Abstract
Objective:
To evaluate Bacille Calmette-Guérin (BCG) effects after clinically isolated syndromes (CIS).
Methods:
In a double-blind, placebo-controlled trial, participants were randomly assigned to receive BCG or placebo and monitored monthly with brain MRI (6 scans). Both groups then entered a preplanned phase with IM interferon-β-1a for 12 months. From month 18 onward, the patients took the disease-modifying therapies (DMTs) that their neurologist considered indicated in an open-label extension phase lasting up to 60 months.
Results:
Of 82 randomized subjects, 73 completed the study (33 vaccinated and 40 placebo). During the initial 6 months, the number of cumulative lesions was significantly lower in vaccinated people. The relative risks were 0.541 (95% confidence interval [CI] 0.308–0.956; p = 0.03) for gadolinium-enhancing lesions (the primary endpoint), 0.364 (95% CI 0.207–0.639; p = 0.001) for new and enlarging T2-hyperintense lesions, and 0.149 (95% CI 0.046–0.416; p = 0.001) for new T1-hypointense lesions. The number of total T1-hypointense lesions was lower in the BCG group at months 6, 12, and 18: mean changes from baseline were −0.09 ± 0.72 vs 0.75 ± 1.81 (p = 0.01), 0.0 ± 0.83 vs 0.88 ± 2.21 (p = 0.08), and −0.21 ± 1.03 vs 1.00 ± 2.49 (p = 0.02). After 60 months, the cumulative probability of clinically definite multiple sclerosis was lower in the BCG + DMT arm (hazard ratio = 0.52, 95% CI 0.27–0.99; p < 0.05), and more vaccinated people remained DMT-free (odds ratio = 0.20, 95% CI 0.04–0.93; p = 0.04).
Conclusions:
Early BCG may benefit CIS and affect its long-term course.
Classification of evidence:
BCG, as compared to placebo, was associated with significantly reduced development of gadolinium-enhancing lesions in people with CIS for a 6-month period before starting immunomodulating therapy (Class I evidence).
The majority of multiple sclerosis (MS) cases start with a first demyelinating episode (usually referred to as clinically isolated syndrome [CIS]) that is generally reversible. Approximately half of these cases convert to clinically definite MS (CDMS) within 2 years of the diagnosis and have substantial risk of disability, while about 10% of people with CIS remain free of further neurologic events, even in the presence of MRI compatible with MS.1–4 Interferon (IFN)–β and glatiramer acetate in subjects with CIS proved to be beneficial for conversion to CDMS.5–8 Concerning disability, recent works showed that a delay in the administration of IFN-β up to 2 years from the first clinical event did not affect long-term disease progression.9–11
In a previous pilot study, Bacille Calmette-Guérin (BCG) vaccine was safe and possibly effective in reducing MRI activity of patients with relapsing-remitting MS.12 There are also data that BCG vaccination might decrease the risk of developing persistent T1-hypointense lesions over a 24-month follow-up, suggesting long-term beneficial effects of the vaccine.13 Though underlying mechanisms remain largely unknown, there is empiric evidence from other immunopathologic conditions (especially type 1 diabetes and asthma) that an adjuvant approach exerts favorable immunomodulatory effects.14–17 Considering the above evidence and because the adjuvant approach is inexpensive, safe, and handy, we thought BCG appropriate for people with CIS.
METHODS
Classification of evidence.
This study provides Class I evidence that BCG vaccination, as compared to placebo, was associated with significantly reduced development of gadolinium (Gd)–enhancing lesions in patients with CIS for a 6-month period prior to starting immunomodulating therapy.
Standard protocol approvals, registration, and patient consents.
Individuals with a first clinical event suggestive of MS were enrolled between January 2003 and June 2006 at 4 Italian MS Centers: Center for Experimental Neurological Therapies, Ospedale S. Andrea-site, and Department of Neurology and Psychiatry, “Sapienza” University of Rome; Fondazione Don Carlo Gnocchi, IRCCS, Milan; and Department of Neurological Sciences, Federico II University, Naples. The study is registered at the ClinicalTrial.gov Web site with the identifier NCT00202410. The trial was conducted according to Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was approved by the local ethics committees and each patient provided written informed consent.
Patients.
Inclusion criteria were a single clinical episode suggestive of MS with either monofocal (optic neuritis, brainstem-cerebellar syndrome, spinal cord involvement) or multifocal onset; a baseline brain MRI scan supporting a diagnosis of MS (i.e., with at least 2 clinically silent T2-hyperintense lesions); age between 18 and 50 years; no steroid treatment in the 2 months prior to inclusion in the study; and no disease-modifying therapy (DMT). Exclusion criteria were systemic diseases or suspected tuberculosis (as resulting from Mantoux reaction or chest x-ray), pregnancy (women of childbearing potential agreed to use contraception), or breastfeeding.
Protocol.
Participants were randomly assigned to receive BCG vaccine or placebo and monitored monthly with brain MRI scans for 6 months. Then all the individuals entered a preplanned follow-up phase of 12 months with IM IFN-β-1a (which was indicated in first demyelinating events when the study was designed). A prospectively planned open-label extension of the trial was conducted up to 60 months from vaccine or placebo (from month 18 onward the patients were treated with the DMT that their neurologist in charge considered indicated).
Participants underwent physical and neurologic examinations, including rating of disability by the Expanded Disability Status Scale (EDSS) score,18 routine blood tests, additional tests to rule out alternative conditions mimicking MS, ECG, x-ray chest, and brain MRI scan. Moreover, Mantoux reaction (intradermal injection of purified protein derivative [5 units] of Mycobacterium tuberculosis [Statens Institut, Copenhagen, Denmark]) was performed to exclude hyperergic subjects. After providing informed consent, subjects were randomly assigned (1:1 ratio) to receive a single intracutaneous dose of 0.1 mL freeze-dried BCG (1 mg/ML; Pasteur) or placebo (a sham injection) within 90 days of onset of the first clinical event.
A list of randomization numbers and corresponding treatment numbers was computer-generated before the start of the study. Participants, assessing neurologists (a 2-physician treating and assessing model was used: the treating physician was responsible for supervision of study drug administration and for recording adverse events and safety assessments; the assessing physician was exclusively responsible for all neurologic assessments), and neuroradiologists were masked to treatment allocation for the double-blind phase of the study (the 6 months following vaccine or placebo and the preplanned follow-up of 12 months during which all patients were treated with IM IFN-β-1a [Avonex; Biogen Idec Inc., Weston, MA] 30 μg/week IM). Follow-up to 60 months from vaccine or placebo was the unblinded extension of the trial.
Subjects underwent serial clinical examination (monthly at the same time point as MRI during the first 6 months, every 3 months during the preplanned follow-up from month 6 to month 18, and every 6 months during the open-label extension to 60 months) aimed at assessing a relapse (the appearance of new symptoms or worsening of previous symptoms/signs associated with changes in the neurologic examination lasting longer than 24 hours) or any adverse event (any untoward medical occurrence regardless of its causal relationship to the study treatment). The severity of the adverse events was graded as follows: mild (minimal or no required treatment and no interference with the patient's daily activities); moderate (low level of inconvenience or concern, may require therapeutic measures and cause some interference with functioning); severe (interruption of a patient's usual daily activities and requirement of systemic drug therapy or other treatment, usually incapacitating); life-threatening (immediate risk of death). During 6 months after vaccine or placebo, subjects were imaged with monthly Gd-enhanced MRI of brain. MRI was performed in all patients with a 1.5T magnet (Philips Gyroscan NT 1.5; Philips, Guildford, UK). Proton density and T2-weighted (T2W) conventional spin-echo (CSE) (repetition time [TR] 2,000 ms; echo time [TE] 20/90 ms), fast fluid-attenuated inversion recovery (TR 6,000 ms; TE 150 ms), and T1-weighted (T1W) CSE (TR = 550 ms; TE = /12 ms) were acquired in the axial plane with 5-mm contiguous slices, and a field of view = 240 mm, matrix = 256 × 256. The hard copy was analyzed by 2 experienced neuroradiologists working in pairs (when there was a disagreement, a third senior neuroradiologist reviewed the images and a final consensus was reached), who detected the number of Gd-enhancing lesions, T2-hyperintense lesions, and T1-hypointense lesions. A T1-hyponintese lesion was defined as any region with low signal intensity relative to the surrounding white matter, corresponding to a hyperintense lesion on T2W images and not associated with the presence of an acute enhancement on T1W postcontrast MRI.19 An additional 2 brain MRI scans were performed 12 and 18 months after vaccination or placebo.
Outcome measures.
The primary endpoint was the cumulative mean number of total (new and persisting) Gd-enhancing lesions during the initial 6 months. Secondary endpoints included other MRI outcome measures (mean number of new and enlarging T2-hyperintense lesions and mean number of new T1-hypointense lesions during the first 6 months, as well as mean number of Gd-enhancing lesions, new and enlarging T2-hyperintense lesions, and new T1-hypointense lesions at the months 12 and 18; mean changes in the number of total T1-hypointense lesions from baseline to months 6, 12, and 18); mean number of relapses at months 6 and 18; clinical endpoints measured after 60 months of follow-up (cumulative probability of CDMS conversion, mean number of relapses, mean EDSS score, and number of subjects free of DMT); and adverse events.
Statistical analysis.
The calculation of sample size was performed considering our previous study with BCG on patients with relapsing-remitting MS12,13: we assume that a sample size of 35 patients per group had 80% power and an αvalue of 5% to detect a treatment effect of 65% reduction or more on the number of total (new and persisting) Gd-enhancing lesions seen on 6 monthly MRI scans. The level of evidence was Class I, according to the classification scheme requirements of the American Academy of Neurology. A negative binomial regression model was adopted to compare the rate of total number of Gd-enhancing lesions, new and enlarging T2-hyperintense lesions, and new T1-hypointense lesions over the first 6 months after BCG or placebo (relative risk [RR]). Negative binomial regression was chosen when the assumption of equality of the mean and variance in the Poisson model did not hold true. These statistical analyses were adjusted for the following baseline variables: age, sex, EDSS, Gd-enhancing lesions, T2-hyperintense lesions, T1-hypointense lesions, clinical forms at onset. The cumulative probability of CDMS was calculated for each group according to the Kaplan-Meier product-limit method and compared with use of the Mantel log-rank test; the same outcome measure was also calculated through a multivariate analysis according to the Cox model. Moreover, a regression logistic model on DMT-free subjects (follow-up to 60 months) was performed. The above multivariate analyses were adjusted for baseline variables. MRI endpoints were also assessed with Fisher exact and Mann-Whitney U test to compare categorical variables and distribution of lesions. Clinical efficacy analyses are reported for the intention-to-treat population, defined as all patients who were randomized and evaluated at baseline. All statistical analyses were 2-sided, with an α value of 0.05; no adjustment (type I error rate) for multiple comparisons was made.
RESULTS
Patient disposition and characteristics.
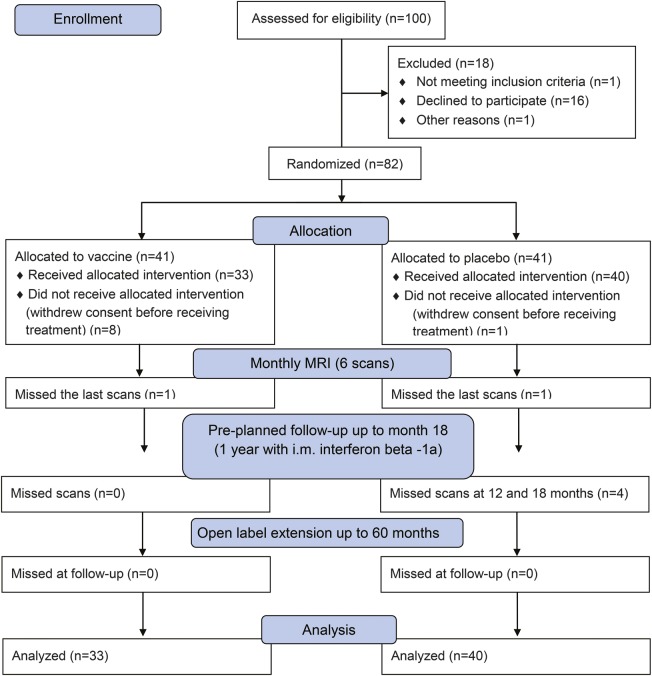
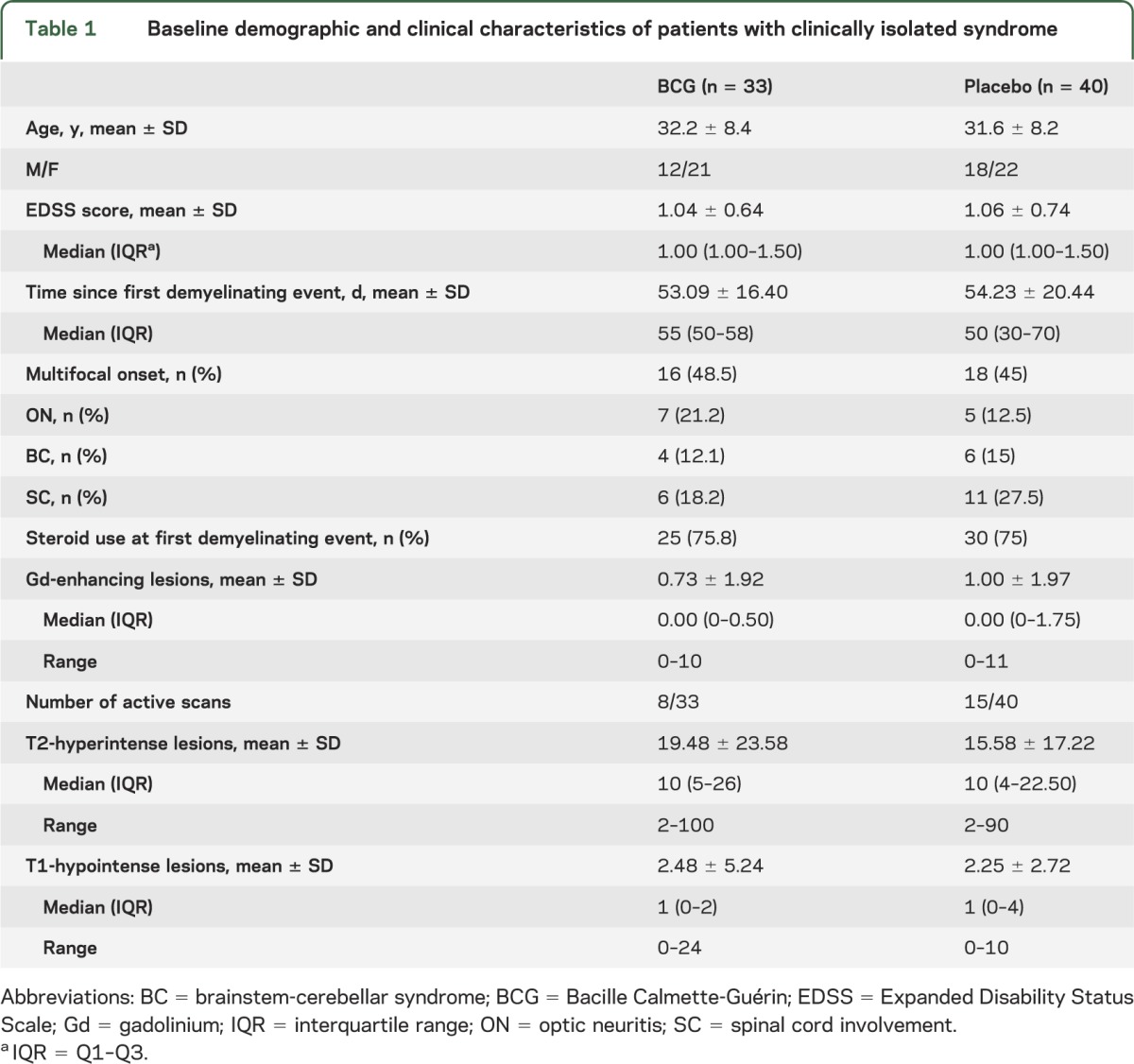
A total of 100 individuals with CIS were assessed for eligibility. Eighty-two were deemed eligible and were randomly assigned to receive vaccine or placebo. Nine subjects withdrew consent before receiving treatment, with the remaining 33 assigned to vaccine and 40 to placebo: all subjects completed the phase of monthly MRI, except for 1 vaccinated and 1 placebo individual who missed the last scans; all subjects completed the preplanned follow-up with IM IFN-β-1a (4 out of 40 individuals of placebo arm missed scans at 12 and 18 months) and the open-label extension of the trial up to 60 months (figure 1). Demographic, clinical, and MRI characteristics were comparable between the 2 groups at baseline: none of the comparisons gave significant differences (p > 0.18 in all cases). A trend toward more active scans and more subjects with spinal cord involvement was present in the placebo group (table 1).
Figure 1. Flow of patients through the trial.
Table 1.
Baseline demographic and clinical characteristics of patients with clinically isolated syndrome
Outcome measures.
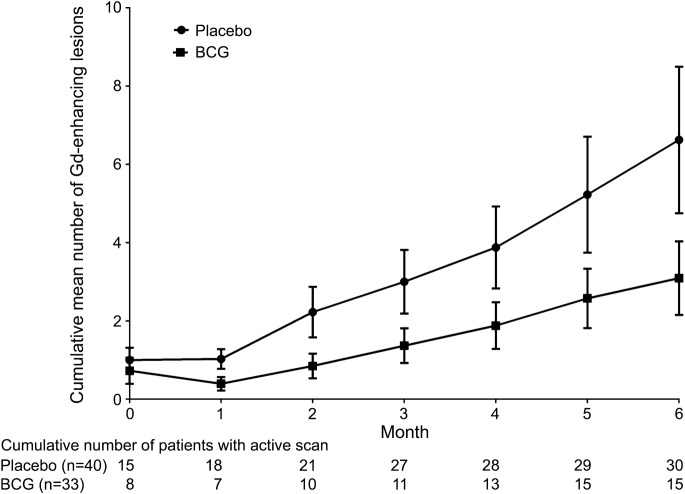
Disease activity was reduced in the treated group during the 6-month follow-up (table 2, figure 2). For the primary endpoint, the rate of cumulative mean number of total Gd-enhancing lesions was lower in the BCG (3.09 ± 5.40) compared to the placebo group (6.62 ± 11.84), in spite of wide variances in both study arms (figure e-1 on the Neurology® Web site at www.neurology.org). The RR was 0.541 (95% confidence interval [CI] 0.308–0.956; p = 0.03), adjusted for the baseline variables. Table e-1 shows that 45.5% of vaccinated subjects vs 75% of placebo subjects developed 1 or more new Gd-enhancing lesions and met criteria of dissemination in time for MS diagnosis20 (p = 0.02). The risk difference was 29.5% (95% CI 7.9%–51.2%) and the number needed to treat was 3.39. The BCG group had also a lower cumulative mean number of new and enlarging T2-hyperintense (RR = 0.364, 95% CI 0.207–0.639; p = 0.001) and new T1-hypointense (RR = 0.149, 95% CI 0.046–0.416; p = 0.001) lesions during the 6-month follow-up (table 2). No adverse event occurred after 6 months except for local reaction to inoculation in 3 subjects who were vaccinated. The number of relapses was 5/40 (12.5%) in placebo vs 2/33 (6.06%) in BCG arm (risk difference 6.44%, 95% CI 6.65%–19.53%; p = not significant).
Table 2.
Disease activity at MRI and T1-hypointense lesions after 6-month scans following vaccination or placebo
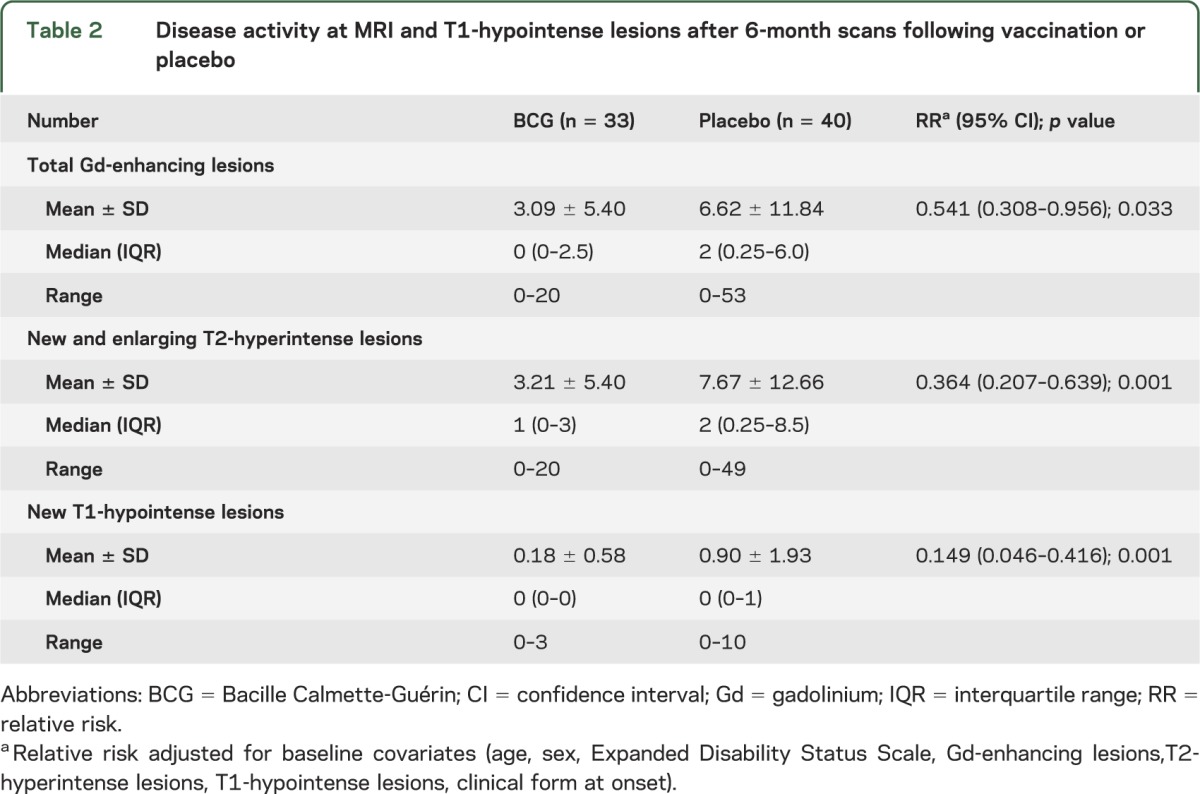
Figure 2. Cumulative mean number of total gadolinium-enhancing lesions on MRI during the first 6 months.
The p values were <0.05 at months 1, 3, 4, and 6; the difference was near-significant at months 2 (p = 0.07) and 5 (p = 0.09; Mann-Whitney U test). BCG = Bacille Calmette-Guérin; Gd = gadolinium; bars = standard error.
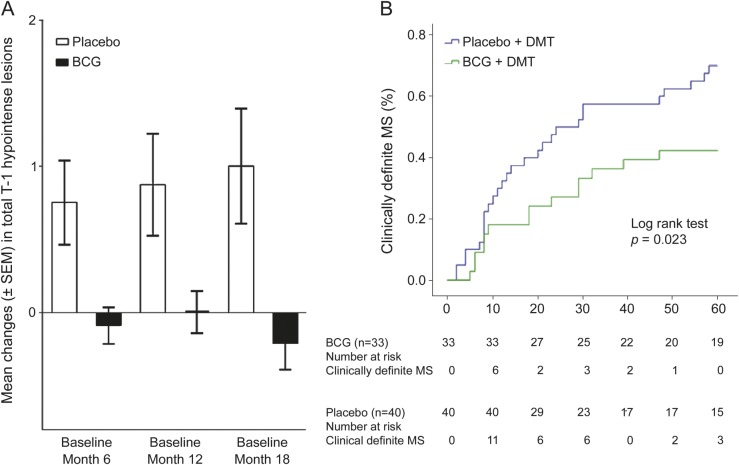
Data from single scans at months 12 and 18 are presented in table e-2. MRI activity did not differ between the BCG and placebo group, while the mean number of new T1-hypointense lesions was higher in the placebo group at month 18 (0.20 ± 0.56 vs 0.00 ± 0.00; p = 0.04). At the same time point, the categorical analysis showed a higher percentage of patients with at least 1 new T1-hypointense lesion in the placebo group (12.5% vs 0%, p = 0.03). The mean change in the number of total T1-hypointense lesions from baseline to months 6, 12, and 18 showed virtually no accumulation in vaccinated CIS subjects, compared to an increased load in those receiving placebo: −0.09 ± 0.72 vs 0.75 ± 1.81 (p = 0.01); 0.0 ± 0.83 vs 0.88 ± 2.21 (p = 0.08); and −0.21 ± 1.03 vs 1.00 ± 2.49 (p = 0.02) for each time point (figure 3A). After 18 months, the cumulative number of relapses was 25/40 (62.5%) in placebo vs 10/33 (30.3%) in BCG arm (risk difference 32.2%, 95% CI 10.5%–53.9%; p = 0.01).
Figure 3. Effect of Bacille Calmette-Guérin vaccine across time.
(A) Mean change in the number of total T1-hypointense lesions from baseline to months 6, 12, and 18 from vaccination or placebo in the 2 study arms. The p values were <0.05 at months 6 and 18; the difference was near-significant at month 12 (p = 0.08; Mann-Whitney U test to compare the distribution of lesion changes). (B) Kaplan-Meier estimates of the cumulative probability of the development of clinically definite multiple sclerosis (MS) according to treatment group. BCG = Bacille Calmette-Guérin; DMT = disease-modifying therapy.
During the follow-up at 5 years, we observed a significant difference between BCG + DMT and placebo + DMT arm in the occurrence of the second demyelinating event (conversion to CDMS). At the end of follow-up, more than half of vaccinated individuals remained relapse-free (19/33; 57.6%), compared with 12/40 (30%) in the placebo arm, with an absolute difference of 27.6% (p = 0.018). A log-rank test showed a different pattern of cumulative probability of CDMS in the 2 groups (p = 0.02, figure 3B). A Cox regression model adjusted for baseline data showed that the 5-year cumulative probability of CDMS was lower in the BCG + DMT arm (hazard ratio = 0.52, 95% CI 0.27–0.99; p < 0.05). The mean time free of relapse was 42.94 ± 21.99 months in the BCG group vs 32.45 ± 23.29 in the placebo group (p < 0.05). During the follow-up at 5 years, most patients remained under IFN-β therapy (56/73) or shifted to different DMT (6/73). In a subgroup of subjects with “benign” course (no disability and stability at MRI), the neurologist in charge decided to withdraw IFN-β and left the patient free of any DMT. A regression logistic model showed that the DMT-free subjects were more frequent in the vaccinated than in the nonvaccinated group (8/33 vs 3/40; odds ratio = 0.20, 95% CI 0.04–0.93; p = 0.04, adjusting for baseline characteristics). At the end of the follow-up the mean EDSS, as well as the mean relapse rate, remained low in both study arms: 1.45 ± 0.88 (range 0–3.5) and 1.52 ± 2.34 in BCG + DMT vs 1.40 ± 0.79 (range 0–3.5) and 1.60 ± 1.94 in placebo + DMT.
No major adverse event was recorded during the trial. During the follow-up, the frequency and the nature of adverse events were within the established profile of DMT that the patients took, without differences between vaccinated and nonvaccinated individuals.
DISCUSSION
BCG vaccination appears to have early beneficial effects and possibly long-term action in persons with CIS, supporting previous observations in patients with MS.12,13 Compared with placebo, BCG significantly decreased disease activity at MRI and the number of new T1-hypointense lesions during the first 6 months. In addition, BCG may have longer effects: treated people had less T1-hypointense lesions and cumulative number of relapses after 18 months and showed a reduced risk of conversion to CDMS over 5 years.
The exact mechanisms behind the effects of BCG in neuroinflammation are unclear. Pleiotropic pathways may help explain the action of BCG in MS: antigenic competition and traffic diversion of autoreactive T cells21,22; the immunomodulatory action of effectors, which are usually associated with proinflammatory pathways15,23,24; and the development of regulatory cells that are activated by adjuvant approaches25 and other microbial products,26 or by exposure to parasites,27 providing support to the view (“hygiene hypothesis”) that “Western” habits have facilitated the development of immune disorders in recent decades.28
General issues that may be relevant for the use of BCG in MS regard the limited use and availability of the vaccine in developed countries, including the United States, and the possible need to obtain a Mantoux reaction prior to treatment initiation in future therapies. Specific issues concerning this trial include partial imbalance between the 2 study arms in consent withdrawal (8 in BCG vs 1 in placebo group) and in baseline characteristics (not significant prevalence of active scans and spinal cord syndromes in the placebo group). While we do not have an explanation for the former point, it is of note for the latter that the BCG effect on the outcome measures remained significant when adjusted for baseline covariates. Potential limitations of the open-label phase of the study may fall within those of all open-label, long-term extensions of clinical trials and especially the unblinded assessment. This limits the interpretation of the 60-month follow-up data, in particular those regarding the DMT-free subjects.
With these considerations in mind, and though the comparison must be taken with caution given the peculiarity of our study and the different protocols of each trial, it is worth noting that the absolute risk reduction of CDMS in our study is not lower than that reported with IFN-β or glatiramer acetate.5–9 Interestingly, the curves of probability of CDMS development (figure 3B) show a clearer separation between the 2 study arms after 10 months, when all patients received DMT. This may be due to the absolute low conversion rate during the first months, but an additive effect of vaccine with DMT cannot be ruled out, as it is also suggested by the significant difference between the 2 study arms in the cumulative number of relapses at month 18.
The long-lasting effects of BCG vaccination are important29,30 and will have to be taken into account if and when phase 3 trials will be initiated. In fact, the proper dose and frequency of BCG remain to be determined. Repeated vaccinations may be an option (as recently reported for patients with insulin-dependent diabetes mellitus).31 However, considering the duration of the favorable effects in MS, more “relaxed” vaccination schedules may be possible, hence reassuring against possible harms of too frequent immunizations (hyperergic reactions, and T helper 17–dependent tissue damage).32 Taken as a whole, our results warrant future phase 3 trials in people with CIS, with additional outcome measures (whole brain and gray matter atrophy, among others) especially aimed at verifying a potential neuroprotective effect. Moreover, our findings demonstrate the feasibility and possible benefit of safe, inexpensive, and handy approaches immediately after the first demyelinating episode.
Supplementary Material
Acknowledgment
The authors thank Lucia De Caprio for linguistic revision of the manuscript, Drs. S. Brescianini (Italian National Institute of Health, Rome) and V. Annibali (CENTERS S. Andrea Hospital-site and NESMOS Department, “Sapienza” University of Rome) for assistance with statistical analysis, and Dr. F. Bagnato (Radiology Department, Institute of Imaging Science, Vanderbilt University, Nashville, TN) for comments on the manuscript.
GLOSSARY
- BCG
Bacille Calmette-Guérin
- CDMS
clinically definite multiple sclerosis
- CI
confidence interval
- CIS
clinically isolated syndrome
- CSE
conventional spin-echo
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- Gd
gadolinium
- IFN
interferon
- MS
multiple sclerosis
- RR
relative risk
- T1W
T1-weighted
- T2W
T2-weighted
- TE
echo time
- TR
repetition time
Footnotes
Editorial, page 15
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Drs. Ristori and Salvetti were the principal investigators. Drs. Ristori, Salvetti, Pozzilli, Caputo, and Bresciasmorra conceived and designed the study. Drs. Romano, Cannoni, Visconti, Coarelli, Mendozzi, Lanzillo, Buttinelli, Gasperini, and Frontoni followed up the patients and acquired the clinical data. Drs. Tinelli, Cecconi, and Quarantelli acquired MRI data. All the authors were involved in the assessment and interpretation of the data. The statistical analyses were conducted by Drs. Cannoni and Vanacore. Drs. Ristori, Romano, Vanacore, Pozzilli, and Salvetti wrote the manuscript.
STUDY FUNDING
Supported by the Italian Ministry of Health (RF99.57) and intramural research funds of Center for Experimental Neurological Therapies (CENTERS; also funded, as a special project, by the Fondazione Italiana Sclerosi Multipla [FISM]), Ospedale S. Andrea-site, NESMOS Department and Department of Neurology and Psychiatry, “Sapienza” University of Rome; Fondazione Don Carlo Gnocchi, IRCCS, Milan; and Department of Neurological Sciences, Federico II University, Naples.
DISCLOSURE
G. Ristori, S. Romano, S. Cannoni, A. Visconti, E. Tinelli, L. Mendozzi, P. Cecconi, Dr. Lanzillo, R. Quarantelli, and M. Buttinelli report no disclosures. C. Gasperini has received fees as speaker from Merck Serono, Aventis, Bayer, and Biogen. M. Frontoni and G. Coarelli report no disclosures. D. Caputo has received honoraria for lectures and travel expense refunds from Sanofi-Aventis, Merck-Serono, Bayer-Schering, and Biogen-Dompè. V. Bresciamorra reports no disclosures. N. Vanacore reports no disclosures. C. Pozzilli has received honoraria for consultancy and speaking from Sanofi-Aventis, Biogen, Bayer Schering, Merck Serono, and Novartis, in addition to research grants from Sanofi-Aventis, Merck Serono, and Bayer Schering. M. Salvetti receives research support and has received fees as speaker from Sanofi-Aventis, Biogen, Bayer Schering, and Merck Serono. He also receives research support from the Italian MS Foundation (FISM). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Brex PA, Ciccarelli O, O'Riordan JI, Sailer M, Thompson AJ, Miller DH. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med 2002;346:158–164 [DOI] [PubMed] [Google Scholar]
- 2.Miller D, Barkhof F, Montalban X, Thompson A, Filippi M. Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol 2005;4:281–288 [DOI] [PubMed] [Google Scholar]
- 3.Tintorè M, Rovira A, Rio J, et al. Baseline MRI predicts future attacks and disability in clinically isolated syndromes. Neurology 2006;67:968–972 [DOI] [PubMed] [Google Scholar]
- 4.Fismiku LK, Brex PA, Altman DR, et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain 2008;131:808–817 [DOI] [PubMed] [Google Scholar]
- 5.Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis: CHAMPS Study Group. New Engl J Med 2000;343:898–904 [DOI] [PubMed] [Google Scholar]
- 6.Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet 2001;357:1576–1582 [DOI] [PubMed] [Google Scholar]
- 7.Kappos L, Freedman M, Polman CH, et al. ; for the BENEFIT Study Group Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet 2007;370:389–397 [DOI] [PubMed] [Google Scholar]
- 8.Comi G, Martinelli V, Rodegher M, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomized, double-blind, placebo-controlled trial. Lancet 2009;374:1503–1511 [DOI] [PubMed] [Google Scholar]
- 9.Kappos L, Freedman M, Polman CH, et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol 2009;8:987–997 [DOI] [PubMed] [Google Scholar]
- 10.Kinkel RP, Kollman C, O’Connor P, et al. IM interferon beta1-a delays definite multiple sclerosis 5 years after a first demyelinating event. Neurology 2006;66:678–684 [DOI] [PubMed] [Google Scholar]
- 11.Kinkel RP, Donchev M, Kollman C, et al. Association between immediate initiation of intramuscular interferon beta-1a at the time of a clinically isolated syndrome and long-term outcomes: a 10-year follow-up of the controlled high-risk Avonex multiple prevention study in ongoing neurological surveillance. Arch Neurol 2012;69:183–190 [DOI] [PubMed] [Google Scholar]
- 12.Ristori G, Buzzi MG, Sabatini U, et al. Use of Bacille Calmette-Guèrin (BCG) in multiple sclerosis. Neurology 1999;53:1588–1589 [DOI] [PubMed] [Google Scholar]
- 13.Paolillo A, Buzzi MG, Giugni E, et al. The effect of Bacille Calmette-Guerin on the evolution of the new enhancing lesions to hypointense T1 lesions in relapsing-remitting MS. J Neurol 2003;250:247–248 [DOI] [PubMed] [Google Scholar]
- 14.Kodama S, Kuhtreiber W, Fujimura S, Dale EA, Faustman D. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science 2003;302:1223–1227 [DOI] [PubMed] [Google Scholar]
- 15.Qin Hy, Chaturvedi P, Singh B. In vivo apoptosis of diabetogenic T cells in NOD mice by IFN-gamma/TNF-alpha. Int Immunol 2004;16:1723–1732 [DOI] [PubMed] [Google Scholar]
- 16.Zuany-Amorim C, Sawicka E, Manlius C, et al. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med 2002;8:625–629 [DOI] [PubMed] [Google Scholar]
- 17.El-Zein M, Parent ME, Benedetti A, Rousseau MC. Does BCG vaccination protect against the development of childhood asthma? A systematic review and meta-analysis of epidemiological studies. Int J Epidemiol 2010;39:469–486 [DOI] [PubMed] [Google Scholar]
- 18.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452 [DOI] [PubMed] [Google Scholar]
- 19.Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain 2003;126:1782–1789 [DOI] [PubMed] [Google Scholar]
- 20.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–127 [DOI] [PubMed] [Google Scholar]
- 21.Bach JF. Infections and autoimmune diseases. J Autoimmunity 2005;25(suppl):74–80 [DOI] [PubMed] [Google Scholar]
- 22.Sewell DL, Reinke EK, Co DO, et al. Infection with mycobacterium bovis BCG diverts traffic of myelin oligodendroglial glycoprotein autoantigen-specific T cells away from the central nervous system and ameliorates experimental autoimmune encephalomyelitis. Clin Diagn Lab Immunol 2003;10:564–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faustman D, Davis M. TNF receptor 2 pathways: drug target for autoimmune diseases. Nat Rev Drug Discov 2010;9:482–493 [DOI] [PubMed] [Google Scholar]
- 24.Kahn DA, Archer DC, Gold DP, Kelly CJ. Adjuvant immunotherapy is dependent on inducible nitric oxide synthase. J Exp Med 2001;193:1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakaguchi S. Control of immune responses by naturally arising CD4+ regulatory T cells that express Toll-like receptors. J Exp Med 2003;197:397–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber MS, Benkhoucha M, Lehmann-Horn K, et al. Repetitive pertussis toxin promotes development of regulatory T cells and prevents central nervous system autoimmune disease. PLoS One 2010;5:e16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol 2007;61:97–108 [DOI] [PubMed] [Google Scholar]
- 28.Ristori G, Buttinelli C, Pozzilli C, Fieschi C, Salvetti M. Microbe exposure, innate immunity and autoimmunity. Immunol Today 1999;20:54. [DOI] [PubMed] [Google Scholar]
- 29.Smith SG, Lalor MK, Gorak-Stolinska P, et al. Mycobacterium tuberculosis PPD-induced immune biomarkers measurable in vitro following BCG vaccination of UK adolescents by multiplex bead array and intracellular cytokine staining. BMC Immunol 2010;11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawara M. Recombinant Mycobacterium bovis BCG vector system expressing SIV Gag protein stably and persistently induces antigen-specific humoral immune response concomitant with IFN gamma response, even at three years after immunization. Clin Immunol 2008;129:492–498 [DOI] [PubMed] [Google Scholar]
- 31.Faustman DL, Wang L, Okubo Y, et al. Proof-of-concept, randomized, controlled clinical trial of Bacillus-Calmette-Guerin for treatment of long-term type 1 diabetes. PLoS One 2012;7:e41756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cruz A, Fraga AG, Fountain JJ, et al. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J Exp Med 2010;207:1609–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.