Abstract
Neurofibromatosis type 1 (NF1) results from mutations in the NF1 tumor-suppressor gene, which encodes neurofibromin, a negative regulator of diverse Ras signaling cascades. Arterial stenosis is a nonneoplastic manifestation of NF1 that predisposes some patients to debilitating morbidity and sudden death. Recent murine studies demonstrate that Nf1 heterozygosity (Nf1+/−) in monocytes/macrophages significantly enhances intimal proliferation after arterial injury. However, the downstream Ras effector pathway responsible for this phenotype is unknown. Based on in vitro assays demonstrating enhanced extracellular signal-related kinase (Erk) signaling in Nf1+/− macrophages and vascular smooth muscle cells and in vivo evidence of Erk amplification without alteration of phosphatidylinositol 3-kinase signaling in Nf1+/− neointimas, we tested the hypothesis that Ras-Erk signaling regulates intimal proliferation in a murine model of NF1 arterial stenosis. By using a well-established in vivo model of inflammatory cell migration and standard cell culture, neurofibromin-deficient macrophages demonstrate enhanced sensitivity to growth factor stimulation in vivo and in vitro, which is significantly diminished in the presence of PD0325901, a specific inhibitor of Ras-Erk signaling in phase 2 clinical trials for cancer. After carotid artery injury, Nf1+/− mice demonstrated increased intimal proliferation compared with wild-type mice. Daily administration of PD0325901 significantly reduced Nf1+/− neointima formation to levels of wild-type mice. These studies identify the Ras-Erk pathway in neurofibromin-deficient macrophages as the aberrant pathway responsible for enhanced neointima formation.
Neurofibromatosis type 1 (NF1) results from mutations in the NF1 tumor-suppressor gene, which encodes the protein neurofibromin. Neurofibromin negatively regulates Ras activity in multiple cell types by accelerating the hydrolysis of active Ras-GTP to its inactive diphosphate conformation.1 These loss-of-function mutations accelerate Ras signaling and sensitize vessel wall cells and circulating hematopoietic cells, particularly myeloid progenitors and their differentiated progeny, to growth factors implicated in maintaining vascular wall homeostasis and disease pathogenesis.1–4 Some patients with NF1 are predisposed to intimal proliferation, termed neointima, leading to debilitating arterial stenosis and tissue ischemia that contribute significantly to the premature mortality observed in this population.5
Nf1 heterozygous (Nf1+/−) mice display increased neointima formation, characterized by proliferating vascular smooth muscle cells (VSMCs) and infiltration of bone marrow–derived macrophages after arterial ligation, which is reminiscent of patients with NF1.5,6 Neurofibromin-deficient endothelial cells, VSMCs, and bone marrow–derived myeloid cells demonstrate preferential activation of the Ras-Erk signaling pathway, without corresponding alterations in Ras–phosphatidylinositol 3-kinase signaling, in response to multiple growth factors in vitro.2–4,7 This is an interesting observation because lineage-restricted inactivation of a single Nf1 gene in endothelial cells and/or VSMCs does not replicate the striking neointima observed in Nf1 heterozygous mice. However, we recently demonstrated that lineage-specific inactivation of a single Nf1 gene copy in monocytes/macrophages is sufficient to reproduce the enhanced neointima formation observed in Nf1 heterozygous mice compared with wild-type (WT) mice.8
Based on these observations, we used in vitro and in vivo systems of macrophage function to test the hypothesis that Nf1 heterozygous macrophage function and mobilization to sites of inflammation are directly controlled by Ras-Erk signaling and that use of a specific and long-acting inhibitor of Ras-Erk signaling, under evaluation in multiple phase 1 and 2 clinical trials for cancer and preclinical models of NF1 malignancy,1,9–12 will reduce neointima formation after mechanical injury.
Materials and Methods
Animals
Protocols were approved by the Institutional Animal Care and Use Committee at Indiana University (Indianapolis, IN). Nf1+/− mice were obtained from Tyler Jacks, Ph.D., (Massachusetts Institute of Technology, Cambridge, MA) and backcrossed 13 generations into the C57BL/6J strain. Mice were genotyped by PCR, as previously described.2 Nf1+/− and WT mice (C57BL/6J) were crossed to generate experimental progeny. Male mice, between 12 and 15 weeks of age, were used for experiments.
Carotid Artery Ligation
In five independent experiments, the carotid arteries of Nf1+/− and WT mice were mechanically injured via ligation of the right common carotid artery proximal to the bifurcation, as previously described.2,8 Briefly, experimental and control animals were anesthetized by inhalation of a 2% isoflurane and 98% oxygen admixture. Under a dissecting microscope, the proximal arterial tree was exposed through a midline neck incision and the right common carotid artery was ligated using a 6-0 silk suture. The contralateral carotid artery was sham ligated as a control. Buprenorphine, 15 μg (Reckitt, Richmond, VA), was administered via i.p. injection and mice recovered for 28 days without complication.
Mitogen-Activated Protein Kinase/Erk Kinase (Mek) Inhibitor Administration
Nf1+/− and WT mice were administered 5 or 10 mg PD0325901/kg once daily via oral gavage. Treatment commenced 7 days before arterial injury and continued until tissue was harvested 28 days after arterial ligation. In some experiments, treatment was initiated 7 days after arterial injury and continued through tissue harvest at 28 days after injury. Nf1+/− and WT control mice were administered an equivalent volume of hydroxypropylmethylcellulose (Sigma-Aldrich, St. Louis, MO) as a vehicle control.
Histopathological Characteristics and Morphometric Analysis
After a 28-day recovery period, mice were anesthetized via inhalation of a 2% isoflurane and 98% oxygen admixture. Carotid arteries were perfusion fixed in situ with 10 mL of 0.125 mmol/L adenosine plus 0.0125 mmol/L sodium nitroprusside for 10 minutes, followed by Z-fix solution (Anatech, Battle Creek, MI), as previously described.2,8 By using a dissecting microscope, whole injured and uninjured control carotid arteries were excised, incubated in Z-fix solution at 4°C for 24 hours, and paraffin embedded. On identification of the carotid bifurcation, serial arterial cross sections (7 μm thick) were collected at 200-μm intervals across the length of the artery. Van Gieson staining was performed according to standard methods, and arterial cross sections at 400, 800, and 1200 μm proximal to the ligation were analyzed for neointima formation using ImageJ software version 1.46 (NIH, Bethesda, MD). For each cross section, lumen area, area inside the internal elastic lamina (IEL), and area inside the external elastic lamina (EEL) were measured. To quantify neointima area, intima area (IEL area − lumen area), media area (EEL area − IEL area), and in tima/media (I/M) ratio (intima area/media area) were calculated and reported. Arteries containing significant thrombus (>50% lumen occlusion) at 400 μm were excluded from analysis. Numbers of excluded mice did not differ significantly according to genotype and/or treatment group.
Macrophage Recruitment In Vivo
Nf1+/− and WT mice were challenged with 100 μg lipopolysaccharide (LPS) or PBS by i.p. injection. In separate experiments, mice were sacrificed at 24, 48, 72, and 96 hours after LPS challenge, and peritoneal cells were collected via peritoneal lavage in 5 mL of PBS. Lavage fluid and cells were incubated in red cell lysis buffer for 10 minutes, followed by inactivation with 1 mL of adult bovine serum (ABS). Samples were centrifuged and resuspended in 500 μL of 10% ABS in Dulbecco’s modified Eagle's medium. The total number of lavage cells was determined using a hemocytometer. Cells were then incubated on ice with murine Fc Blocking Reagent (Miltenyi Biotec, Cologne, Germany) for 10 minutes before staining with CD115-phosphatidylethanolamine (eBioscience, San Diego, CA), F4/80-Allophycocyanin (Serotech, Oxford, UK), TER119-Pac Blue (eBioscience), and LIVE/DEAD violet fixable dead cell stain (Invitrogen, Grand Island, NY) for 30 minutes and resuspended in 2% ABS in PBS. Stained samples were acquired on a BD LSRII flow cytometer (BD Biosciences, San Jose, CA) equipped with a 405-nm violet laser, a 488-nm blue laser, and a 633-nm red laser. At least 100,000 events were collected for samples. Data were collected uncompensated and analyzed using FlowJo software, version 8.7.3 (Tree Star, Ashland, OR). Monocyte and macrophage cell numbers were calculated as the product of CD115 or F4/80 as a percentage of live cells and the total lavage cell count and reported as cells/mL of lavage volume. In some experiments, mice were administered 5 mg PD0325901/kg per day via oral gavage beginning 7 days before LPS injection and continued until peritoneal lavage was performed at 96 hours.
Isolation of BM-Derived Macrophages
Bone marrow (BM)–derived macrophages were generated from long bones of 8- to 12-week-old WT and Nf1+/− mice. Briefly, BM cells were flushed into a 50-mL falcon tube using a syringe needle and Iscove’s modified Dulbecco’s medium (IMDM). Cells were collected by centrifugation at 800 × g for 5 minutes (Beckman Coulter, Brea, CA) at room temperature, and red blood cells were lysed with red cell lysis buffer for 5 minutes at room temperature. The cells were centrifuged and resuspended in 5 mL IMDM. Low-density BM cells were isolated by density-gradient centrifugation using Histopaque 1083 (Sigma-Aldrich). For macrophages, low-density BM cells were cultured in complete media consisting of IMDM, 20% fetal bovine serum supplemented with 1% penicillin/streptomycin, and 100 ng/mL macrophage colony-stimulating factor (M-CSF; PeproTech, Rocky Hill, NJ). Cells were used for experiments between three and six passages.
Macrophage Proliferation Assay
Cells were starved in media containing 0.2% bovine serum albumin (BSA) in IMDM without growth factors for 6 to 7 hours. A total of 5 × 104 macrophages were placed in a 96-well plate in 200 μL starvation media in the presence or absence of the indicated concentration of M-CSF. Cells were serum starved for 48 hours and pulsed with 1.0 μCi (0.037 MBq) [3H] thymidine for 6 hours. Cells were harvested using an automated 96-well cell harvester (Brandel, Gaithersburg, MD), and thymidine incorporation was determined as cpm. Where indicated, cells were pretreated with the indicated concentration of PD0325901 or vehicle for 1 hour.
Macrophage Migration Assay
The bottom of Transwell filters (8-μmol/L pore filter; Corning, Tewksbury, MA) was coated with 20 μg/mL fibronectin CH296 peptide for 2 hours at 37°C and rinsed twice with PBS containing 2% BSA. The fibronectin CH296 peptide–coated filters were placed in the lower chamber containing 500 μL complete medium, with or without 100 ng/mL M-CSF. A total of 2.5 × 105 macrophages were resuspended in 100 μL IMDM and allowed to migrate toward the bottom of the top chamber. After 20 hours of incubation at 37°C, nonmigrated cells in the upper chamber were removed with a cotton swab. The migrated cells that attached to the bottom surface of the membrane were stained with 0.1% crystal violet dissolved in 0.1 mol/L borate, pH 9.0, and 2% ethanol for 5 minutes at room temperature. The number of migrated cells per membrane was determined in five random fields with an inverted microscope using a 20× objective lens. Where indicated, cells were pretreated with the indicated concentration of PD0325901 or vehicle for 1 hour.
Macrophage Adhesion Assay
Flat-bottom, 96-well polystyrene plates (BD Biosciences) were coated with 20 μg/mL fibronectin fragment CH296 in PBS for 1 hour at 37°C. Wells were washed once with PBS, incubated with 20 mg/mL BSA for 1 hour at 37°C to block non-specific sites, and again washed twice with PBS. For examination of cell adhesion on the coated surface, 1 × 105 cells were added to each well and incubated at 37°C for 60 minutes. At the end of the incubation, unbound cells were removed by aspiration and wells were washed twice with cold PBS. Adherent cells were fixed with 3.5% formaldehyde and stained with 0.1% crystal violet. The stain was eluted with 10% acetic acid, and absorbance was determined at 600 nm using a microplate reader (Spectramax 250; Molecular Devices, Sunnyvale, CA). Where indicated, cells were pretreated with the indicated concentration of PD0325901 or vehicle for 1 hour.
Western Blot Analysis
Peritoneal cells from Nf1+/− and WT mice treated with either PD0325901 or vehicle control were harvested via peritoneal lavage at 96 hours after stimulation with 100 μg LPS. Cells were lysed in lysis buffer, and equal amounts of protein lysates were fractionated on a 4% to 20% SDS-PAGE gel. The separated protein was electrophoretically transferred onto a nitrocellulose membrane, and Western blot analysis was performed using anti–phosphorlyated-Erk and anti-Erk antibodies (Cell Signaling, Beverly, MA). Separately, BM-derived macrophages from WT and NF1+/− mice were isolated and starved overnight in serum- and growth factor–free medium and stimulated with 100 ng/mL M-CSF for 5 minutes in the presence or absence of PD0325901. Cells were harvested and lysed, as described, and Western blot analysis was performed using anti–phosphorlyated-Erk and anti-Erk antibodies.
Statistical Analysis
All values are presented as means ± SEM. Intima area and I/M ratio analysis for each treatment group were assessed by one-way analysis of variance with a Tukey’s post hoc test using GraphPad Prism, version 6.0b (GraphPad Software, San Diego, CA). P < 0.05 was considered significant.
Results
Ras-Mek-Erk Signaling Regulates Neurofibromin-Deficient and WT Macrophage Function
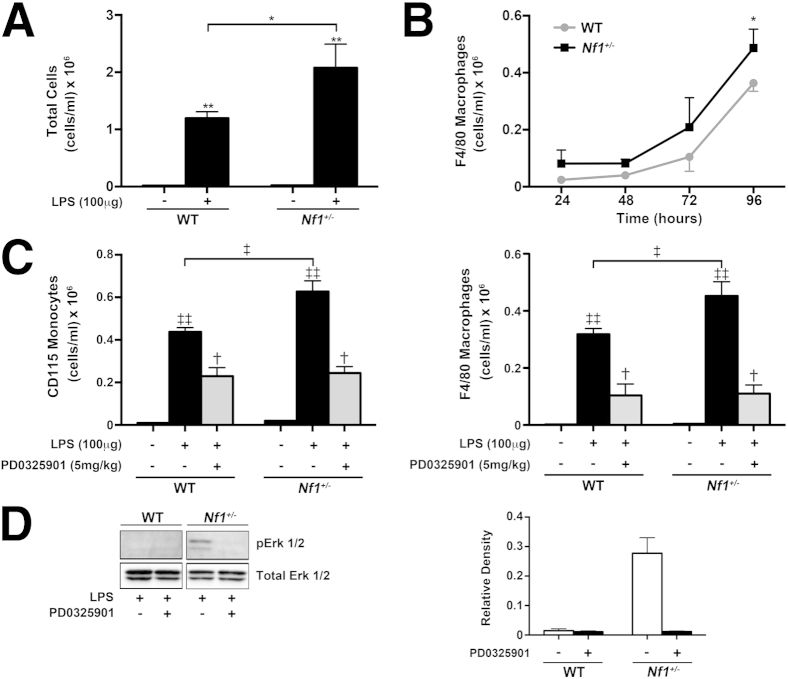
To assess monocyte and macrophage response to growth factors associated with arterial disease, we used a well-established model of LPS-induced peritonitis in Nf1+/− and WT mice. After i.p. injection of 100 μg LPS, Nf1 heterozygous mice more readily mobilized hematopoietic cells to the peritoneum at 96 hours when compared with WT mice (Figure 1A). Analysis of the lavage demonstrated a more robust recruitment of CD115-positive monocytes and F4/80-positive macrophages to the peritoneum of Nf1+/− mice, compared with WT mice (Figure 1, B and C). The preferential mobilization of Nf1+/− monocytes and macrophages was effectively suppressed with daily administration of PD0325901 (Figure 1C), a specific and potent inhibitor of Ras-Erk signaling1 (Figure 1D). Not surprisingly, recruitment of WT monocytes and macrophages was suppressed by PD0325901, thus demonstrating that the Ras-Erk signaling pathway regulates the recruitment of monocytes/macrophages to sites of inflammation in nonmutant animals.
Figure 1.
Ras-Erk signaling regulates neurofibromin-deficient and WT macrophage function in vivo. A: Total cell count from the peritoneum of WT and Nf1+/− at 96 hours after i.p. injection of 100 μg LPS. Data represent means ± SEM cell count per mL (n = 4 to 5). B: Recruitment of WT and Nf1+/− macrophages to the peritoneum after i.p. injection of 100 μg LPS over 96 hours. Data represent means ± SEM cell count per mL (n = 4 to 5). C: Recruitment of WT and Nf1+/− monocytes and macrophages to the peritoneum at 96 hours after i.p. injection of 100 μg LPS in the presence or absence of PD0325901. Data represent means ± SEM cell count per mL (n = 5). D: Erk activity in LPS-elicited peritoneal macrophages isolated from WT and Nf1+/− mice administered daily 5 mg PD0325901/kg or placebo. Western blot analyses for Erk phosphorylation and total Erk are shown. Quantitative densitometry ± SEM is reported as a ratio for phosphorylated-Erk density/total-Erk density. The data are from a single experiment and represent a replicate of three total experiments. ∗P < 0.001 for WT versus Nf1+/− macrophages; ∗∗P < 0.001 for LPS-stimulated WT and Nf1+/− total cell count versus placebo-stimulated WT and Nf1+/− total cell counts; †P < 0.01 for LPS-stimulated WT and Nf1+/− monocytes and macrophages versus LPS-stimulated WT and Nf1+/− monocytes and macrophages in the presence of PD0325901; ‡P < 0.001 for LPS-stimulated WT versus LPS-stimulated Nf1+/− monocytes and macrophages; and ‡‡P < 0.001 for LPS-stimulated WT and Nf1+/− monocytes and macrophages versus placebo-stimulated WT and Nf1+/− monocytes and macrophages.
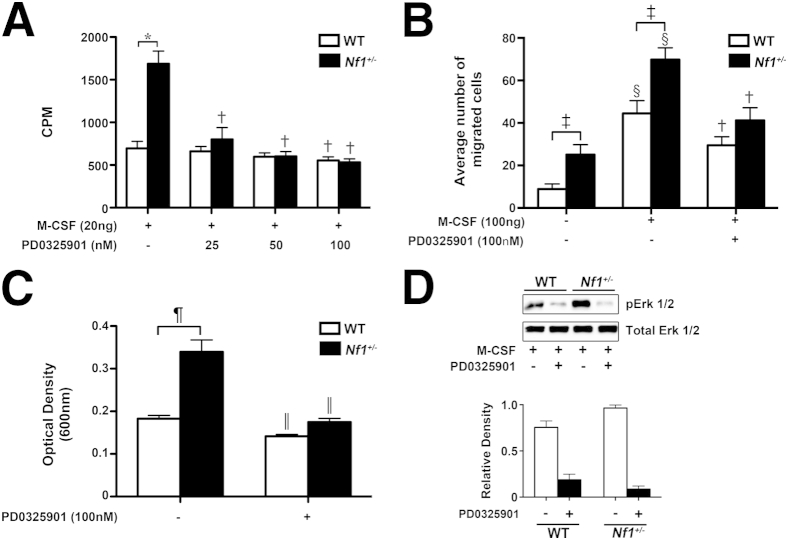
To assess the regulatory role of Ras-Erk signaling in macrophage function, cultured macrophages were stimulated with M-CSF, a growth factor implicated in cardiovascular disease and arterial stenosis,13 in the presence or absence of PD0325901. Neurofibromin-deficient macrophages demonstrated increased proliferation, migration, and adhesion in response to single growth factor stimulation via Ras-Erk activation when compared with WT macrophages (Figure 2, A–D), which is consistent with previous reports.2,8 In the presence of nanomolar concentrations of PD0325901, Nf1+/− macrophage function was significantly reduced to levels of WT macrophages in vitro (Figure 2, A–C). WT macrophages also demonstrated an impaired response to M-CSF in the presence of PD0325901; however, this response was modest when compared with Nf1+/− macrophages (Figure 2, A–C).
Figure 2.
PD0325901 inhibits neurofibromin-deficient and WT macrophage function in vitro. WT and Nf1+/− macrophage proliferation (A) and migration (B) in response to M-CSF in the presence of PD0325901, where indicated. C: Adhesion to fibronectin in the presence of PD0325901, where indicated. A: Data represent thymidine incorporation reported as means ± SEM cpm (n = 6). B: Data represent average ± SEM number of migrated cells per high-power field (n = 6). C: Data represent means ± SEM optical density (n = 5). D: Erk activity in WT and Nf1+/− cultured macrophages stimulated with M-CSF in the presence or absence of 100 nmol/L PD0325901. Western blots for Erk phosphorylation and total Erk are shown. Quantitative densitometry ± SEM is reported as a ratio for phosphorylated-Erk density/total-Erk density. The data are from a single experiment and represent a replicate of three other experiments. Black bars, Nf1+/−; white bars, WT. ∗P < 0.001 for WT macrophages stimulated with M-CSF versus Nf1+/− macrophages stimulated with M-CSF; †P < 0.001 for WT and Nf1+/− macrophages stimulated with M-CSF versus WT and Nf1+/− macrophages stimulated with M-CSF in the presence of PD0325901; ‡P < 0.001 for WT macrophages stimulated with PBS versus Nf1+/− macrophages stimulated with PBS; §P < 0.001 for WT and Nf1+/− macrophages stimulated with PBS versus WT and Nf1+/− macrophages stimulated with M-CSF; ¶P < 0.001 for WT versus Nf1+/− macrophages; and ‖P < 0.001 for WT and Nf1+/− macrophages versus WT and Nf1+/− macrophages in the presence of PD0325901.
Daily Administration of PD0325901 Prevents Neointima Formation in Nf1+/− Mice after Arterial Injury
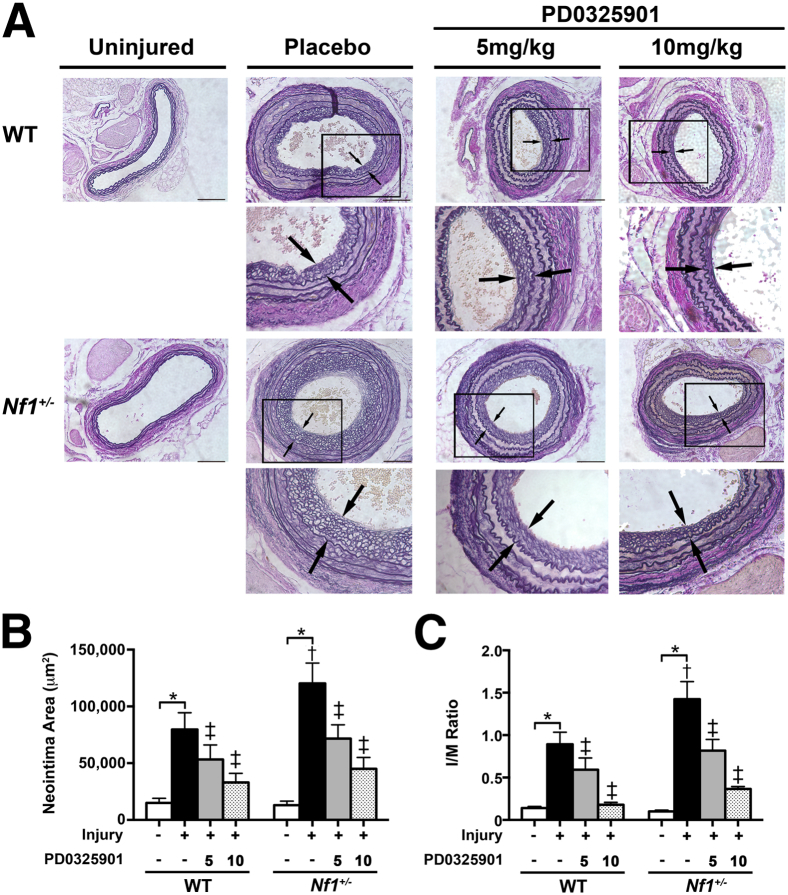
To assess the role of Ras-Erk signaling in Nf1+/− and WT neointima formation, Nf1+/− and WT mice were administered daily oral 5 or 10 mg PD0325901/kg per day for 1 week before arterial injury; this was continued until arteries were harvested. This dose range effectively inhibits downstream Ras-Mek-Erk signaling for 24 hours in multiple bone marrow lineages, as demonstrated in several animal models.1,10 After arterial ligation of the right common carotid artery, Nf1+/− mice demonstrated enhanced neointima formation when compared with WT mice, which is consistent with previous findings2,6,8 (Figure 3, A–C). Daily administration of PD0325901 significantly reduced Nf1+/− neointima formation in a dose-dependent manner, returning arterial stenosis and remodeling to levels observed in WT mice. Consistent with previous in vitro and in vivo observations that Erk signaling is preferentially activated in neurofibromin-deficient myeloid cells, vascular wall cells, and neointimas,1,10,14 Nf1+/− neointimas expressed similar phosphorylated-AKT staining when compared with WT neointimas, regardless of treatment group, thus demonstrating the specificity of PD0325901 (data not shown). The observation that PD0325901 moderately reduced neointima formation in WT mice suggests Ras-Mek-Erk signaling maintains the vascular wall in unperturbed states and regulates the response of the vascular wall to injury. Recent reports using a murine model of myeloproliferative dysplasia have demonstrated that PD0325901 partially corrects aberrant myelopoiesis and ineffective erythropoiesis observed in this model.1,10 However, examination of the bone marrow from Nf1+/− and WT mice in each treatment group did not differ in cellularity or myeloid/erythroid ratio, signifying that the reduction of neointima size observed in each treatment group is more likely due to effects on differentiated macrophage function outside the bone marrow compartment, rather than through alterations in developmental hematopoiesis (data not shown).
Figure 3.
Daily administration of PD0325901 prevents neointima formation in Nf1+/− mice after arterial injury. A: Representative photomicrographs of Van Gieson–stained carotid arteries from WT and Nf1+/− mice 28 days after no injury, injury, and placebo treatment or injury and 5 or 10 mg PD0325901/kg per day treatment. Arrows indicate neointima boundaries; boxes, the area of injured artery that is magnified below. Scale bar = 100 μm. Quantification of neointima area (B) and I/M ratio (C) of uninjured and injured carotid arteries from WT and Nf1+/− mice treated with either placebo or PD0325901. Data represent the means ± SEM neointima area of three arterial cross sections (400, 800, and 1200 μm proximal to the ligation) (n = 12 to 15). ∗P < 0.01 for WT and Nf1+/− uninjured versus WT and Nf1+/− injured with placebo treatment; †P < 0.01 for WT injured with placebo treatment versus Nf1+/− injured with placebo treatment; and ‡P < 0.01 for WT and Nf1+/− injured with placebo treatment versus WT and Nf1+/− injured with 5 or 10 mg PD0325901/kg per day treatment.
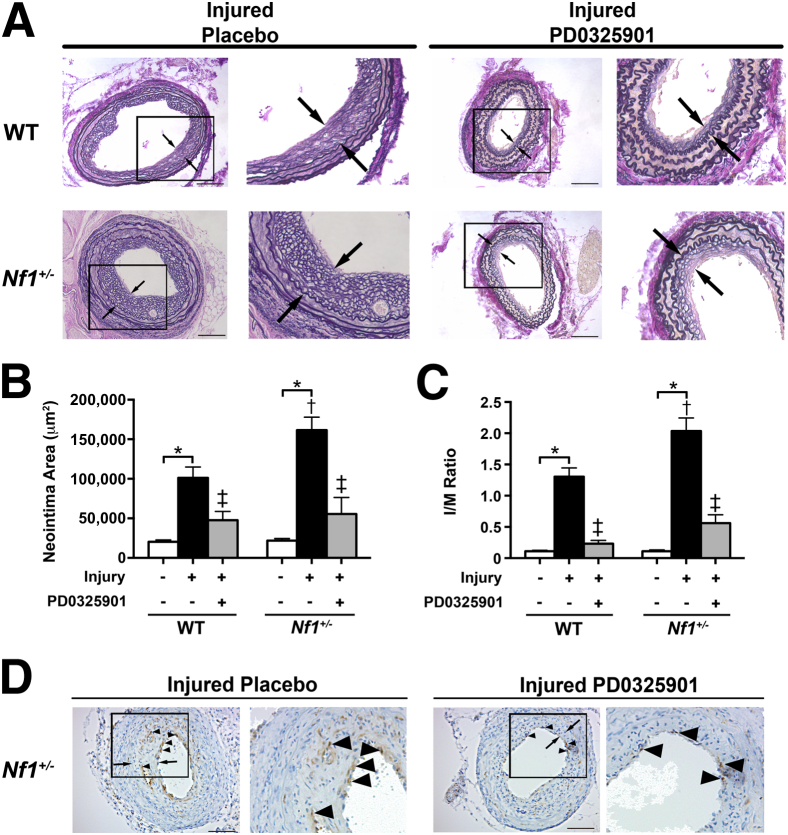
Initiation of Mek Inhibition after Arterial Injury Reduces Neointima Formation in Nf1+/− Mice
To test whether inhibition of the Mek-Erk signaling axis is efficacious in reducing neointima formation after arterial remodeling has been initiated, Nf1+/− and WT mice were administered either daily 5 mg PD0325901/kg or vehicle, commencing on day 7 after arterial injury and continuing through day 28 after injury. Morphometric analysis of injured and control arteries from Nf1+/− and WT mice demonstrated that initiation of treatment 7 days after arterial injury significantly reduced neointima area and I/M ratio in Nf1+/− and WT mice when compared with vehicle-treated mice (Figure 4, A–C). Although neointima formation in both Nf1+/− and WT mice was slightly increased in the vehicle-treatment group, reduction of Nf1+/− and WT neointima formation was greater when treatment was initiated after arterial injury. In addition, Nf1+/− neointimas from mice administered PD0325901 demonstrated fewer infiltrating macrophages when compared with vehicle-treated mice (Figure 4D).
Figure 4.
Initiation of Mek inhibition after arterial injury reduces neointima formation in Nf1+/− mice. A: Representative photomicrographs of Van Gieson–stained carotid arteries from WT and Nf1+/− mice 28 days after injury and placebo treatment or injury and 5 mg PD0325901/kg per day treatment. Arrows indicate neointima boundaries; boxes, area of injured artery that is magnified to the right. Scale bar = 100 μm. Quantification of neointima area (B) and I/M ratio (C) of uninjured and injured carotid arteries from WT and Nf1+/− mice treated with either placebo or PD0325901. Data represent the means ± SEM neointima area of three arterial cross sections (400, 800, and 1200 μm proximal to the ligation) (n = 8 to 10). ∗P < 0.01 for WT and Nf1+/− uninjured versus WT and Nf1+/− injured with placebo treatment; †P < 0.01 for WT injured with placebo treatment versus Nf1+/− injured with placebo treatment; ‡P < 0.01 for WT and Nf1+/− injured with placebo treatment versus WT and Nf1+/− injured with PD0325901 treatment. D: Representative photomicrograph of injured carotid artery cross section from placebo and PD0325901-treated Nf1+/− mice stained with anti-Mac3 antibody (brown) and counterstained with hematoxylin (blue). Arrows indicate neointima boundaries; arrowheads, positive Mac3 staining; box, the area of injured artery that is magnified to the right. Scale bar = 100 μm.
Discussion
Mounting evidence suggests that Ras signaling partially regulates vascular wall homeostasis and that perturbation in Ras signaling results in a variety of congenital and acquired cardiovascular disease manifestations.15,16 Recent animal studies demonstrate that amplification of Ras signaling enhances neointima formation, whereas inhibition of the canonical Ras-Mek-Erk pathway reduces neointima formation and VSMC proliferation and migration in vivo.15,17 Neurofibromin, the protein product of the NF1 gene, accelerates the hydrolysis of Ras-GTP to its inactive conformation to negatively regulate Ras signaling. Thus, even partial loss of neurofibromin expression leads to Ras acceleration and increased cell proliferation and accumulation in both unperturbed and diseased states. In support of this hypothesis, arterial cross sections from neurofibromin-deficient mice express increased Erk signaling within the neointima layer, compared with WT controls. The signaling is effectively restored with early administration of imatinib mesylate, an inhibitor of multiple signaling pathways, including the platelet-derived growth factor-BB receptor, after arterial injury.6
Macrophages are early and important cellular effectors of cardiovascular remodeling and neointima formation.13,18,19 Macrophages secrete growth factors and matrix metalloproteinases that stimulate vascular smooth muscle cell proliferation and migration, and are implicated in cardiovascular disease pathogenesis.13,19 Although macrophages comprise a small percentage of the total cell mass within Nf1+/− and WT neointimas, neurofibromin-deficient macrophages significantly exaggerate the proliferative response of Nf1+/− and WT VSMCs via the Ras-Mek-Erk signaling cascade when compared with WT macrophages.2 These previous observations support our recent report demonstrating that heterozygous inactivation of the Nf1 gene in monocytes/macrophages alone completely reproduced the neointima phenotype observed in Nf1+/− mice.8
Herein, we show that neurofibromin-deficient monocytes and macrophages are hypersensitive to inflammatory states and growth factor stimulation, which is mediated via the Ras-Mek-Erk signaling axis. Furthermore, we show that daily administration of PD0325901 to specifically inhibit Ras-Mek-Erk signaling significantly reduced Nf1+/− neointimas in a dose-dependent manner and resulted in a modest reduction of WT neointimas. Administration of PD0325901 at 1 week after arterial injury further reduced neointima formation in Nf1+/− and WT mice, indicating a therapeutic window may exist. PD0325901 is a next-generation Mek inhibitor and has been used as an experimental therapeutic agent in preclinical testing for multiple NF1-related manifestations and several phase 1 and 2 clinical trials for cancer.9,11,12 In particular, PD0325901 has shown significant efficacy in preclinical models of juvenile myelomonocytic leukemia, a common malignancy associated with Ras mutations in myeloid progenitor cells.1,10 Thus, Ras-Erk signaling in neurofibromin-deficient monocytes/macrophages may be an important molecular target for the development of novel therapeutics to prevent or treat NF1 vasculopathy.
Footnotes
Supported by Pediatric Scientist Development Program (National Institute of Child Health and Human Development) grant K12HD000850 (B.K.S.) and NIH grants P50 NS052606 (D.A.I.) and TLI RR025759 (B.D.D., and A. Shakhar, P.I.).
References
- 1.Chang T., Krisman K., Theobald E.H., Xu J., Akutagawa J., Lauchle J.O., Kogan S., Braun B.S., Shannon K. Sustained MEK inhibition abrogates myeloproliferative disease in Nf1 mutant mice. J Clin Invest. 2013;123:335–339. doi: 10.1172/JCI63193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lasater E.A., Li F., Bessler W.K., Estes M.L., Vemula S., Hingtgen C.M., Dinauer M.C., Kapur R., Conway S.J., Ingram D.A., Jr Genetic and cellular evidence of vascular inflammation in neurofibromin-deficient mice and humans. J Clin Invest. 2010;120:859–870. doi: 10.1172/JCI41443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li F., Munchhof A.M., White H.A., Mead L.E., Krier T.R., Fenoglio A., Chen S., Wu X., Cai S., Yang F.C., Ingram D.A. Neurofibromin is a novel regulator of RAS-induced signals in primary vascular smooth muscle cells. Hum Mol Genet. 2006;15:1921–1930. doi: 10.1093/hmg/ddl114. [DOI] [PubMed] [Google Scholar]
- 4.Munchhof A.M., Li F., White H.A., Mead L.E., Krier T.R., Fenoglio A., Li X., Yuan J., Yang F.C., Ingram D.A. Neurofibroma-associated growth factors activate a distinct signaling network to alter the function of neurofibromin-deficient endothelial cells. Hum Mol Genet. 2006;15:1858–1869. doi: 10.1093/hmg/ddl108. [DOI] [PubMed] [Google Scholar]
- 5.Friedman J.M., Arbiser J., Epstein J.A., Gutmann D.H., Huot S.J., Lin A.E., McManus B., Korf B.R. Cardiovascular disease in neurofibromatosis 1: report of the NF1 Cardiovascular Task Force. Genet Med. 2002;4:105–111. doi: 10.1097/00125817-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Lasater E.A., Bessler W.K., Mead L.E., Horn W.E., Clapp D.W., Conway S.J., Ingram D.A., Li F. Nf1+/- mice have increased neointima formation via hyperactivation of a Gleevec sensitive molecular pathway. Hum Mol Genet. 2008;17:2336–2344. doi: 10.1093/hmg/ddn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y.Y., Vik T.A., Ryder J.W., Srour E.F., Jacks T., Shannon K., Clapp D.W. Nf1 regulates hematopoietic progenitor cell growth and ras signaling in response to multiple cytokines. J Exp Med. 1998;187:1893–1902. doi: 10.1084/jem.187.11.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stansfield B.K., Bessler W.K., Mali R., Mund J.A., Downing B., Li F., Sarchet K.N., DiStasi M.R., Conway S.J., Kapur R., Ingram D.A., Jr. Heterozygous inactivation of the Nf1 gene in myeloid cells enhances neointima formation via a rosuvastatin-sensitive cellular pathway. Hum Mol Genet. 2013;22:977–988. doi: 10.1093/hmg/dds502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jessen W.J., Miller S.J., Jousma E., Wu J., Rizvi T.A., Brundage M.E., Eaves D., Widemann B., Kim M.O., Dombi E., Sabo J., Hardiman Dudley A., Niwa-Kawakita M., Page G.P., Giovannini M., Aronow B.J., Cripe T.P., Ratner N. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J Clin Invest. 2013;123:340–347. doi: 10.1172/JCI60578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyubynska N., Gorman M.F., Lauchle J.O., Hong W.X., Akutagawa J.K., Shannon K., Braun B.S. A MEK inhibitor abrogates myeloproliferative disease in Kras mutant mice. Sci Transl Med. 2011;3:76ra27. doi: 10.1126/scitranslmed.3001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boasberg P.D., Redfern C.H., Daniels G.A., Bodkin D., Garrett C.R., Ricart A.D. Pilot study of PD-0325901 in previously treated patients with advanced melanoma, breast cancer, and colon cancer. Cancer Chemother Pharmacol. 2011;68:547–552. doi: 10.1007/s00280-011-1620-1. [DOI] [PubMed] [Google Scholar]
- 12.Haura E.B., Ricart A.D., Larson T.G., Stella P.J., Bazhenova L., Miller V.A., Cohen R.B., Eisenberg P.D., Selaru P., Wilner K.D., Gadgeel S.M. A phase II study of PD-0325901, an oral MEK inhibitor, in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res. 2010;16:2450–2457. doi: 10.1158/1078-0432.CCR-09-1920. [DOI] [PubMed] [Google Scholar]
- 13.Shiba Y., Takahashi M., Yoshioka T., Yajima N., Morimoto H., Izawa A., Ise H., Hatake K., Motoyoshi K., Ikeda U. M-CSF accelerates neointimal formation in the early phase after vascular injury in mice: the critical role of the SDF-1-CXCR4 system. Arterioscler Thromb Vasc Biol. 2007;27:283–289. doi: 10.1161/01.ATV.0000250606.70669.14. [DOI] [PubMed] [Google Scholar]
- 14.Le D.T., Kong N., Zhu Y., Lauchle J.O., Aiyigari A., Braun B.S., Wang E., Kogan S.C., Le Beau M.M., Parada L., Shannon K.M. Somatic inactivation of Nf1 in hematopoietic cells results in a progressive myeloproliferative disorder. Blood. 2004;103:4243–4250. doi: 10.1182/blood-2003-08-2650. [DOI] [PubMed] [Google Scholar]
- 15.Dong L.H., Wen J.K., Liu G., McNutt M.A., Miao S.B., Gao R., Zheng B., Zhang H., Han M. Blockade of the Ras-extracellular signal-regulated kinase 1/2 pathway is involved in smooth muscle 22 alpha-mediated suppression of vascular smooth muscle cell proliferation and neointima hyperplasia. Arterioscler Thromb Vasc Biol. 2010;30:683–691. doi: 10.1161/ATVBAHA.109.200501. [DOI] [PubMed] [Google Scholar]
- 16.Rauen K.A., Schoyer L., McCormick F., Lin A.E., Allanson J.E., Stevenson D.A. Proceedings from the 2009 genetic syndromes of the Ras/MAPK pathway: from bedside to bench and back. Am J Med Genet Part A. 2010;152A:4–24. doi: 10.1002/ajmg.a.33183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin G., Chieh-Hsi Wu J., Li Y.S., Hu Y.L., Shyy J.Y., Chien S. Effects of active and negative mutants of Ras on rat arterial neointima formation. J Surg Res. 2000;94:124–132. doi: 10.1006/jsre.2000.6014. [DOI] [PubMed] [Google Scholar]
- 18.Schober A., Zernecke A., Liehn E.A., von Hundelshausen P., Knarren S., Kuziel W.A., Weber C. Crucial role of the CCL2/CCR2 axis in neointimal hyperplasia after arterial injury in hyperlipidemic mice involves early monocyte recruitment and CCL2 presentation on platelets. Circ Res. 2004;95:1125–1133. doi: 10.1161/01.RES.0000149518.86865.3e. [DOI] [PubMed] [Google Scholar]
- 19.Danenberg H.D., Fishbein I., Epstein H., Waltenberger J., Moerman E., Monkkonen J., Gao J., Gathi I., Reichi R., Golomb G. Systemic depletion of macrophages by liposomal bisphosphonates reduces neointimal formation following balloon-injury in the rat carotid artery. J Cardiovasc Pharmacol. 2003;42:671–679. doi: 10.1097/00005344-200311000-00014. [DOI] [PubMed] [Google Scholar]