after codon 115 and a C insertion after codon 291, both of which did not exist in the parental cells. Subcloning and sequencing the Znt1 gene from these cells revealed that the mutations were on separate alleles, thus precluding expression of functional ZnT1 protein in the ZnS cells.
Zinc Resistance of BHK Cells Lacking Expression of ZnT1, MT, or Both. The BHK cells used in these studies do not express their endogenous Mt genes, presumably because of gene silencing by DNA methylation as observed in several mouse cell lines (19, 20). To derive cell lines that express Mt genes with or without expression of Znt1, the ZnS and ZnR BHK cells were treated with 5-azacytidine and then selected in 3 μM Cd, which kills cells that cannot synthesize MT (19, 20). Alternatively, these cells were transfected with a cosmid carrying the mouse Mt1 and Mt2 genes, and stable clones resistant to 3 μM Cd were selected; clones with one to two copies of the cosmid were chosen for analysis. Several clones of each type were characterized. Activation of the endogenous Mt genes or expression of the transfected Mt genes was documented by measuring MT protein with a 109Cd-binding assay (Table 1). The relative contributions of Mt and Znt1 expression toward protection against zinc toxicity was determined by measuring the number of cells (DNA content) after 4 days of exposure to various concentrations of zinc; results are expressed as the ED50.ZnS cells that express neither Mt nor Znt1 had an ED50 of 23 μM Zn; expression of just Mt genes increased ED50 to 96 μM (average of five clones; see Table 1), expression of just Znt1 (ZnR cells) increased it to 161 μM, and expression of both Znt1 and Mt genes increased ED50 to 184 μM (average of seven clones; Table 1). Thus, either Znt1 or Mt genes can confer resistance to zinc in the absence of the other gene, but Mt expression provides little extra protection in the presence of Znt1.
Table 1. Effect of MT and ZnT1 expression on zinc toxicity.
| Cell line* | Zinc,* μM | Zinc bound to MT,† fmol per cell | Zinc toxicity‡ (ED50), μM |
|---|---|---|---|
| ZnS (3286) | 30 | None | 23 |
| ZnS-Aza c1 | 75 | 0.33 | 86 |
| ZnS-Aza c2 | 75 | 0.30 | 80 |
| ZnS-Aza c3 | 75 | 0.27 | 108 |
| ZnS-3121 c2 | 75 | 0.52 | 98 |
| ZnS-3121 c3 | 75 | 0.92 | 134 |
| ZnR (3038) | 125 | None | 161 |
| ZnR-Aza c4 | 125 | 0.27 | 215 |
| ZnR-Aza c5 | 125 | 0.13 | 190 |
| ZnR-Aza c6 | 125 | 0.16 | 192 |
| ZnR-3121 c7 | 125 | 0.08 | 158 |
| ZnR-3121 c10 | 125 | 0.71 | 204 |
| ZnR-3121 c11 | 125 | 0.27 | 160 |
| ZnR-3121 c12 | 125 | 0.29 | 172 |
Cell clones with Mt gene expression were isolated after treatment with azacytidine (Aza) or transfection with the cosmid (3121). Cells were grown in the presence of the indicated concentration of zinc for 16-18 h to measure MT.
MT protein was assayed with a cadmium-binding assay and expressed as MT-bound zinc. For comparison, when grown in 4 μM Zn, ZnS and ZnR cells have ≈0.30 and 0.25 fmol Zn per cell, respectively. Both cells have ≈0.6 fmol Zn per cell when grown in the 75 or 125 μM Zn, respectively. Thus, most of these clones that express MT increase total zinc content by 50-100%.
Zinc toxicity was measured as shown in Fig. 2.
Roles of ZnT1 and MT in Regulation of MTF-1. The effect of Znt1 and Mt gene expression on the zinc pool that regulates the transcription factor MTF-1 was determined by taking advantage of a stably integrated MRE-βGeo reporter gene, which has a basal promoter with five copies of a metal response element recognized by MTF-1 driving the expression of a β-galactosidase-neomycin phosphotransferase fusion protein (13). The β-galactosidase activity is an indirect measure of “free” zinc, the amount of zinc available to activate MTF-1. ZnS cells have a high basal expression of β-galactosidase and they are maximally induced by ≈25 μM Zn (Fig. 1, curve a). Mt gene expression in the absence of Znt1 lowered the basal expression and shifted the maximal induction to higher zinc concentrations (Fig. 1, curve b). The average expression of β-galactosidase in the absence of added zinc for the five clones that express Mt (Table 1) was 47% of that observed with optimal zinc, compared with 68% for the ZnS cells. The amount of MT protein (based on 109Cd binding) in clone ZnS-3121-c2 (see Table 1) doubled after induction with zinc, in agreement with the 2-fold increase in reporter gene expression in those cells. In contrast, the presence of Znt1 lowered basal activity of the reporter gene to ≈5% of maximum and shifted peak induction to ≈120 μM zinc (Fig. 1, curve c). Addition of Mt genes to these cells (ZnR-3121 c10) lowered the basal activity a little more, but had essentially no effect on the induction by zinc (Fig. 1, curve d). Zinc induced MT protein ≈8-fold in these cells (data not shown). Extra copies of Znt1 gene (see below) completely silenced the reporter gene in normal medium, and optimal induction was shifted to ≈200 μM Zn (Fig. 1, curve e).
Fig. 1.
Effects of zinc on expression of MRE-βGeo in cells expressing ZnT1, MT, or both. BHK cells were grown in 24-well dishes until nearly confluent and then treated with the indicated amounts of ZnSO4 for 18 h before assay of β-galactosidase. Data are expressed as percent maximum activity. Curve a, ZnS cells that lack Znt1 and Mt gene expression. Curve b, ZnS cells with mouse Mt genes (3121-c2). Curve c, ZnR cells that express Znt1. Curve d, ZnR cells with mouse Mt genes (3121-c10). Curve e, ZnVR cells with amplified Znt1 genes but no Mt gene expression.
Selection and Properties of Zinc-Resistant BHK Cells. After chronic selection of ZnR cells with increasing concentrations of zinc, cells that could grow in 600 μM Zn were isolated (see Materials and Methods). When these cells (referred to as very resistant, ZnVR) were diluted directly from medium containing 600 μM Zn, they grew optimally in medium containing ≈200 μM Zn; growth was retarded at higher concentrations, and most cells died in excess of 800 μM Zn (Fig. 2A, curve d). Surprisingly, growth was also inhibited when these cells were shifted to normal medium containing 4 μM Zn. If the cells were grown in the absence of added zinc for 5 days before being tested, growth in the normal medium was restored but they lost their ability to grow in >500 μM Zn (Fig. 2 A, curve c). Similar results were obtained after propagating the cells without added zinc for up to a month (data not shown).
Fig. 2.
Effects of zinc on cell growth and reporter gene expression. (A and B) BHK cells were diluted and grown for 5 days in various concentrations of ZnSO4; then either DNA content (A) or β-galactosidase (B) activity per well was measured as described in Materials and Methods and plotted as percent maximum. Curve a, ZnS cells. Curve b, ZnR cells. Curve c, ZnVR cells selected for growth in 600 μM ZnSO4 and then grown without ZnSO4 for 5 days before zinc challenge. Curve d, ZnVR cells selected for growth in 600 μM ZnSO4 and transferred directly to the medium containing various amounts of ZnSO4. (C) Hepa 1A cells were grown for 5 days in various concentrations of ZnSO4; then DNA content was measured and plotted as percent maximum. Curve e, Hepa 1A cells. Curve f, Hepa 1A cells selected for growth in 500 μM ZnSO4 and then grown for 20 days without ZnSO4 before being testing. Curve g, Hepa 1A cells selected for growth in 500 μM ZnSO4 and then transferred directly medium containing various amounts of ZnSO4.
The induction of MRE-βGeo in the ZnVR cells was shifted to the right relative to the ZnR cells (Fig. 2B, compare curves b and d). The original ZnR line shows half-maximal induction of β-galactosidase activity at ≈50 μM Zn compared with ≈150 μM for ZnVR cells transferred either directly from 600 μM Zn or withdrawn from selection for 5 days before being tested. Note that there was little induction of β-galactosidase in the ZnVR cells with <50 μM Zn (Fig. 2B, curves c and d). Because β-galactosidase activity per culture is presented, the decline in activity at high concentrations reflects the impact of zinc on cell growth. The maximal β-galactosidase activity per cell was achieved at the point of maximal growth; this specific activity was maintained at higher concentrations, despite poor growth (data not shown).
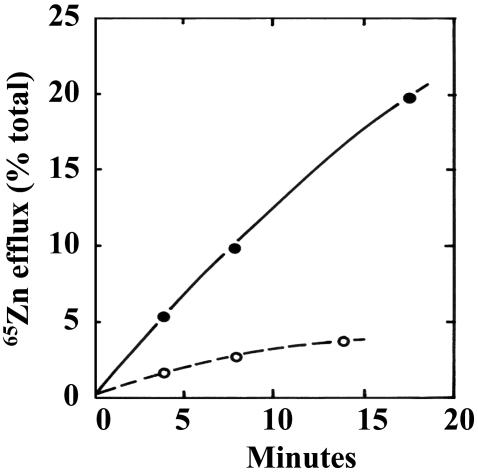
To assess whether the increased resistance to zinc was due to enhanced zinc efflux or intracellular sequestration of zinc, the ZnR and ZnVR cells were grown for 2 days in 65Zn to equilibrate intracellular pools of zinc. Then the cells were washed to remove extracellular 65Zn. Fresh medium was added, and the amount of 65Zn efflux was measured. The ZnVR cells had 0.19 fmol Zn per cell compared with 0.26 fmol zinc per cell for ZnR cells; however, the ZnVR cells had a 4-fold greater efflux of 65Zn efflux (Fig. 3).
Fig. 3.
Zinc efflux by ZnR and ZnVR cells. ZnR cells (○) and ZnVR cells resistant to 600 μM zinc sulfate (•) were growth for 2 days in medium containing 4 μM 65Zn. Then the media was removed, the cells were washed twice in PBS, and medium containing 4 μM unlabeled zinc was added. The amount of 65Zn in the medium and the cells were measured at various times thereafter. The percentage of total 65Zn (cells plus medium) in the medium is shown.
The Znt1 Gene Is Amplified. Because the properties of these ZnVR cells resemble those of BHK cells transfected with plasmids allowing overexpression of rat Znt1 cDNA (3), the relative number of endogenous Znt1 genes was assessed by a quantitative Southern blot procedure. Equal amounts of DNA from the original ZnR cells and the ZnVR cells were digested with BamHI and hybridized with rat Znt1 cDNA probe. The upper band (Fig. 4A) represents the Znt1 gene of ZnR cells; lanes 2-5 represent the Znt1 gene in DNA of ZnVR cells collected during growth in 600 μM zinc or after 5, 18, or 37 days of withdrawal, respectively. The signal is clearly more intense and nearly constant in all of the samples from ZnVR cells. The blot was also hybridized with an Mt1 gene probe to show that it was not amplified (Fig. 4A, lower band). Lane 6 contains a 10-fold dilution of DNA from the ZnVR cells, which allows a visual estimation of Znt1 gene amplification (≈10-fold). In a different isolate of ZnVR cells, ≈15-fold Znt1 gene amplification was observed, but in this case most of the genes were lost after 3 weeks of growth without selection (Fig. 4B).
Fig. 4.
Determination of gene copy number by Southern blot. (A) DNA (6 μg, lanes 1-5; 0.6 μg, lane 6) from ZnR cells was digested with BamH1, electrophoresed, and hybridized with probes for both Znt1 (Z1) and Mt. Lane 1, DNA from nonselected cells. Lane 2, DNA from ZnVR cells taken directly of medium with 600 μM Zn. Lanes 3-5, DNA from ZnVR cells withdrawn from selection for 5, 18, or 37 days, respectively. Lane 6, 1/10th the amount of DNA as in lane 2. (B) DNA from a different population of ZnVR cells was hybridized with Znt1 probe. Lane 1, ZnVR. Lane 2, same cells after 3 weeks of growth without added zinc. (C) DNA (6 μg) from mouse DKO7 cells was digested with EcoR1 and hybridized with a Znt1 gene probe. Lanes 1 and 2, nonselected cells. Lanes 3-6, DNA from a ZnVR population and three subclones. (D) Same blot as C but hybridized with a Znt2 (Z2) gene probe, which confirms equal loading. (E) DNA from mouse Hepa 1A cells was digested with EcoR1 and hybridized with Mt1 gene probe. Lane 1, nonselected cells. Lane 2, ZnVR cells. Lane 3, ZnVR cells after 20 days without selection. Lane 4, 1/10th the amount of DNA in lane 2. (F) Same blot as E but hybridized with a Znt1 gene probe. M, λ HindIII markers.
In addition to gene amplification, Znt1 mRNA was induced ≈15-fold by zinc as revealed by Northern blot analysis of mRNA from ZnVR cells grown in the presence of 600 μM zinc or withdrawn from zinc for 5 days (Fig. 5).
Fig. 5.
Northern blot of RNA from ZnVR BHK cells. RNA was isolated from ZnVR BHK cells (lanes 1-5) or withdrawn from zinc selection for 5 days (lanes 6-10). Lanes 1 and 6 have 18 μg of total RNA; subsequent lanes contain 2-fold dilutions of RNA. Arrow indicates the position of 28S rRNA.
To assess whether Znt1 gene amplification was special property of BHK cells, mouse cells were also gradually selected with increasing concentration of zinc. For one experiment, the cells were derived from an Mtf1-null mouse embryo (16), which lacks the transcription factor necessary for Mt gene induction by metals. The parental cells grow poorly in the presence of >80 μM Zn; however, after chronic selection, cells able to grow in 400 μM Zn were established. The Southern blot procedure shows that the Znt1 gene was also amplified in these cells (Fig. 4C); lanes 1 and 2 represent the Znt1 gene in control cells, and lanes 3-6 represent the Znt1 gene in a population of zinc-resistant mouse cells and three subclones. To confirm equal loading of DNA, the same blot was hybridized with a rat Znt2 gene probe (Fig. 4D). The extent of Znt1 gene amplification appears similar to that observed in the BHK cells.
In another experiment, mouse Hepa1A cells, which were derived from a mouse hepatoma, were used as the starting material. Unlike the cells described above, these cells can synthesize MT, and, as a consequence, they are more resistant to zinc toxicity; the ED50 was ≈200 μM Zn (Fig. 2C). After months of selection, a population of cells that grew optimally at 300 μM Zn was obtained with an ED50 at ≈650 μM Zn; whereas, after withdrawal from zinc selection for 20 days, ED50 fell to 300 μM Zn (Fig. 4C). Southern blots of DNA from the original Hepa1A cells and the zinc-resistant cells did not reveal any change in Znt1 gene copy number (Fig. 4F); however, the endogenous Mt1 genes were amplified ≈10-fold (Fig. 4E, compare lanes 1 and 2). This amplification of Mt1 genes correlated with a ≈4-fold increase in the maximum amount of Mt1 mRNA (from 4,200 to 15,000 molecules per cell) that could be induced by zinc. Most of the amplified Mt1 genes were lost after 20 days of growth without zinc selection (Fig. 4E, compare lanes 2 and 3).
Thus, it appears that Mt genes are preferentially amplified if they can be expressed. To further substantiate this idea, the BHK (ZnR-Aza-c4) cells in which the endogenous Mt genes were reactivated by treatment with 5-azacytidine were subjected to selection with increasing concentrations of zinc until the population could grow in 400 μM Zn. In this case, the Mt genes were preferentially amplified (≈5-fold) instead of the Znt1 genes (data not shown).
Discussion
In these experiments the roles played by ZnT1 and MT in regulation of intracellular zinc levels and protection against zinc toxicity in BHK cells were addressed. Both gene products are present at low levels in most cells, and both can be induced by the zinc-responsive transcription factor MTF-1 in response to challenge with a variety of heavy metals (7, 21). The mechanisms of protection by the two proteins are completely different. Induction of Mt gene expression results in synthesis of apo-MT, which binds zinc with high affinity (Kd ≈ 10-12 M), resulting in that accumulation of Zn7-MT. Cells that can induce MT can increase the maximum total cellular zinc content considerably (in these experiments by 50-100%), but after amplification of Mt genes (by selection or transfection), maximum zinc content can reach 10 times that tolerated by cells that cannot make MT (22). In contrast, ZnT1 facilitates zinc efflux, thereby lowering total cellular zinc content (3). Thus, as excess zinc enters a cell the two proteins compete with each other for handling zinc disposal. The genetic experiments described here, in which resistance to zinc toxicity was compared in BHK cells expressing neither ZnT1 nor MT, either alone or both, suggest that Znt1 gene expression has the largest protective effect, but Mt gene expression also provides some protection. These results are consistent with the observation that only Znt1 and Znt2 cDNAs were recovered when ZnS cells were transfected with a cDNA expression library and selected for ability to grow in 100 μM Zn (3). However, the relative role of these genes in providing protection against zinc toxicity may differ in other cell types; e.g., inactivation of both Znt1 alleles in mouse embryonic stem cells has a negligible effect on sensitivity to zinc toxicity (unpublished observations), suggesting that other gene products, possibly including MT, play a more important role in those cells.
When cells are suddenly exposed to high levels of zinc, these two protective mechanisms probably work in concert. As cellular zinc levels increase, zinc efflux by means of preexisting ZnT1 predominates, initially because all existing MT would already be saturated with metals; i.e., significant pools of apo-MT do not exist. The rise in zinc would also activate MTF-1, which would initiate transcription of the Mt1, Mt2, and Znt1 genes. As the newly synthesized apo-MT is released from the ribosome, it would bind available zinc because of its high affinity. Then, as ZnT1 accumulated, it would help lower intracellular zinc concentrations. An advantage of this dual system is that under conditions in which zinc levels fluctuate between scarcity and overabundance, cells would be able to sequester zinc during periods of abundance to help satisfy essential needs during shortage.
The interplay between MT and ZnT1 helps explain the consequences of their expression on the pool of free zinc that is available to regulate MTF-1. In the absence of either ZnT1 or MT, MRE-βGeo is almost fully induced in fnormal medium, suggesting that the free pool of zinc keeps MTF-1 almost fully activated. Two functional copies of the Znt1 gene lower the free zinc pool such that MRE-βGeo is expressed at ≈5% of maximum, whereas 20 copies lowers it to <1%. Interestingly, restoration of Mt gene expression, in the absence of Znt1 (e.g., the ZnS-3121c2 cells), lowers the free zinc pool only slightly, despite the high affinity of MT for zinc. I estimate that these cells synthesize more apo-MT than all of the other apo-metalloproteins combined, and they all compete for available zinc. Thus, one might predict that because of its high affinity for zinc, apo-MT would not only deplete the free pool of zinc but also compete with essential apo-metalloproteins; however, neither is the case (Fig. 1). Perhaps, when unopposed by ZnT1, zinc influx by the SLC39 class of zinc transporters (ZIP proteins, ref. 4) maintains a large pool of free zinc that provides sufficient zinc for all of the newly synthesized zinc-binding proteins. Expression of ZnT1 lowers the free pool of zinc, but MT still does not compete with other zinc-binding proteins for available zinc (21).
Gradual selection for zinc resistance results in ≈10-fold amplification of the Znt1 gene in the BHK cells and the Mtf1-null mouse cells, but not in mouse Hepa 1A cells, where Mt genes were amplified instead. BHK cells have been very useful for studying zinc regulation because their endogenous Mt genes are not expressed. The absence of Mt gene expression is apparently because of gene silencing rather than lack of transacting factors because metals regulate the stably integrated MRE-βGeo reporter gene appropriately. It was established previously that overexpression of MTs provides resistance to many different heavy metals, including zinc (15), and amplification of Mt1 gene in the Hepa 1A cells described here confirms that result. Thus, amplification of the Znt1 gene may occur preferentially in cells that cannot express their Mt genes. The preferential amplification of Mt genes over Znt1 genes when both are expressed could reflect (i) the close linkage of Mt1 and Mt2 genes (7 kb apart) and consequent amplification of both genes, (ii) DNA sequences surrounding the Mt locus that facilitate amplification, or (iii) an energetic advantage of binding zinc rather than exporting it. Nevertheless, the amplification of Znt1 genes provides clear genetic evidence that the rate of zinc efflux is limited by Znt1 gene expression. Consequently, enhancing Znt1 gene expression provides an effective means of protection against chronically elevated levels of extracellular zinc. Transport of zinc in intracellular organelles is another potential mechanism reducing zinc toxicity as evidenced by the fact that overexpression of ZnT2 (18) or ZnT4 (unpublished observations) confers resistance in mammalian cells, and expression of ZRC1 confers resistance in yeast (4). Furthermore, there are several other members of the SLC30 gene family that could have similar properties (14). Amplification of these genes would be reasonable candidates if there were no changes in Znt1 or Mt genes.
The size of the amplification units surrounding either the Znt1 or Mt1 gene has not been determined for any of the cell lines described here; however, the availability of mouse genome sequence would make that determination much easier than before. Previous studies of Mt gene amplification in response to chronic cadmium exposure revealed both unstable and stable forms of amplification (22). The stable forms typically represent integration of the amplified units into an endogenous chromosome, whereas the unstable forms usually exist as small chromosomal fragments, called “double minutes” because of their appearance in metaphase spreads (23). The Mt1 gene amplification described here was unstable. Both stable and unstable Znt1 gene amplification was observed in different populations of ZnVR BHK cells, but the location of the amplified units has not been examined. Gene amplification is thought to require breakdown of normal DNA replication checkpoints (24). The genetic changes leading to transformation of BHK cells is unknown, but the Mtf1-null mouse embryo cells were transformed with simian virus 40 T-antigen, a known suppressor of p53, which would facilitate gene amplification (25).
A downside of Znt1 gene amplification is loss of zinc responsiveness in the physiological range. This loss is illustrated by the absence of induction of MRE-βGeo reporter gene in ZnVR cells with <50 μM Zn, whereas near-maximal induction occurs in the ZnR cells at this concentration. Presumably, the rate of zinc efflux is so high that even after 5 days of growth in normal media there is no change in intracellular zinc after a 10- to 20-fold change in extracellular zinc. Furthermore, cell growth was inhibited when ZnVR BHK cells were switched directly from 600 to 4 μM Zn, but this phenomenon dissipated after several days of adaptation in normal media. Because zinc induces transcription of Znt1 (21), during continuous exposure to 600 μM Zn, the levels of Znt1 mRNA and protein are substantially higher than during growth in normal media. This elevated level of zinc efflux potential would exacerbate a direct switch from high to low zinc concentrations. The reverse situation also occurs. After a few days of withdrawal from growth in 600 μM Zn, all of the cells die if they are reexposed to 600 μM Zn. However, they can be stepped to 300 μM Zn for 1 day and then stepped to 600 μM Zn. A likely interpretation is that the intermediate exposure provides sufficient inductive signal to MTF-1 to enhance Znt1 transcription, which is necessary for accumulation of ZnT1 protein and maximal resistance.
Acknowledgments
I thank my colleagues for many helpful suggestions during the preparation of this manuscript. This work was supported in part by National Institutes of Health Grant DK 53013.
Abbreviations: BHK, baby hamster kidney; MT, metallothionein; MTF-1, metal-regulated transcription factor 1; ZnT1, zinc transporter 1; ZnS, zinc-sensitive cells; ZnR, zinc-resistant cells; ZnVR, cells very resistant to zinc challenge.
References
- 1.Vallee, B. W. & Falchuk, K. H. (1993) Physiol. Rev. 73, 79-118. [DOI] [PubMed] [Google Scholar]
- 2.Berg, J. M. & Shi, Y. (1996) Science 271, 1081-1085. [DOI] [PubMed] [Google Scholar]
- 3.Palmiter, R. D. & Findley, S. D. (1995) EMBO J. 14, 639-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaither, L. A. & Eide, D. J. (2001) BioMetals 14, 251-270. [DOI] [PubMed] [Google Scholar]
- 5.Hamer, D. H. (1986) Annu. Rev. Biochem. 55, 913-955. [DOI] [PubMed] [Google Scholar]
- 6.Cousins, R. J. & McMahon, R. J. (2000) J. Nutr. 130, Suppl. 5S, 1384S-1387S. [DOI] [PubMed] [Google Scholar]
- 7.Heschel, R., Radtke, F., Georgiev, O., Stark, G., Aguet, M. & Schaffner, W. (1994) EMBO J. 13, 2870-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichtlen, P. & Schaffner, W. (2001) BioEssays 23, 1010-1017. [DOI] [PubMed] [Google Scholar]
- 9.Giedroc, D. P., Chen, X. & Apuy, J. L. (2001) Antioxid. Redox Signal. 3, 577-596. [DOI] [PubMed] [Google Scholar]
- 10.Sheline, C. T., Behrens, M. M. & Choi, D. W. (2000) J. Neurosci. 20, 3139-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi, D. W. & Koh, J. Y. (1998) Annu. Rev. Neurosci. 21, 347-375. [DOI] [PubMed] [Google Scholar]
- 12.Kim, A. H., Sheline, C. T., Tian, M., Higashi, T., McMahon, R. J., Cousins, R. J. & Choi, D. W. (2000) Brain Res. 886, 99-107. [DOI] [PubMed] [Google Scholar]
- 13.Palmiter, R. D. (1994) Proc. Natl. Acad. Sci. USA 91, 1219-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmiter, R. D. & Huang, L. (2003) Pflügers Arch. Eur. J. Physiol. 447, 744-751. [DOI] [PubMed] [Google Scholar]
- 15.Durnam, D. M. & Palmiter, R. D. (1984) Mol. Cell. Biol. 4, 484-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radtke, F., Georgiev, O., Muller, H., Brugnera, E. & Schaffner, W. (1995) Nucleic Acids Res. 23, 2277-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eaton, D. L. & Cherian M. G. (1991) Meth. Enz. 205, 83-88. [DOI] [PubMed] [Google Scholar]
- 18.Palmiter, R. D., Cole, T. B. & Findley, S. D. (1996) EMBO J. 15, 1784-1791. [PMC free article] [PubMed] [Google Scholar]
- 19.Compere, S. J. & Palmiter, R. D. (1981) Cell 25, 233-240. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman, M. W., Beach, L. R. & Palmiter, R. D. (1983) Cell 35, 207-214. [DOI] [PubMed] [Google Scholar]
- 21.Langmade, S. J., Ravindra, R., Caniels, P. J. & Andrews, G. K. (2000) J. Biol. Chem. 275, 34803-34809. [DOI] [PubMed] [Google Scholar]
- 21.Palmiter, R. D. (1995) Toxicol. Appl. Pharmicol. 135, 139-146. [DOI] [PubMed] [Google Scholar]
- 22.Beach, L. R., Mayo, K. E., Durnam, D. M. & Palmiter, R. D. (1981) in Developmental Biology Using Purified Genes: Proceedings of the ICN-UCLA Symposia on Developmental Biology Using Purified Genes, held in Keystone, Colorado, on March 15-20, 1981, ICN-UCLA Symposia on Molecular and Cellular Biology, eds. Brown D. & Fox, C. F. (Academic, New York), Vol. 23, pp. 239-248. [Google Scholar]
- 23.Schimke, R. T. (1986) Cancer 57, 1912-1917. [DOI] [PubMed] [Google Scholar]
- 24.Tlsty, T. D. (1990) Proc. Natl. Acad. Sci. USA 87, 3132-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu, C., Mills, K. D., Ferguson, D. O., Lee, C., Manis, J., Fleming, J., Gao, Y., Morton, C. C. & Alt, F. W. (2002) Cell 109, 811-821. [DOI] [PubMed] [Google Scholar]