Abstract
The clinical effects of treatment withn β-adrenoceptor (β-AR) agonists and antagonists in heart failure vary with duration of therapy, as do the effects of β-AR agonists in asthma. Therefore, we hypothesized that chronic effects of “β-blockers” in asthma may differ from those observed acutely. We tested this hypothesis in an antigen (ovalbumin)-driven murine model of asthma. Airway resistance responses (Raw) to the muscarinic agonist methacholine were measured by using the forced oscillation technique. In comparison with nontreated asthmatic mice, we observed that: (i) The β-AR antagonists nadolol or carvedilol, given as a single i.v. injection (acute treatment) 15 min before methacholine, increased methacholine-elicited peak Raw values by 33.7% and 67.7% (P < 0.05), respectively; when either drug was administered for 28 days (chronic treatment), the peak Raw values were decreased by 43% (P < 0.05) and 22.9% (P < 0.05), respectively. (ii) Chronic treatment with nadolol or carvedilol significantly increased β-AR densities in lung membranes by 719% and 828%, respectively. (iii) Alprenolol, a β-blocker with partial agonist properties at β-ARs, behaved as a β-AR agonist, and acutely reduced peak Raw value by 75.7% (P < 0.05); chronically, it did not alter Raw.(iv) Salbutamol, a β-AR partial agonist, acutely decreased peak Raw by 41.1%; chronically, it did not alter Raw. (v) None of the β-blockers produced significant changes in eosinophil number recovered in bronchoalveolar lavage. These results suggest that β-AR agonists and β-blockers with inverse agonist properties may exert reciprocating effects on cellular signaling dependent on duration of administration.
Keywords: β-blockers, sympathomimetics, airway resistance, inverse agonist
Pharmacological modulation of β-adrenoreceptor (β-AR) function with drugs classified as β-AR antagonists and inverse agonists (“β-blockers”) accomplishes important therapeutic objectives in diverse disease processes such as hypertension, angina, congestive heart failure (CHF), and glaucoma. Recently, there has been a major paradigm shift in the treatment of CHF as a result of the observation that duration of treatment in this syndrome determines whether the therapeutic effects of β-AR ligands are beneficial or detrimental. In heart failure, β-AR agonists produce short-term hemodynamic improvements, whereas they have been found to increase mortality rates when administered chronically (**, 2, 3). Conversely, β-blockers, a class of drugs previously contraindicated in heart failure, produce symptomatic worsening at the onset of therapy, yet some members of this class improve both hemodynamics and mortality with chronic use (4-6).
Although asthma and CHF are disease processes that appear unrelated, both are influenced by ligands interacting with β-ARs. In asthmatic patients, acute administration of β-AR agonists typically produces bronchodilation (7) and bronchoprotection, i.e., attenuation of the airway narrowing elicited by constrictor agonists or exercise and prevention of the occurrence of antigen-induced early asthmatic reaction. On the other hand, chronic administration of β-AR agonists in asthmatic individuals may cause clinically detrimental effects, including an enhanced degree of unspecific airway responsiveness (8) and airway inflammation (9, 10), loss of bronchoprotection, and ultimately, increased risk of death. In this connection, epidemiological studies have demonstrated a positive correlation between the chronic use of short-acting β-AR agonists and asthma mortality (11, 12). Recently, a large trial with the long-acting β2-AR agonist, salmeterol, was stopped because of increased death rates and serious “asthma related” events among the African-American subgroup on long-term salmeterol (13). Taken together, these data suggest the possibility that the administration of β-AR ligands in asthmatic patients might cause either beneficial or detrimental effects depending on duration of therapy, in a fashion similar to that observed in CHF.
Presently, the administration of β-blockers is contraindicated in asthmatics (14) because of the observation that single-dose administration of nonselective β-blockers may cause airway narrowing in asthmatic patients, presumably because of sympathetic modulation of airway smooth muscle tone. Interestingly, results from a metaanalysis suggest that the use of cardioselective β-blockers (those with preference for the β1-AR subtype) appears to be safe in patients with chronic airway obstruction (15). Presumably, this is due to the lack of antagonist effect at the predominant subtype in the lung, the β2-AR. However, the effects of long-term administration of nonselective β-blockers to asthmatic subjects or in animal models of asthma have never been examined, perhaps because of the implicit assumption that the long-term effects of these drugs are similar to those produced during short-term administration (16, 17).
Based on the observation that the clinical effects of β-AR ligand treatments in heart failure vary dramatically with duration of therapy, we hypothesized that the long-term effects of β-blockers in asthma may be different from those observed during short-term treatment. We tested this hypothesis in an antigen-driven murine model of asthma. We found that the β-blockers nadolol and carvedilol, administered for 28 days, decreased significantly the maximal airway constrictor response to cholinergic stimulation and increased significantly β-AR densities in lung tissue. The degree of inhibition produced by 28 days of nadolol treatment was similar to that obtained with a single dose of the β2-AR agonist, salbutamol. These results suggest that β-AR agonists and certain β-blockers may exert reciprocating effects on cellular signaling that depend on the duration of treatment.
Methods
Animals. Balb/cJ mice aged 6 weeks (The Jackson Laboratory) were housed under specific pathogen-free conditions and fed a chicken ovalbumin-free diet. The Animal Research Ethics Boards of both the University of Houston and Baylor College of Medicine approved all experiments reported in this investigation.
Experimental Design. The effects of administration of the β-blockers carvedilol (GlaxoSmithKline, King of Prussia, PA), nadolol (Sigma), alprenolol (Sigma), and of salbutamol (Sigma), a β2-AR partial agonist, were examined in a murine model of human asthma (18). The results obtained in drug-treated animals were compared with those obtained in vehicle-treated counterparts in experiments performed in close temporal relationship.
Antigen Sensitization and Challenge Protocols. Mice were systemically sensitized with ovalbumin adsorbed to aluminum hydroxide and respiratory challenged with the same antigen (18). A group of ovalbumin-sensitized saline-challenged mice served as controls for systemic sensitization and respiratory challenges with ovalbumin. In this report, ovalbumin-sensitized and ovalbumin-challenged mice, and ovalbumin-sensitized saline-challenged mice will be referred to as asthmatic and control mice, respectively.
Drug Administration. The drugs used were salbutamol (a β2-AR partial agonist), alprenolol (a β1/β2 antagonist with partial β2 agonist activity; refs. 19-21), nadolol and carvedilol (both β1/β2 antagonist with inverse agonist activity at the β2-AR' refs. 22 and 23). To examine the effects of duration of β-AR ligand therapy on the phenotype of the murine model of asthma, the experimental drugs were administered either acutely or chronically to different groups of asthmatic mice. Asthmatic mice on acute therapy were given a single i.v. bolus infusion of either β-AR ligand or normal saline on protocol day 28, 15 min before airway responsiveness to methacholine was determined. The doses of carvedilol, nadolol, alprenolol, and salbutamol administered to the mice were 24 mg·kg-1, 72 mg·kg-1, 72 mg·kg-1, and 1.2 mg·kg-1, respectively. Asthmatic mice on chronic therapy were treated with β-AR ligand from protocol days 1 to 28. Those on β-blocker therapy had free access to chow treated with carvedilol, nadolol, or alprenolol at concentrations of 2,400 ppm, 250 ppm, or 7,200 ppm, respectively. These concentrations were chosen because they had previously been shown to produce effects in mice (24, 25). The i.v. bolus doses were calculated to approximate the loading dose required to achieve similar blood levels to those obtained at steady-state with chronic treatment. The nontreated asthmatic mice were fed normal chow. Salbutamol was delivered for 28 days at a dose of 0.5 mg·kg-1·day-1 by using an osmotic minipump (Alzet no. 2004, Durect, Cupertino, CA).
Animal Preparation, Lung Physiology, and Bronchoalveolar Lavage. On protocol day 28, mice were anesthetized, tracheotomized, and connected to a computer-controlled small animal ventilator (Flexivent, Scientific Respiratory Equipment, Montreal) (18). Airway resistance (Raw) was measured by using the forced oscillation technique (18). The cellular composition of bronchoalveolar lavage fluid (BALF) was determined as reported (18). In nontreated asthmatic mice, the degree of airway responsiveness and the number of eosinophils recovered in BALF were significantly higher compared to the ovalbumin-sensitized saline-challenged (control) mice. However, we observed that the degree of airway responsiveness and the number of eosinophils recovered in BALF were lower in nontreated asthmatic mice studied in close temporal relationship with mice receiving acute β-blocker treatments than in those obtained in nontreated asthmatic mice studied concomitantly with mice on chronic β-blocker therapy.
Airway Smooth Muscle Activation. To induce airway constriction, a solution containing 150 μg·ml-1 of acetyl-β-methylcholine chloride (methacholine) (Sigma) was infused i.v. at constant rates, using a syringe infusion pump (Raze Scientific Instruments, Stanford, CT). The methacholine infusion was started at 0.008 ml·min-1, and its rate was doubled stepwise up to a maximum of 0.136 ml·min-1. Each methacholine dose was administered until a plateau was reached, during which data were sampled at 1-min intervals for 3-5 min and then averaged.
Determination of β-AR Density by Radioligand Binding. Plasma membranes were prepared from homogenized lung tissue as described (26). β-AR density was determined by incubation with increasing concentrations (5-7,500 pmol) of (-)3-[125I]iodocyanopindolol (Amersham Pharmacia). Propranolol (100 μM) (Sigma) was used to determine nonspecific binding. All experiments were performed in triplicate.
Data Analysis. The complex input impedance of the respiratory system was computed as described (27), and the value of the real part of respiratory system impedance at 19.75 Hz was taken to reflect the magnitude of Raw. To examine the degree of airway responsiveness (AR) of each animal, the values for Raw as a function of methacholine doses were plotted. The largest value for Raw obtained in response to methacholine stimulation was referred to as peak Raw. For mice that achieved a plateau in the methacholine dose-Raw response curve, the ED50 was calculated by linear interpolation using PRISM4 (GraphPad, San Diego). However, the methacholine dose-response curves illustrated in Fig. 1 are not constrained to a fit. Specific binding was calculated by using the total - nonspecific binding data and fitted to a hyperbolic equation to estimate KD and Bmax.
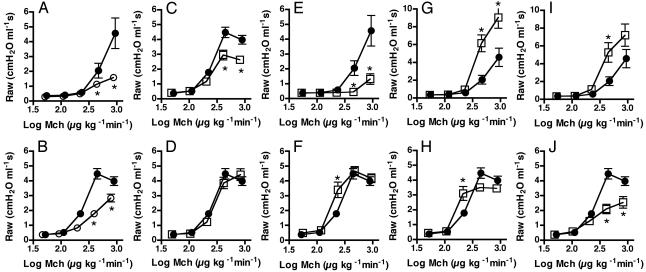
Fig. 1.
Effects of treatments with β-AR ligands on airway responsiveness to methacholine in a murine model of asthma. Asthmatic mice received either a single i.v. bolus injection 15 min before methacholine (Mch) challenge (acute; Upper) or were treated for 28 days (chronic; Lower) with salbutamol via osmotic minipump (C and D), alprenolol in food (E and F), carvedilol in food (G and H), or nadolol in food (I and J). Controls received either saline i.v. or normal mouse chow and were run in parallel (A and B). Average methacholine dose-airway resistance relationships were obtained in control mice (Ctrl, open circles, n = 6-21), nontreated asthmatic mice (NTX, filled circles, n = 7-25), and asthmatic mice treated with the β-AR ligands (open squares, n = 8-19). Values are mean ± SEM. Note the change in the scale of the y axis for G and I. *, P < 0.05 compared with NTX; #, P < 0.05 compared with Ctrl (ANOVA).
Statistical Analysis. Results were expressed as mean ± SEM. Comparisons between results obtained for β-AR ligand-treated and nontreated mice were performed by using the ANOVA for multiple groups or Student's t test for comparing two groups. The Bonferroni test was used to examine statistical differences between experimental groups. The effects of acute drug treatments on baseline respiratory system mechanics were assessed by using the two-tailed paired t test. A value of P < 0.05 was considered statistically significant.
Results
The experimental treatments were overall well tolerated. The mortality rate was 5%, evenly distributed among the experimental and control groups, and mainly related to anesthetic compound overdose or airway instrumentation.
Airway Responsiveness to Methacholine. For nontreated asthmatic mice, the values for Raw at high doses of methacholine were significantly enhanced compared with those in nontreated (ovalbumin-sensitized saline-challenged) control mice (Fig. 1 A and B), indicating that the murine model used in the present study exhibited airway hyperresponsiveness, a key manifestation of airway dysfunction in human asthma.
The administration of a single i.v. bolus of salbutamol to asthmatic mice produced a marked attenuation of the model's AR, i.e., bronchoprotection, as reflected by significant decreases in the values for Raw at methacholine doses ≥408 μg·kg-1·min-1 (Fig. 1C) and in the values for peak Raw (Fig. 2A) relative to those obtained in vehicle-treated asthmatic mice. When salbutamol was delivered for 28 days, its bronchoprotective effect was not observed (Figs. 1D and 2 A).
Fig. 2.
Effects of administration of β-AR ligands on the peak airway responsiveness to cholinergic stimulation. Peak Raw was determined for each mouse by examining the individual methacholine dose-response curves and choosing the highest Raw value produced by any of the methacholine doses (most often the next to last dose, 408 μg·kg-1·min-1). Shown are the mean peak Raw ± SEM after treatments with the β-AR agonist salbutamol (A), after acute treatments (B), and after chronic treatment with β-blockers (C), in comparison with nontreated asthmatic mice (NTX; black bars, n = 7-25) and control mice (Ctrl; white bars, n = 6-21). Values are mean ± SEM for the peak Raw to methacholine of n = 8-19 mice. Note the change in the scale of the y axis for B. *, P < 0.05 compared to NTX; #, P < 0.05 compared to Ctrl (ANOVA).
β-blocker treatments caused variations in AR that depended on the specific drug and duration of administration. When asthmatic mice were given a single i.v. bolus of alprenolol, a β-blocker possessing partial agonist activity (19-21), their AR was diminished, as indicated by significant decreases in both the values for Raw at methacholine doses ≥408 μg·kg-1·min-1 (P < 0.05) (Fig. 1E) and in value for peak Raw (Fig. 2B) compared with those obtained in nontreated counterparts. When asthmatic mice were exposed to alprenolol for 28 days, their average methacholine dose-response relationship was similar to that obtained in nontreated asthmatic mice, except for the average value for Raw at a methacholine dose of 204 μg·kg-1·min-1, which was significantly higher in the former than in the latter (P < 0.05) (Fig. 1F). The alprenolol-induced changes in AR (Fig. 1 E and F) were qualitatively similar to those caused by salbutamol (Fig. 1 C and D) and exhibited the same dependence on duration of administration, suggesting that alprenolol possessed partial agonist properties in our mice.
Unlike the attenuation of AR associated with acute salbutamol or alprenolol treatments, a single i.v. bolus of carvedilol or nadolol, enhanced AR, as shown by the elevated values for Raw at methacholine doses ≥408 μg·kg-1·min-1 (Fig. 1 G and I) and for peak Raw (Fig. 2B) observed in mice treated with either β-blocker. In contrast to the lack of effects on AR associated with chronic administration of salbutamol or alprenolol, carvedilol delivered chronically decreased peak Raw and the airway constrictor response at high doses of methacholine and shifted the methacholine dose-airway response relationship to the left of that obtained in the nontreated asthmatic group (Figs. 1H and 2C). The values for ED50 in long-term carvedilol-treated mice were significantly lower than in nontreated mice (ED50: 164 ± 1.0 vs. 242 ± 1.0 μg·kg-1·min-1 for long-term carvedilol and nontreated mice, respectively; P < 0.01). Chronic delivery of nadolol also produced a decrease in AR, which was more pronounced than that caused by long-term carvedilol treatment. Indeed, the methacholine dose-Raw response relationship obtained in mice on chronic nadolol treatment was similar to that obtained in mice on acute salbutamol treatment (Figs. 1J and 2C). Neither acute salbutamol nor chronic nadolol treatments altered the values of ED50 relative to those in asthma mice.
Airway Resistance in the Nonconstricted State. Airway resistance values in the nonconstricted (“baseline”) state for the nontreated acute and chronic asthmatic mice were 0.31 ± 0.03 cm H2O ml-1·s and 0.39 ± 0.01 cm H2O ml-1·s, respectively, and 0.37 ± 0.01 cm H2O ml-1·s in nontreated control mice. In the nonconstricted state, the acute effects of β-AR ligands were determined before and after the 15-min drug administration, allowing for each mouse to serve as its own control. Results obtained in asthmatic mice on long-term β-AR ligand treatments were compared with those obtained in nontreated asthmatic mice.
β-AR ligands produced variations in baseline Raw that depended on the ligand and duration of administration. Whereas a single i.v. bolus infusion of salbutamol or alprenolol increased the average values for Raw by 10 ± 2% and 7 ± 2%, respectively, compared to preinfusion (P < 0.02, paired t test), an i.v. bolus of nadolol, carvedilol, or saline caused no significant changes in Raw.
Among mice on long-term therapies, salbutamol did not alter the magnitude of baseline Raw, whereas alprenolol increased the average value for baseline Raw by 18 ± 8% (P < 0.05) and nadolol diminished it by 20 ± 8% (P < 0.05) relative to that in nontreated asthmatic mice. Chronic carvedilol treatment caused no significant changes in baseline Raw.
β-AR Receptor Densities. Radioligand binding assays using whole lung membranes and (-)3-[125I]-iodocyanopindolol as the radiolabel revealed that lung membranes from asthmatic mice had a significantly decreased density of β-ARs compared to control mice (Table 1). Acute treatment with a single i.v. bolus of salbutamol or alprenolol restored the density back to levels comparable to control mice. However, with chronic salbutamol or alprenolol treatment (28 days), this effect was lost. Acute treatment with nadolol or carvedilol had no effect on receptor density. However, chronic treatment with either of these ligands produced a significant up-regulation of β-AR densities (Table 1; P < 0.05). The apparent KD values in the nadolol and carvedilol treatment groups were increased in both the acute, and to a larger extent, the chronic groups (Table 1).
Table 1. Determination of β-AR density by radioligand binding.
| 15 min
|
28 days
|
|||
|---|---|---|---|---|
| Treatment | Bmax, fmol·mg−1 protein | KD, pM | Bmax, fmol·mg−1 protein | KD, pM |
| Ctrl | 286.8 ± 88.02 | 107.9 ± 43.67 | 286.8 ± 88.02 | 107.9 ± 43.67 |
| NTX | 109.2 ± 9.72* | 193.6 ± 20.66 | 109.2 ± 9.72* | 193.6 ± 20.66 |
| Salbutamol | 256.5 ± 29.24† | 228.8 ± 33.07 | 97.0 ± 23.02 | 225.4 ± 41.79 |
| Alprenolol | 299.5 ± 12.19† | 453.6 ± 86.33 | 179.2 ± 53.05 | 290.9 ± 55.07 |
| Carvedilol | 86.3 ± 19.42 | 565.2 ± 192.8† | 904.1 ± 43.46† | 1444.0 ± 202.0† |
| Nadolol | 181.9 ± 48.28 | 695.1 ± 286.3† | 785.5 ± 154.8† | 1591.6 ± 335.0† |
β-AR density was measured in membranes prepared from lung tissue using the β-AR radioligand (−)3-[125I]-iodocyanopindolol in increasing concentrations (5-7,500 pM). Samples were run in triplicate and values are mean ± SEM of n = 3-5 animals in each group. Please note, the 15-min and 28-day time point refers to duration of drug treatment. All mice were killed at the same age; and thus for vehicle-treated groups (Ctrl and NTX), the groups were identical and the results were pooled.
P < 0.05 compared to Ctrl.
P < 0.05 compared to NTX (Student's t test).
Bronchoalveolar Lavage Cellularity. Asthmatic mice exhibited a significant increase in the number of eosinophils and lymphocytes in BALF relative to those obtained in control mice (Fig. 3, which is published as supporting information on the PNAS web site). The number of neutrophils was increased in nontreated asthmatic mice compared to mice that served as controls for chronic β-blocker treatments. In comparison to their notable effects on airway responsiveness, the β-AR ligands tested had very little effect on BALF cellularity. Whereas acute salbutamol administration did not alter the cellular composition of BALF, chronic salbutamol treatment produced a significant increase in the number of eosinophils relative to that in nontreated asthmatic mice. A single bolus infusion of alprenolol produced a significant increase in the number of macrophages; when alprenolol was administered chronically, no changes in BALF cellularity were observed. Nadolol, delivered either acutely or chronically, produced significant elevations in the number of neutrophils in BALF relative to those in nontreated asthmatic mice. Carvedilol, irrespective of duration of treatment, had no effects on BALF cellularity.
Discussion
Here we examine the effects of duration of treatment with nonselective β-blockers on an animal model of asthma. Alprenolol, carvedilol, and nadolol have affinity for and block β1-AR and β2-AR. Furthermore, alprenolol has been shown to be a partial β2-agonist (19-21), whereas nadolol and carvedilol have been shown to possess inverse agonist properties in β2-AR systems (23, 28). We also investigated the effects of salbutamol, a β2-AR partial agonist currently used in the treatment of asthma. The main findings of the present study are: (i) carvedilol and nadolol exhibited opposite effects on the degree of AR, which depended on duration of administration: acutely, these compounds enhanced AR; whereas chronically, they diminished it. (ii) The acute administration of the drugs with agonist properties, salbutamol or alprenolol, decreased AR, whereas AR was not altered when either drug was given chronically. (iii) The effect of reducing AR correlated to the ligand's ability to increase β-AR receptor density in the lungs. Thus, salbutamol and alprenolol produced an increase in receptor density after a single dose, but had no effect on β-AR density with chronic treatment; and carvedilol and nadolol had no acute effect on receptor density, but produced significant up-regulation of β-AR density with chronic treatment. These data indicate that there is a temporal component to the effects of the β-AR ligands with agonist properties, salbutamol and alprenolol, and the ligands with inverse agonist properties, nadolol and carvedilol.
Effect of β-AR Ligands on Airway Responsiveness to Methacholine and Receptor Densities. Airway hyperresponsiveness is of paramount clinical importance in human asthma because exaggerated airway narrowing in response to unspecific or specific stimuli may cause respiratory failure and death. Thus, pharmacological modulation of AR in asthmatic subjects is an important therapeutic objective. We have used a murine model of asthma that, although not reproducing the entire complexity of the human condition (29, 30), exhibits several key features of asthma (31), such as enhanced degree of AR, increased BALF cellularity, and heterogeneous airway narrowing during induced constriction. Also, the asthma model shows decreased numbers of β-ARs in lung membranes compared to control mice. A generalized β-AR dysfunction in asthma is a controversial theory that has been proposed to explain some of the symptoms (32). Our data suggest that the β-AR ligands' ability to reduce airway hyperresponsiveness positively correlates with their ability to restore the decreased β-AR number (see below), and confirm that β-AR ligands have the capacity to alter the degree of AR in this murine model. Depending on duration of administration, both nadolol and carvedilol altered airway constrictor responses to methacholine. When administered acutely, they increased AR, whereas chronically, they significantly decreased the maximal constrictor response to methacholine. Whereas the acute effects of nadolol and carvedilol are in line with the reported effects of single-dose administration of nonselective β-blockers such as propranolol or timolol to asthmatic subjects (33) and various animal models (34-37), the long-term effects of these drugs on airway responsiveness represent a major finding that could have therapeutic implications. It is noteworthy that a similar time-dependent opposite effect has also been observed with certain β-blockers in the treatment of CHF. For example, metoprolol produces a significant decrease in left ventricular ejection fraction (LVEF), an index of cardiac contractility, after 1 day of treatment, but causes significant increases in LVEF after 90 days of treatment (38). Carvedilol and metoprolol, β-blockers recently approved for treatment of CHF, have been shown to possess inverse agonist properties in cardiac myocytes isolated from failing human hearts, whereas bucindolol, a compound that elicited no overall beneficial effect in CHF, has been shown to behave as a partial agonist in the myocytes of almost half of the patients (28). Thus, both in our murine model of asthma and in the treatment of patients with CHF, there appears to be acute detrimental effects of β-AR inverse agonists that convert to beneficial effects during chronic dosing.
The mechanism(s) that account for changes in AR associated with chronic carvedilol or nadolol therapy could result from changes occurring at any step in the signal transduction cascade elicited by a ligand-receptor interaction, or from mechanical factors influencing the degree of shortening of the activated airway smooth muscle (39). Given the reported reciprocity of agonist versus inverse agonist effects on many cellular functions (40), we examined the changes in levels of receptor protein produced by the ligand treatments. Chronic carvedilol or nadolol treatment increased the number of membrane-associated β-ARs (Table 1). This change in β-AR number could result in bronchoprotection against the spasmogen either by simply increasing available receptors for endogenous adrenaline, or by increasing the number of constitutively active β-ARs. Complementary results were found by McGraw et al. (41), who showed that transgenic overexpression of β2-ARs in airway smooth muscle cells results in enhanced adenylyl cyclase activity at baseline and in response to agonist, and diminished airway responsiveness to inhaled methacholine in the mouse. Combined, these results suggest that β2-AR numbers on airway smooth muscle cells represent a limiting factor of the signal transduction pathway. However, this cannot be the complete explanation because, in our study, a majority of the receptors would be occupied by the antagonist/inverse agonist and would presumably be less available for activation by circulating adrenaline and would not be constitutively active. We observe that the apparent KD of (-)3-[125I]-iodocyanopindolol is approximately a log unit higher in membranes from carvedilol- or nadolol-treated mice, suggesting competition for the receptor by circulating drug. It is difficult to speculate what the physiological result is of concomitant receptor up-regulation and the presence of a competing blocker. Also, it is possible that the up-regulation of receptor numbers is not the only mechanism, or even the main mechanism, responsible for the reduced airway responsiveness. In this regard, work by others has suggested that it is a “crosstalk” between the β-AR system and Gq-phospholipase C-coupled receptors that underlies the potentially detrimental effect of chronic β-AR agonist use (42). Notwithstanding our lack of insight into the underlying mechanisms, chronic inverse agonist treatment may have therapeutic value. A recently concluded phase II trial compared the combined administration of the μ-opioid receptor agonist, oxycodone, and the μ-opioid receptor antagonist/inverse agonist, naltrexone, versus administering oxycodone alone. The data demonstrated that the combination therapy prevented the tolerance observed when chronically administering the agonist alone, and this resulted in superior pain relief after 3 weeks of treatment.†† Because μ-opioid receptors are, like the β2-ARs, G protein-coupled receptors, and their desensitization are regulated by related kinases, perhaps this strategy may also be useful in asthma therapy. The up-regulation produced by carvedilol and nadolol can “protect” the agonist response, and supplementing chronic β2-AR agonist therapy in asthma with an appropriate β-blocker may prevent some of the negative effects associated with chronic β2-AR agonist therapy in asthma. In this study, nadolol did not alter the position of the methacholine dose-Raw characteristics, whereas carvedilol caused a small (<2-fold) but significant decrease in the ED50 relative to that obtained in nontreated mice. This difference in carvedilol and nadolol related effects on airway responsiveness are not readily explainable. In transgenic mice with cardiac overexpression of the human β2-AR, nadolol has a greater intrinsic activity as an inverse agonist than carvedilol (22, 23), a difference that could account for nadolol's more pronounced decrease in maximal airway constrictor response and the lack of shift in the average methacholine dose-Raw relationship compared to carvedilol's effects. In this regard, the effects of alprenolol, carvedilol, and nadolol on reducing maximal airway constrictor responses correlate with their negative intrinsic efficacy, suggesting that the effect is mediated via the β2-AR. Nevertheless, the effect of carvedilol can be viewed as either beneficial by reducing the maximal constrictor response, or detrimental by left-shifting the methacholine responses. Similar to salbutamol, alprenolol decreased airway responsiveness when given as a single bolus infusion and did not alter AR when delivered for 28 days. When the drugs were administered acutely, both salbutamol and alprenolol restored the decreased β-AR density observed in vehicle-treated asthma mice relative to controls (Table 1). It should be noted that the drugs were given 15 min before the start of the methacholine challenge, and by the time the lungs were collected for radioligand binding assays, the drugs were circulating systemically for ≈2 h. Similar findings of relative short-term agonists exposures producing increased gene transcription of the receptor or receptor density have previously been reported for the dopamine D2 receptor and the β2-AR (43, 44). However, with chronic administration, the receptor numbers decreased back to the levels in the control asthma mice, presumably due to classic desensitization mechanisms.
Effects of β-AR Ligands on Airways in the Nonconstricted State. The average values for Raw obtained in nontreated asthmatic mice in the nonconstricted state were similar to those previously reported (18, 45).
In humans, short-term administration of salbutamol usually causes an increase in maximal expiratory flows or specific airway conductance reflecting functional antagonism to a small para-sympathetic tone. In contrast, in our murine model of asthma, a single i.v. bolus of salbutamol or alprenolol caused a small but significant increase in Raw, the mechanism of which is presently unknown.
These seemingly paradoxical results associated with acute salbutamol or alprenolol administration, i.e., an enhanced Raw in the nonconstricted state in the presence of an attenuated airway constrictor response to methacholine could be reconciled, in part, by the existence of a serial distribution of β-AR subtypes along the murine airways, in which the β1 and β2 subtypes are predominant in the trachea and peripheral airways, respectively (46). This is in marked contrast to the human airway, which expresses predominantly the β2 subtype along its entirety. The magnitude of Raw in the nonconstricted state is dominated by the geometry of the trachea and main bronchi, which in mice express β1-ARs. During methacholine stimulation, the magnitude of Raw is strongly influenced by the geometry of the distal portion of the airway tree as it undergoes severe narrowing, the peripheral airway mostly expressing β2-ARs. Thus, if a β-AR ligand produced different effects on β1-ARs and on β2-ARs, these could have resulted in a qualitatively dissimilar modulation of Raw in the nonconstricted state relative to that during contractile agonist-induced airway narrowing.
Effects of β-AR Ligands on BALF Cellularity. Chronic salbutamol administration increased the number of eosinophils in BALF relative to that in nontreated asthmatic animals. This finding is in agreement with the reported increase in eosinophil influx into the airways (9, 10) of asthmatics during the regular use of inhaled salbutamol. However, overall, the effects of β2-AR ligands on BALF cellularity were modest in comparison to their effects on AR, suggesting that the latter were independent of the severity of airway inflammation as reflected by BALF cellularity.
In summary, our results show that, in a murine model of asthma, the administration of β2-AR inverse agonists produces opposite effects on airway responsiveness that depend on the duration of therapy. There was an apparent reciprocity between the effects of the inverse agonists, nadolol and carvedilol, on airway responsiveness and the effects of the agonists, salbutamol and alprenolol. This finding parallels results obtained with regards to cardiac contractility and mortality with short-term and long-term effects of β-AR agonists and β-AR inverse-agonists in the treatment of CHF (1, 2, 28, 38). Within the limitations of extrapolating from a murine model to human asthma (30), these results provide a second disease model where compounds producing an acutely detrimental effect may provide a therapeutically beneficial effect with chronic administration (16), and indicate that the chronic effect of the ligands cannot be predicted from their acute effects. As pharmaceutical research continues to strive for more rapid drug discovery cycles, it may mean that compounds useful for chronic diseases are being missed because long-term effects of drugs may not be studied.
Acknowledgments
We thank GlaxoSmithKline for the generous gift of carvedilol chow and Gill Fleetwood for her excellent scientific input. In addition, we thank Mrs. Claudia Gerken for technical assistance. The research was supported by grants from the National Institutes of Health (to F.R.S., B.J.K., P.F.K.C., and R.A.B.), the American Heart Association (to R.A.B.), the Sandler Program for Asthma Research (to R.A.B.), and GlaxoSmithKline (to R.A.B.).
Abbreviations: AR, airway responsiveness; β-AR, β-adrenoceptor; BALF, bronchoalveolar lavage fluid; CHF, congestive heart failure.
**Dies, F., Krell, M. J., Whitlow, P., Liang, C.-S., Goldenberg, I., Applefeld, M. M. & Gilbert, E. M. (1986) Circulation 74, Suppl. II, 38 (abstr.).
Footnotes
Chindalore, V., Craven, R., Alftine, C., Butera, P., Yu, K. & Friedmann, N., 23rd American Pain Society Annual Meeting, May 6-9, 2004, Vancouver, Canada, poster 854 (abstr.).
References
- 1.Jessup, M. & Brozena, S. (2003) N. Engl. J. Med. 348, 2007-2018. [DOI] [PubMed] [Google Scholar]
- 2.The Xamoterol in Severe Heart Failure Study Group (1990) Lancet 336, 1-6. [PubMed] [Google Scholar]
- 3.Lambertz, H., Meyer, J. & Erbel, R. (1984) Circulation 69, 298-305. [DOI] [PubMed] [Google Scholar]
- 4.Packer, M., Bristow, M. R., Cohn, J. N., Colucci, W. S., Fowler, M. B., Gilbert, E. M. & Shusterman, N. H. (1996) N. Engl. J. Med. 334, 1349-1355. [DOI] [PubMed] [Google Scholar]
- 5.Foody, J. M., Farrell, M. H. & Krumholz, H. M. (2002) J. Am. Med. Assoc. 287, 883-889. [DOI] [PubMed] [Google Scholar]
- 6.Lechat, P., Packer, M., Chalon, S., Cucherat, M., Arab, T. & Boissel, J. P. (1998) Circulation 98, 1184-1191. [DOI] [PubMed] [Google Scholar]
- 7.Nelson, H. S. (1995) N. Engl. J. Med. 333, 499-506. [DOI] [PubMed] [Google Scholar]
- 8.Drazen, J. M., Israel, E., Boushey, H. A., Chinchilli, V. M., Fahy, J. V., Fish, J. E., Lazarus, S. C., Lemanske, R. F., Martin, R. J., Peters, S. P., et al. (1996) N. Engl. J. Med. 335, 841-847. [DOI] [PubMed] [Google Scholar]
- 9.Gauvreau, G. M., Jordana, M., Watson, R. M., Cockroft, D. W. & O'Byrne, P. M. (1997) Am. J. Respir. Crit. Care Med. 156, 1738-1745. [DOI] [PubMed] [Google Scholar]
- 10.Manolitsas, N. D., Wang, J., Devalia, J. L., Trigg, C. J., McAulay, A. E. & Davies, R. J. (1995) Am. J. Respir. Crit. Care Med. 151, 1925-1930. [DOI] [PubMed] [Google Scholar]
- 11.Spitzer, W. O., Suissa, S., Ernst, P., Horwitz, R. I., Habbick, B., Cockcroft, D., Boivin, J. F., McNutt, M., Buist, A. S. & Rebuck, A. S. (1992) N. Engl. J. Med. 326, 501-506. [DOI] [PubMed] [Google Scholar]
- 12.Suissa, S., Ernst, P., Boivin, J. F., Horwitz, R. I., Habbick, B., Cockroft, D., Blais, L., McNutt, M., Buist, A. S. & Spitzer, W. O. (1994) Am. J. Respir. Crit. Care Med. 149, 604-610. [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration (January 23, 2003) FDA Talk Paper (TO3-06), www.fda.gov/bbs/topics/ANSWERS/2003/ANS01192.html.
- 14.Barnes, P. J. (1997) Drug-Induced Asthma (Lippincott-Raven, Philadelphia).
- 15.Salpeter, S. R., Ormiston, T. M. & Salpeter, E. E. (2002) Ann. Intern. Med. 137, 715-725. [DOI] [PubMed] [Google Scholar]
- 16.Bond, R. A. (2001) Trends Pharmacol. Sci. 22, 273-276. [DOI] [PubMed] [Google Scholar]
- 17.Bond, R. A. (2002) Nat. Rev. Drug. Discovery 1, 825-829. [DOI] [PubMed] [Google Scholar]
- 18.Evans, K. L., Bond, R. A., Corry, D. B. & Shardonofsky, F. R. (2003) J. Appl. Physiol. 94, 245-252. [DOI] [PubMed] [Google Scholar]
- 19.Brodde, O. E., Daul, A., Stuka, N., O'Hara, N. & Borchard, U. (1985) Naunyn Schmiedebergs Arch. Pharmacol. 328, 417-422. [DOI] [PubMed] [Google Scholar]
- 20.Jasper, J. R., Michel, M. C. & Insel, P. A. (1990) Mol. Pharmacol. 37, 44-49. [PubMed] [Google Scholar]
- 21.Lima, J. J. (1996) J. Recept. Signal Transduct. Res. 16, 357-372. [DOI] [PubMed] [Google Scholar]
- 22.Bond, R. A., Leff, P., Johnson, T. D., Milano, C. A., Rockman, H. A., McMinn, T. R., Apparsundaram, S., Hyek, M. F., Kenakin, T. P., Allen, L. F., et al. (1995) Nature 374, 272-276. [DOI] [PubMed] [Google Scholar]
- 23.Nagaraja, S., Iyer, S., Liu, X., Eichberg, J. & Bond, R. A. (1999) Br. J. Pharmacol. 127, 1099-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callaerts-Vegh, Z., Evans, K. L., Shipley, G. L., Davies, P. J., Cuba, D. L., Gurji, H. A., Giles, H. & Bond, R. A. (2003) Br. J. Pharmacol. 138, 1505-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richard, D., Boily, P., Dufresne, M. C. & Lecompte, M. (1988) Can. J. Physiol. Pharmacol. 66, 1297-1302. [DOI] [PubMed] [Google Scholar]
- 26.Mak, J. C., Nishikawa, M., Shirasaki, H., Miyayasu, K. & Barnes, P. J. (1995) J. Clin. Invest. 96, 99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuessler, T. F. & Bates, J. H. (1995) IEEE Trans. Biomed. Eng. 42, 860-866. [DOI] [PubMed] [Google Scholar]
- 28.Maack, C., Cremers, B., Flesch, M., Hoper, A., Sudkamp, M. & Bohm, M. (2000) Br. J. Pharmacol. 130, 1131-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelfan, E. W. & Persson, C. G. A. (2002) Am. J. Respir. Crit. Med. 166, 5-8. [Google Scholar]
- 30.Persson, C. G., Erjefalt, J. S., Korsgren, M. & Sundler, F. (1997) Trends Pharmacol. Sci. 18, 465-467. [DOI] [PubMed] [Google Scholar]
- 31.Hessel, E. M., Van Oosterhout, A. J., Hofstra, C. L., De Bie, J. J., Garssen, J., Van Loveren, H., Verheyen, A. K., Savelkoul, H. F. & Nijkamp, F. P. (1995) Eur. J. Pharmacol. 293, 401-412. [DOI] [PubMed] [Google Scholar]
- 32.Szentivanyi, A. (1968) J. Allergy 42, 202-232. [Google Scholar]
- 33.Vatrella, A., Parrella, R., Pelaia, G., Biscione, G. L., Tranfa, C. M., De Sarro, G. B., Bariffi, F. & Marsico, S. A. (2001) Eur. J. Clin. Pharmacol. 57, 99-104. [DOI] [PubMed] [Google Scholar]
- 34.Boskabady, M. H. & Snashall, P. D. (2000) Respirology 5, 111-118. [DOI] [PubMed] [Google Scholar]
- 35.Devereux, G., Fishwick, K., Aiken, T. C., Bourke, S. J. & Hendrick, D. J. (1998) Br. J. Clin. Pharmacol. 46, 79-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latimer, K. M. & Ruffin, R. E. (1990) Eur. J. Clin. Pharmacol. 39, 441-445. [DOI] [PubMed] [Google Scholar]
- 37.Ruffin, R. E., McIntyre, E. L., Latimer, K. M., Ward, H. E., Crockett, A. J. & Alpers, J. H. (1982) Br. J. Clin. Pharmacol. 13, 325S-335S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall, S. A., Cigarroa, C. G., Marcoux, L., Risser, R. C., Grayburn, P. A. & Eichhorn, E. J. (1995) J. Am. Coll. Cardiol. 25, 1154-1161. [DOI] [PubMed] [Google Scholar]
- 39.Moreno, R. H., Hogg, J. C. & Pare, P. D. (1986) Am. Rev. Respir. Dis. 133, 1171-1180. [DOI] [PubMed] [Google Scholar]
- 40.Milligan, G. & Bond, R. A. (1997) Trends Pharmacol. Sci. 18, 468-474. [DOI] [PubMed] [Google Scholar]
- 41.McGraw, D. W., Fukuda, N., James, P. F., Forbes, S. L., Woo, A. L., Lingrel, J. B., Witte, D. P., Matthay, M. A. & Liggett, S. B. (2001) Am. J. Physiol. Lung Cell Mol. Physiol. 281, L895-L903. [DOI] [PubMed] [Google Scholar]
- 42.McGraw, D. W., Almoosa, K. F., Paul, R. J., Kobilka, B. K. & Liggett, S. B. (2003) J. Clin. Invest. 112, 619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Filtz, T. M., Artymyshyn, R. P., Guan, W. & Molinoff, P. B. (1993) Mol. Pharmacol. 44, 371-379. [PubMed] [Google Scholar]
- 44.Collins, S., Bouvier, M., Bolanowski, M. A., Caron, M. G. & Lefkowitz, R. J. (1989) Proc. Natl. Acad. Sci. USA 86, 4853-4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomioka, S., Bates, J. H. & Irvin, C. G. (2002) J. Appl. Physiol. 93, 263-270. [DOI] [PubMed] [Google Scholar]
- 46.Henry, P. J., Rigby, P. J. & Goldie, R. G. (1990) Br. J. Pharmacol. 99, 136-144. [DOI] [PMC free article] [PubMed] [Google Scholar]