Abstract
Nucleus accumbens-associated protein 1 (NAC1), encoded by the NACC1 gene, is a transcription co-regulator that plays a multifaceted role in promoting tumorigenesis. However, the NAC1-regulated transcriptome has not been comprehensively defined. In this study, we compared the global gene expression profiles of NAC1-overexpressing SKOV3 ovarian cancer cells and NAC1-knockdown SKOV3 cells. We found that NAC1 knockdown was associated with up-regulation of apoptotic genes and down-regulation of genes involved in cell movement, proliferation, Notch signaling, and epithelial-mesenchymal transition. Among NAC1-regulated genes, FOXQ1 was further characterized because it is involved in cell motility and epithelial-mesenchymal transition. NAC1 knockdown decreased FOXQ1 expression and promoter activity. Similarly, inactivation of NAC1 by expression of a dominant-negative construct of NAC1 suppressed FOXQ1 expression. Ectopic expression of NAC1 in NACC1 null cells induced FOXQ1 expression. NAC1 knockdown resulted in decreased cell motility and invasion, whereas constitutive expression of FOXQ1 rescued motility in cells after NAC1 silencing. Moreover, in silico analysis revealed a significant co–up-regulation of NAC1 and FOXQ1 in ovarian carcinoma tissues. On the basis of transcription profiling, we report a group of NAC1-regulated genes that may participate in multiple cancer-related pathways. We further demonstrate that NAC1 is essential and sufficient for activation of FOXQ1 transcription and that the role of NAC1 in cell motility is mediated, at least in part, by FOXQ1.
Nucleus accumbens-associated protein 1 (NAC1) is a member of the bric-a-brac–tramtrack–broad (BTB) family of proteins that functions as a transcriptional co-regulator. However, unlike other BTB family proteins, NAC1 lacks a DNA-binding domain. Therefore, NAC1 is thought to form a complex with other DNA-binding co-factors to form a higher-order transcription complex.1 NAC1 participates in various biological processes, including maintenance of pluripotency in embryonic stem cells,2 regulation of acute psychomotor stimulant responses in mice,3 control of bony patterning in murine vertebral column,4 and promotion of tumor development.5 On the basis of analysis of The Cancer Genome Atlas (TCGA) ovarian cancer data, we identified NACC1, the gene encoding NAC1, as one of the most significant genes that shows a positive correlation between DNA and RNA copy number in human cancers,6 suggesting that NAC1 plays a driver role in cancer development. In fact, increased expression of NAC1 was found to be associated with disease aggressiveness, development of chemoresistance, and tumor recurrence in several types of human cancer, including ovarian, endometrial, and cervical carcinomas.5,7–14
Experimentally, abundant NAC1 protein is essential for migration and motility of cancer cells,13,15 maintenance of cell survival,5,9 prevention of cell senescence,16 and activation of autophagy in the presence of cisplatin through the high-mobility group B1 pathway.17 Containing a nuclear localization signal, NAC1 is predominantly located in the nucleus.18 However, cytosolic NAC1 can also be detected, especially during mitosis,19 suggesting that NAC1 may be involved in transcription-independent cellular events. To this end, we recently have demonstrated that NAC1 interacts with actin through the conserved BTB domain and that the NAC1-actin complex is crucial for effective cytokinesis in cancer cells.15
Given the significant and diverse roles of NAC1 in cancer biology, it is important to identify the NAC1-regulated transcriptome and determine whether any of these transcripts contribute to the pathogenesis of ovarian cancer. In this study, we used the SKOV3 cell line as the discovery cell model and compared the transcriptomes of NAC1-expressing SKOV3 cells and control, NAC1-knockdown SKOV3 cells. We further investigated the role of a NAC1-regulated gene, FOXQ1, in mediating cell motility and invasion.
Materials and Methods
Cell Lines and Culture Conditions
Cancer cell lines used in this study, including SKOV3, OVCAR5, and HeLa, were purchased from the ATCC (Manassas, VA). Cells were maintained in RPMI-1640 (Mediatech, Manassas, VA) supplemented with 5% (v/v) fetal bovine serum (Cellgro; Mediatech) and penicillin-streptomycin (Invitrogen, Grand Island, NY). The HeLa N130 tetracycline-controlled transactivator inducible cell line was described in our previous study.7 These cells can be induced to express the NAC1 deletion mutant (N130) that contains only the BTB domain, which disrupts NAC1 function in cells after removal of doxycycline from the culture medium.7 Primary culture of lung fibroblasts derived from NAC1 knockout mice was described in our previous study.14,15 Briefly, lungs were minced and incubated with 10 μg/mL of collagenase at 37°C to isolate single cells. Primary cultures of mouse ovarian surface epithelial (OSE) cells were isolated by scraping the ovarian surface followed by trypsin digestion of the scraped cells. Cells were cultured in RPMI-1640 supplemented with 10% (v/v) fetal bovine serum and penicillin-streptomycin.
Plasmid DNA Constructs and Transfection
To generate the FOXQ1 expression vector, we first amplified the protein-coding sequence of FOXQ1 by PCR (primers: forward, 5′-ATGAAGTTGGAGGTGTTCGT-3′; reverse, 5′-AGGCTAGGAGCGTCTCCA-3′) and cloned the PCR product into the pLentiToV5HisPuro expression vector. The NAC1 expression plasmid pcDNA6/NAC1-V5 was previously described.5,7 Two siRNAs that independently target NACC1 and one control siRNA (Stealth RNAi siRNA Negative Control Med GC) were purchased from Invitrogen. The target sequences of the NAC1 siRNAs were 5′-ACAUGAUGGGUGUGGAGCAUGGCUU-3′ and 5′-CAGCAGAUCCUCAGCUUCUGCUACA-3′. Transfection of siRNAs was performed using Lipofectamine RNAiMAX (Invitrogen). The shRNA plasmid targeting NAC1 (5′-GTACACCATGTACAGCATGAT-3′) and the control plasmid (pLKO.1-puro vector) were obtained from Open Biosystems (Thermo Scientific, Waltham, MA). For the pGL3-FOXQ1 promoter construct, we cloned the potential FOXQ1 promoter region (−1368 to +120 bp) into the pGL3-basic vector (Promega, Madison, WI).
Microarray Analysis of Gene Expression
SKOV3 cells were treated with either NAC1 shRNA or control shRNA lentivirus, and cells were harvested 24 or 48 hours after transduction. Total RNA was isolated using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany), and RNA samples were hybridized onto the Human HT-12 v3 Expression BeadChip (Illumina, San Diego, CA). By comparing the global gene expression profiles of NAC1 shRNA-treated and control cells, we selected genes with a false discovery rate (FDR) <0.002 at either time point for further analysis. Up- and down-regulated genes were defined by the regulation status at a given time point with the lowest FDR. Genes with a >1.5-fold change compared with the control group were further examined by Ingenuity Pathway Analysis (Ingenuity Systems, Redwood City, CA). This analysis is based on expected causal relationships between input genes and biological functions. The expected causal relationships are derived from the literature compiled in the Ingenuity Knowledge Base and allow a prediction for each function based on the direction of change in gene expression.
qPCR
First-strand cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Relative transcription levels were measured using the CFX96 Real-Time PCR Detection System (Bio-Rad) and quantified by fluorescence intensity of SYBR Green I (Invitrogen) staining. The real-time quantitative PCR (qPCR) primers used in this study are listed in Table 1. Averages in the CT of duplicate measurements were obtained. The relative gene expression level was calculated by the difference in CT between the gene of interest and the control gene, GAPDH, which was used as an internal reference for data normalization.
Table 1.
Oligonucleotide Primers Used for qPCR
| Primer | Sequence |
|---|---|
| NAC1 F | 5′-AAGCTGAGGATCTGCTGGAA-3′ |
| NAC1 R | 5′-CCAGACACTGCAGATGGAGA-3′ |
| FOXQ1 F | 5′-CTCAACGACTGCTTCGTCAA-3′ |
| FOXQ1 R | 5′-GTGTACTCGCTGTTGGGGTT-3′ |
| NOTCH1 F | 5′-CCGCAGTTGTGCTCCTGAA-3′ |
| NOTCH1 R | 5′-ACCTTGGCGGTCTCGTAGCT-3′ |
| FOXA2 F | 5′-TGTTGCTCACGGAGGAGTAG-3′ |
| FOXA2 R | 5′-TTAAAGTATGCTGGGAGCGG-3′ |
| CDKN1A F | 5′-CATGGGTTCTGACGGACATC-3′ |
| CDKN1A R | 5′-TGCCGAAGTCAGTTCCTTGT-3′ |
| Jagged1 F | 5′-ACTGTCAGGTTGAACGGTGTC-3′ |
| Jagged1 R | 5′-ATCGTGCTGCCTTTCAGTTT-3′ |
| IGFBP6 F | 5′-GCCTGCTTGGGGTTTACTCT-3′ |
| IGFBP6 R | 5′-ATCCGCCCAAGGACGAC-3′ |
| DBNL R | 5′-TTGAGCTCCTCCACCATCTC-3′ |
| DBNL F | 5′GAGAAGTCCCCGACCGACT-3′ |
| NCOA6 F | 5′-AGTCGCAGTCCTGCTTGTTT-3′ |
| NCOA6 R | 5′-CGATCTTCTCGACCTGCTTC-3′ |
| DVL1 F | 5′-ACGCTCCTTCTCACGGC-3′ |
| DVL1 R | 5′-CGGGCTTTAGCTATGGCAG-3′ |
| KBTBD8 F | 5′-TGTAAGGCCGCTAGTGAACA-3′ |
| KBTBD8 R | 5′-CGATGAAGGACAGTTGACAGA-3′ |
| IDH1 F | 5′-TCCGTCACTTGGTGTGTAGG-3′ |
| IDH1 R | 5′-GGCTTGTGAGTGGATGGGTA-3′ |
| SNF2H F | 5′-ACCTGCTGCTCAGAAGACTCCAACT-3′ |
| SNF2H R | 5′-GTCGGTAATCGCCAACGGATAGTA-3′ |
| m-FOXQ1 F | 5′-AGCGAAGGAACACTTTTGGA-3′ |
| m-FOXQ1 R | 5′-GGAAGACAAGCGAGGAATGA-3′ |
| GAPDH F | 5′-TTGGTATCGTGGAAGGACTC-3′ |
| GAPDH R | 5′-ACAGTCTTCTGGGTGGCAGT-3′ |
Western Blot Analysis
Protein lysates were suspended in Laemmli buffer, separated in a 4% to 15% SDS-PAGE Mini-Protean TGX Gel (Bio-Rad), and transferred to an 0.2-μm Trans-Blot polyvinylidene difluoride membrane (Bio-Rad) using a semidry transfer apparatus (Bio-Rad). The membrane was incubated with the appropriate antibodies and then in enhanced chemiluminescence developing solution (Thermo Scientific) for signal development. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. The antibodies used in this study were anti-NAC1,7 anti-FOXQ1 (AV39755; Sigma, St. Louis, MO), anti-GAPDH (G9545; Sigma), donkey anti-mouse IgG horseradish peroxidase (715-035-150; Jackson ImmunoResearch, West Grove, PA), and donkey anti-rabbit IgG horseradish peroxidase (711-035-152; Jackson ImmunoResearch).
Luciferase Reporter Assay
SKOV3 and HeLa cells were first transfected with NAC1-targeting siRNA or control siRNA. After 24 hours, cells were transfected with control pGL3 plasmid or the pGL3-FOXQ1 promoter firefly luciferase constructs and the pRL-Renilla reporter plasmid (Promega) using Lipofectamine LTX and Plus Reagent (Invitrogen). The firefly and Renilla luciferase activities were measured 24 hours after transfection using a luminometer (PerkinElmer, Waltham, MA) and the Dual-Glo luciferase reagent (Promega). Firefly luciferase activity was normalized to Renilla luciferase activity.
Migration and Invasion Assay
Transwell assays were performed to quantify the migration and invasion of SKOV3 cells. Uncoated inserts (354578; BD Biosciences, San Jose, CA) were used for migration assays, and inserts coated with Matrigel (354480; BD Biosciences) were used for invasion assays. Duplicate wells were used for each experimental condition. Cells were seeded on top of the inserts, inserts were cultured overnight and fixed with 4% formaldehyde and stained with 0.1% crystal violet, and cells that had migrated or invaded to the underside of the membrane were visualized. Cells were counted in five individual fields on each insert.
In Silico Analysis of Co-Expression of NAC1 and Its Target Genes
The Z-scores of mRNA expression data from the TCGA ovarian cancer study were retrieved from the Cancer Genomics Data Server (CGDS) through the cBioPortal for Cancer Genomics (http://www.cbioportal.org, last accessed May 11, 2012), hosted by Memorial-Sloan-Kettering Cancer Center using the CGDS-R software package version 1.1.19 (http://cran.r-project.org/web/packages/cgdsr/index.html, last accessed May 11, 2012). Z-scores were available for 489 ovarian high-grade serous carcinomas, whose mRNA expression data were produced on the same platform (Agilent Technologies, Santa Clara, CA). The scores were calculated using tumors diploid for each gene as the reference population, and individual overexpressed and underexpressed genes were defined by Z-scores >1.65 or <−1.65, respectively. We also assessed the tendency for co-occurrence of NAC1 and its potential regulated genes by calculating the odds ratios (ORs).
Statistical Analysis
Two-tailed t-tests were used to determine significance of differences between groups. Data are presented as means ± SD. P < 0.05 was considered statistically significant. For co-occurrence tests performed in the TCGA ovarian cancer data set, we followed the criteria used in cBioPortal for Cancer Genomics, and two genes were considered to have a tendency toward co-occurrence if the OR was >1.5. The significance of co-occurrence was further tested using a one-sided Fisher's exact test.
Results
Identification of NACC1-Regulated Genes in Ovarian Cancer Cells
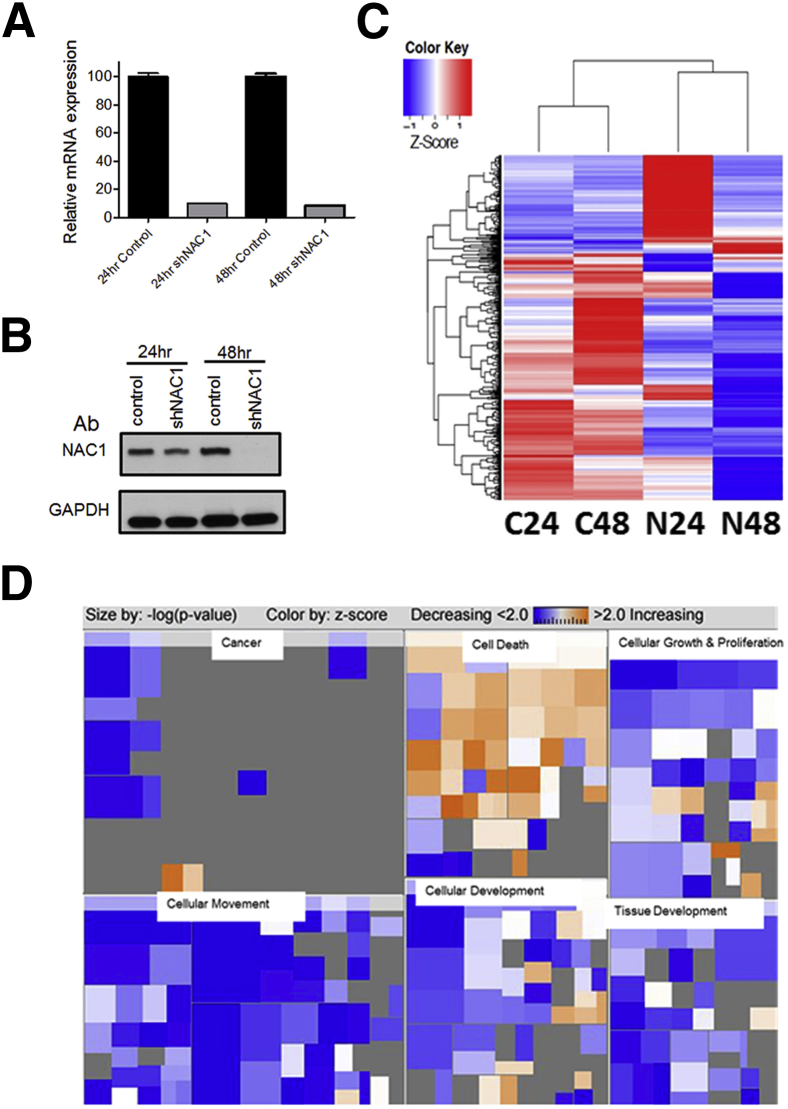
To identify the potential NAC1-regulated genes, we performed shRNA-mediated knockdown and microarray analysis of SKOV3 cells, which overexpress NAC1. Total RNA and proteins were isolated from the cells 24 and 48 hours after NACC1 knockdown, and both Western blotting and qPCR were performed to validate NACC1 knockdown efficiency. As expected, we found significant down-regulation of NACC1 mRNA at both 24 and 48 hours (Figure 1A) and significant inhibition of NAC1 protein expression 48 hours after NAC1 shRNA treatment (Figure 1B), indicating the robustness of our knockdown approach. Affymetrix GeneChip microarray experiments were then performed, and specific transcriptome alterations were detected in NACC1 knockdown cells compared with control cells. This comparison revealed significant changes (fold change, >1.5; FDR, <0.002) in expression of 710 genes at the transcript level (Figure 1C and Supplemental Tables S1 and S2). Nearly 70% (493 of 710) of these genes were down-regulated as a result of shRNA-mediated knockdown of NACC1, indicating that expression of most genes was induced by NAC1. Ingenuity pathway analysis of these 710 genes demonstrated that genes down-regulated by NACC1 knockdown were enriched in pathways related to cell movement, cell and tissue development, and growth and proliferation, whereas genes up-regulated by NACC1 knockdown were enriched in the pathway related to cell death (Figure 1D and Supplemental Tables S3 and S4).
Figure 1.
Identification of NAC1-regulated genes. A: qPCR was performed to determine the robustness of shRNA-mediated NAC1 knockdown. NAC1 mRNA level was normalized to GAPDH. B: Western blot analysis of NAC1 protein expression after shRNA-mediated NAC1 knockdown. GAPDH was used as the loading control. C: Genome-wide transcriptional array analysis identified genes differentially expressed between SKOV3 cells treated with NAC1 shRNA virus (N24, N48) and control virus (C24, C48) at 24 and 48 hours. A threshold ratio of 1.5 was used to select candidate NAC1-regulated genes. Columns represent experimental samples, and rows represent genes. The expression level of each gene in an individual specimen is shown as a pseudocolor gradient based on the normalized, relative expression level of each gene, where red indicates overexpression and blue indicates underexpression. D: Pathway analysis of genes affected by NAC1 knockdown. A heat map shows predicted trends of the six most significantly associated categories of biological function. Each square in the heat map represents a specific biological function, and related biological functions are grouped into major categories. The size of the squares reflects the log (P value) of the biological function, with larger size indicating higher statistical significance. The color of the squares is graded from orange to blue, with orange indicating a positive Z-score (increased biological function predicted) and blue a negative Z-score. Biological functions for which a direction could not be predicted based on the current Ingenuity Knowledge Base are shown in gray.
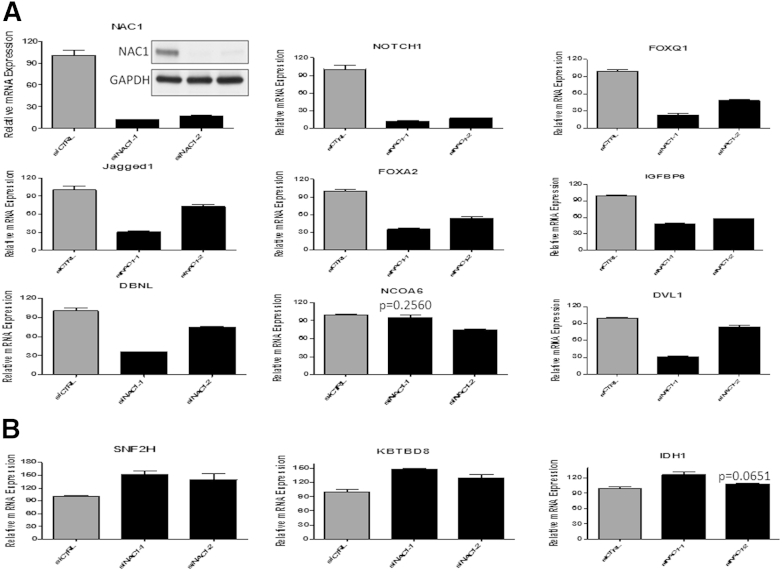
To validate the microarray results, qPCR was performed using two independent NACC1 siRNAs for 11 genes selected based on prior knowledge of their involvement in cancer-associated pathways. All genes were validated by both siRNAs (P < 0.05) (Figure 2), with the exception of NCOA6 and IDH1, which could be confirmed by only one siRNA. These genes include those whose transcription was supported by NACC1, such as FOXQ1 FOXA2, IGFBP6, NOTCH1, JAGGED1, DBNL, and DVL1 (Figure 2A), and those whose transcription was repressed by NACC1, including SNF2H and IDH1 (Figure 2B). In this study, we further validated representative members of the Notch pathway by either NAC1 knockdown or ectopic expression of NAC1 in other cell lines, including SKOV3TR (a paclitaxel-resistant SKOV3 variant), OVCAR5, and OSE4. Because Notch3 has been known to play an important role in the pathogenesis of ovarian high-grade serous carcinoma,20–23 we also included Notch3 in the analysis. As shown in Supplemental Figure S1, NACC1 knockdown resulted in decreased levels of Jagged1 and Notch3 mRNA expression in both SKOV3TR and OVCAR5 cells, whereas ectopic expression of NAC1 in OSE4 cells led to increased levels of Jagged1, Notch1, and Notch3 mRNA.
Figure 2.
Validation of NAC1-regulated genes (A, down-regulated; B, up-regulated) by qPCR using two independent siRNAs. qPCR was performed to confirm the candidate NAC1 target genes identified by microarray analysis. SKOV3 cells were treated with two different NAC1 siRNAs, and NAC1 knockdown efficiency was validated at both mRNA and protein levels. Relative mRNA levels of candidate genes were determined using GAPDH as a reference gene for data normalization. All results are presented as means ± SD. All except two of the candidate genes could be validated (P < 0.05) by both siRNAs.
FOXQ1 Expression Is Transcriptionally Regulated by NAC1
Among the NACC1-regulated genes, FOXQ1 was selected for further study because of its reported functions in cancer and stem cell biology.24–26 We first determined whether NAC1 was necessary and/or sufficient to regulate FOXQ1 expression. As shown in Figure 3, both NACC1 siRNAs inhibited FOXQ1 protein expression (Figure 3A and Supplemental Figure S2), and ectopic expression of NAC1 in mouse ovarian surface epithelium and lung fibroblasts established from NACC1−/− mice significantly increased FOXQ1 expression (Figure 3, B and C, and Supplemental Figure S3). Because BTB domain–mediated protein dimerization is known to contribute to the biological functions of NAC1, we tested whether disruption of NAC1 dimerization by N130 affected FOXQ1 expression. As shown in Figure 3D, induction of N130 resulted in significant suppression of FOXQ1 transcript levels in HeLa cells at different time points, particularly at 6 hours after N130 induction. To assess whether NAC1 regulates FOXQ1 transcription through FOXQ1 promoter activity, we performed a FOXQ1 promoter reporter assay. Reporter plasmid that contained the FOXQ1 promoter sequence was transfected into NAC1-overexpressing SKOV3 and HeLa cells. Suppression of NAC1 expression by two different NACC1 siRNAs significantly reduced the luciferase activity associated with the FOXQ1 promoter reporter (Figure 4).
Figure 3.
NAC1 is both required and sufficient for FOXQ1 expression. A: Western blot analysis demonstrates a decrease in FOXQ1 protein levels 48 hours after NAC1 knockdown in both SKOV3 and HeLa cells. GAPDH was used as the loading control. B: Western blot analysis shows an increase in FOXQ1 protein levels 48 hours after ectopic NAC1 expression in NACC1−/− mouse ovarian surface epithelium. GAPDH was used as a loading control. C: qPCR demonstrates that FOXQ1 expression was induced 24 hours after transfection of a NAC1 expression construct into NACC1−/− mouse fibroblasts. D: Expression of N130, the truncated form of NAC1 comprising only the BTB domain, down-regulates FOXQ1 transcript level in HeLa cells compared with noninduced cells at different time points.
Figure 4.
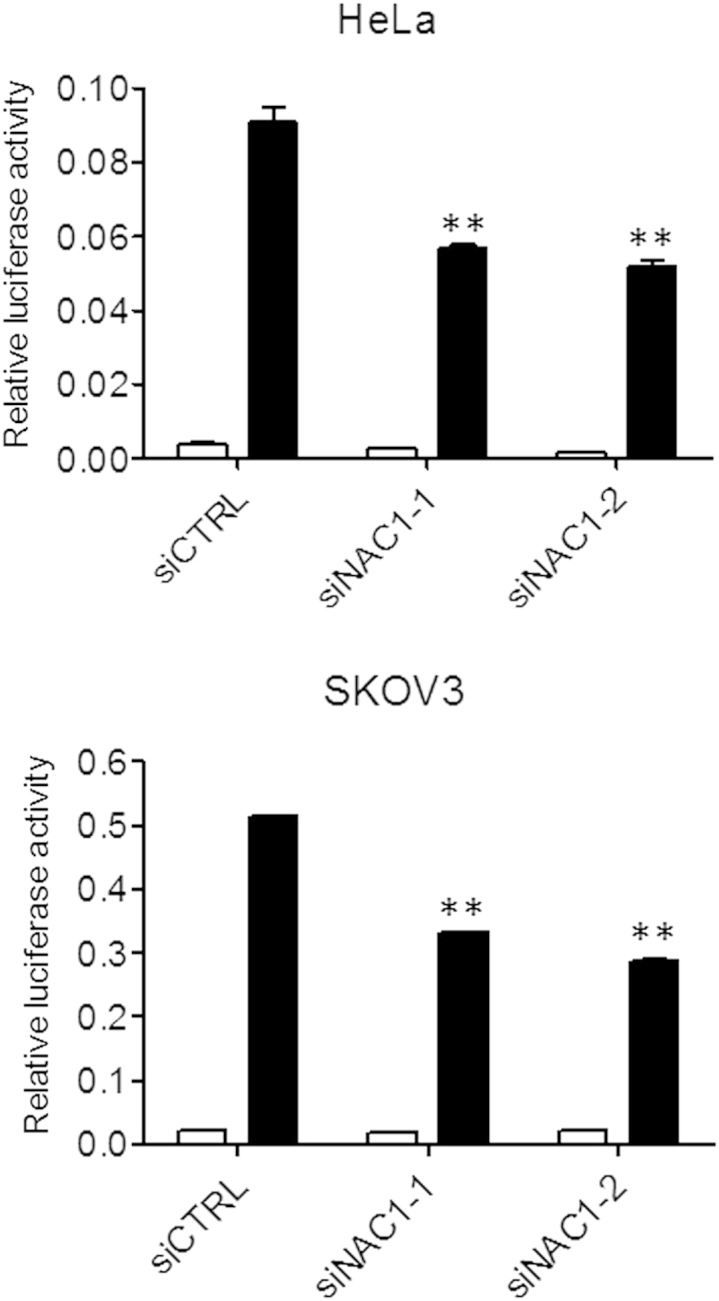
NAC1 regulates FOXQ1 promoter activity. HeLa or SKOV3 cells were first transfected with two independent NAC1 siRNAs to knock down NAC1 expression. One day later, the pGL3-FOXQ1 (black bars) promoter construct or pGL3 control plasmid (white bars) was transfected into the cells. Luciferase activity was measure 24 hours after transfection of the promoter construct. FOXQ1 promoter activity in NAC1 siRNA-treated cells is significantly lower than in the control siRNA-treated cells. ∗∗P < 0.01.
FOXQ1 Mediates NAC1-Induced Cell Motility and Invasion
Next, we addressed the role of FOXQ1 in mediating the biological functions of NAC1. Because NAC1 is involved in cell proliferation, drug resistance, and migration and invasion, we investigated whether any of these phenotypes were mediated by FOXQ1 in NAC1-overexpressing cancer cells. A rescue assay was performed to determine the salvage effect of ectopic expression of FOXQ1 in cells with siRNA-mediated NAC1 knockdown using two independent siRNAs. Our data demonstrate that ectopic expression of FOXQ1 did not rescue cell proliferation or restore paclitaxel resistance in NAC1-knockdown cells (data not shown). However, ectopic expression of FOXQ1 significantly increased cell migration and invasion compared with control cells without engineered expression of FOXQ1, suggesting that FOXQ1, at least in part, mediates cell motility induced by NAC1 (Figure 5).
Figure 5.
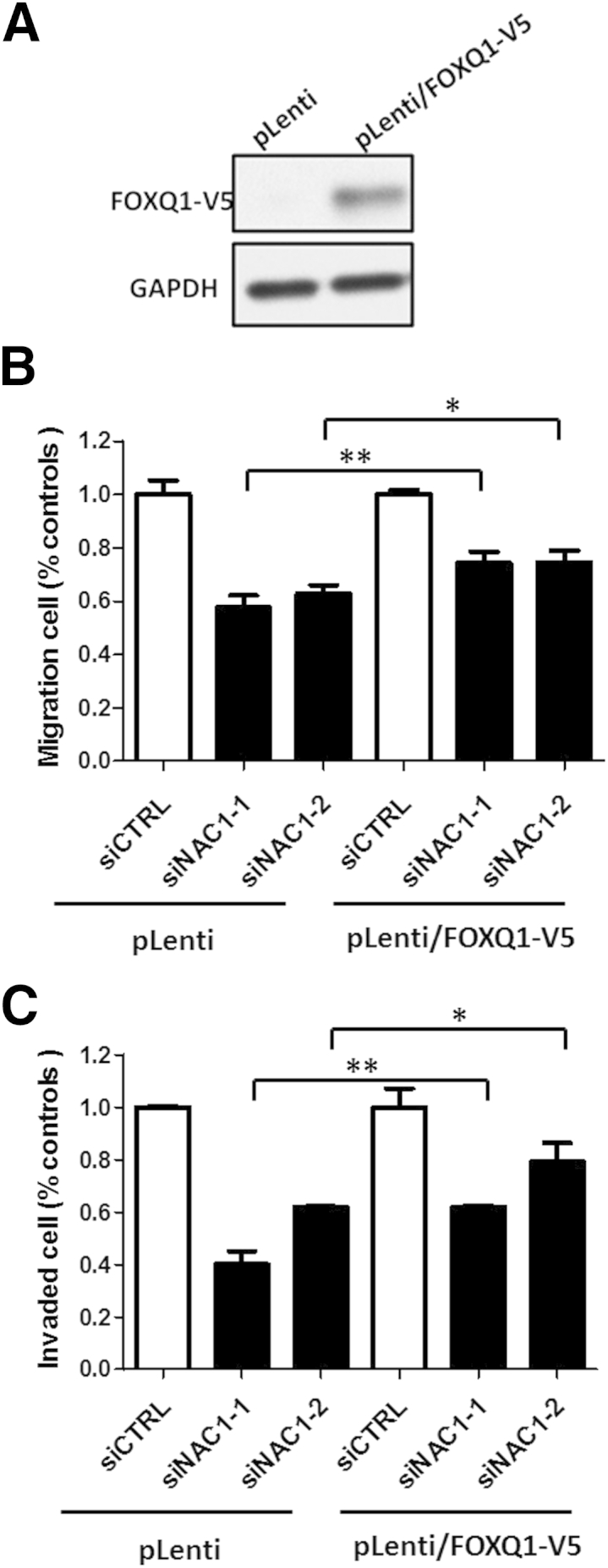
Ectopic expression of FOXQ1 increases motility of NAC1-knockdown cells. A:FOXQ1 cDNA tagged with a V5 epitope was cloned into the pLenti lentiviral vector. SKOV3 cells were transfected with the pLenti/FOXQ1-V5 or control plasmid. FOXQ1 protein expression was analyzed by Western blot using an anti-V5 antibody. GAPDH was used as a loading control. B: NAC1 siRNA- or control siRNA-treated SKOV3 cells were transfected with pLenti/FOXQ1-V5 or control plasmid, and migration (B) and invasion (C) were measured in Transwell assays. Compared with control cells (siCTRL), NAC1 siRNA-treated cells have a reduced capacity to migrate or invade in both pLenti- and pLenti/FOXQ1-V5 transfected groups (P < 0.001). Ectopic FOXQ1 expression increases the migration and invasiveness of NAC1-knockdown cells. For each group, data were normalized to control siRNA-treated samples. The error bars represent one SD. ∗P < 0.05, ∗∗P < 0.01.
Correlation of NACC1 and Target Gene Expression in Ovarian Cancer Tissues
The TCGA ovarian high-grade serous carcinoma data set allowed us to perform statistical analysis to determine the co-occurrence of aberrant expression of NACC1 and its target genes in 489 ovarian high-grade serous carcinoma tissues. Of 493 genes that were potentially up-regulated and the 217 genes that were potentially down-regulated by NACC1, gene expression data were available from the cBioPortal for Cancer Genomics for 477 up-regulated and 210 down-regulated genes (Supplemental Tables S5 and S6). Of the 477 potentially up-regulated genes, 125 (26.2%) had a tendency toward linked up-regulation with NACC1 (OR = >1.5), and 52 (10.9%) of these associations were statistically significant (P < 0.05). Notably, there was significant co-occurrence of NACC1 and FOXQ1 up-regulation (OR = 6, P = 0.0002). Of the 210 genes that were potentially down-regulated by NAC1, 67 (31.9%) had a tendency toward co-occurrence with NACC1 overexpression, and 50 (23.8%) of these associations were statistically significant. The ORs and P values of co-occurrence with NACC1 up-regulation and down-regulation for each gene are shown in Supplemental Tables S5 and S6, respectively, and the pattern of co-regulation for representative genes is shown in Supplemental Figure S4.
Discussion
Evidence is accumulating to indicate a multifaceted role of NAC1 in cancer pathogenesis, including promotion of cell survival and proliferation, acquisition of resistance to chemotherapeutic agents, enhancement of cell motility, and autophagy. These multiple functions are thought to be related to the transcriptional regulatory activity of NAC1. Although NAC1 lacks a DNA-binding domain, it may actively participate in transcriptional regulation by interacting with a specific transcription complex. On the basis of comparison of the transcriptomes of NAC1-knockdown and control SKOV3 ovarian cancer cells, we identified a set of genes whose expression levels depend on the NAC1 expression level. Moreover, we were able to validate NAC1 regulation of representative target genes, confirming the robustness of the microarray analysis for discovering NAC1-regulated genes. Interestingly, a substantial number of NAC1 downstream candidate genes, including FOXQ1, exhibited a pattern of co-expression with NACC1 in a large cohort of human ovarian high-grade serous carcinoma tissues. This result further supports the idea that NAC1 may regulate these target genes at the tissue level.
The current study has suggested a regulatory role of NAC1 in several well-known cancer-associated pathways, including those involved in cell proliferation, apoptosis, Notch signaling, cell motility and invasion, and epithelial-mesenchymal transition. Among these, potential activation of Notch signaling by NAC1 is of particular interest. Our current data indicate that expression of Notch receptor and its ligand Jagged1 depends on NAC1 in at least one of the cell lines tested (Figure 2A and Supplemental Figure S1). It is well established that overexpression of the Notch receptor and Jagged1 activates Notch signaling, a molecular pathway that controls cell fate during development and that aberration of this pathway contributes to cancer development. We have demonstrated that Jagged1 is the primary Notch ligand in ovarian cancer and that the Jagged1-Notch interaction constitutes a juxtacrine loop, promoting proliferation and dissemination of ovarian cancer cells within the intraperitoneal cavity.23 Inactivation of the Notch pathway results in growth inhibition and induction of apoptosis in ovarian cancer cells, suggesting that targeting the Notch signaling pathway may represent a new therapeutic approach for ovarian cancer.21,22,27 Thus, our results indicating that NAC1 is necessary for maintaining the expression of the Notch receptor and Jagged1 in SKOV3 or OVCAR5 ovarian cancer cells and that NAC1 expression is sufficient to up-regulate Notch receptor and Jagged1 in OSE4 cells provide new insight into the complex regulatory network underlying Notch signaling.
In this study, we found that expression of several members of the FOX family, including FOXQ1, FOXA2, FOXJ2, and FOXS1, decreases after NAC1 knockdown (Supplemental Table S1), suggesting that their expression depends on NAC1. Several members of the FOX gene family are known to have a plethora of biological functions, including embryonic development and cell cycle progression.28,29 In this study, we demonstrate that NAC1 is not only essential but also sufficient to maintain FOXQ1 expression. Because FOXQ1 has been established as an important gene in epithelial-mesenchymal transition,24–26 and its up-regulation is associated with poor prognosis in colorectal, breast, and non–small cell lung carcinomas, NAC1 may also contribute to epithelial-mesenchymal transition and aggressiveness of disease through up-regulation of FOXQ1. To this end, we have recently demonstrated that ovarian carcinoma cells express a higher level of FOXQ1 than normal epithelial cells.26 Silencing of FOXQ1 expression using a shRNA-mediated knockdown approach altered gene expression of several cell cycle regulators, leading to suppressed cell proliferation and reduced cell motility and invasion. Consistent with previous reports, FOXQ1 up-regulated epithelial markers and down-regulated mesenchymal cell markers, indicating that FOXQ1 is involved in epithelial-mesenchymal transition in ovarian cancer cells. Of note, in the present study, we found that NAC1 dimerization is required to sustain FOXQ1 expression, suggesting inhibition of NAC1 dimerization as a possible strategy to suppress FOXQ1 expression.
This study also raises several important questions to be further addressed. For example, it is important to determine which other NAC1-regulated genes mediate the key biological functions of NAC1, such as resistance to chemotherapeutic agents and pluripotency of stem cells. It is plausible that a subset of NAC1-regulated genes cooperates to mediate unique functions of NAC1. As evidenced by our data for FOXQ1 (Figure 5), although ectopic expression of FOXQ1 abated the suppressive effect of NAC1 knockdown on cellular motility, the rescue effect was modest at best, suggesting that other NAC1 downstream genes may be also involved in this phenotype. In fact, pathway analysis demonstrated that several NAC1-regulated genes, including IGFBP6,30 CXCL1,31 DNBL,32 ROCK2,33 and ADAMTS1,34 are also involved in cellular motility and invasion.
In conclusion, we identified the NAC1-regulated genes in ovarian cancer cells and found a significant correlation between the expression levels of NAC1 and its downstream target genes in human cancer tissues. This compendium of NAC1 targets is fundamental for future studies aimed at delineating the biological roles of NAC1 in the pathogenesis of tumor development. The fact that NAC1 regulates expression of multiple tumor-associated genes and pathways indicates that NAC1 may serve as one of the master molecular switches in promoting oncogenesis in human cancer. Targeting the NAC1 pathway by, for example, using small molecule compounds to prevent NAC1 dimerization may have translational implications in designing new therapeutics to treat cancers that exhibit NAC1 up-regulation.
Footnotes
Supported by NIH/National Cancer Institute grants CA103937, CA148826, and U24CA160036 (I.S.).
A.L.H. is a fellow of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; process No. 10417/12-5).
Supplemental Data
qPCR shows alterations in gene expression of NAC1, Jagged1, Notch1, and Notch3 in different cell lines after NAC1 gene knockdown or ectopic expression of NAC1. A: Paclitaxel-resistant SKOV3TR cells derived from parental SKOV3. B: OVCAR5 ovarian cancer cells. C: OSE4 cells derived from normal human ovarian surface epithelium. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Densitometry measurement of NAC1 protein expression using Chemi-DOC XRS (Bio-Rad, Hercules, CA). The bar graphs indicate the relative density of the FOXQ1 protein bands from each experimental group. FOXQ1 expression was normalized to GAPDH using Image Lab software version 4.0.1 (Bio-Rad). ∗∗P < 0.001.
FOXQ1 expression on NAC1−/− mouse OSE cells and lung fibroblasts. A: OSE cell culture was established from NAC1−/− mouse. OSE cells were transfected with NAC1-V5 or control plasmid. qPCR demonstrates that the expression of FOXQ1 is significantly increased 48 hours after transfection of NAC1-V5 plasmid. B: Western blot analysis shows expression of NAC1-V5 in pcDNA6-NAC1-V5 transfected NAC1−/− mouse lung fibroblasts. In those cells, the protein level of FOXQ1 is also increased compared with the control pcDNA6 (vector only) transfected cells. GAPDH serves as the loading control.
Co-expression of NAC1 and NAC1-regulated genes in 489 ovarian high-grade serous carcinomas. A: Co-occurrence of NAC1 up-regulation and expression of representative NAC1 up-regulated genes. B: Co-occurrence of NAC1 up-regulation and expression of representative NAC1 down-regulated genes.
Supplemental Data
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2013.09.024.
References
- 1.Stead M.A., Carr S.B., Wright S.C. Structure of the human Nac1 POZ domain. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009;65:445–449. doi: 10.1107/S1744309109012214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J.R.S., Chu J., Shen X., Levasseur D.N., Theunissen T.W., Orkin S.H. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 3.Mackler S., Pacchioni A., Degnan R., Homan Y., Conti A.C., Kalivas P., Blendy J.A. Requirement for the POZ/BTB protein NAC1 in acute but not chronic psychomotor stimulant response. Behav Brain Res. 2008;187:48–55. doi: 10.1016/j.bbr.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap K.L., Sysa-Shah P., Bolon B., Wu R.C., Gao M., Herlinger A.L., Wang F., Faiola F., Huso D., Gabrielson K., Wang T.L., Wang J., Shih Ie M. Loss of NAC1 expression is associated with defective bony patterning in the murine vertebral axis. PLoS One. 2013;8:e69099. doi: 10.1371/journal.pone.0069099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jinawath N., Vasoontara C., Yap K.L., Thiaville M.M., Nakayama K., Wang T.L., Shih I.M. NAC-1, a potential stem cell pluripotency factor, contributes to paclitaxel resistance in ovarian cancer through inactivating Gadd45 pathway. Oncogene. 2009;28:1941–1948. doi: 10.1038/onc.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shih Ie M., Nakayama K., Wu G., Nakayama N., Zhang J., Wang T.L. Amplification of the ch19p13.2 NACC1 locus in ovarian high-grade serous carcinoma. Mod Pathol. 2011;24:638–645. doi: 10.1038/modpathol.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayama K., Nakayama N., Davidson B., Sheu J.J., Jinawath N., Santillan A., Salani R., Bristow R.E., Morin P.J., Kurman R.J., Wang T.L., Shih Ie M. A BTB/POZ protein, NAC-1, is related to tumor recurrence and is essential for tumor growth and survival. Proc Natl Acad Sci U S A. 2006;103:18739–18744. doi: 10.1073/pnas.0604083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson B., Berner A., Trope C.G., Wang T.L., Shih Ie M. Expression and clinical role of the bric-a-brac tramtrack broad complex/poxvirus and zinc protein NAC-1 in ovarian carcinoma effusions. Hum Pathol. 2007;38:1030–1036. doi: 10.1016/j.humpath.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama K., Nakayama N., Wang T.-L., Shih I.-M. NAC-1 controls cell growth and survival by repressing transcription of Gadd45GIP1, a candidate tumor suppressor. Cancer Res. 2007;67:8058–8064. doi: 10.1158/0008-5472.CAN-07-1357. [DOI] [PubMed] [Google Scholar]
- 10.Yeasmin S., Nakayama K., Ishibashi M., Katagiri A., Iida K., Purwana I.N., Nakayama N., Miyazaki K. Expression of the bric-a-brac tramtrack broad complex protein NAC-1 in cervical carcinomas seems to correlate with poorer prognosis. Clin Cancer Res. 2008;14:1686–1691. doi: 10.1158/1078-0432.CCR-07-4085. [DOI] [PubMed] [Google Scholar]
- 11.Ishibashi M., Nakayama K., Yeasmin S., Katagiri A., Iida K., Nakayama N., Fukumoto M., Miyazaki K. A BTB/POZ gene: NAC-1, a tumor recurrence-associated gene, as a potential target for taxol resistance in ovarian cancer. Clin Cancer Res. 2008;14:3149–3155. doi: 10.1158/1078-0432.CCR-07-4358. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa M., Nakayama K., Yeasmin S., Katagiri A., Iida K., Nakayama N., Miyazaki K. NAC1, a potential stem cell pluripotency factor expression in normal endometrium, endometrial hyperplasia and endometrial carcinoma. Int J Oncol. 2010;36:1097–1103. doi: 10.3892/ijo_00000591. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama K., Rahman M.T., Rahman M., Yeasmin S., Ishikawa M., Katagiri A., Iida K., Nakayama N., Miyazaki K. Biological role and prognostic significance of NAC1 in ovarian cancer. Gynecol Oncol. 2010;119:469–478. doi: 10.1016/j.ygyno.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Yeasmin S., Nakayama K., Rahman M.T., Rahman M., Ishikawa M., Katagiri A., Iida K., Nakayama N., Otuski Y., Kobayashi H., Nakayama S., Miyazaki K. Biological and clinical significance of NAC1 expression in cervical carcinomas: a comparative study between squamous cell carcinomas and adenocarcinomas/adenosquamous carcinomas. Hum Pathol. 2012;43:506–519. doi: 10.1016/j.humpath.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Yap K.L., Fraley S.I., Thiaville M.M., Jinawath N., Nakayama K., Wang J., Wang T.L., Wirtz D., Shih Ie M. NAC1 is an actin-binding protein that is essential for effective cytokinesis in cancer cells. Cancer Res. 2012;72:4085–4096. doi: 10.1158/0008-5472.CAN-12-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Cheng Y., Ren X., Hori T., Huber-Keener K.J., Zhang L., Yap K.L., Liu D., Shantz L., Qin Z.H., Zhang S., Wang J., Wang H.G., Shih Ie M., Yang J.M. Dysfunction of nucleus accumbens-1 activates cellular senescence and inhibits tumor cell proliferation and oncogenesis. Cancer Res. 2012;72:4262–4275. doi: 10.1158/0008-5472.CAN-12-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y., Cheng Y., Ren X., Zhang L., Yap K.L., Wu H., Patel R., Liu D., Qin Z.H., Shih I.M., Yang J.M. NAC1 modulates sensitivity of ovarian cancer cells to cisplatin by altering the HMGB1-mediated autophagic response. Oncogene. 2012;31:1055–1064. doi: 10.1038/onc.2011.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okazaki K., Nakayama N., Nariai Y., Kato H., Nakayama K., Miyazaki K., Maruyama R., Kosugi S., Urano T., Sakashita G. Nuclear localization signal in a cancer-related transcriptional regulator protein NAC1. Carcinogenesis. 2012;33:1854–1862. doi: 10.1093/carcin/bgs193. [DOI] [PubMed] [Google Scholar]
- 19.Wu P.H., Hung S.H., Ren T., Shih Ie M., Tseng Y. Cell cycle-dependent alteration in NAC1 nuclear body dynamics and morphology. Phys Biol. 2011;8:015005. doi: 10.1088/1478-3975/8/1/015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J.T., Chen X., Trope C.G., Davidson B., Shih Ie M., Wang T.L. Notch3 overexpression is related to the recurrence of ovarian cancer and confers resistance to carboplatin. Am J Pathol. 2010;177:1087–1094. doi: 10.2353/ajpath.2010.100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J.T., Li M., Nakayama N., Davidson B., Eberhart C.G., Kurman R.J., Shih I.-M., Wang T.-L. Notch-3 gene amplification in ovarian cancer. Cancer Res. 2006;66:6312–6318. doi: 10.1158/0008-5472.CAN-05-3610. [DOI] [PubMed] [Google Scholar]
- 23.Choi J.H., Park J.T., Davidson B., Morin P.J., Shih Ie M., Wang T.L. Jagged-1 and Notch3 juxtacrine loop regulates ovarian tumor growth and adhesion. Cancer Res. 2008;68:5716–5723. doi: 10.1158/0008-5472.CAN-08-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H., Meng F., Liu G., Zhang B., Zhu J., Wu F., Ethier S.P., Miller F., Wu G. Forkhead transcription factor foxq1 promotes epithelial-mesenchymal transition and breast cancer metastasis. Cancer Res. 2011;71:1292–1301. doi: 10.1158/0008-5472.CAN-10-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mani S.A., Guo W., Liao M.J., Eaton E.N., Ayyanan A., Zhou A.Y., Brooks M., Reinhard F., Zhang C.C., Shipitsin M., Campbell L.L., Polyak K., Brisken C., Yang J., Weinberg R.A. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao M., Shih I.M., Wang T.L. The role of forkhead box Q1 transcription factor in ovarian epithelial carcinomas. Int J Mol Sci. 2012;13:13881–13893. doi: 10.3390/ijms131113881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shih I.M., Wang T.L. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res. 2007;67:1879–1882. doi: 10.1158/0008-5472.CAN-06-3958. [DOI] [PubMed] [Google Scholar]
- 28.Hannenhalli S., Kaestner K.H. The evolution of Fox genes and their role in development and disease. Nat Rev Genet. 2009;10:233–240. doi: 10.1038/nrg2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benayoun B.A., Caburet S., Veitia R.A. Forkhead transcription factors: key players in health and disease. Trends Genet. 2011;27:224–232. doi: 10.1016/j.tig.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Fu P., Thompson J.A., Bach L.A. Promotion of cancer cell migration: an insulin-like growth factor (IGF)-independent action of IGF-binding protein-6. J Biol Chem. 2007;282:22298–22306. doi: 10.1074/jbc.M703066200. [DOI] [PubMed] [Google Scholar]
- 31.Kuo P.L., Shen K.H., Hung S.H., Hsu Y.L. CXCL1/GROalpha increases cell migration and invasion of prostate cancer by decreasing fibulin-1 expression through NF-kappaB/HDAC1 epigenetic regulation. Carcinogenesis. 2012;33:2477–2487. doi: 10.1093/carcin/bgs299. [DOI] [PubMed] [Google Scholar]
- 32.Schymeinsky J., Sperandio M., Walzog B. The mammalian actin-binding protein 1 (mAbp1): a novel molecular player in leukocyte biology. Trends Cell Biol. 2011;21:247–255. doi: 10.1016/j.tcb.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Vigil D., Kim T.Y., Plachco A., Garton A.J., Castaldo L., Pachter J.A., Dong H., Chen X., Tokar B., Campbell S.L., Der C.J. ROCK1 and ROCK2 are required for non-small cell lung cancer anchorage-independent growth and invasion. Cancer Res. 2012;72:5338–5347. doi: 10.1158/0008-5472.CAN-11-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyan S.W., Hsu C.H., Peng K.L., Chen C.C., Kuo W.H., Lee E.Y., Shew J.Y., Chang K.J., Juan L.J., Lee W.H. Breast cancer cells induce stromal fibroblasts to secrete ADAMTS1 for cancer invasion through an epigenetic change. PLoS One. 2012;7:e35128. doi: 10.1371/journal.pone.0035128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
qPCR shows alterations in gene expression of NAC1, Jagged1, Notch1, and Notch3 in different cell lines after NAC1 gene knockdown or ectopic expression of NAC1. A: Paclitaxel-resistant SKOV3TR cells derived from parental SKOV3. B: OVCAR5 ovarian cancer cells. C: OSE4 cells derived from normal human ovarian surface epithelium. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Densitometry measurement of NAC1 protein expression using Chemi-DOC XRS (Bio-Rad, Hercules, CA). The bar graphs indicate the relative density of the FOXQ1 protein bands from each experimental group. FOXQ1 expression was normalized to GAPDH using Image Lab software version 4.0.1 (Bio-Rad). ∗∗P < 0.001.
FOXQ1 expression on NAC1−/− mouse OSE cells and lung fibroblasts. A: OSE cell culture was established from NAC1−/− mouse. OSE cells were transfected with NAC1-V5 or control plasmid. qPCR demonstrates that the expression of FOXQ1 is significantly increased 48 hours after transfection of NAC1-V5 plasmid. B: Western blot analysis shows expression of NAC1-V5 in pcDNA6-NAC1-V5 transfected NAC1−/− mouse lung fibroblasts. In those cells, the protein level of FOXQ1 is also increased compared with the control pcDNA6 (vector only) transfected cells. GAPDH serves as the loading control.
Co-expression of NAC1 and NAC1-regulated genes in 489 ovarian high-grade serous carcinomas. A: Co-occurrence of NAC1 up-regulation and expression of representative NAC1 up-regulated genes. B: Co-occurrence of NAC1 up-regulation and expression of representative NAC1 down-regulated genes.