Abstract
Melanoma is a tumor where virulence is conferred on transition from flat (radial) to three-dimensional (tumorigenic) growth. Virulence of tumorigenic growth is governed by numerous attributes, including presence of self-renewing stem-like cells and related formation of patterned networks associated with the melanoma mitogen, laminin, a phenomenon known as vasculogenic mimicry. Vasculogenic mimicry is posited to contribute to melanoma perfusion and nutrition in vivo; we hypothesized that it may also play a role in stem cell–driven spheroid formation in vitro. Using a model of melanoma in vitro tumorigenesis, laminin-associated networks developed in association with three-dimensional melanoma spheroids. Real-time PCR analysis of laminin subunits showed that spheroids formed from anchorage-independent melanoma cells expressed increased α4 and β1 laminin chains and α4 laminin expression was confirmed by in situ hybridization. Association of laminin networks with melanoma stem cell–associated nestin and vascular endothelial growth factor receptor-1 also was documented. Moreover, knockdown of nestin gene expression impaired laminin expression and network formation within spheroids. Laminin networks were remarkably similar to those observed in melanoma xenografts in mice and to those seen in patient melanomas. These data indicate that vasculogenic mimicry–like laminin networks, in addition to their genesis in vivo, are integral to the extracellular architecture of melanoma spheroids in vitro, where they may serve as stimulatory scaffolds to support three-dimensional growth.
Vasculogenic mimicry (VM) is a phenomenon that may be observed in aggressive melanoma cell lines that contain subpopulations of cells with an embryonic-like phenotype.1 First described by Maniotis, Hendrix, and colleagues in 1999,2 VM is defined by expression of endothelial genes by primitive tumor cells in association with net-like or tubular patterns of extracellular matrix deposition.3,4 VM-like networks within melanoma are PAS-positive and, in patients, are associated with poor prognosis independent of Breslow depth.5,6 In addition to PAS positivity, laminin expression is also an important biomarker associated with VM.1,7 Inhibition of the laminin γ2 chain reduced the expression of vasculogenic mimicry–associated genes in melanoma cells.8 Furthermore, when nonaggressive melanoma cells were plated onto collagen matrices preconditioned with laminin networks made by aggressive melanoma cells, they formed tubules and networks along the existing laminin scaffolds.8 Genes involved in angiogenesis and vasculogenesis were up-regulated in aggressive melanoma cells.9 These included vascular endothelial growth factor-C (VEGF-C), vascular endothelial (VE)-cadherin (CD144), tyrosine kinase with Ig and epidermal growth factor homology domains-1 (TIE-1), the laminin-5 γ2 chain, and ephrin-A2. The amount of VE-cadherin and TIE-1 seen on Western blots was greater in highly aggressive, as opposed to less aggressive, melanoma cell lines.10 CD31 was not present. Melanoma cells with VE-cadherin knockdown lost the ability to form VM-like networks,10 implicating their crucial role in VM.
We have previously confirmed laminin chain β2 networks in melanoma xenografts to be dependent on expression of vascular endothelial growth factor receptor-1 (VEGFR-1) by stem-like melanoma cells that coexpress endothelial markers VE-cadherin and TIE-1, but not CD31.11 VM, as described by Hendrix and colleagues,1,3 may be promoted by a hypoxia-driven in vivo pathway whereby aggressive cancer cells contribute to perfusion during periods of rapid growth via true anastomoses with authentic tumor vessels or by transporting fluid from sites of intralesional vascular leakage.12,13
Laminin is a heterotrimeric protein composed of various combinations of one of five α, three β, and three γ subunits. Laminin serves as an important basement membrane protein, with laminin 411 (α4, β1, γ1) involved in formation of vascular basement membranes. Various laminin isoforms are expressed by melanoma cell lines and patient melanomas.8,14,15 Throughout this paper, unless a specific laminin chain or heterotrimer is designated, we use laminin as a generic term for the protein family or when the specific laminin isoforms are unknown. Moreover, studies have implicated a correlation between laminin expression and melanoma virulence, although the biological basis for this association remains conjectural. It is known, however, that extracellular laminin (either secreted by keratinocytes, endothelial cells, or added exogenously) promotes melanoma mitogenesis, growth, and migration.16–20 Indeed, particular regions within laminin chains have been found to enhance melanoma metastasis to organs such as lung and liver.21,22
In this study, we used melanoma spheriods as a model for three-dimensional tumorigenesis in an effort to study laminin-associated VM.23–26 Although previous pioneering work has established the ability of several melanoma cell lines to produce VM on radial proliferation on laminin gels,2 examination of three-dimensional models that recapitulate the expansile tumorigenic growth phase of melanoma is lacking. Melanoma spheroids have been used as assays for stem cell activity and have been implicated in melanoma self-renewal and tumorigenesis.27,28 We hypothesized that not only is VM integral for melanoma nutrition and perfusion but also plays an important role in stem cell–driven spheroid formation, a potential three-dimensional in vitro surrogate for early tumorigenic growth. The data presented support this hypothesis and establish melanoma spheroids as a novel model for exploring the pathobiology of VM.
Materials and Methods
Cell Lines and Cell Culture
Human melanoma cell A2058 and A375 were originally obtained from American Type Culture Collection (Manassas, VA). Melanoma cells were grown in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone Laboratories, Logan, UT) and 200 mmol/L l-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin, and maintained at 37°C, 5% CO2. Viable cells were counted by Trypan blue exclusion assay under a hemocytometer.
Melanoma Spheroid Cultures
Liquefied Matrigel (50 μL; Millipore, Billerica, MA) was spotted onto a Petri dish and allowed to set at 37°C for 2 hours; 5 × 103 A2058 viable cells per 10 μL complete medium were loaded over Matrigel, incubated at 37°C, 5% CO2, for 4 hours for attachment, and then overlaid with complete medium and cultured for 7 to 10 days. Melanoma spheroids over Matrigel were fixed with formalin and embedded. Perpendicular sections were used for histological analysis.
Melanoma spheroid culture in suspension was performed at low cell-plating density (2000 viable cells per 6-well or 1000 viable cells per 24-well) in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and penicillin/streptomycin/glutamine in the ultra-low attachment plate (Corning, Acton, MA) at 37°C containing 5% CO2 for 14 to 21 days. Spheroids were fed with 0.5 mL of fresh medium twice weekly. They were collected, fixed, and processed for histological analysis using a Cellient Automated Cell Block System (Hologic, Marlborough, MA). Epithelial growth factor and fibroblast growth factor-2 were not used for Matrigel or suspension cultures because they may alter nestin expression.29,30 Methylcellulose was used in the media for all spheroid cultures to prevent aggregation.31
shRNA Gene Knockdown
A lentivirus-based shRNA method was used to knock down the gene expression of nestin. shRNA clones (TRCN000014728, TRCN000014729, TRCN000014730, TRCN000014731, and TRCN000014732) specifically targeting nestin were purchased (Sigma-Aldrich). shRNA lentivirus was produced in HEK293T cells by cotransfecting shRNA lentiviral backbone and packaging vectors psPAX2, pMD2.VSV-G as described before.32 Supernatant was collected and filtered through 0.45-μm filters and used to transfect target cells. A nontargeting, scramble vector (SHC002; Sigma-Aldrich) was used as vector control. Efficacy of nestin gene knockdown (nestin KD) was confirmed by quantitative real-time PCR and Western blot. Among the five shRNA clones examined, TRCN000014728, which targeted the 3′-untranslated region of nestin, had the highest KD efficiency and was routinely used in this study.
Quantitative Real-Time RT-PCR
Total RNA from A2058 adherent cells and spheroid-forming cells, including nestin KD and vector controls, was extracted using RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was reverse transcribed from total RNA using the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). Relative expression of each of the eight laminin α and β chain isoforms were compared between the spheroid-forming and adherent cells using real-time RT-PCR Applied Biosystems 7300 system (Foster City, CA) and the 2−ΔΔCt method of analysis33 with >2-fold changes considered significant. Similarly, nestin expression was analyzed in the nestin KD and vector control spheroids compared to glyceraldehyde 3-phosphate dehydrogenase. The primers of laminin chains α1, α3 to α5, and β1 to β3, as seen in Table 1, were ordered for SyBr Green real-time PCR, partly based on the sequences of Oikawa et al.14 TaqMan real-time PCR primers of human nestin (Hs00707120_s1), matrix metalloproteinase 2 (MMP2) (Hs01548727), and laminin α2 (Hs01124081_m1) were purchased from Applied Biosystems.
Table 1.
Laminin Primer Sequences
| Laminin | Forward | Reverse |
|---|---|---|
| alpha 1 | 5′-CAGACTTTGGATGAAGATTTCC-3′ | 5′-AGTTCAAGGGTGGCATTTTG-3′ |
| alpha 3 | 5′-AGTCGACTAAGTTTCCCTCC-3′ | 5′-CAAGGCTCCACTTCAGTTGTG-3′ |
| alpha 4 | 5′-CTGGATCAGCTTCGTACGGT-3′ | 5′-GAGACTTGGATCTTGCTGGC-3′ |
| alpha 5 | 5′-AACAACTTCGCCGAGGGCTG-3′ | 5′-AGTGGGTTCCCAAAGAATCC-3′ |
| beta 1 | 5′-CAGCAGCTTCTGAGGAAACC-3′ | 5′-CAATATATTCTGCCTCCCCG-3′ |
| beta 2 | 5′-TGGCTTCTTTGGGCTCAGCA-3′ | 5′-ACTGTTGGGGTCACAAGGAG-3′ |
| beta 3 | 5′-CGGGCTGCGACAAGGCATCA-3′ | 5′-CACCGGGTAGCGATTACAGTA-3′ |
Western Blot Analysis
Subconfluent cell cultures and medium were collected for Western blot analysis. Cells were resuspended in cell lysate buffer (Cell Signaling, Danvers, MA) containing proteinase inhibitor and phenylmethylsulfonyl fluoride. Cell lysate protein (100 μg) and 50 μL of cell culture medium (cell concentration of 2 × 105/mL) were loaded onto SDS-PAGE gel along with molecular weight markers. Proteins were separated at a constant 100 V for 3.5 hours and transferred to a nitrocellulose membrane at a constant 340 mA for 1.5 hours at 4°C. The membrane was blocked with 5% nonfat milk in Tris-buffered saline–Tween 20 at room temperature for 1 hour, incubated with primary nestin antibody at 1:1000 dilution at 4°C overnight, and incubated with horseradish peroxidase–conjugated secondary antibodies at room temperature for 1 hour with actin as the loading control. Signal was developed using chemiluminescent substrate (Thermo Scientific, Rockford, IL) at room temperature for 5 minutes and detected by film. Antibodies for Western blot analysis were commercially available for mouse anti-nestin (#AB5922; Millipore), and mouse anti-actin (#ab6276; Abcam, Cambridge, MA).
Immunohistochemistry
Immunohistochemical studies used 5-μm sections of formalin-fixed, paraffin-embedded tissue. All slides were deparaffinized and rehydrated, and for laminin staining, underwent antigen retrieval with 60 μg/mL proteinase K at 37°C for 30 minutes. For the remaining antibodies, heat-based antigen retrieval was performed in citrate buffer. Sections were incubated with primary antibody at 4°C overnight and then incubated with peroxidase-conjugated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) at room temperature for 60 minutes, followed by detection with NovaRED Substrate (Vector Laboratories) for nonfluorescent immunohistochemistry. For immunofluorescent staining, slides were incubated with Alexa Fluor 594 donkey anti-goat IgG and Alexa Fluor 488 donkey anti-rabbit IgG secondary antibodies at room temperature for 60 minutes. Rabbit anti-human polyclonal laminin (1:7500) (#Z0097; Dako, Carpinteria, CA), rabbit anti-human nestin (1:1000) (#5326; Millipore), rabbit anti-human VE-cadherin (1:20) (#2500; Cell Signaling Technology, Beverly, MA), rabbit anti-human CD31 (1:100) (#00055; Bethyl, Montgomery, TX), and goat anti-human VEGFR-1 (1:20) (#AF321; R&D Systems, Minneapolis, MN) primary antibodies were used.
In Situ Hybridization
Laminin RNA probes were prepared with the primer pair 5′-TAATACGACTCACTATAGGGA-3′ and 5′-CCCGCAGTTGCACACTGCACAG-3′, and used to generate the DNA template for antisense and sense RNA probes spanning 282 bp of human laminin α4 cDNA. RNA probe labeling with digoxigenin and in situ hybridizations were performed as previously described.34
Melanoma Xenografts
NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were maintained in accordance with the institutional guidelines of Harvard Medical School, and experiments were performed according to the approved experimental protocols. Human to mouse melanoma xenografts were performed as described11 with injection of 103 to 104 A375 melanoma cells into NSG mice.
Patient Melanomas
Eleven human melanomas (five radial growth phase and six vertical growth phase) were obtained from the Pathology Archives of Brigham and Women’s Hospital. The radial growth phase lesions were all Clark levels I and II, and the vertical growth phase lesions were all Clark levels III and IV. Patient samples were obtained with approval from the institutional review board of Brigham and Women’s Hospital.
Results
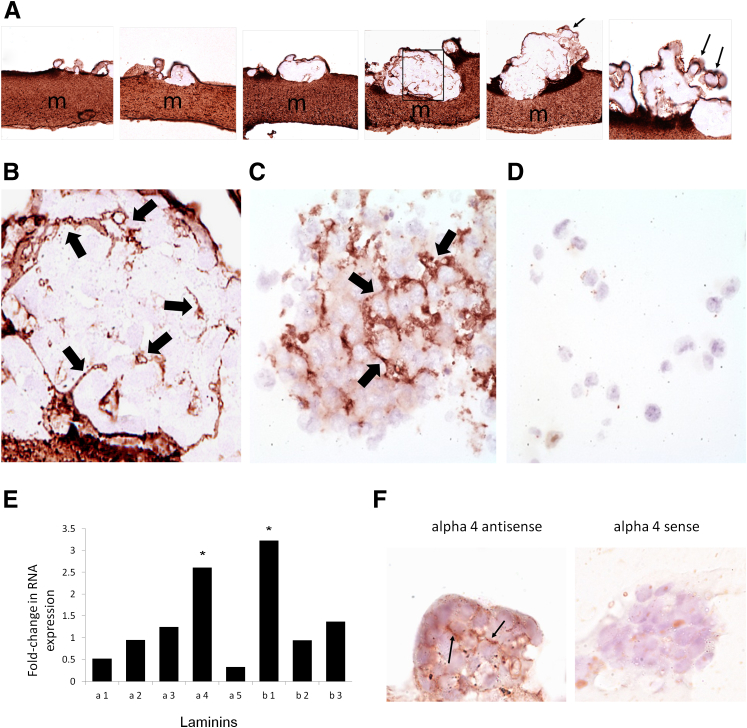
Melanoma spheroids derived from both A2058 and A375 cell lines formed within 2 weeks from cells growing on Matrigel and within 3 weeks from suspension cultures. Matrigel-associated spheroids appeared to develop from small aggregates of cells that progressively proliferated along three-dimensional planes (Figure 1A). Laminin staining (Figure 1) revealed variably complex patterned networks within Matrigel-associated spheroids (Figure 1, A and B) as well as in spheroids derived from suspension culture (Figure 1C). By contrast, adherent cells not grown on Matrigel failed to form spheroids or stain positively for detectable laminin by immunohistochemistry (Figure 1D). RT-PCR of laminin subunits confirmed that A2058 Matrigel-derived spheroids expressed a different laminin subunit mRNA profile from non–spheroid-forming adherent cells (Figure 1E). Specifically, spheroid-forming cells showed a greater than twofold increase in laminin chains α4 and β1. Laminin α4 production by spheroids was further confirmed by in situ hybridization with a probe specific for the laminin α4 subunit (Figure 1F).
Figure 1.
Laminin immunohistochemistry of developing melanoma A2058 spheroids (A) grown on laminin-containing Matrigel matrices (m) shows progressive development of internal patterned networks and small lumen-like spaces (region enclosed by the box is enlarged in B). Laminin also formed a mantle that enveloped the spheroids (arrows) and that appeared to associate with budding of minispheroids into the culture suspension (A, arrows). A2058 and A375 melanoma spheroids grown free in suspension also exhibited immunohistochemical laminin networks (C, arrows), unlike single adherent melanoma cells (D). RT-PCR of specific laminin α and β subunit chains confirmed that A2058 melanoma spheroid-forming cells expressed a different laminin subunit profile from A2058 melanoma adherent cells with increased expression of the α4 and β1 chains (E). In situ hybridization for the laminin α4 chain mRNA on A2058 melanoma spheroids confirms this finding (F, arrows indicate laminin expression). Asterisks indicate a greater than twofold increase in expression. Original magnification: ×400 (A and F); ×1600 (B, C, and D).
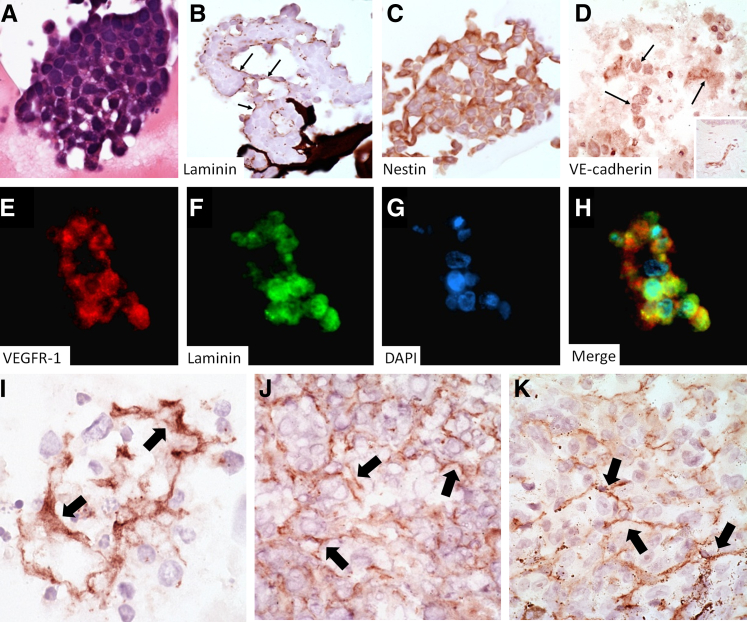
H&E evaluation of Matrigel-associated spheroids revealed occasional foci of lumen-like formations (Figure 2A). These lumen-like structures consisted of circular to ovoid zones lined by a laminin-positive mantle (Figure 2B) that was focally continuous with laminin networks (Figure 1B) and stained positively for the vasculogenesis and stem-cell–associated biomarker nestin (Figure 2C), as well as for VE-cadherin (Figure 2D), whereas the mantle stained negatively for CD31 (not shown). VEGFR-1 and laminin immunofluorescence double labeling required steam antigen-retrieval and revealed colocalization of these two markers in cell cords with evidence of cytoplasmic laminin not generally appreciated by immunohistochemistry with proteinase K antigen retrieval (Figure 2, E–H). Overall, laminin-positive networks in spheroids were similar to those seen in mouse melanoma xenografts and in human melanomas (Figure 2, I–K). Networks in patient melanomas were restricted to the vertical growth phase of patient melanomas and were detected in only 17% of vertical growth phase lesions examined.
Figure 2.
H&E staining of spheroids shows lumen-like spaces (A) that stain positively for laminin by immunohistochemistry (B, arrows) and the associated angiogenesis/vasculogenesis markers nestin (C), VE-cadherin (D, arrows indicate positive expression, inset is a VE-cadherin stain of a blood vessel in normal skin), and VEGFR-1, whose expression overlaps with laminin by fluorescent immunohistochemistry (VEGFR-1, E; laminin, F; DAPI, G; merged image, H). The immunohistochemical laminin networks seen in melanoma spheroids (I) are similar to those seen in melanoma xenografts (J) and patient melanomas (K); arrows indicate laminin expression. Original magnification: ×400 (A–K); ×100 (inset).
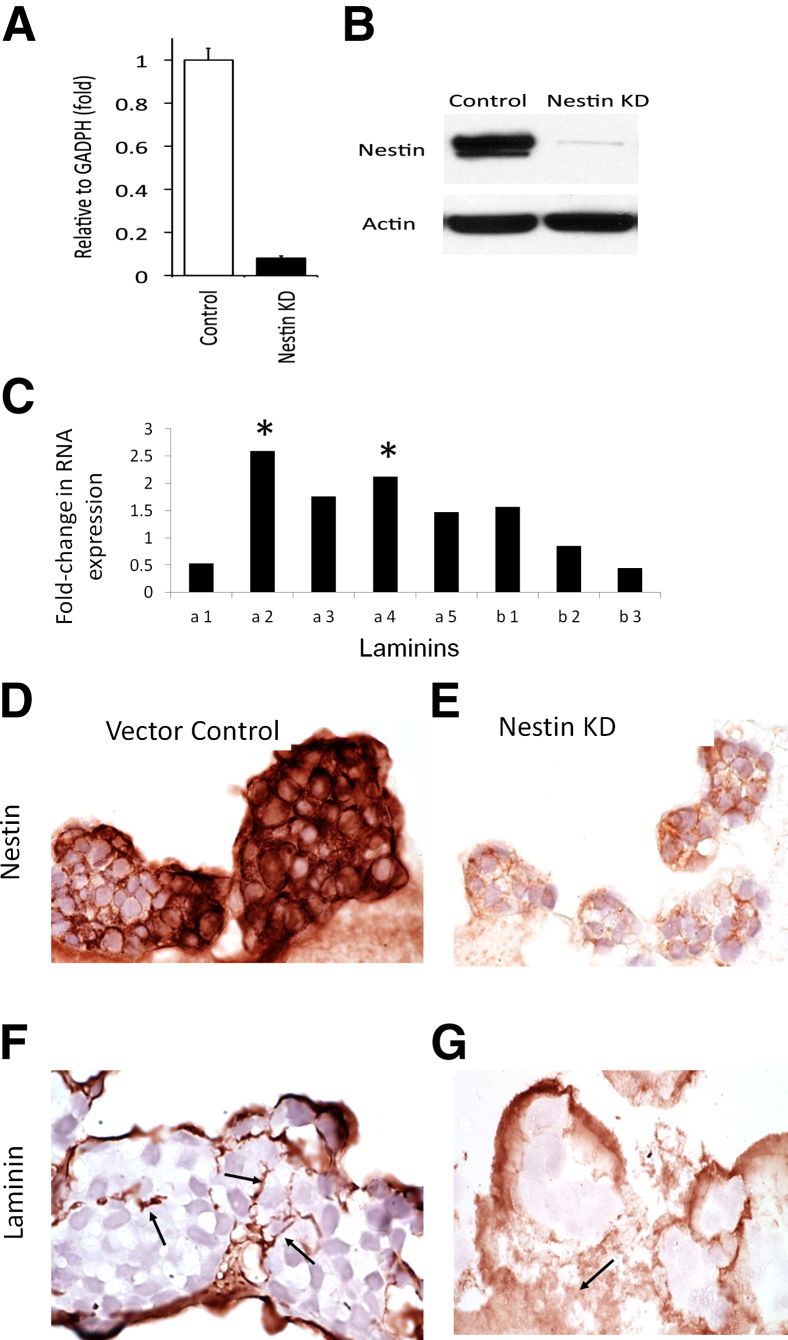
To assess depletion of a known melanoma stem cell–associated marker, nestin,35 on laminin network formation, we evaluated the effects of nestin gene KD on spheroid laminin network formation by the A2058 melanoma cell line (Figure 3). Nestin KD was confirmed by RT-PCR (Figure 3A) and Western blotting (Figure 3B). For most laminin chains, including α4 and β1, there was increased expression in vector control cells compared to KD cells (Figure 3C). As compared to wild-type controls, nestin KD immunohistochemistry (Figure 3, D and E), showed markedly diminished to absent formation of laminin networks in size-matched spheroids (Figure 3, F and G). Because tubular structures were an infrequent finding in the wild-type spheroids, it was difficult to tell whether there was a marked change in these structures on nestin KD. Additionally, nestin KD did not alter MMP2 mRNA expression in A2058 melanoma cells (data not shown).
Figure 3.
Nestin KD A2058 melanoma cells show a decrease in nestin RNA by PCR (A) and protein by Western blot (B). Vector control melanoma cells show increased expression of multiple laminin chains, including α4 and β1; α2 and α4 are significantly increased compared to nestin KD (C). Similarly sized spheroids show increased nestin expression by immunohistochemistry in vector controls (D) compared to nestin KD spheroids (E) and have well-formed laminin mantles in controls (F, arrows) compared to poorly formed wispy laminin networks in nestin KD (G, arrows). Asterisks indicate greater than twofold increase in expression. Original magnification: ×400 (D–G).
Discussion
In this study, we demonstrated that: i) laminin networks resembling VM develop in melanoma spheroids in vitro; ii) spheroids with laminin networks and lumen-like structures contain subpopulations of cells that preferentially express VM and stem cell–associated biomarkers VE-cadherin, VEGFR-1, and nestin; iii) KD of the melanoma stem cell biomarker nestin diminishes laminin network formation; and iv) spheroid laminin networks are morphologically similar to xenograft and patient melanoma networks.
It has been well known since the 1980s that certain melanoma cell lines, or subpopulations of cells within these cell lines, form spheroids in vitro.36 Melanoma formation of spheroids is believed by many to serve as a model for a subpopulation of self-renewing stem-like melanoma cells that through asymmetrical division produce cellular diversity during three-dimensional tumorigenesis akin to the nodular vertical growth phase of melanoma in vivo.25,26,37,38 Melanoma spheroids exhibit more chemoresistance than their counterparts that grow in monolayers,26,39 and this characteristic is considered to further reflect stem cell–like behavior.25,38 However, spheroids are not universally accepted as a system with pure clonally derived colonies, and there is a debate regarding the use of spheroids as a model system for stem cell–like tumorigenesis.31,40 In theory, spheroids could also arise from cell–cell aggregation, although in our hands, melanoma spheroids preferentially express stem cell markers (unpublished data), and methylcellulose is regularly used to prevent aggregation.31 The tendency of melanoma cells to form spheroids may also be due to the microenviroment in which they are grown.41,42 Cancer spheroids are known to form preferentially in association with Matrigel, and this was also true in the present study for A2058 melanoma cells.43 In one study, when Matrigel was injected along with melanoma cells into NOD/SCID mice, the melanoma cells had a higher growth rate and showed increased tumorigenicity.44 Matrigel contains structural proteins such as laminin, a known melanoma mitogen,16–20 as well as collagen and other growth factors.45 Laminin subunit β2 has recently been shown to be produced in human melanoma xenografts where it is intimately associated with stem-like cells.11 The capability of generating laminin networks by melanoma spheroids, both associated and unassociated with Matrigel, and the spatial relationship of these networks to stem-like cells further emphasizes the potential interdependence of stem cell–driven three-dimensional melanoma growth and key mediators in the extracellular microenvironment. Although MMP2 has been shown to be indispensable for VM,8 in our study, expression of stem cell–associated nestin appeared to be required for VM formation, prompting ongoing inquiry into potential biological interplay between nestin and MMP gene expression (unpublished data). Nestin has been shown to be related to other stem-like cell marker pathways that are associated with VM, specifically Notch4/Nodal and cyclic adenosine monophosphate (cAMP)/exchange protein activated by cAMP (Epac)/Ras related protein 1 (Rap1). Notch4 and Nodal have been found to be associated with VM and aggressive behavior in melanoma cells,46 murine xenografts and patient melanomas.34 Nodal inhibition is associated with up-regulation of nestin in embryonic stem cells, implying a relationship between these proteins. The cAMP/Epac/Rap1 pathway inhibits VM in melanoma cells,47 although individually, cAMP48 and Rap149 may increase nestin expression. Thus, although nestin expression appears to impact on pathways that influence VM formation, the complexity of these interactions will require further study.
The posited functions of VM in vivo are vascular perfusion, fluid transport, and nutrition, all critical requirements at early stages of invasive melanoma tumorigenesis when expansile intradermal growth may not be fully supported by conventional angiogenesis. However, the spheroid model is an in vitro surrogate for tumorigenic growth devoid of dependency on angiogenesis. Accordingly, laminin networks within melanoma spheroids could provide insight into an additional benefit of VM, namely provision of a three-dimensional stimulatory scaffold to support tumorigenic growth.
We noted increased expression of laminin chains α4 and β1 in melanoma spheroids. Laminin 411 is typically expressed in the basement membrane of blood vessels, consistent with VM-like activity by spheroid-forming cells. This notion is further substantiated by biomarkers indicative of expression of endothelial and stem cell–related proteins. In contrast to melanoma spheroids, it may be difficult to distinguish whether the laminin in VM within human melanomas and melanoma xenografts is derived from endothelial cells, adjacent keratinocytes, or melanoma cells per se. Accordingly, melanoma spheroids, particularly when unassociated with Matrigel, provide novel insight into the biology of VM-like network formation and now provide a model for further elucidation of this phenomenon. It is interesting that different laminin chains and heterotrimers have been found to be expressed by different melanoma cell lines and patient samples.8,14,15 Thus, there seems to be heterogeneity in the subsets of laminin proteins involved in VM in melanoma.
In our experience, laminin network expression by advanced primary cutaneous melanomas in patients is not a ubiquitous finding. Indeed, of six vertical growth phase lesions in this study, only one showed striking network formation. Most melanomas begin as a superficial growth phase closely associated with the laminin-rich dermal–epidermal junction. In this phase, cells proliferate radially along an extrinsic laminin gradient produced predominantly by basal keratinocytes.50 Invasive cells eventually acquire the capacity to grow within the dermis vertically in three-dimensional spherical nodules. During early phases of this vertical (tumorigenic) growth before the ingrowth of authentic vessels, metabolic factors [eg, hypoxia-inducible factor 1α (HIF-1α)] drive VM.51 This scenario could explain why some established invasive cutaneous melanomas, replete with networks of authentic tumor vessels and evolving over many months to years, may show a paucity of residual VM-like laminin networks, although these networks are easily detected in human melanoma xenografts11 that grow rapidly over several weeks. The ability to study laminin networks and the cells that produce them in spheroids will hopefully now provide translational insights into the roles of VM in patient melanomas.
Footnotes
Supported by NIH grant SPORE 5P50CA093683-08, NIH grant 5R01CA138231-05, and BWH Biomedical Research Institute Microseed grant. A.R.L. is supported by NIH Training grant T32AR007098-38.
Disclosures: G.F.M. holds stock options or bond holdings in Bristol-Meyers-Squibb. G.F.M. receives funding from Bristol-Myers Squibb for work on melanoma biomarkers that are unrelated to this work.
References
- 1.Hendrix M.J., Seftor E.A., Hess A.R., Seftor R.E. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 2.Maniotis A.J., Folberg R., Hess A., Seftor E.A., Gardner L.M., Pe’er J., Trent J.M., Meltzer P.S., Hendrix M.J. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seftor R.E., Hess A.R., Seftor E.A., Kirschmann D.A., Hardy K.M., Margaryan N.V., Hendrix M.J. Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Pathol. 2012;181:1115–1125. doi: 10.1016/j.ajpath.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folberg R., Maniotis A.J. Vasculogenic mimicry. APMIS. 2004;112:508–525. doi: 10.1111/j.1600-0463.2004.apm11207-0810.x. [DOI] [PubMed] [Google Scholar]
- 5.Thies A., Mangold U., Moll I., Schumacher U. PAS-positive loops and networks as a prognostic indicator in cutaneous malignant melanoma. J Pathol. 2001;195:537–542. doi: 10.1002/path.988. [DOI] [PubMed] [Google Scholar]
- 6.Warso M.A., Maniotis A.J., Chen X., Majumdar D., Patel M.K., Shilkaitis A., Gupta T.K., Folberg R. Prognostic significance of periodic acid-Schiff-positive patterns in primary cutaneous melanoma. Clin Cancer Res. 2001;7:473–477. [PubMed] [Google Scholar]
- 7.Iyengar B., Singh A.V. Embryonic vasculogenesis in nodular melanomas and tumour differentiation. Pathol Oncol Res. 2011;17:569–577. doi: 10.1007/s12253-010-9350-y. [DOI] [PubMed] [Google Scholar]
- 8.Seftor R.E., Seftor E.A., Koshikawa N., Meltzer P.S., Gardner L.M., Bilban M., Stetler-Stevenson W.G., Quaranta V., Hendrix M.J. Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61:6322–6327. [PubMed] [Google Scholar]
- 9.Seftor E.A., Meltzer P.S., Schatteman G.C., Gruman L.M., Hess A.R., Kirschmann D.A., Seftor R.E., Hendrix M.J. Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: role in vasculogenic mimicry. Crit Rev Oncol Hematol. 2002;44:17–27. doi: 10.1016/s1040-8428(01)00199-8. [DOI] [PubMed] [Google Scholar]
- 10.Hendrix M.J., Seftor E.A., Meltzer P.S., Gardner L.M., Hess A.R., Kirschmann D.A., Schatteman G.C., Seftor R.E. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci U S A. 2001;98:8018–8023. doi: 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank N.Y., Schatton T., Kim S., Zhan Q., Wilson B.J., Ma J., Saab K.R., Osherov V., Widlund H.R., Gasser M., Waaga-Gasser A.M., Kupper T.S., Murphy G.F., Frank M.H. VEGFR-1 expressed by malignant melanoma-initiating cells is required for tumor growth. Cancer Res. 2011;71:1474–1485. doi: 10.1158/0008-5472.CAN-10-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruf W., Seftor E.A., Petrovan R.J., Weiss R.M., Gruman L.M., Margaryan N.V., Seftor R.E., Miyagi Y., Hendrix M.J. Differential role of tissue factor pathway inhibitors 1 and 2 in melanoma vasculogenic mimicry. Cancer Res. 2003;63:5381–5389. [PubMed] [Google Scholar]
- 13.Mihic-Probst D., Ikenberg K., Tinguely M., Schraml P., Behnke S., Seifert B., Civenni G., Sommer L., Moch H., Dummer R. Tumor cell plasticity and angiogenesis in human melanomas. PloS One. 2012;7:e33571. doi: 10.1371/journal.pone.0033571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oikawa Y., Hansson J., Sasaki T., Rousselle P., Domogatskaya A., Rodin S., Tryggyason K., Patarroyo M. Melanoma cells produce multiple laminin isoforms and strongly migrate on alpha5 laminin(s) via several integrin receptors. Exp Cell Res. 2011;317:1119–1133. doi: 10.1016/j.yexcr.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Chen H.B., Chen L., Zhung J.K., Chow V.W., Wu B.Q., Wang Z.H., Cheng S.B., Chew E.C. Expression of laminin in metastatic melanoma cell lines with different metastatic potential. Anticancer Res. 2001;21:505–508. [PubMed] [Google Scholar]
- 16.Akalu A., Roth J.M., Caunt M., Policarpio D., Liebes L., Brooks P.C. Inhibition of angiogenesis and tumor metastasis by targeting a matrix immobilized cryptic extracellular matrix epitope in laminin. Cancer Res. 2007;67:4353–4363. doi: 10.1158/0008-5472.CAN-06-0482. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji T., Kawada Y., Kai-Murozono M., Komatsu S., Han S.A., Takeuchi K., Mizushima H., Miyazaki K., Irimura T. Regulation of melanoma cell migration and invasion by laminin-5 and alpha3beta1 integrin (VLA-3) Clin Exp Metastasis. 2002;19:127–134. doi: 10.1023/a:1014573204062. [DOI] [PubMed] [Google Scholar]
- 18.Saito N., Hamada J., Furukawa H., Tsutsumida A., Oyama A., Funayama E., Saito A., Tsuji T., Tada M., Moriuchi T., Yamamoto Y. Laminin-421 produced by lymphatic endothelial cells induces chemotaxis for human melanoma cells. Pigment Cell Melanoma Res. 2009;22:601–610. doi: 10.1111/j.1755-148X.2009.00590.x. [DOI] [PubMed] [Google Scholar]
- 19.Chung H., Suh E.K., Han I.O., Oh E.S. Keratinocyte-derived laminin-332 promotes adhesion and migration in melanocytes and melanoma. J Biol Chem. 2011;286:13438–13447. doi: 10.1074/jbc.M110.166751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mortarini R., Gismondi A., Maggioni A., Santoni A., Herlyn M., Anichini A. Mitogenic activity of laminin on human melanoma and melanocytes: different signal requirements and role of beta 1 integrins. Cancer Res. 1995;55:4702–4710. [PubMed] [Google Scholar]
- 21.Kuratomi Y., Nomizu M., Tanaka K., Ponce M.L., Komiyama S., Kleinman H.K., Yamada Y. Laminin gamma 1 chain peptide, C-16 (KAFDITYVRLKF), promotes migration, MMP-9 secretion, and pulmonary metastasis of B16-F10 mouse melanoma cells. Br J Cancer. 2002;86:1169–1173. doi: 10.1038/sj.bjc.6600187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim W.H., Nomizu M., Song S.Y., Tanaka K., Kuratomi Y., Kleinman H.K., Yamada Y. Laminin-alpha1-chain sequence Leu-Gln-Val-Gln-Leu-Ser-Ile-Arg (LQVQLSIR) enhances murine melanoma cell metastases. Int J Cancer. 1998;77:632–639. doi: 10.1002/(sici)1097-0215(19980812)77:4<632::aid-ijc25>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Rappa G., Mercapide J., Anzanello F., Prasmickaite L., Xi Y., Ju J., Fodstad O., Lorico A. Growth of cancer cell lines under stem cell-like conditions has the potential to unveil therapeutic targets. Exp Cell Res. 2008;314:2110–2122. doi: 10.1016/j.yexcr.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Na Y.R., Seok S.H., Kim D.J., Han J.H., Kim T.H., Jung H., Lee B.H., Park J.H. Isolation and characterization of spheroid cells from human malignant melanoma cell line WM-266-4. Tumour Biol. 2009;30:300–309. doi: 10.1159/000261073. [DOI] [PubMed] [Google Scholar]
- 25.Fang D., Nguyen T.K., Leishear K., Finko R., Kulp A.N., Hotz S., Van Belle P.A., Xu X., Elder D.E., Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 26.Kalirai H., Damato B.E., Coupland S.E. Uveal melanoma cell lines contain stem-like cells that self-renew, produce differentiated progeny, and survive chemotherapy. Invest Ophthalmol Vis Sci. 2011;52:8458–8466. doi: 10.1167/iovs.11-7379. [DOI] [PubMed] [Google Scholar]
- 27.Schatton T., Murphy G.F., Frank N.Y., Yamaura K., Waaga-Gasser A.M., Gasser M., Zhan Q., Jordan S., Duncan L.M., Weishaupt C., Fuhlbrigge R.C., Kupper T.S., Sayegh M.H., Frank M.H. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boiko A.D., Razorenova O.V., van de Rijn M., Swetter S.M., Johnson D.L., Ly D.P., Butler P.D., Yang G.P., Joshua B., Kaplan M.J., Longaker M.T., Weissman I.L. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang K.W., Huang Y.L., Wong Z.R., Su P.H., Huang B.M., Ju T.K., Yang H. Fibroblast growth factor-2 up-regulates the expression of nestin through the Ras-Raf-ERK-Sp1 signaling axis in C6 glioma cells. Biochem Biophys Res Commun. 2013;434:854–860. doi: 10.1016/j.bbrc.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 30.Delcroix G.J., Curtis K.M., Schiller P.C., Montero-Menei C.N. EGF and bFGF pre-treatment enhances neural specification and the response to neuronal commitment of MIAMI cells. Differentiation. 2010;80:213–227. doi: 10.1016/j.diff.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Kuch V., Schreiber C., Thiele W., Umansky V., Sleeman J.P. Tumor-initiating properties of breast cancer and melanoma cells in vivo are not invariably reflected by spheroid formation in vitro, but can be increased by long-term culturing as adherent monolayers. Int J Cancer. 2012;132:E95–E105. doi: 10.1002/ijc.27785. [DOI] [PubMed] [Google Scholar]
- 32.Girouard S.D., Laga A.C., Mihm M.C., Scolyer R.A., Thompson J.F., Zhan Q., Widlund H.R., Lee C.W., Murphy G.F. SOX2 contributes to melanoma cell invasion. Lab Invest. 2011;92:362–370. doi: 10.1038/labinvest.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.McAllister J.C., Zhan Q., Weishaupt C., Hsu M.Y., Murphy G.F. The embryonic morphogen, Nodal, is associated with channel-like structures in human malignant melanoma xenografts. J Cutan Pathol. 2010;37(Suppl 1):19–25. doi: 10.1111/j.1600-0560.2010.01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein W., Wu B., Zhao S., Wu H., Klein-Szanto A., Tahan S. Increased expression of stem cell markers in malignant melanoma. Mod Pathol. 2007;20:102–107. doi: 10.1038/modpathol.3800720. [DOI] [PubMed] [Google Scholar]
- 36.Rofstad E.K., Wahl A., Davies Cde L., Brustad T. Growth characteristics of human melanoma multicellular spheroids in liquid-overlay culture: comparisons with the parent tumour xenografts. Cell Tissue Kinet. 1986;19:205–216. doi: 10.1111/j.1365-2184.1986.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 37.Ramgolam K., Lauriol J., Lalou C., Lauden L., Michel L., de la Grange P., Khatib A.M., Aoudjit R., Charron D., Alcaide-Loridan C., Al-Daccak R. Melanoma spheroids grown under neural crest cell conditions are highly plastic migratory/invasive tumor cells endowed with immunomodulator function. PloS One. 2011;6:e18784. doi: 10.1371/journal.pone.0018784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santini R., Vinci M.C., Pandolfi S., Penachioni J.Y., Montagnani V., Olivito B., Gattai R., Pimpinelli N., Gerlini G., Borgognoni L., Stecca B. Hedgehog-GLI signaling drives self-renewal and tumorigenicity of human melanoma-initiating cells. Stem Cells. 2012;30:1808–1818. doi: 10.1002/stem.1160. [DOI] [PubMed] [Google Scholar]
- 39.Smalley K.S., Haass N.K., Brafford P.A., Lioni M., Flaherty K.T., Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–1144. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- 40.Schatton T., Frank M.H. The in vitro spheroid melanoma cell culture assay: cues on tumor initiation? J Invest Dermatol. 2010;130:1769–1771. doi: 10.1038/jid.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sztiller-Sikorska M., Koprowska K., Jakubowska J., Zalesna I., Stasiak M., Duechler M., Czyz M.E. Sphere formation and self-renewal capacity of melanoma cells is affected by the microenvironment. Melanoma Res. 2012;22:215–224. doi: 10.1097/CMR.0b013e3283531317. [DOI] [PubMed] [Google Scholar]
- 42.Ghislin S., Deshayes F., Lauriol J., Middendorp S., Martins I., Al-Daccak R., Alcaide-Loridan C. Plasticity of melanoma cells induced by neural cell crest conditions and three-dimensional growth. Melanoma Res. 2012;22:184–194. doi: 10.1097/CMR.0b013e328351e7c4. [DOI] [PubMed] [Google Scholar]
- 43.Harma V., Virtanen J., Makela R., Happonen A., Mpindi J.P., Knuuttila M., Kohonen P., Lotjonen J., Kallioniemi O., Nees M. A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PloS One. 2010;5:e10431. doi: 10.1371/journal.pone.0010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quintana E., Shackleton M., Sabel M.S., Fullen D.R., Johnson T.M., Morrison S.J. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleinman H.K., McGarvey M.L., Liotta L.A., Robey P.G., Tryggvason K., Martin G.R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 46.Hardy K.M., Kirshmann D.A., Seftor E.A., Margaryan N.V., Postovit L.M., Strizzi L., Hendrix M.J. Regulation of the embryonic morphogen Nodal by Notch4 facilitates manifestation of the aggressive melanoma phenotype. Cancer Res. 2010;70:10340–10350. doi: 10.1158/0008-5472.CAN-10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lissitzky J.C., Parriaux D., Ristorcelli E., Verine A., Lombardo D., Verrando P. Cyclic AMP signaling as a mediator of vasculogenic mimicry in aggressive human melanoma cells in vitro. Cancer Res. 2009;69:802–809. doi: 10.1158/0008-5472.CAN-08-2391. [DOI] [PubMed] [Google Scholar]
- 48.Rooney G.E., Howard L., O’Brien T., Widebank A.J., Barry F.P. Elevation of cAMP in mesenchymal stem cells transiently upregulates neural markers rather than inducing neural differentiation. Stem Cells Dev. 2009;18:387–398. doi: 10.1089/scd.2008.0080. [DOI] [PubMed] [Google Scholar]
- 49.Perez-Martinez L., Jaworski D.M. Tissue inhibitor of metalloproteinase-2 promotes neuronal differentiation by acting as an anti-mitogenic signal. J Neurosci. 2005;25:4917–4929. doi: 10.1523/JNEUROSCI.5066-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nomura K., Sugawara T., Sato T., Sawamura D., Hashimoto I., Sugita Y., Uitto J. Expression of laminin, type IV procollagen and 230 kDa bullous pemphigoid antigen genes by keratinocytes and fibroblasts in culture: application of the polymerase chain reaction for detection of small amounts of messenger RNA. Arch Dermatological Res. 1994;286:408–413. doi: 10.1007/BF00371801. [DOI] [PubMed] [Google Scholar]
- 51.Zhang S., Li M., Zhang D., Xu S., Wang X., Liu Z., Zhao X., Sun B. Hypoxia influences linearly patterned programmed cell necrosis and tumor blood supply patterns formation in melanoma. Laboratory Invest. 2009;89:575–586. doi: 10.1038/labinvest.2009.20. [DOI] [PubMed] [Google Scholar]