Abstract
Background
There is considerable variation in psychological reactions to natural disasters, with responses ranging from relatively mild and transitory symptoms to severe and persistent posttraumatic stress (PTS). Some survivors also report post-traumatic growth (PTG), or positive psychological changes due to the experience and processing of the disaster and its aftermath. Gene-environment interaction (GxE) studies could offer new insight into the factors underlying variability in post-disaster psychological responses. However, few studies have explored GxE in a disaster context.
Methods
We examined whether ten common variants in seven genes (BDNF, CACNA1C, CRHR1, FKBP5, OXTR, RGS2, SLC6A4) modified associations between Hurricane Katrina exposure and PTS and PTG. Data were from a prospective study of 205 low-income non-Hispanic Black parents residing in New Orleans prior to and following Hurricane Katrina.
Results
We found a significant association (after correction) between RGS2 (rs4606; p=0.0044) and PTG, which was mainly driven by a cross-over GxE (p=0.006), rather than a main genetic effect (p=0.071). The G (minor allele) was associated with lower PTG scores for low levels of Hurricane exposure and higher PTG scores for moderate and high levels of exposure. We also found a nominally significant association between variation in FKBP5 (rs1306780, p=0.0113) and PTG, though this result did not survive correction for multiple testing.
Limitations
Although the inclusion of low-income non-Hispanic Black parents allowed us to examine GxE among a highly vulnerable group, our findings may not generalize to other populations or groups experiencing other natural disasters. Moreover, not all participants invited to participate in the genetic study provided saliva.
Conclusions
To our knowledge, this is the first study to identify GxE in the context of post-traumatic growth. Future studies are needed to clarify the role of GxE in PTS and PTG and post-disaster psychological responses, especially among vulnerable populations.
Keywords: Genes, Adversity, Hurricane, Post-traumatic stress, Post-traumatic growth, resilience
Introduction
Each year, an estimated 500 events throughout the world meet the Red Cross definition of a natural disaster (Norris et al., 2005). People exposed to natural disasters have a greater risk of experiencing mental health problems, including post-traumatic stress disorder (PTSD; Galea et al., 2007, Neria et al., 2008). PTSD is a debilitating condition characterized by re-experiencing, avoidance and numbing, and hyperarousal symptoms (American Psychiatric Association, 1994). One-fifth to one-third of natural disaster survivors experience PTSD (Galea et al., 2007, Galea et al., 2008). Women, African Americans, and low-income populations have been found in some studies to have at least two times the odds of experiencing disaster-related PTSD relative to their counterparts (e.g., males, Whites, and those with higher income; Galea et al., 2008, Brewin et al., 2000, Norris et al., 2002).
However, there is considerable variation in levels of posttraumatic stress (PTS), or symptoms of PTSD, in the aftermath of disasters. Even among vulnerable groups, survivors’ responses range from relatively mild, acute, and transitory to severe and persistent PTS symptoms that meet diagnostic criteria for PTSD (Galea et al., 2008). Greater exposure to disaster-related stressors and lower social support have been shown to predict more severe PTS and PTSD (Norris et al., 2002). In addition, some survivors also experience positive responses following exposure to traumatic events (Bonanno, 2004), including posttraumatic growth (PTG), or self-reported positive psychological changes induced by the experience and processing of a traumatic event and its aftermath (Tedeschi and Calhoun, 1995). These positive changes, which often co-occur with PTSD (Lowe et al., 2013), include improved interpersonal relationships, a greater sense of new possibilities, increased personal strength, heightened spirituality, and an enhanced appreciation for life. Although less is known about PTG, recent studies suggest that upwards of 50% of survivors of natural disasters experience some degree of PTG (Tang, 2006, Xu and Liao, 2011, Yu et al., 2010), with PTG being more common among older adults, non-Hispanic Blacks, and those exposed to a greater number of stressors (Lowe et al., 2013).
Although genetic factors contribute to variation in response to trauma (Dunn et al., 2011, Koenen et al., 2009a), only a handful of studies have examined gene-environment interaction (GxE) as a determinant of PTSD and anxiety-related outcomes in the context of a natural disaster (Kilpatrick et al., 2007, Koenen et al., 2009b, Amstadter et al., 2009, Pietrzak et al., 2013). Kilpatrick and colleagues found a three-way interaction between 5-HTTLPR genotype, hurricane exposure, and social support, with the highest levels of PTSD detected among those with the short/short (s/s) genotype, low social support, and high hurricane exposure (Kilpatrick et al., 2007). A similar finding was observed in the same study for RGS2, a gene that encodes the regulator of G-protein signaling 2; here, the highest levels of post-hurricane PTSD were observed among people with two copies of the RGS2 C (major) allele, low social support, and high exposure to the hurricane as well as other potentially traumatic events(Amstadter et al., 2009).
Although this literature suggests that GxE may play a role in the etiology of post-disaster PTSD, these studies have several limitations. First, most were cross-sectional and unable to examine GxE at different time points post-disaster. Second, all lacked pre-disaster data, even though prior studies have shown that pre-disaster factors (e.g., mental health status, degree of social support) are among the strongest predictors of post-disaster psychological responses (Ginexi et al., 2000, Norris et al., 2002, Sullivan et al., 2013). As a result, it remains unclear to what extent GxE predicts psychopathology beyond pre-disaster factors. Third, most examined only one gene, rather than multiple potentially important genes. Finally, no studies to our knowledge examined GxE for PTG.
The current study overcomes these limitations by examining whether ten common variants in seven genes (BDNF, CACNA1C, CRHR1, FKBP5, OXTR, RGS2, SLC6A4), identified as related to psychiatric phenotypes, modified the association between level of exposure to Hurricane Katrina (“Katrina”) and degree of PTS and PTG. We used data from an on-going prospective study of 1,019 (259 genotyped) low-income non-Hispanic Black parents who resided in New Orleans prior to Katrina, which made landfall as a Category 3 storm on August 29, 2005 and led to extensive property damage and population displacement(Knabb et al., 2005, U.S. Department of Commerce, 2006). Being part of an ongoing study uniquely positioned us to prospectively examine the relationship between exposure to Katrina and subsequent outcomes, after adjusting for pre-storm characteristics. Because of our modest sample size, we restricted our investigation to genetic loci previously associated with PTSD (BDNF, FKBP5, RGS2, SLC6A4) or an associated mood and anxiety disorder (CACNA1C, CRHR1, OXTR), either alone or in interaction with an environmental insult. We hypothesized that the highest levels of PTS would be observed among individuals with both high-risk genetic variants and higher hurricane exposure, after adjusting for pre-disaster factors. Given the lack of research on PTG, we made no a priori hypotheses for this outcome.
Methods
Sample and Procedures
Data were from a sample of participants (Lowe et al., 2010, Rhodes et al., 2010) in the New Orleans site of the Opening Doors Study, a multi-site national study designed to examine whether modest performance-based scholarships promoted academic achievement, health, and well-being of low income parents attending community college (Richburg-Hayes and Brock, 2009). To be eligible for the study, students had to be between the ages of 18 and 34, be a parent of at least one dependent child under 19, have a household income under 200% of the federal poverty level, and have a high school diploma or equivalent. In 2004 and 2005 (Time1, or T1), 1,019 participants completed a brief survey, which assessed demographic, physical, and mental health information. Prior to Katrina, 492 participants had been enrolled long enough to complete a more extensive 12-month pre-disaster follow-up survey (T2). Between May 2006 and March 2007 (T3), 402 (81.7%) participants who completed the T2 survey were successfully located and completed a post-disaster interview by phone with a trained interviewer. The T3 interview was similar to T2, but also included an inventory of hurricane exposure and a measure of PTS. Between April 2009 and March 2010, trained researchers administered an additional post-disaster survey by phone (T4) to 409 participants (83.1% of the T2 sample; 348 of these respondents also completed the T3 survey). The T4 survey included the same measures as T3 plus a measure of PTG.
All T4 participants were invited to provide a saliva sample for the purpose of genomic analysis; 259 of these respondents did so. There were no significant differences between respondents who provided a sample and those who did not with respect to nearly all social-demographic characteristics. However, respondents in the genetic sample had slightly higher levels of T1 social support (genetic sample mean=3.25; SD=0.46; non-genetic sample mean=3.17; SD=0.44; p=0.32).
Measures
Predictors: Severity of exposure to Hurricane Katrina
Exposure to Katrina was measured at T3 using an 8-item scale jointly designed by the Washington Post, the Kaiser Family Foundation, and the Harvard School of Public Health (Brodie et al., 2006). Participants indicated whether they experienced any of the following conditions in the immediate aftermath of the storm: (1) no fresh water to drink, (2) no food to eat, (3) felt their life was in danger, (4) lacked necessary medicine, (5) lacked necessary medical care, (6) had a family member who lacked necessary medical care, (7) lacked knowledge of safety of their children, and (8) lacked knowledge of safety of their other families members. Exposures were summed to create a total score. For the 101 individuals missing data on Hurricane Katrina exposure at T3, we used their responses to this scale at T4. Exposure reports were modestly correlated (r=0.43) among the 104 participants with data at both T3 and T4; although some respondents over-reported (n=38) (i.e., reported higher levels of exposure at T4 compared to T3) or under-reported exposure (n=38), the average difference between the two reports of exposure was small (mean difference=0.119; SD=2.26).
Outcomes: Post-Traumatic Stress (PTS) Symptoms and Post-Traumatic Growth (PTG)
Post-traumatic stress symptoms (PTS) resulting from Katrina were measured at T3 and T4 using the Impact of Events Scale–Revised, which is a 22-item self-report inventory capturing the major symptoms of PTSD, specifically intense fear, horror or helplessness, re-experiencing, avoidance/numbing, and hyperarousal (Weiss and Marmar, 1997). Participants indicated how often during the past week they were distressed or bothered by experiences related to Katrina. Response options were on a 5-point Likert scale, ranging from 0 (not at all) to 4 (extremely). The scale has demonstrated good psychometric properties (Creamer et al., 2003) (T3 Cronbach’s α =0.95, T4 α=0.95).
Post-traumatic growth (PTG) was measured at T4 using the 21-item Post-Traumatic Growth Inventory (Tedeschi and Calhoun, 1996), which has been used in previous disaster studies (Tang, 2001) and had good internal consistency reliability in our sample (α=0.93). Participants rated their extent of changes as a result of Katrina on a 5-point scale (0=not at all; 4=extremely). Sample items included “I have a greater appreciation for the value of my own life,” and “I am better able to accept the way things work out.”
Covariates
All models controlled for T1 covariates: sex; age (18–25 vs. 26–35); social support, as measured by the Social Provisions Scale (Cutrona and Russell, 1987), and psychological distress, as measured by the K6 (Kessler et al., 2002). We also adjusted for the age of participants’ youngest child assessed at T2 (0=ages 0–2; 1=ages 3–5; 2 ages 6–11; 3=ages 12–20). These variables have been shown in previous studies to predict level of exposure to a natural disaster or psychological outcomes (Norris et al., 2002). We also adjusted for the wave when exposure was collected.
Genetic Polymorphisms
We examined ten polymorphisms in seven genes, which have been previously associated with PTSD or an associated psychiatric outcome either alone or in interaction with an environmental insult (e.g., child maltreatment). In addition, 40 markers were genotyped as part of an ancestry informative marker set (AIMs) used to assess population structure in this sample.
1. BDNF (rs6265, chromosome 11p14)
Brain-derived neurotrophic factor (BDNF) is a small secreted protein that influences neuronal growth and differentiation during development and neuronal survival, function, and plasticity in adulthood (Lewin and Barde, 1996). BDNF has been implicated in a wide range of anxiety and mood disorders (Martinowich et al., 2007, Rakofsky et al., 2012, Frielingsdorf et al., 2010). A missense variant (A/G SNP) in the BDNF gene, located at codon 66, changes the amino acid from valine (val) to methionine (met). This Val66Met polymorphism has been linked in some studies to an elevated risk for PTSD(Zhang et al., 2013).
2. CACNA1C (rs1006737, chromosome 12p13)
CACNA1C is the gene encoding the alpha-1C subunit of the L-type voltage-gated calcium channel. L-type calcium channels facilitate the entry of calcium ions into the cell during membrane polarization and are essential for fear learning and extinction (Davis and Bauer, 2012). Prior studies have identified genome-wide significant associations between CACNA1C SNPs and risk for bipolar disorder (Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011, Ferreira et al.), schizophrenia (Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium, 2011, Nyegaard et al., 2010, Hamshere et al., 2013), recurrent major depression (Green et al., 2010), as well as shared risk across these and other disorders (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013).
3. CRHR1 (rs12944712; chromosome 17q21)
Corticotrophin releasing hormone (CRH) is a hormone involved in regulating the stress response via the hypothalamic-pituitary-adrenal (HPA) axis. Multiple variants in the gene encoding the CRH type 1 receptor (CRHR1) have been associated with depression and anxiety both alone and in interaction with exposure to child abuse and trauma(Bradley et al., 2008, Amstadter et al., 2011).
4. FKBP5 (rs1360780, rs9296158, and rs9470080, chromosome 6p21)
FKBP5 encodes a negative regulator of glucocorticoid receptor function. We examined 3 intronic SNPs, rs1360780 (C/T), rs9296158 (A/G), and rs9470080 (C/T), which have been reported to predict peritraumatic dissociation (Koenen et al., 2005) and to moderate the effect of exposure to child maltreatment on PTSD symptoms (Binder et al., 2008) or PTSD diagnoses (Xie et al., 2010).
5. OXTR (rs53576, and rs2254298, chromosome 3p25)
Oxytocin is a neuropeptide hormone linked to multiple social behaviors (e.g., attachment, interpersonal trust) and stress reactions in mammals (Gimpl and Fahrenholz, 2001). Two SNPs in OXTR, the gene that encodes the oxytocin receptor, have been linked to social dysfunction (Tost et al.) and found to moderate the relationship between childhood maltreatment or disadvantage on adolescent social and separation anxiety (Thompson et al., 2011) as well as adult emotion dysregulation and attachment style (Bradley et al., 2011).
6. RGS2 (rs4606, chromosome 1q31)
Regulator of G-protein signaling 2 modulates neurotransmitter response by accelerating the deactivation of G proteins. The rs4606 SNP has been associated with variation in RGS2 expression (Semplicini et al., 2006). Prior studies have linked rs4606 to panic disorder (Leygraf et al., 2006, Otawa et al., 2011), generalized anxiety disorder (Koenen et al., 2009b), and anxiety-related phenotypes (Smoller et al., 2008) and have also found that this SNP modifies the effect of social support and hurricane exposure on PTSD symptoms (Amstadter et al., 2009).
7. SLC6A4 (variable number tandem repeat VNTR and rs25531, chromosome 17.q11–17.q12)
SLC6A4 encodes the serotonin transporter, which plays a central role in serotonergic neurotransmission. A VNTR polymorphism (5-HTTLPR) located in the promoter region of the serotonin transporter gene (SLC6A4) has been commonly studied in GxE research (Caspi et al., 2003, Risch et al., 2009, Karg et al., 2011, Dunn et al., 2011) and consists of a short (S; 16 repeats) and long allele (L; 18 repeats); 5HTTLPR has been linked to PTSD and depression following trauma exposure (Kilpatrick et al., 2007, Xie et al., 2009, Grabe et al., 2009). The L allele is associated with increased serotonin transporter expression. A functional A/G SNP has also been identified in the repeat region; 5HTTLPR L alleles that carry the G allele of rs25531 appear to be functionally similar to S alleles (Hu et al., 2005). In accordance with prior studies, we combined VNTR and SNP genotypes and analyzed the number of minor (LA) alleles (i.e., long VNTR with the A SNP).
Genotyping and Quality Control
DNA was extracted from saliva using Oragene DNA purification kits (DNA Genotek Inc., Ottawa, Ontario, Canada). SNP and 5-HTTLPR genotyping were performed at the Massachusetts General Hospital Center for Human Genetic Research.
SNP genotyping was performed in 267 samples (including 8 duplicates) using the Sequenom iPLEX Gold® chemistry and the MassARRAY® system. The concordance of the 8 duplicate samples was 0.99.
5-HTTLPR genotyping was performed on 264 samples (including 5 duplicates) using Applied Biosystems® instruments and reagents. The concordance of the 5 duplicate samples was 1.0. Genomic DNA was amplified using the following primers: 5-HTTLPR-F 6FAM-GGCGTTGCCGCTCTGAATGC, 5-HTTLPR-R GAGGGACTGAGCTGGACAACCAC. The long allele appears as a product of about 528 base pairs (bp) while the short allele is about 483 bp. The genotype of rs25531 imbedded in the VNTR was determined by digesting the VNTR PCR product with the restriction enzyme, HpaII. Post-digestion, products appear at either 335 bp (long VNTR with the A SNP), 292 bp (short VNTR with the A SNP), or 163 bp (G SNP from either the long or short form of the VNTR). The final genotype is determined from the results of both the digested and undigested PCR product.
During quality control, we removed 5 AIMs and one SNP (rs2267735, in the ADCYAP1R gene) that had a call rate of less than 95%. We also removed 5 participants who had a call rate of less than 95%. In addition, we also removed 5 AIMs that failed Hardy Weinberg equilibrium (p<10−6). Thus, our final analytic sample included 254 participants with information on 39 SNPs (9 SNPs, 30 AIMs) plus the 5-HTTLPR. All SNPs had a minor allele frequency (MAF) greater than 0.05. We performed principal components analysis on the 30 AIMs to assess the population substructure and selected 207 non-Hispanic Black subjects based on the top two principal components. The final analysis of T4 PTS and PTG included 205 participants for whom hurricane exposure was recorded. The analysis of PTS at T3 included 163 participants.
Data Analyses
Our multiple linear regression analyses proceeded in two steps. We first examined the effect of Katrina exposure on the three outcomes (T3/T4 PTS; T4 PTG), after adjusting for covariates. We then tested the association between each variant and the outcomes using a joint test for a main genetic effect and GxE (Kraft et al., 2007). The joint test compares fit statistics (e.g., deviance) from two nested models (i.e., model with exposure effects is compared to model with exposure and genetic effects and the GxE interaction). Prior studies have found that the joint test has more power to detect GxE compared to the traditional test of effect modification using the cross-product interaction term (Kraft et al., 2007). In each model the genetic variant was coded additively as the number of minor alleles. Permutation was used to establish a significance threshold that accounted for multiple testing (p=0.0042 for PTS and p=0.0062 for PTG). These thresholds were derived using permutation, separately for each phenotype, to account for the correlation among the SNPs and the correlation between PTS T3 and T4. We generated 10,000 sets of permuted phenotypes, computed the p-value for the joint test, and recorded the minimum p-value across SNPs within each set and phenotype. We then computed the 5th percentile of the minimum p-value to establish the significance threshold accounting for multiple testing. For PTS, the permutation maintained the correlation between T3 and T4 to account for the fact that two time-points were tested. For each significant finding, we conducted post-hoc analyses to determine whether a main genetic effect or GxE was driving the association. All analyses were conducted in PLINK version 1.07 (Purcell et al., 2007) or the R package (R Development Core Team, 2011). A cube root transformation was applied to PTS at T3 and T4 to address non-normality.
Results
Hurricane Exposure, PTS, and PTG
The sample was diverse with respect to levels of exposure to Hurricane Katrina (mean=3.09 exposures; sd=2.17). About one-third of participants (29.76%; n=61) experienced 0 or 1 exposure, one-third (29.76%; n=61) experienced 2 or 3 exposures, and the remaining 40.49% (n=83) experienced 4 or more exposures. Level of exposure was negatively associated with social support (p=0.0001), such that the lowest social support scores (indicating less social support) were found among participants with 4 or more Katrina-related exposures (refer to Table 1). No other covariates were associated with exposure.
Table 1.
Demographic characteristics of the Hurricane Katrina genetic analytic sample (n=205)
| Total Sample | Exposure to Hurricane Katrina
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–1 Exposures | 2–3 Exposures | 4+ Exposures | p-value | ||||||
|
| |||||||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | ||
| Social support | 3.25 | (0.44) | 3.33 | (0.38) | 3.37 | (0.42) | 3.09 | (0.47) | 0.0001 |
| Mental health status | 4.83 | (4.00) | 4.04 | (3.12) | 4.58 | (3.89) | 5.58 | (4.43) | 0.06 |
| Age | 25.82 | (4.39) | 25.20 | (3.85) | 25.44 | (4.26) | 26.55 | (4.78) | 0.14 |
| N | % | N | % | N | % | N | % | ||
| Gender | 0.91 | ||||||||
| Male | 8 | 3.90 | 2 | 0.98 | 3 | 1.46 | 3 | 1.46 | |
| Female | 197 | 96.10 | 59 | 28.78 | 58 | 28.29 | 80 | 39.02 | |
| Number of children | 0.72 | ||||||||
| No children | 49 | 41.18 | 17 | 14.29 | 15 | 12.61 | 17 | 14.29 | |
| Child between 0–5 | 33 | 27.73 | 11 | 9.24 | 9 | 7.56 | 13 | 10.92 | |
| Child between 6–10 | 30 | 25.21 | 10 | 8.4 | 7 | 5.88 | 13 | 10.92 | |
| Child between 11–17 | 7 | 5.82 | 1 | 0.84 | 1 | 0.84 | 5 | 4.2 | |
All covariates were assessed at baseline. Number of children was coded according to the lowest age of the child, if there were multiple children. Not all rows will sum to 100% or total N due to missing data.
Higher levels of stress exposure were associated with higher levels of PTS at T3 (p=8×10−5) and T4 (p=2×10−8), after adjustment for wave when exposure was collected. This effect persisted for both T3 (p=0.0015; r2=0.064) and T4 (p=1.1×10−6; r2=0.117) after adjusting for all other covariates. However, level of Katrina exposure was unrelated to PTG (p=0.10; r2=0.015).
Interaction between Genetic Variants and Hurricane Exposure on PTS and PTG
As shown in Table 2, we found evidence of two nominally significant genetic and GxE effects; one of these results (for RGS2) survived correction for multiple testing.
Table 2.
Association between exposure to Hurricane Katrina, genetic variation, and gene-by-environment interaction (GxE) on post-traumatic stress symptoms (PTS) and post-traumatic growth (PTG) (n=205)
| A1/A2 | MAF | p-value joint test | Main Effect of G | Effect of G x Exposure | |||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | (SE) | p-value | Beta | (SE) | p-value | ||||
| Post Traumatic Growth (Time 4) | |||||||||
| BDNF (rs6265) | T/C | 0.05 | 0.6777 | 1.17 | 3.67 | 0.750 | −1.52 | 1.85 | 0.411 |
| CACNA1C (rs1006737) | A/G | 0.46 | 0.9991 | 0.03 | 1.73 | 0.988 | 0.03 | 0.80 | 0.968 |
| CRHR1 (rs12944712) | A/G | 0.23 | 0.2773 | 0.19 | 1.85 | 0.917 | −1.36 | 0.85 | 0.110 |
| FKBP5 (rs1360780) | T/C | 0.40 | 0.0113 | 4.78 | 1.61 | 0.003 | −0.46 | 0.75 | 0.537 |
| FKBP5 (rs9296158) | A/G | 0.44 | 0.0606 | 3.73 | 1.60 | 0.021 | −0.39 | 0.78 | 0.614 |
| FKBP5 (rs9470080) | T/C | 0.44 | 0.0673 | 3.68 | 1.62 | 0.024 | −0.42 | 0.78 | 0.591 |
| OXTR (rs53576) | A/G | 0.19 | 0.7006 | −1.26 | 2.09 | 0.547 | −0.59 | 1.00 | 0.555 |
| OXTR (rs2254298) | A/G | 0.25 | 0.8524 | 1.00 | 1.95 | 0.610 | −0.24 | 0.98 | 0.807 |
| RGS2 (rs4606) | G/C | 0.35 | 0.0044 | 3.28 | 1.80 | 0.071 | 2.25 | 0.81 | 0.006 |
| 5-HTTLPR | 1/0 | 0.44 | 0.0819 | −0.03 | 1.68 | 0.988 | 1.77 | 0.78 | 0.025 |
| Post Traumatic Stress (Time 3) | |||||||||
| BDNF (rs6265) | T/C | 0.05 | 0.7852 | −0.03 | 0.04 | 0.543 | −0.01 | 0.02 | 0.736 |
| CACNA1C (rs1006737) | A/G | 0.46 | 0.9847 | 0.00 | 0.02 | 0.910 | 0.00 | 0.01 | 0.893 |
| CRHR1 (rs12944712) | A/G | 0.23 | 0.7963 | 0.00 | 0.02 | 0.980 | 0.01 | 0.01 | 0.501 |
| FKBP5 (rs1360780) | T/C | 0.40 | 0.9972 | 0.00 | 0.02 | 0.948 | 0.00 | 0.01 | 0.971 |
| FKBP5 (rs9296158) | A/G | 0.44 | 0.9310 | −0.01 | 0.02 | 0.787 | 0.00 | 0.01 | 0.792 |
| FKBP5 (rs9470080) | T/C | 0.44 | 0.9912 | 0.00 | 0.02 | 0.894 | 0.00 | 0.01 | 0.992 |
| OXTR (rs53576) | A/G | 0.19 | 0.9091 | 0.00 | 0.02 | 0.972 | −0.01 | 0.01 | 0.664 |
| OXTR (rs2254298) | A/G | 0.25 | 0.4532 | 0.00 | 0.02 | 0.915 | −0.01 | 0.01 | 0.211 |
| RGS2 (rs4606) | G/C | 0.35 | 0.2017 | −0.04 | 0.02 | 0.076 | 0.00 | 0.01 | 0.806 |
| 5-HTTLPR | 1/0 | 0.44 | 0.8155 | 0.00 | 0.02 | 0.884 | −0.01 | 0.01 | 0.535 |
| Post Traumatic Stress (Time 4) | |||||||||
| BDNF (rs6265) | T/C | 0.05 | 0.9118 | −0.05 | 0.19 | 0.768 | −0.03 | 0.09 | 0.755 |
| CACNA1C (rs1006737) | A/G | 0.46 | 0.1521 | 0.17 | 0.09 | 0.053 | −0.01 | 0.04 | 0.890 |
| CRHR1 (rs12944712) | A/G | 0.23 | 0.1135 | 0.06 | 0.09 | 0.502 | 0.08 | 0.04 | 0.049 |
| FKBP5 (rs1360780) | T/C | 0.40 | 0.9014 | 0.04 | 0.08 | 0.659 | 0.00 | 0.04 | 0.910 |
| FKBP5 (rs9296158) | A/G | 0.44 | 0.8610 | 0.02 | 0.08 | 0.766 | 0.02 | 0.04 | 0.646 |
| FKBP5 (rs9470080) | T/C | 0.44 | 0.9525 | 0.02 | 0.08 | 0.819 | 0.01 | 0.04 | 0.832 |
| OXTR (rs53576) | A/G | 0.19 | 0.9946 | −0.01 | 0.10 | 0.928 | 0.00 | 0.05 | 0.958 |
| OXTR (rs2254298) | A/G | 0.25 | 0.6797 | −0.03 | 0.10 | 0.798 | −0.04 | 0.05 | 0.401 |
| RGS2 (rs4606) | G/C | 0.35 | 0.4125 | 0.12 | 0.09 | 0.206 | 0.02 | 0.04 | 0.672 |
| 5-HTTLPR | 1/0 | 0.44 | 0.4977 | 0.09 | 0.08 | 0.272 | 0.02 | 0.04 | 0.664 |
A1 and A2 are the minor and major alleles, respectively. The minor allele was modeled additively in each model. All p-values are unadjusted for multiple testing. The p-value cut-off for the joint test after adjusting for multiple testing is p=0.005 for each phenotype. All models include adjustments for the following covariates: sex (0=male; 1=female); age (0=ages 18–25; 1=ages 26–35); social support, as measured by the Social Provisions Scale(Cutrona and Russell, 1987), psychological distress, as measured by the K6(Kessler et al., 2002), age of participants’ youngest child assessed at T2 (0=ages 0–2; 1=ages 3–5; 2 ages 6–11; 3=ages 12–20), and wave when exposure was collected. 5-HTTLPR was coded as 0=long A (minor allele); 1=long G or short A (no one in our sample was classified as short G).
First we found a significant association between RGS2 (rs4606; p=0.0044) and PTG, which was mainly driven by a cross-over GxE (p=0.006), rather than a main genetic effect (p=0.071). At low levels of Katrina exposure, the G allele (minor allele) was associated with lower PTG (indicating less PTG). However, at moderate and high exposure, the G allele was associated with higher PTG scores (indicating more PTG; refer to Figure 1). This result withstood correction for multiple testing. In this model, the GxE explained 3.9% of the variability in PTG.
Figure 1. Association between exposure to Hurricane Katrina and RGS2 (rs4606) on post-traumatic growth (PTG) (n=205).
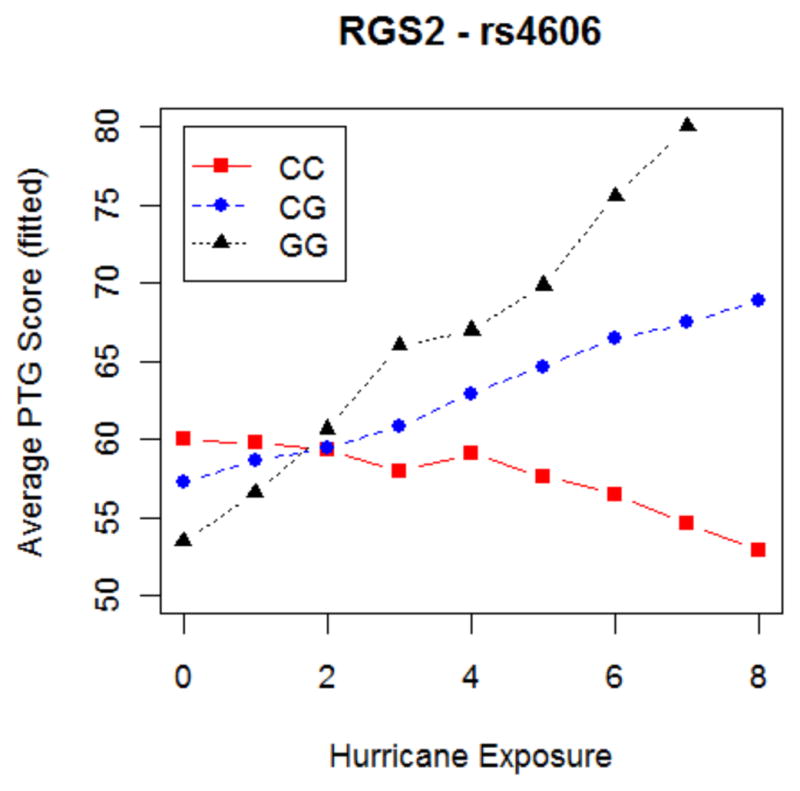
The figure displays the relationship between number of Hurricane Katrina exposures (x-axis) and levels of post-traumatic growth (y-axis). The findings for RGS2 withstood correction for multiple testing (p=0.0044). In our sample, 82 individuals were homozygous for the RGS2 rs4606 C allele (CC genotype), 104 were heterozygotes (GC genotype), and 19 were homozygous for the G allele (GG genotype).
Second, we found a nominally significant association between FKBP5 (rs1306780, p=0.0113) and PTG, with the T allele conferring a higher likelihood of experiencing PTG. The joint effect was driven by a genetic main effect (p=0.003) rather than GxE (p=0.537). This result did not withstand correction for multiple testing.
We did not find any significant associations between the genetic variants and T3 or T4 PTS (all p-values > 0.05).
Discussion
In this prospective analysis of low-income non-Hispanic Black parents, we find the first evidence of gene-environment interaction (GxE) for post-traumatic growth (PTG). At low levels of exposure to Hurricane Katrina, G allele (minor allele) homozygotes at rs4606 (RGS2) had the lowest level of PTG. However, at moderate and high levels of hurricane exposure, G allele homozygotes had the highest level of PTG. This cross-over interaction result, which appeared to be driven more by a GxE than a main effect for RGS2, withstood a correction for multiple testing. We also found an association between FKBP5 (rs1306780, p=0.0113) and PTG. However, this finding did not survive correction for multiple testing. We found no association between any of the genetic polymorphisms we examined and post-traumatic stress symptoms (PTS). Post-hoc power analyses suggest we had 75% power to detect a GxE that explained 5% or greater of the variation in T4 PTSD, with the genetic main effect explaining 0.2% of the variation and exposure to Hurricane Katrina explaining 12% of the variation.
Given the lack of prior GxE research on PTG, it is challenging to put this RGS2 finding in context. Prior studies have implicated the RGS2 rs4606 variant in a number of anxiety disorders and anxiety-related phenotypes, including panic disorder, PTSD, generalized anxiety disorder, and anxious temperament (Koenen et al., 2009b, Leygraf et al., 2006, Smoller et al., 2008, Amstadter et al., 2009, Otawa et al., 2011), although results have been inconsistent (Hettema et al., 2013). A study of adults exposed to Hurricane Andrew found significant interactions between rs4606 and level of hurricane exposure, with C allele homozygotes (major allele) with low social support and high hurricane exposure (as well as other potentially traumatic events) having the highest level of lifetime PTSD (Amstadter et al., 2009). That is, the rs4606 G allele associated in our study with greater PTG among individuals with high levels of Katrina exposure was associated with reduced PTSD risk among those with high levels of exposure to Hurricane Andrew in this prior study. The G allele has also been associated with behavioral inhibition, a temperamental and familial risk factor for anxiety disorders, and with increased limbic reactivity to emotional stimuli (Smoller et al., 2008). Future research is needed to replicate the rs4606 finding in the context of both natural disasters as well as other traumatic life events.
Several limitations must be noted. First, although the inclusion of low-income non-Hispanic Black parents allowed us to examine GxE among a highly vulnerable group, our findings may not generalize to other populations or groups experiencing other natural disasters. Second, not all participants invited to participate in the genetic study provided saliva. However, only minor differences were observed in baseline social support between genetic participants and non-participants.
Despite these limitations, our study has substantial strengths. We limited our analyses to genetic variants previously implicated in PTSD and related phenotypes and we applied correction for multiple testing. We also examined GxE in the context of PTG, which is largely understudied (Kim-Cohen and Turkewitz, 2012), and two continuous phenotypes, which provide greater power compared to categorical outcomes (Dunn et al., 2011). We also examined these relationships in a sample of vulnerable non-Hispanic Black respondents, who are often excluded from genetic research. We were also able to control for pre-disaster factors that could have confounded the GxE relationships.
Conclusion
In summary, our results suggest that RGS2 plays a role in PTG. Future studies are needed to understand which genetic as well as social and psychological determinants explain the variation in response to natural disasters and which populations are most vulnerable (or resilient) to developing PTSD and experiencing PTG following exposure to trauma.
Acknowledgments
The study was funded by NIH grant R01HD046162, and the National Science Foundation, the MacArthur Foundation, the Center for Economic Policy Studies at Princeton University, and the Robert Wood Johnson Health and Society Program.
Footnotes
Contributors:
The roles and responsibilities of authors are as follows: Dr. Dunn and Solovieff had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Smoller, Koenen, Waters, and Rhodes conceived of the design of the analysis. Dr. Dunn supervised the analysis, drafted the initial manuscript, and approved the final version. Ms. Gallagher and Mr. Chaponis completed genotyping and guided the write-up of the genetic analyses. Dr. Rosand helped with initial interpretation of results, and revisions to the manuscript.
Conflicts of interest:
None
Role of the Funding Source
See Acknowledgements
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Amstadter AB, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, Gelernter J. Variant in RGS2 moderates posttraumatic stress symptoms following potentially traumatic event exposure. Journal of Anxiety Disorders. 2009;23:369–373. doi: 10.1016/j.janxdis.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Nugent NR, Yang BZ, Miller A, Siburian R, Moorjani P, Haddad S, Basu A, Fagerness J, Saxe G, Smoller JW, Koenen KC. Corticotrophin-releasing hormone type 1 receptor gene (CRHR1) variants predict posttraumatic stress disorder onset and course in pediatric injury patients. Disease Markers. 2011;30:89–99. doi: 10.3233/DMA-2011-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Journal of the American Medical Association. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno GA. Loss, trauma, and human resilience: Have we underestimated the human capacity to thrive after extremely aversive events. American Psychologist. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- Bradley B, Westen D, Mercer KB, Binder EB, Jovanovic T, Crain D, Wingo A, Heim C. Association between childhood maltreatment and adult emotional dysregulation in a low-income, urban, African American sample: Moderation by oxytocin receptor gene. Development and Psychopathology. 2011;23:439–452. doi: 10.1017/S0954579411000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells SF, Kessler RC. Influence of child abuse on adult depression: Moderation by the corticotropin-releasing hormone receptor gene. Archives of General Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology. 2000;68:748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Brodie M, Welzien E, Altman DG, Blendon RJ, Benson JM. Experiences of Hurricane Katrina evacuees in Houston shelters: Implications for future planning. American Journal of Public Health. 2006;96:1402–1408. doi: 10.2105/AJPH.2005.084475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington HL, Mcclay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Creamer M, Bell R, Failla S. Psychometric properties of the Impact of Event Scale-Revised. Behavior Research and Therapy. 2003;41:1489–1496. doi: 10.1016/j.brat.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: A genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrona CE, Russell DW, editors. The provisions of social relationships and adaptation to stress. Greenwich, CT: JAI Press; 1987. [Google Scholar]
- Davis SE, Bauer EP. L-type voltage-gated calcium channels in the basolateral amydgala are necessary for fear extinction. Journal of Neuroscience. 2012;32:13582–13586. doi: 10.1523/JNEUROSCI.0809-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn EC, Uddin M, Subramanian SV, Smoller JW, Galea S, Koenen KC. Gene-environment interaction (GxE) research in youth depression: A systematic review with recommendations for future research. Journal of Child Psychology and Psychiatry. 2011;52:1223–1238. doi: 10.1111/j.1469-7610.2011.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MA, O’donovan MC, Meng Y, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nature Genetics. 40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frielingsdorf H, Bath KG, Soliman F, Difede JA, Casey BJ, Lee FS. Variant brain-derived neurotrophic factor Val66Met endophenotypes: Implications for posttraumatic stress disorder. Annals of the New York Academy of Sciences. 2010;1208:150–157. doi: 10.1111/j.1749-6632.2010.05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea S, Brewin CR, Gruber M, Jones RT, King DW, King LA, Mcnally RJ, Ursano RJ, Petukhova M, Kessler RC. Exposure to Hurricane-Related Stressors and Mental Illness After Hurricane Katrina. Arch Gen Psychiatry. 2007;64:1427–1434. doi: 10.1001/archpsyc.64.12.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea S, Tracy M, Norris F, Coffey SF. Financial and social circumstances and the incidence and course of PTSD in Mississippi during the first two years after Hurricane Katrina. Journal of Traumatic Stress. 2008;21:357–368. doi: 10.1002/jts.20355. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Phsyiological Review. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Ginexi EM, Weihs K, Simmens SJ, Hoyt DR. Natural Disaster and Depression: A Prospective Investigation of Reactions to the 1993 Midwest Floods. American Journal of Community Psychology. 2000;28:495–518. doi: 10.1023/a:1005188515149. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Spitzer C, Schwahn C, Marcinek A, Frahnow A, Barnow S, Lucht M, Freyberger HJ, John U. Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to posttraumatic stress disorder in the general population. Archives of General Psychiatry. 2009;166:926–933. doi: 10.1176/appi.ajp.2009.08101542. [DOI] [PubMed] [Google Scholar]
- Green EK, Grozeva D, Jones I, et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Molecular Psychiatry. 2010;15:1016–1022. doi: 10.1038/mp.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamshere ML, Walters JTR, Smith R, Richards AL, Green E, et al. Genome-wide significant associations in schizophrenia to ITIH3/4, CACNA1C and SDCCAG8, and extensive replication of associations reported by the Schizophrenia PGC. Molecular Psychiatry. 2013;18:708–712. doi: 10.1038/mp.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, Sun C, Chen X, Kendler KS. Genetic association study between RGS2 and anxiety-related phenotypes. Psychiatric Genetics. 2013 doi: 10.1097/YPG.0b013e32835d70b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation fo the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcoholism: Clinical and Experimental Research. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychological Medicine. 2002;32:959–976. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggeiro KJ, Acierno R, Galea S, Resnick HS, Roitzsch J, Boyle J, Gelernter J. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. American Journal of Psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Turkewitz R. Resilience and measured gene-environment interactions. Development and Psychopathology. 2012;24:1297–1306. doi: 10.1017/S0954579412000715. [DOI] [PubMed] [Google Scholar]
- Knabb RD, Rhome JR, Brown DP. Tropical Cyclone Report Hurricane Katrina 23–30 August 2005. National Hurricane Center; 2005. [Google Scholar]
- Koenen KC, Amstadter AB, Nugent NR. Gene-environment interaction in posttraumatic stress disorder: An update. Journal of Traumatic Stress. 2009a;22:416–426. doi: 10.1002/jts.20435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Amstadter AB, Ruggerio KJ, Acierno R, Galea S, Kilpatrick DG, Gelernter J. RGS2 and generalized anxiety disorder in an epidemiologic sample of hurricane-exposed adults. Depression and Anxiety. 2009b;26:309–315. doi: 10.1002/da.20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Saxe G, Purcell S, Smoller JW, Bartholomew D, Miller A, Hall E, Kaplow J, Bosquet M, Moulton S, Baldwin C. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Molecular Psychiatry. 2005;10:1058–1059. doi: 10.1038/sj.mp.4001727. [DOI] [PubMed] [Google Scholar]
- Kraft P, Yen YC, Stram DO, Morrison JM, Gauderman WJ. Exploiting gene-environment interaction to detect genetic associations. Human Heredity. 2007;63:111–119. doi: 10.1159/000099183. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annual Review of Neuroscience. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Leygraf A, Huhoff C, Freitag C, Willis-Owen SaG, Krakowitzky P, Fritze J, Franke P, Bandelow B, Fimmers R, Flint J, Deckert J. RGS 2 gene polymorphisms as modulators of anxiety in humans. Journal of Neural Transmisson. 2006;113:1921–1925. doi: 10.1007/s00702-006-0484-8. [DOI] [PubMed] [Google Scholar]
- Lowe SR, Chan CS, Rhodes JE. Pre-hurricane perceived social support protects against psychological distress: A longitudinal analysis of low income mothers. Journal of Consulting and Clinical Psychology. 2010;78:551–560. doi: 10.1037/a0018317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SR, Manove EE, Rhodes JE. Posttraumatic stress and posttraumatic growth among low-income mothers who survived Hurricane Katrina. Journal of Consulting and Clinical Psychology. 2013 doi: 10.1037/a0033252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nature Neuroscience. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Neria Y, Nandi A, Galea S. Post-traumatic stress disorder following disasters: a systematic review. Psychological Medicine. 2008;38:467–480. doi: 10.1017/S0033291707001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris FH, Baker CK, Murphy AD, Kaniasty K. Social Support Mobilization and Deterioration after Mexico’s 1999 Flood: Effects of Context, Gender, and Time. American Journal of Community Psychology. 2005;36:15–28. doi: 10.1007/s10464-005-6230-9. [DOI] [PubMed] [Google Scholar]
- Norris FH, Friedman MJ, Watson PJ, Byrne CM, Diaz E, Kaniasty K. 60,000 disaster victims speak: Part I. An empirical review of the empirical literature, 1981–2001. Psychiatry: Interpersonal and Biological Processes. 2002;65:207–239. doi: 10.1521/psyc.65.3.207.20173. [DOI] [PubMed] [Google Scholar]
- Nyegaard M, Demontis D, Foldager L, Hedemand A, Flint TJ, et al. CACNA1C (rs1006737) is associated with schizophrenia. Molecular Psychiatry. 2010;15:119–121. doi: 10.1038/mp.2009.69. [DOI] [PubMed] [Google Scholar]
- Otawa T, Shimada T, Kawamura Y, Sugaya N, Yoshida E, Inoue K, Yashuda S, et al. Association of RGS2 variants with panic disorder in a Japanese population. American Journal of Medical Genetics Part B. 2011;156B:430–434. doi: 10.1002/ajmg.b.31178. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Galea S, Southwick SM, Gelernter J. Examining the relation between the serotonin transporter 5-HTTLPR genotype x trauma exposure interaction on a contemporary phenotypic model of posttraumatic stress symptomatology: A pilot study. Journal of Affective Disorders. 2013:148. doi: 10.1016/j.jad.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychiatric Gwas Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nature Genetics. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MaR, Bender D, Maller J, Sklar P, De Bakker PIW, Daly MJ, Sham PC. PLINK: a toolset for whole-genome association and population-based linkage analysis. American Journal of Human Genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Rakofsky JJ, Ressler KJ, Dunlop BW. BDNF function as a potential mediator of bipolar disorder and post-traumatic stress disorder comorbidity. Molecular Psychiatry. 2012;17:22–35. doi: 10.1038/mp.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J, Chan C, Paxson C, Rouse CE, Waters M, Fussell E. The impact of hurricane katrina on the metal and physical health of low-income parents in New Orleans. American Journal of Orthopsychiatry. 2010;80:233–243. doi: 10.1111/j.1939-0025.2010.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richburg-Hayes L, Brock T. Rewarding persistence: Effects of a performance-based scholarship program for low-income parents. New York: MDRC; 2009. [Google Scholar]
- Risch N, Herrell R, Lehner T, Kung-Yee L, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta analysis. Journal of the American Medical Association. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Psychiatric Genome-Wide Association Study (Gwas) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nature Genetics. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semplicini A, Lenzini L, Sartori M, Papparella I, Calo LA, Pagnin E, Strapazzon G, Benna C, Costa R, Avogaro A, Ceolotto G, Pessina AC. Reduced expression of regulator of G-protein signaling 2 (RGS2) in hypertensive patients increases calcium mobilization and ERK1/2 phosphorylation induced by angiotensin II. Journal of Hypertension. 2006;24:1115–1124. doi: 10.1097/01.hjh.0000226202.80689.8f. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Paulus MP, Fagerness JA, Purcell S, Yamaki LH, Hirshfeld-Becker DR, Biederman J, Rosenbaum JF, Gelernter J, Stein MB. Influence of RGS2 on anxiety-related temperament, personality, and brain function. Archives of General Psychiatry. 2008;65:298–308. doi: 10.1001/archgenpsychiatry.2007.48. [DOI] [PubMed] [Google Scholar]
- Sullivan G, Vasterling JJ, Han X, Tharp AT, Davis T, Deitch EA, Constans JI. Preexisting mental illness and risk for developing a new disorder after Hurricane Katrina. Journal of Nervous and Mental Disease. 2013;201:161–166. doi: 10.1097/NMD.0b013e31827f636d. [DOI] [PubMed] [Google Scholar]
- Tang CS. Positive and negative post-disaster psychological adjustment among adult survivors of the Southeast Asian earthquke-tsunami. Journal of Psychosomatic Research. 2006;61:699–705. doi: 10.1016/j.jpsychores.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Tang CS-K. Posttraumatic Growth of Southeast Asian Survivors with Physical Injuries: Six Months after the 2004 Southeast Asian Earthquake-Tsunami. The Australasian Journal of Disaster and Trauma Studies. 2001;2007-1 [Google Scholar]
- Tedeschi RG, Calhoun LG. Trauma and transformation: Growing in the aftermath of suffering. Thousand Oaks, CA: Sage; 1995. [Google Scholar]
- Tedeschi RG, Calhoun LG. The posttraumatic growth inventory: Measuring the positive legacy of trauma. Journal of Traumatic Stress. 1996;9:455–471. doi: 10.1007/BF02103658. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Parker KJ, Fallmayer JF, Waugh CE, Gotlib IH. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology. 2011;36:144–147. doi: 10.1016/j.psyneuen.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindberg A. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proceedings of the National Academy of Science. 107:13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Commerce. Gulf coast recovery: 17 months after the hurricanes. 2006. [Google Scholar]
- Weiss DS, Marmar CR. The impact of events scale-revised. In: WILSON JP, KEAN TM, editors. Assessing psychological trauma and PTSD: A practitioner’s handbook. New York, NY: Guilford; 1997. [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, Weiss RD, Farrer L, Gelernter J. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR polymorphism on posttraumatic stress disorder diagnosis in 2 independent populations. Archives of General Psychiatry. 2009;66:1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liao Q. Prevalence and predictors of posttraumatic growth among adult survivors one year following 2008 Sichuan earthquake. Journal of Affective Disorders. 2011;133:247–280. doi: 10.1016/j.jad.2011.03.034. [DOI] [PubMed] [Google Scholar]
- Yu X, Lau JTF, Zhang J, Mak WWS, Choi KC, Lui WWS, Zhang J, Chan EYY. Posttraumatic growth and reduced suicidal ideation among adolescents at month 1 after the Sichuan earthquke. Journal of Affective Disorders. 2010;123:327–331. doi: 10.1016/j.jad.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Zhang L, Benedek DM, Fullerton CS, Forsten RD, Naifeh JA, Li XX, Hu XZ, Li H, Jia M, Xing GQ, Benevides KN, Ursano RJ. PTSD risk is associated with BDNF Val66Met and BDNF overexpression. Molecular Psychiatry. 2013 doi: 10.1038/mp.2012.180. [DOI] [PubMed] [Google Scholar]


