Abstract
Objective:
We investigated whether severe, MRI-visible perivascular spaces (PVS) in the cerebral hemisphere white matter (centrum semiovale) are more common in patients with pathology-proven cerebral amyloid angiopathy (CAA) than in those with pathology-proven non–CAA-related intracerebral hemorrhage (ICH).
Methods:
Using a validated 4-point scale on axial T2-weighted MRI, we compared PVS in patients with pathology-proven CAA to PVS in those with spontaneous ICH but no histopathologic evidence of CAA. In a preliminary analysis restricted to patients with T2*-weighted gradient-recalled echo MRI, we also investigated whether including severe centrum semiovale PVS increases the sensitivity of existing diagnostic criteria for probable CAA.
Results:
Fourteen patients with CAA and 10 patients with non–CAA-related ICH were included. Eight of the patients with CAA were admitted for symptomatic, spontaneous lobar ICH, 1 because of ischemic stroke, 1 with transient focal neurologic episodes, and 4 due to cognitive decline. Severe (>20) centrum semiovale PVS were more frequent in patients with CAA compared to controls (12/14 [85.7%; 95% confidence interval (CI): 57.2%–98.2%] vs 0/10 [1-sided 95% CI: 0%–30.8%], p < 0.0005); this was robust to adjustment for age. The original Boston criteria for probable CAA showed a sensitivity of 76.9% (95% CI: 46.2%–95%), which increased to 92.3% (95% CI: 64%–99.8%), without loss of specificity, after including severe centrum semiovale PVS.
Conclusions:
Severe centrum semiovale PVS on MRI may be a promising new neuroimaging marker for the in vivo diagnosis of CAA. However, our findings are preliminary and require confirmation and external validation in larger cohorts of pathology-proven CAA.
Sporadic cerebral amyloid angiopathy (CAA),1 caused by progressive vascular amyloid-β deposition in small cortical and leptomeningeal vessels, is a major and increasingly frequent cause of lobar intracerebral hemorrhage (ICH) and cognitive impairment in older patients.2–4 CAA is associated with neuroimaging markers, including lobar ICH and strictly lobar cerebral microbleeds, which allow its clinical-radiologic diagnosis using the Boston criteria.2,5,6 However, these criteria have limited sensitivity in some studies; new noninvasive diagnostic markers of CAA are therefore needed, particularly to identify early disease.2,3
Recently, perivascular spaces (PVS) have emerged as a potential MRI marker of the presence and severity of cerebral small-vessel disease.7–12 PVS are interstitial fluid-filled cavities surrounding small penetrating vessels, which function to allow interstitial fluid and solute efflux from the brain.13–15 CAA almost invariably accompanies Alzheimer disease (AD), and in this setting vascular amyloid-β is associated with enlargement of PVS.16–19 However, whether PVS are associated with sporadic CAA is not known. We hypothesized that progressive amyloid-β deposition in CAA impairs interstitial fluid drainage, causing severe, MRI-visible PVS in the cerebral hemisphere white matter (centrum semiovale). To test this hypothesis, we compared the prevalence of severe centrum semiovale PVS in patients with pathology-proven sporadic CAA to the prevalence in patients with pathology-proven non–CAA-related spontaneous ICH. In a preliminary analysis, we also investigated whether including severe centrum semiovale PVS increases the sensitivity of existing diagnostic criteria for CAA.5
METHODS
Study participants and data collection.
We included all eligible patients from 4 stroke centers identified retrospectively by a systematic keyword search of pathology reports. Cases were defined as subjects with both pathology-proven CAA (from routinely collected brain biopsy, biopsy at hematoma evacuation, or autopsy) and adequate T2-weighted MRI sequences. Controls were defined as subjects with spontaneous, symptomatic ICH, no pathologic evidence of CAA, and adequate T2-weighted MRI.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the National Hospital for Neurology and Neurosurgery and Institute of Neurology Joint Research Ethics Committee and the Commission d’Ethique Biomedicale Hospitalo Facultaire of the Faculte de Medicine (Universite Catholique de Louvain).
Pathologic analysis.
Brain biopsies or hematoma evacuation samples were fixed in 10% formalin, embedded in paraffin, and processed for paraffin sectioning. Routine hematoxylin & eosin staining was performed for morphologic assessment, and the presence or absence of vascular amyloid-β deposition was confirmed by immunohistochemical detection. Samples not containing any assessable vessels were excluded from analysis. We assessed CAA presence and severity in all available vessels (in all cases from solid tissue fragments except in one case from isolated vessels); we also determined, wherever possible, whether vessels were leptomeningeal or parenchymal. CAA severity was graded using the modified Vonsattel grading system20,21; all included CAA cases had a severity of at least grade 2 (i.e., replacement of the whole vessel wall by amyloid-β), in line with previous recommendations.21 Non-CAA cases were defined as absence of vascular amyloid-β deposition.
MRI data and analysis.
Mandatory imaging included axial T2-weighted sequences in all patients (slice thickness: 5 mm, field strength: 1.5T) and, whenever available, T2*-weighted gradient-recalled echo (T2*-GRE) sequences. MRIs were reviewed blinded to clinical details, histopathologic diagnosis, and microbleeds status. PVS were defined and rated on T2-weighted MRIs, according to STandards for ReportIng Vascular changes on nEuroimaging (STRIVE),12 by a trained observer using a validated 4-point visual rating scale (0 = no PVS, 1 = ≤ 10 PVS, 2 = 11–20 PVS, 3 = 21–40 PVS, and 4 = > 40 PVS) in the basal ganglia and centrum semiovale (cerebral hemisphere white matter).9,22 Intrarater reliability testing of the PVS scale using an independent dataset of ICH scans (n = 30) showed an intrarater Cohen kappa of 0.91 for basal ganglia PVS and 0.82 for centrum semiovale PVS.
Statistics.
We prespecified a definition of severe centrum semiovale PVS as >20 (grade 3). Although arbitrary, this definition is in line with the most severe category of white matter PVS used in a previous study (and found to relate to vascular risk factors)23 and provided 2 numerically balanced categories consistent with our aim of increasing the sensitivity of CAA diagnosis. We compared both the crude and age-standardized prevalence of severe PVS between groups using the Fisher exact test. We calculated the sensitivity and specificity after the addition of severe centrum semiovale PVS to the original Boston diagnostic criteria for CAA (including strictly lobar cerebral microbleeds),5 in line with Standards for Reporting of Diagnostic Accuracy (STARD) guidelines.24 Significance level was set at 0.05 for all analyses. Statistical analyses were carried out using STATA (Version 11.2, StataCorp.).
RESULTS
Fourteen CAA patients and 10 non-CAA ICH patients were included (table 1) out of 56 screened; 30 patients were excluded because of no MRI available, 1 non-CAA patient because of no evidence of ICH on MRI, and 1 non-CAA patient because the tissue sample contained no assessable vessels. Eight of the CAA patients were admitted with symptomatic, spontaneous lobar ICH, 1 with ischemic stroke, 1 with transient focal neurologic episodes, and 4 with cognitive decline (1 of these also had radiologic evidence of a previous ICH). All non-CAA patients were admitted with symptomatic, spontaneous ICH. Twenty-three of the 24 available pathologic samples contained at least 2 assessable parenchymal or leptomeningeal vessels. There were 23 solid tissue samples including vessels and 1 sample including only isolated vessels. All 14 CAA cases contained >10 assessable vessels: 12 cases with both cortical and leptomeningeal vessels and 2 with only leptomeningeal vessels. Of the 10 non-CAA cases, 4 contained >10 assessable vessels while the other 6 contained a median number of 2 assessable vessels (interquartile range 2–3); 1 case contained both cortical and leptomeningeal vessels, 5 only cortical vessels, and 3 only leptomeningeal vessels. In the case containing only isolated vessels, their origin could not be determined. The amount of available pathologic tissue was generally greater in the CAA cases than the non-CAA cases.
Table 1.
Patient and imaging characteristics
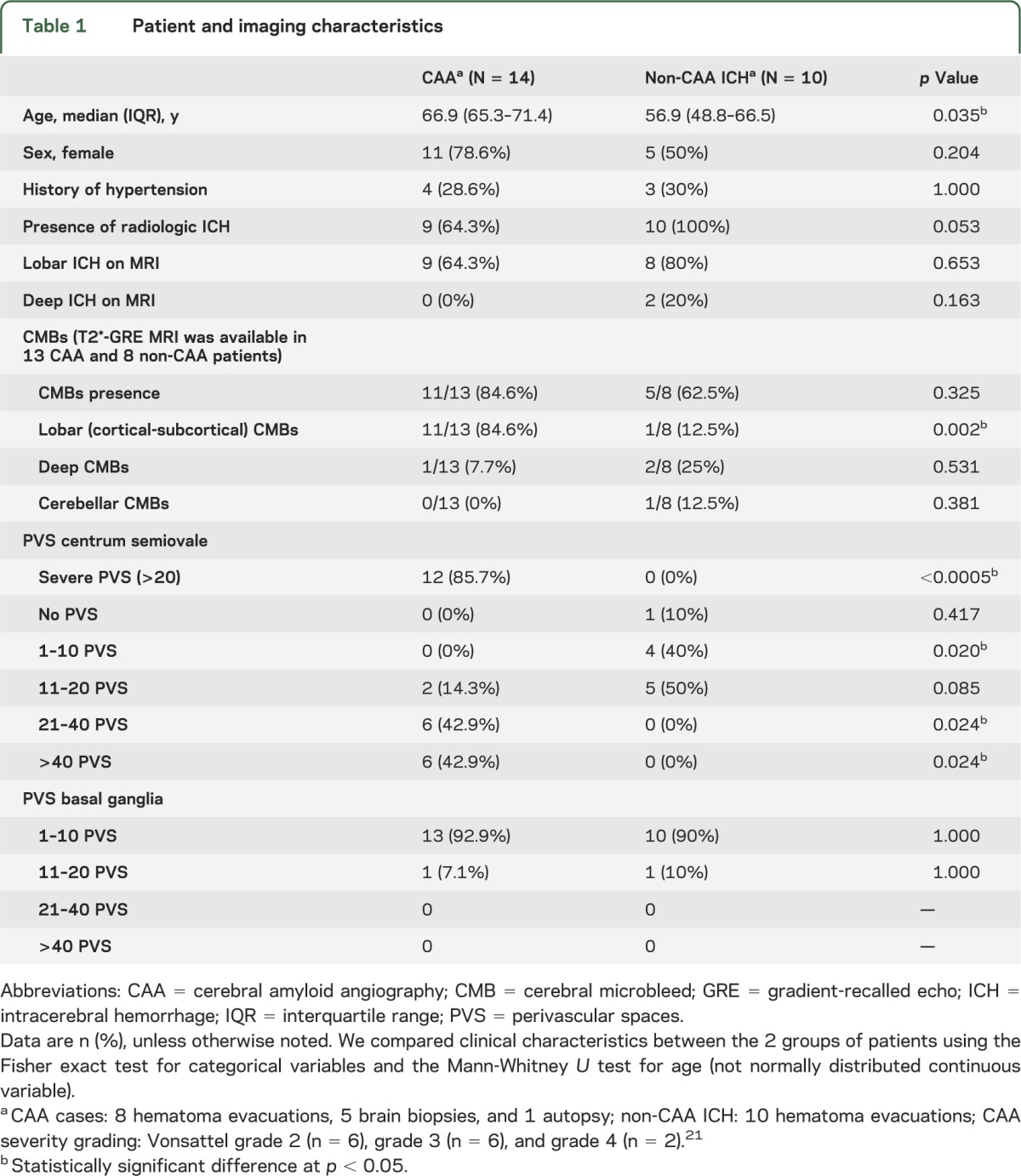
Severe (>20) centrum semiovale PVS were detected in 85.7% (95% confidence interval [CI]: 57.2%–98.2%) of patients with histopathologically confirmed CAA but in none (1-sided 95% CI: 0%–30.8%) of the controls with histopathologically confirmed non-CAA ICH (p < 0.0005) (figure). The age-standardized prevalences of severe centrum semiovale PVS were 85.7% (95% CI: 65.7%–100%) in the CAA group vs 0% in the non-CAA ICH group. The prevalence of the most severe category of centrum semiovale PVS (>40) was also higher in CAA than non-CAA ICH (table 1). Within the CAA group, 7/8 (87.5%; 95% CI: 47.4%–99.7%) patients who presented with symptomatic lobar ICH had severe centrum semiovale PVS (p < 0.0005 compared with the non-CAA ICH group). There was no difference in the prevalence of any basal ganglia PVS category between the CAA and non-CAA ICH groups.
Figure. MRI-visible white matter perivascular spaces in CAA and non-CAA ICH.
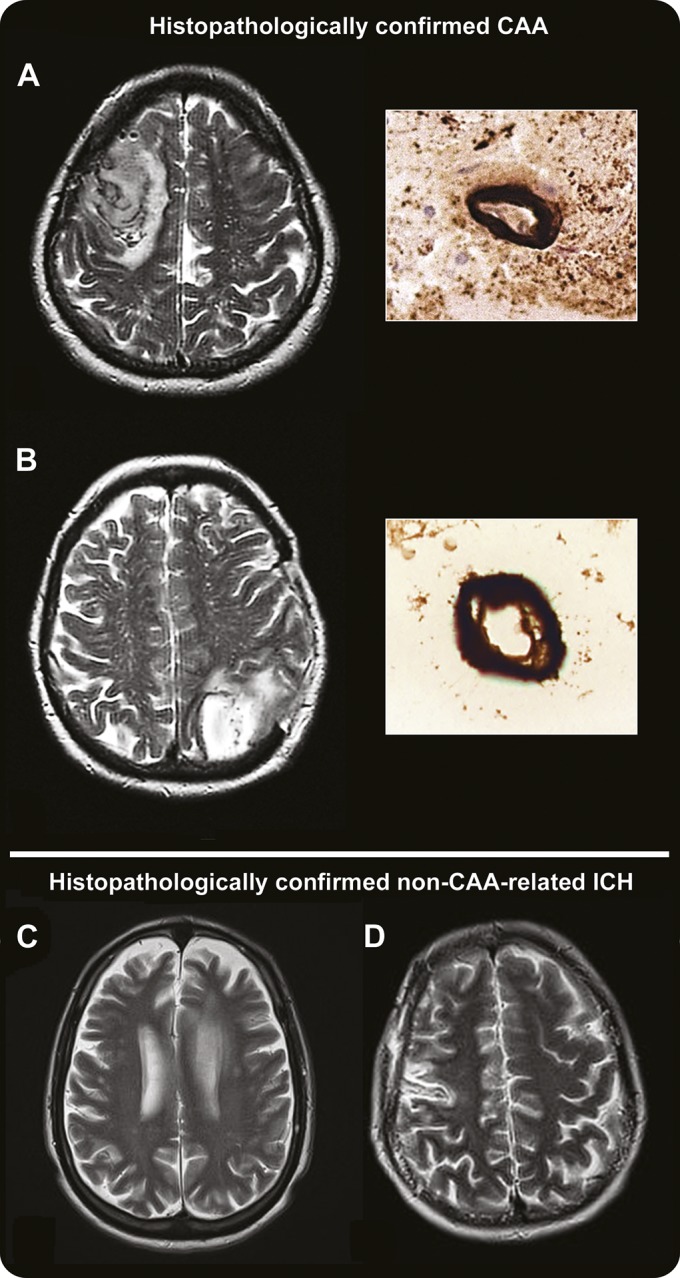
Perivascular spaces (PVS) are defined as small sharply delineated structures of (or close to) CSF intensity measuring <3 mm and following the course of perforating or medullary vessels in basal ganglia and centrum semiovale. In (A) and (B), the left panels show dot-like hyperintensities (florid PVS) on representative axial T2-weighted MRI at 1.5T in the centrum semiovale in patients with histopathologically confirmed cerebral amyloid angiopathy (CAA); the right panels show the corresponding pathology (immunostaining for amyloid-β), with dense amyloid deposition spanning the entire vessel wall of a cortical (A, right panel) and a leptomeningeal arteriole (B, right panel). (C) and (D) show axial T2-weighted MRI from 2 of the non-CAA cases, showing a paucity of centrum semiovale PVS. ICH = intracerebral hemorrhage.
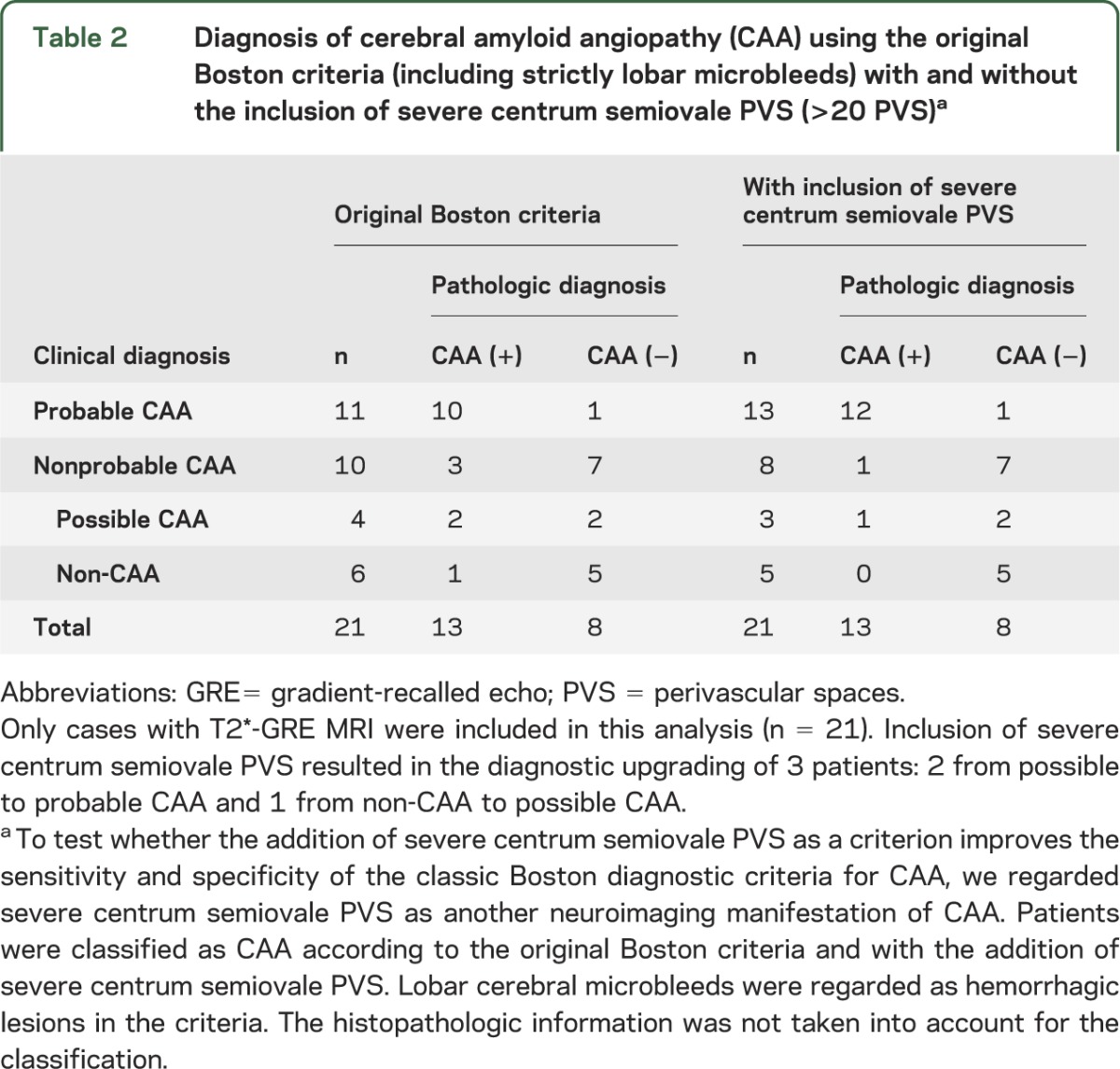
T2*-GRE MRI was available in 13 CAA and 8 non-CAA ICH patients. Adding severe centrum semiovale PVS (>20 PVS) to the original Boston criteria for CAA resulted in the diagnostic upgrading of 3 patients (table 2). The original Boston criteria showed a sensitivity of 92.3% (95% CI: 64%–99.8%) for possible or probable CAA diagnosis, while inclusion of severe centrum semiovale PVS increased their sensitivity to 100% (95% CI: 75.3%–100%), without loss of specificity (62.5%; 95% CI: 24.5%–91.5%). For probable CAA, the original Boston criteria had a sensitivity of 76.9% (95% CI: 46.2%–95%); inclusion of severe centrum semiovale PVS increased this to 92.3% (95% CI: 64%–99.8%). The specificity for probable CAA was 87.5% (95% CI 47.3%–99.7%) for both criteria.
Table 2.
Diagnosis of cerebral amyloid angiopathy (CAA) using the original Boston criteria (including strictly lobar microbleeds) with and without the inclusion of severe centrum semiovale PVS (>20 PVS)a
DISCUSSION
We have shown that severe PVS in the centrum semiovale white matter are more common in subjects with pathology-proven sporadic CAA than control non-CAA subjects. These findings suggest that in this patient population severe centrum semiovale PVS may be a promising new neuroimaging marker to improve the sensitivity of in vivo diagnosis of CAA. However, the sensitivity and specificity of severe centrum semiovale PVS require external validation, ideally in a larger independent cohort.
PVS are a key route for the drainage of interstitial fluid and solutes (including soluble amyloid-β) from the brain. Progressive amyloid-β deposition in small cortical and leptomeningeal arteries in CAA could gradually impair perivascular drainage, causing retrograde dilation of PVS in the underlying white matter of the centrum semiovale either by blocking bulk flow or by diminishing the pulsatility of small vessels (required for efficient interstitial fluid drainage) due to smooth muscle cell loss.13,25 Impaired perivascular drainage could then further exacerbate vascular and perivascular amyloid-β deposition, creating a “feed-forward loop.”10,15 Consistent with this hypothesis, a postmortem study of AD found that amyloid-β blocks perivascular drainage pathways in CAA associated with AD, and the degree of enlargement of white matter PVS correlates with CAA severity.16 Moreover, a recent MRI study in ICH patients reported that very severe centrum semiovale PVS were associated with CAA defined according to clinical-radiologic criteria.7 However, more studies are needed to definitively determine the pathologic basis of MRI-visible PVS.
We found no increased prevalence of basal ganglia PVS in CAA, consistent with deep perforating arteries being largely spared by amyloid-β deposition.4 Previous studies report that neuroimaging markers of hypertensive arteriopathy (lacunes, deep cerebral microbleeds, and white matter changes) are more strongly linked to basal ganglia than centrum semiovale PVS, also suggesting that the distribution of PVS may reflect the underlying arteriopathy.7,9–11 Since lobar ICHs are more likely to be surgically evacuated, our sample of non-CAA ICH is biased toward lobar ICH, which may contribute to the lack of severe basal ganglia PVS in the non-CAA group. However, non-CAA ICH cases more often had milder (1–10) centrum semiovale PVS compared to CAA cases.
Limitations of our study include the small cohort and selection bias due to the requirement for T2-weighted MRI. Because we included routinely collected hematoma evacuation samples, which may be suboptimal in comparison to postmortem examinations, it was not possible to definitively assess whether vessels were parenchymal or leptomeningeal in all patients. Due to limited tissue sampling, it is possible that some non-CAA ICH patients might have had undetected CAA. Moreover, the amount of available tissue (and number of assessable cortical and leptomeningeal vessels) was generally higher in the CAA group, which could have led to a greater chance of sampling error in the non-CAA group. However, misclassification and small sample size would tend to bias toward a null result. Although CAA patients were older than non-CAA patients, the difference in severe centrum semiovale PVS was robust to adjustment for age; furthermore, the CAA group had a higher prevalence of PVS only in the centrum semiovale: confounding by age would be expected to increase PVS severity in both the centrum semiovale and the basal ganglia.7,10,23 Finally, although the PVS raters were blinded to clinical and histopathologic information as well as microbleeds status, it was impossible to blind them to ICH presence.
Our findings are preliminary and hypothesis-generating, requiring external validation in larger independent cohorts with pathologic verification of CAA. Nevertheless, if confirmed, PVS in the centrum semiovale may help improve the sensitivity of MRI for in vivo diagnosis of sporadic CAA in an appropriate clinical context. Further studies are also needed to determine whether centrum semiovale PVS precede other imaging features of CAA,26 which might help identify CAA earlier in its natural history.
GLOSSARY
- AD
Alzheimer disease
- CAA
cerebral amyloid angiopathy
- CI
confidence interval
- GRE
gradient-recalled echo
- ICH
intracerebral hemorrhage
- PVS
perivascular spaces
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. A. Charidimou and M. Burnell. A. Charidimou: project design, data collection, data analysis, write-up. Z. Jaunmuktane: data collection, histology review and classification, critical revisions. J-C. Baron: project design, data collection, critical revisions. M. Burnell: data analysis, data interpretation, critical revisions. P. Varlet: data collection, critical revisions. A. Peeters: data collection, critical revisions. J. Xuereb: data collection, histology review and classification, critical revisions. R. Jäger: imaging analysis advice, critical revisions. S. Brandner: data collection, histology review and classification, critical revisions. D. J. Werring: project design, data interpretation, supervision, obtaining funding, write-up.
STUDY FUNDING
Part of this work was undertaken at UCLH/UCL, which received a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme.
DISCLOSURE
A. Charidimou receives research support from the Greek State Scholarship Foundation, the Stroke Association, and the British Heart Foundation. Z. Jaunmuktane, J.-C. Baron, M. Burnell, P. Varlet, A. Peeters, J. Xuereb, R. Jäger, and S. Brandner report no disclosures. D. J. Werring receives research support from the Department of Health/Higher Education Funding Council for England (Clinical Senior Lectureship Award), the Stroke Association, and the British Heart Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689–701 [DOI] [PubMed] [Google Scholar]
- 2.Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry 2012;83:124–137 [DOI] [PubMed] [Google Scholar]
- 3.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol 2011;70:871–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke 1987;18:311–324 [DOI] [PubMed] [Google Scholar]
- 5.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–539 [DOI] [PubMed] [Google Scholar]
- 6.van Rooden S, van der Grond J, van den Boom R, et al. Descriptive analysis of the Boston criteria applied to a Dutch-type cerebral amyloid angiopathy population. Stroke 2009;40:3022–3027 [DOI] [PubMed] [Google Scholar]
- 7.Charidimou A, Meegahage R, Fox Z, et al. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: a multicentre MRI cohort study. J Neurol Neurosurg Psychiatry 2013;84:624–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cumurciuc R, Guichard JP, Reizine D, Gray F, Bousser MG, Chabriat H. Dilation of Virchow-Robin spaces in CADASIL. Eur J Neurol 2006;13:187–190 [DOI] [PubMed] [Google Scholar]
- 9.Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 2010;41:450–454 [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Ramirez S, Pontes-Neto OM, Dumas AP, et al. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology 2013;80:1551–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouhl RP, van Oostenbrugge RJ, Knottnerus IL, Staals JE, Lodder J. Virchow-Robin spaces relate to cerebral small vessel disease severity. J Neurol 2008;255:692–696 [DOI] [PubMed] [Google Scholar]
- 12.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marin-Padilla M, Knopman DS. Developmental aspects of the intracerebral microvasculature and perivascular spaces: insights into brain response to late-life diseases. J Neuropathol Exp Neurol 2011;70:1060–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol 2009;117:1–14 [DOI] [PubMed] [Google Scholar]
- 15.Arbel-Ornath M, Hudry E, Eikermann-Haerter K, et al. Interstitial fluid drainage is impaired in ischemic stroke and Alzheimer's disease mouse models. Acta Neuropathol 2013;126:353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roher AE, Kuo YM, Esh C, et al. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer's disease. Mol Med 2003;9:112–122 [PMC free article] [PubMed] [Google Scholar]
- 17.Preston SD, Steart PV, Wilkinson A, Nicoll JA, Weller RO. Capillary and arterial cerebral amyloid angiopathy in Alzheimer's disease: defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol Appl Neurobiol 2003;29:106–117 [DOI] [PubMed] [Google Scholar]
- 18.Attems J, Jellinger K, Thal DR, Van Nostrand W. Review: sporadic cerebral amyloid angiopathy. Neuropathol Appl Neurobiol 2011;37:75–93 [DOI] [PubMed] [Google Scholar]
- 19.Weller RO, Massey A, Newman TA, Hutchings M, Kuo YM, Roher AE. Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer's disease. Am J Pathol 1998;153:725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP., Jr Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol 1991;30:637–649 [DOI] [PubMed] [Google Scholar]
- 21.Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke 1997;28:1418–1422 [DOI] [PubMed] [Google Scholar]
- 22.Maclullich AM, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry 2004;75:1519–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke 2010;41:2483–2490 [DOI] [PubMed] [Google Scholar]
- 24.Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 2003;138:W1–W12 [DOI] [PubMed] [Google Scholar]
- 25.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathol 2008;18:253–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deramecourt V, Slade JY, Oakley AE, et al. Staging and natural history of cerebrovascular pathology in dementia. Neurology 2012;78:1043–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]


