Abstract
The novel pandemic influenza A (H1N1) caused an epidemic of critical illness, and some patients developed severe acute respiratory distress syndrome (ARDS) or severe cardiopulmonary failure despite the use of conventional management. Extracorporeal membrane oxygenation (ECMO) support may successfully rescue these severely ill patients. We demonstrate the causative role of H1N1 in refractory ARDS of a previously healthy 15-year-old man who presented to the intensive care unit with a hypoxic and persistent cardiogenic shock refractory to conventional management as the leading symptom of influenza A. Because of compromised cardiopulmonary function, venovenous ECMO was applied 24 h after admission. Despite that the patient was manifesting heart failure, we decided the placement of venovenous ECMO because we believed that the real problem was the uncontrollable hypoxia and hypercapnia. A normal left ventricular ejection fraction was documented on a 2D echocardiography on day 2. The patient, after 6 days of ECMO, recovered completely and was successfully weaned from the mechanical ventilator on the 9th day after admission. The patient was discharged from the hospital on the 15th day. This experience showed that ECMO can be lifesaving for severe H1N1 infection also in patients with atypical clinical presentation of influenza.
Keywords: extra-corporeal life support, H1N1-flu, lung recovery, myocardial recovery, resuscitation
Introduction
The novel influenza A (H1N1) pandemic caused an epidemic of critical illness [1–6]. Although most of the confirmed cases have been self-limited and uncomplicated febrile respiratory illness, there have been severe infections with complications or mortality as well. A proportion of these patients presented with, or developed, severe acute respiratory distress syndrome (ARDS). In some severe cases, extracorporeal membrane oxygenation (ECMO) was commenced for the treatment of refractory hypoxemia, hypercapnia, or both, which occurred despite mechanical ventilation and rescue ARDS therapies [7]. Here, we report the first case of atypical clinical presentation of H1N1 infection that was successfully treated with ECMO in Florence.
Case report
An otherwise healthy 15-year-old white man presented to our emergency department with fever, lethargy, and emesis, after 1 week of flu-like symptoms. His medical history was significant for childhood asthma, for which he required no medication. Medications on admission included amoxicillin, levofloxacin, and paracetamol and started 6 days earlier for a flu-like syndrome.
Examination on arrival was significant for altered mental status, ataxia, and hypotension with mottled extremities. His vital signs were as follows: temperature, 39.8 °C; blood pressure, 80/40 mmHg; pulse rate, 115 beats/minute; respiratory rate, 30 breaths/minute; and oxygenation saturation, 92% with supplemental oxygen. He had diminished breath sounds and bilateral lower-extremity edema. The patient became more hypotensive and lost consciousness; his respiratory status rapidly progressed to respiratory failure (PaO2 /FiO2, 111.9; PaCO2, 113 mmHg). The patient was intubated on the day of admission. Ceftriaxone, azithromycin, meropenem, and caspofungin were given empirically. Laboratory test revealed a white blood cell count of 23,800 m/L, creatinine phosphokinase at a peak level of 1776 U/L, troponin I at 4.14 ng/dL, lactate dehydrogenase at 547 U/L, brain natriuretic peptide at 6361 pg/mL, creatinine at 2.03 mg/dL, C-reactive protein at 61 mg/L, and procalcitonin at 250.12 ng/mL. Liver and thyroid function tests were within normal limits. A computerized tomography (CT) scan of the brain was normal.
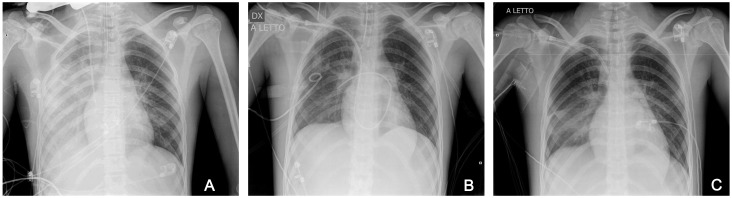
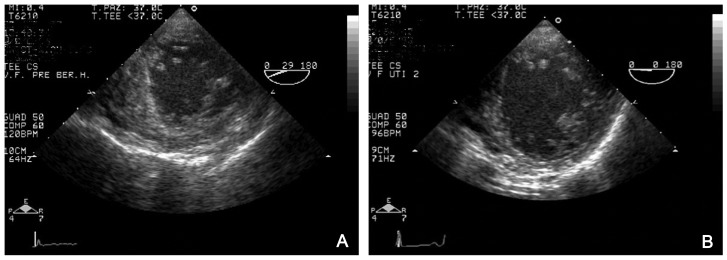
Chest radiography revealed consolidation and collapse of the right lower lobe (Fig. 1A). Transthoracic cardiac ultrasonography revealed poor cardiac contractility with an estimated left ventricular ejection fraction (LVEF) of 31% (Fig. 2A).
Fig. 1.
Evolution of chest X-ray shows diffuse airspace disease with interstizial alveolar infiltrate/consolidation on ICU admission (A), on day 4 after ECMO a partial resolution of the finding (B), and near complete resolution of the pulmonary infiltrates at ICU discharge (C)
Fig. 2.
Serial echocardiograms obtained in patient in term of LV function and motion. (A) Before ECMO. (B) After ECMO
After few hours of arrival in intensive care unit (ICU), the patient became oliguric and was in acute renal failure. A new thoracic X-ray revealed marked evolution in interstitial and alveolar bilateral infiltrates, consolidation in progression. CT scan showed consolidations on both lower lobes with bilateral pleural effusion.
The patient was well oxygenated and adequately ventilated (ventilator setting was 100% oxygen; positive end-expiratory pressure [PEEP], 15 mmHg; and peak inspiratory pressure [PIP], 45 mmHg), but remained hemodynamically unstable with poor perfusion on maximal medical therapy (see Table I). Given the reasonable likelihood of recovery of cardiac function, we chose ECMO support. We were unsure whether venovenous (VV) ECMO or venoarterial (VA) ECMO should be applied, but in this case, despite the severe left ventricular (LV) dysfunction, VV ECMO was adopted because we believed that the real problem was the uncontrollable hypoxia and hypercapnia. ECMO implantation was attempted via femoro-jugular access with our innovative cannulation technique (χ-configuration) as previously reported [8]. The scope was to maximize extracorporeal blood oxygenation albeit setting the mechanical ventilation in a very protective manner.
Table I.
Hemodynamic parameters of the refractory severe CS in H1N1 patient treated with extracorporeal life support
| 1 h before ECMO |
12 h after ECMO |
Day 2 on ECMO |
Day 5 on ECMO |
12 h after ECMO explantation |
|
|---|---|---|---|---|---|
| Hemodynamic variables | |||||
| MAP (mmHg) | 40 | 80 | 82 | 95 | 80 |
| Spontaneous cardiac index (L/min·m−2)a | 1.8 | 2.2 | 3.4 | 3.8 | 3.8 |
| Left ventricular ejection fraction (%)b | 20 | 30 | 40 | 50 | 60 |
| ECMO assistance (L/min) | – | 5 | 5.5 | 3.5 | – |
| Organ perfusion variable | |||||
| PaO2/FiO2 (mmHg) | 48 | 315 | 321 | 222 | 246 |
| SvO2 (%) | 47.8 | 86.0 | |||
| Plasma lactate concentration (mmol/L) | 6.7 | 2.9 | 1.4 | 1.5 | 1.1 |
| Serum creatinine concentration (mg/dL) | 2.5 | 1.48 | 1.11 | 0.76 | 0.62 |
| Prothrombine time (%) | 34 | 47 | 54 | 70 | 69 |
| Doses of vasoactive drugs | |||||
| Epinephrine (μg/kg/min) | 1.5 | 0.8 | – | – | – |
| Norepinephrine (μg/kg/min) | 1.9 | 0.5 | 0.2 | – | – |
| Dobutamine (μg/kg/min) | 20 | 10 | 7 | – | |
|
aEvaluation using transthoracic echocardiography was
performed by the same operator. bExpressed as percentage of normal values | |||||
The patient’s data course is reported in Table II. Our ventilation strategy during ECMO was as follows: once maximal ECMO-flow was achieved, ventilation was gradually reduced to avoid ventilation-induced lung injury (VILI). Protective ventilator settings were set to pressure control, PIP ≤ 25 cmH2O, and PEEP between 10 and 15 cmH2O, depending on pressure–volume curves. Values were all calculated with ventilator’s built-in application (Draeger Evita XL, Draeger Medical AG, Germany). PEEP was set to 2 cmH2O above the lower inflection point curve. Controlled respiratory frequency was reduced to 8–10 breaths/min. Inspired oxygen fraction was reduced to 0.5 or lower, whenever possible [9]. ECMO flow was constantly adjusted to keep arterial saturation of 95% with normal CO2 levels.
Table II.
Respiratory and hemodynamic parameters during ICU stay and ECMO
| 1 h before ECMO |
12 h after ECMO initiation |
On ECMO Day 2 |
Pre-ECMO weaning |
48 h after ECLS explanation |
|
|---|---|---|---|---|---|
| Hemodynamic values | |||||
| Cardiac output (L/min)/cardiac index (L/min·m2) |
3.11/1.8 | 3.80/2.2 | 5.88/3.4 | 6.57/3.8 | – |
| MAP (mmHg) | 40 | 80 | 82 | 95 | 80 |
| Mechanical ventilation setting | |||||
| Modality | CV | BiPAP | BiPAP | BiPAP | CPAP |
| FiO2 | 100% | 40% | 30% | 50% | 40% |
| PIP (cmH2O) | 45 | 24 | 25 | 20 | 16 |
| PEEP (cmH2O) | 15 | 12 | 10 | 12 | 8 |
| Tidal volume (mL) | 350 | 75 | 200 | 720 | 670 |
| Breaths/min | 16 | 7 | 8 | 9 | 14 |
| Pulmonary compliance (mL/cm2) | 18.7 | 16.8 | 20.6 | 64.5 | – |
| Oxygenation index | 92.8 | ||||
| Murray score | 4 | – | – | – | – |
| ECMO setting | |||||
| ECMO flow (L/min and L/min·m2) | – | 5/2.89 | 5.5/3.17 | 3.5/2.02 | – |
| ECMO gas flow (L/min) | – | 5 | 4 | – | |
| ECMO FiO2 (%) | – | 100 | 100 | 100 | – |
| Laboratory values | |||||
| WBC count (N·1000/mL) | 23.8 | 18.4 | 6.9 | 6.2 | 6.7 |
| Hb (g/dL) | 11.8 | 9.5 | 11.2 | 11.9 | 11.3 |
| Platelet count (N·1000/mL) | 109 | 92 | 62 | 91 | 163 |
| Procalcitonin (ng/mL) | 250.12 | 190.25 | 96.63 | 8.65 | 2.97 |
| Serum creatinine (mg/dL) | 2.5 | 1.68 | 1.11 | 0.76 | 0.62 |
| Blood gas values/organ perfusion | |||||
| Radial artery blood samples | |||||
| pH | 7.05 | 7.48 | 7.47 | 7.49 | 7.46 |
| pCO2 (mmHg) | 96 | 41.4 | 38 | 35.6 | 37.8 |
| pO2 (mmHg) | 48.5 | 126 | 96.4 | 111 | 98.7 |
| sO2 (%) | 83 | 99.7 | 99.3 | 99.5 | 99.9 |
| Lac (mmol/L) | 6.7 | 2.9 | 1.4 | 1.5 | 1.1 |
| BE (mml/L) | –12 | 1.7 | 3.5 | 2.2 | 1.3 |
| HCO3 (mml/L) | 29 | 26.3 | 27.6 | 25.8 | 26.7 |
| Pulmonary artery blood samples | |||||
| pCO2 (mmHg) | – | 42.4 | 42.6 | 43.8 | – |
| pO2 (mmHg) | – | 78.6 | 88.8 | 89.2 | – |
| sO2 (%) | – | 94.5 | 97.4 | 95.1 | – |
| Central venous samples | |||||
| pCO2 (mmHg) | – | 51.3 | 52.5 | 53.6 | |
| pO2 (mmHg) | – | 43.5 | 44.7 | 45.3 | |
| ScvO2 (%) | – | 66.5 | 74.6 | 73.8 | |
| Pre-oxygenator samples | |||||
| pCO2 (mmHg) | – | 50.6 | 51.4 | 55.2 | – |
| pO2 (mmHg) | – | 46.5 | 48.8 | 46.5 | – |
| sO2 (%) | – | 66.5 | 74.6 | 74.4 | – |
| Post-oxygenator samples | |||||
| pCO2 (mmHg) | – | 31.2 | 32.6 | – | |
| pO2 (mmHg) | – | 412.6 | 424 | – | |
| sO2 (%) | – | 100 | 100 | 100 | – |
| BRF (%) (preoxy
sO2–ScO2)/ (postoxy sO2–ScO2)·100 |
– | 5.54 | 5.07 | 1.95 | – |
| Patients BSA = 1.73 m2. All reported data are the mean of 3 consecutive measures.Abbreviations: CV = controlled volume; BiPAP = bi-levels positive airways pressure; CPAP = continuous positive airways pressure; FiO2 = fraction inspired oxygen; MV = respiratory minute volume; TV = tidal volume; PEEP = positive end-expiratory pressure; PIP = peak inspiratory pressure; CO = cardiac output; BSA = body surface area; CI = cardiac index; ECMO = extracorporeal membrane oxygenation; paCO2 = arterial carbon dioxide tension; paO2 = arterial oxygen tension; WBC = white blood cells; Hb = hemoglobin concentration; SO2 = oxygen saturation; ctO2 = oxygen content; Lac = lactate concentration; ABE = base excess; HCO3= bicarbonate concentration; ScvO2 = central-venous oxygen saturation; BRF = blood recirculation fraction with calculation formula [8] | |||||
On day 1, real-time reverse transcriptase–polymerase chain reaction of respiratory sample for H1N1 virus showed positive results. The patient was treated with 150 mg oseltamivir twice daily, and antibiotics were reduced to Ceftriazone alone. During ICU stay, hydrocortisone (200 mg/day) was given due to refractory septic shock.
During ECMO assistance, inotropic agents were rapidly discontinued (see Table I). On day 2, hemodynamic and transesophageal echocardiography (TEE) data showed significant recovery of the LV function (see Table II).
Lungs were evaluated periodically by CT scan, thoracic Rx, and daily lung ultrasound examinations [10].
The patient remained stable on ECMO without any significant complications during the complete course.
On day 4 of ECMO, lung functions significantly improved: pulmonary compliance reached 60 cmH2O, with Rx, and CT and lung ultrasounds findings were improving dramatically (cleaning of infiltrates, Fig. 1B). VV ECMO weaning was started, and support was reduced by progressive extracorporeal blood-flow lessening as previous reported [8]. On day 6, ECMO was removed, and on day 8, the patient was extubated.
A transthoracic echocardiogram 4 days after ECMO explantation showed an LVEF of 65% and good left ventricular contractility (Fig. 2B); chest radiographs (Fig. 1C) and CT revealed significant clearing of the parenchymal infiltrates. The patient was discharged from the intensive care unit and transferred to the medical ward. Forty-eight hours later, the patient was transferred to the step-down unit. The rest of the patient’s hospital course was unremarkable, and he was discharged on hospital day 15.
A 1-month follow-up chest radiograph and respiratory functional tests revealed normal lungs. At a 6-month follow-up, the patient was well and showed complete and stable cardiac recovery without neurological sequelae: his ejection fraction remained stable without any sign of regional dyssynergy. At a 12-month follow-up, the patient demonstrated complete and stable pulmonary recovery without sequelae: his respiratory function, blood gas analysis, and CT remained stable without any sign of regional lesions. He was required no further hospitalization.
Discussion
Seasonal and novel (H1N1-09) influenza commonly present with a constellation of symptoms known as influenza-like illness (fever, cough, sore throat, myalgias). Pulmonary complications, pneumonia and acute respiratory distress syndrome, are the major reported causes of morbidity and mortality associated. Patients with these complications require intensive care with mechanical ventilation and possibly extracorporeal life support.
Complications in extra-respiratory tissues such as encephalopathy, myocarditis, and myopathy occur occasionally [11]. The association of a severe influenza-like illness followed by the development of myocardial dysfunction or cardiomyopathy has been described in epidemiological studies [12].
The fact that influenza A (H1N1) can develop in healthy patients and evolve in few hours to a severe ARDS with a refractory hypoxemia needing recourse to ECMO in 5% to 20% of patients is new [13, 14].
Although viral myocarditis and heart failure are well-recognized and feared complications of seasonal influenza A infection, only limited information on H1N1-induced heart failure has been reported so far. Also fulminant myocarditis secondary to seasonal influenza infection has been well described but has not been reported with H1N1 influenza virus. Emerging but limited data on H1N1-associated cardiac dysfunction is available [15–23].
We found only two largest retrospective studies in the literature: Martin et al. [24] have identified reversible cardiac dysfunction in six (2 men and 4 women, age range 23 to 51 years) out of 123 cases with influenza A (H1N1) infection, and the Japanese Circulation Society [25] reported 15 patients (9 men and 6 women, mean age 42.4 ± 20.8 years). However, none of these cases initially presented with symptoms of acute heart failure.
In our own experience, all other cases of 2009 influenza H1N1 that had been admitted to our ICU (49 cases) presented with flu-like symptoms and signs of respiratory tract infection.
As mentioned before, influenza is a well-documented cause of myocarditis in both children and adults. There is a wide clinical spectrum of presentation that varies from completely asymptomatic to cardiogenic shock, with dyspnea, arrhythmias, and congestive heart failure along the continuum. Some patients more commonly present with abdominal pain, diarrhea, and lethargy [26].
The case presentation of H1N1 influenza discussed here is of interest because the patient did not present with a classic influenza-like illness in the initial phase of the infection. This atypical presentation could lead to a delay in diagnosis. The patient had signs and symptoms of cardiogenic hypoperfusion; he had neurologic and gastrointestinal symptoms at initial presentation. It is notable that the patient did not present with dyspnea or cough, which is the most common clinical symptom in patients with myocarditis and influenza, respectively. So this nonspecific clinical presentation and the lack of pulmonary symptoms may make diagnosis difficult. Most patients complained of not upper respiratory symptoms, but only systemic symptoms, but early recognition of this syndrome could facilitate initiation of both timely and appropriate therapy. In our case, VV ECMO was chosen as a mechanical support against uncontrollable hypoxia and hypercapnia. It was also the right supportive therapy for heart failure. So we encountered a young patient with influenza acute heart failure who was rescued by utilization of ECMO. Immediate application of ECMO is the key to saving patient with such a syndrome.
Conclusions
Relevant clinical conclusion can be drawn from this report.
Firstly, influenza H1N1 infection can occur with cardiac dysfunction without flu-like symptoms. It is important to recognize that patient with heart failure might have influenza infection, with the need for testing for H1N1 infection because early diagnosis is required for adequate treatment.
Second, extracorporeal membrane oxygenation may produce excellent results in such critically patients and should be considered before end-stage multiorgan failure develops.
Funding Statement
Funding sources: None.
Footnotes
Conflict of interest: None.
Authors’ contributions: MB, MC, GS and AP organized the ECLS/ECMO Service and collected patient’s data. MB and GDL reviewed the literature. MB, GDL and GH wrote the draft of article. MB, MC and GDL performed ECMO insertion procedure and managed the case here reported.
Contributor Information
Massimo Bonacchi,
Marco Ciapetti,
Gabriella Di Lascio,
Guy Harmelin,
Guido Sani,
Adriano Peris,
References
- 1.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009 Jun 18;360(25):2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Outbreak of swine-origin influenza A (H1N1) virus infection - Mexico, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009 May 8;58(17):467–470. [PubMed] [Google Scholar]
- 3.Editorial Team. Pandemic phase level 4: human cases of the novel influenza A/H1N1 strain confirmed in Scotland and Spain. Euro Surveill. 2009 Apr 30;14(17) [PubMed] [Google Scholar]
- 4.Wkly Epidemiol Rec, New influenza A(H1N1) virus infections: global surveillance summary, May 2009, 2009May15; 84(20): 173–179 [PubMed] [Google Scholar]
- 5.Wkly Epidemiol Rec, New influenza A(H1N1) virus – Update, 2009May8; 84(19): 171–172 [PubMed] [Google Scholar]
- 6.World Health Organization, DG statement following the meeting of the Emergency Committee, 2009; http://www.who.int/csr/disease/swineflu/4th_meeting_ihr/en/index.html, [accessed: September 9, 2009]
- 7.Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, Jackson A, McGuinness S, Nair P, Pellegrino V, Pettilä V, Plunkett B, Pye R, Torzillo P, Webb S, Wilson M, Ziegenfuss M. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009 Nov 4;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 8.Bonacchi M, Harmelin G, Peris A, Sani G. A novel strategy to improve systemic oxygenation in venovenous extracorporeal membrane oxygenation: the "χ-configuration". J Thorac Cardiovasc Surg. 2011 Nov;142(5):1197–1204. doi: 10.1016/j.jtcvs.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 9.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005 Oct 20;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 10.Peris A, Cianchi G, Biondi S, Bonizzoli M, Pasquini A, Bonacchi M, Ciapetti M, Zagli G, Bacci S, Lazzeri C, Bernardo P, Mascitelli E, Sani G, Gensini GF. Extracorporeal life support for management of refractory cardiac or respiratory failure: initial experience in a tertiary centre. Scand J Trauma Resusc Emerg Med. 2010 May 21;18:28. doi: 10.1186/1757-7241-18-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mamas MA, Fraser D, Neyses L. Cardiovascular manifestations associated with influenza virus infection. Int J Cardiol. 2008 Nov 28;130(3):304–309. doi: 10.1016/j.ijcard.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Onitsuka H, Imamura T, Miyamoto N, Shibata Y, Kashiwagi T, Ayabe T, Kawagoe J, Matsuda J, Ishikawa T, Unoki T, Takenaga M, Fukunaga T, Nakagawa S, Koiwaya Y, Eto T. Clinical manifestations of influenza a myocarditis during the influenza epidemic of winter 1998-1999. J Cardiol. 2001 Jun;37(6):315–323. [PubMed] [Google Scholar]
- 13.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler RA. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009 Nov 4;302(17):1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 14.The Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, Jackson A, McGuinness S, Nair P, Pellegrino V, Pettilä V, Plunkett B, Pye R, Torzillo P, Webb S, Wilson M, Ziegenfuss M. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009 Nov 4;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 15.Papadopoulos N, Ahmad Ael-S, Marinos S, Moritz A, Zierer A. Extracorporeal membrane oxygenation for influenza-associated acute respiratory distress syndrome. Thorac Cardiovasc Surg. 2013 Sep;61(6):516–521. doi: 10.1055/s-0032-1330923. [DOI] [PubMed] [Google Scholar]
- 16.Mytton OT, Rutter PD, Mak M, Stanton EA, Sachedina N, Donaldson LJ. Mortality due to pandemic (H1N1) 2009 influenza in England: a comparison of the first and second waves. Epidemiol Infect. 2012 Sep;140(9):1533–1541. doi: 10.1017/S0950268811001968. [DOI] [PubMed] [Google Scholar]
- 17.Suojaranta-Ylinen R, Salmenperä M, Kuitunen A. (A)H1N1-virusinfektioon liittyvae myokardiitti. Finnanest. 2009;42:443–445. [Google Scholar]
- 18.Wiegand JA, Torgersen C, Bloechlinger S, Takala J, Dünser MW. Influenza A(H1N1) infection and severe cardiac dysfunction in adults: A case series. Wien Klin Wochenschr. 2011 Feb;123(3-4):120–123. doi: 10.1007/s00508-010-1520-0. [DOI] [PubMed] [Google Scholar]
- 19.Oda T, Yasunaga H, Tsutsumi Y, Shojima T, Zaima Y, Nishino H, Ito S, Todo K. A child with influenza A (H1N1)-associated myocarditis rescued by extracorporeal membrane oxygenation. J Artif Organs. 2010 Dec;13(4):232–234. doi: 10.1007/s10047-010-0523-y. [DOI] [PubMed] [Google Scholar]
- 20.Bratincsák A, El-Said HG, Bradley JS, Shayan K, Grossfeld PD, Cannavino CR. Fulminant myocarditis associated with pandemic H1N1 influenza A virus in children. J Am Coll Cardiol. 2010 Mar 2;55(9):928–929. doi: 10.1016/j.jacc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Khouzam RN, Parizianu C, Hafiz AM, Chawla S, Schwartz R. Fulminant myocarditis associated with novel H1N1 influenza A. Heart Lung. 2011 Nov-Dec;40(6):566–568. doi: 10.1016/j.hrtlng.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Kumar K, Guirgis M, Zieroth S, Lo E, Menkis AH, Arora RC, Freed DH. Influenza myocarditis and myositis: case presentation and review of the literature. Can J Cardiol. 2011 Jul-Aug;27(4):514–522. doi: 10.1016/j.cjca.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Chan K, Meek D, Chakravorty I. Unusual association of ST-T abnormalities, myocarditis and cardiomyopathy with H1N1 influenza in pregnancy: two case reports and review of the literature. J Med Case Rep. 2011 Jul 14;5:314. doi: 10.1186/1752-1947-5-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin SS, Hollingsworth CL, Norfolk SG, Wolfe CR, Hollingsworth JW. Reversible cardiac dysfunction associated with pandemic 2009 influenza A(H1N1) Chest. 2010 May;137(5):1195–1197. doi: 10.1378/chest.10-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ukimura A, Izumi T, Matsumori A. A national survey on myocarditis associated with the 2009 influenza A (H1N1) pandemic in Japan. Circ J. 2010 Oct;74(10):2193–2199. doi: 10.1253/circj.cj-10-0452. [DOI] [PubMed] [Google Scholar]
- 26.Vashist S, Singh GK. Acute myocarditis in children: current concepts and management. Curr Treat Options Cardiovasc Med. 2009 Oct;11(5):383–391. doi: 10.1007/s11936-009-0039-z. [DOI] [PubMed] [Google Scholar]