Abstract
Context
Traumatic experiences in early childhood are associated with increased risk for developing stress-related disorders, which are linked to structural brain abnormalities. However, it is unclear if these volumetric brain changes are present before disease onset or reflect the consequences of the disease progression.
Objective
To identify structural abnormalities in the nonhuman primate brain that may predict increased risk for stress-related neuropsychiatric disorders in humans.
Design
Rhesus monkeys were divided in two groups at birth: a group raised with their mothers and other juvenile and adult animals (mother-reared, MR), and a group raised with three age-matched monkeys only (peer-reared, PR). Anatomical brain images were acquired in juvenile male and female rhesus monkeys using magnetic resonance imaging.
Main Outcome Measures
Volumetric measures of the anterior cingulate cortex, medial prefrontal cortex, hippocampus, corpus callosum and cerebellar vermis were compared between MR (n=15) and PR animals (n=13).
Results
Compared to MR monkeys, we found an enlarged vermis, dorsomedial prefrontal and dorsal anterior cingulate cortex in PR monkeys but no differences in the corpus callosum and hippocampus.
Conclusions
Peer-rearing during infancy induces enlargements in stress-sensitive brain regions. These changes may be a structural phenotype for an increased risk to stress-related neuropsychiatric disorders in humans.
Keywords: stress, development, anterior cingulate cortex, medial prefrontal cortex, cerebellar vermis, nonhuman primate
Traumatic experiences during early childhood have been consistently associated with increased risk for developing stress-related neuropsychiatric disorders later in life, including depression, anxiety, and substance abuse disorders1, 2. Brain imaging studies have reported morphological changes in healthy adults exposed to early life stress3, children exposed to abuse4, and in children and adults with maltreatment-related posttraumatic stress disorders (PTSD)5, 6. These findings suggest that regional brain abnormalities may be present before the onset of the disease, and thus represent a risk factor for subsequent stress-related neuropsychiatric disorders. However, retrospective clinical studies can be difficult to interpret because of the variety of events that can be considered as traumatic experiences, the timing and intensity differences to which individuals were exposed to the stressors, and the unknown effects of medications. Moreover, the long-term effects of stress exposure may be delayed or expressed only when the vulnerable brain system reaches maturation7. It is therefore important to clearly define and control for the type and duration of the stressor, the developmental stage during stress exposure, and the developmental time point at the time of brain measurement to clarify whether the reported brain abnormalities were present before the illness onset or reflected the consequences of the disease progression.
Evidence from human and preclinical studies indicates that the hippocampus (HC), the cerebellum (CB), prefrontal cortex (PFC) and the corpus callosum (CC) are particularly susceptible to stress2. These regions have a long period of postnatal development and/or relative high levels of glucocorticoid receptors (GRs) and, in the case of the HC, some degree of postnatal neurogenesis, which may contribute to their stress vulnerability2, 8, 9.
Not only are the above brain regions vulnerable to stress, together with many of their prominent connections, including the anterior cingulate (ACC), they have been implicated in emotional regulation and stress reactivity10-12, suggesting a risk factor for stress-related neuropsychiatric disorders later in life. In support of this conjecture, decreased volumes of both rostral and dorsal ACC have been found in adults exposed to early life stress3, 13, while reduced HC volume has been demonstrated in adults exposed to chronic stress14. Further, CB-vermis (CB-V) abnormalities have been reported in adults abused during childhood15, while smaller CC is seen in neglected children4, 16.
Preclinical and human studies have shown that early life stress induces both acute and long-term dysregulations of the hypothalamic-pituitary-adrenal (HPA) axis17 and the serotonin (5-HT) system18. Dysfunctions of both systems have been widely implicated in mood and anxiety disorders19, and the two systems are known to have reciprocal influences20. Together these data suggest that abnormal brain development following early life stress may, at least in part, be related to a dysregulation of the HPA axis and 5-HT system.
The goal of the present study was to determine if exposure to an adverse environment during infancy leads to long-term morphological changes in vulnerable brain structures in nonhuman primates. Comparative studies have shown a high degree of homology between the human and nonhuman primate brain, especially in prefrontal cortical regions21. Further, compared to rodents, primate PFC and CB display higher levels of GRs22, highlighting the importance of using nonhuman primates reared in a well controlled environment to model the consequences of early life stress on human brain development.
To this aim, we acquired anatomical brain images in juvenile rhesus monkeys that were either reared with their mothers and social group (mother-reared, MR), or with three infants of about the same age (peer-reared, PR). Peer-rearing is an established model of early-life adversity that has been shown to alter the HPA axis and the 5-HT system, and to induce high levels of anxiety throughout development into adulthood23-29.
We hypothesized that stress sensitive brain regions including the medial PFC (mPFC, which includes the rostral ACC), dACC, HC, CB-V and CC will be smaller in juvenile PR compared to MR monkeys. To control for the specificity of any changes seen in prefrontal regions, the most posterior part of the cingulate cortex (PCC) and the entire prefrontal lobe (PFL) were also measured. Finally, because peer-rearing has been shown to induce long-term changes in the HPA axis and 5-HT system25, 27, 28, we hypothesized that baseline activity of the HPA axis and 5-HT system at the time of the study would be related to regional structural changes. As such, plasma levels of cortisol and cerebrospinal fluid (CSF) levels of the main 5-HT metabolite (5-hydroxyindole-aceticacid, 5-HIAA) were determined.
METHODS
SUBJECTS
Twenty eight rhesus monkeys (Macaca mulatta) between 23 and 32 months of age were used for the study. The rhesus monkeys (representing two birth cohorts) were born and housed at the National Institutes of Health (NIH) Animal Center in Poolesville, MD. Subjects were randomly divided at birth into two groups, which resulted in different social and rearing experiences early in life. MR monkeys (n = 15; 7 males, 8 females) were reared for the first six months of life with their biological or cross-fostered mothers (n = 4) and fathers in social groups comprised of eight to twelve adult females (about half of whom had same-aged infants) and two adult males. PR monkeys (n = 13; 7 females, 6 males) were separated from their mothers and housed in an incubator for the first 14 days of life. From day 14 until day 37, they were placed alone in a nursery cage and provided a blanket and a terry cloth–covered, rocking surrogate. At 37 days of age, they were placed in a cage with three other age-mates with whom they had continuous access. Peer-rearing condition deprives animals of parental input and the opportunity to learn appropriate social behaviors and context during early development and is considered a model for early-life stress (both rearing conditions have been described in detail elsewhere30). After six months, PR and MR monkeys were raised together in a big social group that included adult, juvenile and infant monkeys until the time of the study. At the time of the study, the rhesus monkeys were between 2 and 2.5 years old, an age range considered to correspond to 6 to 8 years of age in children31. The animals were transported in groups of four to the National Institute on Drug Abuse (NIDA, Baltimore), where they were paired-housed for about one month during which the MRI data were acquired. Paired-housing allowed social interactions with familiar animals and thus, limited the stress to the new environment.
Protocols were approved by the institutional Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism, NIDA and the National Institute of Child Health and Human Development, NIH.
NEUROCHEMICAL SAMPLING AND ANALYSES
One week before the shipment, CSF and blood samples were collected from each monkey. Blood samples (2 ml) were drawn from the femoral vein, and CSF samples (2 ml) were removed via cisternal puncture, both under ketamine anesthesia (10 mg/kg, IM). CSF samples data from two females (one MR and one PR) were not analyzed due to contamination. All samples were collected between 11:30 am and 14:30 pm, within 15 min of investigator’s entrance into the housing facility for capture and sampling. Cisternal CSF samples were immediately aliquoted into polypropylene tubes and frozen in liquid nitrogen. Blood samples were placed on wet ice and centrifuged at 4°C for 20 min. Next, the plasma was aliquoted and frozen in liquid nitrogen. CSF and plasma samples were stored at -80°C until assay. The plasma cortisol level was assessed using a coat-a-count radioimmunoassay kit from Siemens Diagnostics (tkco5). Simultaneous determination of 5-HIAA in CSF was performed with high-performance liquid chromatography using electrochemical detection, as previously described in24.
BRAIN IMAGE ACQUISITION AND ANALYSES
Each monkey was initially anesthetized with ketamine (10 mg/kg, IM). Anesthesia was maintained throughout the study with continuous infusion of propofol (30-50 mg/kg/h, IV). An individually molded thermoplastic face mask was secured to a custom-made monkey head-holder, reducing head movements during scans. Vital signs were monitored continuously during the study.
Images were acquired on a 3.0 Tesla Siemens Allegra (Siemens Medical Solutions, Inc., Malvern, Pa, U.S.A.). The parameters for the 3D T1 MPRAGE acquisition are as follows: TR/TE/TI: 2500/3.49/1000ms, 1 slab of 224 slices: 0.60mm thickness, 0.30mm spacing, flip angle 8°, and matrix 256x256. The acquisition was run at 4NEX using Nova DR dual surface coils. The 3D slab was placed over the entire brain, centered and angled on the anterior - posterior commissures (AC/PC) line.
Image processing was preformed with ANALYZE 7.5 (Biomedical Imaging, Mayo Foundation). For manual tracings of the regions of interest (ROIs), T1-weighted images were converted to cubic voxel dimensions of 0.391 mm. All scans were then oriented into a standardized oblique plane to eliminate any bias in slice angle. In the standardized orientation, the transaxial plane was parallel to the AC/PC line and perpendicular to the interhemispheric fissure.
ANATOMICAL SUBDIVISIONS
ROIs were defined using the atlas of Saleem and Logothetis32 and were measured by one rater blind to subject gender and rearing condition; a second independent rater performed identical measurements to establish reliability and accuracy of the measurement, which was calculated for every ROI on each brain side. The minimum value for inter- and intra-rater reliability, calculated as intra-class correlation coefficients, was between 0.89 and 0.97. Manual tracing for the PFC, cingulate cortex (CingC), and HC were performed in the coronal plane, edited in the sagittal and/or the axial planes and then re-edited in the coronal view; the CC and CB-V were drawn using the mid-sagittal view and the intracranial volumes (ICV) were traced on the axial view.
ICVs were calculated by tracing each axial slice excluding the skull and the dura. A semi-automated, threshold-based, region–growing algorithm was used to outline the brain in each axial slice. ICV was defined and subsequently measured as all gray and white matter tissue and CSF volumes in both hemispheres, including part of the midbrain. The inferior border of the pons was chosen for demarcation because it is readily and reliably identifiable on monkey brain images33.
ROI DEFINITIONS
The CingC ROI (Figure 1a, b) comprised the entire CingC above the CC. The coronal slice containing the initial appearance of the genu of the CC was selected as the anterior boundary, while the posterior boundary was the last coronal slice containing the most posterior part of the splenium of CC. The CingC was divided further into two subregions, the dACC and the posterior part of CingC (PCC). The posterior border of the dACC was defined on the coronal view as the slice previous to that where the arcuate hypothalamic nucleus was apparent. The subsequent slice marked the anterior border of the PCC (Figure 3a, vertical line).
Figure 1.
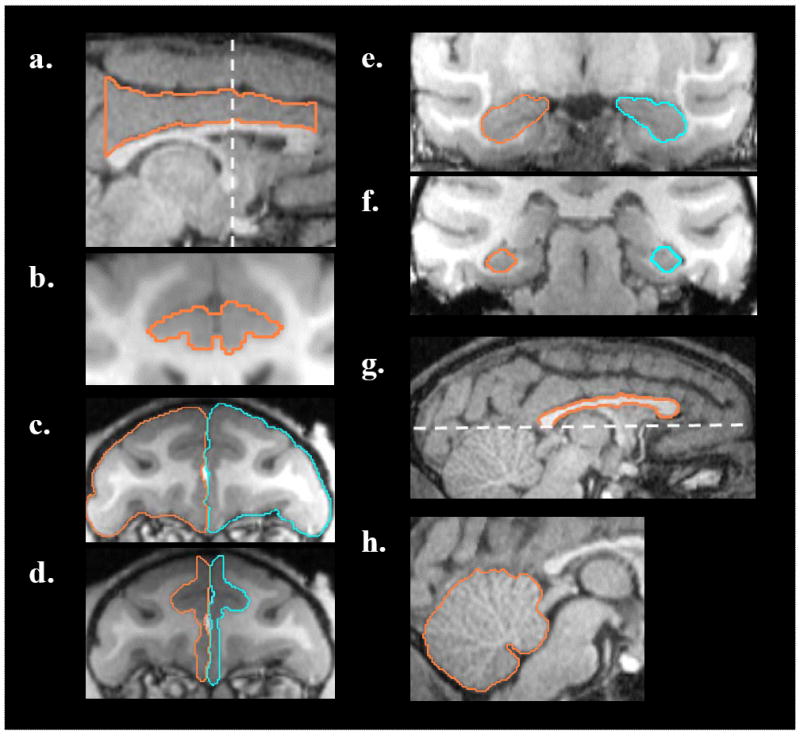
Representative T1-weighted magnetic resonance images of two-year old rhesus monkeys to illustrate the ROI boundaries use to trace regional volumes. CingC, (a) sagittal and (b) coronal views, respectively. The vertical dashed line on the sagittal view indicates the division of the CingC into dACC and PCC. PFC(c) and mPFC (d), coronal view. Dashed line on (g) indicates the ventral boundary for the DmPFC. Anterior (e) and posterior (f) slices of HC, coronal view. CC (g) and CB-V (h), sagittal view. Right site – orange tracing line; left site - blue tracing line.
The PFL volume was traced on coronal slices (Figure 1c) in each hemisphere separately and included all gray and white matter tissue up to the most anterior coronal section containing grey matter. The posterior boundary was the coronal slice before the initial appearance of the genu of CC34. The mPFC (Figure 1d) in each hemisphere was defined as the grey matter located along the medial wall of the PFL, with the posterior boundary based on the PFL tracing. Due to the difficulty in distinguishing the boundaries between grey and white matter in the most anterior slices, the anterior boundary was defined in the coronal view as the tenth slice posterior to the first coronal slice containing only grey matter. In one animal, the mPFC could not be traced due to the image artifacts. In addition, to better characterize the mPFC subdivision that included the rACC, we excluded the most ventral part from the measurement and analyzed the more dorsal part (DmPFC)34. Therefore, the mPFC was divided further into dorsal and ventral subdivisions by tracing a horizontal line parallel to the edge of the splenium of the CC on the coronal and sagittal view (Figure 1g). This line marked the first axial slice (without the appearance of splenium) to be included in the ventral subdivision of mPFC. The PCC and PFL volumes were measured to be able to control for the specificity of our results in the mPFC and dACC.
The HC (Figure 1e, f) on each brain side were measured on coronal sections in an anterior-posterior direction. The most anterior coronal slice used for the analyses was defined on the sagittal slice where the HC length was maximal and the rostral end of the HC was present at the most anterior point. The most posterior slice was identified as a slice in which the HC first appeared adjacent to the trigone of the lateral ventricle33. Two 0.6 mm slices anterior to this location were excluded from the determination of the HC volume due to the lack of reliable boundaries for distinguishing the amygdala from the HC. Volume measurements in our study were performed using a region drawing technique that relies on gray/white matter segmentation. Compared to other methods, such as Cavalieri’s method, which still requires an identification of landmarks clearly visible on a T1-weighted MR image, the region drawing technique used is more time consuming. A stereological approach eliminates the need to draw regions on each MR image section and is free from mathematical bias. Nevertheless, it has been shown that manual segmentation is a highly reliable and reproducible method for volumetric measurements. Moreover, comparison between the results obtained by both methods reveal that both approaches yield high repeatability and precision35.
For the CC analysis (Figure 1g), the midsagittal area of the CC was measured in its entirety, and based on its length, was divided further into three equal subdivisions: the anterior CC (including the genu, rostrum and the rostral body), the middle CC (including the anterior and posterior midbody) and the posterior CC (including the isthmus and splenium)36. Since the CC was often difficult to define using the midsagittal slice, the adjacent slice in the right hemisphere was used. The subregion analyses was conducted based on previous findings in maltreatment-related PTSD children5 and rhesus monkeys reared in isolation37 showing that the middle and/or posterior part of the CC was more affected.
Finally, the CB-V (Figure 1h) was drawn from the midsagittal slice38. When the ROI was defined as an area rather then a volume, we normalized the measured area by dividing the square root of the area by the cubic root of the ICV to bring all measures to the same geometric dimensionality36.
DATA ANALYSES
StatView 5.0.1 (SAS Institute, Inc., Cary, NC) was used for all statistical analyses. Kolmogorov-Smirnov Normality test was used to insure the normal distribution of each measurement. To assess the effects of rearing condition and gender on each ROI, a two-way Analysis of Variance (ANOVA) was conducted with rearing condition (MR or PR) and gender (male or female) as independent variables. When ROIs were measured in both hemispheres, repeated measures ANOVA with hemisphere as within subject variable was used. Pearson correlation (R) was applied to investigate the relationship between ROI volumes and baseline levels of 5-HIAA and cortisol. Effects of rearing condition and gender on baseline levels of 5-HIAA and cortisol, in addition to ICV, weight and age were assessed using two-way ANOVA. All data are presented as mean ± standard error of the mean (SEM), with significance testing set as two-tailed analysis with α=0.05. Correction for multiple comparisons was not applied, but p values for each statistical analysis are provided to the reader.
RESULTS
PHYSIOLOGICAL CHARACTERISTICS
We found no gender or rearing differences in age (p>0.17, p>0.35, respectively), weight (p>0.29, p>0.33), baseline plasma cortisol (p>0.42, p>0.51) or CSF 5-HIAA levels (p>0.62, p>0.23) (Table 1, Figure 2).
Table 1.
Physiological data in MR and PR monkeys.
| MR monkeys | PR monkeys | Statistical analyses | |
|---|---|---|---|
| Age (months) | 27.40 ± 0.90 | 26.39 ± 0.33 | F1,24 = 0.89, n.s. |
| Weight (kg) | 3.61 ± 0.13 | 3.44 ± 0.09 | F1,24 = 0.96, n.s. |
| 5-HIAA (pmol/ml) | 268.18 ± 16.18 | 241.28 ± 13.85 | F1,24 = 1.51, n.s. |
| Cortisol (ug/dl) | 33.02 ± 1.22 | 34.62 ± 1.80 | F1,24 = 0.43, n.s. |
Results are presented as mean ± S.E.M., n.s.: not significant.
Figure 2.
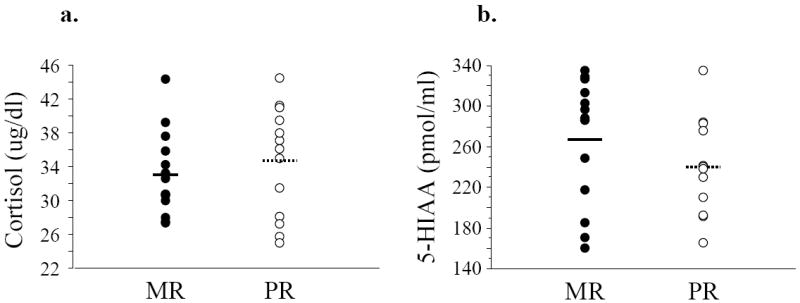
Cortisol (a) and 5-HIAA (b) scatter plots of individual subjects in the MR and PR group, with means plotted as horizontal lines.
BRAIN ANATOMICAL MEASURES
Peer-rearing did not affect total ICV (p>0.49; Table 2), although as expected, male rhesus monkeys had bigger brain volumes (97097.75 ± 1828.03 mm3) compared to females (87190.06 ± 1510.26mm3) F1,24=17.76, p<0.001. Due to this gender difference in ICV, regional volume data were normalized to individual ICV.
Table 2.
Normalized and absolute volumes of brain areas measured in MR and PR groups.
| Brain areas | Normalized volume (#) | Absolute volume (mm3) | Absolute volume Difference (%) | Statistical analyses for normalized values | ||
|---|---|---|---|---|---|---|
| MR monkeys | PR monkeys | MR monkeys | PR monkeys | |||
| ICV | 91,121.10 ± 1,904.22 | 92,561.94 ± 2,413.57 | 1.61 | |||
| dACC | 5.29 ± 0.13 | 5.74 ± 0.17 | 481.98 ± 15.84 | 531.71 ± 21.87 | 10.32 | F1,24 = 4.46, p<0.05 |
| PCC | 10.81 ± 0.27 | 10.93 ± 0.22 | 984.99 ± 32.91 | 1,011.61 ± 34.23 | 2.70 | F1,24 = 0.07, n.s |
| Right mPFC | 7.59 ± 0.14 | 7.99 ± 0.23 | 692.13 ± 19.16 | 741.37 ± 23.37 | 7.11 | F1,23 = 2.20, n.s |
| Left mPFC | 7.53 ± 0.14 | 7.90 ± 0.23 | 685.99 ± 18.90 | 734.04 ± 23.87 | 7.00 | F1,23 = 1.87, n.s. |
| Right DmPFC | 6.12 ± 0.13 | 6.64 ± 0.18 | 557.97 ± 16.43 | 616.35 ± 16.78 | 10.46 | F1,23 = 6.37, p<0.05 |
| Left DmPFC | 6.15 ± 0.12 | 6.67 ± 0.16 | 560.57 ± 15.4 | 618.74 ± 16.93 | 10.38 | F1,23 = 6.88, p<0.05 |
| Right PFL | 35.31 ± 0.50 | 36.13 ± 0.91 | 3,218.09 ± 80.31 | 3,336.38 ± 100.03 | 3.68 | F1,24 = 0.52, n.s. |
| Left PFL | 36.42 ± 0.50 | 36.68 ± 0.81 | 3,317.83 ± 79.04 | 3,393.01 ± 109.48 | 2.27 | F1,24 = 0.08, n.s. |
| Right HC | 5.31 ± 0.11 | 5.18 ± 0.12 | 481.66 ± 8.45 | 479.28 ± 16.06 | -0.49 | F1,24 = 0.48, n.s. |
| Left HC | 5.16 ± 0.10 | 5.04 ± 0.12 | 467.77 ± 6.90 | 465.82 ± 13.44 | -0.42 | F1,24 = 0.52, n.s. |
| CC | 125.21 ± 1.20 | 125.28 ± 1.81 | 31.79 ± 0.82 | 32.21 ± 1.15 | 1.32 | F1,24 = 0.03, n.s. |
| CB-V | 254.66 ± 2.09 | 264.43 ± 1.56 | 131.99 ± 2.31 | 142.93 ± 2.66 | 8.29 | F1,24 = 14.33, p<0.01 |
ICV: intracranial volume, dACC: dorsal anterior cingulate cortex, PCC: posterior cingulate cortex, mPFC: medial prefrontal cortex, DmPFC: dorsomedial prefrontal cortex, PFL: prefrontal lobe, HC: hippocampus, CC: corpus callosum, CB-V: cerebellar vermis.
Results are presented as mean ± S.E.M.; n.s.: not significant.
Normalized values were calculated as ROI volume/ICV. CB-V and CC were defined as areas rather than volumes, therefore data were normalized by dividing the square root of the area by the cubic root of the ICV to bring all measures to the same geometric dimensionality36. All normalized values were multiplied by 1000.
Cingulate Cortex
PR monkeys had larger dACC volumes compared to MR monkeys F1,24=4.46, p<0.05; Table 2. As expected, there was no effect of rearing on PCC (p>0.79; Table 2), nor were there any gender effects (dACC: p>0.26; PCC: p>0.72).
Prefrontal Cortex
The mPFC analyses showed a rearing x gender x hemisphere interaction F1,23=4.91, p<0.04, but separate analyses for each hemisphere did not demonstrate any significant effect of rearing (right: p>0.15, left: p>0.19) or gender (right: p>0.88, left p>0.96). Further analyses of the dorsal subdivision of the mPFC showed greater DmPFC size in PR monkeys compared to MR monkeys, F1,23=6.92, p<0.02, (Table 2). There was also a rearing x gender by hemisphere interaction F1,23=5.12, p<0.04, therefore separate analyses for each hemisphere were conducted. In both the right F1,23=6.37, p<0.02 and left F1,23=6.88, p<0.02 hemisphere, PR monkeys showed greater DmPFC, although there was no effect of gender (right: p>0.20; left; p>0.22). Analyses of the PFL showed a smaller size of this region in right hemisphere compared with the left F1,24=11.41, p<0.003 (Table 2). There was also a rearing x gender x hemisphere interaction F1,24=7.24, p<0.02, but separate analyses for each hemisphere revealed no effect of rearing (right: p>0.47, left: p>0.77) or gender (right: p>0.98, left: p>0.68).
Hippocampus
Rearing condition and gender did not influence HC volume (p>0.47; p>0.57, respectively, Table 2), although the right HC was larger than the left F1,24=15.41, p<0.001.
Cerebellar vermis
PR monkeys showed a larger CB-V area than MR monkeys F1,24=14.33, p<0.001 (Table 2), but there was no effect of gender in this area (p>0.11).
Corpus Callosum
There was a significant main effect of sub-regions F2,48=179.82, p<0.001 and gender F1,24 =4.87, p<0.04 on CC volume. Separate analyses for each sub-regions showed larger middle F1,24 =5.28, p<0.04 and posterior F1,24 =9.37, p<0.01 CC in females compared to males, but no difference in the anterior CC (p>0.53). The total CC area and studied CC sub-regions were not affected by rearing condition (p>0.87, Table 2).
Correlation with baseline plasma cortisol and CSF 5-HIAA levels
We found a significant correlation between the right DmPFC volume and 5-HIAA levels R=0.4, p<0.05 (Table 3). However, correlation between the left DmPFC volume and 5-HIAA levels did not reached significance (p>0.06). None of the other volumes measured correlated with baseline cortisol or 5-HIAA levels.
Table 3.
Correlation between normalized volumes and baseline 5-HIAA and cortisol levels.
| Brain areas | 5-HIAA (pmol/ml) | Cortisol (ug/dl) |
|---|---|---|
| dACC | R= 0.16, n.s. | R= 0.18, n.s. |
| Right DmPFC | R= 0.40, p<0.05 | R= 0.10, n.s. |
| Left DmPFC | R= 0.37, n.s. | R= 0.12, n.s. |
| CB-V | R= 0.01, n.s. | R= 0.25, n.s. |
n.s.: not significant.
COMMENTS
Our data demonstrate that peer-rearing, a well-established model of early life stress in nonhuman primates has long-term consequences on morphological brain development. The fact that the data were collected several months following cessation of the stress exposure, when the monkeys were still juvenile, allowed us to investigate a) the long-term consequences of stress on brain morphology and b) brain abnormalities that are present independent of the influence of hormonal changes that occur during and following adolescence. Among two-year old rhesus monkeys exposed to such an adverse environment during infancy, we observed an increase in size in the dorsal part of the mPFC, the dACC and the CB-V, but no difference in the CC and HC volumes. There were also no changes in the PCC, PFL or ICV size, suggesting that the reported changes were not the consequence of a generalized abnormal brain development but specific effects on brain regions particularly vulnerable to early life stress exposure.
The main finding of the present study is an increased DmPFC and dACC volumes in PR monkeys compared to control animals. Since the DmPFC measure included the rostral part of anterior cingulate gyrus, these data suggest that the entire ACC might have been sensitive to early-life stress exposure. In support to the specificity of this result, we found no differences in the PCC.
Previous findings in humans report reduced rACC volume in healthy subjects exposed to early life stress3, and reduced dACC in adults with abuse-related PTSD13. However, recent data demonstrate an increased volume of the middle inferior PFC grey matter (including the rACC) in pediatric PTSD34, support our findings in nonhuman primates and suggest that developmental differences may be important.
On a cellular level, the abnormal ACC development could be mediated by several processes, including altered neuronal organization, increased number/size of neurons or synapses, or non-neuronal changes involving glia or both. Molecular changes in the ACC have been found in children and adolescent with maltreatment-related PTSD39, in adults PTSD40 and in monkey reared under variable foraging demand during infancy41. These MR spectroscopy studies report a decreased N-acetyl-aspartate (NAA)/creatine (Cr) ratio, suggesting a neuronal/axonal loss. However, increased choline/Cr and myo-inositol (MI)/Cr ratios in the ACC have also been found in PTSD42. Because MI is considered a marker for glial cell, the authors suggest that in PTSD alterations in glial cells occur and may precede NAA decline. Even though structural MRI studies are unable to address specific mechanisms that underlie brain volume increases, these findings suggest that glial cell abnormalities might play an important role in the increased ACC volume reported herein.
Numerous human studies have reported diminished HC volume compared to healthy controls in patients affected by mood and anxiety disorders, and antidepressant treatments have been shown to increase adult hippocampal neurogenesis8. In the present study, we found no HC differences as a function of early-life rearing history.
However, findings from rodent studies revealed that early life stress may not have an immediate effect on the HC but induces changes over time that become manifest only in later phases of development43. Consistent with the delayed effects of stress on HC volume, decreased HC volume has been observed in adults and not in children5, 6. Moreover, no changes in the HC were reported in eighteen-month old male rhesus monkeys raised in isolation for the first year of life37. Thus, it is possible that anatomical effects not evident in our two-year old cohort, may be detected at a later stage in development.
Another brain area that was proposed to be sensitive to the effects of early life stress exposure was the CB-V. Similar to the changes reported in the DmPFC and dACC, an increase in CB-V area was seen in PR monkeys compared to controls. It should be mentioned that like the HC, CB-V has a protracted period of postnatal ontogeny and high density of GRs and may therefore be particularly vulnerable to the effects of stress hormones2, 22. Imaging studies have described vermal abnormalities in resting blood flow in adults exposed to abuse during childhood15 and the role of the CB-V in emotional regulation and anxiety is supported by evidence from case studies of various cerebellar pathologies12. Our findings, in combination with the data described above, supports the hypothesis that early life stress exerts deleterious effects on the development of the CB-V, suggesting that the CB in general may play an important role in mood and anxiety disorders.
We found no difference between PR and MR animals in any of the CC measures. In contrast, previous findings showed a reduced CC size in eighteen-month old male rhesus monkeys reared in isolation for the first year of life37. It is possible that CC changes were present six months earlier in development, but that they were not detectable in two-year old rhesus monkeys. Another explanation could be that a longer period of stress exposure is necessary to induce these changes. However, it should be pointed out that white matter measures in both studies were restricted to the CC and thus provide very limited information on changes in connectivity between and within hemispheres. Since cognitive and emotional regulation relies on interactions between and within multiple brain networks rather than activity within single brain regions, further investigations using diffusion tensor imaging and functional connectivity analyses may provide a better understanding of how early life stress influences the neural circuits of anxiety and stress.
Several maturational processes, including synaptogenesis, synaptic pruning, myelination and in some regions neurogenesis contribute to postnatal brain development. By influencing the levels of neurohormones, neurotransmitters and neurotrophic factors, chronic stress is considered to lead to adverse brain development1. Although peer-rearing did not affect baseline levels of 5-HIAA and cortisol in juvenile monkeys in our study, it is possible that more subtle changes occurred in specific brain regions. In support to this hypothesis, we found a correlation between 5-HIAA levels and the right DmPFC volume, suggesting a relationship between 5-HT levels and DmPFC development. However, since 5-HIAA level in the CSF reflects the net result of several processes in the brain including 5-HT synthesis, release and reuptake, and does not provide information regarding specific regional changes, future studies will be important to clarify this finding.
There are several limitations in this study. It is likely that the limited number of animals available led to a reduced statistical power to investigate rearing by gender interactions or correlations with physiological measures (e.g. the correlation between left DmPFC size and 5-HIAA did not reach the level of statistical significance). In addition, we did not correct for multiple comparisons, therefore current findings should be considered as preliminary, with future studies necessary to confirm these results. Future investigations should also include behavioral measures of stress reactivity and/or anxiety, which would add important information on the functional consequences of the observed structural changes.
In summary, evidence is presented that peer-rearing, a model of early-life stress exposure in nonhuman primates, has long-term consequences on brain development. Increased volume of DmPFC, dACC and CB-V were identified in juvenile rhesus monkeys, suggesting that these brain regions may be particularly sensitive to exposure to an adverse environment during infancy. Taken together, our data suggest that enlarged DmPFC, dACC and CB-V may be a structural phenotype during childhood for an increased risk of developing stress-related neuropsychiatric disorders in humans.
Acknowledgments
We thank Eric Singley, Stephen Lindell, and Melanie Schwandt for physiological data collection and analyses, Eliscia Smith for assisting during MR scans, and Dan Stein for insightful comments on an earlier draft of the manuscript.
Funding/Support: This work was supported by the Intramural Research Programs of the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and National Institute of Child Health and Human Development.
Footnotes
Author contributions
S.S. planned and conducted the study, analyzed the data, prepared the figures and wrote the manuscript in consultation with E.S. and S.C. S.C. conducted the study and analyzed the MRI data. S.J.S. provided access to the nonhuman primates and shared with JDH the oversight and design of the subjects’ early rearing experiences. JDH also provided input concerning the design and oversight of the physiological data collection. C.S.B. provided access to physiological data and their analyses. All authors reviewed and edited the manuscript. E.S. supervised the project.
Financial Disclosure: None reported.
Contributor Information
Svetlana Chefer, Email: SCHEFER@intra.nida.nih.gov.
Stephen J. Suomi, Email: suomis@lce.nichd.nih.gov.
J. Dee Higley, Email: dee_higley@byu.edu.
Christina S. Barr, Email: cbarr@mail.nih.gov.
Elliot Stein, Email: EStein@intra.nida.nih.gov.
References
- 1.De Bellis MD. The psychobiology of neglect. Child Maltreat. 2005;10(2):150–172. doi: 10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- 2.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27(1-2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 3.Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, Niaura R, Clark CR, McFarlane A, Bryant R, Gordon E, Williams LM. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59(10):975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56(2):80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 5.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52(11):1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 6.Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: A meta-analysis. Hippocampus. 2008 doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 8.Banasr M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol Disord Drug Targets. 2007;6(5):311–320. doi: 10.2174/187152707783220929. [DOI] [PubMed] [Google Scholar]
- 9.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 11.Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann N Y Acad Sci. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- 12.Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum - insights from the clinic. Cerebellum. 2007;6(3):254–267. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- 13.Kitayama N, Quinn S, Bremner JD. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J Affect Disord. 2006;90(2-3):171–174. doi: 10.1016/j.jad.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35(2):795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson CM, Teicher MH, Polcari A, Renshaw PF. Abnormal T2 relaxation time in the cerebellar vermis of adults sexually abused in childhood: potential role of the vermis in stress-enhanced risk for drug abuse. Psychoneuroendocrinology. 2002;27(1-2):231–244. doi: 10.1016/s0306-4530(01)00047-6. [DOI] [PubMed] [Google Scholar]
- 16.De Bellis MD, Keshavan MS. Sex differences in brain maturation in maltreatment-related pediatric posttraumatic stress disorder. Neurosci Biobehav Rev. 2003;27(1-2):103–117. doi: 10.1016/s0149-7634(03)00013-7. [DOI] [PubMed] [Google Scholar]
- 17.De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. A.E. Bennett Research Award Developmental traumatology Part I: Biological stress systems. Biol Psychiatry. 1999;45(10):1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 18.Vazquez DM, Eskandari R, Zimmer CA, Levine S, Lopez JF. Brain 5-HT receptor system in the stressed infant rat: implications for vulnerability to substance abuse. Psychoneuroendocrinology. 2002;27(1-2):245–272. doi: 10.1016/s0306-4530(01)00048-8. [DOI] [PubMed] [Google Scholar]
- 19.Gordon JA, Hen R. The serotonergic system and anxiety. Neuromolecular Med. 2004;5(1):27–40. doi: 10.1385/NMM:5:1:027. [DOI] [PubMed] [Google Scholar]
- 20.Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2002;14(11):911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- 21.Preuss TM. Primate Origins, Developments in Primatology: Progress and Prospects. New York: Springer; 2006. Evolutionary specialization of primate brain systems; pp. 625–666. [Google Scholar]
- 22.Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20(12):4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004;55(7):733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Erickson K, Gabry KE, Lindell S, Champoux M, Schulkin J, Gold P, Suomi SJ, Higley JD. Social withdrawal behaviors in nonhuman primates and changes in neuroendocrine and monoamine concentrations during a separation paradigm. Dev Psychobiol. 2005;46(4):331–339. doi: 10.1002/dev.20061. [DOI] [PubMed] [Google Scholar]
- 25.Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, Higley JD. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcohol Clin Exp Res. 2000;24(5):644–650. [PubMed] [Google Scholar]
- 26.Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Sci U S A. 1991;88(16):7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychiatry. 1992;32(2):127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- 28.Shannon C, Schwandt ML, Champoux M, Shoaf SE, Suomi SJ, Linnoila M, Higley JD. Maternal absence and stability of individual differences in CSF 5-HIAA concentrations in rhesus monkey infants. Am J Psychiatry. 2005;162(9):1658–1664. doi: 10.1176/appi.ajp.162.9.1658. [DOI] [PubMed] [Google Scholar]
- 29.Spinelli S, Schwandt ML, Lindell SG, Newman TK, Heilig M, Suomi SJ, Higley JD, Goldman D, Barr CS. Association between the recombinant human serotonin transporter linked promoter region polymorphism and behavior in rhesus macaques during a separation paradigm. Dev Psychopathol. 2007;19(4):977–987. doi: 10.1017/S095457940700048X. [DOI] [PubMed] [Google Scholar]
- 30.Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. Am J Primatol. 1998;46(4):311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 31.Malkova L, Heuer E, Saunders RC. Longitudinal magnetic resonance imaging study of rhesus monkey brain development. Eur J Neurosci. 2006;24(11):3204–3212. doi: 10.1111/j.1460-9568.2006.05175.x. [DOI] [PubMed] [Google Scholar]
- 32.Saleem KS, Logothetis NK. A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in stereotaxic coordinates. Academic Press; 2006. [Google Scholar]
- 33.Lyons DM, Yang C, Sawyer-Glover AM, Moseley ME, Schatzberg AF. Early life stress and inherited variation in monkey hippocampal volumes. Arch Gen Psychiatry. 2001;58(12):1145–1151. doi: 10.1001/archpsyc.58.12.1145. [DOI] [PubMed] [Google Scholar]
- 34.Richert KA, Carrion VG, Karchemskiy A, Reiss AL. Regional differences of the prefrontal cortex in pediatric PTSD: an MRI study. Depress Anxiety. 2006;23(1):17–25. doi: 10.1002/da.20131. [DOI] [PubMed] [Google Scholar]
- 35.Sheline YI, Black KJ, Lin DY, Christensen GE, Gado MH, Brunsden BS, Vannier MW. Stereological MRI volumetry of the frontal lobe. Psychiatry Res. 1996;67(3):203–214. doi: 10.1016/0925-4927(96)02831-4. [DOI] [PubMed] [Google Scholar]
- 36.Phillips KA, Sherwood CC, Lilak AL. Corpus callosum morphology in capuchin monkeys is influenced by sex and handedness. PLoS ONE. 2007;2(8):e792. doi: 10.1371/journal.pone.0000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812(1-2):38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- 38.Mostofsky SH, Mazzocco MM, Aakalu G, Warsofsky IS, Denckla MB, Reiss AL. Decreased cerebellar posterior vermis size in fragile X syndrome: correlation with neurocognitive performance. Neurology. 1998;50(1):121–130. doi: 10.1212/wnl.50.1.121. [DOI] [PubMed] [Google Scholar]
- 39.De Bellis MD, Keshavan MS, Spencer S, Hall J. N-Acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. Am J Psychiatry. 2000;157(7):1175–1177. doi: 10.1176/appi.ajp.157.7.1175. [DOI] [PubMed] [Google Scholar]
- 40.Ham BJ, Chey J, Yoon SJ, Sung Y, Jeong DU, Ju Kim S, Sim ME, Choi N, Choi IG, Renshaw PF, Lyoo IK. Decreased N-acetyl-aspartate levels in anterior cingulate and hippocampus in subjects with post-traumatic stress disorder: a proton magnetic resonance spectroscopy study. Eur J Neurosci. 2007;25(1):324–329. doi: 10.1111/j.1460-9568.2006.05253.x. [DOI] [PubMed] [Google Scholar]
- 41.Mathew SJ, Shungu DC, Mao X, Smith EL, Perera GM, Kegeles LS, Perera T, Lisanby SH, Rosenblum LA, Gorman JM, Coplan JD. A magnetic resonance spectroscopic imaging study of adult nonhuman primates exposed to early-life stressors. Biol Psychiatry. 2003;54(7):727–735. doi: 10.1016/s0006-3223(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 42.Seedat S, Videen JS, Kennedy CM, Stein MB. Single voxel proton magnetic resonance spectroscopy in women with and without intimate partner violence-related posttraumatic stress disorder. Psychiatry Res. 2005;139(3):249–258. doi: 10.1016/j.pscychresns.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29(11):1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]


