Abstract
We have previously shown that mammary epithelial specific expression of the activated erbB2 allele under the control of the endogenous promoter in mice resulted in the formation of mammary adenocarcinomas. To assess whether mammary tumorigenesis in this model is influenced by the developmental window of expression, we generated mice that expressed the activated erbB2 allele in the germ line. Although we were able to derive viable transgenic mice that were heterozygous for the activated erbB2 allele, mice homozygous for the activated erbB2 allele died at 12.5 days of embryogenesis. These two separate lines of mice expressed activated erbB2 at equal levels in the mammary gland. Surprisingly, unlike the tumor-prone mice expressing activated ErbB2 in the mammary epithelium, mice with the germ-line erbB2 allele failed to develop tumors. Gene expression analysis of the preneoplastic mammary glands revealed that there were a number of luminal epithelial markers expressed at higher levels in the tumor-prone mice. These data suggest either an expansion of a susceptible population in the tumor-prone mice or the loss of this population in the tumor-resistant mice. Taken together, these observations suggest that the temporal pattern of expression of activated ErbB2 is a critical determinant in mammary tumorigenesis. These results strongly suggest that there are feedback mechanisms present that can compensate for the expression of a potent oncogene.
Amplification and overexpression of the Neu (ErbB2, HER2) protooncogene has been observed in 20-30% of human breast cancers and correlates with a poor prognosis for the patient (1, 2). Direct evidence supporting a role for the various epidermal growth factor receptor family members and their ligands in mammary tumorigenesis is derived from observations made with transgenic mice (3). For example, mammary-specific expression of activated ErbB2 results in the rapid induction of mammary tumors (4-6). Although mammary epithelial expression of the activated erbB2 oncogene is capable of efficiently inducing multifocal mammary tumors, no comparable activating mutations have been detected in the transmembrane domain of human ErbB2 (7). Thus, the primary mechanism by which ErbB2 induces mammary tumorigenesis in human breast cancer is through overexpression of the wild-type receptor. Interestingly, focal mammary tumors arose in mice expressing the wild-type receptor under the control of the mouse mammary tumor virus (MMTV) promoter after a long latency period (8). Tumor progression in these mice is associated with the activation of ErbB2 tyrosine kinase activity caused by somatic activating mutations in the transgene in 70% of the mammary tumors analyzed (9-11). These mutations are confined to the cysteine-rich region of the receptor located in juxtatransmembrane domain (9). Further analyses revealed that these cysteine alterations promote the formation of intermolecular cysteine bridges between ErbB2 monomers, resulting in receptor dimerization and activation (11). Although these transgenic studies suggested that activation of erbB2 is a critical step in tumor progression, they relied on a strong viral promoter for transgene expression. In an attempt to more closely mimic the events involved in human ErbB2-induced mammary tumor progression, we derived transgenic mice that carried a Cre inducible activated erbB2 allele under the transcriptional control of the endogenous erbB2 promoter (12). In contrast to the rapid tumor progression observed in the MMTV strains, focal mammary tumors arose only after an extended latency period. Tumor progression was associated with a dramatic elevation of both ErbB2 protein and transcript. Remarkably, the elevated expression of ErbB2 was correlated with genomic amplification of the activated erbB2 allele (12). Thus, like human breast cancers, amplification of erbB2 appears to be a critical event in mammary tumor progression in this unique mouse model.
Although these studies suggested that somatic activation of ErbB2 could predispose these mice to development of mammary tumors, one important issue that remained to be addressed was whether the developmental timing of activated ErbB2 expression could perturb the kinetics of mammary tumor induction. To assess the effect of introducing a potent gain-of-function mutant of erbB2 in the germ line of mice on both tumorigenesis and development, we derived mice that carried activated erbB2 in the germ line. Surprisingly, viable mice heterozygous for the germ-line-activated erbB2 were obtained at the expected ratio. However, mice homozygous for the germ-line-activated allele died at 12.5 days postcoitum (dpc) due to defects in both cardiac and neural development. Although heterozygous mice bearing either the germ-line- or mammary-specific activation of ErbB2 expressed comparable levels of erbB2 transcript in their mammary glands, mice bearing germ-line activation of the identical erbB2 allele were completely resistant to mammary tumor development, in contrast to the mammary tumor-prone phenotype of the conditional activated strains (12). These observations suggest that developmental window of expression of erbB2 is a critical factor in mammary tumor development.
Materials and Methods
Generation and Characterization of Mice. The derivation of the conditionally activated mice has been described in detail (12). To generate mice that expressed NeuNT under the control of the endogenous promoter in the germ line, a construct expressing Cre recombinase under the control of the chicken β-actin promoter was microinjected without linearization into embryos derived from mice bearing the loxP-neo-loxP-NeuNT allele controlled by the endogenous ErbB2 promoter. The resulting mice were examined for the presence of the excised recombinant allele through Southern analysis. Mammary gland analysis was completed as described (12). For embryo analysis, the extra-embryonic tissue surrounding the embryos was dissected free, and the visceral yolk sac was retained for genotyping. The embryos were either fixed or flash frozen for protein or RNA extraction. The in situ analysis for Phox2a was completed as published (13).
Immunoblotting. Western blotting was conducted as described (12). To detect ErbB2, the AB3 antibody (Oncogene Science) was used.
Array Analysis. To compare the conditional and germ-line activation of ErbB2, RNA was obtained from the mammary glands of 10 virgin mice for each genotype through a standard CsCl gradient method. The RNA was pooled into two sets from five mice each and was compared through an Affymetrix array. Data were the analyzed by using the Affymetrix microarray suite and the data mining tool to discard genes with marginal results and low P values. Final results were filtered for fold changes using a 3-fold difference as a cutoff point, and selected results are shown.
Quantitative RT-PCR. For quantitative RT-PCR, the reverse transcription, PCR, and quantification were carried out in a single capillary by using the LightCycler RNA Amplification Kit SYBR Green (Roche Diagnostics). The suggested protocol was followed in the RT-PCR reactions by using 200 ng of total RNA. The primers used to amplify WDNM1 were as follows; 5′-TCT TTG TTC TGG TAG CTT TGA TTT-3′ and 5′-GTT TGC AGG CAT GAC CAC AG-3′. The primers used to amplify ε casein were 5′-CTT TTG GCC GTT GCT CTT G-3′ and 5′-TTG CTG TAT CGT TTC ATT TTG TTC-3′. The primers used to amplify cea10 were 5′-TGG TAC AAG GGA AAC AGT GG-3′ and 5′-CAA GGA GGG TAA AAG TGA GG-3′. The primers used to amplify erbB2 were 5′-CCC AGA TCT CCA CTG GCT CC-3′ and 5′-TTC AGG GTT CTC CAC AGC ACC-3′ for both mouse and rat and 5′-AAC CAC GTC AAG ATT ACA GAT-3′ and 5′-AAA TCA GGG ATC TCC CGG-3′ for rat specific transcripts. The PCR was stopped while all samples were in the log-linear phase of amplification, and the product was subject to a melting curve analysis. To standardize the level of RNA in these samples, RT-PCR was also completed for GAPDH by using the following primers; 5′-TCA TGA CCA CAG TGG ATG CC-3′ and 5′-GGA GTT GCT GTT GAA GTC GC-3′.
Results
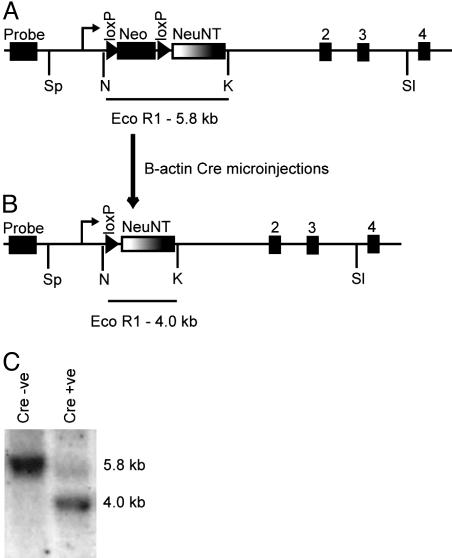
Transgenic Mice Homozygous for the Germ-Line-Activated erbB2 Allele Die at 12.5 Days of Embryogenesis. To explore whether expression of an activated erbB2 allele could be tolerated during embryogenesis, single-cell embryos with one copy of the Cre inducible erbB2 recombinant allele (Fig. 1A) were microinjected with a plasmid containing Cre Recombinase under the control of the chicken β-actin promoter. The β-actin promoter is expressed at the two-cell phase in the mouse embryo and should direct excision in the germ line to place activated erbB2 under the control of the endogenous promoter in all tissues in which it is normally expressed (Fig. 1B) (14). In several of the Cre-injected progeny, there was complete excision of the loxP flanked sequence in a Southern analysis (Fig. 1C). Subsequent breeding of these mice confirmed that one copy of the activated erbB2 allele could be passed through the germ line without any obvious phenotypic abnormality.
Fig. 1.
Generation of mice expressing NeuNT under the control of the endogenous promoter in the germ line. To create mice expressing the activated erbBB2 allele under the control of the endogenous promoter, mice containing a loxP neomycin loxP NeuNT sequence in place of exon 1 of the endogenous erbB2 allele (A) were interbred (12). The single-cell embryos from this cross were then microinjected with a circular β-actin Cre plasmid to excise the loxP flanked sequence resulting in the germ-line NeuNT allele (ErbB2WT/NT) (B). The excision of the neomycin cassette can be detected through a shift in size of the EcoR1 fragment detected in a Southern analysis using the activated erbB2 cDNA as a probe (C). Without the addition of Cre recombinase, there is a single band at 5.8 kb. However, after Cre-mediated excision, this band shifts to 4.0 kb, placing the activated erbB2 cDNA under the control of the endogenous promoter.
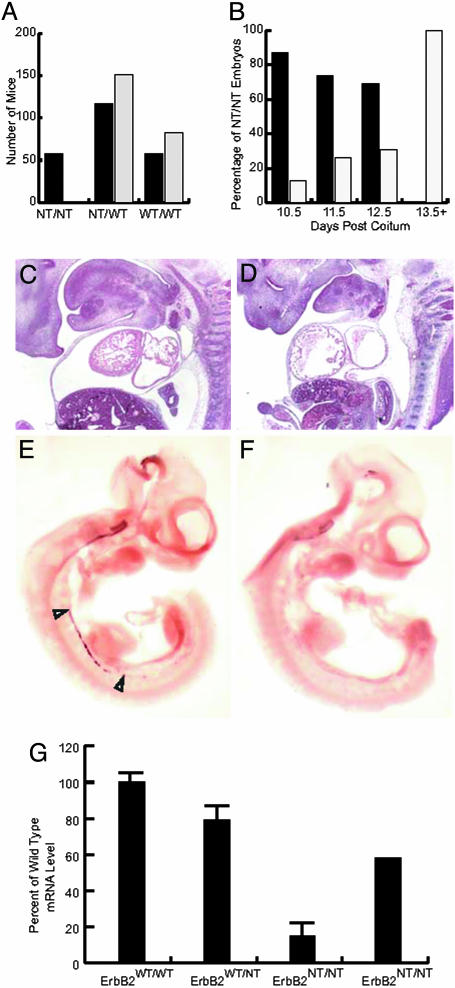
To assess whether mice carrying two copies of the activated erbB2 allele were compatible with viability, the heterozygous germ-line-activated ErbB2 mice (ErbB2WT/NT) were interbred to generate homozygous offspring. The results showed that mice homozygous for the germ-line-activated erbB2 allele (ErbB2NT/NT) were not detected (Fig. 2A). To determine when the ErbB2NT/NT mice were succumbing to embryonic lethality, embryos were harvested from 10.5 to 13.5 dpc. This revealed that we were able to generate the expected number of viable ErbB2NT/NT embryos at 10.5 dpc (Fig. 2B), but that by 11.5 and 12.5 dpc, a proportion of these embryos were dying. After 12.5 dpc, we were unable to detect viable ErbB2NT/NT embryos (Fig. 2B).
Fig. 2.
Embryonic lethality in ErbB2NT/NT mice at 12.5 dpc due to cardiac and neural defects. After interbreeding ErbB2WT/NT mice, the progeny were geno-typed, and the expected number of each genotype is shown with black bars. The observed genotypes are shown (gray bars), indicating that no viable ErbB2NT/NT mice were detected (A). On genotyping embryos from timed matings, we noted that no viable ErbB2NT/NT embryos were observed after 12.5 dpc. Examining only the ErbB2NT/NT genotype, the percentage of viable and dead or dying embryos is shown. The percentage of viable ErbB2NT/NT embryos (black bars) and the number of ErbB2NT/NT embryos that showed signs of being resorbed (gray bars) are shown from 10.5 to 13.5 dpc (B)(n = 58). Sections of the ErbB2WT/NT (C) and ErbB2NT/NT (D) embryos were examined at 12.5 dpc and illustrated that there was a defect in cardiac trabeculation in the ErbB2NT/NT embryos (D). Moreover, when the sympathetic chain ganglia was examined through a phox2a in situ analysis, it was clear that the heterozygous control was developing normally (E), whereas the ErbB2NT/NT embryos lacked proper development (F). The level of expression of the erbB2 transcript was examined in 10.5-dpc embryos through a quantitative RT-PCR analysis (G). When compared against the ErbB2WT/WT control, it was observed that the level of both ErbB2WT/NT and ErB2NT/NT transcript was reduced. Moreover, when the wild-type and heterozygous embryos were compared, an expected transcript level for the ErbB2NT/NT was generated that was far higher than the observed levels. Error bars denote standard deviation measured on four samples per genotype repeated three times and standardized to a GAPDH control.
To investigate the cause of the embryonic lethality observed in the ErbB2NT/NT embryos, embryos were harvested at 12.5 dpc. Although the ErbB2WT/WT and ErbB2WT/NT embryos had normal cardiac trabeculation (Fig. 2C), the ErbB2NT/NT embryos had a reduction in the amount of trabeculation (Fig. 2D). In many respects, this phenotype resembled the trabecular defects observed in erbB2 null mice (15). However, unlike the germ-line ablation, which lacked trabeculae entirely, the ErbB2NT/NT embryos have small trabeculae, which likely allowed the embryos to survive for 2 days longer than their null counterparts. We also examined whether this strain exhibited defects in the peripheral nervous system. Using expression of phox2a as a marker of neural development, we performed in situ hybridization on 11.5-dpc embryos with phox2a-specific probes. In both the ErbB2WT/WT and ErbB2WT/NT embryos, the presence of phox2a expression in the developing sympathetic chain ganglia was clearly present (Fig. 2E). However, the presence of phox2a expression could not be detected in the ErbB2NT/NT embryos (Fig. 2F). These results suggest that the expression of an activated erbB2 allele under the control of the endogenous promoter results in both cardiac and neurological defects reminiscent of the erbB2 null mice. To exclude the possibility that these phenotypes reflected loss of expression of activated erbB2 allele, we measured the levels of activated erbB2 transcript in 11.5-dpc embryos. The results revealed that transcripts specific to activated erbB2 could be detected, albeit at lower-than-expected levels (Fig. 2G). Taken together, these observations suggest that germ-line expression of activated erbB2 can be tolerated only in mice heterozygous for the activated erbB2 allele.
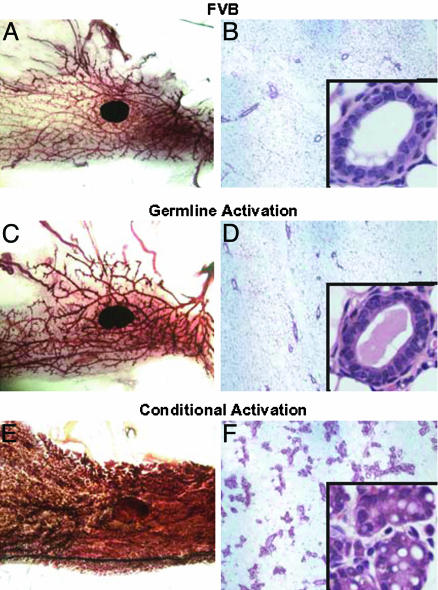
The Developmental Window of Activated erbB2 Expression Determines Mammary Tumor Susceptibility. One expected phenotype of the ErbB2WT/NT mice was the development of mammary epithelial hyperplasias and tumors, because mammary-specific activation of the same activated erbB2 allele resulted in development of tumors (12). To examine whether the ErbB2WT/NT mice exhibited mammary epithelial abnormalities, we compared the mammary gland morphology of mice carrying either the germ-line erbB2 allele or mice carrying mammary-specific activation of the same erbB2 allele. In contrast to the precocious lobualveolar hyperplasias observed in mice expressing the conditional activated erbB2 allele in the mammary epithelium (Fig. 3 E and F), age-matched mammary glands derived from ErbB2WT/NT mammary glands (Fig. 3 C and D) exhibited morphologies indistinguishable from wild type Freund leukemia virus/B strain mice (Fig. 3 A and B). Given that the same recombinant allele expressing activated erbB2 under the control of the endogenous promoter was present in both strains, these results indicate that differences in either the temporal or spatial expression patterns are capable of causing striking changes in the susceptibility of the mammary gland to hyperplasias. Although it is possible that developmental abnormalities could have arisen in the germ-line ErbB2WT/NT mice, it was noted that these mice were fully capable of lactating, suggesting that the mammary gland differentiated normally.
Fig. 3.
Effect of NeuNT expression on mammary gland development. Mammary glands from adult mice were compared to assess the effect of activated ErbB2 expression under the control of the endogenous promoter through both whole mounts and hematoxylin/eosin-stained histology. Wild-type (A and B), germ-line activation (C and D), and conditional activation (E and F) models were compared in this analysis. The wild-type and germ-line activation mammary glands are similar, whereas the conditional activation of ErbB2 results in a hyperplastic gland. The hematoxylin/eosin-stained sections of these whole mounts reinforce the differences in ductal density between the samples. Higher magnification (Insets) of these sections reveals no striking differences in ductal architecture between the wild-type and germ-line activation, whereas the hyperplasia in the conditional activation is readily observed. Whole-mount photomicrographs were taken at ×1.4, low-magnification histology at ×50, and Insets at ×400.
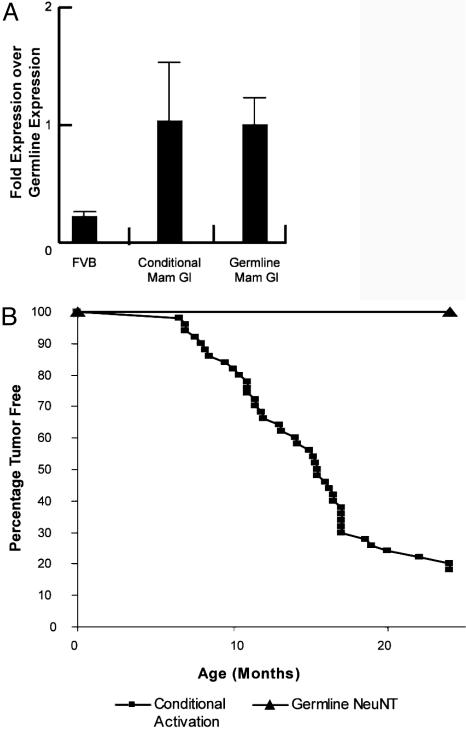
To preclude the possibility that the difference in hyperplasia susceptibility between these strains was due to differences in the levels of expression, we measured the levels of activated erbB2 transcript with activated erbB2 allele specific primers. The results showed that the mammary gland derived from either conditional or germ-line ErbB2WT/NT mice expressed equivalent levels of activated erbB2 transcript (Fig. 4A), illustrating that the dramatic difference in the mammary phenotype cannot be accounted for by different levels of activated erbB2 transcript.
Fig. 4.
Lack of tumors in ErbB2WT/NT mice. Given the differences in mammary gland morphology between the germ-line and conditional models, the level of erbB2 transcripts were measured by quantitative RT-PCR with primers specific for the activated erbB2 allele. After setting the level of the germ-line expression to 1, it was clear that the conditional and germ-line models expressed the activated erbB2 allele at identical levels (A). Error bars represent standard deviation measured in four samples after repeating the analysis three times and standardizing to GAPDH. To determine whether the ErbB2WT/NT mice were susceptible to tumors, 25 mice were observed for >2 years and remained tumor free in all tissues (B). This is in stark contrast to the conditional model, where the tumor latency is 15.9 months for 50% of female mice.
To explore whether the expression pattern impacted the ability of these strains to develop mammary tumors, virgin female mice with either the conditionally activated erbB2 allele or the germ-line ErbB2WT/NT were monitored for mammary tumors. Mice with the mammary-specific activation developed mammary tumors with an average latency period of 15.9 months with >90% affected by 2 years (Fig. 4B). In contrast, over a 2-year observation period, mice carrying the germ-line ErbB2WT/NT allele failed to develop tumors. Moreover, because ErbB2 has been implicated in other cancers, these mice were observed for tumor formation in all tissues for 2 years and were found to be completely tumor free.
Gene Expression Profiling Reveals a Distinctive Tumor-Prone Signature. To explore the molecular basis for the differential response of the mammary epithelium to activated erbB2 in these strains, we compared the gene expression profiles of RNA derived from mammary gland the conditionally activated erbB2 mice to the ErbB2WT/NT strain. Ten virgin mammary glands from each line were compared through a set of Affymetrix gene chip analyses. In Table 1, the genes that are expressed at a higher level in the conditional model in both repeats of the gene chip experiment are shown with the average fold increase in a positive value. Accordingly, the genes expressed at a higher level in the ErbB2NT/WT mammary gland are shown as a negative average fold increase.
Table 1. Expression profile of germ-line and conditionally activated erbB2 mammary glands.
| Fold change | Gene name | Ontology | GenBank accession no. |
|---|---|---|---|
| Differentation | |||
| 21.1 | ε Casein | Stimulated by hormones | V00740 |
| 11.7 | Fatty Acid B.Protein | Differentiated mammary marker | X14961 |
| 11.3 | WAP | V00856 | |
| 8.9 | Glycaml | Elevated in tumor model | M93428 |
| 8.6 | Glycoprotein | Z22552 | |
| 8.3 | Connexin-30 | Differentiation marker | Z70023 |
| 4.8 | Connexin-26 | Expressed in pregnancy | M81445 |
| 4.3 | α-lactalbumin | Stimulated by hormones | M87863 |
| 3.2 | Butyrophilin | Milk protein | U67065 |
| Potential neoplastic markers | |||
| 8.3 | MRP8 | Elevated in breast cancer (24) | M83218 |
| 8.0 | Cea10 related tag | Some cea proteins deregulated in tumors | AV381191 |
| 5.3 | Cea10 | Some cea proteins deregulated in tumors | D38422 |
| 4.9 | Glycerol kinase | Raf induces expression in MCF10a | U48403 |
| 5.1 | WDNMI | Elevated in ErbB2 and Ras tumors (17) | X93037 |
| 4.4 | lactotransferrin | Elevated in ErbB2 and Ras tumors (17) | J03298 |
| 4.3 | CAII | Inhibitors have antitumor properties | M25944 |
| 3.7 | Ceruloplasmin | Copper transporter, some breast cancer evidence | U49430 |
| 9.2 | MRP14 | See MRP8 | M83219 |
| 3.0 | κ-casein | Elevated in ErbB2 and Ras tumors (17) | M10114 |
| 2.8 | CRBPI | Elevated in ErbB2 and Ras tumors (17) | X60367 |
| 7.0 | CAB1 | Coamplified with ErbB2 | X82457 |
| 3.0 | Mat8 | Elevated in ErbB2 and Ras tumors (17) | |
| Tumor suppressors | |||
| −3.9 | EBI-1 | Enhances antitumor immunity | L31580 |
| −3.4 | Neuronatin | Identified as a tumor supressor | X83569 |
| −3.1 | Similar to 53BP1 | Similar to a p53-binding protein | A1593047 |
| Others | |||
| 4.9 | β-1-globin | V00722 | |
| 3.7 | Arginase II | AF032466 | |
| −4.0 | Slfn1 | Growth regulatory genes | AF099972 |
| −4.1 | ALDR | Induced by retinoic acid | Z48670 |
| −4.3 | MUP V | Urinary protein | M16360 |
| −4.6 | Similar to PKC | AV336804 | |
| −4.9 | Retinal oxidase | Absent in MCF7 cells | AB017482 |
| −6.7 | Adrenergic receptor | X72862 | |
| −8.9 | Reelin | Up-regulated in esophageal cancer | U24703 |
Mammary glands from both the conditional and germ-line NeuNT mice were compared through Affymetrix chip analysis. A portion of the results of this analysis is shown after using a 3-fold change as the cutoff. Genes that were expressed at a higher level in the conditional model are shown as a positive-fold elevation, whereas genes expressed at higher levels in the germ-line model are shown as a negative-fold elevation. The fold change in gene expression, gene name, a brief ontology, and the accession number are shown.
One category of genes that were consistently up-regulated in mammary glands derived from the conditionally activated model are markers of mammary gland differentiation and lactation that are observed includes genes such as ε casein (21-fold increase), wap (11-fold increase) connexin 26, connexin 30, and α-lactalbumin (4-fold increase). Included in this list of differentiation markers is glycam1 (9-fold increase), which was previously observed to be elevated in tumors arising in the conditional activation of neu (16). The preneoplastic markers are another class consistently up-regulated in the conditional activated mammary epithelium and are also frequently up-regulated in Ras or Neu initiated mammary tumors (17) and include WDNM1 (5.1-fold increase), lactotransferrin (4.4-fold increase), κ-casein (3.0-fold increase), and CRBP1 (2.8-fold increase) (Table 1).
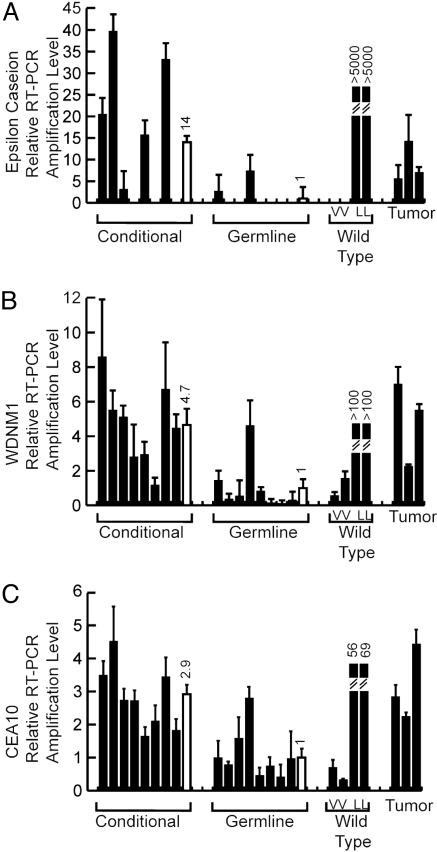
To confirm the accuracy of the gene expression data, quantitative RT-PCR was performed for various samples from the conditional and germ-line activation models. In addition, ErbB2-induced tumors and wild-type virgin and lactating mammary glands were included as controls. The quantification for ε casein, WDNM1, and cea10 is shown, illustrating that the trends revealed by the gene chip were accurate (Fig. 5). Elevated levels of these transcripts were also observed in ErbB2 tumors and lactating mammary tissue. Significantly, these markers were expressed at elevated levels in mammary epithelium before overt tumor formation, suggesting that the expression of these genes in virgin epithelium represents an expansion of cell type targeted by ErbB2.
Fig. 5.
Confirmation of the expression analysis. To confirm the gene expression data, quantitative RT-PCR was performed for three target genes. ε casein (A), WDNM1 (B), and CEA10 (C) were tested for expression, and the results of three repeats standardized to GAPDH are shown for each of eight samples for both the conditional and germ-line expression of the activated erbB2 allele (black bars). The average of these samples is shown with the open bar and is stated above the bar. These data are also compared to the virgin (V) and lactating (L) wild-type controls in addition to the conditional tumor control.
Discussion
The ability of an activated oncogene to induce tumors depends on multiple factors. Here we demonstrate that the temporal timing of expression of an activated oncogene is a critical parameter in tumor induction. Previous studies with activation of an identical activated erbB2 allele in the mammary epithelium revealed that these mice developed focal mammary carcinomas with a long latency period (12). To explore whether timing of expression of the erbB2 oncogene would affect tumorigenesis, we induced activation of an identical activated erbB2 allele in single-cell embryos. Surprisingly, mice heterozygous for the activated erbB2 had no obvious phenotype, but we were not able to derive mice that were homozygous for the activated erbB2 allele (Figs. 1 and 2). Examination of the ErbB2NT/NT embryos revealed that embryonic lethality was occurring at 12.5 dpc because of defects in cardiac trabeculation and development of the nervous system. Interestingly, expression of the activated erbB2 allele allowed the ErbB2NT/NT embryos to proceed past the point of embryonic lethality observed in the ErbB2 null mice but was not sufficient to rescue embryonic lethality. Importantly, when the wild-type erbB2 cDNA was used, the resulting homozygous mice were viable (13). These results suggest that normal signaling from ErbB2 is required for development of the mouse. Further, when the expected level of erbB2 mRNA was calculated, the level of observed erbB2 expression was far lower than expected in the homozygous sample (Fig. 2G). Previous results using the same targeting strategy for several alternate erbB2 alleles resulted in unchanged levels of erbB2 mRNA, although a reduction in protein levels was noted (13). The reduction of erbb2 mRNA in the ErbB2NT/NT model suggests that expression of activated erbB2 has triggered a feedback loop that regulates the promoter activity of erbB2.
Although two copies of the activated erbB2 allele were not tolerated during embryonic development, ErbB2WT/NT mice exhibited no immediate phenotype. Given that the mammary-specific activation of the same activated erbB2 allele resulted in the induction of mammary carcinomas (12), we anticipated that germ-line transmission of activated erbB2 allele would result in a similar phenotype in the ErbB2WT/NT mice. However, both histological and phenotypic analyses revealed that these animals failed to develop mammary hyperplasias or tumors (Figs. 3 and 4). The dramatic difference in tumor phenotype between the two strains was not due to changes in activated erbB2 transcript, because both mammary samples expressed equivalent levels of erbB2 (Fig. 4A).
One possible explanation for the absence of tumors in the germ-line-activated erbB2 strains is that, because of its expression in the embryonic state, there is adaptation of mammary epithelium to constitutive erbB2 signaling. These results are reminiscent of experiments using Rous sarcoma virus (RSV) (18). Infection of newly hatched chicks with RSV resulted in a sarcoma at the site of injection and was associated with expression of active v-src. However, with an in ovo infection, there was no corresponding sarcoma development (18, 19). Interestingly, in the chicks infected in ovo, v-src was detected to be both expressed and active despite the lack of sarcoma formation (19). Given that the germ-line expression of activated erbB2 occurs in the embryonic state and that conditional activation would result in excision and activation of activated erbB2 in the postnatal mouse, there are striking similarities in these results. Further support for this view stems from observations with patients carrying germ-line activated fibroblast growth factor receptor mutations. Although these patients exhibit a variety of developmental defects, they do not show a greater predisposition to develop cancer (20). These observations strongly argue that the developmental window of expression of an activated oncogene can profoundly affect its oncogenic potential.
Another possible mechanism by which embryonic expression of erbB2 may influence tumor development is by indirectly affecting the epithelial target population for erbB2-mediated transformation. Comparison of gene expression profiles of virgin mammary glands between the mammary-specific and germ-line-activated ErbB2WT/NT revealed substantial differences in the expression of a number of genes. In particular, the expression of a number of luminal epithelial markers such WMND1 and κ casein is dramatically down-regulated in the germ-line strains (Table 1 and Fig. 5). Significantly, elevated expression of these markers is frequently observed in Ras- and ErbB2-initiated tumors and during luminal differentiation (17), suggesting that there is a deficiency in the luminal cell type that is targeted for transformation by activated erbB2 in the ErbB2WT/NT strain. Consistent with these observations, the ErbB2WT/NT mice fail to exhibit the precocious lobular hyperplasias characteristic of the conditional activated erbB2 strain (Fig. 3). These data suggest that germ-line expression of activated erbB2 may have altered the normal course of mammary epithelial differentiation. The future elucidation of the precise mechanism by which germ-line expression of erbB2 confers resistance to tumor induction will provide important insight into the molecular mechanism of erbB2-induced tumorigenesis. Interestingly, ovexpression of the ErbB2 by various keratin promoters in transgenic mice has resulted in developmental abnormalities in both the skin and hair follicles (21), skin hyperplasia (22), and squamous cell carcinomas (23). The lack of tumors in the mammary gland, skin, or any of the other tissues that would normally express ErbB2 suggests there is a general resistance in these mice to ErbB2-mediated tumorigenesis. In the future, modulation of this pathway would have clear benefits for treatment of ErbB2 positive tumors.
Acknowledgments
We appreciate the technical support of Carrie Merola-Weir and Monica Graham. This work was supported by the Canadian Institutes of Health Research and Canadian Breast Cancer Research Alliance. E.R.A. was supported by a U.S. Department of Defense Breast Cancer Predoctoral Scholarship during this research (DAMD17-99-1-9285).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: dpc, days post coitum.
References
- 1.Slamon, D. J., Clark, G. M., Wong, S. G., Levin, W. J., Ullrich, A. & McGuire, W. L. (1987) Science 235, 177-182. [DOI] [PubMed] [Google Scholar]
- 2.Slamon, D. J., Godolphin, W., Jones, L. A., Holt, J. A., Wong, S. G., Keith, D. E., Levin, W. J., Stuart, S. G., Udove, J., Ullrich, A. & Press, M. F. (1989) Science 244, 707-712. [DOI] [PubMed] [Google Scholar]
- 3.Dankort, D. L. & Muller, W. J. (2000) Oncogene 19, 1038-1044. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard, L., Lamarre, L., Tremblay, P. J. & Jolicoeur, P. (1989) Cell 57, 931-936. [DOI] [PubMed] [Google Scholar]
- 5.Guy, C. T., Cardiff, R. D. & Muller, W. J. (1996) J. Biol. Chem. 271, 7673-7678. [DOI] [PubMed] [Google Scholar]
- 6.Muller, W. J., Sinn, E., Pattengale, P. K., Wallace, R. & Leder, P. (1988) Cell 54, 105-115. [DOI] [PubMed] [Google Scholar]
- 7.Lemoine, N. R., Staddon, S., Dickson, C., Barnes, D. M. & Gullick, W. J. (1990) Oncogene 5, 237-239. [PubMed] [Google Scholar]
- 8.Guy, C. T., Webster, M. A., Schaller, M., Parsons, T. J., Cardiff, R. D. & Muller, W. J. (1992) Proc. Natl. Acad. Sci. USA 89, 10578-10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel, P. M., Dankort, D. L., Hardy, W. R. & Muller, W. J. (1994) Mol. Cell. Biol. 14, 7068-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel, P. M. & Muller, W. J. (1996) Proc. Natl. Acad. Sci. USA 93, 8878-8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel, P. M., Ryan, E. D., Cardiff, R. D. & Muller, W. J. (1999) EMBO J. 18, 2149-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrechek, E. R., Hardy, W. R., Siegel, P. M., Rudnicki, M. A., Cardiff, R. D. & Muller, W. J. (2000) Proc. Natl. Acad. Sci. USA 97, 3444-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan, R., Hardy, W. R., Laing, M. A., Hardy, S. E. & Muller, W. J. (2002) Mol. Cell. Biol. 22, 1073-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araki, K., Araki, M., Miyazaki, J. & Vassalli, P. (1995) Proc. Natl. Acad. Sci. USA 92, 160-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, K. F., Simon, H., Chen, H., Bates, B., Hung, M. C. & Hauser, C. (1995) Nature 378, 394-398. [DOI] [PubMed] [Google Scholar]
- 16.Andrechek, E. R., Laing, M. A., Girgis-Gabardo, A. A., Siegel, P. M., Cardiff, R. D. & Muller, W. J. (2003) Cancer Res. 63, 4920-4926. [PubMed] [Google Scholar]
- 17.Morrison, B. W. & Leder, P. (1994) Oncogene 9, 3417-3426. [PubMed] [Google Scholar]
- 18.Dolberg, D. S. & Bissell, M. J. (1984) Nature 309, 552-556. [DOI] [PubMed] [Google Scholar]
- 19.Howlett, A. R., Carter, V. C., Martin, G. S. & Bissell, M. J. (1988) Proc. Natl. Acad. Sci. USA 85, 7587-7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Moerlooze, L. & Dickson, C. (1997) Curr. Opin. Genet. Dev. 7, 378-385. [DOI] [PubMed] [Google Scholar]
- 21.Xie, W., Wu, X., Chow, L. T., Chin, E., Paterson, A. J. & Kudlow, J. E. (1998) Cell Growth Differ. 9, 313-325. [PubMed] [Google Scholar]
- 22.Xie, W., Chow, L. T., Paterson, A. J., Chin, E. & Kudlow, J. E. (1999) Oncogene 18, 3593-3607. [DOI] [PubMed] [Google Scholar]
- 23.Kiguchi, K., Bol, D., Carbajal, S., Beltran, L., Moats, S., Chan, K., Jorcano, J. & DiGiovanni, J. (2000) Oncogene 19, 4243-4254. [DOI] [PubMed] [Google Scholar]
- 24.Bera, T. K., Lee, S., Salvatore, G., Lee, B. & Pastan, I. (2001) Mol. Med. 7, 509-516. [PMC free article] [PubMed] [Google Scholar]