Abstract
Interactions between CD83 and its ligand(s) can up-regulate immune responses. M2-CD83 cells, derived by transfecting the M2 clone of mouse melanoma K1735 cells to express mouse CD83, were rejected by syngeneic mice, unless they were injected with a CD83Ig fusion protein. Rejection was mediated by CD4+ and CD8+ T cells plus natural killer cells, whereas rejection of M2-1D8 cells, which express anti-CD137 single-chain variable region fragments (scFv), occurs in the absence of CD8+ T cells. Furthermore, the tumor specificity of the immunity induced by the two cell lines differed. Immunization with live or mitomycin C-treated M2-CD83 cells prevented outgrowth of transplanted M2-WT cells and had therapeutic efficacy against established M2-WT tumors. A highly metastatic clone of K1735 cells, SW1-C, and its subline SW1-P2, which expresses an activating transcription factor 2-driven peptide, were then studied because they have particularly low immunogenicity. Neither SW1-C nor SW1-P2 cells became rejectable after expression of CD83 or anti-CD137 scFv. However, outgrowth of cells from either line was delayed in mice immunized against M2-CD83 or M2-1D8 cells, and immunization with a mixture of mitomycin C-treated cells from M2-CD83 plus M2-1D8 prevented tumor formation by SW1-P2 cells in five of five and by SW1-C cells in three of five mice. We conclude that M2 cells expressing CD83 can induce a tumor-destructive immune response also against SW1 cells and that this response can be made more effective by combining them with M2 cells expressing anti-CD137 scFv. A similar approach may be therapeutically beneficial against certain human cancers.
Keywords: tumor vaccines, immunotherapy, antigen presentation, activating transcription factor 2
CD83 is a marker of activated human dendritic cells (1). In humans, CD83 ligands are primarily expressed on resting monocytes and a subpopulation of activated T cells (2). Transfection of cells from the human B cell line T51 to express CD83 increases their ability to stimulate proliferation of allogeneic peripheral blood mononuclear cells, including CD8+ cytotoxic T lymphocytes, and this effect is abolished in the presence of a soluble CD83 fusion protein with an Ig “tail” (3).
In mice, the ligands for CD83 are primarily expressed on B cells (4), and fusion proteins that express the extracellular part of mouse CD83 (mCD83) inhibit antigen-specific T cell proliferation and IL-2 secretion in cultures of mouse spleen (4). Injection of a CD83 mouse Ig fusion protein can facilitate the outgrowth of transplanted cells from the P815 mastocytoma and decrease a tumor-directed cytotoxic T lymphocyte response (3). Furthermore, cells from the M2 clone of mouse melanoma K1735 cells transfected to express human CD83 were shown to be rejected by syngeneic mice, some of which also rejected a subsequent transplant of nontransfected (WT) cells from the M2 clone (3).
Although mCD83 and human CD83 share 63% amino acid identity (5), an immune response to human antigens may contribute to the immunogenicity of mouse tumor cells expressing human CD83. Therefore, we have now immunized mice with M2 cells expressing mCD83. Furthermore, we have used mitomycin C (MMC)-treated rather than live tumor cells as immunogen in many of our experiments to make them more relevant as models for potential human application. This method also facilitates comparisons between immunizing tumor cells that express CD83 or an irrelevant molecule.
To make our tumor model more demanding, we extended our studies to a different K1735 clone, SW1-C, and the SW1-P2 line, which had been obtained by transfecting SW1 cells to express a 50-aa peptide derived from the NH2-terminal domain of activating transcription factor type 2 (ATF-2) (6, 7). Expression of ATF-2, which belongs to the ATF/CREB (cAMP-response element-binding protein) family, within the nucleus rather than the cytoplasm is associated with poor prognosis of melanoma (8). SW1-P2 cells express less ATF-2 in the nucleus and more in the cytoplasm than SW1-C cells, and this is correlated with decreased malignancy and increased ability to undergo apoptosis after exposure to radiation or certain chemicals (6).
As we now show, neither SW1-C nor SW1-P2 cells became immunogenic after transfection to express CD83. Neither did transfection to express anti-CD137 single-chain variable region fragments (scFv) cause SW1-C or SW1-P2 cells to be rejected, whereas similarly transfected M2 cells are efficacious as therapeutic vaccines against established WT M2 tumors (9). However, outgrowth of both SW1-C and SW1-P2 was delayed in mice immunized with MMC-treated M2 cells that expressed either CD83 or anti-CD137 scFv, and it could be prevented in most mice concomitantly immunized with both M2-CD83 and M2-1D8 cells, probably because these cells engaged the immune system in different ways.
Materials and Methods
Tumor Lines. K1735 is a melanoma of C3H/HeN origin from which clones M2 and SW1 were isolated (10, 11). SW1-P2 was derived by transfecting SW1 cells to express an ATF-2-derived peptide (7). The SW1-C and SW1-P2 cell lines were chosen because pilot tests revealed that they were not rejected by immunocompetent mice after transfection to express anti-CD137 scFv derived from hybridoma 1D8 (data not shown), a procedure that makes cells from the M2 clone highly immunogenic (9). Ag104 is a spontaneous sarcoma of C3H/HeN originally obtained from H. Schreiber (University of Chicago, Chicago) and has low immunogenicity (12). Tumor cells transplanted to mice were derived from in vitro cultures, and suspensions comprising >90% live tumor cells were prepared by exposing cultures to 0.01% versene for 5 min.
Vectors and Transfection of Cells. The mCD83 gene was amplified from anti-CD3 mAb-activated mouse spleen cells by using primers GTGTCGCAGCGCTCCAGCC and GGCATTCAGGCACACTGATC (5). An amplified cDNA fragment was first cloned into pGEM-T easy vector (Promega) and verified by DNA sequencing, after which the mCD83 gene was cloned into pLNCX2 vector (CLONTECH) and pLenti6/V5 vector (Invitrogen). Transfection of a packaging cell line and infection of target cell lines (including cells from the M2 clone of K1735 cells) were performed according to manufacturer's instructions.
To produce the mCD83 Ig fusion protein, the mCD83 extracellular domain (ECD) was amplified from the mCD83 gene by using primers AAGCTTCCAGCCATGTCGCAAGGCCTC and GGATCCGCCCTGTACTTCCTG. The amplified fragment was first cloned to PCR-TOPO vector (Invitrogen) and verified by DNA sequencing. mCD83-ECD was cloned into pD18-mIgG vector and was transfected to COS-7 cells to produce mCD83-ECD-mIgG fusion protein, which was subsequently purified with protein A Sepharose 4B (Sigma).
A CD83-human Ig fusion protein was generated by cloning a human tail (13) in the place of the murine tail (3). It was applied for screening hybridomas for production of anti-CD83 antibody and for fluorescence-activated cell sorter analysis with FITC-labeled goat anti-human Ig used as a second reagent.
Target cells transfected with mCD83 gene were detected with rat anti-mCD83 mAb 7A1 (obtained as described below) and R-phycoerythrin-labeled goat anti-rat Ig. Transfected cells are referred to by the name of the respective clone or subline followed by the transfected gene, e.g., SW1-P2-CD83 cells were derived from SW1-P2 and stably express CD83 at their surface. As one control, M2 cells were transfected with an irrelevant gene (mouse anti-human CD28 scFv) and are referred to as M2-control (9). M2-1D8 cells that express anti-CD137 scFv from hybridoma 1D8 were constructed as described in ref. 9.
For most experiments, tumor cells were sterilized by in vitro exposure to MMC (Sigma). Tumor cells were washed once with PBS and then incubated with 50 μg of MMC per 107 cells for 1 h at 37°C (14), after which they were washed four times with PBS before use in vivo (as vaccines) or in vitro (to stimulate T cell responses).
Antibodies. For in vitro studies, we used R-phycoerythrin-, FITC-, or Biotin-conjugated anti-mouse CD4 mAb GK1.5, similarly conjugated anti-mouse CD8 mAb 53-6.7, R-phycoerythrin-conjugated anti-mouse CD19 and anti-mouse CD11c, as well as PC5-conjugated anti-mouse CD45 and anti-mouse natural killer (NK) mAb, all of which were purchased from Pharmingen. R-phycoerythrin-conjugated goat F(ab′)2 anti-human IgG was purchased from BioSource International (Camarillo, CA). For in vivo studies, we used mAb 169-4 (anti-CD8) produced by a rat hybridoma from R. Mittler (Emory University, Atlanta), anti-CD4 mAb GK1.5 produced by a rat hybridoma from American Type Culture Collection, rabbit anti-asialo GM1 antibodies bought from Wako Pure Chemical (Richmond, VA), and purified rat IgG bought from Sigma and Rockland (Gilbertsville, PA).
To obtain anti-CD83 mAbs, a Lewis rat was first immunized by intramuscular injection of 200 μg of mCD83-ECD-mIgG fusion protein mixed with TiterMax (CytRx, Norcross, GA) and then injected three times s.c. with 150 μg of mCD83-ECD-mIgG every second week. The immunized rat was given a booster 2 weeks after the last immunization by i.p. injection 10 days and i.v. injection 3 days before being killed. Spleen cells were fused with mouse myeloma cells P3-X63-AG8.653 by using standard procedures (15), hybridomas were screened for binding to mCD83-ECD-human IgG fusion protein, and six high-producers were cloned. The highest producer, 7A1, was cultured in protein-free hybridoma medium, (PFHM-II; Invitrogen), and its mAb was purified on Protein G Sepharose 4B (Sigma).
Animal Studies. Six- to 8-week-old female C3H/HeN mice were purchased from Taconic (Charles River Labs, Wilmington, MA). Experiments were carried out in facilities approved by the Association of Laboratory Animal Care under protocols approved by Pacific Northwest Research Institute's Animal Committee. Mice (five per group unless otherwise stated) were immunized by transplantation (s.c. on one side of the back) with 2 × 106 live or MMC-treated tumor cells and challenged with 106 or 2 × 106 live WT cells on the other side of the back. Tumor size was assessed by measuring the two largest perpendicular diameters with calipers and reported as average tumor area (mm2) ± SD. To investigate therapeutic activity, mice with s.c. WT tumors on one side of the back (≈20-mm2 surface area) were immunized by s.c. injection of live or MMC-treated tumor cells (2 × 106 cells per mouse) on the contralateral side of the back. Mice were followed with respect to tumor growth and survival. Mice whose tumors had regressed were observed for a minimum of 120 days and checked for evidence of autoimmunity, including weight loss and depigmentation.
To investigate the in vivo effect of mCD83mIg fusion protein, 2 × 106 M2-CD83 cells per mouse were transplanted s.c. and 100 μg of mCD83mIg fusion protein per mouse was injected i.p. on the day of tumor transplantation, followed by the same dose every third day for a total of four injections. In a parallel experiment, each mouse was instead injected i.p. with 100 μg of rat anti-mCD83 mAb (rat Ig being used as control) on the day of transplantation of M2-CD83 cells (2 × 106 per mouse).
In Vivo Depletion of CD4+ and/or CD8+ T Lymphocytes and of NK Cells. T cells were depleted as described (16) by injecting mice i.p. three times with mAb to CD4 (GK1.5, rat IgG2b) or CD8 (169-4, rat IgG 2a) or with a mixture of the two at 0.5 mg per mouse for 3 consecutive days. This was followed by 0.5 mg of each mAb every third day. NK cells were depleted by injection of anti-asialo GM1 antibodies at 30 μl per mouse i.p. every fourth day. On day 12, spleen cells from each group were analyzed by fluorescence-activated cell sorter to verify that the depletions were efficient. On day 13, each mouse was transplanted s.c. with 2 × 106 M2-CD83 cells and followed for tumor outgrowth.
Phenotypical and Functional Characterization of Lymphoid Cells. Tumors were removed for studies by immunohistochemistry 4-8 days after transplantation of 2 × 106 tumor cells. Frozen sections were cut at 10 μm and stained by using a Vector Laboratories ABC kit according to the manufacturer's instructions and stained to detect CD4+ and CD8+ T cells and NK cells. The sections were counterstained with hematoxylin/eosin.
To study tumor-draining lymph node and spleen cells in vitro, suspensions were prepared mechanically, and the spleen cell suspensions were incubated with red blood cell lysing buffer (Sigma) for 5 min at room temperature. To obtain tumor-infiltrating lymphoid cells, tumors were cut in 1-mm2 pieces and digested for 3 h at room temperature in 1 mM Hepes Hank's buffer (5 units per ml collagenase/0.1% hyaluronidase/0.01% DNase) with enzymes purchased from Invitrogen. The suspensions were filtered, the cells were collected by centrifugation, and the erythrocytes were lysed. After being washed with PBS, the cells were added to a mouse lymphocyte isolation buffer (Cedarlane Laboratories) and centrifuged at 200 × g for 20 min, after which the lymphocyte band was collected and washed with PBS.
Experiments were included in which spleen cells were cultivated in vitro for 4 days in a six-well plate coated with 10 μg/ml anti-CD3 mAb (PN IM2767 from Immunotech) and 10 units per ml IL-2 (Roche) followed by flow cytometry analysis for expression of certain surface markers.
A T cell proliferation assay was performed as described in ref. 9. Spleen cells were seeded into 96-well flat-bottom plates (1 × 105 cells per well) together with 5 × 105 syngeneic MMC-treated spleen cells and MMC-treated tumor cells. After incubation for 72 h, triplicate cultures were pulsed for 16-18 h with 1 μCi (1 Ci = 37 GBq) of [3H] thymidine (Amersham Pharmacia), and its uptake was then measured.
Flow cytometry analyses (EPICS XL, Coulter) were performed as described in ref. 17. To measure the production of IFN-γ by spleen cells, enzyme-linked immunospot kits (ELISPOT; Cell Sciences, Norwood, MA) were used according to the manufacturer's protocol, and the plates were counted by an image analyzer at Fred Hutchinson Cancer Research Center (courtesy of C. Yee).
Results
Transfected Cells Express CD83 at Their Surface. Fig. 1 A-C demonstrates expression of CD83 on M2-CD83, SW1-P2-CD83, and SW1-C-CD83 cells and lack of expression on cells from the corresponding WT lines according to flow cytometry. A similar experiment demonstrated strong expression of CD83 on Ag104-CD83 cells (data not shown).
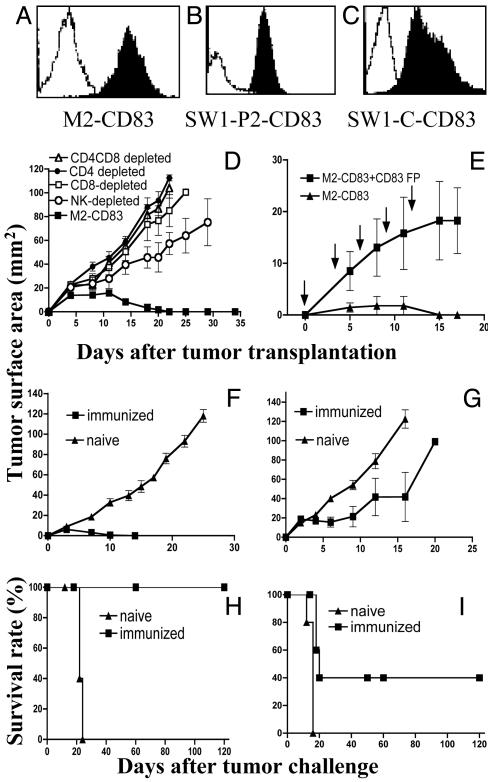
Fig. 1.
(A-C) Flow cytometry data showing expression of CD83 on transfected (filled area) versus WT (open area) cells. (D) Growth of M2-CD83 cells in mice depleted of the indicated cell populations. (E) Outgrowth of M2-CD83 cells in mice injected i.p. with 100 μg of mCD83 fusion protein (CD83 FP) at the time of tumor transplantation and repeated as indicated. The control group was injected with PBS. (F and H) Regression of M2-WT cells. (G and I) Regression of Ag104 cells in some mice immunized by live M2-CD83 cells (n = 5 mice per group).
M2-CD83 Cells Are Rejected by a Mechanism That Involves CD4+ and CD8+ T Cells and NK Cells and Depends on an Interaction Between CD83 and Its Ligands. M2-CD83 cells are regularly rejected when transplanted (2 × 106 cells per mouse) to syngeneic immunocompetent mice (data not shown). To investigate the roles of CD4+ and CD8+ T cells and NK cells in the regression of M2-CD83 tumors, we injected naive mice with antibodies to remove the respective cell populations. Control mice were injected with rat IgG. Twelve days later, when flow cytometry analysis of spleen cells from similarly injected mice showed that the targeted cell populations were depleted, 2 × 106 M2-CD83 cells were transplanted s.c. M2-CD83 cells were rejected by mice injected with rat IgG with similar growth kinetics as in untreated mice. Injection of mAbs to CD8 plus CD4, CD8, CD4, or NK cells allowed the M2-CD83 cells to grow progressively in all mice (Fig. 1D), although they grew more slowly in mice with depleted NK cell populations than in mice lacking CD4+ and/or CD8+ T cells. These findings are different from those obtained in similar experiments with M2-1D8 cells for which CD8+ T cells are not needed for tumor rejection (9).
To test whether an interaction between CD83 and its ligand(s) is responsible for the regression of M2-CD83 in immunocompetent mice, we investigated whether regression could be prevented by injection of mCD83 Ig. As shown in Fig. 1E, 2 × 106 M2-CD83 cells grew in mice that had been repeatedly injected with mCD83Ig and not in the controls. An experiment was also performed in which the experimental mice were injected with rat anti-mCD83 mAb 7A1 and the controls with rat IgG. M2-CD83 cells grew in all of five mice receiving anti-mCD83 mAb and were rejected in the control group (data not shown).
Systemic Antitumor Immunity in Mice That Rejected M2-CD83 Cells. Fig. 1F shows that mice that first rejected M2-CD83 also rejected a challenge dose of 2 × 106 M2-WT cells. In contrast, M2-WT cells grew in and killed all naive mice. Outgrowth of cells from the syngeneic Ag104 sarcoma was retarded (Fig. 1G). All five mice whose M2-CD83 tumors regressed survived without evidence of recurrence of the WT tumors (Fig. 1H), as did two of five immunized mice challenged with Ag104 cells (Fig. 1I). This experiment was repeated twice with similar results. The observed rejection of Ag104 by some of the mice immunized against M2-CD83 cells is in contrast to findings obtained with mice that had been immunized against M2-1D8 expressing anti-CD137 scFv in which a challenge dose of WT cells from Ag104 grows progressively while M2 cells are rejected (9). There was no evidence of toxicity in mice immunized against M2-CD83 cells, including no depigmentation of the skin as observed in approximately one-third of the mice that had been repeatedly immunized against M2-1D8 cells (9).
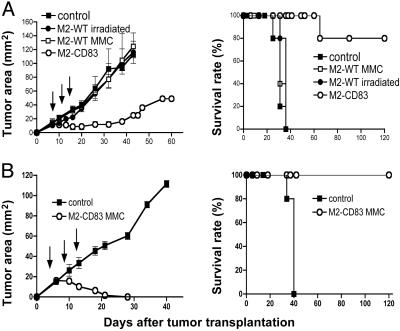
Therapeutic Efficacy Against M2-WT Tumors. Fig. 2A shows that live M2-CD83 cells can be used as a therapeutic vaccine against established s.c. M2-WT tumors. Immunization was started when the tumors had ≈20 mm2 of surface area, and it was repeated three times as indicated by arrows. In contrast, M2-WT tumors grew as well in mice immunized against irradiated (12,000 rads) or MMC-treated M2-WT cells as in mice injected with PBS. Four of five mice vaccinated by transplantation of M2-CD83 cells were cured and remained tumor-free during an observation period of 120 days. There were no signs of toxicity and no depigmentation. The experiment was repeated with similar results.
Fig. 2.
Therapeutic efficacy against s.c. established M2-WT tumors by immunization with live (A) or MMC-treated (B) M2-CD83 cells as indicated. (Left) Tumor growth. (Right) Survival rates. (n = 5 mice per group.)
We next studied the immunogenicity of M2-CD83 cells that had been sterilized by in vitro treatment with MMC or irradiation (12,000 rads). In both cases, the sterilized tumor cells prevented outgrowth of a challenge dose of M2-WT cells (data not shown). Experiments were then performed in which mice with M2-WT tumors were repeatedly immunized with MMC-treated M2-CD83 cells, starting when the tumors had ≈20 mm2 of surface area. Although the WT tumors grew in all controls, they were rejected in all five immunized mice (Fig. 2B), which survived tumor-free and without signs of toxicity over an observation period of 120 days. The experiment was repeated with similar results.
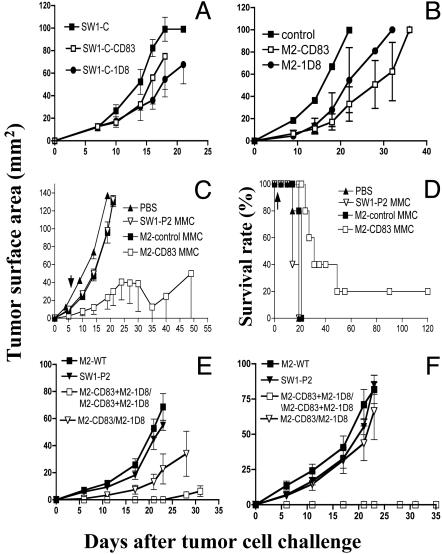
Transfection of SW1-C Cells or SW1-P2 Cells to Express CD83 (or 1D8) Does Not Make Them Immunogenic. Our next step was to perform experiments similar to those with M2-WT cells but with cells from the SW1 clone (11), here referred to as SW1-C. Like M2, this clone is derived from K1735 cells. We also worked with its SW1-P2 line, which expresses an ATF-2-derived peptide (7). In contrast to M2-CD83, both SW1-C-CD83 and SW1-C-1D8 cells grew progressively in naive syngeneic mice (Fig. 3A), as did SW1-P2 cells transfected to express either CD83 or anti-CD137 scFv (data not shown). Outgrowth of SW1-C cells was delayed but not prevented in mice immunized four times against MMC-treated cells from M2-CD83 or M2-1D8 (Fig. 3B); the delay was less in mice immunized one to three times. Outgrowth of WT cells from SW1-P2 was inhibited to a greater extent in mice that had been similarly immunized (Fig. 3 C and D), and one of five mice rejected the WT tumor cells and survived tumor-free. Immunization with M2-1D8 cells, which are effective for therapeutic vaccination against M2-WT tumors (9), delayed but did not prevent outgrowth of SW1-C cells or SW1-P2 cells (data not shown), and immunization with MMC-treated M2-WT, M2-control, or SW1-C -WT cells had no effect.
Fig. 3.
(A) Tumor formation by SW1-C-CD83, SW1-C-1D8, and SW1-C transplanted (106 cells per mouse) to naive mice. (B) Tumor formation by SW1-C (106 cells per mouse) to mice that had been immunized four times with MMC-treated cells (2 × 106 per mouse) from either M2-CD83 or M2-1D8. (C and D) Tumor formation by SW1-P2 cells (106 per mouse) transplanted to mice (C) and survival of mice (D) that had been immunized three times, 7 days apart, with MMC-treated M2-CD83 cells (2 × 106 per mouse) and reimmunized as indicated by arrows. (E and F) Inhibited outgrowth of SW1-C (E) and SW1-P2 (F) in mice immunized twice, 1 week apart, by a mixture of M2-CD83 and M2-1D8 cells (106 of each per mouse). (n = 5 mice per group.)
Rejection of WT Cells from SW1-C and SW1-P2 by Mice Immunized by a Mixture of MMC-Treated M2-CD83 and M2-1D8 Cells. Based on data in Fig. 1 and previous studies on M2 cells transfected to express the anti-CD137 scFv vector 1D8 (9), we hypothesized that, because the immunological mechanisms engaged by M2-CD83 cells and M2-1D8 cells appear to be different, they may complement each other. Experiments were thus performed by concomitantly immunizing mice with a mixture of cells from the two cell lines. Fig. 3E shows that immunization by a mixture of M2-CD83 and M2-1D8 cells prevented the take of SW1-C cells in three of five mice and delayed it in the two others, and Fig. 3F shows that it prevented the take of SW1-P2 cells in all immunized mice. In contrast, there was no protection against a challenge with SW1-P2 cells and only a modest effect against SW1-C cells if M2-CD83 cells were given first, followed 1 week later by M2-1D8 cells. Immunization with MMC-treated WT cells from M2 or SW1-P2 was ineffective (Fig. 3 E and F). This experiment was repeated with similar findings.
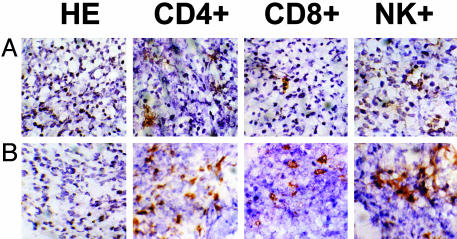
M2-CD83 Attracts and Stimulates CD4 and CD8+ T Cells and NK and B Cells. Respectively, Fig. 4 A and B shows sections of M2-WT and M2-CD83 tumors established from cells (2 × 106 per mouse) that had been transplanted 6 days previously, when the M2-CD83 tumors started to regress. There was a heavy influx of both CD4+ and CD8+ T cells into the M2-CD83 tumors and an increase in the number of infiltrating NK cells. Flow cytometry data in Table 1 demonstrate significant increases in the numbers of CD4+ and CD8+ T cells among tumor-infiltrating lymphoid cells from M2-CD83 tumors as compared with M2-WT tumors, and the frequency of tumor-infiltrating lymphoid cells, which stained for IFN-γ, almost doubled in the M2-CD83 tumors. There were also increased numbers of CD4+ and CD8+ T cells in spleens from mice transplanted with M2-CD83 cells and a dramatic increase of IFN-γ-positive CD4 cells in spleens from the M2-CD83 group. This experiment was repeated with similar results.
Fig. 4.
Immunostained sections of M2-WT (A) and M2-CD83 tumors (B) harvested 6 days after transplantation to naive mice. (Magnification, ×64.)
Table 1. Flow cytometry analyses of spleen (Sp) cells and tumor-infiltrating lymphoid cells (TIL).
| Cell population | M2-WT | M2-CD83 |
|---|---|---|
| Sp CD4+/CD45+ | 15.1 ± 0.3 | 16.9 ± 0.2 |
| Sp CD8+/CD45+ | 9.6 ± 0.1 | 11.3 ± 0.1 |
| Sp CD4+ IFN-γ | 1.0 ± 0.1 | 5.9 ± 0.5 |
| TIL CD4+/CD45+ | 4.6 ± 0.4 | 17.1 ± 0.5 |
| TIL CD8+/CD45+ | 2.2 ± 0.4 | 42.0 ± 0.2 |
| TIL IFN-γ | 34.2 ± 2.1 | 59.2 ± 2.8 |
| TIL TNF | 45.5 ± 1.1 | 54.3 ± 0.6 |
Data are given as mean ± SD from a total of three determinations for each entry. TNF, tumor necrosis factor.
In other experiments spleens were harvested 6 days after transplantation of 2 × 106 M2-CD83 cells and cultured for 4 days. For comparison, spleens were obtained from naive mice and, in one experiment, from a mouse transplanted 6 days previously with the same dose of M2-WT cells. Table 2 shows that there was a consistent increase in CD19+ cells, which are most likely B cells, in the spleens of mice that were rejecting M2-CD83 tumors. There was no difference in the number of CD19+ cells in mice with M2-WT tumors as compared to naive mice, and neither were there any differences in the frequency of spleen cells expressing the other markers that were analyzed.
Table 2. Composition of spleen cell populations after cultivation in vitro for 4 days and analyzed by flow cytometry.
| Cells | Naive | M2-WT | M2-CD83 |
|---|---|---|---|
| CD83L | 11.5; 10.8 | NT | 21.5; 16.7 |
| CD19 | 8.2; 9.3 | NT; 11.6 | 28.9; 27.9 |
| CD4 | 46.3; 46.6 | NT; 48.1 | 42.2; 43.4 |
| CD8 | NT; 34.1 | NT; 25.1 | NT; 17.7 |
| CD11b | NT; 4.2 | NT; 5.1 | NT; 6.0 |
| CD11c | 8.4; 6.0 | NT; 10.2 | 8.6; 7.8 |
| CD14 | 1.0; 0.8 | NT; 0.8 | 1.2; 0.9 |
Data are the result of two experiments. NT, not tested.
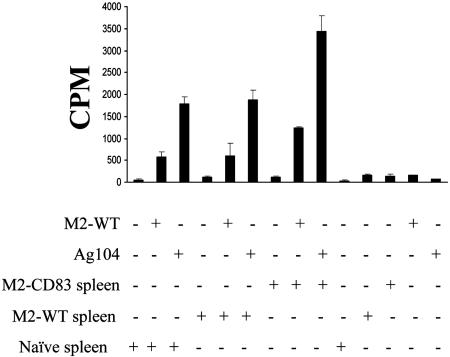
Proliferation of spleen cells from mice that had rejected M2-CD83 cells, as compared with spleen cells from naive mice or mice immunized against M2-WT cells (Fig. 5), doubled, both when the spleen cells were combined with MMC-treated M2-WT cells and with Ag104-WT cells. Experiments in which the spleen cells were labeled with 5(6)-carboxyfluorescein diacetate revealed that both CD4+ and CD8+ T cells proliferated (data not shown). According to enzyme-linked immunospot assays, there was an increased number of IFN-γ-producing T cells from mice that had been immunized against M2-CD83 cells compared with mice that were naive or had been immunized against M2-WT cells. This was the case both when the T cells were combined with M2-WT and with Ag104-WT cells (data not shown). The in vitro data thus correlated with the in vivo demonstration indicating that some antigens are shared by M2 and Ag104.
Fig. 5.
Proliferation of splenocytes from mice that either were naive or had been immunized three times, 7 days apart, with MMC-treated M2-WT or M2-CD83 cells (2 × 106 per mouse). The spleen cells were cocultured with MMC-treated M2-WT or Ag104-WT cells for 3 days.
Discussion
M2-CD83 cells, which express CD83 at their surface, can induce systemic immunity and cause the rejection of M2-WT tumors. Although Scholler et al. (3) reported that K1735 cells from the M2 clone transfected to express CD83 are immunogenic, the CD83 gene was of human origin. This makes their data hard to interpret, given that transfection of M2 cells to express another human molecule, the melanoma antigen p97, has been shown to increase their immunogenicity (18). We now demonstrate that immunization with M2 cells that express mCD83 causes the rejection of transplanted M2-WT cells. Furthermore, it does so more effectively than when the M2 cells express human CD83. Thus, 2 × 106 M2-WT cells were rapidly rejected by 100% of mice that had previously rejected M2 cells expressing mCD83 (Fig. 1 F and H), whereas rejection was slower and did not occur in all mice that had rejected a similar dose of M2 cells expressing human CD83 (3). Syngeneic mice were found to reject transplanted M2-CD83 cells by a mechanism that involves CD4+ and CD8+ T cells and NK cells. This finding is noteworthy, given that CD8+ T cells are not needed for rejection of M2-1D8 cells, which express anti-CD137 scFv (9). Another difference between the two systems is that the outgrowth of Ag104-WT cells was inhibited in mice that had been immunized against M2-CD83 cells but not in mice immunized against M2-1D8 cells (9). In vitro data on T cell proliferation and IFN-γ production parallel the respective in vivo findings and support the notion that M2 and Ag104 share tumor rejection antigens. Independent evidence that M2 and Ag104 share some tumor antigens comes from the demonstration of cytolytic activity against both cell lines, which was mediated by a cytotoxic T lymphocyte line generated against M2 cells that expressed human p97 together with the costimulatory ligand CD80 (19).
Our data thus indicate that transfection of M2 cells to express either anti-CD137 scFv (9) or CD83 are two independent approaches to induce an antitumor response and suggest that these may be combined for more effective vaccination, as has been demonstrated in other systems for certain immunological signals (20-22). We now show that immunization by a mixture of M2-CD83 and M2-1D8 cells caused rejection of subsequently transplanted SW1-C WT cells (three of five mice) and SW1-P2 cells (five of five mice), whereas immunization with either line alone had only modest antitumor activity. Furthermore, the two cell lines had to be given together. Differences observed between SW1-C cells and SW1-P2 cells may be attributed to changes elicited by altered ATF-2 expression, which may affect cell surface expression of select antigens and/or processing of the peptides expressed.
Immunization with CD83-transfected tumor cells that have been sterilized by exposure to MMC can be applied as efficiently as their live counterparts to treat mice with established M2-WT tumors. The fact that treatment with MMC does not abolish tumor immunogenicity facilitates comparisons between various experimental and control groups.
There was significant infiltration of CD4+ and CD8+ T cells in M2-CD83 tumors harvested shortly before they started to regress, and the frequency of CD4+ T cells that contained IFN-γ was increased among tumor-infiltrating lymphoid cells from M2-CD83 as compared to M2-WT tumors. Furthermore, an increased number of CD19+ cells, most likely B lymphocytes, was detected in the spleens of mice transplanted with M2-CD83 cells. These findings are relevant in view of the demonstration that expression of CD83 facilitates the development of CD4+ T cells (23) and that mouse B lymphocytes express CD83 ligand(s) (4).
Sensitization against cells from the human B cell line T51 that had been transfected to express CD83 has been shown to induce the proliferation of allogeneic peripheral blood mononuclear cells and facilitate the generation of cytotoxic T lymphocytes unless a human CD83Ig fusion protein is added to the system (3). We have now demonstrated that administration of either mCD83 Ig or anti-CD83 mAb prevented rejection of transplanted M2-CD83 cells. Outgrowth of M2-WT cells was facilitated as well (data not shown) as is the outgrowth of P815 mastocytoma cells in mice given human CD83Ig (3). We hypothesize that immune responses to tumor antigens (and probably to other antigens as well) are stimulated by interaction between CD83 and its ligand(s) on B cells (4) and perhaps also on monocytes which, in humans, express the ligand (2). By regulating this interaction upwards or downwards, novel and effective approaches may be identified to modify immune responses in both autoimmunity and cancer.
We postulate that MMC-treated melanoma cells that have been transfected to express CD83 will have efficacy as therapeutic vaccines against small tumors established from certain melanomas (and perhaps some other tumors) in humans. Our data with the SW1 clone of melanoma K1735 cells show, however, that all tumors do not become immunogenic by this approach, perhaps as a result of inhibitory mechanisms engaged by molecules that are made by some tumors (24, 25). One needs, therefore, to ascertain that a tumor cell-based vaccine can stimulate the generation and expansion of tumor-selective CD8+ T cells and CD4 cells of the Th1 type. However, an even better approach is probably to construct a gene-based vaccine that combines antigen(s) expressed by a patient's tumor with CD83 and that also involves additional immunostimulatory molecules, such as anti-CD137 scFv.
Acknowledgments
We thank Anindita Bhoumik, who established SW1 cultures, and Janice Pullman for valuable technical assistance. This work was supported by National Institutes of Health Grants CA79490 (to K.E.H.) and CA85780 (to I.H.), Specialized Programs of Research Excellence Grants PA50 CA83636 (to I.H. and K.E.H.) and CA51995 (to Z.R.). S.Y., who is a visiting research fellow from Shanghai International Joint Cancer Institute, was also supported from China by an International Exchange Award to Y.G. from the Shanghai Commission of Science and Technology.
Abbreviations: mCD83, mouse CD83; NK, natural killer; ECD, extracellular domain; MMC, mitomycin C; scFv, single-chain variable region fragments; ATF-2, activating transcription factor type 2.
References
- 1.Banchereau, J., Briere, F., Caux, C., Davoust, J., Lebecque, S., Liu, J. Y., Pulendran, B. & Palucka, K. (2000) Annu. Rev. Immunol. 18, 767-811. [DOI] [PubMed] [Google Scholar]
- 2.Scholler, N., Hayden-Ledbetter, M., Hellstrom, K. E., Hellstrom, I. & Ledbetter, J. A. (2001) J. Immunol. 166, 3865-3872. [DOI] [PubMed] [Google Scholar]
- 3.Scholler, N., Hayden-Ledbetter, M., Dahlin, A., Hellstrom, I., Hellstrom, K. E. & Ledbetter, J. A. (2002) J. Immunol. 168, 2599-2602. [DOI] [PubMed] [Google Scholar]
- 4.Cramer, S. O., Trumpfheller, U. C., Mehlhoop, U., More, S., Fleischer, B. & von Bonin, A. (2000) Int. Immunol. 12, 1347-1351. [DOI] [PubMed] [Google Scholar]
- 5.Twist, C. J., Beier, D. R., Disteche, C. M., Edelhoff, S. & Tedder, T. F. (1998) Immunogenetics 48, 383-393. [DOI] [PubMed] [Google Scholar]
- 6.Bhoumik, A., Ivanov, V. & Ronai, Z. (2001) Clin. Cancer Res. 7, 331-342. [PubMed] [Google Scholar]
- 7.Bhoumik, A., Huang, T. G., Ivanov, V., Gangli, L., Quiao, R. F., Woo, S. L., Chen, S. H. & Ronai, Z. (2002) J. Clin. Invest. 110, 643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger, A. J., Kluger, H. M., Li, N., Kielhorn, E., Halaban, R., Ronai, Z. & Rimm, D. L. (2003) Cancer Res. 63, 8301-8307. [PubMed] [Google Scholar]
- 9.Ye, Z., Hellstrom, I., Hayden-Ledbetter, M., Dahlin, A., Ledbetter, J. A. & Hellstrom, K. E. (2002) Nat. Med. 8, 343-348. [DOI] [PubMed] [Google Scholar]
- 10.Fidler, I. J. & Hart, I. R. (1981) Cancer Res. 41, 3266-3267. [PubMed] [Google Scholar]
- 11.Price, J. E., Tarin, D. & Fidler, I. (1988) Cancer Res. 48, 2258-2264. [PubMed] [Google Scholar]
- 12.Ward, P. I., Koeppen, H., Hurteau, T. & Schreiber, H. (1989) J. Exp. Med. 170, 217-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenschow, D. J., Zeng, Y., Thistlethwaite, J. R., Montag, A., Brady, W., Gibson, M. G., Linsley, P. S. & Bluestone, J. A. (1992) Science 257, 789-792. [DOI] [PubMed] [Google Scholar]
- 14.Hara, I., Nagai, H., Miyake, H., Yamanaka, K., Hara, S., Micallef, M. J., Kurimoto, M., Gohji, K., Arakawa, S., Ichihashi, M. & Kamidono, S. (2000) Cancer Gene Ther. 7, 83-90. [DOI] [PubMed] [Google Scholar]
- 15.Yeh, M. Y., Hellström, I., Brown, J. P., Warner, G. A., Hansen, J. A. & Hellström, K. E. (1979) Proc. Natl. Acad. Sci. USA 76, 2927-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, L., Ashe, S., Brady, W. A., Hellström, I., Hellström, K. E., Ledbetter, J. A., McGowan, P. & Linsley, P. S. (1992) Cell 71, 1093-1102. [DOI] [PubMed] [Google Scholar]
- 17.Weston, S. A. & Parish, C. R. (1990) J. Immunol. Methods 133, 87-97. [DOI] [PubMed] [Google Scholar]
- 18.Estin, C. D., Stevenson, U., Kahn, M., Hellström, I. & Hellström, K. E. (1989) J. Natl. Cancer Inst. 81, 445-448. [DOI] [PubMed] [Google Scholar]
- 19.Li, Y., McGowan, P., Hellström, I., Hellström, K. E. & Chen, L. (1994) J. Immunol. 153, 421-428. [PubMed] [Google Scholar]
- 20.Li, Y., Hellström, K. E., Newby, S. A. & Chen, L. (1996) J. Exp. Med. 183, 639-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melero, I., Bach, N., Hellstrom, K. E., Aruffo, A., Mittler, R. S. & Chen, L. (1998) Eur. J. Immunol. 28, 1116-1121. [DOI] [PubMed] [Google Scholar]
- 22.Greiner, J. W., Zeytin, H., Anver, M. R. & Schlom, J. (2002) Cancer Res. 62, 6944-6951. [PubMed] [Google Scholar]
- 23.Fujimoto, Y., Tu, L., Miller, A. S., Bock, C., Fujimoto, M., Doyle, C., Steeger, D. A. & Tedder, T. F. (2002) Cell 108, 755-767. [DOI] [PubMed] [Google Scholar]
- 24.Hahne, M., Rimoldi, D., Schroter, M., Romero, P., Schreier, M., French, L. E., Schneider, P., Bornand, T., Fontana, A., Lienard, D., Cerottini, J.-C. & Tschopp, J. (1996) Science 274, 1363-1366. [DOI] [PubMed] [Google Scholar]
- 25.Dong, H., Strome, S. E., Salomao, D., Tamura, H., Hirano, F., Fllies, D. B., Roche, P. C., Lu, J., Zhu, G., Tamada, K., Lennon, V. A., Celis, E. and Chen, L. (2002) Nat. Med. 8, 793-800. [DOI] [PubMed] [Google Scholar]