Background: Enterococcal bacteriocin BacL1 shows bactericidal activity in the co-presence of BacA.
Results: Recombinant BacL1 alone acts as a d-isoglutamyl-l-lysine endopeptidase against E. faecalis peptidoglycan independently of BacA.
Conclusion: BacL1 is a peptidoglycan hydrolase and potentially lyses viable bacterial cells in the presence of BacA.
Significance: This study of bacterial fratricide mediated by bacteriocin provides new insight into the ecological physiology of bacteria.
Keywords: Bacterial Pathogenesis, Cell Wall, Enzymes, Hydrolases, Peptidoglycan, Enterococcus faecalis, Bacteriocin
Abstract
Enterococcus faecalis strains are commensal bacteria in humans and other animals, and they are also the causative agent of opportunistic infectious diseases. Bacteriocin 41 (Bac41) is produced by certain E. faecalis clinical isolates, and it is active against other E. faecalis strains. Our genetic analyses demonstrated that the extracellular products of the bacL1 and bacA genes, which are encoded in the Bac41 operon, coordinately express the bacteriocin activity against E. faecalis. In this study, we investigated the molecular functions of the BacL1 and BacA proteins. Immunoblotting and N-terminal amino acid sequence analysis revealed that BacL1 and BacA are secreted without any processing. The coincidental treatment with the recombinant BacL1 and BacA showed complete bacteriocin activity against E. faecalis, but neither BacL1 nor BacA protein alone showed the bacteriocin activity. Interestingly, BacL1 alone demonstrated substantial degrading activity against the cell wall fraction of E. faecalis in the absence of BacA. Furthermore, MALDI-TOF MS analysis revealed that BacL1 has a peptidoglycan d-isoglutamyl-l-lysine endopeptidase activity via a NlpC/P60 homology domain. These results collectively suggest that BacL1 serves as a peptidoglycan hydrolase and, when BacA is present, results in the lysis of viable E. faecalis cells.
Introduction
Bacteriocins are antimicrobial proteins or peptides produced by a wide variety of bacteria. Bacteriocins usually show a narrow spectrum of antimicrobial activity that is specifically active against species that are identical or closely related to the strain that is producing the bacteriocin (1). Meanwhile, bacteriocin-producing bacteria also have specific immunity factors that protect the producer strain from being killed by the cognate bacteriocin. The production of bacteriocin is thought to provide a competitive advantage to the producer strain in an ecological niche that has closely related strains present.
Many clinical isolates of Enterococcus faecalis have been reported to produce various bacteriocins (2, 3). These enterococcal bacteriocins are often encoded on a pheromone-responsive conjugative plasmid (4–7). They have been classified into five groups based on the bactericidal spectrum identified in our previous study (6). Class 1 is active against a wide variety of Gram-positive bacteria (6, 8). The β-hemolysin/bacteriocin (cytolysin), which belongs to class 1-type bacteriocins, shows not only bactericidal activity but also hemolytic activity against mammalian cells, and it is associated with virulence in an animal model (9–11). Class 2 is active against a broad spectrum of bacteria, including E. faecalis, the other Streptococcus spp., and Staphylococcus aureus. The class 2 bacteriocins contain the peptide antibiotics AS-48 (12) and bacteriocin 21 (13). Class 3 shows activity against E. faecalis, Enterococcus hirae, and Listeria monocytogenes and includes bacteriocin 31 (6). Class 4 and class 5 show activity only against E. faecalis and E. hirae, respectively (4, 14–16).
Bacteriocin 41 (Bac41)2 is a class 4 type bacteriocin found in the pheromone-responsive plasmid pYI14 of the clinically isolated strain E. faecalis YI14 (4). Our subsequent epidemiologic study showed that more than 50% of E. faecalis clinical strains, but not laboratory strains, produce Bac41 (17). Our previous genetic analysis revealed several features of Bac41. Bac41 is specifically active only against E. faecalis and has no activity against Enterococcus faecium. The determinant of Bac41 is encoded in the EcoRI fragments A (12.6 kbp) and H (3.5 kbp) of pYI14 and consists of six genes, including bacL1, bacL2, bacA, and bacI. The bacteriocin activity of Bac41 is complementarily expressed by two extracellular components: the bacL1- and bacA-encoded proteins, BacL1 and BacA. ORF bacL2 is required for the expression of bacL1 and bacL2 itself (18). ORF bacI is an immunity factor protecting the Bac41-harboring strain from being killed by BacL1 and BacA (4).
BacL1 is a 595-amino acid protein (64.5 kDa) consisting of two domains located in the 3–140 and 163–315 amino acid regions of the amino acid sequence. The domains show homology to the bacteriophage-type peptidoglycan hydrolase and the NlpC/P60 family peptidoglycan hydrolase, respectively (19, 20). A C-terminal, three-repeat structure located in the 329–590 amino acid region of BacL1 shows homology with the bacterial Src homology 3 (SH3) domain that is reported to bind to the bacterial cell wall (21, 22). BacA is a 726-amino acid protein (79.1 kDa) showing a significant degree of homology to YbfG and YkuF of Bacillus subtilis (23). The functions of YbfG and YkuF are unknown, but a putative peptidoglycan-binding domain and a domain similar to the GH25 family peptidoglycan hydrolase are detected in the 81–140 and 208–491 amino acid regions of BacA, respectively (4, 24).
The N termini of BacL1 and BacA are predicted to have a signal peptide, presumed to be secreted in a sec-dependent manner (4). These investigations suggest that BacL1 and BacA are cell wall lytic enzymes able to induce the bacteriolytic killing of target cells. However, the precise molecular functions of BacL1 and BacA remain elusive. In this report, we demonstrate the biochemical analysis of BacL1 and BacA and reveal that BacL1 has the enzymatic activity of a peptidoglycan hydrolase.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Plasmids, oligonucleotides, Media, Antimicrobial Reagents, and Antibodies
The bacterial strains and plasmids used in this study are shown in Table 1. The oligonucleotides used in this study are listed in Table 2. A standard plasmid DNA methodology was used (25). E. faecalis and Escherichia coli strains were grown in Todd-Hewitt broth (THB; Difco) and Luria-Bertani medium (LB; Difco) at 37 °C, respectively (26), unless otherwise noted. E. coli strains were grown in Luria-Bertani medium at 37 °C. The antibiotic concentrations for the selection of Escherichia coli were as follows: ampicillin, 100 μg ml−1; chloramphenicol, 30 μg ml−1. The concentration of chloramphenicol for the selection of E. faecalis was 20 μg ml−1. All antibiotics were obtained from Sigma. Anti-BacL1 and -BacA sera were prepared by immunization of rabbits with recombinant BacL1-His and BacA-His proteins, respectively (Operon Technologies, Alameda, CA). Anti-FLAG antibodies were purchased from Invitrogen.
TABLE 1.
Bacterial strains and plasmids used in this study
| Description | Source/Reference | |
|---|---|---|
| Strains | ||
| E. faecalis OG1S | str; derivative of OG1 | Ref. 26 |
| E. coli DH5α | Host for DNA cloning | Bethesda Research Laboratories |
| E. coli BL21 Rosetta | Host for protein expression | Novagen |
| Plasmids | ||
| pAM401 | E. coli-E. faecalis shuttle plasmid; cat, tet | Ref. 38 |
| pHT1100 | pAM401 containing wild-type Bac41 | Ref. 4 |
| pHT1101 | pAM401 containing Bac41 without bacA | Ref. 4 |
| pMG1106 | pAM401 containing Bac41 without bacL1 | Ref. 4 |
| pAM401::bacL1/bacL2 | pAM401 containing bacL1 and bacL2 | Ref. 4 |
| pAM401::bacL1-his/bacL2 | pAM401 containing bacL1-his and bacL2 | This study |
| pAM401::flag-bacL1-his/bacL2 | pAM401 containing flag-bacL1-his and bacL2 | This study |
| pMGS100 | E. coli-E. faecalis shuttle expression plasmid; cat, tet | Ref. 39 |
| pMGS100::bacA | pMGS100 containing bacA | This study |
| pMGS100::bacA-his | pMGS100 containing bacA-his | This study |
| pMGS100::flag-bacA-his | pMGS100 containing flag-bacA-his | This study |
| pET22b(+) | Expression vector of His-tagged protein in E. coli | Novagen |
| pET::bacA | pET22b (+) containing bacA | This study |
| pET::bacL1 | pET22b (+) containing bacL1 | This study |
| pET::bacL1Δ1 | pET22b (+) containing bacL1 truncate Δ1 | This study |
| pET::bacL1Δ2 | pET22b (+) containing bacL1 truncate Δ2 | This study |
| pET::bacL1Δ1Δ2 | pET22b (+) containing bacL1 truncate Δ1Δ2 | This study |
| pET::bacL1Δ3 | pET22b (+) containing bacL1 truncate Δ3 | This study |
TABLE 2.
Oligonucleotides used in this study
The underlines indicate the following endonuclease recognition sequences: GAATTC, EcoRI, GCCGGC, EagI; TCGCGA, NruI; CATATG, NdeI; CTCGAG, XhoIV.
| Oligonucleotides | Sequence | Generated plasmid | Source/Reference |
|---|---|---|---|
| B9P2842F | CCGGAATTCTAGCAACCGAAAACCACGTTGG | pAM401::bacL1/bacL2 pAM401::bacL1-his/bacL2 pAM401::flag-bacL1-his/bacL2 | Ref. 4 |
| B9P5773R | GCGGAATTCATTGCGCAGCAAATCATTGC | pAM401::bacL1/bacL2 pAM401::bacL1-his/bacL2 pAM401::flag-bacL1-his/bacL2 | Ref. 4 |
| F-His-L1_stop | CACCACCATCACCATCATTAGTACAAATTATATTGCTT | pAM401::bacL1-his/bacL2 pAM401::flag-bacL1-his/bacL2 | This study |
| R-His-L1_stop | ATGATGGTGATGGTGGTGATTAAAGAATCCTTTGCCCC | pAM401::bacL1-his/bacL2 pAM401::flag-bacL1-his/bacL2 | This study |
| F-FLAG-L1_start | GATTATAAAGATGACGATGACAAAAATTACAGTCAAAAAGCAAT | pAM401::flag-bacL1-his/bacL2 | This study |
| R-FLAG-L1_start | TTTGTCATCGTCATCTTTATAATCCATAAACTTCACCTCATATT | pAM401::flag-bacL1-his/bacL2 | This study |
| F-EagI-bacA | TTTTTCGGCCGGCATGGATGAAATGGTTTTA | pMGS100::bacA, pMGS100::bacA-his | This study |
| R-NruI-bacA | ATTTTTTCGCGATTAAGCTAATGCAGCAAAAA | pMGS100::bacA | This study |
| F-FLAG-bacA | AAGATGACGATGACAAAGATGAAATGGTTTTAGGTA | pMGS100::flag-bacA-his | This study |
| F-EagI-FLAG | TTTTTCGGCCGGCATGGATTATAAAGATGACGATGACAAA | pMGS100::flag-bacA-his | This study |
| R-His-bacA | ATGATGGTGATGGTGGTGAGCTAATGCAGCAAAAAATG | pMGS100::bacA-his pMGS100::flag-bacA-his | This study |
| R-NruI-His | ATTTTTTCGCGATTAATGATGGTGATGGTGGTG | pMGS100::bacA-his and pMGS100::flag-bacA-his | This study |
| F-NdeI-bacA | CGCCATATGGATGAAATGGTTTTAGG | pET::bacA | This study |
| R-XhoI-bacA | CCGCTCGAGAGCTAATGCAGCAAAAAATG | pET::bacA | This study |
| F-NdeI-bacL1 | CGCCATATGATGAATTACAGTCAAAAAGC | pET::bacL1 | This study |
| R-XhoI-bacL1 | CCGCTCGAGATTAAAGAATCCTTTGCCCC | pET::bacL1 | This study |
| F-del-L1_Lys1 | AAGTTTATGAATACAGCCCTTTATCTTGAAGG | pET::bacL1Δ1, pET::bacL1Δ1Δ2 | This study |
| R-del-L1_Lys1 | ATAAAGGGCTGTATTCATAAACTTCACCTCAT | pET::bacL1Δ1, pET::bacL1Δ1Δ2 | This study |
| F-del-L1_Lys2 | CGTATTGGTTTTTATCCTGGAGATTCTTCTGG | pET::bacL1Δ2, pET::bacL1Δ1Δ2 | This study |
| R-del-L1_Lys2 | ATCTCCAGGATAAAAACCAATACGTGCGTGAT | pET::bacL1Δ2, pET::bacL1Δ1Δ2 | This study |
| F-del-L1-His_SH3 | GATTCAGTGAATAAAGGATTCTTTAATCACCA | pET::bacL1Δ3 | This study |
| R-del-L1_SH3 | AAAGAATCCTTTATTCACTGAATCTCCTTTTG | pET::bacL1Δ3 | This study |
Construction of Expression Plasmids
The amplification of the respective genes for the plasmid construction was performed by the PCR method using the corresponding primers as indicated in Table 2. The constructed plasmids were sequenced to confirm that the desired sequence had been inserted.
Preparation of Whole Cell and Culture Supernatant Proteins from E. faecalis
Overnight cultures of E. faecalis strains were inoculated into fresh THB broth and incubated at 37 °C for the indicated time. The bacterial pellet was resuspended with distilled water, and the culture supernatant was filtered (0.22 μm; Millipore (Billerica, MA)). Trichloroacetic acid was then added to each sample at a final concentration of 10%. After incubation on ice for 15 min, the supernatants were centrifuged at 10,000 rpm for 10 min. The precipitated protein samples were neutralized with 2 m Tris-base and dissolved in sample buffer. The resulting protein samples were separated with SDS-PAGE and then subjected to CBB staining or immunoblot analysis.
Isolation of Recombinant His6-tagged Proteins
An overnight culture of E. faecalis expressing the recombinant protein was inoculated into 500 ml of fresh THB and incubated at 37 °C for 18 h. The E. coli BL21 Rosetta strains expressing recombinant protein were inoculated into 500 ml of fresh LB and cultured at 37 °C with shaking until an optical density of 0.5–0.7 at 600 nm was obtained. Then isopropyl-β-d-thiogalactoside was added to a final concentration of 0.5 mm for induction, following an additional incubation at 30 °C with shaking for 3 h. The bacterial cells were collected by centrifugation and resuspended in 10 ml of lysis buffer (25 mm Tris-HCl, 150 mm NaCl, 10 mm imidazole, 10 mg ml−1 lysozyme, pH 8.0) with EDTA-free protease inhibitor mixture (Complete MINI EDTA-free, Roche Applied Science) to be enzymatically lysed at 37 °C for 30 min. The bacterial suspension was further lysed by sonication on ice using a sonicator (Ultrasonic Disruptor UD-201; TOMY, Tokyo, Japan) set at power level 6, at 40% duty, for 20 min and then clarified by centrifugation at 15,000 rpm for 10 min. The resulting soluble lysate was added to 1 ml of 50% Ni-NTA nickel chromatography resin (Ni-NTA purification system; Invitrogen) pre-equilibrated with lysis buffer, and the column was washed with 40 ml of wash buffer (25 mm Tris-HCl, 150 mm NaCl, 20 mm imidazole, pH 8.0). The His6-tagged protein was eluted with elution buffer (25 mm Tris-HCl, 150 mm NaCl, 200 mm imidazole, pH 8.0). The eluent containing the His6-tagged protein was subjected to ultracentrifugation using Amicon Ultra (Millipore, Billerica, MA) and resuspended in PBS. The protein concentration was determined by the Bradford method (Bio-Rad protein assay kit) using BSA (Sigma) as the standard.
N-terminal Amino Acid Sequence Analysis
An overnight culture of E. faecalis expressing the recombinant protein was inoculated to 500 ml of fresh THB and incubated at 37 °C for 18 h. The culture supernatant was prepared by centrifugation at 1,300 × g for 10 min at 4 °C and filtered (0.22 μm; Millipore). The supernatant sample was added to 1 ml of 50% Ni-NTA nickel chromatography resin (Ni-NTA purification system; Invitrogen), and the His6-tagged protein was eluted as described above. The His6-tagged protein prepared from the supernatant was separated by SDS-PAGE and transferred to a 0.2-μm pore nitrocellulose membrane (Immobilon-PSQ, Millipore) and stained with CBB. The target band was excised and subjected to Edman degradation amino acid sequence analysis to determine its N-terminal structure (GENOSTAFF, Tokyo, Japan).
Soft Agar Assay and Liquid Phase Bactericidal Assay
The soft agar assay for bacteriocin activity was performed as described previously (27). Briefly, 1 μl of the bacterial culture supernatant or recombinant protein sample was spotted onto THB soft agar (0.75%) containing the indicator strain and was then incubated at 37 °C for 24 h. For the liquid phase bactericidal assay, an overnight culture of indicator strain was diluted with fresh THB and adjusted to an optical density at 600 nm of 0.2, and then the recombinant proteins were added, and the sample was incubated at 37 °C. The change in optical density at 600 nm was monitored by a spectrometer (DU730, Beckman Coulter, Fullerton, CA). For the morphological analysis, the bacterial suspension that had been treated with recombinant protein was sampled and subjected to Gram-staining (Faber G, Nissui, Tokyo, Japan) and then analyzed by microscopy (Axiovert 200, Carl Zeiss, Oberkochen, Germany).
Zymograph Analysis
The zymogram gel was prepared by adding a sample of autoclaved indicator strain, E. faecalis OG1S, to a final optical density of 0.4 at 600 nm. The protein samples were separated by SDS-PAGE using the zymogram gel prepared above. After electrophoresis, the gel was washed with 20 mm Tris-HCl (pH 8.0), 1% Triton X-100 at room temperature with gentle shaking for 1 h and then incubated in 20 mm Tris-HCl (pH 8.0), 0.1% Triton X-100 at 37 °C for 36 h (28). The plaques that were observed on the gel were analyzed by densitometer (GS-800 calibrated densitometer, Bio-Rad).
Preparation of the Cell Wall Fraction
E. faecalis grown in THB at the exponential phase was collected by centrifugation. The bacterial pellet was rinsed with PBS and resuspended in 10 ml of 4% SDS and boiled at 95 °C for 30 min. The pellet was washed with distilled water four times and treated with 0.5 mg ml−1 trypsin (0.1 m Tris-HCl (pH 6.8), 20 mm CaCl2) at 37 °C for 16 h. The sample was further washed with distilled water four times and was resuspended in 10% TCA and incubated at 4 °C for 5 h and then given additional washes with distilled water four times (29). Finally, the cell wall fraction was resuspended with PBS and quantified from the optical density at 600 nm for the cell wall degradation assay. Mutanolysin (Sigma) was used as a positive control for the cell wall degradation enzyme.
Identification of Cleavage Site in Peptidoglycan by Mass Spectrometry
The identification of the enzymatic cleavage site was performed as described previously (30). Briefly, the cell wall fraction prepared as described above was treated with the indicated enzyme. This was followed by the addition of 20% phosphoric acid to adjust the pH to 4.0 and then incubation at 95 °C for 5 min to stop the reaction. The mixture was then added to 1.5 m sodium borate buffer (pH 9.0) and 10 mg solid sodium borohydride and incubated at room temperature for 15 min. The excess borohydride was quenched using 20% phosphoric acid until effervescence disappeared. The muropeptide solution was then adjusted to pH 2.5 using 4 n HCl prior to centrifugation at 10,000 × g for 15 min. The supernatant was passed through a membrane filter (0.22 μm) to remove the insoluble contaminants. Separation of the muropeptides was carried out by reverse-phase HPLC using a Hypersil ODS (5 Å, 250 × 4.6 mm) column. A linear gradient was prepared from 5% (v/v) methanol in 50 mm sodium phosphate buffer (pH 2.5) to 30% (v/v) methanol in 50 mm sodium phosphate buffer (pH 2.8) over 210 min. The muropeptides were detected from their UV absorbance at 206 nm. For MALDI-TOF MS analysis, the HPLC fractions containing the muropeptides of interest were desalted with a C-18 ZipTip (Millipore). The samples (1 μl) were co-spotted with an equal volume of the matrix, a saturated solution of α-cyano-4-hydroxy cinnamic acid in CH3CN/TFA (50:50). Mass spectrometric measurements were performed in a reflextron positive mode on a Biflex iV MALDI-TOF MS instrument (Bruker Daltonics, Billerica, MA). In MALDI postsource decay (PSD) experiments, the timed ion selector was used to select the [M + Na]+ value of the precursor ion. To determine the BacL1 cleavage site of peptidoglycan, each structure of the detected degradation products was compared with the peptidoglycan structure of E. faecalis (29–31).
Binding Assay
2 mg (wet weight) of cell wall fraction prepared as above and 10 μg of recombinant protein were mixed in PBS and incubated for 1 h at 4 °C. After incubation, a part of the mixture was kept for the following analysis. The pellet and the supernatant fractions were separated by centrifugation at 13,000 rpm for 1 min, and the pellet was further washed with PBS three times. Each fraction was subjected to SDS-PAGE and CBB staining. The resulting gel was analyzed by densitometer, and the signal intensities of the bands were quantified by ImageJ (National Institutes of Health, Bethesda, MD). The signal of each band intensity in the pellet or the supernatant fraction was normalized by comparison with that of the total fraction.
RESULTS
Secretion Profile of BacL1 and BacA
The culture supernatant of E. faecalis carrying pHT1100, which encodes the entire Bac41 operon, including the bacL1 and bacA genes, shows bactericidal activity against E. faecalis (Fig. 1A). In contrast, this bactericidal activity was no longer detected in the supernatant of E. faecalis carrying pHT1101 or pMG1106, which are deficient for either the bacA or bacL1 gene from pHT1100, respectively. The mixture of the culture supernatants of E. faecalis (pHT1101 and pMG1106) showed the same bactericidal activity as the culture supernatant of E. faecalis (pHT1100). These facts clearly indicate that BacL1 and BacA are secreted into the culture supernatant and are essential for the bactericidal activity of Bac41.
FIGURE 1.
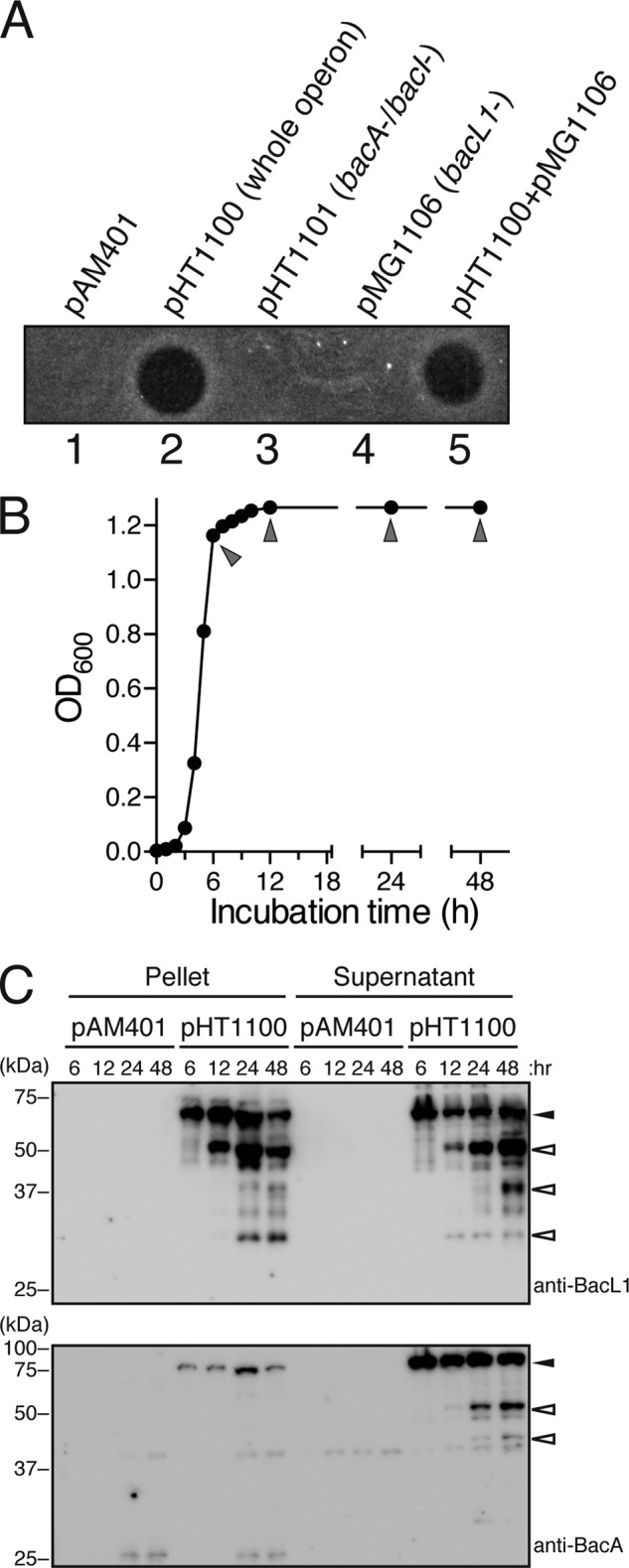
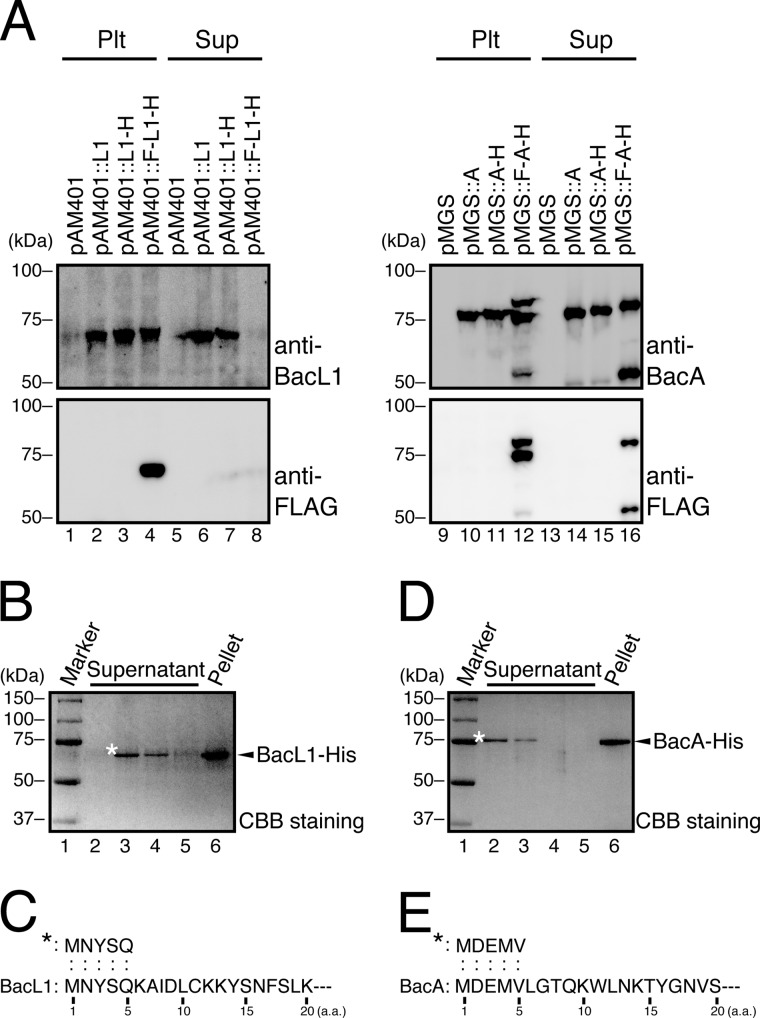
Bacteriocin activity and secreted protein profile in the culture supernatant of E. faecalis carrying Bac41 genes. A, culture supernatants of E. faecalis carrying pAM401 (lane 1), pHT1100 (lane 2), pHT1101 (lane 3), or pMG1106 (lane 4) were spotted onto a THB soft agar plate (0.75%) containing the indicator strain E. faecalis OG1S without any plasmid. A 1:1 mixture of the culture supernatants of cells harboring pHT1101- and pMG1106-carrying E. faecalis strains was spotted in lane 5. The plate was incubated at 37 °C for 24 h, and the formation of halos was evaluated. B, a seed bacterial culture of E. faecalis was inoculated into fresh THB broth and incubated at 37 °C. The turbidity at an optical density of 600 nm was monitored. Arrowheads, time points at which samples of the bacterial culture were collected to prepare protein samples. C, the bacterial pellets and the culture supernatant proteins were prepared from cultures of E. faecalis carrying pAM401 or pHT1100 at the indicated time point during the incubation period. The resulting protein samples were separated with SDS-PAGE and analyzed by immunoblotting using anti-BacL1 (top) and anti-BacA (bottom) serum. Filled arrowheads or open arrowheads indicate the band position of the predicted intact molecule or the degraded product of the respective proteins.
Many bacteriocins in E. faecalis have been reported to be secreted in a sec-dependent manner. The N-terminal signal sequence of the substrate is cleaved during its secretion process. Previously, we predicted the signal sequences for the sec-dependent secretion in the N-terminal amino acid sequence of BacL1 and BacA (4). However, it was not checked whether the N-terminal sequences of these proteins are actually processed during their secretion. Thus, we set out to identify the extracellular forms of BacL1 and BacA to determine whether any processing occurs during their secretion.
We prepared whole intracellular and extracellular proteins from the bacterial pellet and the supernatant fractions, respectively, of E. faecalis carrying pAM401 or pHT1100 at several points during the growth phase (Fig. 1B). No difference was recognized between the whole-protein profile of E. faecalis (pAM401) and E. faecalis (pHT1100) (data not shown). The expression and secretion profile of BacL1 and BacA was analyzed by immunoblotting using anti-BacL1 or anti-BacA sera (Fig. 1C). The signal of BacL1, in either the pellet or supernatant, gradually increased during the later phase of growth. In contrast, the signal of BacA in the pellet or the supernatant was detected even at a relatively early phase in growth, and the extracellular BacA in the supernatant appeared to be degraded during the later period. Degraded products of BacL1 and BacA were also detected.
Unexpectedly, we did not detect any shift in mobility with either BacL1 or BacA because of processing of their N termini, suggesting that BacL1 and BacA were not processed during their secretion. To confirm this conclusion, we tagged the N termini of BacL1 and BacA with the FLAG peptide and examined whether the N-terminal FLAG tag is still detectable after secretion (Fig. 2A). As shown in Fig. 2A, the signal of N-terminally tagged FLAG peptide was detectable. The addition of the FLAG tag at the N terminus of BacL1 drastically interfered with its secretion efficiency.
FIGURE 2.
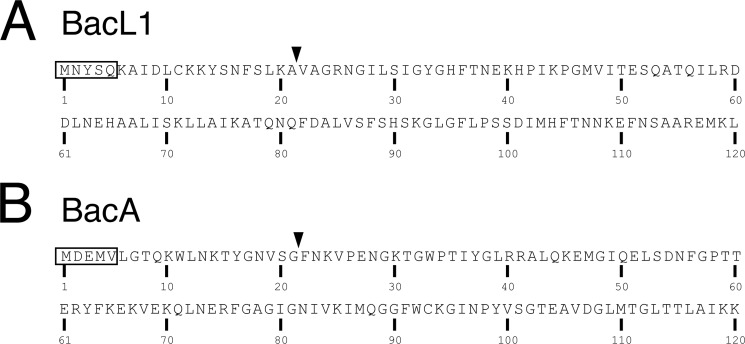
Identification of the secreted forms of BacL1 and BacA in the culture supernatant of E. faecalis. A, the bacterial cell pellets (Plt; lanes 1–4 and 9–12) and the culture supernatant proteins (Sup; lanes 5–8 and 13–16) prepared from the bacterial culture of E. faecalis carrying pAM401 (lanes 1 and 5), pAM401::bacL1/bacL2 (pAM401::L1; lanes 2 and 6), pAM401::bacL1-his/bacL2 (pAM401::L1-H; lanes 3 and 7), pAM401::flag-bacL1-his/bacL2 (pAM401::F-L1-H; lanes 4 and 8), pMGS100 (pMGS; lanes 9 and 13), pMGS100::bacA (pMGS::A; lanes 10 and 14), pMGS100::bacA-his (pMGS::A-H; lanes 11 and 15), or pMGS100::flag-bacA-his (pMGS::F-A-H; lanes 12 and 16) were subjected to immunoblotting analysis using anti-BacL1 serum, anti-BacA serum, or anti-FLAG antibody. B, secreted C-terminal hexahistidine-tagged BacL1 (BacL1-His; lanes 2–6) was purified from the filtered (0.22-μm pore size) culture supernatant of E. faecalis OG1S carrying pAM401::bacL1-his/bacL2, as described under “Experimental Procedures.” Extracellular BacL1-His bound to the immobilized nickel-NTA column was sequentially eluted with 200 mm (lanes 2–4) and 500 mm imidazole (lane 5). Intracellular BacL1-His was purified from the bacterial cell pellet (lane 6). Each sample was separated by SDS-PAGE and stained with CBB. A molecular marker was applied to lane 1. The asterisk indicates the band in lane 3 that was subjected to N-terminal sequence analysis. C, the N-terminal 5 amino acids of secreted BacL1-His were determined by Edman sequencing (upper sequence). The lower sequence shows the predicted amino acid sequence of BacL1, including the N-terminal 20 amino acids from the start methionine that was obtained from DNA sequence data (4). D, secreted C-terminal hexahistidine-tagged BacA (BacA-His; lanes 2–6) was purified from the filtered (0.22-μm) culture supernatant of E. faecalis OG1S carrying pMGS::bacA-his, as described under “Experimental Procedures.” Extracellular BacA-His on the immobilized nickel-NTA column was sequentially eluted with 200 mm (lanes 2–4) and 500 mm imidazole (lane 5). Intracellular BacA-His was purified from the bacterial cell pellet (lane 6). Each sample was separated by SDS-PAGE and stained with CBB. A molecular marker was applied in lane 1. The asterisk indicates the band in lane 2 that was subjected to the N-terminal sequence analysis shown in E. E, the N-terminal 5 amino acids of the secreted BacA-His were revealed by Edman sequencing and are shown in the upper sequence. The lower sequence shows the predicted amino acid sequence of BacA, including the N-terminal 20 amino acids from the start methionine that were obtained from the DNA sequence data (4).
The extracellular or intracellular C-terminal His6-tagged BacL1 (BacL1-His) was purified from the culture supernatant of E. faecalis (pAM401::bacL1-his) using an Ni-NTA-agarose column. The electrophoretic mobility of the purified extracellular BacL1-His was apparently the same as that of intracellular BacL1-His (Fig. 2B). We directly confirmed this finding by sequencing the N-terminal amino acid sequence of BacL1. The band corresponding to the extracellular BacL1-His was excised and subjected to N-terminal amino acid sequencing by the Edman method. The N-terminal amino acid sequence of extracellular BacL1-His was MNYSQ and identical to the 5-amino acid sequence from the start methionine of the BacL1 ORF (Fig. 2C). Similarly, the extracellular BacA-His was also purified using a Ni-NTA-agarose column to sequence its N-terminal amino acids. Its molecular weight was identical to that of intracellular BacA-His in SDS-PAGE (Fig. 2D). The N-terminal amino acids of secreted BacA (MDEMV) were also identical to the 5-amino acid sequence from the start methionine of the BacA ORF (Fig. 2E). Collectively, these results demonstrate that BacL1 and BacA are secreted without sec-dependent signal peptide cleavage or any N-terminal processing.
Bactericidal Activity of Recombinant BacL1 and BacA
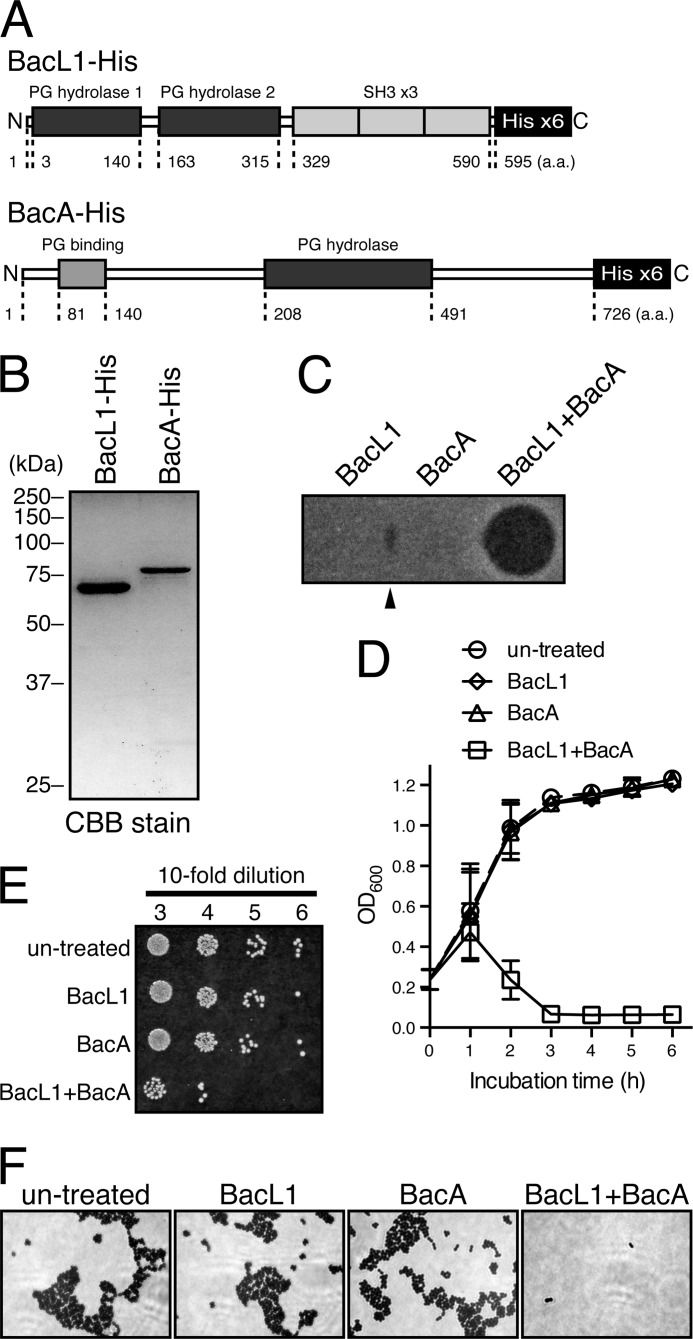
To investigate the molecular function of BacL1 and BacA in detail, we prepared the recombinant BacL1-His (65.3 kDa) and BacA-His (79.9 kDa) (Fig. 3B). In a soft agar assay, the bactericidal activity against the indicator strain E. faecalis was observed when using a mixture of BacL1-His and BacA-His proteins and also between the spotted area of BacL1-His and BacA-His (Fig. 3C). However, neither individual protein was sufficient for the bactericidal activity.
FIGURE 3.
Bacteriocin activity of recombinant BacL1 and BacA proteins. A, conserved domain structures of the recombinant BacL1 and BacA are shown. The hexahistidine tag was added to the C terminus of each wild-type protein for purification with the Ni-NTA system. B, recombinant BacL1-His and BacA-His proteins prepared from E. faecalis OG1S carrying pAM401::bacL1-his/bacL2 and E. coli BL21 Rosetta carrying pET22-bacA-his, respectively, were separated by SDS-PAGE and stained with CBB. C, recombinant BacL1-His (25 ng), BacA-His (25 ng), or a mixture of both proteins (25 ng each) was spotted on the THB soft agar (0.75%) containing the indicator strain E. faecalis OG1S. The plate was incubated at 37 °C for 24 h, and the formation of halos was evaluated. The arrowhead indicates the area in which the bactericidal activity of BacL1 and BacA complemented each other. D, a culture of E. faecalis was diluted with fresh THB broth to adjust the optical density at 600 nm to 0.2. Recombinant BacL1-His (5 μg/ml), BacA-His (5 μg/ml), or a mixture of both proteins (5 μg/ml each) was added into the bacterial suspension and incubated at 37 °C. The turbidity was monitored during the incubation period. The data for each case are presented as the means ± S.E. (error bars) of three independent experiments. E, E. faecalis was treated with BacL1, BacA, or both proteins as in Fig. 3C. After incubation for 6 h, the bacterial suspensions were serially diluted 10-fold with fresh THB broth and then spotted onto a THB agar plate. The plate was incubated at 37 °C for 24 h, and colony formation was evaluated as a measure of bacterial viability. The data for each case are presented as the means ± S.E. of three independent experiments. F, E. faecalis was treated with BacL1, BacA, or both proteins as in C. After incubation for 2 h, the bacterial suspensions were subjected to Gram staining and analyzed by microscopy.
To examine bacteriolysis in the aqueous phase, a suspension of E. faecalis in fresh THB broth had either BacL1-His, BacA-His, or the mixture of both proteins added, and the turbidity of the suspension was monitored (Fig. 3D). In the absence of any treatment, the turbidity of the suspension gradually increased due to bacterial growth. However, when BacL1-His and BacA-His were both added to the bacterial suspension, a remarkable reduction in turbidity was observed after 2 h of incubation, and after 3 h of incubation, the optical density at 600 nm was less than 0.1. Furthermore, the viability of cells co-treated with BacL1-His and BacA-His was greatly reduced compared with untreated cells (Fig. 3E), and the lysed cells did not stain well in Gram staining (Fig. 3F). In contrast, treatment with either BacL1-His or BacA-His alone did not affect bacterial growth (Fig. 3D), and no significant effects on the viability or morphology of the cells were observed when the bacterial suspension was treated with each individual protein (Fig. 3, E and F).
Peptidoglycan Hydrolase Activity of BacL1 and Its Cleavage Site
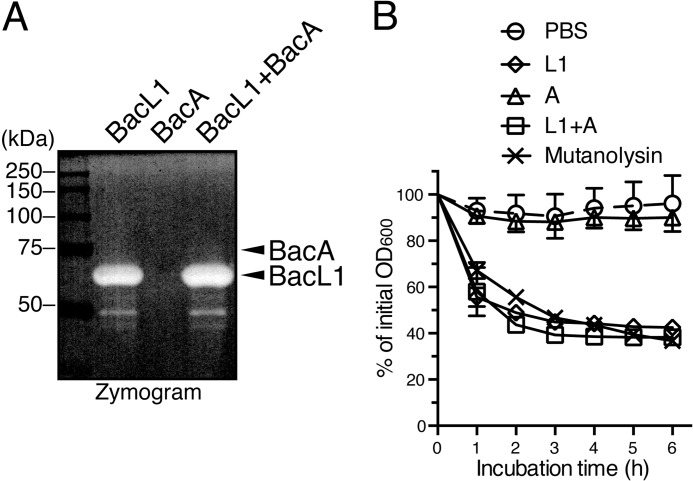
To further investigate the enzymatic activity of BacL1 and BacA, the constructs were subjected to zymography analysis using a gel containing autoclaved E. faecalis cells as a substrate (Fig. 4A). BacL1-His alone was sufficient for bacteriolytic activity in the presence or absence of BacA-His. Furthermore, the purified cell wall fraction was prepared from E. faecalis and treated with BacL1-His, BacA-His, a mixture of the two constructs, and mutanolysin (Fig. 4B). The cell wall treated with BacL1-His alone or with the mixture of BacL1-His and BacA-His was gradually degraded, as after mutanolysin digestion. In contrast, the activity of BacA-His was completely undetectable. These data clearly show that BacL1 alone was sufficient for the degradation of the cell wall component and that it acts as a peptidoglycan hydrolase, although it has no bactericidal activity against viable cells.
FIGURE 4.
Degrading activity of recombinant BacL1 against the cell wall fraction of E. faecalis. A, recombinant BacL1-His (400 ng; lane 1), BacA-His (400 ng; lane 2), and a mixture of both proteins (400 ng each; lane 3) were separated by SDS-PAGE with the zymogram gel containing autoclaved E. faecalis cells, following static incubation at 37 °C for 36 h. The resulting gel was analyzed using a densitometer. The top and bottom arrowheads indicate the band positions of BacA and BacL1, respectively. B, a cell wall fraction prepared from E. faecalis at exponential phase was diluted with fresh PBS. Recombinant BacL1-His (5 μg/ml), BacA-His (5 μg/ml), and a mixture of both proteins (5 μg/ml each) or mutanolysin (1 μg/ml) was added into the cell wall suspension and incubated at 37 °C. The turbidity at optical density of 600 nm was quantified at the indicated times of incubation. The values presented are the percentages of the initial turbidity of the respective samples. The data for each case are presented as the means ± S.E. (error bars) of three independent experiments.
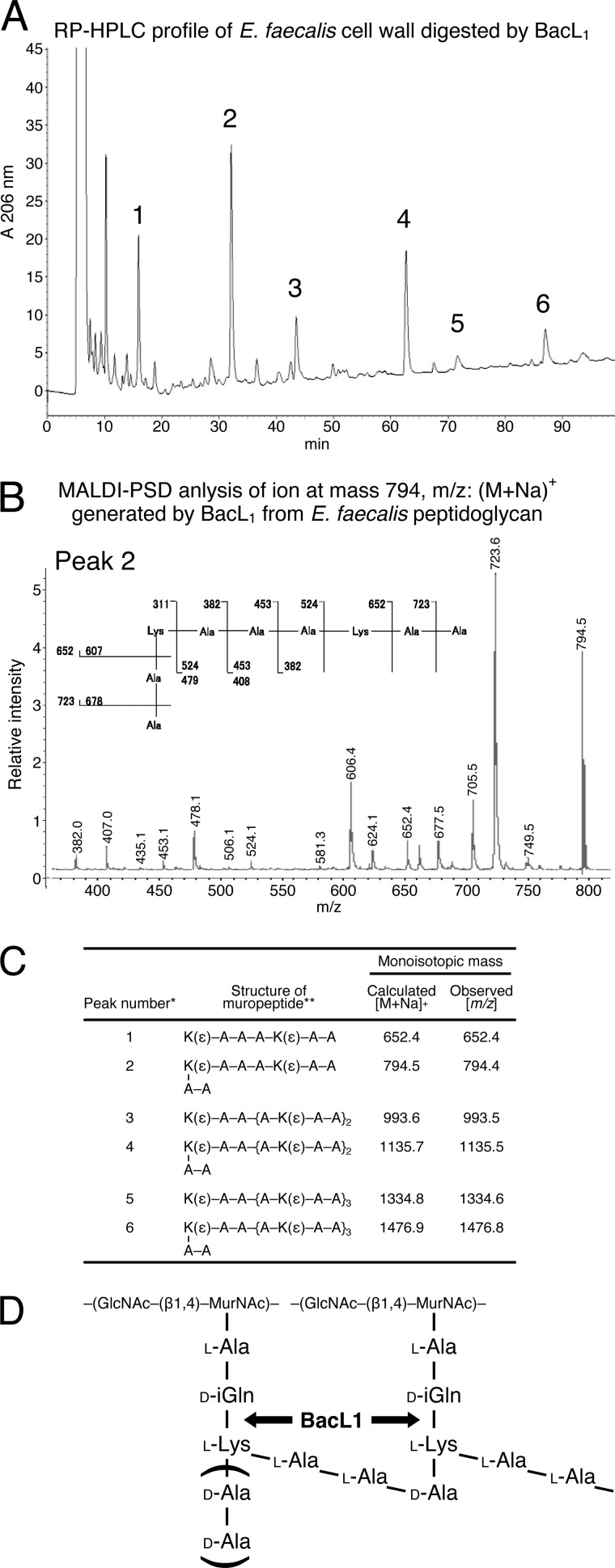
Thus, BacL1 appears to act as a peptidoglycan-degrading enzyme. To determine the BacL1 cleavage site, the soluble product generated from BacL1-treated peptidoglycan was separated by reverse-phase HPLC on an ODS-Hypersil column (Fig. 5A). The HPLC fractions were analyzed by MALDI-PSD analysis to determine their molecular structures as described under “Experimental Procedures” (Fig. 5, B and C). The data indicate that BacL1 has an endopeptidase activity that hydrolyzes the peptide bond between d-isoglutamine and l-lysine in the stem peptide (Fig. 5D).
FIGURE 5.
Cleavage site of E. faecalis peptidoglycan by BacL1. A, the E. faecalis cell wall fraction was incubated with wild-type BacL1-His. The solubilized peptidoglycan component was separated on a C18 reverse-phase HPLC column, as described under “Experimental Procedures.” The numbers above the peaks indicate the fractions isolated for the following MALDI-PSD MS analysis. B, the compound corresponding to the purified peak 2 of reverse-phase HPLC in A was subjected to MALDI-PSD MS. C, possible muropeptide structures and their calculated masses [M + Na]+ are shown as observed mass (m/z) in MADLI-PSD analysis (B). *, peak numbers refer to the HPLC chromatogram (A). **, K, lysine; A, alanine. D, predicted cleavage site of BacL1 in E. faecalis peptidoglycan (filled arrows).
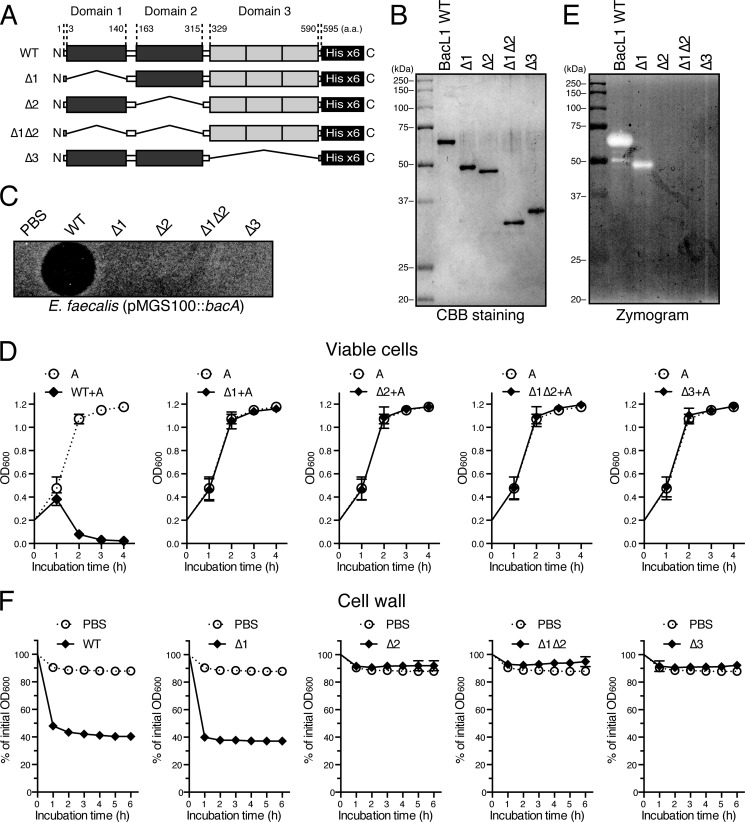
Domain Analysis of BacL1
To determine the molecular function of BacL1, we constructed truncated derivatives of the recombinant BacL1-His proteins. BacL1Δ1 (49.9 kDa), BacL1Δ2 (48.4 kDa), BacL1Δ1Δ2 (33.0 kDa), and BacL1Δ3 (37.7 kDa) had a deletion in domain 1 or domain 2, in both domain 1 and 2, or in domain 3, respectively (Fig. 6, A and B). Unlike the wild-type BacL1-His, each truncated protein no longer showed bactericidal activity against viable E. faecalis in a soft agar assay or a liquid bacteriolytic assay, even in the presence of BacA (Fig. 6, C and D). However, in the zymography and cell wall lysis assays, the protein with the truncated domain 1 (BacL1Δ1) still showed similar cell wall-degrading activity as the wild-type BacL1-His (Fig. 6, E and F). Furthermore, treatment of the cell wall fraction with the BacL1Δ1 protein generated the soluble fragments l-Lys-l-Ala2-(d-Ala-l-Lys-l-Ala2)n and l-Lys-(d-Ala2)-l-Ala2-(d-Ala-l-Lys-l-Ala2)n, which were identical to the results obtained with wild-type BacL1 (Fig. 5, B and C). In contrast, these peptides were not generated from the cell wall treated with BacL1Δ2, BacL1Δ1Δ2, or BacL1Δ3 (data not shown), indicating that the d-isoglutamyl-l-lysine endopeptidase activity is dependent on domains 2 and 3 of BacL1.
FIGURE 6.
Domain analysis of BacL1. A, diagrammatic representation of the truncated BacL1 constructs. The wild-type BacL1 (WT) consists of 595 amino acids, with three domain structures in the region of 3–140 (domain 1), 163–315 (domain 2), and 329–590 (domain 3) amino acids, respectively. BacL1Δ1, -Δ2, or -Δ3 is the truncated derivative from which domain 1, 2, or 3 from wild-type BacL1 has been deleted, respectively. BacL1Δ1Δ2 is the derivative from which both domain 1 and 2 have been deleted. The hexahistidine tag was added to the C terminus of each truncated protein for purification with the Ni-NTA system. B, the recombinant truncated BacL1 derivatives (400 ng) were separated by SDS-PAGE and stained with CBB. C, the recombinant truncated BacL1 derivatives (25 ng) were spotted onto the THB soft agar (0.75%) containing the indicator strain E. faecalis carrying pMGS100::bacA, which produces BacA. The plate was incubated at 37 °C for 24 h, and the formation of halos was evaluated. D, a culture of E. faecalis was diluted with fresh THB broth to adjust the optical density at 600 nm to 0.2. The mixture of each truncated BacL1 derivative and BacA-His (5 μg/ml each) was added into the bacterial suspension and incubated at 37 °C. The turbidity was monitored during the incubation period. The result of the BacA-His6-treated sample without any BacL1 derivatives is presented in each graph as the negative control. The data are presented as the means ± S.E. (error bars) of three independent experiments. E, the recombinant truncated BacL1 derivatives (400 ng) were separated by SDS-PAGE on the zymogram gel containing autoclaved E. faecalis cells, followed by static incubation at 37 °C for 36 h. The resulting gel was analyzed using a densitometer. F, a cell wall fraction prepared from E. faecalis at exponential phase was diluted with fresh PBS. The recombinant truncated BacL1 derivatives (5 μg/ml) were added into the cell wall suspension and incubated at 37 °C. The turbidity at 600 nm was quantified at the indicated times after incubation. The values presented are the percentages of the initial turbidity of the respective samples. The PBS-treated sample is presented in each graph as a negative control. The data are presented as the means ± S.E. of three independent experiments.
Cell Wall Targeting Mediated by Repeated SH3 Domain of BacL1
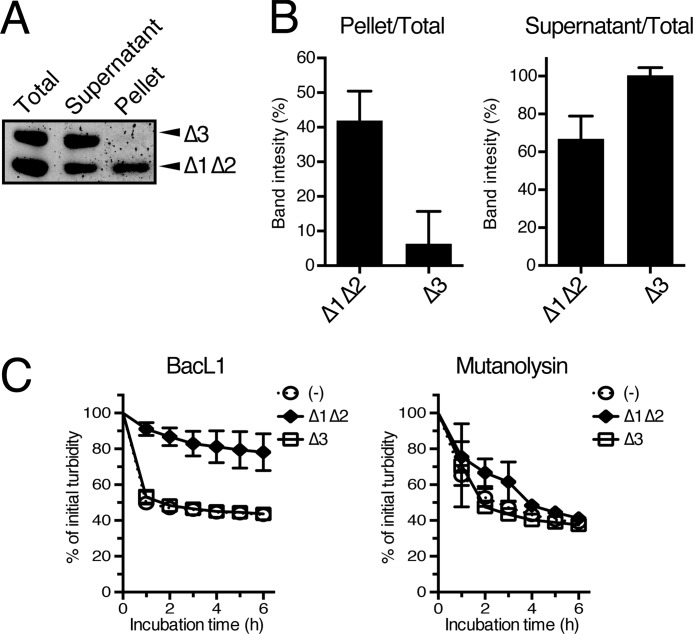
The data shown in Fig. 6 also indicate that the C-terminal repeated domain showing homology with the SH3 domain of ALE-1 of S. aureus is required for both bacteriolytic and cell wall-degrading activity. The repeated SH3 domain of ALE-1, a peptidoglycan hydrolase of S. aureus, has been reported to be a targeting domain that binds to peptidoglycan (22). The SH3 domain of BacL1 (domain 3) presumably also binds to peptidoglycan. We therefore assessed the binding efficiency of the truncated BacL1 proteins to the cell wall fraction of E. faecalis (Fig. 7, A and B).
FIGURE 7.
Binding activity of SH3 domain to the cell wall of E. faecalis. A, a cell wall fraction prepared from E. faecalis at exponential phase (2 mg) was mixed with the recombinant BacL1Δ1Δ2 and BacL1Δ3 (Total). After incubation at 4 °C for 1 h, the cell wall-bound (Pellet) and unbound (Supernatant) fractions were separated by centrifugation and then analyzed by SDS-PAGE followed by CBB staining. The arrowheads indicate the specific band positions of the respective proteins. B, quantified protein levels (percentages) of the pellet or supernatant fractions were determined by defining the relative band intensity of the total fraction as 100%. The data for each case are presented as the means ± S.E. (error bars) of three independent experiments. C, a cell wall fraction prepared from E. faecalis was preincubated with BacL1Δ1Δ2 or BacL1Δ3 at 4 °C for 1 h. After preincubation, wild-type BacL1 (left) or mutanolysin (right) was added, and the turbidity at an optical density of 600 nm was monitored. The values presented are the percentages of the initial turbidity of the respective samples. The data for each case are presented as the means ± S.E. of three independent experiments.
Domain 3 of BacL1 (BacL1Δ1Δ2) showed 40% binding efficiency. In contrast, the binding efficiency of BacL1 with a deleted domain 3 (BacL1Δ3) was less than 10%, showing that domain 3 of BacL1 binds to the substrate. Furthermore, preincubation with BacL1Δ1Δ2 interfered with the cell wall-degrading activity of BacL1, but not of mutanolysin. In contrast, the truncated protein BacL1Δ3 did not affect the degradation activity of BacL1. Collectively, these results suggest that domain 3 of BacL1 works as a cell wall-targeting domain of BacL1.
DISCUSSION
In this study, we investigated the biochemical features of two Bac41 components, BacL1 and BacA, using their recombinant proteins. First, we confirmed that the secreted BacL1 and BacA were the enzymatically active forms. Many enterococcal bacteriocins have an N-terminal sec signal sequence and are translated as preproteins to be secreted by the sec pathway (6, 13–16). The N-terminal sec signal peptide, which consists of N-terminal positively charged residues, a hydrophobic core, and a polar C-terminal cleavable site, is recognized by the sec-dependent secretion machinery (32–34). Upon the translocation via sec machinery, the sec signal peptide at the N terminus of preprotein is cleaved. The mature form of the protein is exported, exerting the bactericidal effect against target cells (34).
Our previous sequence analysis predicted the existence of a signal sequence in the N terminus of BacL1 and BacA (4) (Fig. 8). However, we observed no trace of the cleavage of an N-terminal peptide from BacL1 or BacA (Fig. 1C) and confirmed that the N termini of postsecretion BacL1 and BacA were completely intact (Fig. 2). Thus, the full-length recombinant proteins of BacL1 and BacA were sufficient for the bacteriocin activity in the culture supernatant of E. faecalis (pHT1100), which carries wild-type Bac41-related genes, including bacL1 and bacA (Fig. 3). Based on these observations, we concluded that BacL1 and BacA are secreted without N-terminal processing and that the proteins as they translated are already in an active form.
FIGURE 8.
N-terminal amino acid sequences of BacL1 and BacA. Amino acid sequences of 1–120 residues in BacL1 (A; accession number BAG12399) and BacA (B; accession number BAG12403) are presented (4). Residues in the frame are the resulting sequences from the N-terminal amino acids sequence analysis as in Fig. 2. Arrows indicate the processing site predicted in the previous study (4).
However, we also noted that the N-terminal regions of BacL1 and BacA appear to be involved in the efficiency of their secretion or the cytosolic state, respectively (Fig. 2). The secretion efficiency of BacL1 was strikingly reduced by tagging with the N-terminal FLAG peptide but not by tagging with the C-terminal hexahistidine. In contrast, an N-terminal FLAG tag did not affect the intracellular amount of BacL1 (Fig. 2A), suggesting that the intact N terminus is required for the efficient secretion of BacL1. The N-terminal peptide fusion to BacA also resulted in an unexpected artificial effect (Fig. 2A). The band expected for the intact secreted FLAG-BacA-His in the culture supernatant was observed at the predicted position and behaved similarly to BacA-His or non-tagged BacA (Fig. 2A). However, the cytosolic FLAG-BacA-His was shifted to larger and smaller sizes than predicted from the molecular mass (Fig. 2A). Although we cannot explain this phenomenon, the artificial N-terminal tagging probably affected the cytosolic state of BacA. Hence, the N-terminal regions of BacL1 and BacA appear to play a role in their secretion and in their cytosolic form.
As described above, it does not seem that BacL1 and BacA are secreted in a typical sec-dependent manner. Indeed, the SignalP 4.1 program server does not predict an obvious sec-dependent signal in the BacL1 or BacA sequence. Despite that, the N-terminal amino acid sequence of BacL1 or BacA contains multiple lysine residues and a potential hydrophobic core. Taken together with the result shown in Fig. 2, BacL1 and BacA might be secreted via a sec-dependent pathway but not cleaved by signal peptidase during translocation.
Another possible secretion mechanism for BacL1 and BacA is leakage due to cell disruption. The Streptococcus pneumoniae cytolysin, pneumolysin, is a secreted protein that shows cytotoxicity to mammalian cells. Nevertheless, pneumolysin lacks a signal peptide sequence at its N terminus. The extracellular secretion of pneumolysin is thought to depend on the disruption of cells upon the characteristic autolysis that is induced during stationary or death phase (35). Further investigation is needed to elucidate this issue.
BacL1 contains two distinct peptidoglycan hydrolase domains, referred to as domains 1 and 2 in this paper. Domain analysis showed that the BacL1Δ1 protein, but not BacL1Δ2, was able to degrade peptidoglycan (Fig. 6, E and F) and showed d-isoglutamyl-l-lysine endopeptidase activity (Fig. 5) similar to wild-type BacL1. The data suggested that domain 2 of BacL1, rather than domain 1, is responsible for the d-isoglutamyl-l-lysine endopeptidase activity. When the structural model of BacL1 was analyzed using the Phyre program, domain 2 was predicted to be similar to the NlpC/P60 family peptidoglycan hydrolases. The NlpC/P60 family peptidoglycan hydrolases have been found in various Gram-positive or -negative bacteria (36).
Most Nlp/P60 family proteins are reported to be endopeptidases that cleave the peptide bond between d-isoglutamine and mesodiaminopimelic acid or l-lysine in the stem peptide of peptidoglycan. Indeed, mass spectrometry analysis revealed that BacL1 is an endopeptidase that digests the linkage between d-isoglutamine and l-lysine in the stem peptide of E. faecalis peptidoglycan (Fig. 5). Thus, the endopeptidase activity of domain 2 is likely to play a critical role in the lytic activity against E. faecalis cells.
By contrast, because the truncated BacL1Δ2 with an intact domain 1 did not degrade the E. faecalis cell wall (Fig. 6, E and F), the activity of domain 1 was not detected, despite its significant homology with the bacteriophage-type lysozyme with peptidoglycan hydrolase activity (4, 37). However, domain 1 was still required for bactericidal action against viable cells (Fig. 6, C and D) but not for degradation of the purified cell wall fraction (Fig. 6, C and F). Probably, domain 1 does not have cell wall-degrading activity itself but has an accessory function for the efficient endopeptidase activity of domain 2. Another possibility is that the deletion of domain 2 resulted in a conformational change that interferes with the function of domain 1. Further study is required to understand the role of domain 1 of BacL1 in bactericidal activity against viable cells.
The data in Fig. 3 clearly demonstrate that BacL1 and BacA are both required for bacteriolysis against E. faecalis. Nevertheless, BacL1 still showed cell wall degradation activity, even in the absence of BacA (Fig. 4), indicating that the peptidoglycan hydrolase activity of BacL1 does not immediately result in the bacteriolysis of viable E. faecalis. In addition, BacA has a putative peptidoglycan binding domain (4) and a GH25 family peptidoglycan hydrolase-like domain (24), suggesting that BacA also binds to the target cell wall rather than just being an activator of BacL1. Nevertheless, the binding activity of full-length recombinant BacA-His to peptidoglycan was poorly detected (data not shown). Thus, we need to address the role of BacA in a future study. The molecular mechanism of BacL1-induced bacteriolysis is clearly complex and is associated with additional factors, the state of the target cells, and the unknown function of BacA.
Acknowledgment
We thank E. Kamei for helpful advice about the manuscript.
This work was supported by grants from the Japanese Ministry of Education, Culture, Sport, Science, and Technology (Grant-in-Aid for Young Scientists (B) 25870116, Gunma University Operation Grants) and the Japanese Ministry of Health, Labor, and Welfare (Grants H24-Shinkou-Ippan-010 and H24-Shokuhin-Ippan-008).
- Bac41
- bacteriocin 41
- SH3
- Src homology 3
- THB
- Todd-Hewitt broth
- CBB
- Coomassie Brilliant Blue
- Ni-NTA
- nickel-nitrilotriacetic acid
- PSD
- postsource decay.
REFERENCES
- 1. Jack R. W., Tagg J. R., Ray B. (1995) Bacteriocins of gram-positive bacteria. Microbiol. Rev. 59, 171–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clewell D. B. (1981) Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol. Rev. 45, 409–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ike Y., Hashimoto H., Clewell D. B. (1987) High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J. Clin. Microbiol. 25, 1524–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tomita H., Kamei E., Ike Y. (2008) Cloning and genetic analyses of the bacteriocin 41 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI14. A novel bacteriocin complemented by two extracellular components (lysin and activator). J. Bacteriol. 190, 2075–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ike Y., Clewell D. B. (1992) Evidence that the hemolysin/bacteriocin phenotype of Enterococcus faecalis subsp. zymogenes can be determined by plasmids in different incompatibility groups as well as by the chromosome. J. Bacteriol. 174, 8172–8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tomita H., Fujimoto S., Tanimoto K., Ike Y. (1996) Cloning and genetic organization of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI17. J. Bacteriol. 178, 3585–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fujimoto S., Tomita H., Wakamatsu E., Tanimoto K., Ike Y. (1995) Physical mapping of the conjugative bacteriocin plasmid pPD1 of Enterococcus faecalis and identification of the determinant related to the pheromone response. J. Bacteriol. 177, 5574–5581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franz C. M., van Belkum M. J., Holzapfel W. H., Abriouel H., Gálvez A. (2007) Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol. Rev. 31, 293–310 [DOI] [PubMed] [Google Scholar]
- 9. Ike Y., Hashimoto H., Clewell D. B. (1984) Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect. Immun. 45, 528–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chow J. W., Thal L. A., Perri M. B., Vazquez J. A., Donabedian S. M., Clewell D. B., Zervos M. J. (1993) Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob. Agents Chemother. 37, 2474–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jett B. D., Jensen H. G., Nordquist R. E., Gilmore M. S. (1992) Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect. Immun. 60, 2445–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martínez-Bueno M., Maqueda M., Gálvez A., Samyn B., Van Beeumen J., Coyette J., Valdivia E. (1994) Determination of the gene sequence and the molecular structure of the enterococcal peptide antibiotic AS-48. J. Bacteriol. 176, 6334–6339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tomita H., Fujimoto S., Tanimoto K., Ike Y. (1997) Cloning and genetic and sequence analyses of the bacteriocin 21 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pPD1. J. Bacteriol. 179, 7843–7855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inoue T., Tomita H., Ike Y. (2006) Bac 32, a novel bacteriocin widely disseminated among clinical isolates of Enterococcus faecium. Antimicrob. Agents Chemother. 50, 1202–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Todokoro D., Tomita H., Inoue T., Ike Y. (2006) Genetic analysis of bacteriocin 43 of vancomycin-resistant Enterococcus faecium. Appl. Environ. Microbiol. 72, 6955–6964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamashita H., Tomita H., Inoue T., Ike Y. (2011) Genetic organization and mode of action of a novel bacteriocin, bacteriocin 51. Determinant of VanA-type vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 55, 4352–4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng B., Tomita H., Inoue T., Ike Y. (2009) Isolation of VanB-type Enterococcus faecalis strains from nosocomial infections. First report of the isolation and identification of the pheromone-responsive plasmids pMG2200, encoding VanB-type vancomycin resistance and a Bac41-type bacteriocin, and pMG2201, encoding erythromycin resistance and cytolysin (Hly/Bac). Antimicrob. Agents Chemother. 53, 735–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tomita H., Todokoro D., Ike Y. (2010) Genetic analysis of the bacteriocin 41 encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI14. 3rd International ASM Conference on Enterococci, Abstr. 30B, pp. 37–38, American Society for Microbiology, Washington, D. C [Google Scholar]
- 19. Pecenková T., Benes V., Paces J., Vlcek C., Paces V. (1997) Bacteriophage B103. Complete DNA sequence of its genome and relationship to other Bacillus phages. Gene 199, 157–163 [DOI] [PubMed] [Google Scholar]
- 20. Sheehan M. M., García J. L., López R., García P. (1997) The lytic enzyme of the pneumococcal phage Dp-1. A chimeric lysin of intergeneric origin. Mol. Microbiol. 25, 717–725 [DOI] [PubMed] [Google Scholar]
- 21. Tettelin H., Masignani V., Cieslewicz M. J., Eisen J. A., Peterson S., Wessels M. R., Paulsen I. T., Nelson K. E., Margarit I., Read T. D., Madoff L. C., Wolf A. M., Beanan M. J., Brinkac L. M., Daugherty S. C., DeBoy R. T., Durkin A. S., Kolonay J. F., Madupu R., Lewis M. R., Radune D., Fedorova N. B., Scanlan D., Khouri H., Mulligan S., Carty H. A., Cline R. T., Van Aken S. E., Gill J., Scarselli M., Mora M., Iacobini E. T., Brettoni C., Galli G., Mariani M., Vegni F., Maione D., Rinaudo D., Rappuoli R., Telford J. L., Kasper D. L., Grandi G., Fraser C. M. (2002) Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc. Natl. Acad. Sci. U.S.A. 99, 12391–12396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu J. Z., Fujiwara T., Komatsuzawa H., Sugai M., Sakon J. (2006) Cell wall-targeting domain of glycylglycine endopeptidase distinguishes among peptidoglycan cross-bridges. J. Biol. Chem. 281, 549–558 [DOI] [PubMed] [Google Scholar]
- 23. Kunst F., Ogasawara N., Moszer I., Albertini A. M., Alloni G., Azevedo V., Bertero M. G., Bessières P., Bolotin A., Borchert S., Borriss R., Boursier L., Brans A., Braun M., Brignell S. C., Bron S., Brouillet S., Bruschi C. V., Caldwell B., Capuano V., Carter N. M., Choi S. K., Codani J. J., Connerton I. F., Danchin A. (1997) The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390, 249–256 [DOI] [PubMed] [Google Scholar]
- 24. Martinez-Fleites C., Korczynska J. E., Davies G. J., Cope M. J., Turkenburg J. P., Taylor E. J. (2009) The crystal structure of a family GH25 lysozyme from Bacillus anthracis implies a neighboring-group catalytic mechanism with retention of anomeric configuration. Carbohydr. Res. 344, 1753–1757 [DOI] [PubMed] [Google Scholar]
- 25. Sambrook J. J., Russell D. D. W. (2001) Molecular Cloning: A Laboratory Manual, 3rd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 26. Dunny G. M., Craig R. A., Carron R. L., Clewell D. B. (1979) Plasmid transfer in Streptococcus faecalis. Production of multiple sex pheromones by recipients. Plasmid 2, 454–465 [DOI] [PubMed] [Google Scholar]
- 27. Ike Y., Clewell D. B., Segarra R. A., Gilmore M. S. (1990) Genetic analysis of the pAD1 hemolysin/bacteriocin determinant in Enterococcus faecalis. Tn917 insertional mutagenesis and cloning. J. Bacteriol. 172, 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sugai M., Komatsuzawa H., Miyake Y., Suginaka H. (1991) Visualization of endo-β-N-acetylglucosaminidase, lysozyme, and lysostaphin after polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulphate. Zentralbl Bakteriol. 275, 156–161 [DOI] [PubMed] [Google Scholar]
- 29. Eckert C., Lecerf M., Dubost L., Arthur M., Mesnage S. (2006) Functional analysis of AtlA, the major N-acetylglucosaminidase of Enterococcus faecalis. J. Bacteriol. 188, 8513–8519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kajimura J., Fujiwara T., Yamada S., Suzawa Y., Nishida T., Oyamada Y., Hayashi I., Yamagishi J., Komatsuzawa H., Sugai M. (2005) Identification and molecular characterization of an N-acetylmuramyl-l-alanine amidase Sle1 involved in cell separation of Staphylococcus aureus. Mol. Microbiol. 58, 1087–1101 [DOI] [PubMed] [Google Scholar]
- 31. Uchiyama J., Takemura I., Hayashi I., Matsuzaki S., Satoh M., Ujihara T., Murakami M., Imajoh M., Sugai M., Daibata M. (2011) Characterization of lytic enzyme open reading frame 9 (ORF9) derived from Enterococcus faecalis bacteriophage phiEF24C. Appl. Environ. Microbiol. 77, 580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schneewind O., Missiakas D. M. (2012) Protein secretion and surface display in Gram-positive bacteria. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 1123–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thanassi D. G., Bliska J. B., Christie P. J. (2012) Surface organelles assembled by secretion systems of Gram-negative bacteria. Diversity in structure and function. FEMS Microbiol. Rev. 36, 1046–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feltcher M. E., Braunstein M. (2012) Emerging themes in SecA2-mediated protein export. Nat. Rev. Microbiol. 10, 779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martner A., Dahlgren C., Paton J. C., Wold A. E. (2008) Pneumolysin released during Streptococcus pneumoniae autolysis is a potent activator of intracellular oxygen radical production in neutrophils. Infect. Immun. 76, 4079–4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arai R., Fukui S., Kobayashi N., Sekiguchi J. (2012) Solution structure of IseA, an inhibitor protein of dl-endopeptidases from Bacillus subtilis, reveals a novel fold with a characteristic inhibitory loop. J. Biol. Chem. 287, 44736–44748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pritchard D. G., Dong S., Kirk M. C., Cartee R. T., Baker J. R. (2007) LambdaSa1 and LambdaSa2 prophage lysins of Streptococcus agalactiae. Appl. Environ Microbiol. 73, 7150–7154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wirth R., An F. Y., Clewell D. B. (1986) Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J. Bacteriol. 165, 831–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fujimoto S., Ike Y. (2001) pAM401-based shuttle vectors that enable overexpression of promoterless genes and one-step purification of tag fusion proteins directly from Enterococcus faecalis. Appl. Environ. Microbiol. 67, 1262–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]