Abstract
Mucosal vaccines are thought to confer superior protection against mucosal infectious diseases. In addition, mucosal routes of vaccine delivery preferentially induce the generation of T helper 17 (Th17) cells, which produce the cytokine IL-17. Th17 cells are critical in mediating vaccine-induced immunity against several mucosal infectious diseases. However, IL-17 is also a potent proinflammatory cytokine, and we recently showed that IL-17 mediates immunopathology and lung injury after influenza infection in mice. In the present study, we tested the hypothesis that mucosal pre-exposure to Th17-inducing adjuvants can promote disease exacerbation upon subsequent infection with influenza virus. Mice mucosally pre-exposed to Th17-inducing adjuvants, such as type II heat-labile enterotoxin or cholera toxin, resulted in increased morbidity and exacerbated lung inflammation upon subsequent infection with influenza virus. Furthermore, the increased morbidity was accompanied by increased expression of inflammatory chemokines and increased accumulation of neutrophils. Importantly, blockade of the IL-17 pathway in mice pre-exposed to Th17-inducing adjuvants resulted in attenuation of the inflammatory phenotype seen in influenza-infected mice. Our findings indicate that, before mucosal Th17-inducing adjuvants can be used in vaccine strategies, the short- and long-term detrimental effects of such adjuvants on disease exacerbation and lung injury in response to infections, such as influenza, should be carefully studied.
Mucosal vaccines are thought to induce better mucosal immunity and to confer superior protection against mucosal infectious diseases, compared with systemic routes of immunization.1 This is consistent with the finding that mucosal routes of vaccine delivery preferentially induce the generation of T helper 17 (Th17) cells, which produce the cytokine IL-17.2–5 Accordingly, Th17 cells are implicated as key players in mediating vaccine-induced immunity against a variety of mucosal infectious diseases, including tuberculosis,5,6 bacterial pneumonia,7–9 pertussis (whooping cough),10,11 and inhalational anthrax.4 However, IL-17 is also a potent proinflammatory cytokine implicated in several inflammatory autoimmune diseases, as well as in models of tissue injury.12 For example, acute lung injury is a severe clinical state characterized by noncardiogenic pulmonary edema, capillary leak, and hypoxemia that can be caused by both infectious and noninfectious stimuli, often observed as acute respiratory distress syndrome. The primary mediator of the inflammation associated with acute lung injury is rapid recruitment of neutrophils and induction of damaging reactive oxygen species intermediates. In this regard, our research group recently showed that, in response to influenza infection, IL-17 produced by γδ T cells mediates immunopathology and lung injury.13 In the present study, we tested the hypothesis that mucosal pre-exposure to Th17-inducing adjuvants in mice can promote disease exacerbation upon subsequent influenza infection.
Here we report that mucosal pre-exposure to Th17-inducing adjuvants, such as type II heat-labile enterotoxin5 or cholera toxin,4 results in increased morbidity and exacerbated lung inflammation upon subsequent infection with different influenza A strains. A key role for IL-17 in mediating inflammation in the lung is through induction of proinflammatory chemokines that mediate accumulation of neutrophils.12 In accord, the increased inflammation seen in the present study in the lungs of mucosally pre-exposed, influenza-infected mice was accompanied by increased expression of the inflammatory chemokines CXCL1, CXCL9, CXCL10, and CCL2 and increased accumulation of neutrophils. Importantly, we show that the observed exacerbated inflammatory phenotype and neutrophil accumulation due to mucosal pre-exposure to Th17-inducing adjuvants are IL-17 pathway–dependent, because treatment with IL-17 receptor blocking antibodies or use of IL-17 receptor knockout mice attenuated the inflammatory phenotype. These findings thus indicate that, before mucosal delivery of experimental Th17-inducing adjuvants can be used in vaccine strategies, the detrimental effects on disease exacerbation and lung injury to subsequent infections, including influenza, should be carefully studied.
Materials and Methods
Animals
C57BL/6 wild-type mice were purchased from Taconic Farms (Hudson, NY). IL-17 receptor A (IL-17RA) deficient mice14 were bred and maintained at the University of Pittsburgh. Age- and sex-matched mice between the ages of 6 to 8 weeks were infected in accordance with University of Pittsburgh Institutional Animal Care and Use Committee guidelines.
Mucosal Immunization and Influenza Infection
Unanesthetized mice were vaccinated intranasally with 50 μL of PBS containing 1 μg type II heat-labile enterotoxin (LT-IIb) or 1 μg cholera toxin (CT)5 (Sigma-Aldrich, St. Louis, MO) or PBS alone. On day 3 after immunization, mice were infected with 100 plaque-forming units (PFU) of H1N1 influenza strain A Puerto Rico/8/1934 (A/PR/8/34) in 50 μL of PBS by oropharyngeal aspiration15 or were intranasally infected with 1 × 106 PFU of a novel H1N1 influenza strain (A/California/7/2009)16 or 5 × 103 PFU of a highly pathogenic H5N1 influenza strain (A/Vietnam/1203/2004) in 50 μL of PBS.17 The infective dose used was specific for each virus strain and was determined as the dose that resulted in moderate weight loss and lung injury. In some experiments, mice were treated intraperitoneally with either 500 μg of IL-17RA blocking antibody (αIL-17RA) (a kind gift from Amgen, Thousand Oaks, CA) or isotype control (Bio X Cell, West Lebanon, NH), once a week, starting from the day of infection. In some experiments, 133 mg of 6 kDa early secretory antigenic target (ESAT-61-20) peptide was mixed with 1 μg LT-IIb holotoxin, and 50 μL was used for intranasal immunization.
Flow Cytometry
Lung cell suspensions were prepared for flow cytometry as described previously.5 Single-cell suspensions were stained with fluorochrome-labeled antibodies, collected, and analyzed as described previously.5 Cells were gated on IL-17–producing Thy1.1 expressing lymphocytes,18 and the numbers of IL-17–producing αβ T cells and γδ T cells were calculated.
Immunohistochemical Analysis
Lung lobes were instilled with 10% neutral buffered formalin and embedded in paraffin. Lung sections were stained with H&E stain, and inflammatory features were evaluated by light microscopy at the University of Pittsburgh Research Histology Core facility. For immunofluorescent staining, formalin-fixed lung sections were processed as described previously.5 Sections were probed with biotinylated rat anti–Gr-1 (BD Pharmingen, San Diego, CA) to detect neutrophils in the inflammatory lesions as described previously.5
In Situ Hybridization
Paraffin-embedded tissue specimens were processed as described previously.19 In situ hybridization with 35S-labeled riboprobes against different chemokine mRNAs was performed as described previously.5
qPCR
For quantitative real-time PCR (qPCR), lung tissue from infected and control mice was homogenized and frozen in RLT buffer (Qiagen, Valencia CA). Extraction of RNA, reverse transcription, and amplification were performed as described previously,5 and mRNA levels relative to GAPDH were calculated. Primer and probes sequences were as described previously.20,21 Influenza burden was determined by real-time reverse-transcription PCR (RT-qPCR) for viral M protein mRNA, as described previously.13
Determination of Protein Levels
Protein levels of cytokines and chemokines were measured in lung homogenates using a mouse Luminex assay (Linco; EMD Millipore, Billerica, MA). Myeloperoxidase chlorination and peroxidase activity were determined in lung homogenates using an Invitrogen myeloperoxidase activity assay kit (Life Technologies, Carlsbad, CA).
Statistical Analysis
Comparison of means between sample groups was performed with the two-tailed Student’s t-test using GraphPad Prism software version 5 (GraphPad Software, La Jolla, CA). Differences were considered significant at P ≤ 0.05.
Results
Mucosal Pre-Exposure to LT-IIb Adjuvant Increases Morbidity and Mortality after H1N1 Influenza A/PR/8/34 Infection
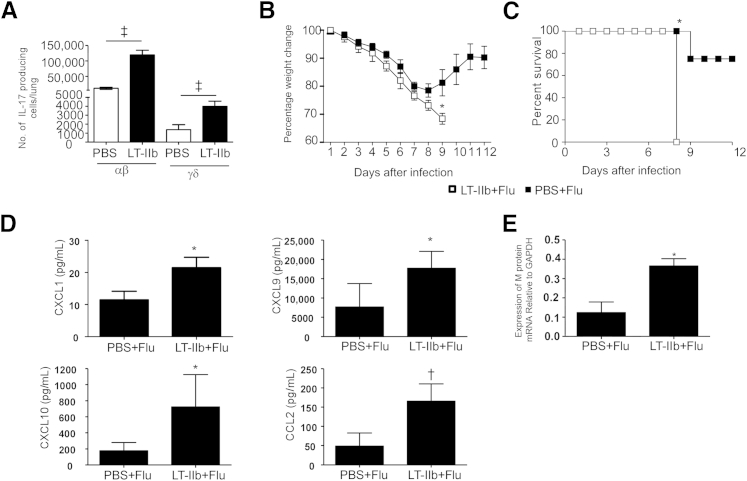
LT-IIb, a potent and well-characterized mucosal adjuvant22 induces, when delivered along with a Mycobacterium tuberculosis–specific antigen, the generation of lung resident antigen–specific Th17 responses.5 Consistent with these findings, when IL-17 Thy1.1 reporter mice18 were mucosally pre-exposed to LT-IIb along with the M. tuberculosis–specific peptide ESAT-61-20, an increase in IL-17 producing cells was observed. Although the majority of the IL-17–producing cells were T cell receptor αβ T cells, a discrete population of IL-17–producing γδ T cells was also detected (Figure 1A). Recently, our research group showed that, after influenza A/PR/8/34 infection, IL-17 has a role to play in mediating lung pathology in mice.13 In the present study, therefore, we investigated whether mucosal pre-exposure to Th17-inducing adjuvants, such as LT-IIb alone, has any effect after subsequent exposure to influenza infection, in which IL-17 is implicated in mediating acute lung injury.13,23
Figure 1.
Mucosal pre-exposure to LT-IIb adjuvant promotes increased morbidity and mortality after H1N1 influenza A/PR/8/34 infection. A: IL-17 Thy1.1 reporter mice were mucosally exposed to ESAT-61-20 peptide in combination with 1 μg LT-IIb adjuvant. On day 6 after exposure, the numbers of Thy1.1+ IL-17-producing αβ+ T cells and γδ+ T cells were determined by flow cytometry. B and C: C57BL/6 mice were mucosally exposed to 1 μg LT-IIb. On day 3 after exposure, mice were infected with 100 PFU of H1N1 influenza strain A/PR/8/34 in 50 μL of PBS. Mice were weighed every day, and percentage weight change was calculated (B) or survival was monitored over time (C). D: CXCL1, CXCL9, CXCL10, and CCL2 protein levels were determined from lung homogenates using a mouse Luminex assay. E: Relative lung viral loads were determined by RT-qPCR of viral M protein mRNA. Data are expressed as means ± SD. n = 4 or 5 mice. ∗P ≤ 0.05, †P ≤ 0.005, and ‡P ≤ 0.0005.
LT-IIb adjuvant alone was delivered mucosally without any antigen, followed by influenza infection. In C57BL/6 mice, exposure mucosally to LT-IIb 3 days before influenza A/PR/8/34 infection resulted in significantly increased weight loss (Figure 1B) and increased mortality (Figure 1C), compared with control mice that were pretreated with PBS before influenza infection. Importantly, mice exposed to LT-IIb and then infected with influenza strain A/PR/8/34 exhibited increased morbidity. In addition, lung homogenates from mice exposed to LT-IIb before influenza infection also exhibited increased expression of the inflammatory chemokines CXCL1, CXCL9, CXCL10, and CCL2 (Figure 1D). These inflammatory chemokines are typical of the cytokine storm associated with increased morbidity seen during severe influenza infections in humans,24,25 suggesting that mucosal pre-exposure to LT-IIb induces exacerbated production of inflammatory chemokines in the influenza-infected lung. In addition, levels of influenza A/PR/8/34 M protein mRNA were increased in lungs of mice exposed to LT-IIb and then infected with influenza, compared with lungs of influenza-infected mice pre-exposed to PBS alone (Figure 1E). These findings suggest that mice pre-exposed to LT-IIb displayed increased susceptibility to A/PR/8/34 virus infection.
Mucosal Pre-Exposure to LT-IIb Adjuvant Promotes Lung Pathology after Influenza A/PR/8/34 Infection
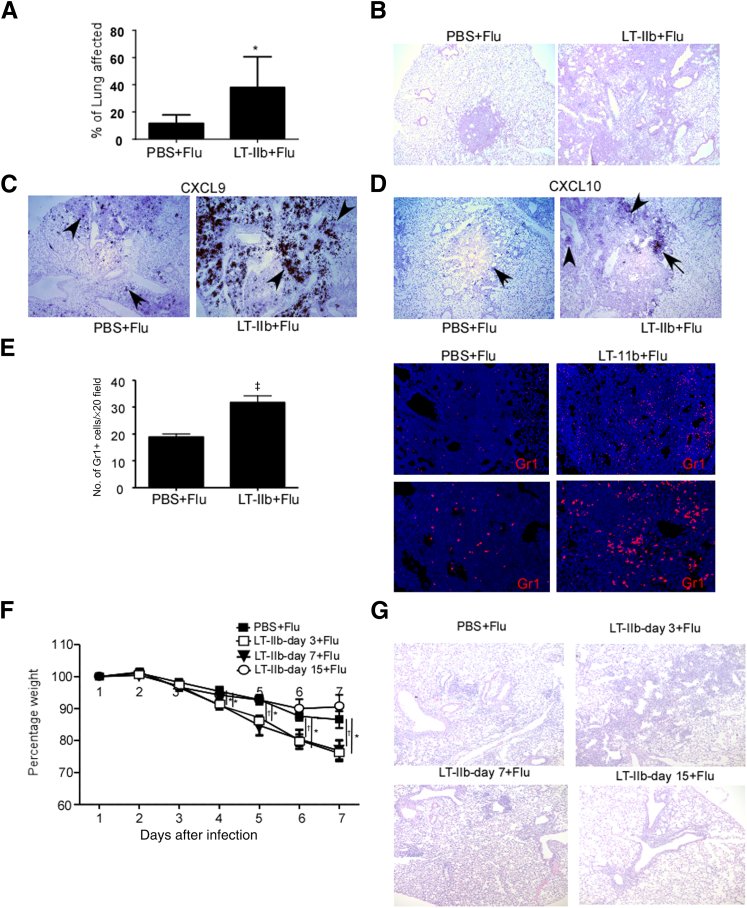
Because our findings suggested that mucosal pre-exposure to Th17-inducing adjuvants increase morbidity and promote disease progression after influenza infection, we next investigated whether the increased morbidity seen in mice exposed to LT-IIb and then infected with influenza is associated with increased pathological responses. Mice exposed to LT-IIb and then infected with A/PR/8/34 virus exhibited severe inflammation in the lung as observed on H&E stained lung sections (Figure 2, A and B), which coincided with increased localization of mRNA for inflammatory chemokines such as CXCL9 and CXCL10 within the inflamed lung (Figure 2, C and D). The increased inflammation observed in influenza-infected mice pre-exposed to LT-IIb coincided with increased accumulation of Gr1+ neutrophils within the inflamed lungs, compared with control PBS pretreated, influenza-infected lungs (Figure 2E). Taken together, these findings indicate that mucosal pre-exposure to Th17-inducing adjuvants can have detrimental effects and may promote disease progression upon subsequent exposure to viral infections.
Figure 2.
Mucosal pre-exposure to LT-IIb adjuvant promotes lung pathology after H1N1 influenza A/PR/8/34 infection. A: C57BL/6 mice were mucosally exposed to 1 μg LT-IIb or PBS and 3 days later were infected with 100 PFU of H1N1 influenza strain A/PR/8/34. B: Formalin-fixed, paraffin-embedded (FFPE) lung sections were stained with H&E, and the percentage of lung affected by inflammation was scored on a scale from 0% to 100%, based on the severity of inflammation. C and D: FFPE lung sections were assayed for CXCL9 (C) and CXCL10 (D) mRNA localization by in situ hybridization, using the corresponding murine chemokine mRNA probes. The arrows and arrowheads indicate expression of CXCL9 and CXCL10 mRNA, respectively. E: FFPE lung sections were analyzed by immunofluorescence using antibodies specific for Gr1 (red). The numbers of Gr1+ cells per ×20 field were counted. F: C57BL/6 mice were mucosally exposed to 1 μg LT-IIb or PBS and 3, 7, or 15 days later were infected with 100 PFU of H1N1 influenza strain A/PR/8/34. Weight loss was monitored in individual mice over time. G: FFPE lung sections were stained with H&E for assessment of inflammation. Data are expressed as means ± SD. n = 4 to 8 mice. ∗P ≤ 0.05, †P ≤ 0.005, and ‡P ≤ 0.0005. Original magnification: ×40 (B–D); ×100 (E, upper row); ×200 (E, lower row).
To address the window of susceptibility after mucosal exposure to Th17 adjuvants within which the detrimental effects are sustained, we exposed mice with LT-IIb either 3, 7, or 15 days before influenza infection. The effect of increased susceptibility to influenza infection in pre-exposed mice was retained for 3 and 7 days after LT-IIb exposure, but was lost by 15 days after LT-IIb exposure (Figure 2F). Consistent with this finding, mice exposed to LT-IIb either 3 or 7 days before influenza infection exhibited considerably worsened lung pathology, whereas mice exposed to LT-IIb 15 days before influenza infection did not demonstrate increased lung pathology (Figure 2G). Taken together, these findings suggest that the detrimental effects of LT-IIb exposure on influenza infection are short-lived.
Mucosal Pre-Exposure to Th17-Inducing Adjuvants Mediates Increased Pathology after Novel Pandemic H1N1 Influenza Infection
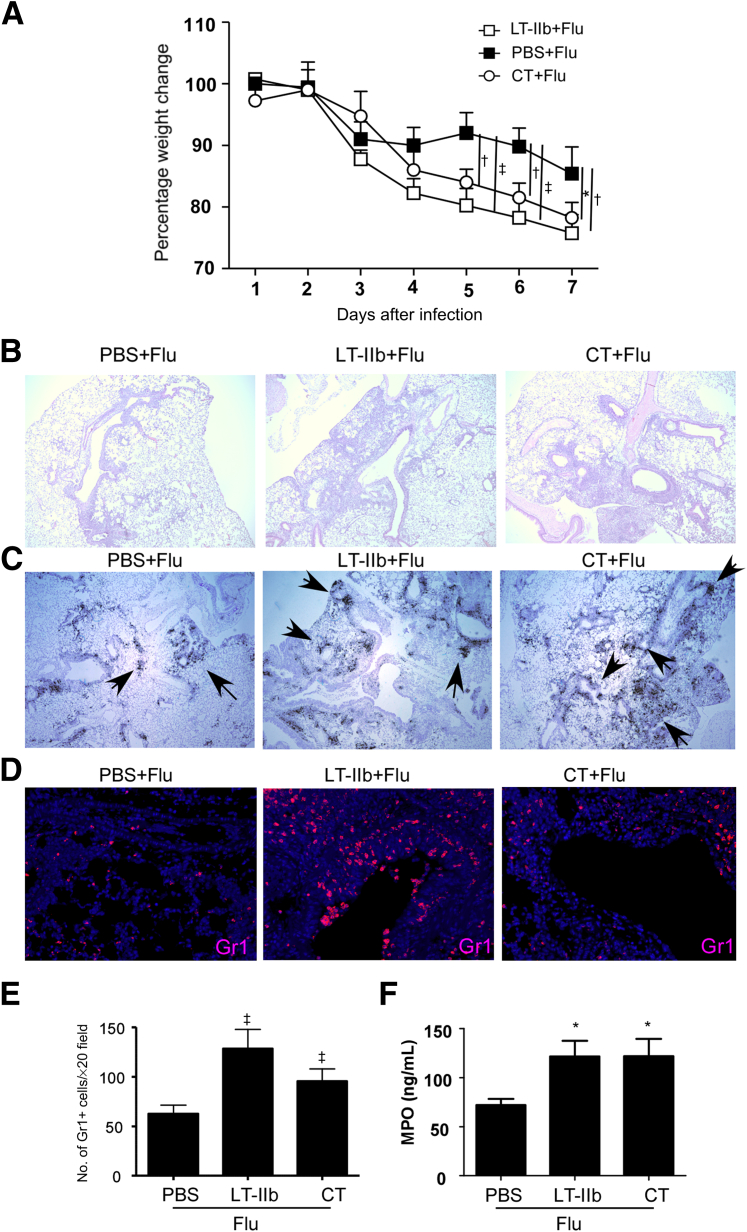
CT, another potent mucosal adjuvant, induces Th17 responses after intranasal delivery.2 When used in an irradiated spore vaccine, CT confers protective responses against inhalational anthrax.4 Next, therefore, we investigated whether the ability to drive inflammatory responses on influenza infection is limited to LT-IIb, or whether mucosal exposure to other Th17-inducing adjuvants (such as CT4) can also similarly induce exacerbated inflammation and increased morbidity upon influenza infection. C57BL/6 mice mucosally pre-exposed to either CT or LT-IIb lost significantly more weight when infected with novel pandemic H1N1 influenza virus, compared with PBS pretreated, H1N1 influenza–infected mice (Figure 3A). The increased weight loss observed in CT and LT-IIb pre-exposed, H1N1 influenza–infected mice also coincided with increased inflammation in the lungs stained with H&E (Figure 3B), increased mRNA expression of CXCL9 (Figure 3C), and increased accumulation of Gr1+ neutrophils within the inflamed lung (Figure 3, D and E). The activity of myeloperoxidase, an enzyme associated with neutrophil activation and generation of reactive oxygen species leading to oxidative damage,26 was also increased significantly in lungs of H1N1 influenza–infected mice pre-exposed to LT-IIb and CT, compared with PBS pretreated, H1N1 influenza–infected control mice (Figure 3F). Taken together, these findings demonstrate that mucosal pre-exposure to Th17-inducing adjuvants, such as LT-IIb and CT, mediates inflammatory pathological responses upon subsequent influenza infection.
Figure 3.
Mucosal pre-exposure to Th17-inducing adjuvants mediates increased pathology after novel pandemic H1N1 influenza infection. A: C57BL/6 mice were mucosally exposed to 1 μg LT-IIb, 1 μg CT, or PBS. On day 3 after exposure, mice were infected with 1 × 106 PFU of novel H1N1 influenza strain A/California/7/2009. Percentage weight change was determined in individual mice over time. B–D: FFPE lung sections were stained with H&E (B) or were assayed for CXCL9 mRNA localization (arrows) by in situ hybridization using a murine CXCL9 mRNA probe (C), and Gr1-accumulating neutrophils were detected by immunohistochemistry (D). E: The numbers of Gr1+ cells per ×20 field were counted. F: The levels of myeloperoxidase activity (MPO) were determined in lung homogenates. Data are expressed as means ± SD. n = 4 or 5 mice. ∗P ≤ 0.05, †P ≤ 0.005, and ‡P ≤ 0.0005. Original magnification: ×40 (B and C); ×200 (D).
IL-17 Induced by Mucosal Pre-Exposure to Th17-Inducing Adjuvants Mediates Pathology after Influenza Infection
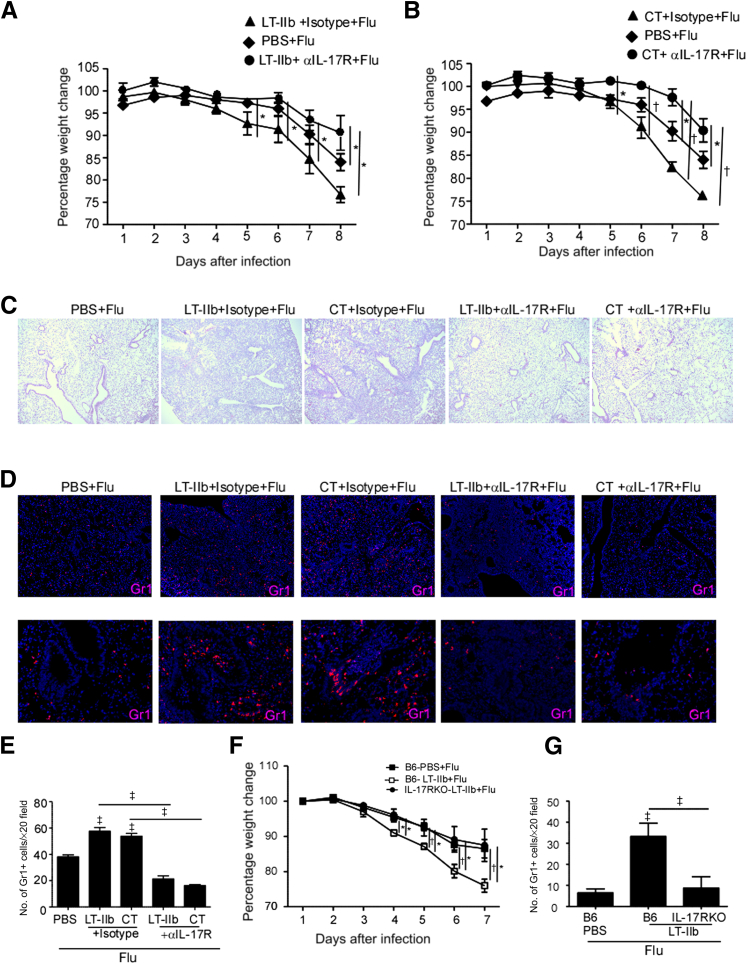
To definitively address whether the increased morbidity due to mucosal pre-exposure to Th17-inducing adjuvants after influenza infection is IL-17 pathway dependent, mice mucosally exposed to either LT-IIb or CT adjuvant were subsequently infected with the highly pathogenic H5N1 influenza virus and then received either isotype control antibody or IL-17RA blocking antibody. Similar to our results with influenza A/PR/8/34 and pandemic H1N1 influenza infection, mice mucosally exposed to CT or LT-IIb and then infected with H5N1 influenza virus lost significantly more weight (Figure 4, A and B) and exhibited severe lung inflammation (Figure 4C), which coincided with increased neutrophil accumulation within the inflamed lung (Figure 4, D and E). Importantly, the increased weight loss, increased lung inflammation, and increased neutrophil accumulation seen in influenza-infected mice mucosally pre-exposed to LT-IIb or CT was significantly attenuated when the mice were treated with IL-17RA blocking antibody (Figure 4, A–E). Similarly, IL-17RA knockout mice exposed to LT-IIb and then infected with H1N1 influenza virus also demonstrated reduced weight loss (Figure 4F), decreased lung inflammation (data not shown), and decreased neutrophil accumulation (Figure 4G), compared with LT-IIb pre-exposed, H1N1 influenza-infected control mice. Interestingly, although the viral titers in mucosally pre-exposed mice trended toward being higher, the overall titers did not differ significantly between the groups (data not shown). Taken together, these findings indicate that mucosal pre-exposure to Th17-inducing adjuvants mediates exacerbates lung inflammation after influenza infection in an IL-17 pathway–dependent manner.
Figure 4.
The IL-17 pathway mediates pathology in mice mucosally exposed to Th17-inducing adjuvants and then infected with influenza virus. A and B: C57BL/6 mice were mucosally exposed to 1 μg LT-IIb, 1 μg CT, or PBS. On day 3 after immunization, mice were infected with 5 × 103 PFU of the highly pathogenic H5N1 influenza strain A/Vietnam/1203/2004. From day 0 of influenza infection, mice were treated with isotype control antibody or with 500 μg IL-17RA neutralizing antibody (αIL-17R). Percentage weight change was determined over time. Both LT- and CT-treated mice were compared with the same PBS-treated mice. C and D: FFPE lung sections were stained with H&E (C) or were analyzed by immunofluorescence using antibodies specific for Gr1 (red) (D). E: The numbers of Gr1+ cells per ×20 field were counted. F: C57BL/6 (B6) or IL-17RA knockout mice were mucosally exposed to 1 μg LT-IIb or PBS. On day 3 after immunization, mice were infected with 1 × 106 PFU of novel H1N1 influenza strain A/California/7/2009, and weight loss was monitored in individual mice over time. G: FFPE lung sections were analyzed by immunofluorescence using antibodies specific for Gr1. The numbers of Gr1+ cells per ×20 field were counted. Data are expressed as means ± SD. n = 4 or 5 mice. ∗P ≤ 0.05, †P ≤ 0.005, and ‡P ≤ 0.0005. Original magnification, ×40 (C); ×100 (D, upper row); ×200 (D, lower row).
Discussion
The emerging consensus is that mucosal vaccines are likely to induce better mucosal immunity and to confer superior protection against infectious diseases, compared with conventional systemic routes of immunization.1 In addition, the identification of the new subset of CD4+ Th17 cells as key players in vaccine-induced immunity against a variety of mucosal infectious diseases5–11 suggests that incorporation of Th17 mucosal adjuvants into vaccine strategies targeting mucosal infectious diseases would be beneficial. However, IL-17 is a potent proinflammatory cytokine implicated as a key player contributing to inflammation and tissue injury.12 Thus, the successful development of Th17 mucosal adjuvants for use in humans will require the generation of tightly controlled antigen-specific Th17 cell responses without induction of the nonspecific IL-17–dependent inflammatory responses.
Our research group has previously demonstrated that delivery of M. tuberculosis antigens in LT-IIb adjuvant induces a persistent lung-resident Th17 population that is protective upon subsequent challenge with M. tuberculosis even 100 days later.5 Thus, mucosal vaccines with Th17-inducing adjuvants are prime candidates for inclusion in future vaccine strategies against global infectious diseases, such as tuberculosis. However, the findings presented here show that ongoing adjuvant-induced IL-17 responses may promote inflammation and exacerbate disease progression if such mucosally exposed individuals subsequently become infected with influenza A, in which IL-17 plays a pathological rather than protective role. Thus, the present findings indicate the importance of examining long-term protective vaccine-induced responses induced by Th17-inducing mucosal adjuvants, along with short-term detrimental effects of vaccine-induced IL-17 responses to infections, including influenza.
Recently, several adjuvants, including oil-in-water nanoemulsions,27 CT,2,4 polyelectrolyte microcapsules,28 heat-labile enterotoxins (such as LT-IIb),5 and heat-killed Klebsiella pneumoniae,9 have been shown to induce potent Th17 responses when delivered mucosally. The ability of these mucosal adjuvants to drive the generation of potent long-term persisting antigen-specific IL-17–producing αβ T cells is critical for the protection induced by the vaccine, and so the mucosal route of vaccination in combination with an appropriate mucosal adjuvant can be an effective strategy to induce lung-resident vaccine-induced Th17 responses. However, the present findings and those of other studies show that innate cells, such as γδ T cells, are also activated by these adjuvants and produce IL-17.29 IL-17 produced by either adaptive or innate immune cells can lead to immediate inflammation upon subsequent infections, including influenza. Our research group has shown that, during influenza A/PR/8/34 infection, the IL-17 pathway plays a role in immunopathology; mice deficient in IL-17 signaling exhibit lesser morbidity, reduced levels of oxidized phospholipids, and decreased lung inflammation, compared with wild-type influenza-infected mice.13 A more recent study similarly demonstrated that infection with pandemic H1N1 influenza infection in IL-17–deficient mice resulted in reduced acute lung injury.23 Interestingly, in that study elevated levels of IL-17 were also found in mild, hospitalized, and critical groups of patients who were confirmed positive for H1N1 influenza A 2009 virus in Beijing.23
A Th17 hypercytokinemia signature was observed in individuals severely infected with influenza in whom high levels of CCL2, CXCL9, and CXCL10 were detected.24,25 Our research group previously reported that IL-17 responsive elements are detected in the promoter regions of both CXCL9 and CXCL10, and that IL-17 can induce expression of these chemokines.6,19 In addition, IL-17 can induce CCL2 production and mediate macrophage recruitment and emphysema in response to cigarette smoke in the lung.30 Thus, the induction of Th17-dependent chemokines such as CXCL9, CXCL10, and CCL2 in mucosally pre-exposed, influenza-infected mice in the present study parallels findings from human studies in which Th17 mediators are associated with more severe disease.24,25 In the present study, the detrimental effects of Th17-inducing adjuvants were short-lived, and it is possible that the inflammatory effects of mucosal delivery of Th17-inducing adjuvants wane over time. Because we delivered mucosal adjuvant alone, prior to influenza infection, we believe that the inflammatory effects mediated by the adjuvant are likely dependent on responsive innate cells producing IL-17 and are short-lived. Before incorporation of Th17 adjuvants into mucosal vaccine strategies, studies should be conducted to specifically address whether the adjuvant induces any detrimental short- or long-term effects against subsequent infections, including influenza.
In summary, the present study demonstrates that mucosal pre-exposure to Th17-inducing adjuvants can induce short-term detrimental effects upon subsequent exposure to stimuli such as influenza infection, highlighting the importance of studying both short-term and long-term effects of Th17-inducing mucosal adjuvants in health and disease.
Acknowledgments
We thank members of the John F. Alcorn Laboratory and the Ted M. Ross Laboratory for technical assistance with influenza infections and Dr. Casey Weaver for IL-17 Thy1.1 reporter mice breeder pairs.
Footnotes
Supported by NIH grants R01AI083541 and R01HL105427 (S.A.K.), R01HL107380 (J.F.A.), R01DE013833 (T.D.C.), and U19-AI091036 (J.R.M.); by the Children’s Hospital of Pittsburgh; and by funds of the Department of Medicine, University of Rochester (J.R.M.).
Disclosure: The IL-17R knockout mice and IL-17RA blocking antibody used in this study were provided by Amgen.
References
- 1.Neutra M.R., Kozlowski P.A. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 2.Lee J.B., Jang J.E., Song M.K., Chang J. Intranasal delivery of cholera toxin induces Th17-dominated T-cell response to bystander antigens. PLoS One. 2009;4:e5190. doi: 10.1371/journal.pone.0005190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zygmunt B.M., Rharbaoui F., Groebe L., Guzman C.A. Intranasal immunization promotes Th17 immune responses. J Immunol. 2009;183:6933–6938. doi: 10.4049/jimmunol.0901144. [DOI] [PubMed] [Google Scholar]
- 4.Datta S.K., Sabet M., Nguyen K.P., Valdez P.A., Gonzalez-Navajas J.M., Islam S., Mihajlov I., Fierer J., Insel P.A., Webster N.J., Guiney D.G., Raz E. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc Natl Acad Sci USA. 2010;107:10638–10643. doi: 10.1073/pnas.1002348107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gopal R., Rangel-Moreno J., Slight S., Lin Y., Nawar H.F., Fallert Junecko B.A., Reinhart T.A., Kolls J., Randall T.D., Connell T.D., Khader S.A. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol. 2013;6:972–984. doi: 10.1038/mi.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khader S.A., Bell G.K., Pearl J.E., Fountain J.J., Rangel-Moreno J., Cilley G.E., Shen F., Eaton S.M., Gaffen S.L., Swain S.L., Locksley R.M., Haynes L., Randall T.D., Cooper A.M. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y.J., Gross J., Bogaert D., Finn A., Bagrade L., Zhang Q., Kolls J.K., Srivastava A., Lundgren A., Forte S., Thompson C.M., Harney K.F., Anderson P.W., Lipsitch M., Malley R. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malley R., Srivastava A., Lipsitch M., Thompson C.M., Watkins C., Tzianabos A., Anderson P.W. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006;74:2187–2195. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen K., McAleer J.P., Lin Y., Paterson D.L., Zheng M., Alcorn J.F., Weaver C.T., Kolls J.K. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity. 2011;35:997–1009. doi: 10.1016/j.immuni.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins S.C., Jarnicki A.G., Lavelle E.C., Mills K.H. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 11.Brereton C.F., Sutton C.E., Ross P.J., Iwakura Y., Pizza M., Rappuoli R., Lavelle E.C., Mills K.H. Escherichia coli heat-labile enterotoxin promotes protective Th17 responses against infection by driving innate IL-1 and IL-23 production. J Immunol. 2011;186:5896–5906. doi: 10.4049/jimmunol.1003789. [DOI] [PubMed] [Google Scholar]
- 12.Kolls J.K., Khader S.A. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010;21:443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowe C.R., Chen K., Pociask D.A., Alcorn J.F., Krivich C., Enelow R.I., Ross T.M., Witztum J.L., Kolls J.K. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009;183:5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye P., Rodriguez F.H., Kanaly S., Stocking K.L., Schurr J., Schwarzenberger P., Oliver P., Huang W., Zhang P., Zhang J., Shellito J.E., Bagby G.J., Nelson S., Charrier K., Peschon J.J., Kolls J.K. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudva A., Scheller E.V., Robinson K.M., Crowe C.R., Choi S.M., Slight S.R., Khader S.A., Dubin P.J., Enelow R.I., Kolls J.K., Alcorn J.F. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol. 2011;186:1666–1674. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khurana S., Verma S., Verma N., Crevar C.J., Carter D.M., Manischewitz J., King L.R., Ross T.M., Golding H. Properly folded bacterially expressed H1N1 hemagglutinin globular head and ectodomain vaccines protect ferrets against H1N1 pandemic influenza virus. PLoS One. 2010;5:e11548. doi: 10.1371/journal.pone.0011548. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Bright R.A., Carter D.M., Crevar C.J., Toapanta F.R., Steckbeck J.D., Cole K.S., Kumar N.M., Pushko P., Smith G., Tumpey T.M., Ross T.M. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS One. 2008;3:e1501. doi: 10.1371/journal.pone.0001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee Y.K., Turner H., Maynard C.L., Oliver J.R., Chen D., Elson C.O., Weaver C.T. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aujla S.J., Chan Y.R., Zheng M., Fei M., Askew D.J., Pociask D.A., Reinhart T.A., McAllister F., Edeal J., Gaus K., Husain S., Kreindler J.L., Dubin P.J., Pilewski J.M., Myerburg M.M., Mason C.A., Iwakura Y., Kolls J.K. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khader S.A., Pearl J.E., Sakamoto K., Gilmartin L., Bell G.K., Jelley-Gibbs D.M., Ghilardi N., deSauvage F., Cooper A.M. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 21.Khader S.A., Rangel-Moreno J., Fountain J.J., Martino C.A., Reiley W.W., Pearl J.E., Winslow G.M., Woodland D.L., Randall T.D., Cooper A.M. In a murine tuberculosis model, the absence of homeostatic chemokines delays granuloma formation and protective immunity. J Immunol. 2009;183:8004–8014. doi: 10.4049/jimmunol.0901937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nawar H.F., Arce S., Russell M.W., Connell T.D. Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities. Infect Immun. 2005;73:1330–1342. doi: 10.1128/IAI.73.3.1330-1342.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C., Yang P., Sun Y., Li T., Wang C., Wang Z., Zou Z., Yan Y., Wang W., Chen Z., Xing L., Tang C., Ju X., Guo F., Deng J., Zhao Y., Tang J., Wang H., Zhao Z., Yin Z., Cao B., Wang X., Jiang C. IL-17 response mediates acute lung injury induced by the 2009 pandemic influenza A (H1N1) virus. Cell Res. 2012;22:528–538. doi: 10.1038/cr.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bermejo-Martin J.F., Ortiz de Lejarazu R., Pumarola T., Rello J., Almansa R., Ramírez P. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13:R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee N.L. Role of cytokines and chemokines in severe and complicated influenza infections. Hong Kong Med J. 2009;8(15 Suppl):38–41. [PubMed] [Google Scholar]
- 26.Heinecke J.W., Li W., Francis G.A., Goldstein J.A. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J Clin Invest. 1993;91:2866–2872. doi: 10.1172/JCI116531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bielinska A.U., Gerber M., Blanco L.P., Makidon P.E., Janczak K.W., Beer M., Swanson B., Baker J.R., Jr. Induction of Th17 cellular immunity with a novel nanoemulsion adjuvant. Crit Rev Immunol. 2010;30:189–199. doi: 10.1615/critrevimmunol.v30.i2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Koker S., Naessens T., De Geest B.G., Bogaert P., Demeester J., De Smedt S., Grooten J. Biodegradable polyelectrolyte microcapsules: antigen delivery tools with Th17 skewing activity after pulmonary delivery. J Immunol. 2010;184:203–211. doi: 10.4049/jimmunol.0803591. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds J.M., Angkasekwinai P., Dong C. IL-17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev. 2010;21:413–423. doi: 10.1016/j.cytogfr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen K., Pociask D.A., McAleer J.P., Chan Y.R., Alcorn J.F., Kreindler J.L., Keyser M.R., Shapiro S.D., Houghton A.M., Kolls J.K., Zheng M. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS One. 2011;6:e20333. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]