Abstract
The clinical features, pathologic changes, and immune repertoire in a remarkable case of chronic varicella-zoster virus (VZV) ganglioneuritis without rash are described.
The clinical features, pathologic changes, and immune repertoire in a remarkable case of chronic varicella-zoster virus (VZV) ganglioneuritis without rash are described.
Case report.
Two years before death, a 39-year-old morbidly obese man experienced 3 episodes of left maxillary-distribution zoster at intervals of 5 and 14 months, respectively, that lasted 2–3 weeks, and was treated with oral antiviral agents. Medical history revealed hypertension and recurrent herpes labialis. Six months before death, he experienced diffuse, increasingly severe headaches. Three months before death, he developed left maxillary-distribution pain, vertigo, blurred vision, and difficulty concentrating; brain MRI revealed 3–4 punctate white matter hyperintensities. CT scan showed diffuse brain swelling without contrast enhancement; CT angiogram was normal. Complete blood count, metabolic panel, HIV serology, and antinuclear antibodies panel were normal/negative. CSF contained 14 leukocytes/μL (81% mononuclear) and 1,615 erythrocytes/μL; CSF protein was 53 mg/dL and glucose was 52 mg/dL. CSF Gram stain and culture and PCR for VZV and herpes simplex virus (HSV) DNA by PCR were negative (lower limit of detection 5 copies/μL). The patient was treated with IV acyclovir, 10 mg/kg 3 times daily for 2 days, followed by oral acyclovir, 800 mg 4 times daily for 8 days. He was found dead in bed 1 month after hospitalization.
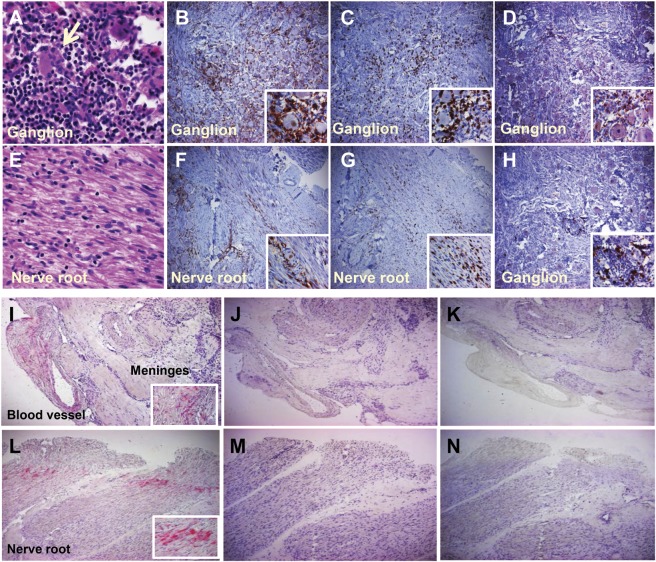
Forensic autopsy revealed severe hypertensive cardiomyopathy. Mild cerebral swelling was noted and the hippocampus contained scant perivascular lymphocytes but no VZV or HSV antigen. Extensive inflammation (figure) was seen in both trigeminal ganglia (figure, A) and adjacent nerve roots (figure, E), greater on the left. Inflammation was dominated by CD4+ (figure, B and F) > CD8+ T cells (figure, E and G) with many CD68+ macrophages (figure, D), rare CD20+ B cells (figure, H), and isolated CD15+ neutrophils.
Figure. Pathologic, immunologic, and virologic findings in varicella-zoster virus ganglioneuritis.
Left trigeminal ganglion and adjacent nerve root stained with hematoxylin & eosin and immunocyte markers. Note dense lymphocytic-predominant inflammatory infiltrate surrounding neurons (arrow) in the ganglion (A) and inflammation in adjacent nerve root (E). CD4+ T cells predominated in the ganglion and nerve root (B, F), occasionally clustered around viable-appearing neurons (inset). CD8+ T cells were also frequently seen in the ganglion and nerve root (C, G, insets) as well as CD68+ macrophages (D, inset). CD20+ B cells (H) were occasionally seen in the ganglion (inset shows an isolated cluster). Magnification ×360 for panels A and B and all insets; ×60 for large panels C–H. Varicella-zoster virus (VZV) antigen is seen at the periphery of the left trigeminal ganglion, in and outside the wall of a meningeal blood vessel (I, red) and the trigeminal nerve root (L) after labeling with anti-VZV antibody, but not after labeling adjacent sections with anti–herpes simplex virus antibody (J and M) or with normal rabbit serum (K and N). Magnification ×60 (large panels); ×360 (insets).
Immunohistochemistry with anti-VZV antibody revealed abundant VZV IE63 antigen at the periphery of both ganglia, primarily in the trigeminal nerve root, adventitia, and wall of a dural artery (figure, I and L), not seen with anti-HSV antibody (figure, J and M) or normal rabbit serum (figure, K and N). Real-time PCR of formalin-fixed tissue amplified VZV and HSV-1 DNA in both ganglia and nerve roots; in the latter, VZV DNA abundance was 84 copies and HSV-1 was 9 copies per 500 ng total DNA. Circle of Willis arteries did not contain VZV DNA. Postmortem CSF did not contain VZV or HSV DNA or anti-VZV or HSV antibody.
Discussion.
A remarkable case of chronic bilateral VZV trigeminal ganglioneuritis with diffuse headache and unilateral maxillary-distribution pain without rash is detailed. The patient had 3 remote episodes of maxillary-distribution zoster, the same site in which chronic pain developed without rash and corresponding to the trigeminal nerve root in which VZV antigen was found. PCR and immunohistochemical studies demonstrated VZV in both trigeminal nerve roots and adventitia and wall of a meningeal artery. VZV as a cause of neurologic disease without rash has been emphasized.1 Most often, recurrent zoster and herpes labialis (which our patient also experienced) would be seen in a severely immunocompromised patient, although recurrent zoster was reported in up to 5.7% of immunocompetent individuals.2 Yet our subject had no history of immunodeficiency or immunomodulatory medications. He also had symptoms consistent with encephalitis, CT-documented brain swelling before death, and mild inflammation in the hippocampus postmortem, although no viral antigen was found. He may have had mild VZV encephalitis that resolved, as in virologically verified VZV-induced limbic encephalitis.3
One other case of chronic VZV ganglionitis without rash in an immunocompetent patient4 has interesting parallels. First, chronic pain was in the maxillary division of the trigeminal nerve. Second, VZV was most abundant in the trigeminal nerve root adjacent to the ganglion in both subjects. Third, in both subjects HSV DNA was found in nerve roots; usually HSV DNA is restricted to ganglia. Note that histologic examination revealed multiple ganglion cells (large neurons) and nodules of Nageotte (foci of prior neuron loss), indicating that tissue was from the interface of ganglion and nerve. This is important since it explains our detection in the nerve root of small amounts of amplifiable HSV DNA. Importantly, copy number of VZV DNA in nerve root was 10-fold that of HSV DNA, corresponding to the presence of VZV antigen.
VZV antigen exclusively in trigeminal nerve roots in both cases suggests that the fundamental lesion accounting for pain originated in nerve root rather than ganglion, a theory proposed by Obersteiner and Redlich and by Nageotte, who described dorsal root inflammation and meningeal thickening to explain severe pain in tabes dorsalis.5
Finally, VZV antigen and inflammation with pain in both cases of chronic VZV ganglioneuritis without rash supports the notion that postherpetic neuralgia may reflect a chronic active VZV ganglionitis.6
Acknowledgments
Acknowledgment: Permission for the studies described in this manuscript was obtained from the patient's wife. The authors thank Marina Hoffman, who reviewed the manuscript for errors in grammar, punctuation, spelling, readability, clarity, and accuracy; and Lori DePriest for word processing and formatting the manuscript.
Footnotes
Author contributions: M. Birlea: drafting/revising the manuscript for content, including medical writing for content; study concept/design; analysis and interpretation of data; contribution of vital reagents. M. Nagel: drafting/revising the manuscript for content, including medical writing for content; study concept/design; analysis and interpretation of data; contribution of vital reagents. N. Khmeleva: acquisition of data. A. Choe: acquisition of data. B.K. DeMasters: interpretation of data. R. Hevner: analysis of data; acquisition of data. P. Boyer: acquisition and analysis of data. K. Lear-Kaul: acquisition and analysis of data. N. Bos: acquisition of data. M. Wellish: acquisition of data. R.J. Cohrs: drafting/revising the manuscript for content, analysis and interpretation of data; contribution of vital reagents/tools/patents; obtaining funding. D. Gilden: drafting/revising the manuscript for content, including medical writing for content; study concept/design; acquisition, analysis, and interpretation of data; contribution of vital reagents, obtaining funding.
Disclosure: M. Birlea was supported by training grant NS007321 to Dr. Gilden from NIH. M. Nagel receives research support from NIH research grant NS067070. N. Khmeleva, A. Choe, B. Kleinschmidt-DeMasters, R. Hevner, P. Boyer, K. Lear-Kaul, N. Bos, and M. Wellish report no disclosures. R. Cohrs receives research support from NIH research grant NS082228 and AG03258. D. Gilden receives research support from NIH research grants AG006127 and AG03258. Dr. Gilden serves as Senior Associate Editor for the Journal of Neurovirology and on the editorial boards of In Vivo, Journal of Virology, Scientific American, Virus Genes, and Neurology. Go to Neurology.org for full disclosures.
References
- 1.Kennedy PG. Zoster sine herpete: it would be rash to ignore it. Neurology 2011;76:416–417 [DOI] [PubMed] [Google Scholar]
- 2.Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc 2011;86:88–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tattevin P, Schortgen F, de Broucker T, Dautheville S, Wolff M. Varicella-zoster virus limbic encephalitis in an immunocompromised patient. Scand J Infect Dis 2001;33:786–788 [DOI] [PubMed] [Google Scholar]
- 4.Hevner RF, Vilela MD, Rostomily RC, et al. An unusual cause of trigeminal-distribution pain and tumour. Lancet Neurol 2003;2:567–571 [DOI] [PubMed] [Google Scholar]
- 5.Wilson SAK. Neurosyphilis. In: Bruce AN, ed. Neurology, Vol. 1 Baltimore: Williams & Wilkins; 1940:507–509 [Google Scholar]
- 6.Gilden DH, Cohrs RJ, Mahalingam R. VZV vasculopathy and postherpetic neuralgia: progress and perspective on antiviral therapy. Neurology 2005;64:21–25 [DOI] [PubMed] [Google Scholar]