Abstract
Tumor-associated macrophages (TAMs) exhibit an M2 macrophage phenotype that suppresses anti-tumor immune responses and often correlates with poor outcomes in patients with cancer. Patients with ovarian cancer frequently present with peritoneal carcinomatosis, but the mechanisms that induce naïve peritoneal macrophages into TAMs are poorly understood. In this study, we found an increased abundance of TAMs in mouse i.p. xenograft models of ovarian cancer that expressed HOXA9, a homeobox gene that is associated with poor prognosis in patients with ovarian cancer. HOXA9 expression in ovarian cancer cells stimulated chemotaxis of peritoneal macrophages and induced macrophages to acquire TAM-like features. These features included induction of the M2 markers, CD163 and CD206, and the immunosuppressive cytokines, IL-10 and chemokine ligand 17, and down-regulation of the immunostimulatory cytokine, IL-12. HOXA9-mediated induction of TAMs was primarily due to the combinatorial effects of HOXA9-induced, tumor-derived transforming growth factor-β2 and chemokine ligand 2 levels. High HOXA9 expression in clinical specimens of ovarian cancer was strongly associated with increased abundance of TAMs and intratumoral T-regulatory cells and decreased abundance of CD8+ tumor-infiltrating lymphocytes. Levels of immunosuppressive cytokines were also elevated in ascites fluid of patients with tumors that highly expressed HOXA9. HOXA9 may, therefore, stimulate ovarian cancer progression by promoting an immunosuppressive microenvironment via paracrine effects on peritoneal macrophages.
It is increasingly recognized that tumor progression is controlled by the dynamic interplay between tumor and stromal cells, such as fibroblasts, endothelial cells, and immune cells.1,2 Among the latter, macrophages are a major component. Macrophages exhibit diverse functional properties in response to different microenvironmental cues. Two polarized macrophage phenotypes have been described that are analogous to the type 1/2 helper T-cell dichotomy of T-cell responses. On one hand, macrophages that are stimulated with microbial agents and interferon-γ exhibit an M1 (classically activated) phenotype and express immunostimulatory cytokines.3,4 On the other hand, stimulation of macrophages with IL-4, IL-10, or IL-13 induces an M2 (alternatively activated) phenotype.3,4 M2 macrophages are often characterized by expression of mannose and scavenger receptors and of immunosuppressive cytokines and chemokines. One important mechanism by which these macrophage-derived factors suppress anti-tumor immunity is by stimulating recruitment of T-regulatory (Treg) cells.3,4 Tumor-associated macrophages (TAMs) derive from circulating monocyte precursors and are recruited to tumors. TAMs are widely thought to exhibit an M2 macrophage phenotype, and are strongly associated with poor outcomes in patients with a wide variety of cancers.5 However, it is unclear whether tissue-specific mechanisms in a particular type of cancer regulate the interaction between tumor cells and macrophages in the tumor microenvironment.
Approximately 75% of patients with epithelial ovarian cancer present with disease that has already disseminated throughout the peritoneal cavity at the time of initial diagnosis.6,7 Ovarian cancer cells typically spread by exfoliating into the peritoneal fluid and implant on the omentum and other peritoneal surfaces.6,7 Peritoneal carcinomatosis is frequently associated with the formation of ascites. The peritoneal cavity normally harbors naïve macrophages that play essential roles in regulating tissue repair and inflammatory responses. Ovarian cancer cells have been demonstrated to polarize macrophages toward an M2 phenotype,8,9 but the molecular mechanisms in ovarian cancer cells that induce this polarization are poorly understood. Because of the unique clinical behavior of ovarian cancer, we hypothesized that interactions between ovarian cancer cells and peritoneal macrophages might be controlled, in part, by tissue-specific mechanisms that are activated in ovarian cancer cells.
Homeobox genes encode transcription factors that control self-renewal and cell differentiation.10,11 The homeobox gene HOXA9 is normally expressed during development of the female reproductive tract, and its expression is tightly regulated in the adult tract.12,13 We recently identified that high HOXA9 expression in ovarian cancer is strongly associated with poor overall survival and that HOXA9 promotes ovarian tumor growth in vivo but not in vitro.14 This suggested that HOXA9 promotes tumor growth by modulating interactions between tumor cells and host cells. In this study, we demonstrate that expression of HOXA9 in ovarian cancer cells stimulates chemotaxis of peritoneal macrophages and elicits TAM-like features. This stimulatory effect of HOXA9 on TAMs was found to be primarily mediated via its induction of tumor-derived transforming growth factor (TGF)-β2 and chemokine ligand (CCL) 2 levels. These results indicate that sustained HOXA9 expression in ovarian cancer may promote disease progression by educating peritoneal macrophages toward a TAM-like phenotype.
Materials and Methods
Abs and Growth Factors
Sources of antibodies (Abs) were as follows: CD68, CD8 (Dako, Glostrup, Denmark), F4/80 (AbD Serotec, Kidlington, UK), CD163 (Leica Microsystems, Buffalo Grove, IL), CD206 (R&D Systems, Minneapolis, MN), FOXP3 (eBioscience, San Diego, CA), HOXA9 (Santa Cruz Biotechnology, Santa Cruz, CA), and secondary Abs (BD Biosciences, San Jose, CA). Recombinant TGF-β2 and CCL2 were purchased from BioLegend (San Diego, CA).
Human Tissue Specimens
Studies using human tissues were reviewed and approved by the University of Texas MD Anderson Cancer Center Institutional Research Board (Houston) and the University of Chicago Institutional Research Board (Chicago, IL). All specimens of human ovarian cancer tissues and ascites were delinked from patient identifiers. All cases were postmenopausal patients with International Federation of Gynecology and Obstetrics (FIGO) stage III/IV, high-grade serous ovarian carcinomas.
Cell Culture
The parental mouse ovarian cancer cell line, MOSEC, has been previously described15 and was provided by Katherine Roby (University of Kansas, Lawrence). MOSEC cell lines that stably express HOX genes and SKOV3ip cell lines that stably express HOXA9 shRNAs have been previously described.13,14 MOSEC and SKOV3ip cell lines were cultured in Dulbecco’s modified Eagle’s medium and McCoy’s 5A medium (Invitrogen, Carlsbad, CA), respectively. The IC-21 mouse peritoneal macrophage cell line was purchased from ATCC (Manassas, VA) and cultured in RPMI 1640 medium (Invitrogen). Normal peritoneal macrophages were isolated from naïve 4-week-old female C57BL/6 mice by pelvic washing with PBS containing 3% fetal bovine serum (FBS). Primary cultures of macrophages were maintained in RPMI 1640 medium. All culture media were supplemented by addition of 10% FBS and penicillin/streptomycin.
Stimulation of Macrophages with Tumor-Conditioned Media
Ovarian cancer cells (1.5 × 106) were seeded in 10-cm dishes and cultured in medium containing 1% FBS for 2 days. Tumor-conditioned medium was collected, filtered, and applied to macrophages. Macrophages were analyzed for expression of surface markers and cytokines at 5 days thereafter.
Chemotaxis Assays
A total of 60,000 tumor cells per well were seeded in the lower chamber of 24-well Transwell chambers (BD Biosciences) and allowed to adhere overnight. A total of 50,000 macrophages per well were seeded in the upper chamber. Migrating macrophages were counted in three random 100× microscopic fields per well at 6 hours after seeding (for IC-21 macrophages) and at 20 hours after seeding (for primary mouse peritoneal macrophages). Three independent experiments were performed for each assay.
Mouse I.P. Xenograft Models
Four-week-old female nude mice were purchased from the National Cancer Institute (Frederick, MD) and inoculated i.p. with 1.5 × 106 cells of MOSEC and 2 × 106 SKOV3ip lines (n = 5 mice per group). Mice were euthanized by CO2 asphyxiation at times indicated in the legend of Figure 1 or when morbid ascites had developed. Cells were collected from ascites by centrifugation. Solid tumor tissue sections were formalin fixed and paraffin embedded.
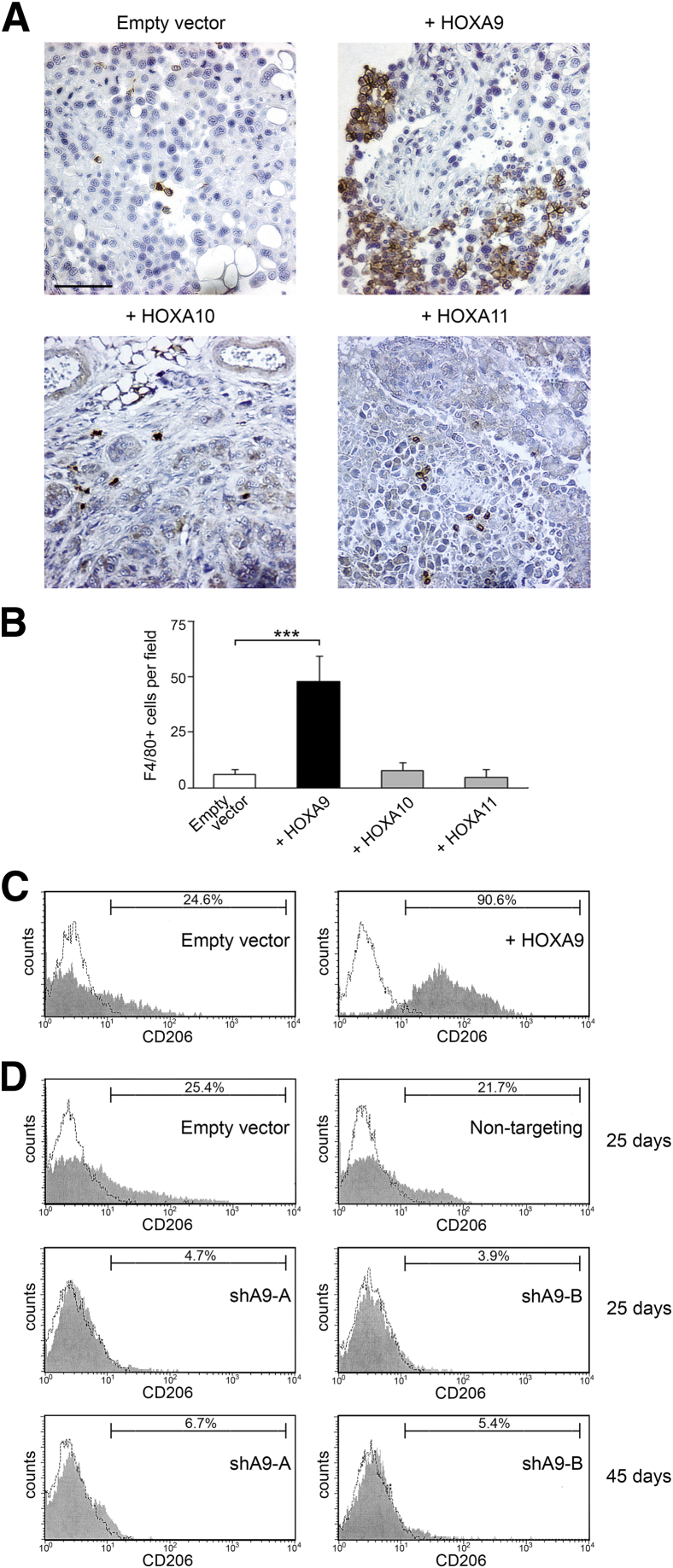
Figure 1.
HOXA9 expression is associated with abundance of M2 macrophages in i.p. xenograft models of ovarian cancer. A–C: Mouse i.p. xenograft models were established from vector-control and HOX-overexpressing MOSEC lines. Mice were sacrificed at 2 months after tumor cell inoculation. A: Representative examples of staining of F4/80+ macrophages in omental implants. Scale bar = 50 μm. B: Average numbers of F4/80+ cells in omental implants were calculated by scoring five random 200× microscopic fields of stained tumor tissue sections of each mouse (n = 5 mice per group). ∗∗∗P < 0.001. C: Ascitic cells of vector-control and +HOXA9 MOSEC xenograft models were stained with Abs to F4/80 and CD206 and evaluated by flow cytometric analysis. Shown are representative examples of the proportions of CD206+ cells within the gated population of F4/80+ macrophages. D: Mouse i.p. xenograft models were established from +HOXA9 control (empty vector and nontargeting) and HOXA9-knockdown (shA9-A and shA9-B) SKOV3ip lines. CD206 expression was evaluated by flow cytometric analysis in ascitic macrophages of control and HOXA9-knockdown groups of mice that were sacrificed at 25 days after tumor cell inoculation. Other HOXA9-knockdown groups of mice were analyzed at 45 days after tumor cell inoculation.
IHC Data
Staining of formalin-fixed, paraffin-embedded tissue sections with Abs was detected by streptavidin-biotin-peroxidase and 3,3′-diaminobenzidine (Dako). Evaluation of staining is described in the figure legends.
Flow Cytometric Analysis
Ascitic cells from xenograft models and cultured peritoneal macrophages were incubated with Abs to F4/80 and CD206 (1:20) for 30 minutes at 4°C, washed, and then incubated with phycoerythrin-conjugated anti-rabbit Ab and peridinin-chlorophyll-protein complex-conjugated anti-rat Ab to detect staining of F4/80 and CD206, respectively. After washing, cells were fixed in 4% paraformaldehyde and staining was analyzed by flow cytometry (FACS Calibur; BD Biosciences).
ELISA Data
Levels of CCL2, TGF-β2, IL-10, and CCL17 in ascites fluid and in tumor-conditioned medium were assayed by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s instructions (R&D Systems).
RT-qPCR
Transcripts were analyzed by using SYBR Green qPCR Master Mix (SA Biosciences, Valencia, CA) and the following primers: human CCL2, 5′-AGAATCACCAGCAGCAAGTGTCC-3′ (forward) and 5′-TCCTGAACCCACTTCTGCTTGG-3′ (reverse); mouse Il10, 5′-CGGGAAGACAATAACTGCACCC-3′ (forward) and 5′-CGGTTAGCAGTATGTTGTCCAGC-3′ (reverse); mouse Ccl17, 5′-CGAGAGTGCTGCCTGGATTACT-3′ (forward) and 5′-GGTCTGCACAGATGAGCTTGCC-3′ (reverse); mouse Il12a, 5′-ACGAGAGTTGCCTGGCTACTAG-3′ (forward) and 5′-CCTCATAGATGCTACCAAGGCAC-3′ (reverse); mouse Mrc1, 5′-GTTCACCTGGAGTGATGGTTCTC-3′ (forward) and 5′-AGGACATGCCAGGGTCACCTTT-3′ (reverse); and mouse Cd163, 5′-GGCTAGACGAAGTCATCTGCAC-3′ (forward) and 5′-CTTCGTTGGTCAGCCTCAGAGA-3′ (reverse). RPL32 (human) and Rpl32 (mouse) transcript levels were used as controls for normalization and were detected using primers previously described.14
ChIP Assay
Chromatin immunoprecipitation (ChIP) assays were performed using the EZ-ChIP Assay kit (Millipore, Temecula, CA). Sheared chromatin was incubated overnight with 1 μg of HOXA9 Ab. DNA was purified from precipitated complexes and used to amplify a 407-bp fragment of the CCL2 promoter using the following primers: forward, 5′-TAACCCAGGCTTGTGCCGAGAT-3′; and reverse, 5′-CTTTGCTGGCTGAGTGTTCACATA-3′. A 166-bp fragment of the GAPDH gene was amplified as an irrelevant control using the following primers: forward, 5′-TACTAGCGGTTTTACGGGCG-3′; and reverse, 5′-TCGAACAGGAGGAGCAGAGAGCGA-3′.
Statistics
Statistical analysis was performed using STATISTICA software version 10 (StatSoft Inc., Tulsa, OK). Values of statistical significance of data were assessed by unpaired two-tailed Student’s t-test, unless noted otherwise. Data represent means ± SD. P < 0.05 was considered significant. Correlation coefficients were determined by Spearman test.
Results
HOXA9 Expression Is Associated with Abundance of M2 Macrophages in I.P. Xenograft Models of Ovarian Cancer
Based on our recent findings that HOXA9 expression in ovarian cancer cells only promotes tumor growth in vivo, but not in vitro,14 we hypothesized that HOXA9 could modulate tumor-host cell interactions. Because the peritoneal cavity is the most commonly involved site in ovarian cancer and is a macrophage-rich environment, we investigated whether HOXA9 expression is associated with increased macrophage infiltration. Macrophages were detected by F4/80 staining in omental implants of mouse i.p. xenograft models generated from control and HOX-overexpressing MOSEC ovarian cancer cell lines that we previously established.13 Although only a few macrophages were detected in tumors derived from vector-control MOSEC cells, significantly more macrophages were detected in tumors derived from MOSEC cells that stably expressed HOXA9 (+HOXA9) (P < 0.001) (Figure 1, A and B). The HOXA10 and HOXA11 genes are expressed in a subset of ovarian cancers and are thought to have evolved by gene duplication from the same ancestral gene as HOXA9.10,13 In contrast to +HOXA9 MOSEC xenografts, the numbers of infiltrating macrophages in MOSEC xenografts that expressed HOXA10 or HOXA11 were not significantly different from those in vector-control xenografts (Figure 1, A and B). These findings indicate that HOXA9 expression in ovarian cancer cells is associated with the abundance of tumor-infiltrating macrophages and that this characteristic is not shared with other related HOX proteins.
To evaluate the phenotype of macrophages in xenograft models, we analyzed the expression of the mannose receptor, CD206, a commonly used mouse M2 macrophage marker, within the population of F4/80+ macrophages in ascites by flow cytometry. As shown in Figure 1C, the proportion of macrophages that expressed CD206 was nearly fourfold higher in the +HOXA9 MOSEC xenograft model than in the vector-control MOSEC model. To confirm these findings in a different model system, we analyzed ascitic macrophages in i.p. xenograft models generated from previously established +HOXA9 control and HOXA9 shRNA-expressing SKOV3ip ovarian cancer cell lines.14 HOXA9 levels in the HOXA9-knockdown SKOV3ip lines (shA9-A and shA9-B) were 25% of the HOXA9 levels in control SKOV3ip lines (that expressed empty vector or nontargeting shRNA).14 We previously found that HOXA9 stimulates ovarian tumor growth in vivo, thereby accelerating the development of morbid ascites and decreasing survival times.14 We, therefore, analyzed ascitic macrophages of sacrificed mice at two different time points after tumor cell inoculation: at 25 days when morbid ascites had developed in the control groups and then at 45 days when morbid ascites had developed in the HOXA9-knockdown groups. At the earlier time point (day 25), the proportions of CD206+ ascitic macrophages in the HOXA9-knockdown groups were 20% to 25% of the proportions found in the control groups (Figure 1D). Even at the later time point (day 45), the HOXA9-knockdown groups still had lower proportions of CD206+ ascitic macrophages than those in the control groups at day 25 (Figure 1D). These observations indicate that high HOXA9 levels in ovarian cancer cells are associated with an increased abundance of M2 macrophages at the equivalent stage of disease.
Secreted Factors Derived from HOXA9-Expressing Ovarian Cancer Cells Directly Stimulate Peritoneal Macrophages to Acquire M2 Features
In addition to macrophages, the tumor stroma is composed of various cellular elements.1,2 We investigated whether HOXA9-expressing ovarian cancer cells directly stimulate peritoneal macrophages to acquire an M2 phenotype independently of effects of other stromal cells. Primary cultures of naïve mouse peritoneal macrophages were stimulated with medium that had been conditioned by equivalent numbers of cells of +HOXA9 control and HOXA9-knockdown SKOV3ip lines. Because the in vitro proliferation rates of control and HOXA9-knockdown SKOV3ip lines are identical,14 this approach also enabled us to compare the direct effects of these tumor lines on macrophages, independently of any differences in tumor cell growth. As shown by flow cytometric analysis in Figure 2A, CD206 expression was more strongly induced in peritoneal macrophages after stimulation with medium conditioned by control SKOV3ip lines than with medium conditioned by HOXA9-knockdown SKOV3ip lines. This difference in CD206 induction was confirmed by quantitative RT-PCR (RT-qPCR) analysis (Figure 2B). Furthermore, expression of the scavenger receptor, CD163, another M2 marker, was more strongly induced in peritoneal macrophages after stimulation with medium conditioned by control SKOV3ip cells than with medium conditioned by HOXA9-knockdown SKOV3ip cells (P < 0.001) (Figure 2B).
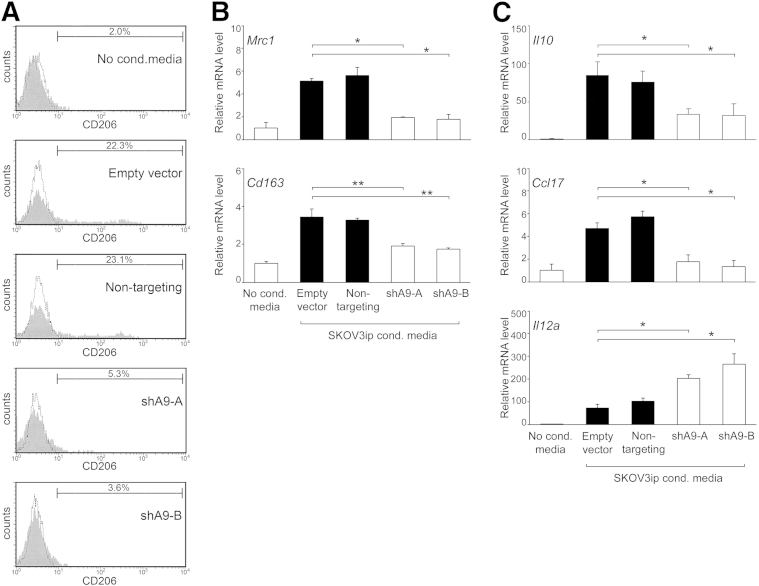
Figure 2.
HOXA9 expression in ovarian cancer cells stimulates peritoneal macrophages to acquire M2 features. Primary cultures of naïve mouse peritoneal macrophages were stimulated with nonconditioned (No. cond.) medium and with medium conditioned by equivalent numbers of cells of +HOXA9 control (empty vector and nontargeting) and HOXA9-knockdown (shA9-A and shA9-B) SKOV3ip lines. At 5 days thereafter, macrophages were evaluated for expression of M2 markers, cytokines, and chemokines. A: Flow cytometric analysis of CD206 expression. B: RT-qPCR analysis of expression of Mrc1 (encoding CD206) and Cd163. C: RT-qPCR analysis of Il10, Ccl17, and Il12a expression. ∗P < 0.005, ∗∗P < 0.001.
M2 macrophage polarization is characterized by elevated expression of immunosuppressive factors, such as IL-10 and CCL17, and reduced expression of immunostimulatory cytokines, such as IL-12.3,4 Expression of Il10 and Ccl17 was more strongly induced in primary mouse peritoneal macrophages after stimulation with medium conditioned by control SKOV3ip cells than with medium conditioned by HOXA9-knockdown SKOV3ip cells (P < 0.01) (Figure 2C). Conversely, IL12a expression was significantly lower in macrophages that were stimulated with control SKOV3ip cell-conditioned medium than in macrophages stimulated with medium conditioned by HOXA9-knockdown SKOV3ip cells (P < 0.01) (Figure 2C). These findings indicate that HOXA9-High ovarian cancer cells are more effective than HOXA9-Low tumor cells in stimulating naïve peritoneal macrophages to acquire features of M2 macrophages.
Stimulatory Effects of HOXA9 on Macrophages Are Not Solely Due to HOXA9-Induced, Tumor-Derived TGF-β2 Levels
Based on our recent finding that the TGFB2 gene is a direct transcriptional target of HOXA9,14 we investigated the possibility that the ability of HOXA9 to induce M2 features in peritoneal macrophages is due to HOXA9-mediated induction of tumor-derived TGF-β2 levels. To investigate this possibility, we compared the effects of control SKOV3ip cells (nontargeting), HOXA9-knockdown SKOV3ip cells (shA9-B), and a previously established TGF-β2–knockdown SKOV3ip line (shTGF-β2), which expresses TGF-β2 at the same low level as HOXA9-knockdown SKOV3ip cells.14 The TGF-β2 levels in media conditioned by nontargeting, shA9-B, and shTGF-β2 SKOV3ip cells were 231 ± 14, 59 ± 7, and 41 ± 24 pg/mL, respectively.14 As shown in Figure 3A, medium conditioned by TGF-β2–knockdown SKOV3ip cells was less effective than medium conditioned by control SKOV3ip cells in inducing CD206 expression in primary mouse peritoneal macrophages. However, medium conditioned by HOXA9-knockdown SKOV3ip cells was even less effective in inducing CD206 expression in primary macrophages (Figure 3A). Similar results were obtained in assays using the peritoneal macrophage cell line, IC-21 (Supplemental Figure S1A). Conversely, we tested the effect of reconstituting TGF-β2 in –HOXA9 tumor cells by using a previously established HOXA9-knockdown SKOV3ip line in which TGFB2 cDNA was stably expressed (shA9-B + TGF-β2).14 This cell line expresses TGF-β2 at the same high level as control SKOV3ip cells (in which TGF-β2 levels in media conditioned by shA9-B + TGF-β2 and nontargeting SKOV3ip cells are 240 ± 35 and 231 ± 14 pg/mL, respectively14). Reconstitution of TGF-β2 in HOXA9-knockdown SKOV3ip cells increased CD206 expression in macrophages, but this induction was only 60% of the level induced by control SKOV3ip cells (Figure 3A). Similarly, stimulation of macrophages with recombinant TGF-β2 at the same concentration as that released by control SKOV3ip cells induced CD206 expression to a level that was only 50% of the level induced by medium conditioned by control SKOV3ip cells (Figure 3A).
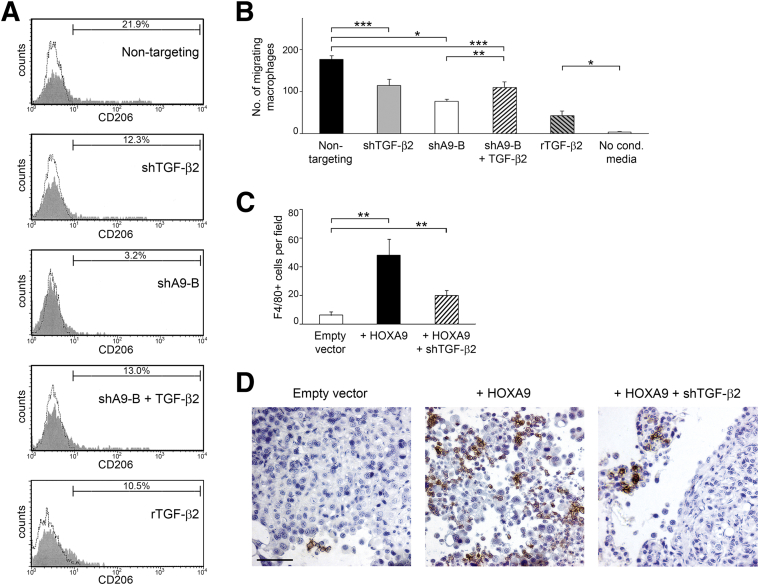
Figure 3.
Stimulatory effects of HOXA9 on macrophages are only partially mediated by HOXA9-induced, tumor-derived TGF-β2 levels. A: Primary cultures of naïve mouse peritoneal macrophages were stimulated with medium conditioned by control (nontargeting), TGF-β2–knockdown (shTGF-β2), and HOXA9-knockdown (shA9-B) SKOV3ip lines and an HOXA9-knockdown line that stably expressed TGF-β2 (shA9-B + TGF-β2). Macrophages were also stimulated with recombinant TGF-β2 (rTGF-β2) at the same concentration as that released by control SKOV3ip cells (240 pg/mL). At 5 days thereafter, macrophages were evaluated for CD206 expression by flow cytometric analysis. B: Chemotaxis of primary mouse peritoneal macrophages toward cells of the indicated SKOV3ip lines was assayed at 20 hours after seeding in Transwell chambers. Macrophages were also stimulated with rTGF-β2, as described for A. Shown are average results of three independent experiments. C and D: Mouse i.p. xenograft models were established from vector-control and +HOXA9 MOSEC lines and a +HOXA9 MOSEC line in which TGF-β2 was knocked down (+HOXA9 + shTGF-β2), and were evaluated at 2 months after tumor cell inoculation. C: Average numbers of F4/80+ cells in omental implants were calculated by scoring five random 200× microscopic fields of stained tumor tissue sections of each mouse (n = 5 mice per group). D: Representative examples of staining of F4/80+ macrophages in omental implants. ∗P < 0.0005, ∗∗P < 0.01, ∗∗∗P < 0.001. Scale bar = 50 μm.
We also evaluated whether macrophage chemotaxis is stimulated by tumor-derived TGF-β2 levels that are induced by HOXA9. Chemotaxis of primary mouse peritoneal macrophages toward SKOV3ip cells was partially inhibited when TGF-β2 was knocked down in SKOV3ip cells, and was more strongly inhibited when HOXA9 was knocked down (Figure 3B). Reconstitution of TGF-β2 in HOXA9-knockdown SKOV3ip cells increased macrophage chemotaxis, but not to the level seen with +HOXA9 control SKOV3ip cells (Figure 3B). Identical results were obtained in chemotaxis assays using IC-21 macrophages (Supplemental Figure S1B). To investigate the effects of inhibiting TGF-β2 in +HOXA9 tumor cells in vivo, we evaluated the numbers of infiltrating macrophages in xenografts generated from vector-control and +HOXA9 MOSEC cell lines and from a previously generated +HOXA9 MOSEC line that stably expressed Tgfb2 shRNA (+HOXA9 + shTGF-β2) (in which TGF-β2 levels in vector-control, +HOXA9, and +HOXA9 + shTGF-β2 MOSEC cells are 105 ± 6, 417 ± 96, and 108 ± 30 pg/mL, respectively14). Compared with +HOXA9 MOSEC xenografts, fewer macrophages were detected in +HOXA9 MOSEC xenografts in which TGF-β2 was knocked down, but the abundance of macrophages was not as low as that seen in vector-control (ie, -HOXA9) xenografts (Figure 3, C and D). Together, our findings indicate that the stimulatory effects of HOXA9 on macrophage chemotaxis and M2 transition are mediated, in part, but not solely, by the ability of HOXA9 to induce tumor-derived TGF-β2 levels.
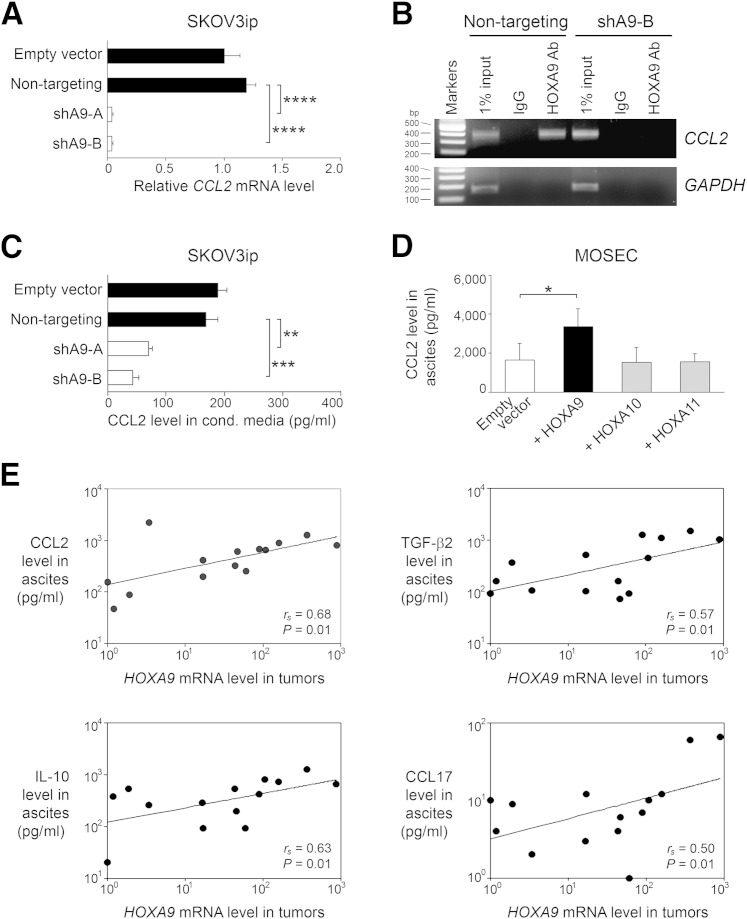
HOXA9 Induces CCL2 Expression in Ovarian Cancer Cells and Correlates with Levels of CCL2, TGF-β2, and M2 Cytokines in Patient Ascites
We recently identified TGF-β2 as one of only few tumor-derived cytokines or chemokines whose expression is induced by HOXA9, and demonstrated that HOXA9 bound to the TGFB2 promoter.14 Another chemokine whose expression was modulated by HOXA9 is CCL2. Knockdown of HOXA9 in SKOV3ip cells significantly down-regulated CCL2 RNA levels (P < 0.0005) (Figure 4A). A putative HOXA9-binding site was identified in the CCL2 promoter at 2.5 kb upstream of the transcription start site. Binding of endogenous HOXA9 to this site was detected by ChIP assays in control SKOV3ip cells, but no binding was detected in HOXA9-knockdown SKOV3ip cells (Figure 4B). Knockdown of HOXA9 in SKOV3ip cells also significantly inhibited CCL2 protein levels (P < 0.01) (Figure 4C). Conversely, CCL2 levels in ascites fluid were elevated in +HOXA9 MOSEC xenograft models compared with vector-control models (P < 0.05), but were not elevated in the +HOXA10 or +HOXA11 xenograft models (Figure 4D). These findings indicate that HOXA9, but not other related HOX proteins, induces CCL2 expression in ovarian cancer cells. Levels of HOXA9 expression in ovarian tumor tissues correlated with CCL2 levels in matching specimens of ascites fluid from the same patients (rs = 0.68, P = 0.01) (Figure 4E). Consistent with our previous finding that HOXA9 induces TGF-β2 expression,14 levels of HOXA9 expression in human tumor specimens also correlated with TGF-β2 levels in the ascites fluid (rs = 0.57, P = 0.01) (Figure 4E). Furthermore, HOXA9 expression levels in tumor specimens correlated with levels of the M2 cytokines, IL-10 and CCL17, in ascites fluid (rs = 0.63 for IL-10 and rs = 0.50 for CCL17, P = 0.01) (Figure 4E).
Figure 4.
HOXA9 induces CCL2 expression in ovarian cancer cells and correlates with levels of CCL2, TGF-β2, and M2 cytokines in patient ascites. A: Relative CCL2 mRNA levels in SKOV3ip lines. B: Detection of binding of endogenous HOXA9 in SKOV3ip cells to the CCL2 promoter by ChIP. Negative controls included IP using cells expressing HOXA9 shRNA (shA9-B) and IgG. GAPDH was amplified as an irrelevant gene control. C: CCL2 levels in media conditioned (cond.) by SKOV3ip lines. Shown are average results of ELISAs using three independent sets of each type of SKOV3ip-conditioned medium. D: CCL2 levels in ascites fluid of mouse i.p. xenograft models established from vector-control and HOX-expressing MOSEC lines (n = 5 mice per group). E: Relative HOXA9 mRNA levels in ovarian tumor tissues and levels of CCL2, TGF-β2, IL-10, and CCL17 in matching specimens of ascites fluid from the same patients (n = 14 cases). Correlations were determined by Spearman test. All cases were postmenopausal patients with FIGO stage III/IV, high-grade serous ovarian carcinoma. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0005.
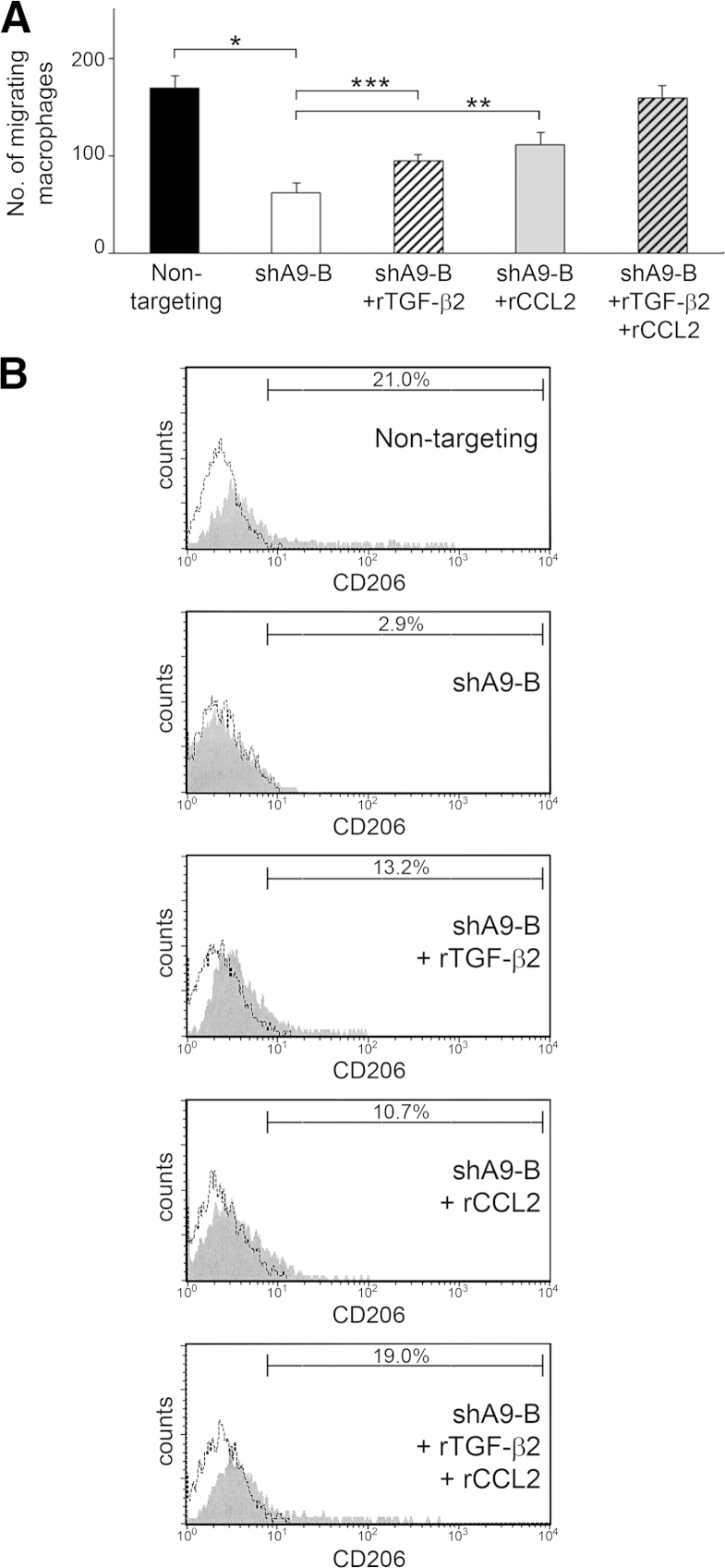
Combination of TGF-β2 and CCL2 Can Recapitulate the Effects of HOXA9 on Macrophages
Because reconstituting TGF-β2 in HOXA9-knockdown tumor cells alone did not completely restore the macrophage-stimulatory effect of HOXA9, we evaluated the effect of reconstituting CCL2. Compared with chemotaxis of primary mouse peritoneal macrophages toward control SKOV3ip cells, macrophage chemotaxis toward HOXA9-knockdown SKOV3ip cells was partially restored by the addition of recombinant CCL2 at the same concentration as that released by control SKOV3ip cells (Figure 5A). Macrophage chemotaxis was almost completely restored by the addition of both CCL2 and TGF-β2 (Figure 5A). Similarly, CD206 induction in primary mouse peritoneal macrophages was partially restored when medium conditioned by HOXA9-knockdown tumor cells was reconstituted with either CCL2 or TGF-β2, but was almost completely restored by the addition of both factors together (Figure 5B). Similar results were obtained in chemotaxis and CD206 induction assays using IC-21 macrophages (Supplemental Figure S2, A and B). These findings indicate that the ability of HOXA9 to stimulate macrophage chemotaxis and M2 transition is largely because of the combinatorial effects of TGF-β2 and CCL2 levels that are induced by HOXA9.
Figure 5.
Combination of TGF-β2 and CCL2 recapitulates the stimulatory effects of HOXA9 on macrophages. A: Chemotaxis of primary mouse peritoneal macrophages toward +HOXA9 control (nontargeting) and HOXA9-knockdown (shA9-B) SKOV3ip cells. Where indicated, recombinant TGF-β2 (rTGF-β2) and CCL2 were added to achieve final concentrations of these factors that were the same as those secreted by nontargeting cells [ie, 240 pg/mL of TGF-β214 and 200 pg/mL of CCL2 (refer to Figure 4C)]. ∗P < 0.0005, ∗∗P < 0.005, and ∗∗∗P < 0.01. B: Primary cultures of naïve mouse peritoneal macrophages were stimulated with medium conditioned by SKOV3ip lines. Where indicated, conditioned medium was reconstituted with TGF-β2 and CCL2, as described for A. At 5 days thereafter, macrophages were evaluated for CD206 expression by flow cytometric analysis.
High HOXA9 Expression in Human Ovarian Cancer Specimens Is Associated with Increased Abundance of M2 Macrophages and Treg Cells and Decreased Abundance of CD8+ TILs
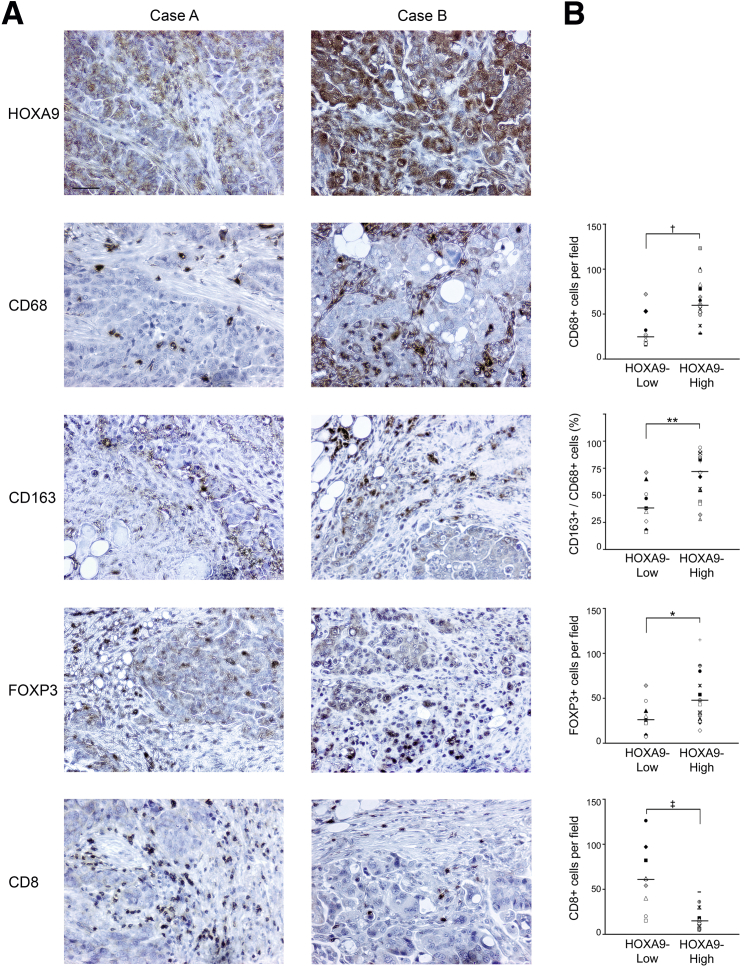
To evaluate whether high HOXA9 levels are associated with increased macrophage infiltration and increased proportions of M2 macrophages in human tumors, we collected a homogenous set of omental metastasis specimens from a group of patients with stage III/IV, high-grade serous ovarian carcinoma and performed immunohistochemical (IHC) analysis. The numbers of macrophages that were detected by CD68 staining in tumors that highly expressed HOXA9 were significantly higher than those detected in tumors that expressed low levels of HOXA9 (P < 0.01) (Figure 6, A and B). CD163 is commonly used as a human M2 macrophage marker.9,16,17 The proportion of macrophages that expressed CD163 was significantly higher in HOXA9-High tumors than in HOXA9-Low tumors (P < 0.01) (Figure 6, A and B). TAMs stimulate recruitment of Treg cells that inhibit effector T-cell activity.3,4 More Treg cells were detected by FOXP3 staining in HOXA9-High tumors than in HOXA9-Low tumors (P < 0.05) (Figure 6, A and B). Conversely, HOXA9-High tumors contained significantly fewer CD8+ tumor-infiltrating lymphocytes (TILs) than HOXA9-Low tumors (P < 0.01) (Figure 6, A and B). These observations indicate that high HOXA9 expression in ovarian cancer is associated with increased abundance of M2 macrophages and Treg cells and with decreased abundance of TILs.
Figure 6.
High HOXA9 expression in human ovarian cancer specimens is associated with increased abundance of M2 macrophages and Treg cells and decreased abundance of CD8+ TILs. A: IHC analysis of omental tumors from patients with FIGO stage III/IV, high-grade serous ovarian carcinoma. Cases with no or weak HOXA9 staining were scored as HOXA9-Low (n = 9). Cases with strong HOXA9 staining were scored as HOXA9-High (n = 16). Shown are representative examples of weak and strong HOXA9 staining in omental tumors of two different cases. Also shown are specimens of the same cases stained with Abs to CD68, CD163, FOXP3, and CD8. Scale bar = 50 μm. B: Numbers of tumor-infiltrating CD68+, CD163+, FOXP3+, and CD8+ cells were counted in five random 200× microscopic fields of stained tissue sections of each case. Average numbers of CD68+, FOXP3+, and CD8+ cells are shown for cases grouped by HOXA9 expression, with each symbol representing an individual case. The percentage of macrophages that were CD163+ was calculated from the numbers of CD163+ and CD68+ cells per field for a given case. The statistical significance of differences between groups of cases was determined by U-test. ∗P < 0.05, ∗∗P < 0.01, †P < 0.005, and ‡P < 0.002.
Discussion
Increasing evidence indicates that cross talk between tumor and stromal cells plays an important role in tumor progression and that this cross talk is regulated by pathways that normally control developmental patterning. For example, the notch, hedgehog, and Wnt signaling pathways are activated in many types of tumors, and these developmental pathways modulate interactions of tumor cells with stromal fibroblasts and endothelial cells.18–20 Homeobox genes are an important class of patterning regulators that have been increasingly reported to modulate tumor progression. Many of the homeobox genes studied have been found to alter tumor cell growth in vitro,21–23 suggesting that these genes control tumor cell–autonomous mechanisms. In this study, we demonstrated that expression of HOXA9, a homeobox gene that is strongly associated with poor prognosis in patients with ovarian cancer, stimulates chemotaxis of peritoneal macrophages and acquisition by macrophages of M2 features. These results indicate that sustained HOXA9 expression in ovarian cancer might promote disease progression by stimulating recruitment of peritoneal macrophages and by educating macrophages toward a TAM-like phenotype.
It is generally accepted that TAMs have a polarized M2 macrophage phenotype. The most well-studied cytokines that induce M2-type polarization are IL-4, IL-10, and IL-13.3,4 TAMs in ovarian tumors have been thought to be primarily regulated by IL-10, because IL-4 and IL-13 were undetectable or detected at only low levels in patient ascites.9,24 Our studies indicate that HOXA9 expression in ovarian cancer cells stimulates macrophage chemotaxis and M2-like polarization via its induction of tumor-derived TGF-β2 and CCL2 levels. CCL2 is known to stimulate monocyte mobilization and has been reported to induce M2-type features.25 However, to our knowledge, our study is the first to report that TGF-β2 stimulates macrophage chemotaxis and M2 polarization. It has been recently demonstrated that the TGFB2 and CCL2 genes are direct transcriptional targets of HOXA9.14,26 Our findings support a model in which the stimulatory effects of HOXA9 on TAMs are largely due to the combinatorial effects of TGF-β2 and CCL2, but the possibility that HOXA9 controls macrophage chemotaxis and polarization by regulating other transcriptional targets cannot be excluded. Presently, it is not possible to conclude that the stimulatory effect of HOXA9 on TAMs is restricted to ovarian cancer. HOXA9 has also been reported to bind the CCL2 promoter in astrocytoma cells,26 raising the possibility that HOXA9 might also exert stimulatory effects on TAMs in cancers of other organ sites. However, HOX proteins function in a context-dependent manner.10 Indeed, HOXA9 has been reported to exert a tumor-suppressive effect in breast cancer.27 A given HOX protein might exert different effects by controlling distinct sets of transcriptional targets in different cell types or contexts.
Because tumor progression is dynamically orchestrated by cross talk between tumor cells, TAMs, and various other cell types, the ability of HOXA9 to induce TGF-β2 and CCL2 levels and to stimulate TAMs could substantially amplify tumor-promoting signals in the tumor microenvironment. CCL2 stimulates monocyte recruitment to tumors and promotes mobilization of mesenchymal stem cells (MSCs).28 We have demonstrated that ovarian tumor–derived TGF-β2 stimulates normal MSCs to transition into cancer-associated fibroblasts (CAFs) that highly express TGF-β2 and IL-6.14 IL-6 has been reported to induce M2-type polarization of macrophages.9,25 HOXA9 might, therefore, stimulate TAMs through the direct effects of tumor-derived TGF-β2 and CCL2 levels on macrophages and promote CAFs that express factors that, in turn, stimulate macrophages in a paracrine manner. Reciprocally, HOXA9-mediated stimulation of macrophages might also promote CAFs because macrophage-derived factors can stimulate MSC mobilization.28
A major mechanism by which TAMs promote tumor progression is by suppressing adaptive immunity. TAMs are characterized by high expression of IL-10 and low levels of IL-12, and these cytokines inhibit and stimulate T-cell proliferation, respectively.3,4 In addition to IL-10, TAMs highly express chemokines, such as CCL17 and CCL22, that recruit Treg cells, which, in turn, suppress effector T-cell activity.3,4 TAM-derived factors also suppress anti-tumor immunity by inducing naïve T-cell anergy, skewing T cells toward a type 2 helper T-cell direction and inhibiting dendritic cell maturation.3,4 Treg cells have been found to contribute to ovarian tumor growth by suppressing tumor-specific immunity and to be predictive of poor survival.29 Conversely, increased numbers of CD8+ TILs are associated with favorable prognosis in patients with ovarian cancer.30 Our study demonstrates that high HOXA9 levels in ovarian cancer are strongly associated with increased abundance of TAMs, with increased numbers of Treg cells, and with decreased numbers of TILs. These observations are consistent with the ability of TAMs to stimulate Treg cell recruitment, and support a model in which the association of HOXA9 with poor outcomes is linked to the ability of HOXA9 to promote a TAM-abundant, immunosuppressive microenvironment. TGF-β signaling controls expression of FOXP3 that plays an essential role in Treg cell differentiation and function.31,32 CCL2 stimulates Treg recruitment to tumors.33 HOXA9-induced levels of TGF-β2 and CCL2 might, therefore, have direct stimulatory effects on Treg cells and indirect effects through TAMs. Reciprocally, increased abundance of Treg cells could promote TAMs because Treg cells highly express IL-4, IL-10, and IL-13.34 Also, TAMs highly express angiogenic factors and matrix metalloproteinases.4,5 We have found that +HOXA9 ovarian tumor xenografts have high microvessel density and increased propensity for i.p. dissemination.14 It is, therefore, possible that the TAM-inducing ability of HOXA9 promotes ovarian tumor progression by stimulating tumor angiogenesis and invasiveness, as well as through immunosuppression.
In summary, our study demonstrates the significance of a developmental gene in regulating tumor cell interactions with macrophages. TAMs represent an attractive target for cancer therapy. Several agents that inhibit CCL2 production or signaling have been evaluated in preclinical studies.35,36 Varieties of agents that inhibit TGF-β signaling have been evaluated in preclinical models and clinical trials and include ligand traps, antisense oligonucleotides, and receptor kinase inhibitors.37 Targeting TAMs has a particularly strong application to ovarian cancer, because it is associated with ascites, which is predominantly composed of macrophages. Our study raises the possibility that a combination of agents that block both CCL2 and TGF-β signaling could be an effective approach to inhibit TAMs in ovarian cancer.
Acknowledgments
We thank Nicolas Barengo for technical assistance and members of the Naora laboratory for discussion.
Footnotes
Supported by Cancer and Prevention Research Institute of Texas grant RP120390 (H.N.), MD Anderson Cancer Center Institutional Research grant (H.N.), NIH grants CA141078 (H.N.) and CA111882 (E.L.), and a Burroughs Wellcome Fund Clinical Scientist Award in Translational Research (E.L.).
Supplemental Data
Stimulatory effects of HOXA9 on macrophages are only partially mediated by HOXA9-induced, tumor-derived TGF-β2 levels. A: IC-21 peritoneal macrophages were stimulated with medium conditioned by control (nontargeting), TGF-β2–knockdown (shTGF-β2), and HOXA9-knockdown (shA9-B) SKOV3ip lines and an HOXA9-knockdown line that stably expressed TGF-β2 (shA9-B + TGF-β2). At 5 days thereafter, IC-21 macrophages were evaluated for CD206 expression by flow cytometric analysis. B: Chemotaxis of IC-21 macrophages toward cells of the indicated SKOV3ip lines was assayed at 6 hours after seeding in Transwell chambers. Shown are average results of three independent experiments. ∗∗P < 0.01, ∗∗∗P < 0.001.
Combination of TGF-β2 and CCL2 recapitulates stimulatory effects of HOXA9 on macrophages. A: Chemotaxis of IC-21 macrophages toward +HOXA9 control (nontargeting) and HOXA9-knockdown (shA9-B) SKOV3ip cells. Where indicated, recombinant TGF-β2 and CCL2 were added to achieve final concentrations of these factors that were the same as those secreted by nontargeting cells: 240 pg/mL TGF-β214 and 200 pg/mL CCL2 (refer to Figure 4C). ∗P < 0.05, ∗∗P < 0.01. B: CD206 expression in IC-21 macrophages after stimulation with medium conditioned by SKOV3ip lines. Where indicated, conditioned medium was reconstituted with TGF-β2 and CCL2, as described for A.
References
- 1.Tlsty T.D., Coussens L.M. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 2.Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 4.Solinas G., Germano G., Mantovani A., Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 5.Pollard J.W. Tumor-educated macrophages promote tumor progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 6.Naora H., Montell D.J. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5:355–366. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- 7.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagemann T., Wilson J., Burke F., Kulbe H., Li N.F., Plüddemann A., Charles K., Gordon S., Balkwill F.R. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 9.Duluc D., Delneste Y., Tan F., Moles M.P., Grimaud L., Lenoir J., Preisser L., Anegon I., Catala L., Ifrah N., Descamps P., Gamelin E., Gascan H., Hebbar M., Jeannin P. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110:4319–4330. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- 10.Pearson J.C., Lemons D., McGinnis W. Modulating HOX gene functions during animal body patterning. Nat Rev Genet. 2005;6:893–904. doi: 10.1038/nrg1726. [DOI] [PubMed] [Google Scholar]
- 11.Samuel S., Naora H. Homeobox gene expression in cancer: insights from developmental regulation and deregulation. Eur J Cancer. 2005;41:2428–2437. doi: 10.1016/j.ejca.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Vitiello D., Kodaman P.H., Taylor H.S. HOX genes in implantation. Semin Reprod Med. 2007;25:431–436. doi: 10.1055/s-2007-991040. [DOI] [PubMed] [Google Scholar]
- 13.Cheng W., Liu J., Yoshida H., Rosen D., Naora H. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med. 2005;11:531–537. doi: 10.1038/nm1230. [DOI] [PubMed] [Google Scholar]
- 14.Ko S.Y., Barengo N., Ladanyi A., Lee J.S., Marini F., Lengyel E., Naora H. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J Clin Invest. 2012;122:3603–3617. doi: 10.1172/JCI62229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roby K.F., Taylor C.C., Sweetwood J.P., Cheng Y., Pace J.L., Tawfik O., Persons D.L., Smith P.G., Terranova P.F. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 16.Shabo I., Stai O., Olsson H., Doré S., Svanvik J. Breast cancer expression of CD163, a macrophage scavenger receptor, is related to early distant recurrence and reduced patient survival. Int J Cancer. 2008;123:780–786. doi: 10.1002/ijc.23527. [DOI] [PubMed] [Google Scholar]
- 17.Pander J., Heusinkveld M., van der Straaten T., Jordanova E.S., Baak-Pablo R., Gelderblom H., Morreau H., van der Burg S.H., Guchelaar H.J., van Hall T. Activation of tumor-promoting type 2 macrophages by EGFR-targeting antibody cetuximab. Clin Cancer Res. 2011;17:5668–5673. doi: 10.1158/1078-0432.CCR-11-0239. [DOI] [PubMed] [Google Scholar]
- 18.Zeng Q., Li S., Chepeha D.B., Giordano T.J., Li J., Zhang H., Polverini P.J., Nor J., Kitajewski J., Wang C.Y. Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell. 2005;8:13–23. doi: 10.1016/j.ccr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Chen W., Tang T., Eastham-Anderson J., Dunlap D., Alicke B., Nannini M., Gould S., Yauch R., Modrusan Z., DuPree K.J., Darbonne W.C., Plowman G., de Sauvage F.J., Callahan C.A. Canonical hedgehog signaling augments tumor angiogenesis by induction of VEGF-A in stromal perivascular cells. Proc Natl Acad Sci U S A. 2011;108:9589–9594. doi: 10.1073/pnas.1017945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luga V., Zhang L., Viloria-Petit A.M., Ogunjimi A.A., Inanlou M.R., Chiu E., Buchanan M., Hosein A.N., Basik M., Wrana J.L. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Muratovska A., Zhou C., He S., Goodyer P., Eccles M.R. Paired-Box genes are frequently expressed in cancer and often required for cancer cell survival. Oncogene. 2003;22:7989–7997. doi: 10.1038/sj.onc.1206766. [DOI] [PubMed] [Google Scholar]
- 22.Tan Y., Cheung M., Pei J., Menges C.W., Godwin A.K., Testa J.R. Upregulation of DLX5 promotes ovarian cancer cell proliferation by enhancing IRS-2-AKT signaling. Cancer Res. 2010;70:9197–9206. doi: 10.1158/0008-5472.CAN-10-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trinh B.Q., Ko S.Y., Barengo N., Lin S.Y., Naora H. Dual functions of the homeoprotein DLX4 in modulating responsiveness of tumor cells to topoisomerase II-targeting drugs. Cancer Res. 2013;73:1000–1010. doi: 10.1158/0008-5472.CAN-12-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matte I., Lane D., Laplante C., Rancourt C., Piché A. Profiling of cytokines in human epithelial ovarian cancer ascites. Am J Cancer Res. 2012;2:566–580. [PMC free article] [PubMed] [Google Scholar]
- 25.Roca H., Varsos Z.S., Sud S., Craig M.J., Ying C., Pienta K.J. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284:34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page S.H., Wright E.K., Jr., Gama L., Clements J.E. Regulation of CCL2 expression by an upstream TALE homeodomain protein-binding site that synergizes with the site created by the A-2578G SNP. PLoS One. 2011;6:e22052. doi: 10.1371/journal.pone.0022052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert P.M., Mouw J.K., Unger M.A., Lakins J.N., Gbegnon M.K., Clemmer V.B., Benezra M., Licht J.D., Boudreau N.J., Tsai K.K., Welm A.L., Feldman M.D., Weber B.L., Weaver V.M. HOXA9 regulates BRCA1 expression to modulate human breast tumor phenotype. J Clin Invest. 2010;120:1535–1550. doi: 10.1172/JCI39534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anton K., Banerjee D., Glod J. Macrophage-associated mesenchymal stem cells assume an activated, migratory, pro-inflammatory phenotype with increased IL-6 and CXCL10 secretion. PLoS One. 2012;7:e35036. doi: 10.1371/journal.pone.0035036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curiel T.J., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P., Evdemon-Hogan M., Conejo-Garcia J.R., Zhang L., Burow M., Zhu Y., Wei S., Kryczek I., Daniel B., Gordon A., Myers L., Lackner A., Disis M.L., Knutson K.L., Chen L., Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 30.Sato E., Olson S.H., Ahn J., Bundy B., Nishikawa H., Qian F., Jungbluth A.A., Frosina D., Gnjatic S., Ambrosone C., Kepner J., Odunsi T., Ritter G., Lele S., Chen Y.T., Ohtani H., Old L.J., Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tone Y., Furuuchi K., Kojima Y., Tykocinski M.L., Greene M.I., Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 32.Zheng Y., Josefowicz S., Chaudhry A., Peng X.P., Forbush K., Rudensky A.Y. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jordan J.T., Sun W., Hussain S.F., DeAngulo G., Prabhu S.S., Heimberger A.B. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57:123–131. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiemessen M.M., Jagger A.L., Evans H.G., van Herwijnen M.J., John S., Taams L.S. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin G., Kawsar H.I., Hirsch S.A., Zeng C., Jia X., Feng Z., Ghosh S.K., Zheng Q.Y., Zhou A., McIntyre T.M., Weinberg A. An antimicrobial peptide regulates tumor-associated macrophage trafficking via the chemokine receptor CCR2, a model for tumorigenesis. PLoS One. 2010;5:e10993. doi: 10.1371/journal.pone.0010993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Germano G., Frapolli R., Belgiovine C., Anselmo A., Pesce S., Liguori M., Erba E., Uboldi S., Zucchetti M., Pasqualini F., Nebuloni M., van Rooijen N., Mortarini R., Beltrame L., Marchini S., Fuso Nerini I., Sanfilippo R., Casali P.G., Pilotti S., Galmarini C.M., Anichini A., Mantovani A., D’Incalci M., Allavena P. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23:249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Connolly E.C., Freimuth J., Akhurst R.J. Complexities of TGF-β targeted cancer therapy. Int J Biol Sci. 2012;8:964–978. doi: 10.7150/ijbs.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stimulatory effects of HOXA9 on macrophages are only partially mediated by HOXA9-induced, tumor-derived TGF-β2 levels. A: IC-21 peritoneal macrophages were stimulated with medium conditioned by control (nontargeting), TGF-β2–knockdown (shTGF-β2), and HOXA9-knockdown (shA9-B) SKOV3ip lines and an HOXA9-knockdown line that stably expressed TGF-β2 (shA9-B + TGF-β2). At 5 days thereafter, IC-21 macrophages were evaluated for CD206 expression by flow cytometric analysis. B: Chemotaxis of IC-21 macrophages toward cells of the indicated SKOV3ip lines was assayed at 6 hours after seeding in Transwell chambers. Shown are average results of three independent experiments. ∗∗P < 0.01, ∗∗∗P < 0.001.
Combination of TGF-β2 and CCL2 recapitulates stimulatory effects of HOXA9 on macrophages. A: Chemotaxis of IC-21 macrophages toward +HOXA9 control (nontargeting) and HOXA9-knockdown (shA9-B) SKOV3ip cells. Where indicated, recombinant TGF-β2 and CCL2 were added to achieve final concentrations of these factors that were the same as those secreted by nontargeting cells: 240 pg/mL TGF-β214 and 200 pg/mL CCL2 (refer to Figure 4C). ∗P < 0.05, ∗∗P < 0.01. B: CD206 expression in IC-21 macrophages after stimulation with medium conditioned by SKOV3ip lines. Where indicated, conditioned medium was reconstituted with TGF-β2 and CCL2, as described for A.