Abstract
Normal and diseased cells release bilayered membrane-bound nanovesicles into interstitial spaces and into bodily fluids. A subgroup of such microvesicles is called exosomes and is described in blood as 30 to 100 nm in diameter and as spherical to cup-shaped nanoparticles with specific surface molecular characteristics (eg, expression of the tetraspanins CD9, CD81, and CD63). Extracellular microvesicles provide local signals (eg, autocrine and paracrine) and distant endocrine signals to cells via the transfer of their contents, which include signal proteins, lipids, miRNAs, and functional mRNAs. Exosomes and related microvesicles also aid cells in exporting less-needed molecules and potentially harmful molecules, including drugs; in the case of neoplasia, the export of chemotherapeutic drugs may facilitate cellular chemoresistance. Cancers have adapted the exosome and related microvesicles as a pathway by which neoplastic cells communicate with each other (autocrine) and with nonneoplastic cells (paracrine and endocrine); via this pathway, cancer suppresses the immune system and establishes a fertile local and distant environment to support neoplastic growth, invasion, and metastases. Because exosomes mirror and bind to the cells from which they arise, they can be used for delivery of drugs, vaccines, and gene therapy, as biomarkers and targets. We review how exosomes and related extracellular microvesicles facilitate the progression and metastases of cancers and describe how these microvesicles may affect clinical care.
Exosomes are a subcategory of bilayer membrane-bound nanovesicles released from normal and diseased cells into interstitial spaces and, in some cases, into bodily fluids. Exosomes also are released by cultured cells into media. Exosomes are defined and separated from other vesicles based on their source, method of isolation, sizes, and surface markers. Exosomes and other vesicles frequently are unrecognized as to their importance to physiological and pathological processes because they are essentially invisible; their small sizes keep them suspended in fluids so that their effects may not be identified in that their contents and functions would seem to be consistent with those of soluble molecules. Exosomes and related microvesicles were once thought to be artifacts and/or cellular trash, but now exosomes are accepted as a component of a newly identified intercellular communication system that can modulate the functions of target cells. The involvement of exosomes and related microvesicles in providing autocrine (ie, local signals between the same cell type, such as cancer cells), paracrine (ie, local signals between different cell types, such as between epithelial cancer cells and stromal cells), and endocrine (ie, distant signals between any types of cells usually carried in bodily fluids, such as blood) signals has led to the frequent use of the term, signalosomes, being applied to these structures.
Exosomes and Related Microvesicles in Cancer
A specific subtype of exosome/vesicle/particle is released by tumor cells (tumor-derived exosomes or TD-exosomes). TD-exosomes/microvesicles may be released into the interstitial space or even directly into lymphatics or into pseudocapillaries formed by tumors. Also, the neovascularity of malignant tumors is thought to be especially leaky and its permeability may be influenced by exosomes, cytokines, and other local molecules, such as vascular endothelial growth factor (VEGF).
The presence of TD-exosomes in patients with malignant tumors leads to several important issues and concepts.1–23
-
1.
Exosomes/microvesicles in the blood of patients with tumors are composed of both exosomes/vesicles from normal cells, diseased cells (comorbid conditions), and TD-exosomes/microvesicles (Table 1). TD-exosomes are characteristic of tumor cells and have different molecular characteristics than microvesicles from other sources.
-
2.
Tumor cells may release more microvesicles than other cells, and TD-exosomes may have easier access to the vascular system and, thus, may be selectively increased in blood compared with microvesicles from other sources.
-
3.
Smaller microvesicles with specific molecular surface characteristics may selectively reach the blood and larger microvesicles may remain in the interstitial space and selectively provide autocrine and paracrine signals to stromal, inflammatory, and endothelial cells.
-
4.
Once specific cellular populations within the tumor are affected by TD/exosomes/microvesicles, various modulatory loops may be established that facilitate the growth, progression, and cellular dissemination of the tumor.
-
5.
Via autocrine interactions, TD-exosomes may stimulate malignant cells to grow, move, and invade the vascular-lymphatic system and disseminate via chemotaxis to nodal and other metastatic sites. Exosomes may establish favorable environments at potential metastatic sites and may aid the survival of neoplastic cells at sites of metastases.
-
6.
As part of establishing specific microenvironments and interacting with various cellular compartments, chemotaxis is likely to be an important, although poorly described, signaling pathway of exosomes.
-
7.
Some tumors frequently induce thrombosis and, hence, may cause an increase in microvesicles/microparticles that are released from platelets. These microvesicles have to be experimentally separated from microvesicles derived from actual malignant cells or other types of cells.
-
8.
TD-exosomes can interact with the normal cells of the immune system to reduce immune surveillance by increasing cells that inhibit immunity, decreasing antigen-presenting cells (APCs), and inhibiting T and natural killer (NK) cells.
-
9.
Exosomes function in removal of waste products, drugs, and/or less necessary molecules from cells and, hence, exosomes may facilitate chemoresistance.
-
10.
Exosomes/microvesicles have been reported to transfer oncogenic features among malignant cells and transiently to nonneoplastic cells, suggesting the probability of lateral transfer of phenotypic features among cells.
-
11.
Immunoseparations suggest that distinct populations of exosomes may have different molecular characteristics and may arise from different intracellular areas of polarized cells.
-
12.
Exosomes may be useful clinically and in translational research by improving the analysis of biomarkers and the delivery of therapeutic and preventive drugs, vaccines, and gene therapy; in addition, they may be useful as targets to improve immunity.
Table 1.
Source of Exosomes in a Patient with a Specific Malignant Process
| Independent of the tumor |
| Exosomes from normal cells |
| Exosomes from cells whose release is caused by nonneoplastic comorbid conditions that are independent of the tumor |
| Dependent on the presence of cancer |
| TD-exosomes from malignant cells |
| Exosomes from uninvolved cells (eg, adjacent uninvolved tissue that appears normal) induced by TD-exosomes |
| Exosomes from normal cells induced by exosomes or other molecules from the cancer (eg, soluble factors); this includes exosomes released from tumor and associated stromal cells |
| Exosomes from immune cells affected by the cancer |
| Exosomes from the cells of a comorbid condition caused by the tumor (eg, disseminated intravascular coagulation caused by the cancer) |
Characteristics of Exosomes
Signaling by exosomes and microvesicles is likely to be affected by their morphological and molecular features. In gaining entrance to the lymphatic-vascular system, smaller nanoparticles are likely to have an advantage because the microvesicles must pass through spaces between endothelial cells via mechanisms that are not adequately defined. Similarly, the molecular characteristics of the external surfaces of exosomes may affect this passage and some exosomes are absorbed by endothelial cells and influence secondary exosomes released from endothelial cells.
Subgroup of Microvesicles
Exosomes typically can be collected from blood via a series of steps of centrifugation to remove larger cellular debris and sometimes by filtration through a 100- to 220-nm filter to exclude larger microvesicles, including apoptotic blebs. Subsequently, exosomes are pelleted by ultracentrifugation at approximately 100,000 × g for 60 minutes, washed, and suspended by ultracentrifugation in a sucrose gradient. After these steps, exosomes isolated from blood typically are described via electron microscopy as spherical to cup shaped, bilayered, membrane-bound nanovesicles ranging from 30 to 100 nm in greatest dimension. They are also described by using Western blot analysis of the exosomal preparations as demonstrating the presence of multiple molecular features, including specific tetraspanins.1–12
When the usual steps are not taken during isolation of exosomes, the nanoparticles presented in preparations frequently are described as microvesicles, exosome-like particles, microparticles, or sometimes oncosomes if the microvesicles transmit oncogenic properties to the target cells. There is confusion because results based on microvesicles are sometimes reported as from exosomes and vice versa. Both the sources of microvesicles and their isolation affect their characteristics: a ≤220-nm filter limits the sizes of microvesicles, and sucrose gradient separations affect sizes via their density and may remove molecules absorbed on exosomes.
If specific microvesicles are involved in intercellular communication, is it important to classify microvesicles based on their sizes and shapes? The effects of size-shape characteristics have not been described adequately, but size, shape, and surface characteristics may affect how exosomes gain access to bodily fluids and deliver signals to specific cells with which they interact.12
Molecules phenotypically expressed on the external surface of microvesicles separate nanovesicles originating from multivesicular bodies (MVBs) from those arising from budding from cellular membranes and, hence, define different subgroups of microvesicles that may have different morphological and molecular characteristics, different sources, and, perhaps, different functions. Those markers identified by using Western blot analysis may not be present on all microvesicles and may not be associated with the microvesicles producing specific functional characteristics.
Thus, the sizes, shapes, and surface molecules of microvesicles may affect their concentration in blood.12 Microvesicles isolated from fluids other than blood may have different morphological features; microvesicles isolated from semen are described as being up to 500 mm and containing intravesicular structures, from spent culture media as biphasic in size (200 to 400 and 600 to 1000 nm), and from saliva as doughnuts with a height/width ratio of 0.04.23–25 Exosomes isolated from ascites are described as being similar to exosomes isolated from matching blood.26
The release of exosomes from normal and diseased (ie, nonneoplastic) cells is affected by multiple factors, including calcium, calcium ionophores, phosphatidylinositol 3-kinase, heat, ischemia, cellular stresses, pH, phorbol esters, and loss of cellular attachment.13,27–33 Hypoxia increases specific exosomes that, in turn, may stimulate angiogenesis.13 As would be expected, factors that affect the formation of MVBs, from which exosomes specifically arise, affect the secretion of exosomes; thus, exosomes are decreased by inhibitors of phosphatidylinositol 3-kinase (wortmannin).
Multiple molecular factors control exosomes, including the small GTPases (Rab) and p53 via TSAP6.34–36 The complex of syndecan 1–syntenin and Alix affects the intraluminal budding of exosomal membranes and, together with an endosomal sorting complex responsible for transport (ESCRT-III), these molecules cleave the membrane buds. This complex may also be involved in growth factor transport37,38 and may be related to control in exosomes of selective protein sorting by ceramide.39 In addition, heparanase, which may aid in the formation of the syndecan-1–syntenin–Alix complex, also stimulates the secretion of classic exosomes.40 Although these molecular features affect the secretion of exosomes, our knowledge of the overall process is rudimentary. This same pathway may also be used by specific viruses in their release from cells.33,36
Kinetics of Exosomes
The kinetics of release of exosomes is relatively rapid. After loading of MCF-7 cells with doxorubicin, exosomes containing doxorubicin showed presence at cellular membranes within 3 hours.23 Similarly, after stimulation of mast cells with alloantigens, exosomes were released within 30 minutes.41 The internalization of exosomes by target cells is a rapid, active process; one-third of dendritic cells (DCs) imported exosomes at 37°C within 2 hours. Internalization requires metabolic energy and is decreased by EDTA, cytochalasin D, and incubation of the target cells at 4°C.31,41,42
Molecular Characteristics of Exosomes
Exosomes are defined by specific molecular features, including the phenotypic expression of specific surface molecules. The commonly used molecular markers of exosomes are the surface tetraspanins, CD9, CD63, CD81, CD82, CD151, and other molecules, including intercellular adhesion molecule-1, αvβ3 (CD51 and CD61), integrin, Alix, externalized phosphatidylserine, milk fat globule-E8/lactoferrin, CD80, CD86, CD96, Rab-5b, and major histocompatibility complex (MHC) class I and MHC class II complexes.7–9,31,32,41–46 Tetraspanins may form a net incorporating different tetraspanins and neighboring molecules, and this net may be incorporated into MVBs and into exosomes.47 Exosomes/microvesicles may vary with their origin; the transferrin receptor is in exosomes from reticulocytes and CD11a and CD54 in exosomes from hematopoietic cells. Exosomes from tumors may express molecular features of the tumor type (eg, melanin A from melanomas5,10–12,17–19,31,33,46,47).
Recent studies using colorectal cells have suggested that when cells are polarized in three-dimensional cultures, two subtypes of exosomes are secreted from different intracellular locations. Specifically, approaches were used via which larger microvesicles are excluded from preparations (ie, filtration through a 100-nm filter and concentration of the specimen through a 5-kDa molecular weight limit membrane). Exosomes were then separated from this preparation by immunoaffinity based on a colonic epithelial cell type–specific molecule, A33, followed by immunoaffinity separation based on Ep-CAM. The two types of exosomes that were identified had slightly >50% of proteins in common; however, there were approximately 20% different proteins in the A33 cell-specific fraction and approximately 25% different proteins in the Ep-CAM fraction. The Ep-CAM fraction seemed to be primarily enriched in apical proteins (eg, CD44 and tetraspanins), whereas the A33 fraction was enriched in basolateral proteins, including clathrin and MHC class I molecules. This observation, if independently confirmed in other cell types, will greatly expand our understanding of exosomes. Similarly, it is likely that there are other subtypes of exosomes (eg, an exosome population involved specifically in cellular waste management).
In that exosomes act in intercellular communication and in exporting waste and less-needed products from cells,48,49 exosomes containing waste products are likely to have surface molecules that instruct phagocytes to remove them.48 For example, the phenotypic surface expression of galectin-5 has been proposed as being important in the removal of reticulocyte exosomes by macrophages.50 Other signals may be analogous to those by which apoptotic cells are phagocytosed.
The release of exosomes from normal and diseased cells provides signals to other cells via their contents, which include a wide range of functional molecules (eg, hundreds of different proteins, mRNAs, miRNAs, and lipids).16,25,51–55 When mRNA contained in murine exosomes from mast cells reacted with human mast cells, murine proteins were produced in the human cells.52
Although miRNAs are a relatively new area of investigation, their impact on human diseases has been demonstrated to be important.56,57 Thus, the observations that exosomes from multiple types of tumors contain miRNAs is noteworthy but controversial, in that some reports indicate that most circulating miRNAs are outside exosomes. Of interest, the controversy is not whether exosomes contain miRNAs, but rather the proportion of circulating miRNAs that are within exosomes.58,59 It would likely be advantageous for all circulating RNAs to be within exosomes. Exosomes are a vehicle that can protect RNAs, but potentially can deliver RNAs to specific target cells in which miRNAs may modulate mRNAs.56 Thus, the exosome is likely to be both a typical chaperone for some miRNAs and their other molecular contents, as well as a specific delivery pathway. As previously indicated, exosomes are invisible unless they are specifically looked for. To support the concept of invisibility, a study challenging the reports indicating that most circulating miRNAs are outside exosomes reported that most circulating miRNAs from serum and saliva are contained within exosomes.60 These studies have used serum and plasma from normal patients; this issue may be more important in exosomes released from tumors rather than exosomes released from nonneoplastic cells. Tumor cells have a high rate of cellular death and when the cells of tumors die, their surviving contents, including RNAs, miRNAs, and argonaute 2 complexes, could be dumped into the interstitial space and picked up by the lymphatic-vascular system. Thus, depending on the rate of death of the malignant cells of specific tumors, it would not be surprising to find miRNAs both inside and outside exosomes, especially if complexed with argonaute 2. Similarly, as discussed, the method of isolation of exosomes may strongly affect the RNAs in tumor-derived exosomes. Specifically, a proportion of exosomes may be destroyed on isolation, releasing their contents into solution.58 The resolution of the question of whether circulation miRNAs are primarily within or outside exosomes will obviously require much more study. The issue may well relate to function in that miRNAs contained in exosomes may be more biologically active with respect to specific uptake by targeted cells.
Exosomes separated from saliva can affect proteins in oral keratinocytes of humans25; thus, exosomes act as an endocrine system and provide autocrine, paracrine, and other signals to target cells. For example, microvesicles can be exchanged among touching cells using tunneling nanotube networks.55,61 Although molecules in exosomes can provide molecular signals to modulate target cells, how target cells accept or reject the hundreds of different molecules contained in microvesicles that could provide different signals is not understood. More is known about the molecular signals that are accepted by target cells than those that are ignored.
How exosomes from different cells target specific cells is yet to be clarified; however, there must be selective interactions of exosomes with target cells, probably via interactions with molecules on the external surface of exosomes with interacting surface molecules on target cells. Exosomes bind to the breast carcinoma cell line, BT-549, via annexins A2 and A6 and the molecules of lipid rafts.32,62 The active uptake of exosomes by DCs can be only partially blocked by monoclonal antibodies to surface molecules, including CD9, CD11a, CD54, CD81, and αvβ3 integrin. Each monoclonal antibody blocked internalization of exosomes by only approximately 30% of control values, but monoclonal antibody to milk fat globule-E8/lactoferrin, a surface molecule that potentiates uptake of apoptotic cells by macrophages, stimulated the uptake of exosomes by approximately 30%.42 How combinations of surface signals potentiate uptake of exosomes remains to be clarified. Of interest, tetraspanins are involved in the fusion of HIV-1 with T cells and facilitate uptake of HIV-1 by DCs and macrophages; thus, understanding the cellular uptake of exosomes might be increased by a better characterization of how cells are infected with viral particles and apoptotic cells are phagocytosed by macrophages.47,63
How do cells decide which molecules to package into exosomes? Waste or less-needed molecules could be packaged into one type of vesicle, but signal molecules into different microvesicles. Alternatively, if both types of molecules are packaged into the same microvesicles, are the waste molecules destined for degradation via molecular modification (eg, ubiquitinated) so that they are ignored by the target cells? The JAB1/CSN5, a component of COP9, seems to play a role in the sorting of proteins that are ubiquitinated into exosomes, and this might be a step in processing of waste molecules.64,65 Other waste molecules may be sorted via the ESCRT-0, ESCRT-I, and ESCRT-II pathways. The tetraspanins, especially CD81, complex with some viral particles (eg, hepatitis C virus) to promote their incorporation into exosomes63; however, viral particles are much more complex than single molecules. Also, ceramide and heparanase seem to play a role in transferring potential signal molecules into exosomes.39,40
Functions of Exosomes in Normal Physiological Characteristics
Exosomes have been isolated from most normal biological fluids, including bronchoalveolar fluid, cerebrospinal fluid, blood, urine, saliva, breast milk, amniotic fluids, semen, and synovial fluid, and diseased fluids, including ascites and pleural effusions.24–26,51,66–69 Thus, exosomes are likely to play important roles in normal physiological features and in diseases. Most roles in normal physiology have not been well characterized, except regarding the functions of exosomes in the immune system.
The Actions of Exosomes in the Normal Immune System
Exosomes play important roles in the intercellular communication among normal immune cells, but communication may typically be dysregulated in disease processes. Most immune cells have been reported to secrete exosomes. During their normal functions, mast cells, DCs, immature APCs, macrophages, T and B lymphocytes, and NK cells use exosomes to communicate.10–12
An excellent example of how exosomes affect the immune system is their involvement in maternal-fetal tolerance.70–76 To protect the fetus, the placenta releases exosomes, some of which phenotypically express Fas ligand, an immunosuppressive and an anti-inflammatory molecule.77–79 In addition, syncytiotrophoblasts secrete exosomes that express ULBP 1 to 5, which are ligands to the NKG2D receptor expressed on γδT, NK, and CD8+ T cells. During pregnancy, an interaction of the NKG2D receptor with its ligands decreases the functions of immune cells.72 Also, the chromosome 19 miRNA cluster, which suppresses immunity and facilitates the growth of some tumors, is present in placental exosomes.75,76 Thus, exosomes are involved in the decreased immunity that is necessary for successful pregnancies.
Exosomes are an important feature in the processing of antigens by APCs. The DCs can use exosomes in the presentation of processed antigens to NK cells and T lymphocytes. In this process, stimulating antigens or antigen peptides are combined in MHC class II complexes present on the surfaces of some exosomes and these MHC class II antigen complexes then stimulate immune cells. The presentation of antigens/peptides by exosomes is much more efficient than if exosomes are not involved.80–83 At least two different subpopulations of DCs are required for optimal stimulation of T cells by exosomes. DCs carrying an MHC class II antigen complex secrete exosomes that carry the complex. These exosomes can be absorbed by CD8−α, CD80+, and CD86+ DCs that can then process the exosomes so that an exosome–HLA class II antigen–peptide complex can be presented by DCs to immune cells, such as CD4+ T cells. The activation of CD4+ T cells can be amplified via transferring the HLA class II antigen–peptide complex to multiple DCs independent of their expression of HLA class II complexes.80,81
CD8α+ DCs control peripheral immune tolerance via modulation of the responses of CD4+ T cells to alloantigens.42 Processing of exosomes by mature DCs is required for stimulation of MHC class I restricted CD8+ T-cell responses. Adjuvant molecules, such as ligands for toll-like receptors 3 and 9, aid in this stimulation.83 In addition, mature B cells may be required for optimally stimulating T cells by exosomes released by DCs.84
Other effects of exosomes on immune cells have been reported; specifically, mast cells secrete exosomes that activate B and T lymphocytes and DCs. Exosomes released in vivo from murine mast cells facilitated the maturation of DCs and increased the ability of DCs to present antigens to T lymphocytes. The heat shock proteins, HSP60 and HSP70, from exosomes have been reported to be important for the presentation of antigens by DCs.41 Exosome-like particles isolated from the spleen can modify CD4+, CD25− T cells and convert them into CD4+, CD25+, Foxp3+ T-regulatory cells (Tregs). Tregs act to inhibit autoreactive T cells and, thus, suppress dysregulated immune responses and minimize autoimmunity.85 Exosomes also are sometimes involved in dysregulation of normal immunity and affect autoimmunity.86 Depending on the source cells and their molecular content, exosomes may suppress or atypically stimulate pathways of the immune system, suggesting an involvement of exosomes in autoimmunity. Exosomes from synovial fibroblasts collected from patients with rheumatoid arthritis, but not osteoarthritis, contain a membrane form of tumor necrosis factor (TNF)-α, which potentiates the signaling of NF-κB and suppresses apoptosis of activated T cells. This may increase the severity of rheumatoid arthritis.87 Alternatively, exosomes from genetically modified immunosuppressive immune DCs [eg, bone marrow–derived DCs (BMDCs)] can reduce autoimmunity. BMDC(s) expressing IL-10 or exposed to IL-10 release exosomes that inhibit both the inflammation and arthritis caused by injections of collagen into mice.88 Similarly, when BMDCs are modified to express transforming growth factor (TGF)-β1, the development of inflammatory bowel disease in mice, secondary to dextran sodium sulfate, is inhibited by exosomes from the TGF-β1 genetically modified BMDCs.89
Normal Functions of Exosomes in the Export of Waste Products, Less-Needed Molecules, and Harmful Molecules
Cells use exosomes to export waste products, molecules no longer as useful as cells differentiate, and molecules, such as drugs, that may be harmful to cells. This pathway can eliminate proteins without signal sequences and permit their secretion. Exosomes are especially important during cellular differentiation when large amounts of molecules must be removed from differentiating cells to optimize their more differentiated functions (eg, metabolic active hematopoietic precursor cells that differentiate to anucleate relatively inactive red blood cells containing primarily hemoglobin).50
Exosomes and Neoplasia
Exosomes are secreted by most types of cells, including those of neoplastic lesions. The microvesicles released from probably all malignant cells are designated as tumor derived (TD). TD-microvesicles have been studied primarily in bodily fluids (eg, blood) and media from cultured cells.
Exosomes/microvesicles in bodily fluids are complex, as is the molecular composition of bodily fluids, because both microvesicles and free molecules in bodily fluids are from normal and diseased cells. Similarly, as noted previously, exosomes and microvesicles, as well as their contents and effects, are invisible unless analyzed as a separate component of fluids. Thus, measurements of the molecular contents of TD-exosomes potentially should be just as sensitive and specific for clinical uses and in translational research as measurements of the same molecules external to exosomes. TD-exosomes/microvesicles mirror the molecular features of the source neoplastic lesions. For example, TD-exosomes in blood from patients with glioblastoma multiforme and high-grade gliomas contain neural markers (eg, LI-NCAM/CD171)88–91 and exosomes from melanomas that express molecules involved in melanin synthesis and other melanoma markers (eg, Melan A/Mart 1).5,46,92
Exosomes Decrease the Immune Surveillance of Tumors
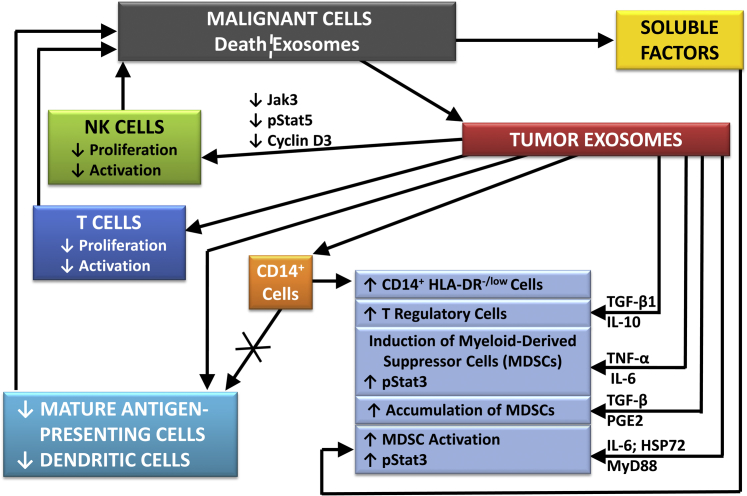
Via its normal functions, the immune system should minimize the development and progression of cancers; however, malignant lesions have been found to inhibit immune surveillance, resulting in their more rapid growth, progression, and dissemination.10–12,93–99 Specifically, tumors have increased growth in vivo after injection of exosomes isolated from matching neoplastic lesions.93 Exosomes ultimately were found to inhibit immunity by several mechanisms, including increases in suppressive immune cells, decreases in the proliferation and cytotoxicity of NK and T cells, and decreases in the number and functions of APCs.10–12 Thus, the decrease in immunity caused by neoplastic lesions is primarily mediated by exosomes released from tumors.93–101
The intercellular signals provided by TD-exosomes to the immune system probably vary with different types of cancers. Oral and ovarian epithelial neoplastic lesions secrete exosomes/microvesicles that contain Fas ligand, Trail, or related molecules (eg, TNFα), which can cause apoptosis of activated T lymphocytes via suppression of CD3ζ and Jak-3.77–79,95 However, some TD-exosomes that increase the growth of their source tumors do not contain Trail or Fas ligand, but interact with NK cells to decrease the secretion of perforin, inhibit Jak-3, and decrease cyclin D3.93 Thus, TD-exosomes are likely to decrease immunity via several mechanisms. For example, exosomes containing TGF-β1 may decrease the expression of the NKG2D receptor via, in part, miRNA-1245, and reduce the activation of NK and CD8+ T cells.96,97
TD-exosomes also decrease immunity by decreasing the numbers and/or activity of APCs, including DCs. Signals from TD-exosomes increase the phosphorylation of Stat 3 and the expression of IL-6 in DCs, decreasing the activity of DCs and the numbers of APCs via inhibiting the differentiation of CD14+ monocytes into mature APCs.98 In addition, CD14+ cells shift to immunosuppressive CD14+ HLA-DR-/low cells, which release TGF-β to inhibit T cells.99,100
TD-exosomes increase myeloid-derived suppressor cells (MDSCs). In humans, the numbers of MDSCs in neoplastic lesions are correlated with the neoplastic progression and decreased patient survival, probably via inhibiting NK cells and CD4+ and CD8+ lymphocytes by MDSCs.101–103 MDSCs (CD11b+, Gr-1+) are increased in neoplastic lesions, spleens, and blood of mice with transplanted syngeneic tumors, and they are decreased in the blood and spleens of mice by removal of transplanted tumors.102 Exosomes increase MDSCs via TGF-β, which increases prostaglandin E2.104 TD-exosomes also increase MDSCs via MyD88, but effects of TLR pathways on MDSCs should be considered carefully because of potential phenotypic changes in cells, induced by long-term cultures.9–11,105,106 In addition to the actions of exosomes, release of cellular soluble mediators, such as granulocyte-macrophage colony-stimulating factor, from neoplastic cells also may increase MDSCs.
Another immune-suppressive cellular population that is increased by exosomes is Tregs. TD-exosomes can induce the differentiation of CD4+, CD25+ T cells to CD4+, CD25+, Foxp3+ Tregs via the phosphorylation of both Stat 3 and SMAD2/3.98,107
Figure 1 summarizes how neoplastic lesions can partially avoid immune surveillance by secreting TD-exosomes, which inhibit the activity of T and NK cells, by decreasing APCs, and by increasing cells that suppress immune surveillance, such as Tregs, MDSCs, and CD14+-HLA-DR-/low cells.
Figure 1.
A scheme summarizing some of the complex effects of TD-exosomes on the immune system and how TD-exosomes suppress immune surveillance by decreasing the proliferation and activation of T and NK cells, decreasing mature antigen presenting cells (APC) and increasing cells such as myeloid-derived suppressor cells and Tregs, which suppress immunity. HSP72, heat shock protein 72; JAK3, Janus kinase 3; MyD88, myeloid differentiation primary response 88; PGE2, prostaglandin E2; pStat3, phosphorylated signal transducer and activator of transcription 3; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α.
Nonimmune Effects of Exosomes on Primary Malignant Lesions
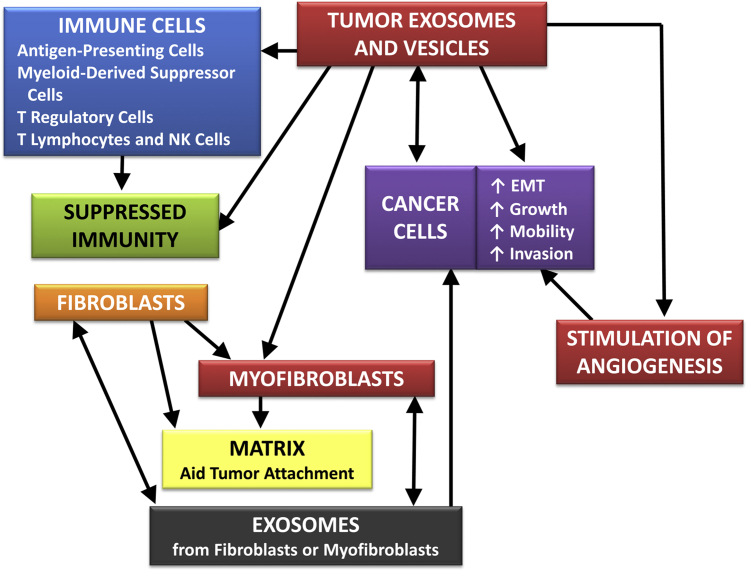
The development, progression, and dissemination of malignant tumors depends on a balance of cellular proliferation and cellular death (eg, apoptosis and autophagy). TD-microvesicles can provide autocrine, paracrine, endocrine, and other signals that can generate fertile environments to support malignant lesions and, hence, stimulate their growth, progression, and metastasis (Figure 2).13–22
Figure 2.
A scheme indicating the potential local effects of TD-exosomes on cancer cells. The divisions of boxes represent potential different exosomal compartments that interact with the microenvironment of the tumor. Paracrine networks are represented between fibroblasts, myofibroblasts, and malignant cells.
An important pathway by which TD-exosomes modulate the local environment of cancers is one that stimulates angiogenesis and, hence, increases the supply of oxygen and nutrients to the growing tumor.1,13,16–18,22,90,91,108,109 Specifically, exosomes secreted by melanomas are reported to facilitate the formation of spheroids by endothelial cells and budding of these spheroids. Also, cytokines, including TGF-β and VEGF, that stimulate angiogenesis are contained in exosomes secreted from malignant cells in hypoxic and similar environments.13,16 Similarly, the blood from patients with glioblastomas and from the media of their short-term cultures has been reported to contain microvesicles with the pro-angiogenic proteins, angiogenin, VEGF, IL-6, and IL-8. The microvesicles were enriched in angiogenic proteins compared with the source glioblastomas, and these microvesicles stimulated increases in the length of endothelial tubes and in angiogenic proteins.90,91,108,109 Also, on exposure to hypoxia, A431 cells secrete exosomes that contain molecules that stimulate angiogenesis and facilitate metastasis.13
TD-exosomes may modulate the local growth of neoplastic lesions via autocrine signals provided by exosomes. Autocrine effects of exosomes have been reported to vary with cellular characteristics and cell type. For example, exosomes from pancreatic cancers increased apoptosis via the Notch pathway if the cell lines were well differentiated, but not if the cell lines were poorly differentiated; thus, exosomal autocrine signals might inhibit the growth via stimulating apoptosis of some pancreatic neoplastic lesions, but not others.110,111 Alternatively, autocrine signals mediated by exosomes from the BT-474 breast cancer cell line, gastric cancer cell lines, and glioma cells increased cellular proliferation.2,90,112 The mechanism of the increased proliferation of the gastric cancer cell lines was thought to be via increased phosphorylation of Akt and extracellular signal–regulated kinase, which, with other downstream molecules, are associated with cellular proliferation. Growth of the glioblastoma cell line, U87, was increased via exosomes derived from primary glioblastomas; although this growth was assumed to be secondary to increased proliferation, decreased apoptosis was not excluded.90 Survivin and heat shock proteins, HSP70 and HSP90, may be increased in exosomes in association with cellular stresses. These proteins may inhibit apoptosis and increase cellular proliferation and invasion so they provide a strong stimulus to the microenvironment that can facilitate the growth and dissemination of primary tumors.31,113
In addition to exosomes, microvesicles of other types may be secreted by neoplastic cells into the local microenvironment of primary tumors. Specifically, PC-3, a prostatic cancer cell line, released microvesicles distinct from exosomes, which stimulated fibroblasts, and the fibroblasts responded by an increased release of microvesicles that stimulated PC-3 cells to migrate and invade. This paracrine loop associated with the CX3CL1 and CX3CR1 was not observed in LNCaP cells, which are not as aggressive as PC-3 cells.14 The variable responses of PC-3 and LNCaP cells to microvesicles from fibroblasts might be due to different molecular features within or on exosomal surfaces, such as the binding complex, type III receptor beta glycan, and its associated ligand, TGF-β, which are expressed strongly in PC-3 cells, but not on or in LNCaP cells.15
TD-exosomes can induce changes in fibroblasts indicative of a transition to myofibroblasts. Fibroblasts/myofibroblasts then may modulate the microenvironment (eg, degradation of the extracellular matrix and increased production of pericellular hyaluronic acid) to facilitate the invasion of neoplastic cells. Also, fibroblasts/myofibroblasts may aid in the induction of epithelial-mesenchymal transitions of malignant cells.15 Thus, TD-exosomes typically increase the growth and progression of primary neoplastic lesions via the induction of phenotypic changes that facilitate more aggressive cellular characteristics.
Exosomes can provide an efficient and specific transfer of molecular signals between cells. If these signals induce oncogenic changes in the cellular phenotype, TD-microvesicles are sometimes referred to as oncosomes. A variant of the epidermal growth factor receptor (EGFR) vIII has oncogenic features via unregulated stimulation of the Akt/mitogen-activated protein kinase pathway that, for example, increases VEGF. Microvesicles from xenografts of glioblastomas have been reported to transfer EGFRvIII to glioma cells lacking this phenotypic feature. Because the EGFRvIII expression remained stable in malignant cells to which EGFRvIII had been transferred, such transfer of oncogenic features could be a mechanism via which oncogenic features can horizontally spread among cells. In response to continuing exposure to exosomes, endothelial cells also transiently developed an EGFRvIII-expressing phenotype, resulting in increased growth and, hence, probably increased angiogenesis. The phenotypic features of endothelial cells associated with increased levels of EGFRvIII were not stable and ended when the exposure to the TD-exosomes containing EGFRvIII ceased.90,91,108,109
Induction of an oncogenic phenotype via larger TD-microvesicles isolated from the MDA-MB-231 breast cancer cell line and the U87 glioma cell line also has been reported. Microvesicles released from these cell lines contained both tissue transglutaminase and fibronectin, a tissue transglutaminase substrate. These microvesicles caused NIH/3T3 fibroblast and benign breast epithelial cells, MCF10A, to develop some aspects of malignant behavior, such as anchorage-independent growth in agar and growth in medium with low concentrations of serum. Maintenance of these features of malignant behavior in nonmalignant cells also required continuous exposure to TD-microvesicles.114,115 Local effects of exosomes on primary neoplastic lesions are demonstrated in Figure 2.
Exosomes Facilitate Metastases
How and where tumors metastasize are affected by multiple molecular features, including functioning genes in primary tumors that stimulate or inhibit metastases.116 TD-exosomes from primary tumors promote the metastatic spread of tumors by inducing features at potential metastatic sites that attract neoplastic cells and build a matrix for their attachment, aid neoplastic cells at the metastatic site to survive, stimulate angiogenesis, and suppress immunity.19–22 Thus, there is likely a role of exosomes in the release of their chemotactic contents or the stimulation of nonneoplastic cells to release chemotactic molecules in attraction of malignant cells to potential sites of metastasis.
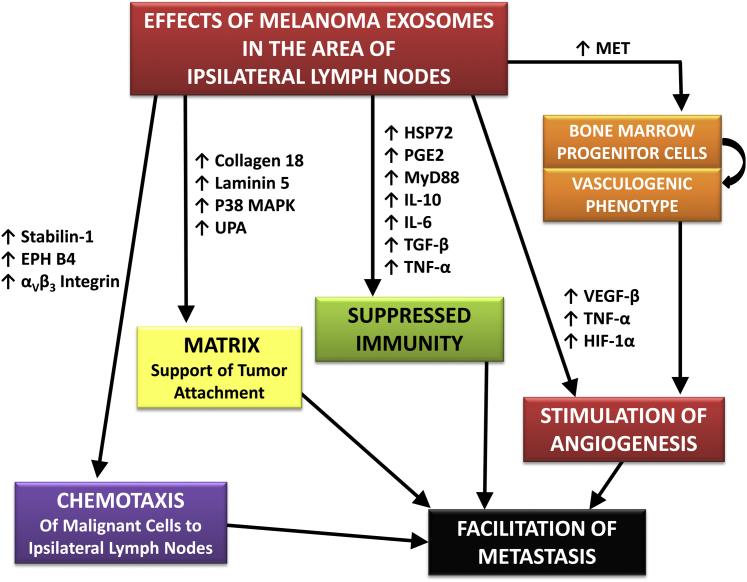
TD-exosomes induce molecular features that cause malignant cells to leave the primary tumor and, via chemotaxis, facilitate the migration of neoplastic cells to sentinel lymph nodes, where exosomes establish a supportive environment that promotes metastases. Specifically, exosomes from melanomas stimulate the production of αvβ3 integrin, ephrin receptor β4, and stabilin 1, which act to recruit melanoma cells to the ipsilateral sentinel lymph node. Exosomes from melanomas also induce the production of collagen 18, laminin 5, mitogen-activated protein kinase (p38), and urokinase plasminogen activator protease, which aid in the production of the matrix necessary to support the growth of metastatic cells. In addition, exosomes also increase nodal metastases by inducing angiogenesis via inducing the expression of VEGFβ, TNFα, and hypoxia-inducible factor 1α19; similarly, exosomes affect the programmed development of some BMDCs via up-regulation of MET to produce a provasculogenic phenotype that facilitates angiogenesis and metastasis.21,22 Figure 3 demonstrates some of the features that facilitate metastases to ipsilateral lymph nodes.
Figure 3.
A model for the effects of TD-exosomes on metastasis based on studies of metastases of melanomas. HSP72, heat shock protein 72; JAK3, Janus kinase 3; MAPK, mitogen-activated protein kinase; MyD88, myeloid differentiation primary response 88; PGE2, prostaglandin E2; pStat3, phosphorylated signal transducer and activator of transcription 3; UPA, urokinase plasminogen activator; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α.
TD-exosomes may interact with soluble factors from tumors to facilitate metastases. For example, when the conditioned media from cultures of cells of a syngeneic pancreatic tumor model were separated into exosomal and soluble components, exosomes were more effective than the soluble factors in increasing metastases to lymph nodes and/or lung; however, the combined soluble and exosomal fractions were more effective in increasing metastases.20 The facilitation of metastases by exosomes was greatly decreased when there was knockdown of CD44 splice variants, especially CD44v6, on which production of matrix depends. This matrix, which supports the attachment and growth of metastatic cells, contained c-Met and urokinase-type plasminogen activator receptor,20 which have been reported to increase metastases.21,22
Much of the work on how exosomes affect metastases is based on the responses of melanomas to TD-exosomes/microvesicles from cell lines grown in vitro in standard two-dimensional cultures. There needs to be increased characterization of the effects of exosomes in vivo in providing autocrine, paracrine, and endocrine signals that modulate the growth, progression, and metastases of tumors other than melanomas.
TD-Exosomes Increase the Resistance of Tumors to Therapies
Exosomes normally function in the export of waste products and less-needed molecules from cells and many harmful products, such as exogenous molecules.4,23,49 In cancers, exosomes also function to export chemotherapeutic drugs, such as cisplatin and doxorubicin; thus, TD-exosomes are used as a pathway of chemoresistance of specific malignant cells to specific drugs. All drugs are not exported from cells. For example, TD-exosomes do not export 5-fluorouracil efficiently. When factors associated with the shedding of exosomes were combined into a vesicle-shedding index, the vesicle shedding index of the National Cancer Institute 60-cell line panel was found to be positively correlated with 50% growth inhibition for most of the 171 compounds of the National Cancer Institute Standard Anticancer Agent Database. Also, the actual shedding rate of microvesicles from six cell lines correlated positively with doxorubicin resistance; however, there was no correlation of vesicle shedding with chemoresistance to 5-fluorouracil.23
The secretion of drugs in exosomes, such as doxorubicin, may involve vacuolar protein sorting 4a, which is important in the secretion of exosomes. Specifically, disruption of vacuolar protein sorting 4a in the erythroleukemic cell line, K562, inhibited the efflux of doxorubicin.117 Thus, exosomes are likely to be a major factor in the chemoresistance of malignant cells to a variety of, but not all, chemotherapeutic drugs.
The Potential Clinical Impact of Exosomes
The molecular features of TD-exosomes mirror many of the molecular features of the tumors from which they arise. TD-exosomes have been reported to contain biomarkers characteristic of tumors, including those of the bladder, brain, colorectum, kidney, and melanomas, so the presence of biomarkers characteristic of tumors in exosomes of biological fluids may aid in clinical decisions and in translational research on biomarkers. Thus, TD-exosomes may be important in clinical decisions, including risk assessment, early detection, and diagnosis, in the prediction of therapeutic efficacy, and in determining prognosis. They may also be important as surrogate end points in evaluating chemotherapeutic, preventive, and novel therapies. Of special importance in translational research is that exosomes are carried in blood and are shed into biological fluids, such as ascites and pleural fluids, all of which can be obtained easily for clinical use. As discussed, the measurements of biomarkers in exosomes from bodily fluids are likely to be just as sensitive and specific as measurements of the same biomarkers in the matching bodily fluids. When specific biomarkers or biomarker panels (signatures) are more concentrated in exosomes, their measurements in the exosomal fraction may be more effective in solving clinical problems than their measurements in matching bodily fluids.90,91,108,109 However, because exosomes and microvesicles and their contents are essentially invisible, use of the exosomes/microvesicles in translational research is in its infancy.
A biomarker of ovarian epithelial tumors, Claudin 4, has been found to be increased in exosomes separated from the blood of patients with ovarian carcinoma,118 and transmembrane protease, serine 2-ETS related gene, δ-catenin, and prostate cancer antigen 3, which are potential biomarkers for cancers of the prostate, have been measured in TD-exosomes isolated from the urine samples of patients with prostate cancer.119 Similarly, miRNAs may aid in the early diagnosis of cancers of the lung via their measurement in TD-exosomes.120 Biomarkers measured in exosomes also may be useful in measuring responses to therapy.
Some TD-exosomes that contain increased concentrations of specific tumor antigens could be used to stimulate DCs to secrete exosomes that initiate cytotoxic T-lymphocytic responses.5,6 The generation of exosomes by DCs in vitro would be an approach that could avoid the negative effects (eg, reduction of immunity) of using TD-exosomes in vivo. To date, the clinical results of using exosomes in cancer therapy have been modest, but there have been few major adverse effects.10–12
Because of the suppression of immune surveillance by TD-exosomes, TD-exosomes could be targeted to reduce the suppression of immunity in patients with cancers. Strategies have been proposed to increase immune reactions to cancer by reducing the transfer to TD-exosomes of molecules that suppress the immune system. Alternatively, molecules that may stimulate immune cells might be introduced into TD-exosomes. Similarly, the function of TD-exosomes in cellular export of drugs could be targeted to increase the effectiveness of many drugs by reducing the release of TD-exosomes via changing local pH or targeting the pathways involving TD-exosomes, such as the VSP4a signaling pathway.10–12,117,121 TD-exosomes also could be decreased in blood using a hemopurifier that selectively removes TD-exosomes from blood by immobilized antibodies that bind to the surface molecules of TD-exosomes.
Because TD-exosomes can be absorbed specifically by neoplastic cells, they could deliver drugs, preventive agents, small molecules (eg, miRNAs), and agents of gene therapy to the cells of specific tumors.122–126 Curcumin is a natural product with anti-tumor and anti-inflammatory features whose bioavailability and therapeutic efficacy is limited because of poor solubility. Its clinical usefulness is improved by the incorporation of curcumin into exosomes, which were much more effective than liposomes containing curcumin in preventing septic shock in a murine model.122–124 Both drugs and miRNAs could be delivered specifically by exosomes to neoplastic cells.125,126
The clinical uses of microvesicles are being studied by commercial and academic organizations as vehicles to deliver chemotherapy, small molecules, agents of gene therapy, and/or prevention to target cells more specifically than systemic administration. Thus, multiple approaches to the selective delivery of therapeutic and preventive agents via biological microvesicles are in active development.
Summary
Exosomes and related microvesicles compose a newly identified method of local and distant intercellular communication. Tumors have hijacked this mechanism of intercellular communication to aid in their growth, progression, and dissemination. TD-exosomes can act to produce a fertile environment to support growth at primary sites of neoplastic lesions and potential sites of metastases. TD-exosomes also may facilitate tumor growth and dissemination by inhibiting immune surveillance and by increasing chemoresistance via removal of chemotherapeutic drugs. Thus, TD-exosomes might be potential targets for therapeutic interventions via their modification or removal. Exosomes and related microvesicles also could serve as specific delivery vehicles to neoplastic lesions of drugs, small molecules, or agents of prevention and gene therapy. In other clinical approaches, TD-exosomes could serve as a subcompartment in which biomarkers could be measured to aid in the early detection and diagnosis of diseases, determining prognosis, prediction of therapeutic efficacy, and therapeutic responses. Such approaches would use the molecular features of TD-exosomes that are different from exosomes of either associated or diseased controls, or from normal individuals.
Footnotes
Supported, in part, by the Susan G. Komen for the Cure grants BCTR0707323 (H.-G.Z.) and BCTR0600484 (W.E.G.), NIH grants R01CA116092, R01CA107181, R01AT004294, and R01CA137037 (H.-G.Z.), the Breast (5P50CA089019), Pancreatic (2P50CA101955), and Cervical (5P50CA098252) Specialized Program of Research Excellence at University of Alabama at Birmingham (W.E.G.), Department of Defense grant PC093309 (W.E.G.), U54 Morehouse School of Medicine/Tuskegee University/University of Alabama at Birmingham Comprehensive Cancer Center Partnership grant 2U54CA118948 (W.E.G.), University of Alabama at Birmingham Skin Diseases Research Center grant P30AR50948-04 (W.E.G.), and University of South Alabama/NIH grant 1R01CA155638-01A1 (W.E.G.).
Disclosure: A guest editor acted as the Editor-in-Chief for this manuscript. No person at the University of Alabama at Birmingham was involved in the peer review process or final disposition for this article.
References
- 1.Tauro B.J., Greening D.W., Mathias R.A., Mathivanan S., Ji H., Simpson R.J. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics. 2013;12:587–598. doi: 10.1074/mcp.M112.021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koga K., Matsumoto K., Akuyoshi T., Kubo M., Yamanaka N., Tasaki A., Nakashima H., Makamura M., Kuroki S., Tanaka M., Katano M. Purification, characterization and biological significance of tumor-derived exosomes. Anticancer Res. 2005;25:3703–3707. [PubMed] [Google Scholar]
- 3.Trams E.G., Lauter C.J., Salem N., Jr., Heine U. Exfoliation of membrane ecto-enzymes in the form of microvesicles. Biochim Biophys Acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 4.Johnstone R.M., Adam J.R., Hammond L.O., Turbide C. Vesicle formation during reticulocyte maturation: association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 5.Wolfers J., Lozier A., Raposo G., Regnault A., Thery C., Masurier C., Flament C., Pouzieux S., Faure F., Tursz T., Angevin E., Amigorena S., Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 6.Clayton A., Court J., Navabi H., Adams M., Mason M.D., Hobot J.A., Newman G.R., Jasani B. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J Immunol Methods. 2001;247:163–174. doi: 10.1016/s0022-1759(00)00321-5. [DOI] [PubMed] [Google Scholar]
- 7.Thery C., Boussac M., Veron P., Ricciardi-Castagnoli P., Raposo G., Garin J., Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 8.Thery C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 9.Escoloa J.M., Kleijmeer M.J., Stoorvogel W., Griffith J.M., Yoshie O., Geuze H.J. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B lymphocytes. J Biol Chem. 1998;273:20121. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H.-G., Grizzle W.E. Exosomes and cancer: a newly described pathway of immune suppression. Clin Cancer Res. 2011;17:1–6. doi: 10.1158/1078-0432.CCR-10-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H.-G., Liu Y., Deng Z.-B., Liu C., Xiang X., Grizzle W.E. Exosomes and immune surveillance of neoplastic lesions: a review. Biotech Histochem. 2012;87:161–168. doi: 10.3109/10520291003659042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H.G., Grizzle W.E. The effects of exosomes and related vesicles on cancer development, progression and dissemination. Emerging Concepts of Tumor Exosomes-Mediated Cell-Cell Communication. In: Zhang H.-G., editor. Springer Science; New York: 2013. pp. 107–129. [Google Scholar]
- 13.Park J.E., Tan H.S., Datta A., Lai R.C., Zhang H., Meng W., Lim S.K., Sze S.K. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9:1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellana D., Zobairi F., Martinez M.C., Panaro M.A., Mitolo V., Freyssinet J.M., Kunzelmann C. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res. 2009;69:785–793. doi: 10.1158/0008-5472.CAN-08-1946. [DOI] [PubMed] [Google Scholar]
- 15.Webber J., Steadman R., Mason M.D., Tabi Z., Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 16.Hong B.S., Cho J.H., Kim H., Choi E.J., Rho S., Kim J., Kim J.H., Choi D.S., Kim Y.K., Hwang D., Gho Y.S. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 2009;10:556. doi: 10.1186/1471-2164-10-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hood J.L., Pan H., Lanza G.M., Wickline S.A., Consortium for Translational Research in Advanced Imaging and Nanomedicine (C-TRAIN) Paracrine induction of endothelium by tumor exosomes. Lab Invest. 2009;89:1317–1328. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gesierich S., Berezovskly I., Ryschich E., Zöller M. Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res. 2006;66:7083–7094. doi: 10.1158/0008-5472.CAN-06-0391. [DOI] [PubMed] [Google Scholar]
- 19.Hood J.L., San R.S., Wickline S.A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 20.Jung T., Castellana D., Klingbell P., Hernández I.C., Vitacolonna M., Orlicky D.J., Roffler S.R., Brodt P., Zöller M. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009;11:1093–1105. doi: 10.1593/neo.09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Péinado H., Alečković M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., Garcia-Santos G., Ghajar C.M., Nitadori-Hoshino A., Hoffman C., Badal K., Garcia B.A., Callahan M.K., Yuan J., Martins V.R., Skog J., Kaplan R.N., Brady M.S., Wolchok J.D., Chapman P.B., Kang Y., Bromberg J., Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H.G., Garcia-Santos G., Peinado H., Lyden D.C. Microenvironmental regulation of metastasis by exosomes. In: Zhang H.-G., editor. Emerging Concepts of Tumor Exosome-Mediated Cell-Cell Communication. Springer Science; New York: 2013. pp. 181–201. [Google Scholar]
- 23.Shedden K., Xie X.T., Chandaroy P., Chang Y.T., Rosania G.R. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–4337. [PubMed] [Google Scholar]
- 24.Poliakov A., Spilman M., Dokland T., Amling C.L., Mobley J.A. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate. 2009;69:159–167. doi: 10.1002/pros.20860. [DOI] [PubMed] [Google Scholar]
- 25.Palanisamy V., Sharma S., Deshpande A., Zhou H., Gimzewski J., Wong D.T. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS One. 2010;5:e8577. doi: 10.1371/journal.pone.0008577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor D.D., Gerçel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer. 2005;92:305–311. doi: 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clayton A., Turkes A., Navabi H., Mason M.D., Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118:3631–3638. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- 28.Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., de Milito A., Coscia C., Iessi E., Logozzi M., Molinari A., Colone M., Tatti M., Sargiacomo M., Fais S. Microenvironment pH is a key factor for exosomes traffic in tumor cells. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taraboletti G., D’Ascenzo S., Giusti I., Marchetti D., Borsotti P., Millimaggi D., Giavazzi R., Pavan A., Dolo V. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia. 2006;8:96–103. doi: 10.1593/neo.05583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savina A., Furlán M., Vical M., Colombo M.I. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 31.Graner M.W., Cumming R.I., Bigner D.D. The heat shock response and chaperones/heat shock proteins in brain tumors: surface expression, release, and possible immune consequences. J Neurosci. 2007;27:11214–11227. doi: 10.1523/JNEUROSCI.3588-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koumangoye R.B., Sakwe A.M., Goodwin J.S., Patel T., Ochieng J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS One. 2011;6:e24234. doi: 10.1371/journal.pone.0024234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenassi M., Cagney G., Liao M., Vaupotič T., Batholomeeusen K., Cheng Y., Krogan N.J., Plemenitaš A., Peterlin B.M. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ cells. Traffic. 2010;11:110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu X., Harris S.L., Levine A.J. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 35.Lespagnol A., Duflaut D., Beekman C., Blanc L., Fiucci G., Marine J.C., Vidal M., Amson R., Telerman A. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 2008;15:1723–1733. doi: 10.1038/cdd.2008.104. [DOI] [PubMed] [Google Scholar]
- 36.Lo Cicero A., Raposo G. The cell biology of exosomes: historical and perspectives. In: Zhang H.-G., editor. Emerging Concepts of Tumor Exosomes-Mediated Cell-Cell Communication. Springer Science+Business Media; New York: 2013. pp. 1–32. [Google Scholar]
- 37.Baietti M.F., Zhang Z., Mortier E., Melchio A., Degeest G., Geeraerts A., Ivarsson Y., Depoortere F., Coomans C., Vermeiren E., Zimmermann P., David G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 38.Wollert T., Hurley J.H. Molecular mechanism of multivesciular body biopsies by ESCRT complexes. Nature. 2010;464:864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trajkovic K., Hsh C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B., Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 40.Thompson C.A., Purushothaman A., Ramani V.C., Vlodavsky I., Sanderson R.D. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem. 2013;288:10093–10099. doi: 10.1074/jbc.C112.444562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skokos D., Botros H.G., Demeure C., Morin J., Peronet R., Birkenmeier G., Boudaly S., Mécheri S. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol. 2003;170:3037–3045. doi: 10.4049/jimmunol.170.6.3037. [DOI] [PubMed] [Google Scholar]
- 42.Morelli A.E., Larregina A.T., Shufesky W.J., Sullivan M.M.L.G., Sullivan D.B.S., Papworth G.D., Zahorchak A.F., Logar A.J., Wang Z., Watkins S.C., Falo L.D., Jr., Thomson A.W. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 43.Van Niel G., Mallegol J., Bevilacqua C., Candalh C., Brugière S., Tomaskovic-Crook E., Heath J.K., Cerf-Besussan N., Heyman M. Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut. 2003;52:1690–1697. doi: 10.1136/gut.52.12.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nazarenko I., Rana S., Baumann A., McAlear J., Hellwig A., Trendelenburg M., Lochnit G., Preissner K.L., Zöller M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 45.Caby M.P., Lankar D., Vincendeau-Scherrer C., Raposo G., Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 46.Logozzi M., De Milito A., Lugini L., Borghi M., Calabrò L., Spada M., Perdicchio M., Marino M.L., Federici C., Iessi E., Brambilla D., Venturi G., Lozupone F., Santinami M., Huber V., Maio M., Rivoltini L., Fais S. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009;4:e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rana S., Zöller M. The Functional Importance of Tetraspanins in Exosomes Emerging Concepts of Tumor Exosomes-Mediated Cell-Cell Communication. In: Zhang Z.-H., editor. Springer Science+Business Media; New York: 2013. pp. 69–106. [Google Scholar]
- 48.Blanc L., De Gassart A., Geminard C., Bette-Bobillo P., Vidal M. Exosome release by reticulocytes—an integral part of the red blood cell differentiation system. Blood Cells Mol Dis. 2005;35:21–26. doi: 10.1016/j.bcmd.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Safaei R., Larson B.J., Cheng T.C., Gibson M.A., Otani S., Naerdemann W., Howell S.B. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4:1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 50.Barrės C., Blanc L., Bette-Bobillo P., André S., Marmoun R., Gabius H.-J., Vidal M. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010;115:696–705. doi: 10.1182/blood-2009-07-231449. [DOI] [PubMed] [Google Scholar]
- 51.Lässer C., Alikhani V.S., Ekström K., Eldh M., Paredes P.T., Bossios A., Sjöstrand M., Gabrielsson S., Lötvall J., Valadi H. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9:9. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 53.Zomer A., Vendrig T., Hopmans E.S., van Eijndhoven M., Middeldorp J.M., Pegtel D.M. Exosomes: fit to deliver small RNA. Commun Integr Biol. 2010;3:447–450. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welton J.L., Khanna S., Giles P.J., Brennan P., Brewis I.A., Staffurth J., Mason M.D., Clayton A. Proteomics analysis of bladder cancer exosomes. Mol Cell Proteomics. 2010;9:1324–1338. doi: 10.1074/mcp.M000063-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrington M.G., Fonteh A.H., Oberins E., Lise P., Cowan R.P., McComb G., Chavez J.N., Rush J., Biringer R.G., Hühmer A.F. The morphology and biochemistry of nanostructures provide evidence for synthesis and signaling functions in human cerebrospinal fluid. Cerebrospinal Fluid Res. 2009;6:10. doi: 10.1186/1743-8454-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McNally L.R., Manne U., Grizzle W.E. Post-transcriptional processing of genetic information and its relation to cancer. Biotech Histochem. 2013;88:365–372. doi: 10.3109/10520295.2012.730152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones J., Grizzle W.E., Wang H., Yates C. MicroRNAs that affect prostate cancer: emphasis on prostate cancer in African Americans. Biotech Histochem. 2013;88:410–424. doi: 10.3109/10520295.2013.807069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turchinovich A., Weiz L., Langheinz A., Burwinkel B. Characterization of extracelluar circulating microRNA. Nucleic Acids Res. 2011;39:7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arroyo J.D., Chevillet J.R., Kroh E.M., Ruf I.K., Pritchard C.C., Gibson D.F., Mitchell P.S., Bennett C.F., Pogosova-Agadjanyan E.L., Stirewalt D.L., Tait J.F., Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gallo A., Tandon M., Alevizos I., Illei G.G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7:e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belting M., Wittrup A. Nanotubes, exosomes, and nucleic acid-binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: implications in health and disease. J Cell Biol. 2008;183:1187–1191. doi: 10.1083/jcb.200810038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hegmans J.P., Bard M.P.L., Hemmes A., Luider T.M., Klejimeer M.J., Prins J.-B., Zitvogel L., Burgers S.A., Hoogsteden H.C., Lambrecht B.N. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Pathol. 2004;164:1807–1815. doi: 10.1016/S0002-9440(10)63739-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montpellier C., Tews B.A., Poitrimole J., Rocha-Perugini V., D’Arienzo V., Potel J., Zhang X.A., Rubinstein E., Dubuisson J., Cocquerel L. Interacting region of CD81 and two of its partners, EWI-2 and EWI-2wint, and their effect on hepatitis C virus infection. J Biol Chem. 2011;286:13954–13965. doi: 10.1074/jbc.M111.220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y., Shah S.V., Xiang X., Wang J., Deng Z.B., Liu C., Zhang L., Wu J., Edmonds T., Jambor C., Kappes J.C., Zhang H.G. COP9-associated CSN5 regulates exosomal protein deubiquitination and sorting. Am J Pathol. 2009;174:1415–1425. doi: 10.2353/ajpath.2009.080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deng Z., Pardi R., Cheadle W., Xiang X., Zhang S., Shah S.V., Grizzle W., Miller D., Mountz J., Zhang H-G. Plant homologue constitutive photomorphogenesis 9 (COP9) signalosome subunit CSN5 regulates innate immune responses in macrophages. Blood. 2011;117:4796–4804. doi: 10.1182/blood-2010-10-314526. [DOI] [PubMed] [Google Scholar]
- 66.Admyre C., Grunewald J., Thyberg S., Gripenbäck S., Tornling G., Eklund A., Scheynius A., Gabrielsson S. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J. 2003;22:578–583. doi: 10.1183/09031936.03.00041703. [DOI] [PubMed] [Google Scholar]
- 67.Skriner K., Adolph K., Jungblut P.R., Burmester G.R. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 2006;54:3809–3814. doi: 10.1002/art.22276. [DOI] [PubMed] [Google Scholar]
- 68.Andre F., Schartz N.E., Movassagh M., Flament C., Pautier P., Morice P., Pomel C., Lhomme C., Escudier B., Le Chevalier T., Tursz T., Amigorena S., Raposo G., Angevin E., Zitvogel L. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 69.Chang S., Lau D.T., Wong W. Breast cancer exosome-like microvesicles and salivary gland cells interplay alters salivary gland cell-derived exosome-like microvesicles in vitro. PLoS One. 2012;7:e33037. doi: 10.1371/journal.pone.0033037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sabapatha A., Gerçel-Taylor C., Taylor D.D. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol. 2006;56:345–355. doi: 10.1111/j.1600-0897.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 71.Taylor D.D., Akyol S., Gerçel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176:1534–1542. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 72.Hedlund M., Stenqvist A.-C., Nagaeva O., Kjellberg L., Wulff M., Baranov V., Mincheva-Nilsson L. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol. 2009;183:340–351. doi: 10.4049/jimmunol.0803477. [DOI] [PubMed] [Google Scholar]
- 73.Mincheva-Nilsson L. Immune cells and molecules in pregnancy: friends or foes to the fetus? Exp Rev Clin Immunol. 2006;2:457–470. doi: 10.1586/1744666X.2.3.457. [DOI] [PubMed] [Google Scholar]
- 74.Toth B., Lok C.A., Böing A., van der Post J.A., Friese K., Nieuwland R. Microparticles and exosomes: impact on normal and complicated pregnancy. Am J Reprod Immunol. 2007;58:389–402. doi: 10.1111/j.1600-0897.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 75.Bullerdiek J., Flor I. Exosome-delivered microRNAs of “chromosome 19 microRNA cluster” as immunomodulation in pregnancy and tumorigenesis. Mol Cytogenet. 2012;5:27. doi: 10.1186/1755-8166-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Donker R., Mouillet J.F., Chu T., Hubel C.A., Stolz D.B., Morelli A.E., Sadovsky Y. The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol Hum Reprod. 2012;18:417–424. doi: 10.1093/molehr/gas013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim S.H., Bianco N., Menon R., Lechman E.R., Shufesky W.J., Morelli A.E., Robbins P.D. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol Ther. 2006;13:289–300. doi: 10.1016/j.ymthe.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 78.Abusamra A.J., Zhong Z., Zheng X., Li M., Ichim T.E., Chin J.L., Min W.P. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis. 2005;35:169–173. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 79.Taylor D.D., Gerçel-Taylor C., Lyons K.S., Stanson J., Whiteside T.L. T-cell apoptosis and suppression of T-cell receptor/CD3-ζ by fas ligand-containing membrane vesicles shed from ovarian tumors. Clin Cancer Res. 2003;9:5113–5119. [PubMed] [Google Scholar]
- 80.Théry C., Duban L., Seguar E., Véron P., Lantz O., Amigorena S. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 81.Vincent-Schneider H., Stumptner-Cuvelette P., Lankar D., Pain S., Raposo G., Benaroch P., Bonnerot C. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol. 2002;14:713–722. doi: 10.1093/intimm/dxf048. [DOI] [PubMed] [Google Scholar]
- 82.Andre F., Chaput N., Schartz N.E.C., Flament C., Aubert N., Bernard J., Lemonnier F., Raposo G., Escudier B., Hsu D.-H., Tursz T., Amigorena S., Angevin E., Zitvogel L. Exosomes are potent cell-free peptide-based vaccine, I: dendritic cell-derived exosomes transfer functional MHC class 1/peptide complexes to dendritic cells. Blood. 2004;172:2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 83.Chaput N., Schartz N.E.C., André F., Taïeb J., Novault S., Bonnaventure P., Aubert N., Bernard J., Lemonnier F., Merad M., Adema G., Adams M., Ferrantini M., Carpentier A.F., Escudier B., Tursz T., Angevin E., Zitvogel L. Exosomes as potent cell-free peptide-based vaccine, II: exosomes in CpG adjuvants efficiently prime naïve Tc1 lymphocytes leading to tumor rejection. J Immunol. 2004;172:2137–2146. doi: 10.4049/jimmunol.172.4.2137. [DOI] [PubMed] [Google Scholar]
- 84.Qazo K.R., Gehrmann U., Jordö E.D., Karlsson M.C.I., Gabrielsson S. Antigen-loaded exosomes alone induce Th 1-type memory through a B cell-dependent mechanism. Blood. 2009;113:2673–2683. doi: 10.1182/blood-2008-04-153536. [DOI] [PubMed] [Google Scholar]
- 85.Wang G.J., Liu Y., Qin A., Shah S.V., Deng Z.B., Xiang X., Cheng Z., Liu C., Wang J., Zhang L., Grizzle W.E., Zhang H.G. Thymus exosomes-like particles induce regulatory T cells. J Immunol. 2008;181:5242–5248. doi: 10.4049/jimmunol.181.8.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang H.G., Liu C., Su K., Yu S., Zhang L., Zhang S., Wang J., Cao X., Grizzle W., Kimberly R.P. A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J Immunol. 2006;176:7385–7393. doi: 10.4049/jimmunol.176.12.7385. [DOI] [PubMed] [Google Scholar]
- 87.Zhang H.G., Grizzle W.E. The effects of exosomes and related vesicles on cancer development, progression and dissemination. In: Zhang H.G., editor. Emerging Concepts for Tumor Exosome-Mediated Cell-Cell Communication. Springer Science; New York, New York: 2013. pp. 107–129. [Google Scholar]
- 88.Kim S.-H., Lechman E.R., Bianco N., Menon R., Keravala A., Nash J., Mi Z., Watkins S.C., Gambotto A., Robbins P.D. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol. 2005;174:6440–6448. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- 89.Cai Z., Zhang W., Yang F., Yu L., Yu Z., Pan J.H., Wang L., Cao X., Wang J. Immunosuppressive exosomes from TGF-β1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells. Cell Res. 2012;22:607–610. doi: 10.1038/cr.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Skog J., Würdinger T., van Rijn S., Meijer D.H., Gainche L., SenaEsteves M., Curry W.T., Jr., Carter B.S., Krichevsky A.M., Breakefield X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Graner M.W., Alzate O., Dechkovskaia A.M., Keene J.D., Sampson J.H., Mitchell D.A., Bigner D.D. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009;23:1541–1557. doi: 10.1096/fj.08-122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao D., Ohlendorf J., Chan Y., Taylor D.D., Rai S.N., Weigel S., Zacharias W., Hao H., McMasters K.M. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One. 2012;7:e46874. doi: 10.1371/journal.pone.0046874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu C., Yu S., Zinn K., Wang J., Zhang L., Yujiang J., Kappes J.C., Barnes S., Kimberly R.P., Grizzle W.E., Zhang H-G. Murine mammary carcinomas exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176:1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 94.Iero M., Valenti R., Huber V., Filipazzi P., Parmiani G., Fais S., Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 95.Kim J.W., Wieckowski E., Taylor D.D., Reichert T.E., Watkins S., Whiteside T.L. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Cancer Res. 2005;11:1010–1020. [PubMed] [Google Scholar]
- 96.Clayton A., Mitchell J.P., Court J., Linnane S., Mason M.D., Taby Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180:7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 97.Espinoza L., Takami A., Yoshioka K., Nakata K., Sato T., Kasahara Y., Nakao S. Human microRNA-1245 down-regulates the NKG2D-mediated functions. 2012;97:1295–1303. doi: 10.3324/haematol.2011.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu S., Liu C., Su K., Wang J., Liu Y., Zhang L., Li C., Cong Y., Kimberly R., Grizzle W.E., Falkson C., Zhang H.G. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 99.Valenti R., Huber V., Filipazzi P., Pilla L., Sovena G., Villa A., Corbelli A., Fais S., Parmiani G., Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-β-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66:9290–9298. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 100.Valenti R., Huber V., Ieor M., Filipazzi P., Parmiani G., Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67:2912–2915. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 101.Xiang X., Poliakov A., Liu C., Liu Y., Deng Z.B., Wang J., Cheng Z., Shah S.V., Wang G.J., Zhang L., Grizzle W.E., Mobley J., Zhang H.G. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124:2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu C., Yu S., Kappes J., Wang J., Grizzle W.E., Zinn K.R., Zhang H.G. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336–4342. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ochoa A.C., Zea A.H., Hernandez C., Rodriguez P.C. Arginase, prostaglandins and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–736s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y., Xiang X., Zhang X., Zhang S., Liu C., Cheng Z., Michalek S.M., Grizzle W.E., Zhang H.G. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol. 2010;176:2490–2499. doi: 10.2353/ajpath.2010.090777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiang X., Liu Y., Zhuang X., Zhang S., Michalek S., Taylor D.D., Grizzle W., Zhang H-G. TLR2-mediated expansion of MDSCs is dependent on the source of tumor exosomes. Am J Pathol. 2010;177:1606–1610. doi: 10.2353/ajpath.2010.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Szajnik M., Czystowska M., Szczepanski M.J., Mandapathil M., Whiteside T.L. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg) PLoS One. 2010;5:e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.AL-Nedawi K., Meehan B., Micallef J., Lhotak V., May L., Guha A., Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 109.Al-Nedawi K., Meehan B., Kerbel R.S., Allison A.C., Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ristorcelli E., Beraud E., Verrando P., Villard C., Lafitte D., Sbarra V., Lombardo D., Verine A. Human tumor nanoparticles induce apoptosis of pancreatic cancer cells. FASEB J. 2008;22:3358–3369. doi: 10.1096/fj.07-102855. [DOI] [PubMed] [Google Scholar]
- 111.Ristorcelli E., Beraud E., Mathieu S., Lombardo D., Verine A. Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. 2009;125:1016–1026. doi: 10.1002/ijc.24375. [DOI] [PubMed] [Google Scholar]
- 112.Qu J.L., Qu X.J., Zhao M.F., GTeng J.E., Zhang Y., Hou K.Z., Jiang Y.H., Yang X.H., Liu Y.P. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Dig Liver Dis. 2009;41:875–880. doi: 10.1016/j.dld.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 113.Khan S., Jutzy J.M.S., Aspe J.R., McGregor D.W., Neidigh J.W., Wall N.R. Survivin is released from cancer cells via exosomes. Apoptosis. 2011;16:1–12. doi: 10.1007/s10495-010-0534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Antonyak M.A., Li B., Boroughs L.K., Johnson J.L., Druso J.E., Bryant K.L., Holowks D.A., Cerione R.A. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci U S A. 2011;12:4852–4857. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deng Z., Cheng Z., Xiang X., Yan J., Zhuang X., Lkiu C., Jiang H., Ju S., Zhang L., Grizzle W., Mobley J., Roman J., Miller D., Zhang H.G. Tumor cell cross talk with tumor-associated leukocytes leads to induction of tumor exosomal fibronectin and promotes tumor progression. Am J Pathol. 2012;180:390–398. doi: 10.1016/j.ajpath.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 116.McNally L.R., Welch D.R., Beck B.H., Stafford L.J., Long J.W., Sellers J.C., Huang Z.Q., Grizzle W.E., Stockard C.R., Nash K.T., Buchsbaum D.J. KISS1 over-expression suppresses metastasis of pancreatic adenocarcinoma in a xenograft mouse model. Clin Exp Metastasis. 2010;27:591–600. doi: 10.1007/s10585-010-9349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen Y.Y., Posada M.M., Blazer L.L., Zhao T., Rosania G.R. The role of the VPS4A-exosome pathway in the intrinsic egress route of a DNA-binding anticancer drug. Pharm Res. 2006;23:1687–1695. doi: 10.1007/s11095-006-9043-0. [DOI] [PubMed] [Google Scholar]
- 118.Li J., Sherman-Baust C.A., Tsai-Turton M., Bristow R.E., Roden R.B., Morin P. Claudin-containing exosomes in the peripheral circulation of women with ovarian cancer. BMC Cancer. 2009;9:244. doi: 10.1186/1471-2407-9-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nilsson J., Skog J., Nordstrand A., Baranov V., Mincheva-Nilsson L., Breakefield X.O., Widmark A. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100:1603–1607. doi: 10.1038/sj.bjc.6605058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rabinowits G., Gercel-Taylor C., Day J.M., Taylor D.D., Kloecker G.H. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10:42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 121.Iessi E., Marino M.L., Lozupone F., Fais S., de Milito A. Tumor acidity and malignancy: novel aspects in the design of anti-tumor therapy. Cancer Ther. 2008;6:55–66. [Google Scholar]
- 122.Zhang H.G., Kim H., Liu C., Yu S., Wang J., Grizzle W.E., Kimberly R.P., Barnes S. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim Biophys Acta. 2007;1773:1116–1123. doi: 10.1016/j.bbamcr.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Narayanan N.K., Nargi D., Randolph C., Narayanan B.A. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer. 2009;125:1–8. doi: 10.1002/ijc.24336. [DOI] [PubMed] [Google Scholar]
- 124.Sun D., Zhuang X., Xiang X., Liu Y., Zhang S., Liu C., Barnes S., Grizzle W., Miller D., Zhang H.G. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun D., Zhuang X., Zhang S., Deng Z.B., Grizzle W., Miller D., Zhang H.G. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv Drug Deliv Rev. 2013;65:342–347. doi: 10.1016/j.addr.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 126.Ohno S.-I., Takanashi M., Sudo K., Ueda S., Ishikawa A., Matsuyama N., Fujita K., Mizutani T., Ohgi T., Ochiya T., Gotoh N., Kuroda M. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]