Background: The fungal roquefortine/meleagrin gene cluster is a source of diverse bioactive molecules.
Results: Novel metabolites of the roquefortine/meleagrin biosynthetic pathway were discovered, and synthetase genes were assigned to biosynthetic reactions.
Conclusion: Distinctive unspecificity of modifying enzymes leads to excessive branching in the pathway, resulting in various intermediates and products.
Significance: Metabolites from the roquefortine/meleagrin gene cluster have potential antimicrobial and chemotherapeutic application.
Keywords: Antibiotics, Anticancer Drug, Fungi, Natural Product Biosynthesis, Secondary Metabolism, Toxins
Abstract
Metabolic profiling and structural elucidation of novel secondary metabolites obtained from derived deletion strains of the filamentous fungus Penicillium chrysogenum were used to reassign various previously ascribed synthetase genes of the roquefortine/meleagrin pathway to their corresponding products. Next to the structural characterization of roquefortine F and neoxaline, which are for the first time reported for P. chrysogenum, we identified the novel metabolite roquefortine L, including its degradation products, harboring remarkable chemical structures. Their biosynthesis is discussed, questioning the exclusive role of glandicoline A as key intermediate in the pathway. The results reveal that further enzymes of this pathway are rather unspecific and catalyze more than one reaction, leading to excessive branching in the pathway with meleagrin and neoxaline as end products of two branches.
Introduction
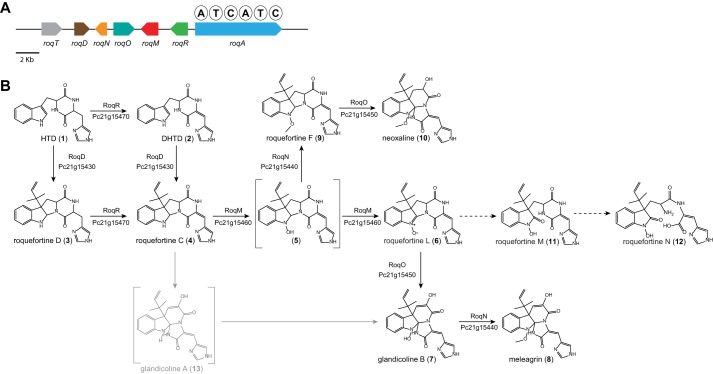
The filamentous fungus Penicillium chrysogenum has been commercially exploited for many decades due to its high production of β-lactam antibiotics such as penicillin G (1). Next to penicillins, secondary metabolites such as roquefortines and glandicolines were isolated from liquid cultures of P. chrysogenum, which show pharmaceutically interesting properties, such as neurotoxic (2), antimicrobial (3, 4), and antitumor (5) activities. They are structurally closely related and arise from the roquefortine/meleagrin pathway, which contains a dimodular nonribosomal peptide synthetase flanked by six associated genes (6, 7). Starting with histidyltryptophanyldiketopiperazine (HTD),5 synthesized by the core synthetase enzyme RoqA using tryptophan and histidine as substrates, RoqD catalyzes the reversed prenylation of HTD at the C-3 of its indole moiety, utilizing dimethylallyl diphosphate to form roquefortine D. At the same time, RoqR, a cytochrome P450 oxidoreductase, oxidizes HTD at its histidinyl moiety to dehydrohistidyltryptophanyldiketo piperazine (DHTD). Both simultaneous reactions of HTD lead to a branch of the roquefortine/meleagrin pathway, one to DHTD via the oxidation by RoqR and further to roquefortine C by dimethylallyl addition of RoqD, and the other via an alteration of the enzymatic order. There, dimethylallyl addition is first performed by RoqD to yield roquefortine D, whereas further oxidation is carried out by RoqR, yielding roquefortine C (see Fig. 1). Although several labeling, silencing, and deletion experiments have been conducted, there is still ambiguity about the subsequent biosynthetic reactions and the genes involved. For instance, roquefortine C is supposed to be converted into glandicoline A and further to glandicoline B with RoqM and RoqO each catalyzing one reaction (6, 7). However, their assignment to a particular reaction is still unclear. In addition, neoxaline was proposed as final product of the pathway, originating from a hydrogenation of meleagrin (8), yet no gene could be found in the roq gene cluster performing that reaction.
FIGURE 1.
Roquefortine/meleagrin biosynthetic gene cluster and proposed corresponding pathway. A, organization of the roquefortine/meleagrin biosynthetic gene cluster. B, proposed roquefortine/meleagrin pathway. Numbers between brackets are compound identifiers used throughout this study. Enzymatic catalyzed reactions are indicated by solid arrows, whereas chemical reactions are indicated by dashed arrows. Structures shown in brackets could not be detected, whereas gray reactions and compounds were previously proposed for various Penicillium species (6, 8, 10).
Here, we describe the quantification, structural identification, and biosynthesis of five previously unidentified metabolites, obtained from highly sensitive comparative metabolite profiling of host and deletion strains. Roquefortine F and neoxaline, next to the three structurally novel compounds, which we named roquefortine L, M, and N, were found to be derived from the roquefortine/meleagrin pathway. These results demonstrate a further branching of this secondary metabolite pathway yielding a variety of intermediates with complex structures and a diverse range of activities.
EXPERIMENTAL PROCEDURES
Host Strains, Media, Grown Conditions, and Plasmid Construction
P. chrysogenum strain DS54555, which lacks both the penicillin cluster genes and the ku70 gene, was used as a host strain for deletion analysis and was kindly supplied by DSM Anti-infective (Delft, The Netherlands). All the strains were grown on YGG medium for protoplast formation and transformation. For analysis, cells were grown on secondary metabolite production medium (7) (glucose, 5.0 g/liter; lactose, 75 g/liter; urea, 4.0 g/liter; Na2SO4, 4.0 g/liter; CH3COONH4, 5.0 g/liter; K2HPO4, 2.12 g/liter; KH2PO4, 5.1 g/liter) for secondary metabolite production using a shaking incubator at 200 rpm for 168 h at 25 °C.
Metabolite Profiling
All strains used for gene assignments were grown in quintuplicate to increase statistical power, according to the procedure described above. Sample preparation was carried out as described previously (7). Metabolomic profiling was performed on an Agilent 1200 capillary pump (Agilent, Santa Clara, CA) coupled to a Surveyor photodiode array detector (Thermo Scientific) and an LTQ-FT-ICR-Ultra mass spectrometer (Thermo Scientific) equipped with an electrospray interface as described earlier (7).
Metabolite Identification
The identity of compound 10 was confirmed by comparing retention time and MS fragmentation spectra to its commercially available standard, purchased from Bio-Connect (Huissen, The Netherlands). Compounds 6, 9, 11, and 12 were identified using NMR after extraction from liquid cultures. 6 was extracted from the roqN deletion strain culture filtrate, which was made alkaline with 25% ammonium hydroxide (pH 10) and extracted with dichloromethane. The alkaline dichloromethane layer was evaporated to dryness, redissolved in water containing 50% acetonitrile, vortexed, centrifuged, and transferred to an autosampler vial for fraction collection via preparative reversed phase LC on an Atlantis T3 column (10 × 100 mm, 5 μm) (Waters, Milford, MA). Compound 9 was extracted following the isolation procedure above except using culture filtrate of the roqO deletion strain, whereas 11 and 12 were obtained from the same culture filtrate after lyophilization and extraction using methanol. The methanol layer was evaporated to dryness, redissolved in water, vortexed, centrifuged, and subjected to repeated semipreparative chromatography as described above. Elemental composition of compounds 6, 9, 11, and 12 was determined using high-resolution MS. NMR spectra were recorded on a Bruker Avance III 700 MHz or 600 MHz spectrometer with sample temperatures ranging from 260 to 300 K, depending on the particular requirements for each sample. By choosing an optimal acquisition temperature, severe line broadening could be avoided, which was observed for various signals due to conformational averaging. For acquisition, samples were dissolved in equal amounts of dimethyl sulfoxide (DMSO) and CDCl3.
Chemical Stability of Compound 6
An aqueous solution of compound 6 was adjusted to pH 2.5 by the addition of formic acid. Metabolite profiling was carried out as described above. Products, formed by a degradation of 6, were compared with extracted standards using HPLC-MS/MS.
RESULTS
Metabolite Profiling of Host and Deletion Strains Leads to Five New Metabolites of the Roquefortine/Meleagrin Pathway
In a previous study, we described the identification of various abundant metabolites and resolved the major enzymatic steps belonging to the roquefortine/meleagrin pathway (7). To identify secondary metabolites originating from the roq gene cluster (Fig. 1A), culture supernatants of the host strain and individually roq gene deletion strains were subjected to comparative metabolite profiling using HPLC-UV-MS (Fig. 2). As host strain, P. chrysogenum DS54555, which is derived from the industrial DS17690 strain lacking the ku70 gene and multiple penicillin biosynthetic genes clusters, was used. Here, we describe the identification and quantification of several less abundant metabolites, roquefortine L (6), roquefortine F (9), neoxaline (10), roquefortine M (11), and roquefortine N (12) (Fig. 1B), that have not been previously considered or structurally characterized, filling missing biosynthetic reaction steps in the roquefortine/meleagrin pathway.
FIGURE 2.
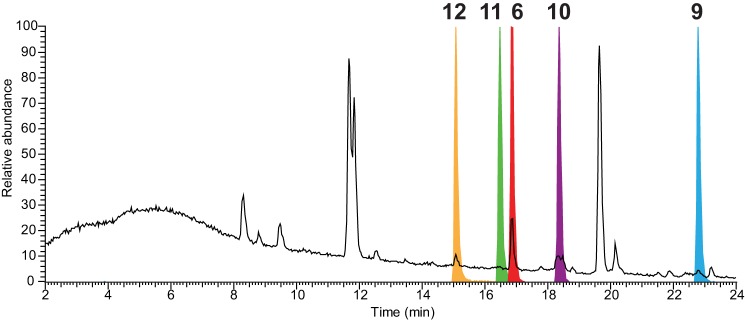
HPLC-MS elution profiles of novel metabolites of the meleagrin/neoxaline pathway. The HPLC-MS total ion chromatogram (black) and normalized extracted ion chromatograms (colored) of the novel secondary metabolites roquefortine N (12, 15.1 min), roquefortine M (11, 16.5 min), roquefortine L (6, 16.8 min), neoxaline (10, 17.8 min), and roquefortine F (9, 22.8 min) from the meleagrin/neoxaline pathway are shown.
Structure Elucidation and Quantification of 6, 11, and 12
Compound 6 is a novel complex metabolite composed of a roquefortine scaffold and a rare nitrone moiety, thus named roquefortine L. The mass-to-charge ratio of its corresponding ion was observed at 404.1706 using HPLC-FT-ICR-MS, representing the protonated molecule [M+H]+ with formula C22H22N5O3 (calc. 404.1717) eluting at 16.8 min. The same ion was previously tentatively identified as glandicoline A (13) (Fig. 1B) as elemental composition and parts of the structure indicated consistency with this compound (7). However, its 1H and 13C NMR data showed high similarity to the diketopiperazine 4, indicating a roquefortine-like core structure. Furthermore, its 1H NMR spectrum revealed two protons at C-8 representing a single bond between C-8 and C-9, which is different from the double bond described for 13 (Table 1). Additionally, C-2 (δC = 146) in the 13C HMBC spectrum indicated a double bond between N-1 and C-2, which was supported by the chemical shift of N-1 (δN = 280) in the 15N HMBC spectrum. As compound 13 was reported from various Penicillium species such as Penicillium albocoremium (8), Penicillium glandicola (9), and P. chrysogenum (10) and proposed as a precursor of 7, host and roq deletion strain chromatograms of P. chrysogenum were further analyzed for the presence of 13. The chromatogram of the ion with m/z 404.1706, representing the protonated molecule [M+H]+ with formula C22H22N5O3 of both compound 6 and compound 13, was extracted in a 5-ppm mass accuracy window. However, no ion possibly corresponding to 13 could be found, whereas 6 was observed at high concentration in the liquid media (Fig. 3). The absence of 13 in host and various P. chrysogenum strains lead to the conclusions that 13 is not produced by P. chrysogenum DS54555.
TABLE 1.
Chemical shifts of 1H, 13C, and 15N NMR of roquefortine L (6) and 1H and 13C NMR of roquefortine F (9) (δ in ppm)
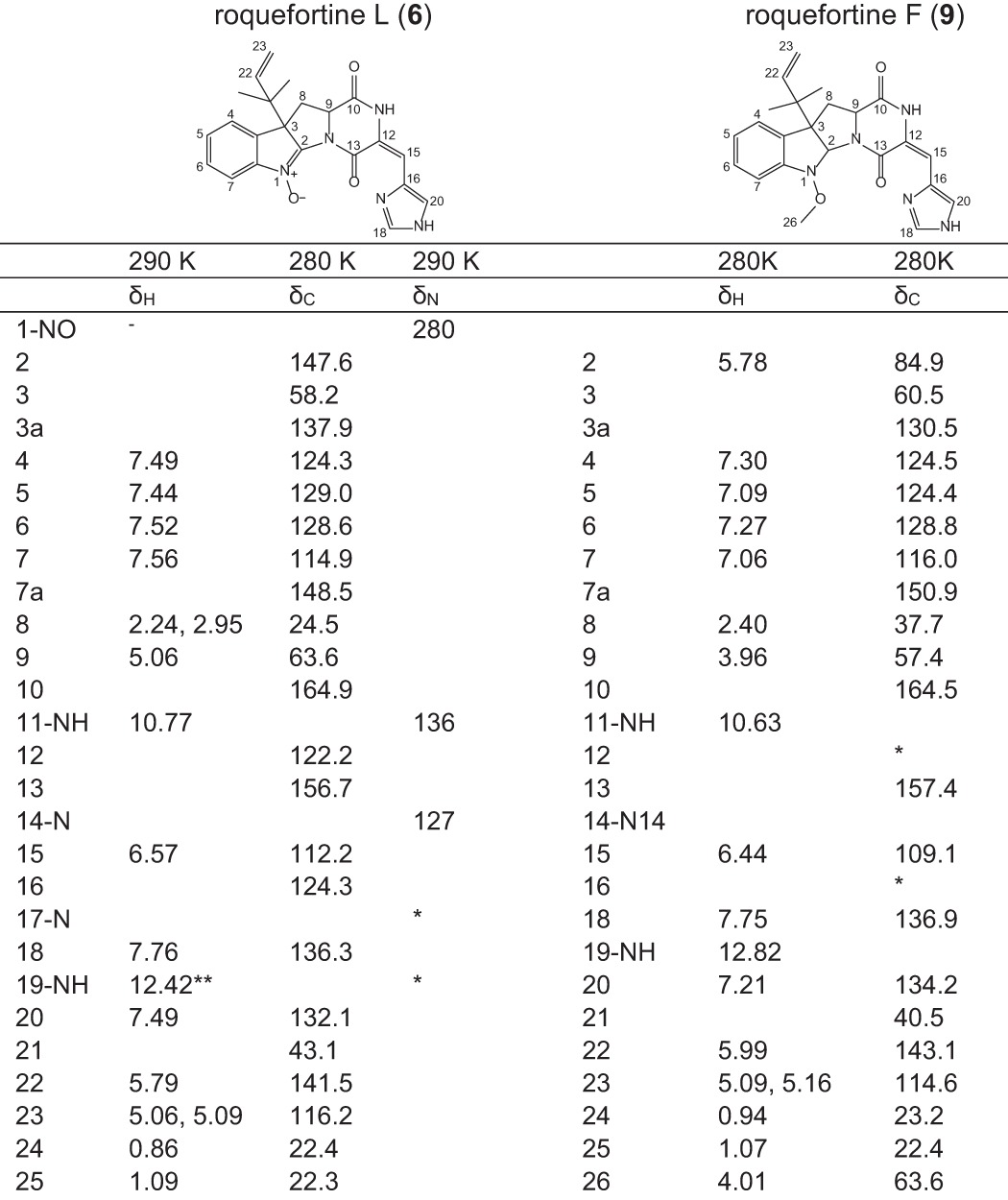
(*) Not observed.
(**) From spectrum at 280 K.
FIGURE 3.
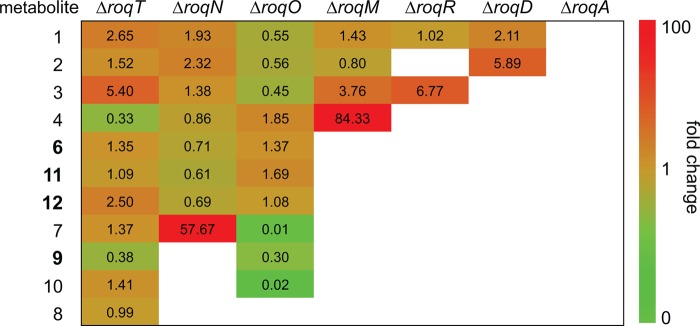
-Fold change of the concentration of secondary metabolites from the roquefortine/meleagrin pathway in deletion strains as compared with the host strain. Numbers in the table represent the internal standard corrected concentrations of secondary metabolites in supernatants of deletion strains obtained from HPLC-UV-MS as compared with their concentration in the supernatant of the host strain P. chrysogenum DS54555. Cell coloring, representing the -fold change, was performed on a logarithmic scale. Red cells indicate a concentration increase, whereas green cells represent a decrease. White cells indicate a complete absence of the metabolite in the deletion sample. Novel metabolites of the roquefortine/meleagrin pathway are shown with bold numbers.
Compounds 11 and 12 are novel compounds based on a roquefortine-like scaffold, thus named roquefortine M and roquefortine N. High-resolution electrospray ionization mass spectrometry of 11 (m/z 422.1814 [M+H]+, calc. 422.1824) and 12 (m/z 440.1918 [M+H]+, calc. 440.1928) established the molecular formula C22H23N5O4 and C22H25N5O5. Their chemical structure was determined using 13C HMBC, 15N HMBC, and 1H NMR (Table 2) showing similar signals as observed for compound 6, which indicates a similar chemical scaffold. However, the significant upfield shift of N-1 (from δN = 280 in 6 to δN = 185 in 11) in the 15N HMBC spectra together with the chemical shift of C-2 (δC = 146 in 6, δC = 172 in 11) in the 13C NMR spectra of compound 11 shows that 11 contains a single bond between N-1 and C-2, with C-2 being a carbonylic carbon. In addition, a comparison between the 15N HMBC spectrum of compounds 11 and 12 revealed that the amide bond between N-14 and C-13 in 11 was hydrolyzed in 12 (δN = 32.0), yielding a primary amine and a carboxyl group. Both compounds commonly occur, together with compound 6, in liquid cultures of P. chrysogenum host and roqT, roqN, and roqO deletion strains (Fig. 3). Their absence in the remaining deletion strain samples concludes the involvement of roqA, roqR, roqD, and roqM in their biosynthesis.
TABLE 2.
Chemical shifts of 1H, 13C, and 15N NMR of roquefortine M (11) and roquefortine N (12) (δ in ppm)
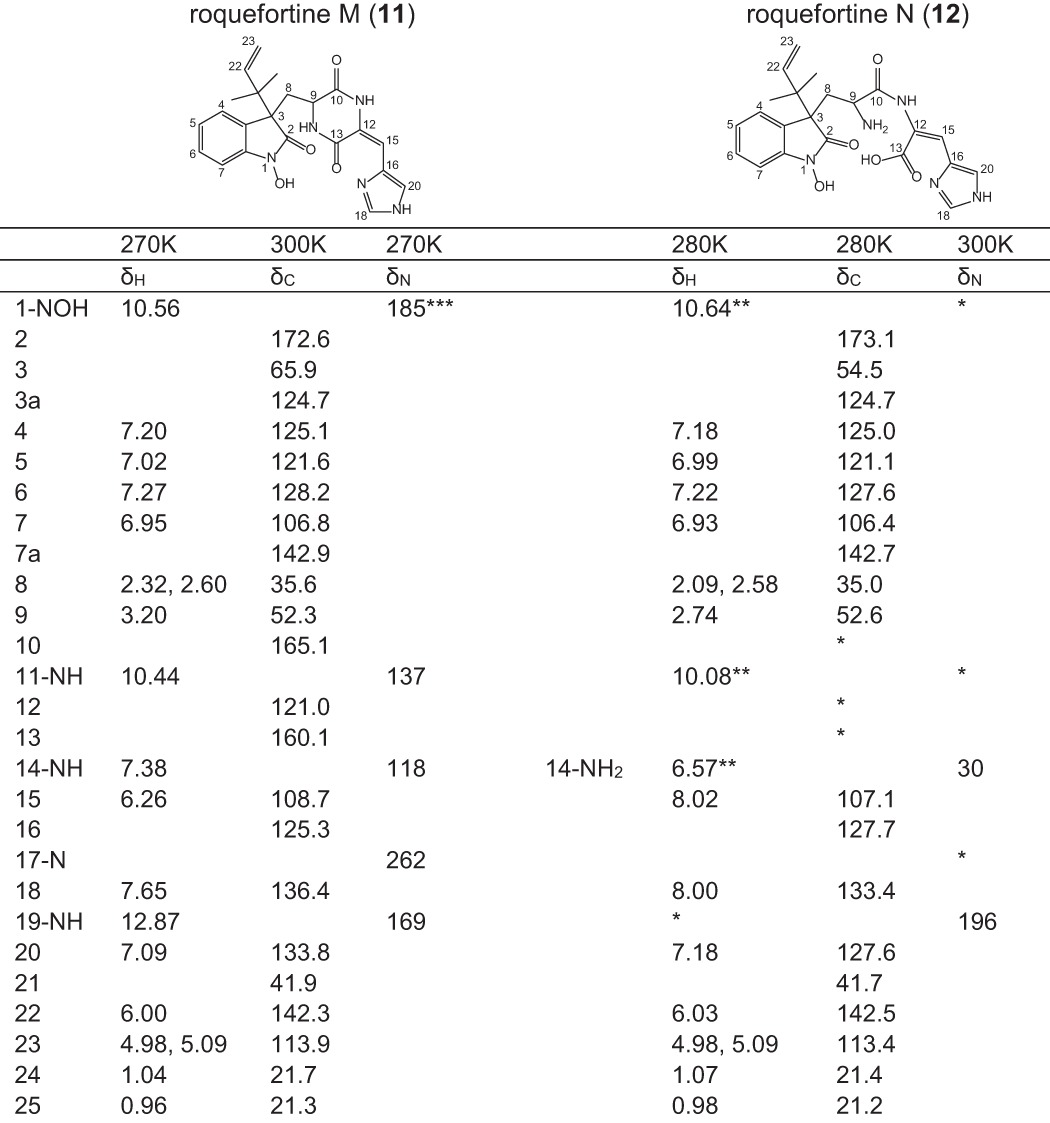
(*) Not observed.
(**) Tentative assignment.
(***) From spectrum at 290 K.
Structure Elucidation and Quantification of 9 and 10
Compound 9 with molecular formula C23H25N5O3, established by high-resolution electrospray ionization mass spectrometry (m/z 420.2015 [M+H]+, calc. 420.2030), was identified as roquefortine F, a metabolite solely reported from a deep ocean sediment-derived Penicillium species (11), using 1H and 13C NMR (Table 1). Its 1H NMR spectrum is very similar to the spectrum of 3 (7), except for a double bond between C-12 and C-15. Furthermore, the presence of C-26 (δC = 63.6) in the 13C NMR spectrum, next to a sharp OCH3 peak (δH = 4.01) and a missing proton on N-1 in the 1H NMR spectrum, fully agrees with a methoxylated N-1 in compound 9. This was supported by the absence of correlations with a carbon or proton in the HMBC spectrum. The concentration of 9, particularly high in the host strain, was found to be reduced to approximately one-third in the deletion strains of roqT and roqO and absent in the remaining deletion strains (Fig. 3). These data suggest that roqO and roqT are the only two genes not involved in the biosynthesis of 9. Compound 10 with molecular formula C23H25N5O4 (m/z 436.1967 [M+H]+, calc. 436.1979) was identified as neoxaline, a metabolite previously isolated from Aspergillus japonicas Fg-551 (12) and Penicillium tulipae (13), by comparing retention time and MS/MS fragments with its commercially available standard. Although the concentration of 10 in host and roqT deletion strain is almost comparable, a 97% decrease was observed in the roqO deletion strain (Fig. 3). In all remaining deletion strains, compound 10 could not be detected, leading to the conclusion that all genes in the roq gene cluster, except roqT, are required for the synthesis of 10.
Chemical Degradation of Compound 6 Leads to Various Products
Nitrones, such as compound 6, are not infinitely stable and degrade already at room temperature in aqueous solution as well as under acidic conditions by incorporation of water (14–16). To determine the resulting degradation products, an aqueous solution of 6 was acidified, and the resulting sample was measured using HPLC-UV-MS (Fig. 4). Next to a 50% decrease of 6, two highly abundant ions were observed in the treated sample corresponding to 11 and 12. Additionally, a third unidentified compound was found eluting at 17.33 min with a mass-to-charge ratio of 422.1823, representing the protonated molecule with the formula C22H24N5O4. These results demonstrate that 11, 12, and an unidentified third compound are produced by degradation of the rather unstable compound 6.
FIGURE 4.
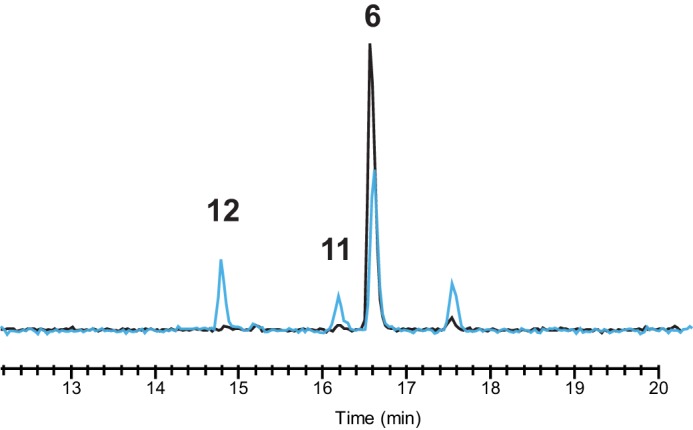
Chemical degradation of compound 6 leads to various products. The total ion chromatograms of pure (black) and degraded (blue) compound 6, after the addition of formic acid, measured on HPLC-MS are shown. Acid-induced degradation leads to the formation of 11 and 12 next to an unidentified compound eluting at 17.33 min with [M+H]+ = 422.1823 and elemental composition C22H23N5O4.
DISCUSSION
Here, we present new insight into the complex biosynthesis of secondary metabolites from the roquefortine/meleagrin pathway. Five novel metabolites were found to originate from the roq gene cluster, obtained from comparative metabolites profiling of the host strain and various deletion strains in combination with NMR- and MS-based structure elucidation. As all five metabolites are produced in a late stage of the pathway, no changes were observed for the biosynthesis of upstream metabolites 1–4, which starts with RoqA taking l-histidine and l-tryptophan as substrates and producing compound 1. Based on the highly significant accumulation of 4 in the roqM deletion strain and the absence of all downstream metabolites 6–12 (Fig. 3), it can be concluded that roqM, encoding a flavin-dependent MAK 1-monooxygenase-like protein, is involved in the conversion of 4 into 6, a novel compound containing an unusual nitrone moiety. Nitrones are widely known due to their free radical-trapping properties and their potential application as therapeutics in age-related diseases (17) such as cancer (18) and ischemic stroke (19). As the chemical scaffold of compound 6 is closely related to the roquefortine group, it was named roquefortine L. Flavin-containing monooxygenases are commonly known to consecutively oxidize drugs and xenobiotics containing a soft nucleophile, such as nitrogen or sulfur (20). In the case of secondary amines, flavin-containing monooxygenases consecutively oxidize the nitrogen, leading to the production of hydroxylamines and nitrones (14–16). A similar mechanism for the synthesis of the nitrone-containing compound 6 is very likely, starting with the oxidation of the secondary amine in the indole part of 4, yielding the hydroxylated intermediate 5 (Fig. 5). Further oxidation on the same nitrogen produces an unstable N,N-dihydroxylated species, which is followed by the loss of water, eventually producing compound 6. However, nitrones are not indefinitely stable and easily degrade at room temperature in aqueous solutions (14–16). Under acidic conditions, compound 6 decomposes by a consecutive incorporation of water leading, among others, to the production of compounds 11 and 12 (Fig. 4 and 6). This decomposition was also observed in NMR experiments after extended storage of a solution of 6 at room temperature. These results suggest that the presence of 11 and 12 in liquid cultures of P. chrysogenum can be attributed to a chemical degradation of 6. Compound 6, with the formula C22H21N5O3, is represented by an ion with a mass-to-charge ratio of 404.1706 and eluting at 16.8 min. The exact same ion was previously tentatively identified as compound 13 (7) as its elemental composition, and parts of the structure indicated consistency with this compound. However, further structure elucidation using various NMR experiments confirmed the structure of 6 instead. This was surprising as compound 13, a proposed key intermediate in the biosynthesis of downstream metabolites such as 7, 8, and 10, was previously tentatively identified in different Penicillium cultures (8, 10). By using a comparable instrumental setup with a similar chromatographic separation method, host and deletion strains of DS54555 were screened for production of 13. Nevertheless, neither 13 nor corresponding degradation products could be detected, whereas 6 was found at high concentrations, leading to the conclusion that 13 is not produced by P. chrysogenum. This is remarkable as 13 was expected as single precursor of 7, modified by RoqO (6). In addition, a deletion of roqO resulted in an up to 98% decrease of 7, whereas the levels of upstream metabolites remained nearly unchanged, indicating that RoqO is indeed involved in the synthesis of 7. Due to the general absence of 13 in the P. chrysogenum-derived samples, compound 7 has to originate from a different biosynthetic route than the previously reported oxidation of 13 on its indole nitrogen (6–8). RoqO, encoding a P450 monooxygenase, closely resembles FtmG (64% identity, 79% similarity at the amino acid level), a cytochrome P450 monooxygenase catalyzing the hydroxylation of fumitremorgin C to dihydroxy-fumitremorgin C (21), compounds that are structurally similar to the roquefortine derivatives. A possible deduced biosynthesis of 7 involves the hydroxylation of 6 on C-9 by RoqO, comparable with the oxidation of fumitremorgin C by FtmG. Subsequent cleavage of the bond between C-9 and N-14 followed by the development of a bond between C-2 and N-11 is postulated to ultimately yield 7, similar to the mechanism previously proposed for 13 from 4 (22). These results, together with the general absence of 13 in P. chrysogenum, lead to the conclusion that 7 and 8 are produced via a different biosynthesis in P. chrysogenum than in other Penicillium strains such as P. tulipae (8), for which a tentatively identified 13 was reported as intermediate.
FIGURE 5.
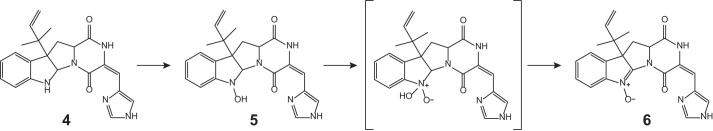
Proposed biosynthesis of compound 6 by RoqM.
FIGURE 6.
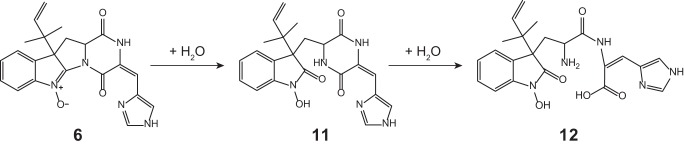
Degradation of compound 6 yielding 11 and 12 by consecutive incorporation of water.
The deletion of roqN resulted in an accumulation of 7 in the liquid medium, whereas metabolites 8, 9, and 10 were absent (Fig. 3). RoqN, a methyltransferase, was previously recognized to catalyze the addition of a methyl group on the hydroxylated nitrogen of 7, producing 8 (6, 7). As 9 contains a methylated hydroxylamine group in the same position as 8, the hydroxylamine containing compound 5, which differs only in a methyl group, is proposed as its direct precursor with RoqN catalyzing the methyl addition to yield 9. These results reveal a further branching of the roquefortine/meleagrin pathway with compounds 6 and 9 being products of 5. In addition, they support the presence of 5, which was proposed based on its involvement in the biosynthesis of 6.
Compound 10 was previously proposed as direct product of 8 by enzymatic hydrogenation (8). However, BLAST analysis did not reveal an enzyme in the roquefortine/meleagrin pathway that is able to perform that reaction (6, 7). Moreover, a 53 times higher concentration of 8 as compared with 10 in the host strain, but the absence of 8 in the roqO deletion strain with 10 still being present, leads to the conclusion that 8 is not a precursor of 10 (Fig. 3). In contrast, due to the high concentration of 9 in the ΔroqO strain and its roquefortine-like structure (roquefortine scaffold with a methoxy group on N-1), compound 9 is proposed as direct precursor of 10 with RoqO catalyzing this reaction, similar to the synthesis of 7 from 6. These results suggest that RoqO is involved in the reactions from 6 into 7 and from 9 into 10 by oxidizing and subsequently converting a roquefortine scaffold into a glandicoline-like structure (Fig. 1B).
In conclusion, these results extend the additional branch of compound 9 leading to the final product 10. Unspecificity, already observed for RoqR and RoqD (7), could now also be observed for RoqO and RoqN, leading to a complex degree of branching in the pathway and a wide palette of compounds. Several of the new compounds identified in the current study were found to be equipped with interesting biological activities. Roquefortine F, previously reported from a deep ocean sediment-derived fungus Penicillium sp., shows moderate cytotoxicity against various tumor cell lines (11). Neoxaline, which was first isolated from A. japonicas Fg-551, stimulates the central nervous system in mice (12) and inhibits cell proliferation (23). Furthermore, it was found to induce cell cycle arrest at the G2/M phase in Jurkat cells (inhibition of tubulin polymerization) (23). Here, the novel metabolites roquefortine L, roquefortine M, and roquefortine N are added to the palette of potential cytotoxic compounds, which demonstrates the potential of engineered industrial P. chrysogenum strains to produce novel bioactive compounds with unusual chemical scaffolds.
Acknowledgments
We thank Drs. H. Menke, W. Heijne, and H. Roubos from the DSM Biotechology Centre for making the DNA microarray data available.
This work was supported by the Perspective Genbiotics program subsidized by Stichting toegepaste wetenschappen (STW) and (co)financed by the Netherlands Metabolomics Centre (NMC), which is a part of the Netherlands Genomics Initiative/Netherlands Organization for Scientific Research.
- HTD
- histidyltryptophanyldiketopiperazine
- DHTD
- dehydrohistidyltryptophanyldiketo piperazine
- HMBC
- heteronuclear multiple bond correlation
- calc.
- calculated.
REFERENCES
- 1. Weber S. S., Polli F., Boer R., Bovenberg R. A., Driessen A. J. (2012) Increased penicillin production in Penicillium chrysogenum production strains via balanced overexpression of isopenicillin N acyltransferase. Appl. Environ. Microbiol. 78, 7107–7113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scott P. M., Merrien M. A., Polonsky J. (1976) Roquefortine and isofumigaclavine A, metabolites from Penicillium roqueforti. Experientia 32, 140–142 [PubMed] [Google Scholar]
- 3. Koolen H. H., Soares E. R., Silva F. M., Souza A. Q., Medeiros L. S., Filho E. R., Almeida R. A., Ribeiro I. A., Pessoa Cdo Ó., Morais M. O., Costa P. M., Souza A. D. (2012) An antimicrobial diketopiperazine alkaloid and co-metabolites from an endophytic strain of Gliocladium isolated from Strychnos cf. toxifera. Nat. Prod. Res. 26, 2013–2019 [DOI] [PubMed] [Google Scholar]
- 4. Clark B., Capon R. J., Lacey E., Tennant S., Gill J. H. (2005) Roquefortine E, a diketopiperazine from an Australian isolate of Gymnoascus reessii. J. Nat. Prod. 68, 1661–1664 [DOI] [PubMed] [Google Scholar]
- 5. Du L., Feng T., Zhao B., Li D., Cai S., Zhu T., Wang F., Xiao X., Gu Q. (2010) Alkaloids from a deep ocean sediment-derived fungus Penicillium sp., and their antitumor activities. J. Antibiot. 63, 165–170 [DOI] [PubMed] [Google Scholar]
- 6. García-Estrada C., Ullán R. V., Albillos S. M., Fernández-Bodega M. Á., Durek P., von Döhren H., Martín J. F. (2011) A single cluster of coregulated genes encodes the biosynthesis of the mycotoxins roquefortine C and meleagrin in Penicillium chrysogenum. Chem. Biol. 18, 1499–1512 [DOI] [PubMed] [Google Scholar]
- 7. Ali H., Ries M. I., Nijland J. G., Lankhorst P. P., Hankemeier T., Bovenberg R. A., Vreeken R. J., Driessen A. J. (2013) A branched biosynthetic pathway is involved in production of roquefortine and related compounds in Penicillium chrysogenum. PLoS One 8, e65328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Overy D. P., Nielsen K. F., Smedsgaard J. (2005) Roquefortine/oxaline biosynthesis pathway metabolites in Penicillium ser. Corymbifera: in planta production and implications for competitive fitness. J. Chem. Ecol. 31, 2373–2390 [DOI] [PubMed] [Google Scholar]
- 9. Kozlovskii A. G., Vinokurova N. G., Reshetilova T. A., Sakharovskii V. G., Baskunov B. P., Seleznev S. G. (1994) New metabolites of Penicillium glandicola var. glandicola - glandicoline A and glandicoline B. Appl. Biochem. Microbiol. 30, 334–337 [Google Scholar]
- 10. Vinokurova N. G., Boichenko L. V., Arinbasarov M. U. (2003) Production of alkaloids by fungi of the genus Penicillium grown on wheat grain. Appl. Biochem. Microbiol. 39, 403–406 [Google Scholar]
- 11. Du L., Li D., Zhu T., Cai S., Wang F., Xiao X., Gu Q. (2009) New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron 65, 1033–1039 [Google Scholar]
- 12. Hirano A., Iwai Y., Masuma R., Tei K., Omura S. (1979) Neoxaline, a new alkaloid produced by Aspergillus japonicus. Production, isolation and properties. J. Antibiot. 32, 781–785 [DOI] [PubMed] [Google Scholar]
- 13. Overy D. P., Phipps R. K., Frydenvang K., Larsen T. O. (2006) epi-Neoxaline, a chemotaxonomic marker for Penicillium tulipae. Biochem. Syst. Ecol. 34, 345–348 [Google Scholar]
- 14. Cashman J. R., Xiong Y. N., Xu L., Janowsky A. (1999) N-Oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication. J. Pharmacol. Exp. Ther. 288, 1251–1260 [PubMed] [Google Scholar]
- 15. Sun H., Ehlhardt W. J., Kulanthaivel P., Lanza D. L., Reilly C. A., Yost G. S. (2007) Dehydrogenation of indoline by cytochrome P450 enzymes: a novel “aromatase” process. J. Pharmacol. Exp. Ther. 322, 843–851 [DOI] [PubMed] [Google Scholar]
- 16. Rodriguez R. J., Proteau P. J., Marquez B. L., Hetherington C. L., Buckholz C. J., O'Connell K. L. (1999) Flavin-containing monooxygenase-mediated metabolism of N-deacetyl ketoconazole by rat hepatic microsomes. Drug Metab. Dispos. 27, 880–886 [PubMed] [Google Scholar]
- 17. Floyd R. A., Kopke R. D., Choi C. H., Foster S. B., Doblas S., Towner R. A. (2008) Nitrones as therapeutics. Free Radic. Biol. Med. 45, 1361–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Floyd R. A., Chandru H. K., He T., Towner R. (2011) Anti-cancer activity of nitrones and observations on mechanism of action. Anticancer Agents Med. Chem. 11, 373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maples K. R., Green A. R., Floyd R. A. (2004) Nitrone-related therapeutics: potential of NXY-059 for the treatment of acute ischaemic stroke. CNS Drugs 18, 1071–1084 [DOI] [PubMed] [Google Scholar]
- 20. Krueger S. K., Williams D. E. (2005) Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol. Ther. 106, 357–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kato N., Suzuki H., Takagi H., Asami Y., Kakeya H., Uramoto M., Usui T., Takahashi S., Sugimoto Y., Osada H. (2009) Identification of cytochrome P450s required for fumitremorgin biosynthesis in Aspergillus fumigatus. Chembiochem. 10, 920–928 [DOI] [PubMed] [Google Scholar]
- 22. Steyn P. S., Vleggaar R. (1983) Roquefortine, an intermediate in the biosynthesis of oxaline in cultures of Penicillium oxalicum. J. Chem. Soc. Chem. Commun. 10, 560–561 [Google Scholar]
- 23. Koizumi Y., Arai M., Tomoda H., Omura S. (2004) Oxaline, a fungal alkaloid, arrests the cell cycle in M phase by inhibition of tubulin polymerization. Biochim. Biophys. Acta 1693, 47–55 [DOI] [PubMed] [Google Scholar]