Abstract
Objective
To summarize the current literature on racial and gender disparities in critical care and the mechanisms underlying these disparities in the course of acute critical illness.
Data Sources
MEDLINE search on the published literature addressing racial, ethnic, or gender disparities in acute critical illness such as sepsis, acute lung injury, pneumonia, venous thromboembolism, and cardiac arrest.
Study Selection
Clinical studies that evaluated general critically ill patient populations in the United States as well as specific critical care conditions were reviewed with a focus on studies evaluating factors and contributors to health disparities.
Data Extraction
Study findings are presented according to their association with the incidence, clinical presentation, management, and outcomes in acute critical illness.
Data Synthesis
This review presents potential contributors for racial and gender disparities related to genetic susceptibility, comorbidities, preventive health services, socioeconomic factors, cultural differences, and access to care. The data is organized along the course of acute critical illness.
Conclusions
The literature to date shows that disparities in critical care are most likely multifactorial involving individual, community, and hospital-level factors at several points in the continuum of acute critical illness. The data presented identify potential targets as interventions to reduce disparities in critical care and future avenues for research.
Keywords: health disparities, critical illness, organ dysfunction, outcomes
Introduction
There are racial, gender, and socioeconomic disparities in the most common causes of morbidity and mortality in the US (1). Racial and ethnic minorities seem less likely to receive appropriate medical care in the hospital ward and intensive care unit (ICU) (2) (3) (4). Since racial and ethnic identity is a measure of the social and historical heritage from a particular country, this review will focus on US-based disparities in health. Eliminating health disparities is the top priority of multiple regulatory bodies and initiatives in the US (e.g., the National Institutes of Health (5), the Institute of Medicine (IOM), the Patient Protection Affordable Care Act (6)). The Committee on Comparative Effectiveness Research has declared racial and ethnic disparities in healthcare delivery to be one of the nation’s top research priorities (7). In this review, the term ‘health disparity’ is used, as per the IOM report, to describe any differences in health status, health outcomes, and healthcare use that reflect a gap in the quality of care delivered and may be caused by societal inequities (e.g., differential socioeconomic status (SES)) and patient, provider, or system-level factors that result in differential treatment (2).
A body of literature shows that health disparities indeed exist in our critically ill patients (8) (9). To date, most of this literature is descriptive without interventional studies to reduce health disparities in these patients. Therefore, the purpose of this review is to summarize the current state of the critical care literature on the possible contributors underlying health disparities, where they exist in the course of acute critical illness, and identify potential targets for intervention (Figure 1).
Figure 1. Healthcare Disparities along the Continuum of Acute Critical Illness.
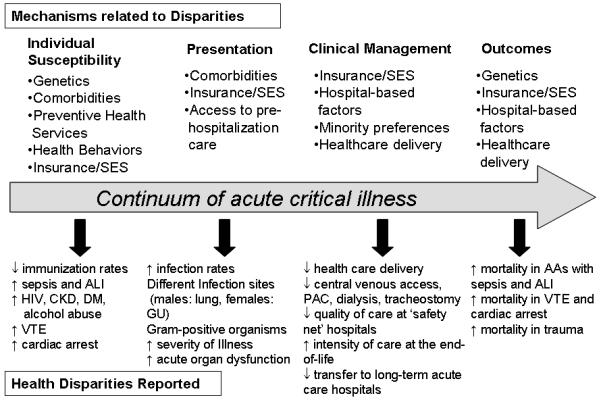
AA, African American; ALI, acute lung injury; CKD, chronic kidney disease; DM, diabetes mellitus; GU, genitourinary; HIV, human immunodeficiency virus; PAC, pulmonary artery catheterization; SES, socioeconomic status; VTE, venous thromboembolism
Health Disparities in the course of Acute Critical Illnesses
Critical illness is unique in the acuity of its presentation and the compressed time frame for disease development and course. Nevertheless, health disparities are documented during the entire course of acute critical illness from the individual susceptibility to acute illness in the community, to the clinical presentation and medical management in the hospital, and subsequent outcomes (Figure 1). Since the factors involved in health disparities differ depending on where these disparities occur along the course of acute critical illness, a clear understanding of where these disparities exist is pivotal to devising future interventions to improve outcomes for all patients regardless of gender, race, and SES.
Traditionally, race was defined as a biological construct affected by genetic makeup and ancestry (10). Race is now recognized to be a social construct and a marker for social class, culture, environmental differences or as proxy for SES (10, 11). Even though self-report is considered the optimal method for collecting racial data (10), research studies have assigned race based on skin color, direct observation, proxy report or extraction from medical records. Furthermore, race and ethnicity are often coded differently in state vital statistics, medical records, or the US Census (12). For this review, we will report race as defined by each study but will specify how race was defined to facilitate interpretation by the readers (Tables 1-4).
Disparities in the incidence of acute critical illness (supplemental digital content, Table 1)
Men and African Americans have a higher incidence of conditions that require ICU-level of care. Males have a higher incidence of sepsis (13) (14) (15) and noncardiogenic acute respiratory failure (16) (17). Further data is needed to determine whether gender is an independent risk factor for venous thromboembolism (VTE) as studies show either higher rates of first VTE in women (18), men (19), or no differences at all (20) (21). However, when looking at particular age groups, there seems to be a higher risk of VTE among women of childbearing age (20) (19) and elderly males (21) (22) (23). Males also seem to have higher rates of recurrence (24) (25) but not all studies support these findings (26).
The racial and gender differences in sepsis are documented in multiple observational and randomized controlled trials (27) (14) (28) (29) (30). African Americans and other non-whites have a higher incidence of sepsis compared to whites (31) (13). Even after adjusting for differences in poverty and region of residence, African American race remains independently associated with higher incidence of sepsis (31). African Americans also have higher age-adjusted rates of cardiac arrest in or out of the hospital (32) (33) (34), acute lung injury (ALI) (35), noncardiogenic acute respiratory failure (16), and VTE (17) (36) (18). It is unlikely that the different rates of VTE are due to diagnostic or ascertainment bias since nationwide data show no differences in use of ventilation-perfusion lung scans, venous ultrasonography, or contrast venography by race or gender (37) (38) .
Contributors to disparities in incidence
Genetic Susceptibility
Differences in hormonal milieu and healthcare access do not fully explain the racial or gender disparities in sepsis (14, 39) (40). Genetic variations may alter the host immunological response and modulate the risk for infection and acute critical illness (41) (42) (43) (44) (45). Several variants of genes involved in inflammatory and innate responses to infection show different allelic frequencies by race and gender in ALI and sepsis, suggesting that race and gender may have variable inherent response to infection. For instance, polymorphisms in lipopolysaccharide binding protein and tumor-necrosis factor B are associated with higher risk of sepsis in males (46) (47) (48) (49). Allelic variants in myosin light chain kinase confer higher risk of sepsis and ALI in African Americans (50) (51).
However, genetic epidemiological studies in critical illness have been limited by including mostly white patients, small sample size, misclassification of syndromal phenotypes like sepsis and ALI, variable methods for adjustment of population admixture, and difficulty in defining race genetically(52) (53) (54) (55) (56).
Genetic susceptibility alone does not fully explain the differences in prevalence of common acute critical illness. For instance, African Americans do not carry the most common mutations associated with higher risk of VTE (36) (57) (58). However, since genetic conditions account only for a small proportion of VTE in general, it is likely that other unknown genetic markers might be more prevalent among African Americans and account for these racial differences.
Chronic Comorbid Conditions
Racial differences in comorbid conditions prior to hospital admission may increase the susceptibility to acute critical illness (13) (31) (14) (40). African Americans have higher rates of comorbidities that increase the risk of acute respiratory failure such as sepsis and acute renal failure (13) (31). Non-white patients have more comorbidities that alter immune function (e.g., HIV, alcohol abuse, chronic renal failure, and diabetes mellitus) (40) (59) and higher rates of acute organ dysfunction and progression to severe sepsis (40). Consistently, studies show that African American septic patients are not only younger but more likely to have diabetes, chronic renal failure, obesity, and HIV. Remarkably, 12% of African Americans with sepsis had HIV compared to only 0.7% of whites (59).
African Americans also have higher rates of comorbidities that increase the risk of VTE such as obesity, hypertension, diabetes, and kidney disease while surgery, trauma, and infections are more prevalent in whites (36) (58).
Socioeconomic Status, Health Behaviors, and Access to Care
Considerably evidence indicates that health status, services, and mortality differ by SES, race, and ethnicity in the US (60) (61). A lower SES and minority race are associated with poor health (62), lower rates of health insurance, less access to preventive health services and primary care (63). SES is a confounder of racial differences in health and part of the causal pathway by which race affects health (64). However, many studies on health disparities fail to adjust for SES partly because SES data is not reported or routinely collected in the US (65) (66). Additionally, different SES indicators capture varying aspects of health risk and may be inaccurate (10) (67). For instance, the use of aggregate geographic measures such as zip codes or census track do not reflect the impact of individual- and household-level factors on health outcomes (68) (69).
Differences in SES and environmental factors such as education, poverty, and segregation contribute significantly to poor health habits and lack of care of comorbid conditions in patients from the minority groups that places them at higher risk for acute critical illness and poor health status prior to hospital admission (70). Minorities with lower SES and those who live in areas with higher rates of poverty have higher incidence of acute critical illness (71) (72) and severe sepsis (31).
Racism is a known cause of health disparities outside of critical care and a possible, but less studied, contributor to disparities in acute critical illness (73) (74). Racial bias leads to disparities in healthcare through residential racial segregation (75) that adversely affects health by limiting education, employment, quality of life, healthy lifestyles, access to medical care, and increasing exposure to environmental toxins and stressors (76) (74). However, precise measures of racial discrimination are not available and most studies are based on surveys and respondent-perceived attitudes and behaviors.
Disparities in clinical presentation (supplemental digital content, Table 2)
African American males develop sepsis at a younger age and are more likely to require ICU admission (13) (15). Males are more likely to have pulmonary infections which represent 40% of sepsis cases while females are more likely to develop sepsis from genitourinary sources which only account for 10% of cases (14) (27) (40). In addition, even after controlling for the source of infection, males are more likely to have sepsis from Gram-positive organisms (40). Similarly, African Americans are more likely to have Gram-positive infections independent of the infection source (24) and higher proportion of invasive pneumococcal disease in adults < 65 years compared to whites (77). This has significant clinical repercussions since Gram-positive infections are less responsive to therapy and lead to more severe illness (78) and higher rates of acute organ failure (40) (79) (80). In the study by Mayr (77), African Americans had higher infection-related hospitalization rates and increased risk of developing acute organ dysfunction (e.g., acute renal failure, acute respiratory failure). The study by Esper also showed that African American and other race patients were more likely to develop at least 1 acute organ dysfunction in sepsis (severe sepsis) when compared with whites (35% vs. 37% vs. 29%, p < 0.01) (40).
African Americans do not only present with acute critical illness at a younger age but they also have more physiological derangements and a higher severity of illness on admission to the hospital and the ICU (81) (82) (31) (83) (84). Furthermore, they also have cardiac arrests at a younger age with longer time for return of spontaneous circulation, less shockable rhythms, delayed defibrillation, and less bystander-initiated cardiopulmonary resuscitation (CPR) (85) (86) (87).
Contributors to disparities in clinical presentation
Access to Care and Preventive Health Services
The higher rates of invasive pneumococcal disease in African Americans are most likely due to their lower rates of pneumococcal vaccination (88) (77). Indeed, among patients hospitalized with community-acquired pneumonia (CAP), prior pneumococcal vaccination is associated with increased survival, decreased risk of respiratory failure, decreased length-of-stay (LOS), and ICU admission (89) (90). The poor often do not have health insurance and this may contribute to a greater burden of comorbidities, delays in seeking medical care and diagnosing at-risk conditions for critical illness, a higher severity of illness and a higher risk of acute organ failure on hospital presentation (81).
Chronic Comorbid Conditions
Several chronic medical conditions differ by race prior to the time of in-hospital cardiac arrest (85). Compared to whites, African Americans are sicker at the time of the arrest and have higher rates of renal insufficiency and hemodialysis, diabetes mellitus, baseline central nervous system depression, stroke, pneumonia, sepsis, and major trauma.
Alcohol abuse plays an important role in acute critical illness. Alcohol-use disorder is more common among African Americans, Hispanics and Native American critically ill patients (91). Critically ill patients with alcohol-use disorder have higher severity of illness, rates of sepsis, ALI, organ dysfunction, pneumonia and postoperative complications (92) (93, 94) (95) (96) (97). However, evidence attributing the racial disparities in acute critical illness to drug- or alcohol-related disorders is currently unavailable.
Disparities in clinical management (supplemental digital content, Table 3)
The care delivered to critically ill African American patients may differ from care delivered to white patients before, during, and after the ICU stay (81). Compared to whites with the same diagnosis, non-whites are admitted to the ICU less often even after controlling for hospital and insurance plans (3) (98). African Americans are less likely to be admitted to cardiac care units (99), wait longer to be admitted to an ICU from the emergency room (ER) (100), and are less likely to receive interventions such as dialysis, tracheostomy, central venous access, and pulmonary artery catheterization even after adjusting for severity of illness (101) (81). Non-whites with sepsis and mechanically ventilated African American patients are less likely to be discharged to another medical facility after an ICU stay (13) (40) or to long-term acute care hospitals (102).
While this is certainly concerning for treatment bias, it is also important to note that differential treatment may reflect differences in the incidence of critical illness, the clinical need, or personal preferences for medical care. For example, while minorities tend to present with greater severity of illness, they may also be younger and have better recovery of organ failures. We need additional studies that examine the racial and gender utilization of critical care interventions that account for variable incidence of critical illness, clinical indication, access, and personal preferences to assess this potential contributor to racial disparities in critical care.
Contributors to disparities in clinical management
Healthcare Delivery and Processes of Care
A significant component of the racial differences in delivery of care can be attributed to the type of institutions that predominantly serves minority patients. Hospitals caring for greater number of African American patients are more likely to be urban and nonprofit and to care for patients on Medicaid (103). These ‘safety net’ hospitals have more financial constraints that limit quality improvement initiatives and lack the infrastructure for the delivery of high-quality of care (104). This is clearly documented in the treatment of CAP (84) (105). Mayr showed that African Americans with CAP are less likely to receive guideline-concordant antibiotics within a 4 hour window than whites but these racial differences were due to differences in healthcare delivery across hospitals but not within the same institution (84). In contrast, at institutions like the VA system with equal access to care, African American and whites patients with CAP were equally likely to receive guideline-concordant antibiotics either in the medical wards or the ICU (106). In a study by Pines, African American patients waited longer in the ER for an ICU bed than other patients in the same hospital; indicating within-hospital racial differences. However, for non-ICU patients, the difference in ER LOS was explained by between-hospital differences: hospitals with a higher proportion of ER visits by African Americans had longer ER stays than hospitals with lower number of ER visits by African Americans. Overall, the data to date indicate that institutional differences in where minorities seek medical care contribute to the quality gap in healthcare (107) (108).
Socioeconomic Status and Access to Care
The poor often do not have health insurance and have less access to costly care or procedures during hospitalization. This is of great concern since African American septic patients are four times more likely to be uninsured than whites (59). Self-pay patients receive the lowest intensity of care such as radiographic and surgical procedures, consultations, and ICU care (3). Indeed, the availability of medical insurance is a determinant of intensity of critical care services in critically ill patients (109). A recent US study showed that within the same hospital, uninsured critically ill patients had less use of common critical care procedures compared to those with health insurance even after adjusting for severity of illness (110). Race and medical insurance status may also affect post-ICU care and long-term acute care utilization (40) (102). However, it is not yet clear whether these differences in access to long-term acute care contribute to disparities in long-term outcomes.
Differences in Preferences for Care
The ICU is a common site for the delivery of end-of-life care (111). African Americans and Hispanics prefer more intensive level of care at the end-of-life (112) (113) (114). They are less likely to discontinue mechanical ventilation (115), have advance directives and do-not-resuscitate orders, or pursue palliation (116) (117) (118). The preferences for end-of-life care are influenced by the race, ethnicity, and cultural beliefs of the patient or surrogate (119) (120). African American patients and their surrogates have more optimistic views about critical illness, prognosis, and treatment efficacy that are associated with more aggressive care at the end-of-life (121) (122) (123). Survival is minimally prolonged even with a higher intensity of care at the end-of-life (112). The discrepancy between increased preferences for aggressive care at the end-of-life in certain racial populations without improved outcomes raises the question of whether there might be physician and patient miscommunication about therapeutic options and benefits at the end-of-life. Indeed, a study by Muni suggests that race and ethnicity may contribute to discordance between physicians’ communication with patients and surrogates (120).
Disparities in outcomes from acute critical illness (supplemental digital content, Table 4)
Mortality
Due to differences in study design, patient population, and case mix, the data on the influence of gender and race on the outcomes of critical illness has yielded conflicting results. Overall, males and African Americans have higher mortality from sepsis (13) (14, 124) (59) and ALI (83) (125). African Americans have higher age-adjusted mortality rates from VTE (17) (57) (126) (127), and lower rates of successful resuscitation from in-hospital and out-of-hospital cardiac arrest (32) (33) (34), less post-resuscitation survival (85) (86) (87), lower risk-adjusted rates of 1-year survival and higher readmission rates (128). Data from the ARDS Network and the trauma registries show that even though ARDS mortality is declining (129), Hispanic patients have higher mortality than whites after adjustment for severity of injury and comorbidities (130). There is inconclusive data on gender-based differences in mortality from VTE and further research is needed to address this question. While some studies show a higher mortality in men (17), particularly among the elderly(131), others show no gender differences at all (23) (57).
Even though there are gender and racial differences in mortality, there does not appear to be a disparity in the case-fatality rates in males and African Americans with sepsis (13) (14, 124) (59), ALI (83), VTE (127), or acute respiratory failure (16) except for recent data in trauma-related ALI showing increased case-fatality rates among Hispanic patients (130). The literature so far suggests that the likelihood of dying once critically ill septic patients are hospitalized does not differ by race or gender and it argues that perhaps the main contributors to disparities are chronic disease, outpatient care, and access to but not delivery of care. This is supported by the studies on CAP that show similar clinical outcomes even though there are differences in the quality of care for CAP (84) (105) .
Most studies on racial disparities in critically illness have focused on African Americans and not on other ethnic or racial groups. A recent study by Cooke showed that even though both African American and other race patients had higher risk of acute respiratory failure, only patients from the other race group with at least 2 organ failures had higher case fatality rates compared to whites (16). A study by Melamed reported significant sepsis-associated mortality in American Indians/Alaska natives, and Hispanics (132). It is unclear in this study whether the racial difference in sepsis-related mortality is due to different mortality in sepsis or different incidence of sepsis which carries a high mortality.
The interpretation of mortality data in critical care is hindered by several methodological issues. Even though minorities are admitted with more physiological derangements, not many studies adjusted for severity of illness in their outcome analysis. In a recent multicenter study by Erickson, African American patients had a higher hospital mortality than whites; however, after adjusting for severity of illness, there were no differences in mortality or ICU LOS (82). This is consistent with epidemiological data in sepsis (13), ALI (83), and general ICU populations (52) that showed no difference in severity-adjusted hospital mortality between African Americans and whites.
Another problem is overestimation of mortality rates from census undercount (11). US Census data is commonly used to calculate the denominators for mortality rates but while the overall undercount for the US population is small, it is larger for African Americans, Hispanics, and reservation Indians. Understanding the racial, gender and age differences in mortality after critical illness is further hampered by the under-enrollment of minorities, women, and the elderly in critical care research. Even though recent pooled data from 3 ARDS Network studies indicated no differences in the likelihood of enrollment by race or ethnicity, men and patients ≥ 75 years of age were less likely to be enrolled due to medical comorbidities (83) (133). Lower consent rates of racial and ethnic minorities are also documented in trauma research (134).
Functional Outcomes
Relatively few studies have examined disparities in other outcomes related to critical care such as health-related quality-of-life, cognitive, psychological, and physical disability. The limited data indicate that minorities are at higher risk of poor functional outcomes. Compared to whites, African Americans and Hispanics have worse outcomes related to quality-of-life, emotional and neurobehavioral complications, less community integration, and are less likely to receive treatment and be employed after traumatic brain injury (135). Minority patients also have higher levels of post-traumatic stress disorder (PTSD) after trauma (136). The literature in this area is scant and it is unclear whether the PTSD can be attributed to greater severity of injury that is often reported in minority patients, or to events before and after hospitalization.
Contributors to disparities in outcomes
Genetic Susceptibility
Even though the genetic makeup does not seem to account for a large proportion of the racial and genetic differences in health, genetic markers may contribute to adverse outcomes. In sepsis, polymorphisms of lipopolysaccharide binding protein and tumor-necrosis factor B are associated with non-survivors and higher hospital mortality in males (46) (48) (49). Furthermore, a functional polymorphism of the Duffy antigen/receptor gene has been recently associated with worse clinical outcomes among African American patients with ALI (137).
Healthcare Delivery and Processes of Care
The racial disparities in survival for in-hospital cardiac arrest may be due to differences in care provided by the institutions in which African Americans receive medical care before, during, and after cardiac arrest (138). Prior to in-hospital cardiac arrest, African Americans are more likely to be admitted to an un-monitored bed, receive care in a hospital with > 500 beds, and to have delayed defibrillation (> 2 min) (85). The survival data in in-hospital cardiac arrest strongly supports that disparities in healthcare are due to African Americans receiving care in lower-quality hospitals with the lowest survival rates (31) (84) (103) (139). Other possible mechanisms such as racial differences in resuscitation-related variables (e.g., time to subsequent defibrillations, quality of chest compressions, number and timing of resuscitation medications) have been proposed and need further study (85).
In addition, the association of African American race with lower rates of long-term survival and higher re-admission rates (128) raises the possibility of differences in care after discharge. Further research is needed to determine whether there are racial differences in discharge destination, cardiac catheterization and implantation of cardioverter-defibrillator during the index hospitalization, or access to follow-up outpatient care.
Socioeconomic Status and Access to Care
Race and the availability of medical insurance are predictors of mortality in critically ill and trauma patients (109) (140). Within the same hospital, uninsured critically ill patients have an increased 30-day mortality compared to those with health insurance even after adjusting for severity of illness (110). In a retrospective study by O’Brien, the lack of health insurance remained associated with sepsis-associated mortality after stratifying the hospitals based on rates of sepsis-associated admission or mortality by age (18-64 y/o and ≥65 y/o) (141). Sepsis-associated mortality was not explained by sociodemographic factors, comorbidities or hospital rates of sepsis-related admission or deaths.
The association between insurance status and worse mortality in critical illness does not necessarily imply treatment bias in hospital care of acute critical illness. Insurance status and access to care prior to or after hospital presentation may contribute more to short- and long-term mortality differences than treatment received in the hospital. For example, in VTE, Schneider found that whites were significantly more likely to be covered by Medicare (p < 0.001) and African Americans were more likely to be uninsured, self-pay, or have charity care (p<0.02) (127). In addition, there was a higher incidence and mortality from VTE in African Americans. However, the in-hospital case fatality was similar between race groups, suggesting that the racial differences in mortality from VTE are independent of hospital care practices and that the disparity may occur prior to the index hospitalization for VTE.
Addressing Health Disparities in the ICU
The contributors to health disparities in acute critical illnesses are diverse including host differences in genetic predisposition and chronic conditions and variable delivery of care before, during, and after hospitalization for acute injury (Figures 1 and 2). SES is among one of the strongest determinants of disparities in health. Research on racial disparities in critical care should seek to understand how socioeconomic and environmental factors combine with clinical determinants to affect the distribution and outcomes of critical illness (142). Furthermore, we need public policies with a focus on community interventions to improve the health conditions and access to healthcare of minorities prior to the development of acute critical illness and the need for in-patient care.
Figure 2. Factors related to Healthcare Disparities in Acute Critical Illness.
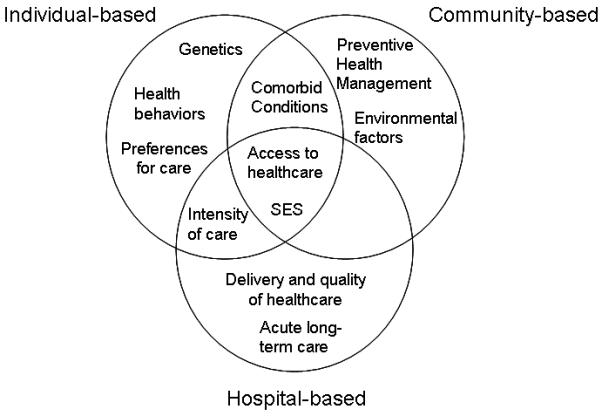
SES, socioeconomic status
No studies have yet addressed interventions to reduce racial and ethnic disparities in the ICU, but the literature to date identifies potential targets along the course of acute critical illness. The Affordable Care Act (6) provides the opportunity to address some of the areas that affect health disparities (e.g., access to healthcare). Even though the genetic makeup cannot be altered, genetic susceptibility may allow for risk prognostication and identification of high risk individuals for preventive strategies. To decrease the susceptibility to critical illness and reduce disparities in disease prevention, presentation, management and outcomes, changes in healthcare policy should involve interventions at several levels. Community-based interventions should address CPR training, increase immunization rates in African Americans, and management of chronic diseases. Of particular importance is to improve SES and environmental factors such as education, segregation, and poverty that directly affect health behaviors, lifestyles, and health status. Regional-based interventions to increase access to health insurance and medical care may improve the care of chronic pre-existing conditions, allow patients to seek medical care early on in their critical illness, and minimize differential access to post-acute care by minorities. Hospital-based interventions should focus on management of acute organ dysfunction and increase quality improvement strategies at institutions with limited resources. Standard admission criteria across long-term acute care hospitals may also improve access to post-acute care by minorities. Lastly, addressing preferences for care and palliation in patients at the end-of-life early on during the ICU admission may provide appropriate medical care and options to patients and families according to their cultural beliefs.
Conclusions
The disparities in acute critical illness are likely multifactorial and involve individual, community, and hospital-level factors at several points in the course of critical illness. An understanding of where and what type of disparities exist in critical care is vital prior to embarking on any intervention to reduce health disparities. The literature presented in this review identifies potential targets for interventions and future areas of research.
Supplementary Material
Acknowledgments
Funding for this Manuscript: None.
MNG is supported by the following grants from the NHLBI: R01 HL086667, UO1 HL108712, and R01 AG035117. GSM is supported by grants from NCATS (UL1 TR000454), NHLBI (R21 HL110044), NIAAA (P50 AA013757) and FDA (R01 FD003440).
Footnotes
Disclosures:
Dr. Greg served as a board member for Cumberland Pharmaceuticals, Astra Zeneca, Pulsion Medical Systems, and Agennix; consulted for Astra Zeneca; and received support for article research from the National Institutes of Health. Dr. Greg’s institution received grant support from NIH and FDA. Dr. Gong is employed by Montefiore Med Ctr; lectured for Beth Isreal Deaconess Med Ctr. Columbia University, Mt Sinai Hospital (Grand Rounds lectures); and received support for article research from NIH. Dr. Gong’s institution received grant support from NIH. Dr. Soto disclosed that she does not have any potential conflicts of interest.
Supplemental Digital Content
The supplemental material includes 4 separate tables describing results from studies that have evaluated disparities in the incidence, clinical presentation, management and outcomes from acute critical illness.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wong MD, Shapiro MF, Boscardin WJ, et al. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347(20):1585–1592. doi: 10.1056/NEJMsa012979. [DOI] [PubMed] [Google Scholar]
- 2.Smedley BDSA, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. 2003. [PubMed] [Google Scholar]
- 3.Yergan J, Flood AB, Diehr P, et al. Relationship between patient source of payment and the intensity of hospital services. Med Care. 1988;26(11):1111–1114. doi: 10.1097/00005650-198811000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Ferraro KF, Shippee TP. Black and white chains of risk for hospitalization over 20 years. J Health Soc Behav. 2008;49(2):193–207. doi: 10.1177/002214650804900206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. [Accessed March 20, 2012];Healthy People 2010. Available at: http://www.healthypeople.org/
- 6. [Accessed March 29, 2013];HR 3590 (111th): Patient protection and affordable care act. Available at: http://www.govtrack.us/congress/bills/111/hr3590/text.
- 7.Institute of Medicine (U.S.) Committee on Comparative Effectiveness Research Prioritization. Initial national priorities for comparative effectiveness research. National Academic Press; Washington, D.C.: 2009. [Google Scholar]
- 8.Foreman MG, Willsie SK. Health care disparities in critical illness. Clin Chest Med. 2006;27(3):473–486. vii. doi: 10.1016/j.ccm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Martin GS. Healthcare disparities in critically ill patients. In: Vincent JL, editor. Yearbook of Intensive Care and Emergency Medicine. Springer-Verlag; Berlin, Germany: 2006. pp. 778–785. [Google Scholar]
- 10.Kaplan JB, Bennett T. Use of race and ethnicity in biomedical publication. JAMA. 2003;289(20):2709–2716. doi: 10.1001/jama.289.20.2709. [DOI] [PubMed] [Google Scholar]
- 11.Williams DR. Race/ethnicity and socioeconomic status: measurement and methodological issues. Int J Health Serv. 1996;26(3):483–505. doi: 10.2190/U9QT-7B7Y-HQ15-JT14. [DOI] [PubMed] [Google Scholar]
- 12.Laws MB, Heckscher RA. Racial and ethnic identification practices in public health data systems in New England. Public Health Rep. 2002;117(1):50–61. doi: 10.1016/S0033-3549(04)50108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 14.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. et al: [DOI] [PubMed] [Google Scholar]
- 15.Dombrovskiy VY, Martin AA, Sunderram J, et al. Facing the challenge: decreasing case fatality rates in severe sepsis despite increasing hospitalizations. Crit Care Med. 2005;33(11):2555–2562. doi: 10.1097/01.ccm.0000186748.64438.7b. [DOI] [PubMed] [Google Scholar]
- 16.Cooke CR, Erickson SE, Eisner MD, et al. Trends in the incidence of noncardiogenic acute respiratory failure: the role of race. Crit Care Med. 2012;40(5):1532–1538. doi: 10.1097/CCM.0b013e31824518f2. et al: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163(14):1711–1717. doi: 10.1001/archinte.163.14.1711. [DOI] [PubMed] [Google Scholar]
- 18.White RH, Zhou H, Murin S, et al. Effect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996. Thromb Haemost. 2005;93(2):298–305. doi: 10.1160/TH04-08-0506. [DOI] [PubMed] [Google Scholar]
- 19.Silverstein MD, Heit JA, Mohr DN, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 20.Anderson FA, Jr., Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151(5):933–938. [PubMed] [Google Scholar]
- 21.Cushman MTA, Heckbert SR, et al. Incidence rates, case fatality, and recurrence rates of deep venous thrombosis and pulmonary embolus: the Longitudinal Investigation of Thromboembolism Etiology (LITE) Thromb Haemost. 2001;86(Suppl1) [Google Scholar]
- 22.Kniffin WD, Jr., Baron JA, Barrett J, et al. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med. 1994;154(8):861–866. [PubMed] [Google Scholar]
- 23.Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117(1):19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 24.McRae S, Tran H, Schulman S, et al. Effect of patient’s sex on risk of recurrent venous thromboembolism: a meta-analysis. Lancet. 2006;368(9533):371–378. doi: 10.1016/S0140-6736(06)69110-1. [DOI] [PubMed] [Google Scholar]
- 25.Kyrle PA, Minar E, Bialonczyk C, et al. The risk of recurrent venous thromboembolism in men and women. N Engl J Med. 2004;350(25):2558–2563. doi: 10.1056/NEJMoa032959. [DOI] [PubMed] [Google Scholar]
- 26.Agnelli G, Becattini C, Prandoni P. Recurrent venous thromboembolism in men and women. N Engl J Med. 2004;351(19):2015–2018. doi: 10.1056/NEJM200411043511919. [DOI] [PubMed] [Google Scholar]
- 27.Sands KE, Bates DW, Lanken PN, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278(3):234–240. [PubMed] [Google Scholar]
- 28.Sundararajan V, Macisaac CM, Presneill JJ, et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33(1):71–80. doi: 10.1097/01.ccm.0000150027.98160.80. [DOI] [PubMed] [Google Scholar]
- 29.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 30.Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288(7):862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 31.Barnato AE, Alexander SL, Linde-Zwirble WT, et al. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med. 2008;177(3):279–284. doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker LB, Han BH, Meyer PM, et al. Racial differences in the incidence of cardiac arrest and subsequent survival. The CPR Chicago Project. N Engl J Med. 1993;329(9):600–606. doi: 10.1056/NEJM199308263290902. [DOI] [PubMed] [Google Scholar]
- 33.Galea S, Blaney S, Nandi A, et al. Explaining racial disparities in incidence of and survival from out-of-hospital cardiac arrest. Am J Epidemiol. 2007;166(5):534–543. doi: 10.1093/aje/kwm102. [DOI] [PubMed] [Google Scholar]
- 34.Ehlenbach WJ, Barnato AE, Curtis JR, et al. Epidemiologic study of in-hospital cardiopulmonary resuscitation in the elderly. N Engl J Med. 2009;361(1):22–31. doi: 10.1056/NEJMoa0810245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erickson S, Cooke CR, Eisner MD, et al. Effect of race on the incidence of acute lung injury. Am J Respir Crit Care Med. 2009;179:A5096. [Google Scholar]
- 36.Heit JA, Beckman MG, Bockenstedt PL, et al. Comparison of characteristics from White- and Black-Americans with venous thromboembolism: a cross-sectional study. Am J Hematol. 2010;85(7):467–471. doi: 10.1002/ajh.21735. [DOI] [PubMed] [Google Scholar]
- 37.Stein PD, Hull RD, Patel KC, et al. Venous thromboembolic disease: comparison of the diagnostic process in blacks and whites. Arch Intern Med. 2003;163(15):1843–1848. doi: 10.1001/archinte.163.15.1843. [DOI] [PubMed] [Google Scholar]
- 38.Stein PD, Hull RD, Patel KC, et al. Venous thromboembolic disease: comparison of the diagnostic process in men and women. Arch Intern Med. 2003;163(14):1689–1694. doi: 10.1001/archinte.163.14.1689. [DOI] [PubMed] [Google Scholar]
- 39.Oberholzer A, Keel M, Zellweger R, et al. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000;48(5):932–937. doi: 10.1097/00005373-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Esper AM, Moss M, Lewis CA, et al. The role of infection and comorbidity: Factors that influence disparities in sepsis. Crit Care Med. 2006;34(10):2576–2582. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorensen TI, Nielsen GG, Andersen PK, et al. Genetic and environmental influences on premature death in adult adoptees. N Engl J Med. 1988;318(12):727–732. doi: 10.1056/NEJM198803243181202. [DOI] [PubMed] [Google Scholar]
- 42.Ness RB, Haggerty CL, Harger G, et al. Differential distribution of allelic variants in cytokine genes among African Americans and White Americans. Am J Epidemiol. 2004;160(11):1033–1038. doi: 10.1093/aje/kwh325. [DOI] [PubMed] [Google Scholar]
- 43.Yim JJ, Ding L, Schaffer AA, et al. A microsatellite polymorphism in intron 2 of human Toll-like receptor 2 gene: functional implications and racial differences. FEMS Immunol Med Microbiol. 2004;40(2):163–169. doi: 10.1016/S0928-8244(03)00342-0. [DOI] [PubMed] [Google Scholar]
- 44.Ferwerda B, McCall MB, Alonso S, et al. TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc Natl Acad Sci USA. 2007;104(42):16645–16650. doi: 10.1073/pnas.0704828104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong MN, Wei Z, Xu LL, et al. Polymorphism in the surfactant protein-B gene, gender, and the risk of direct pulmonary injury and ARDS. Chest. 2004;125(1):203–211. doi: 10.1378/chest.125.1.203. [DOI] [PubMed] [Google Scholar]
- 46.Schroder J, Kahlke V, Book M, et al. Gender differences in sepsis: genetically determined? Shock. 2000;14(3):307–310. [PubMed] [Google Scholar]
- 47.Watanabe E, Buchman TG, Hirasawa H, et al. Association between lymphotoxin-alpha (tumor necrosis factor-beta) intron polymorphism and predisposition to severe sepsis is modified by gender and age. Crit Care Med. 2010;38(1):181–193. doi: 10.1097/CCM.0b013e3181bc805d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schroder J, Kahlke V, Staubach KH, et al. Gender differences in human sepsis. Arch Surg. 1998;133(11):1200–1205. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 49.Hubacek JA, Stuber F, Frohlich D, et al. Gene variants of the bactericidal/permeability increasing protein and lipopolysaccharide binding protein in sepsis patients: gender-specific genetic predisposition to sepsis. Crit Care Med. 2001;29(3):557–561. doi: 10.1097/00003246-200103000-00015. [DOI] [PubMed] [Google Scholar]
- 50.Gao L, Grant A, Halder I, et al. Novel polymorphisms in the myosin light chain kinase gene confer risk for acute lung injury. Am J Respir Cell Mol Biol. 2006;34(4):487–495. doi: 10.1165/rcmb.2005-0404OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Christie JD, Ma SF, Aplenc R, et al. Variation in the myosin light chain kinase gene is associated with development of acute lung injury after major trauma. Crit Care Med. 2008;36(10):2794–2800. doi: 10.1097/ccm.0b013e318186b843. [DOI] [PubMed] [Google Scholar]
- 52.Gao L, Barnes KC. Recent advances in genetic predisposition to clinical acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;296(5):L713–725. doi: 10.1152/ajplung.90269.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barnes KC. Genetic determinants and ethnic disparities in sepsis-associated acute lung injury. Proc Am Thorac Soc. 2005;2(3):195–201. doi: 10.1513/pats.200502-013AC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer NJ, Garcia JG. Wading into the genomic pool to unravel acute lung injury genetics. Proc Am Thorac Soc. 2007;4(1):69–76. doi: 10.1513/pats.200609-157JG. [DOI] [PubMed] [Google Scholar]
- 55.Garcia JG, Moreno Vinasco L. Genomic insights into acute inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(6):L1113–1117. doi: 10.1152/ajplung.00266.2006. [DOI] [PubMed] [Google Scholar]
- 56.Yende S, Kammerer CM, Angus DC. Genetics and proteomics: deciphering gene association studies in critical illness. Crit Care. 2006;10(4):227. doi: 10.1186/cc5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang Y, Sampson B, Pack S, et al. Ethnic differences in out-of-hospital fatal pulmonary embolism. Circulation. 2011;123(20):2219–2225. doi: 10.1161/CIRCULATIONAHA.110.976134. [DOI] [PubMed] [Google Scholar]
- 58.Dowling NF, Austin H, Dilley A, et al. The epidemiology of venous thromboembolism in Caucasians and African-Americans: the GATE Study. J Thromb Haemost. 2003;1(1):80–87. doi: 10.1046/j.1538-7836.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- 59.Dombrovskiy VY, Martin AA, Sunderram J, et al. Occurrence and outcomes of sepsis: influence of race. Crit Care Med. 2007;35(3):763–768. doi: 10.1097/01.CCM.0000256726.80998.BF. [DOI] [PubMed] [Google Scholar]
- 60.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294(22):2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 61.Krieger N, Chen JT, Waterman PD, et al. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312–323. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Epstein AM, Ayanian JZ. Racial disparities in medical care. N Engl J Med. 2001;344(19):1471–1473. doi: 10.1056/NEJM200105103441911. [DOI] [PubMed] [Google Scholar]
- 63.Collins K, Hughes DL, Doty M, et al. Diverse Communities, Common Concerns: Assessing Health Care Quality for Minority Americans. Findings From the Commonwealth Fund 2001 Health Care Quality Survey. The Commonwealth Fund; New York: 2002. [Google Scholar]
- 64.Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann NY Acad Sci. 1999;896:173–188. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- 65.Krieger N, Chen JT, Ebel G. Can we monitor socioeconomic inequalities in health? A survey of U.S. health departments’ data collection and reporting practices. Public Health Rep. 1997;112(6):481–491. [PMC free article] [PubMed] [Google Scholar]
- 66.Williams DR. Missed opportunities in monitoring socioeconomic status. Public Health Rep. 1997;112(6):492–494. [PMC free article] [PubMed] [Google Scholar]
- 67.Krieger N, Fee E. Social class: the missing link in U.S. health data. Int J Health Serv. 1994;24(1):25–44. doi: 10.2190/2JG7-YMD5-WCP2-XXNT. [DOI] [PubMed] [Google Scholar]
- 68.Krieger N, Waterman P, Chen JT, et al. Zip code caveat: bias due to spatiotemporal mismatches between zip codes and US census-defined geographic areas--the Public Health Disparities Geocoding Project. Am J Public Health. 2002;92(7):1100–1102. doi: 10.2105/ajph.92.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soobader MJLF, Hadden W, et al. Using aggregate geographic data to proxy Individual socioeconomic status: does size matter? Am J Public Health. 2001;91:632–636. doi: 10.2105/ajph.91.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lillie-Blanton MMR, Taylor AK, et al. Latina and African American Women: Continuing disparities in health. Int J of Health Ser. 1993;23(3):555–584. doi: 10.2190/MNCJ-NB8E-M0WA-1FGM. [DOI] [PubMed] [Google Scholar]
- 71.Kim C, Diez Roux AV, Hofer TP, et al. Area socioeconomic status and mortality after coronary artery bypass graft surgery: the role of hospital volume. Am Heart J. 2007;154(2):385–390. doi: 10.1016/j.ahj.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 72.Rao SV, Schulman KA, Curtis LH, et al. Socioeconomic status and outcome following acute myocardial infarction in elderly patients. Arch Intern Med. 2004;164(10):1128–1133. doi: 10.1001/archinte.164.10.1128. [DOI] [PubMed] [Google Scholar]
- 73.Williams DR, Sternthal M. Understanding racial-ethnic disparities in health: sociological contributions. J Health Soc Behav. 2010;51(Suppl):S15–27. doi: 10.1177/0022146510383838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shavers VL, Fagan P, Jones D, et al. The state of research on racial/ethnic discrimination in the receipt of health care. Am J Public Health. 2012;102(5):953–966. doi: 10.2105/AJPH.2012.300773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Massey XLDB. Issues of racial segregation. Washington Press; 1993. [Google Scholar]
- 76.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mayr FB, Yende S, Linde-Zwirble WT, et al. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA. 2010;303(24):2495–2503. doi: 10.1001/jama.2010.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Opal SM, Cohen J. Clinical gram-positive sepsis: does it fundamentally differ from gram-negative bacterial sepsis? Crit Care Med. 1999;27(8):1608–1616. doi: 10.1097/00003246-199908000-00039. [DOI] [PubMed] [Google Scholar]
- 79.Alberti C, Brun-Buisson C, Goodman SV, et al. Influence of systemic inflammatory response syndrome and sepsis on outcome of critically ill infected patients. Am J Respir Crit Care Med. 2003;168(1):77–84. doi: 10.1164/rccm.200208-785OC. [DOI] [PubMed] [Google Scholar]
- 80.Guidet B, Aegerter P, Gauzit R, et al. Incidence and impact of organ dysfunctions associated with sepsis. Chest. 2005;127(3):942–951. doi: 10.1378/chest.127.3.942. [DOI] [PubMed] [Google Scholar]
- 81.Williams JF, Zimmerman JE, Wagner DP, et al. African-American and white patients admitted to the intensive care unit: is there a difference in therapy and outcome? Crit Care Med. 1995;23(4):626–636. doi: 10.1097/00003246-199504000-00009. [DOI] [PubMed] [Google Scholar]
- 82.Erickson SE, Vasilevskis EE, Kuzniewicz MW, et al. The effect of race and ethnicity on outcomes among patients in the intensive care unit: a comprehensive study involving socioeconomic status and resuscitation preferences. Crit Care Med. 2011;39(3):429–435. doi: 10.1097/CCM.0b013e318206b3af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Erickson SE, Shlipak MG, Martin GS, et al. Racial and ethnic disparities in mortality from acute lung injury. Crit Care Med. 2009;37(1):1–6. doi: 10.1097/CCM.0b013e31819292ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mayr FB, Yende S, D’Angelo G, et al. Do hospitals provide lower quality of care to black patients for pneumonia? Crit Care Med. 2010;38(3):759–765. doi: 10.1097/CCM.0b013e3181c8fd58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chan PS, Nichol G, Krumholz HM, et al. Racial differences in survival after in-hospital cardiac arrest. JAMA. 2009;302(11):1195–1201. doi: 10.1001/jama.2009.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilde ET, Robbins LS, Pressley JC. Racial differences in out-of-hospital cardiac arrest survival and treatment. Emerg Med J. 2012;29(5):415–419. doi: 10.1136/emj.2010.109736. [DOI] [PubMed] [Google Scholar]
- 87.Ebell MH, Afonso AM. Pre-arrest predictors of failure to survive after in-hospital cardiopulmonary resuscitation: a meta-analysis. Fam Pract. 2011;28(5):505–515. doi: 10.1093/fampra/cmr023. [DOI] [PubMed] [Google Scholar]
- 88.The Centers for Disease Control and Prevention [Accessed March 28, 2013]; Available at: http://www.cdc.gov/mmwr/mmwr_wk/wk_cvol.html.
- 89.Fisman DN, Abrutyn E, Spaude KA, et al. Prior pneumococcal vaccination is associated with reduced death, complications, and length of stay among hospitalized adults with community-acquired pneumonia. Clin Infect Dis. 2006;42(8):1093–1101. doi: 10.1086/501354. [DOI] [PubMed] [Google Scholar]
- 90.Johnstone J, Marrie TJ, Eurich DT, et al. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167(18):1938–1943. doi: 10.1001/archinte.167.18.1938. [DOI] [PubMed] [Google Scholar]
- 91.De Wit M, Best AM, Gennings C, et al. Alcohol use disorders increase the risk for mechanical ventilation in medical patients. Alcohol Clin Exp Res. 2007;31(7):1224–1230. doi: 10.1111/j.1530-0277.2007.00421.x. [DOI] [PubMed] [Google Scholar]
- 92.O’Brien JM, Jr., Lu B, Ali NA, et al. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 2007;35(2):345–350. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- 93.Fernandez-Sola J, Junque A, Estruch R, et al. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med. 1995;155(15):1649–1654. doi: 10.1001/archinte.1995.00430150137014. [DOI] [PubMed] [Google Scholar]
- 94.Bercault N, Boulain T. Mortality rate attributable to ventilator-associated nosocomial pneumonia in an adult intensive care unit: a prospective case-control study. Crit Care Med. 2001;29(12):2303–2309. doi: 10.1097/00003246-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 95.Tonnesen H, Kehlet H. Preoperative alcoholism and postoperative morbidity. Br J Surg. 1999;86(7):869–874. doi: 10.1046/j.1365-2168.1999.01181.x. [DOI] [PubMed] [Google Scholar]
- 96.Moss M, Bucher B, Moore FA, et al. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275(1):50–54. [PubMed] [Google Scholar]
- 97.Moss M, Parsons PE, Steinberg KP, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31(3):869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 98.Yergan J, Flood AB, LoGerfo JP, et al. Relationship between patient race and the intensity of hospital services. Med Care. 1987;25(7):592–603. doi: 10.1097/00005650-198707000-00003. [DOI] [PubMed] [Google Scholar]
- 99.Shippee TP, Ferraro KF, Thorpe RJ. Racial disparity in access to cardiac intensive care over 20 years. Ethn Health. 2011;16(2):145–165. doi: 10.1080/13557858.2010.544292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pines JM, Russell Localio A, Hollander JE. Racial disparities in emergency department length of stay for admitted patients in the United States. Acad Emerg Med. 2009;16(5):403–410. doi: 10.1111/j.1553-2712.2009.00381.x. [DOI] [PubMed] [Google Scholar]
- 101.Rapoport J, Teres D, Steingrub J, et al. Patient characteristics and ICU organizational factors that influence frequency of pulmonary artery catheterization. JAMA. 2000;283(19):2559–2567. doi: 10.1001/jama.283.19.2559. [DOI] [PubMed] [Google Scholar]
- 102.Lane-Fall MB, Iwashyna TJ, Cooke CR, et al. Insurance and racial differences in long-term acute care utilization after critical illness. Crit Care Med. 2012;40(4):1143–1149. doi: 10.1097/CCM.0b013e318237706b. [DOI] [PubMed] [Google Scholar]
- 103.Jha AK, Orav EJ, Li Z, et al. Concentration and quality of hospitals that care for elderly black patients. Arch Intern Med. 2007;167(11):1177–1182. doi: 10.1001/archinte.167.11.1177. [DOI] [PubMed] [Google Scholar]
- 104.Amarasingham R, Plantinga L, Diener-West M, et al. Clinical information technologies and inpatient outcomes: a multiple hospital study. Arch Intern Med. 2009;169(2):108–114. doi: 10.1001/archinternmed.2008.520. [DOI] [PubMed] [Google Scholar]
- 105.Frei CR, Attridge RT, Mortensen EM, et al. Guideline-concordant antibiotic use and survival among patients with community-acquired pneumonia admitted to the intensive care unit. Clin Ther. 2010;32(2):293–299. doi: 10.1016/j.clinthera.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 106.Frei CR, Mortensen EM, Copeland LA, et al. Disparities of care for African-Americans and Caucasians with community-acquired pneumonia: a retrospective cohort study. BMC Health Serv Res. 2010;10:143. doi: 10.1186/1472-6963-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hasnain-Wynia R, Baker DW, Nerenz D, et al. Disparities in health care are driven by where minority patients seek care: examination of the hospital quality alliance measures. Arch Intern Med. 2007;167(12):1233–1239. doi: 10.1001/archinte.167.12.1233. [DOI] [PubMed] [Google Scholar]
- 108.Hausmann LR, Ibrahim SA, Mehrotra A, et al. Racial and ethnic disparities in pneumonia treatment and mortality. Med Care. 2009;47(9):1009–1017. doi: 10.1097/MLR.0b013e3181a80fdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fowler RA, Noyahr LA, Thornton JD, et al. An official American Thoracic Society systematic review: the association between health insurance status and access, care delivery, and outcomes for patients who are critically ill. Am J Respir Crit Care Med. 2010;181(9):1003–1011. doi: 10.1164/rccm.200902-0281ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lyon SM, Benson NM, Cooke CR, et al. The effect of insurance status on mortality and procedural use in critically ill patients. Am J Respir Crit Care Med. 2011;184(7):809–815. doi: 10.1164/rccm.201101-0089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Angus DC, Barnato AE, Linde-Zwirble WT, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638–643. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- 112.Barnato AE, Chang CC, Farrell MH, et al. Is survival better at hospitals with higher “end-of-life” treatment intensity? Med Care. 2010;48(2):125–132. doi: 10.1097/MLR.0b013e3181c161e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garrett JM, Harris RP, Norburn JK, et al. Life-sustaining treatments during terminal illness: who wants what? J Gen Intern Med. 1993;8(7):361–368. doi: 10.1007/BF02600073. [DOI] [PubMed] [Google Scholar]
- 114.Caralis PV, Davis B, Wright K, et al. The influence of ethnicity and race on attitudes toward advance directives, life-prolonging treatments, and euthanasia. J Clin Ethics. 1993;4(2):155–165. [PubMed] [Google Scholar]
- 115.Diringer MN, Edwards DF, Aiyagari V, et al. Factors associated with withdrawal of mechanical ventilation in a neurology/neurosurgery intensive care unit. Crit Care Med. 2001;29(9):1792–1797. doi: 10.1097/00003246-200109000-00023. [DOI] [PubMed] [Google Scholar]
- 116.Degenholtz HB, Thomas SB, Miller MJ. Race and the intensive care unit: disparities and preferences for end-of-life care. Crit Care Med. 2003;31(5 Suppl):S373–378. doi: 10.1097/01.CCM.0000065121.62144.0D. [DOI] [PubMed] [Google Scholar]
- 117.Volandes AE, Paasche-Orlow M, Gillick MR, et al. Health literacy not race predicts end-of-life care preferences. J Palliat Med. 2008;11(5):754–762. doi: 10.1089/jpm.2007.0224. [DOI] [PubMed] [Google Scholar]
- 118.Loggers ET, Maciejewski PK, Paulk E, et al. Racial differences in predictors of intensive end-of-life care in patients with advanced cancer. J Clin Oncol. 2009;27(33):5559–5564. doi: 10.1200/JCO.2009.22.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kwak J, Haley WE. Current research findings on end-of-life decision making among racially or ethnically diverse groups. Gerontologist. 2005;45(5):634–641. doi: 10.1093/geront/45.5.634. [DOI] [PubMed] [Google Scholar]
- 120.Muni S, Engelberg RA, Treece PD, et al. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest. 2011;139(5):1025–1033. doi: 10.1378/chest.10-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.True G, Phipps EJ, Braitman LE, et al. Treatment preferences and advance care planning at end of life: the role of ethnicity and spiritual coping in cancer patients. Ann Behav Med. 2005;30(2):174–179. doi: 10.1207/s15324796abm3002_10. [DOI] [PubMed] [Google Scholar]
- 122.Ford D, Zapka J, Gebregziabher M, et al. Factors associated with illness perception among critically ill patients and surrogates. Chest. 2010;138(1):59–67. doi: 10.1378/chest.09-2124. [DOI] [PubMed] [Google Scholar]
- 123.Phelps AC, Maciejewski PK, Nilsson M, et al. Religious coping and use of intensive life-prolonging care near death in patients with advanced cancer. JAMA. 2009;301(11):1140–1147. doi: 10.1001/jama.2009.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 125.Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979-1996) Crit Care Med. 2002;30(8):1679–1685. doi: 10.1097/00003246-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 126.Ibrahim SA, Stone RA, Obrosky DS, et al. Racial differences in 30-day mortality for pulmonary embolism. Am J Public Health. 2006;96(12):2161–2164. doi: 10.2105/AJPH.2005.078618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schneider D, Lilienfeld DE, Im W. The epidemiology of pulmonary embolism: racial contrasts in incidence and in-hospital case fatality. J Natl Med Assoc. 2006;98(12):1967–1972. [PMC free article] [PubMed] [Google Scholar]
- 128.Chan PS, Nallamothu BK, Krumholz HM, et al. Long-term outcomes in elderly survivors of in-hospital cardiac arrest. N Engl J Med. 2013;368(11):1019–1026. doi: 10.1056/NEJMoa1200657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Erickson SE, Martin GS, Davis JL, et al. Recent trends in acute lung injury mortality: 1996-2005. Crit Care Med. 2009;37(5):1574–1579. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ryb GE, Cooper C. Race/ethnicity and acute respiratory distress syndrome: a National Trauma Data Bank study. J Natl Med Assoc. 2010;102(10):865–869. doi: 10.1016/s0027-9684(15)30700-8. [DOI] [PubMed] [Google Scholar]
- 131.Siddique RM, Siddique MI, Connors AF, Jr., et al. Thirty-day case-fatality rates for pulmonary embolism in the elderly. Arch Intern Med. 1996;156(20):2343–2347. [PubMed] [Google Scholar]
- 132.Melamed A, Sorvillo FJ. The burden of sepsis-associated mortality in the United States from 1999 to 2005: an analysis of multiple-cause-of-death data. Crit Care. 2009;13(1):R28. doi: 10.1186/cc7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cooke CR, Erickson SE, Watkins TR, et al. Age-, sex-, and race-based differences among patients enrolled versus not enrolled in acute lung injury clinical trials. Crit Care Med. 2010;38(6):1450–1457. doi: 10.1097/CCM.0b013e3181de451b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sugarman J, Sitlani C, Andrusiek D, et al. Is the enrollment of racial and ethnic minorities in research in the emergency setting equitable? Resuscitation. 2009;80(6):644–649. doi: 10.1016/j.resuscitation.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gary KW, Arango-Lasprilla JC, Stevens LF. Do racial/ethnic differences exist in post-injury outcomes after TBI? A comprehensive review of the literature. Brain Inj. 2009;23(10):775–789. doi: 10.1080/02699050903200563. [DOI] [PubMed] [Google Scholar]
- 136.Santos MR, Russo J, Aisenberg G, et al. Ethnic/Racial diversity and posttraumatic distress in the acute care medical setting. Psychiatry. 2008;71(3):234–245. doi: 10.1521/psyc.2008.71.3.234. [DOI] [PubMed] [Google Scholar]
- 137.Kangelaris KN, Sapru A, Calfee CS, et al. The association between a Darc gene polymorphism and clinical outcomes in African Americans with acute lung injury. Chest. 2012;141(5):1160–1169. doi: 10.1378/chest.11-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Virnig BA, Lurie N, Huang Z, et al. Racial variation in quality of care among Medicare+Choice enrollees. Health Aff (Millwood) 2002;21:224–230. doi: 10.1377/hlthaff.21.6.224. [DOI] [PubMed] [Google Scholar]
- 139.Werner RM, Goldman LE, Dudley RA. Comparison of change in quality of care between safety-net and non-safety-net hospitals. JAMA. 2008;299(18):2180–2187. doi: 10.1001/jama.299.18.2180. [DOI] [PubMed] [Google Scholar]
- 140.Haider AH, Chang DC, Efron DT, et al. Race and insurance status as risk factors for trauma mortality. Arch Surg. 2008;143(10):945–949. doi: 10.1001/archsurg.143.10.945. [DOI] [PubMed] [Google Scholar]
- 141.O’Brien JM, Jr., Lu B, Ali NA, et al. Insurance type and sepsis-associated hospitalizations and sepsis-associated mortality among US adults: a retrospective cohort study. Crit Care. 2011;15(3):R130. doi: 10.1186/cc10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Williams DR, Mohammed SA, Leavell J, et al. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann NY Acad Sci. 2010;1186:69–101. doi: 10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


