Abstract
Objective
To determine how extracorporeal membrane oxygenation affects cerebral blood flow velocity and to determine whether specific changes in cerebral blood flow velocity may be associated with neurologic injury.
Design
Prospective, observational study.
Setting
PICU in a tertiary care academic center.
Patients
Children (age less than or equal to 18 yr) requiring extracorporeal membrane oxygenation support.
Interventions
None.
Measurements and Main Results
Eighteen patients (age 3.8 ± 7.2 years; venovenous neck, n = 5; venoarterial neck, n = 8; venoarterial chest, n = 5) requiring extracorporeal membrane oxygenation underwent daily transcranial Doppler ultrasound measurements of cerebral blood flow velocity in bilateral middle cerebral arteries. Cerebral blood flow velocity measurements were recorded as a percentage of age and gender normal value. On extracorporeal membrane oxygenation, cerebral blood flow velocities in patients not suffering clinically evident neurologic injury were decreased with systolic flow velocity (Vs) 54% ± 3% predicted and mean flow velocity (Vm) 52% ± 4% predicted. After decannulation, Vs and Vm were higher than while on extracorporeal membrane oxygenation at 73% ± 3% predicted (p = 0.0007 vs. value on extracorporeal membrane oxygenation) and 64% ± 4% predicted (p = 0.01 vs. value on extracorporeal membrane oxygenation).
Patients who developed clinically evident cerebral hemorrhage had higher Vs, diastolic flow velocity (Vd), and Vm compared with those who did not: 123% ± 8% predicted, 130% ± 18% predicted, 127% ± 9% predicted (p < compared to values in children not suffering neurological injury). Supranormal flow velocities were noted 2–6 days before clinical recognition of cerebral hemorrhage in all four patients. There were no significant differences in mean arterial blood pressure, circuit flow, or hematocrit between the children who suffered cerebral hemorrhage and those who did not. Partial pressure of carbon dioxide was lower in the group of patients who experienced cerebral hemorrhage than in those who did not (38 ± 2 vs. 44 ± 1 mm Hg, p = 0.03).
Conclusion
In children who did not suffer clinically apparent neurologic injury, cerebral blood flow velocities were lower than normal while on extracorporeal membrane oxygenation support and increased after decannulation. However, children who developed cerebral hemorrhage had higher than normal cerebral blood flow velocities noted for days prior to clinical recognition of bleeding. Cerebral blood flow velocity measurement may represent a portable, noninvasive way to predict cerebral complications of extracorporeal membrane oxygenation and deserves further study.
Keywords: extracorporal membrane oxygenation, intracranial hemorrhage, neurologic complications, transcranial Doppler ultrasound
Extracorporeal membrane oxygenation (ECMO) is used in children with refractory cardiorespiratory failure as a life-saving measure. Children in the Extracorporeal Life Support Organization Registry treated with ECMO survived to hospital discharge in 53% to 65% of cases (1). Neurologic complications such as intracranial hemorrhage and ischemia are major causes of death and long-term disability in ECMO patients. Following acute neurologic injury (ANI) on ECMO, only 36% of children survive to hospital discharge and 6% to 13% suffer severe neurologic disability (2–4). Another 9% to 26% experience more minor motor difficulties or cognitive delays (3, 4). Current neurological monitoring techniques are insufficient to predict which critically ill children receiving ECMO therapy will suffer neurologic injury. Even after a clinical suspicion of neurologic injury in a patient on ECMO has been raised, diagnosis can be difficult. Infants with an open fontanelle may undergo sonography to assist with a diagnosis, but sensitivity for intraparenchymal hemorrhage and/or cerebral ischemia is limited (5). In order to make a diagnosis of ANI in older children and adolescents, transport to the radiology department for a head computed tomography (CT) is often required. Transport of the critically ill, unstable patient on ECMO is logistically challenging and can put the patient at risk of clinical deterioration.
Transcranial Doppler ultrasound (TCD) is a sensitive, noninvasive, portable, real-time tool for monitoring cerebral blood flow velocity (CBFV). Increased intracranial pressure (ICP) and decreased cerebral perfusion pressure give rise to typical changes in the TCD waveform (i.e., decreased diastolic velocity and increased pulsatility index [pulsatility index (PI) = systolic velocity – diastolic velocity/mean velocity (6–8)]. There are no reports that evaluate the CBFVs of pediatric patients requiring ECMO therapy.
We hypothesize that TCD has the potential to assist in the screening and diagnosis of ANI in children on ECMO. We therefore designed a prospective, observational study to test the hypothesis that variations in CBFV measurements will be noted in children who suffer clinically evident neurologic complications while on ECMO therapy.
MATERIALS AND METHODS
Study Design
This was a prospective, observational study of children who underwent ECMO in a tertiary care children’s hospital from January 2011 to December 2011. Inclusion criteria were: age less than or equal to 18 yr old and need for ECMO support. Exclusion criteria were: previously diagnosed disease known to alter CBFV measurements (sickle cell disease, moyamoya, etc.). If patients lacked an acoustic window allowing for adequate TCD examination, they were withdrawn from the study. Written informed consent was signed by a parent or guardian for all subjects before enrollment in the study. All participants suffered severe respiratory or cardiac failure despite conventional treatment and met institutional criteria for ECMO. For patients deemed candidates for venovenous ECMO, cannulation of the right internal jugular vein with an appropriately sized cannula was undertaken. For patients requiring venoarterial ECMO, cannulation of the right internal jugular vein and right common carotid artery (venoarterial neck) or of the right atrium and aorta (venoarterial chest) was performed. The ECMO circuit consisted of the following: one-fourth or three-eighths-inch tubing (Super Tygon, Akron, OH) with a silicone reservoir, a bladderbox (Better Bladder, Oyster Bay, NY), a membrane oxygenator (Quadrox D, Hirrlingen, Germany), a heat exchanger (Cincinnati sub-zero, Cincinnati, OH), and a pump (Jostra, Hirrlingen, Germany). All subjects underwent standard care for ECMO patients while participating in this study. Heparin infusions were titrated to achieve full systemic anticoagulation with goal partial thromboplastin times (PTT) of 60–80 s and anti-factor Xa levels of 0.4–0.7 U/mL. Patient temperature was maintained at 37°C by the ECMO circuit. Caregivers were blinded to the results of the TCD examinations and no changes in clinical care were undertaken based on the ultrasound results. Clinical data known to affect CBFV measurements such as mean arterial blood pressure (MABP), ECMO circuit flow (mL/kg/minute), partial pressure of carbon dioxide (PaCO2), and hematocrit were noted at the time of each TCD recording. Results of head ultrasounds and CTs performed as part of routine care were also recorded.
Transcranial Doppler Measurements
Participants underwent TCD measurement of systolic, diastolic, and mean flow velocity (Vs, Vd, Vm) in bilateral middle cerebral arteries (MCAs) within 24 hours of ECMO initiation and daily thereafter. The same measurements were taken 24–48 hours following decannulation in those patients able to be removed from ECMO support. For the TCD examination, acoustic windows in the temporal region were identified by anatomic landmarks for each patient. Flow velocity measurements were recorded every 1 mm along the entire course of the vessel according to previously described methods (9). In order to be able to compare the CBFVs of the entire study cohort made up of children of various ages and both genders, measurements of flow velocities were recorded as a percentage of age and gender normal value rather than an absolute value (10, 11). The median CBFV value for each individual over time was used to calculate the median and interquartile range CBFV for the entire group. Flow velocity in the distal internal carotid artery proximal to its entry into the skull was also evaluated in order to calculate the Vs/Vica index, or the Lindegaard ratio (LR) (12). Under the assumption that increased flow velocity in the internal carotid artery is secondary to increased blood flow, a Vs/Vica ratio < 3 represents hyperemia and a ratio > 3 represents MCA narrowing. For this study, the LR was recorded as a whole number. The pulsatility index was derived by the TCD unit for each set of measurements according to the following equation: PI = Vs – Vd/Vm. TCD studies were performed by a single, trained ultrasonographer at the bedside using a 2-MHz pulsed probe and commercially available TCD ultrasonography unit (Sonara Digital TCD; CareFusion, Middleton, WI).
Outcome Measures
The primary outcome was development of clinically evident ANI while on ECMO therapy. Participants underwent daily bedside assessment of neurologic examination by caregivers. As part of routine care, infants with an open fontanelle underwent daily head ultrasounds while on ECMO therapy. Older children underwent head CT if clinical suspicion of neurologic injury was raised based on changes in neurologic examination. All imaging studies were performed at the discretion of the treating clinicians and interpreted by pediatric radiologists. The secondary outcome was neurologic performance at hospital discharge based on the pediatric cerebral performance category (PCPC) (13). The PCPC is a scale developed to assess cognitive abilities of pediatric patients. The six categories in this scale are: 1) normal with age appropriate functioning; 2) mild disability; 3) moderate disability; 4) severe disability with dependence on others for all daily activities; 5) coma or vegetative state; and 6) death (14). Good neurologic outcome was defined as PCPC of 1 or 2 at discharge from hospital and poor neurologic outcome was defined as PCPC of 3–6 at discharge from hospital.
Statistical Analysis
Descriptive analysis was conducted to examine patient characteristics. Serial measurements of CBFV were compared by two-way analysis of variance with group (venovenous ECMO, venoarterial ECMO with peripheral neck cannulation, venoarterial ECMO with central/chest cannulation) and time as the factors. Significance of differences was evaluated with Bonferroni posttest. Mann-Whitney U tests were used to compare CBFV, MABP, circuit flow, PaCO2, hematocrit (Hct), PI, PTT, anti-factor Xa levels, and platelet count of patients suffering ANI with those who did not. Values are reported as the median (interquartile range). Statistical significance was assumed with a p ≤ 0.05. Statistical analysis was performed using Prism 5 (GraphPad Software, San Diego, CA).
RESULTS
Patient Characteristics
Nineteen sequential patients were screened and enrolled, with one withdrawn due to lack of acoustic window to allow for adequate TCD measurements. Eighteen patients were included in the analysis. Demographic and clinical characteristics of the 18 evaluated patients are presented in Table 1. Individual patient characteristics are presented in Table 2. Overall survival rate was 56%. In regards to clinically evident neurologic injury, four patients (22%) suffered cerebral hemorrhage and one patient (5%) suffered diffuse cerebral ischemia while on ECMO. All other study participants had grossly normal neurologic examination throughout their hospital course and did not undergo neurologic imaging. Based on the dichotomized PCPC score, all survivors had good neurologic outcome. Causes of death in the study patients included severe, clinically evident ANI (five patients), refractory septic shock (one patient), and withdrawal of support due to irreversible medical condition (two patients).
TABLE 1.
Clinical Data of Patients on Extracorporeal Membrane Oxygenation (n = 18)
| Patient Characteristics | |
|---|---|
| Mean age in yr (range) | 3.8 (2 d to 18 yr) |
| Male, n (%) | 10 (56) |
| Female, n (%) | 8 (44) |
| Race, n (%) | |
| White | 12 (67) |
| Black | 5 (28) |
| Hispanic | 1 (5) |
| Indication for extracorporeal membrane oxygenation, n (%) | |
| Cardiac failure | 8 (44) |
| Respiratory failure | 8 (44) |
| Cardiac arrest/extracorporeal cardiopulmonary resuscitation |
2 (12) |
| Type of extracorporeal membrane oxygenation, n (%) | |
| Venovenous neck | 5 (28) |
| Venoarterial neck | 8 (44) |
| Venoarterial chest | 5 (28) |
| Neurologic complications, n (%) | |
| Cerebral hemorrhage | 4 (22) |
| Diffuse cerebral ischemia | 1 (5) |
| Survival, n (%) | |
| Yes | 10 (56) |
| No | 8 (44) |
| Cause of death, n (%) | |
| Cerebral hemorrhage | 4 (50) |
| Diffuse cerebral ischemia | 1 (12.5) |
| Refractory septic shock | 1 (12.5) |
| Irreversible condition | 2 (25) |
TABLE 2.
Patient Diagnosis, Age, ECMO Type, ECMO Duration, and Neurologic Injury Characteristics
| Patient | Primary Diagnosis | Age | ECMO Type |
ECMO Duration (d) |
Clinically Evident Neurologic Injury |
|---|---|---|---|---|---|
| 1 | Status asthmaticus | 18 mo | VV neck | 3 | No |
| 2 | Myocarditis | 12 mo | VA neck | 5 | No |
| 3 | Heterotaxy, junctional ectopic tachycardia |
2 mo | VA chest | 2 | No |
| 4 | Hypoplastic left heart | 7 mo | VA chest | 7 | Right anterior temporal hemorrhage, bilateral punctuate hemorrhages near caudate, small left frontal SDH noted on ECMO day 7 |
| 5 | Congenital diaphragmatic hernia |
2 d | VA neck | 5 | No |
| 6 | Pulmonary hypertension | 2 mo | VV neck | 2 | No |
| 7 | Pneumonia | 17 yr | VV neck | 22 | No |
| 8 | Sepsis | 4 wk | VA neck | 3 | No |
| 9 | Transposition of the great arteries |
2 mo | VA chest | 8 | Right frontal SDH 13 mm thick, small left frontal SDH, diffuse edema noted on ECMO day 8 |
| 10 | Pulmonary hemorrhage | 16 yr | VA neck | 2 | No |
| 11 | Tetrology of fallot | 6 mo | VA neck | 7 | Right frontal SDH 23 mm thick, left frontal SDH 21 mm thick, diffuse edema noted on ECMO day 7 |
| 12 | Coarctation of aorta | 2 mo | VA chest | 2 | No |
| 13 | Atrioventricular canal | 6 wk | VA neck | 10 | Diffuse hypoxic ischemic injury noted on ECMO day 10 |
| 14 | Congenital diaphragmatic hernia |
2 d | VA neck | 4 | No |
| 15 | Atrioventricular canal | 15 mo | VA chest | 6 | No |
| 16 | Cystic fibrosis | 17 yr | VV neck | 9 | No |
| 17 | Cardiomyopathy | 3 mo | VA neck | 11 | No |
| 18 | Pneumonia | 18 yr | VV neck | 3 | Bilateral frontal SDH, bilateral intraparenchymal hemorrhages noted on ECMO day 3 |
ECMO = extracorporeal membrane oxygenation; VV = venovenous; VA = venoarterial; SDH = subdural hemorrhage.
Cerebral Blood Flow Velocity
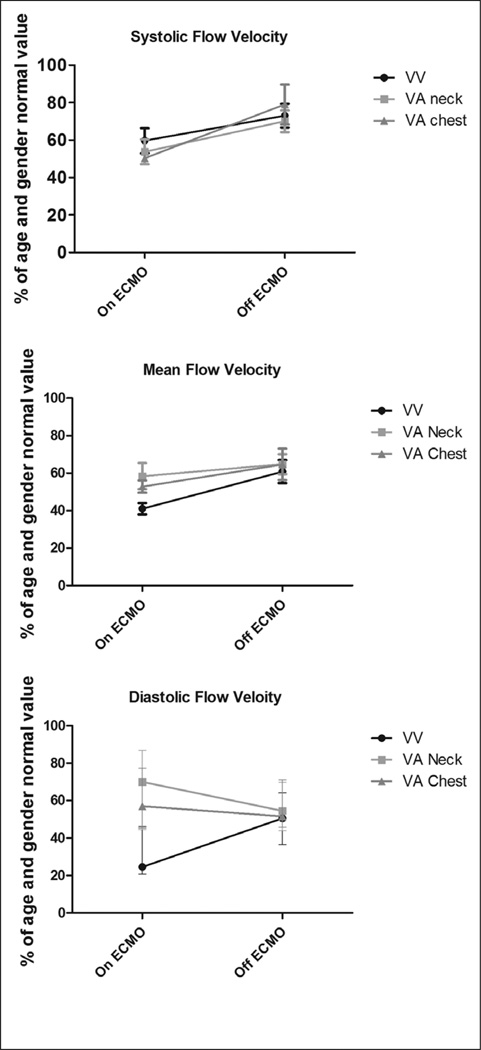
In patients not suffering clinically evident ANI, CBFV changed relatively little from day to day with coefficient of variation < 0.10 for all measurements for each individual. In these participants, measured CBFVs in the bilateral MCAs were significantly reduced compared with age and gender normal values while on ECMO therapy. Systolic flow velocity (Vs) was 54% ± 3% of the predicted value and mean flow velocity (Vm) was 52% ± 4% of the predicted value. After decannulation from ECMO support, Vs was significantly higher than while on ECMO at 73% ± 3% of the predicted value (p = 0.0007). Vm was also significantly higher than while on ECMO at 64% ± 4% of the predicted value (p = 0.01) (Fig. 1). It should be noted that both Vs and Vm remained lower than age and gender normal values even after removal from ECMO. The type of ECMO support (venovenous, venoarterial) and location of cannulation (peripheral, central) were not associated with differences in systolic or mean flow velocities (Vs – p = 0.84, Vm – p = 0.31). In contrast to Vm and Vs measurements, Vd were 64% ± 3% of the predicted value while on support and were relatively unchanged at 57% ± 4% of the predicted value (p = 0.94) following decannulation in those children undergoing venoarterial ECMO. In patients undergoing venovenous ECMO, Vd was 33% ± 5% of predicted on ECMO and significantly increased to 51% ± 3% of predicted after removal from ECMO (p = 0.01) (Fig. 1). Additionally, no significant differences between right and left MCA CBFVs (Vs, Vm, Vd) were noted in those children cannulated in the neck for venoarterial or venovenous ECMO (p > 0.66 for all values, data not shown).
Figure 1.
Percentage of age and gender normal value of systolic flow velocity (Vs), mean flow velocity (Vm), and diastolic flow velocity (Vd) on and following removal from extracorporeal membrane oxygenation (ECMO) support in children not suffering acute neurologic injury in each of the three patient groups (venovenous [VV] ECMO, venoarterial [VA] ECMO with neck cannulation, VA ECMO with chest cannulation). Values represent the median and interquartile range for each group. p = 0.0007 for Vs on vs. off ECMO. p = 0.01 for Vm on vs. off ECMO. p = 0.94 for Vd on vs. off VA ECMO. p = 0.01 for Vd on vs. off VV ECMO.
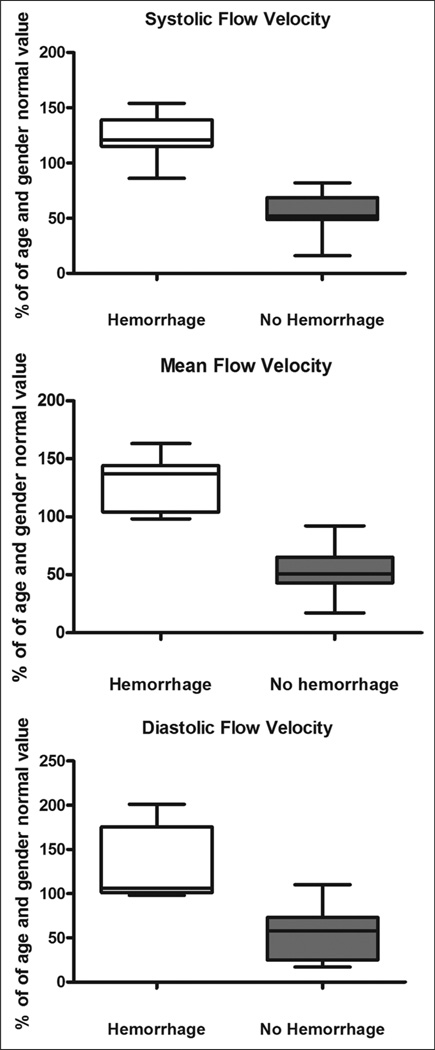
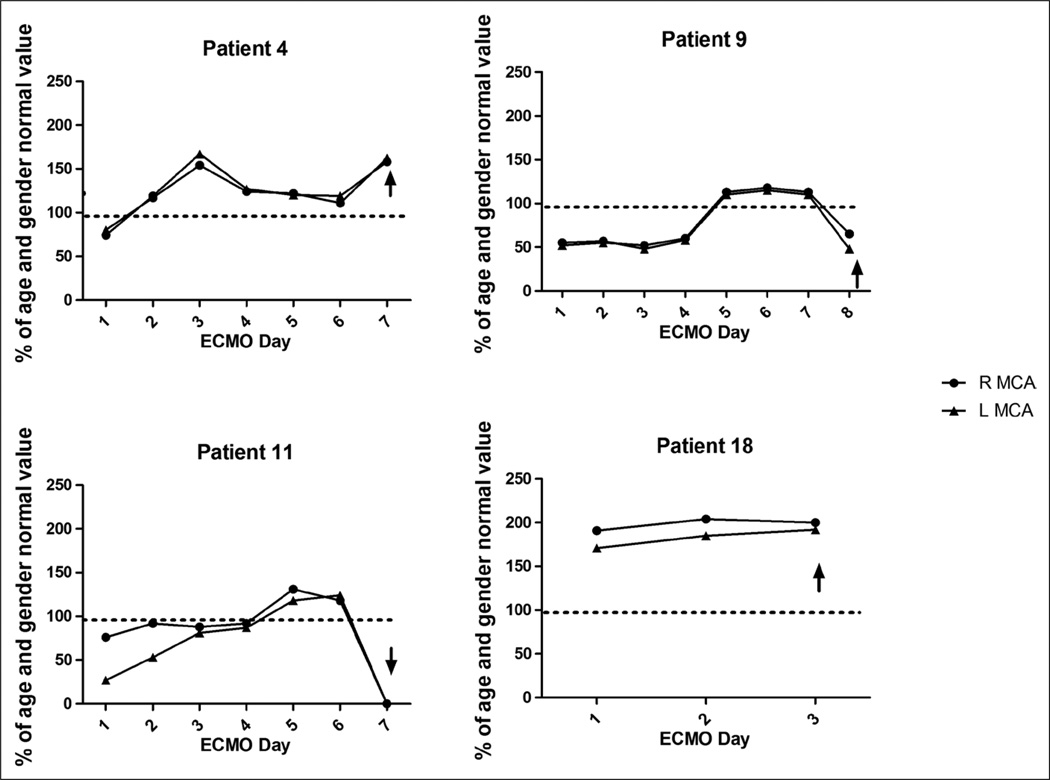
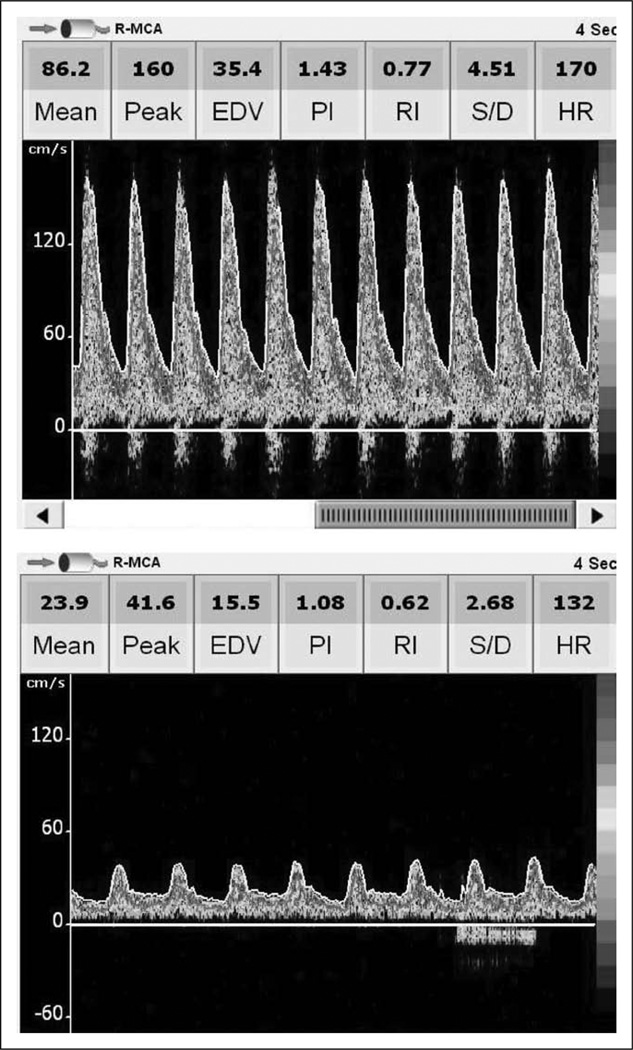
Four children (venovenous ECMO n = 1, venoarterial ECMO with neck cannulation n = 1, venoarterial ECMO with chest cannulation n = 2) suffered clinically evident cerebral hemorrhage while undergoing ECMO therapy. In these children, Vs was 123% ± 8% of the predicted value, Vd was 130% ± 18% of the predicted value, and Vm was 127% ± 9% of the predicted value (p ≤ 0.0001 for all values compared with CBFVs in patients not suffering clinically evident ANI). These findings are depicted in Figure 2. In all four patients, these supranormal CBFVs were noted for 2–6 days prior to clinical recognition of ANI and were sustained at these elevated values over that time frame (Fig. 3). In all four patients, the LR was less than 3 for all measurements, suggesting hyperemia as the cause of elevated CBFV. Actual TCD images from a patient experiencing cerebral hemorrhage are compared with those of an age- and gender-matched individual on ECMO who did not suffer ANI in Figure 4. Median values of MABP, circuit flow, and hematocrit were not significantly different between the patients suffering cerebral hemorrhage and those who did not (MABP: 62 [54–71] vs. 59 [48–74] mm Hg, p = 0.41, circuit flow: 80 [66–128] vs. 83 [66–125] mL/kg/minute, p = 0.93, hematocrit: 39 [37–40] vs. 39 [36–41] %, p = 0.49). PaCO2 was significantly lower in the group of patients suffering cerebral hemorrhage compared with those who did not (37 (33–42) vs. 44 (37–49) mm Hg, p = 0.03). During the entire duration of ECMO therapy, there were no significant differences in PTTs, anti-factor Xa levels, or platelet counts between the children suffering cerebral hemorrhage and those who did not (PTT: 62 (53–76) vs. 66 (56–79) s, p = 0.09, anti-factor Xa: 0.6 (0.35–0.65) vs. 0.6 (0.4–0.7) U/mL, p = 0.65, platelets: 156 (112–178) vs. 162 (116–184) K/cu mm, p = 0.74).
Figure 2.
Percentage of age and gender normal value of systolic flow velocity (Vs), mean flow velocity (Vm), and diastolic flow velocity (Vd) in children suffering cerebral hemorrhage compared with those who did not suffer cerebral hemorrhage. Values represent the median and interquartile range for each group. p ≤ 0.0001 for all values.
Figure 3.
Percentage of age and gender normal value of systolic flow velocity (Vs) on each extracorporeal membrane oxygenation (ECMO) day for each of the four patients ultimately experiencing cerebral hemorrhage. The arrow represents the time of clinical recognition of neurologic injury. R MCA = right middle cerebral artery; L MCA = left middle cerebral artery.
Figure 4.
Transcranial Doppler ultrasound images of a patient suffering massive cerebral hemorrhage on extracorporeal membrane oxygenation (ECMO) (top) compared with an age- and gender-matched patient who did not suffer acute neurologic injury on ECMO (bottom). Note the markedly increased systolic, diastolic, and mean flow velocities in the child suffering cerebral hemorrhage. The image of the patient suffering massive cerebral hemorrhage was taken 4 days prior to any clinical recognition of bleeding. Both patients were on venoarterial ECMO (central cannulation) due to complications of congenital heart disease. Mean arterial blood pressure was 60 mm Hg, ECMO circuit flow was 100 mL/kg/minute, and PaCO2 was 35 mm Hg for both patients at the time these images were taken. R-MCA = right middle cerebral artery; EDV = end diastolic velocity; PI = pulsatility index; RI = resistive index; S/D = systolic/diastolic; HR = heart rate.
Only one patient suffered clinically evident diffuse cerebral ischemia without hemorrhage during this study. In this patient, CBFVs were somewhat lower than in the children who did not suffer clinically evident neurologic injury. Vs measured 46% ± 7% of the predicted value, Vd measured 46% ± 4% of the predicted value, and Vm measured 46% ± 3% of the predicted value.
Among all patients suffering clinically evident ANI, the right MCA PI was elevated at 1.5 ± 0.2 compared with 1 ± 0.1 in the group of patients not suffering ANI (p = 0.03). The left MCA PI was also elevated in the group suffering clinically apparent ANI at 1.7 ± 0.2 compared with 1.1 ± 0.1 in the group without recognized neurologic injury (p = 0.003). In children experiencing cerebral hemorrhage, PI elevations were noted 2–5 days prior to clinical recognition of bleed. In the patient suffering diffuse cerebral ischemia, PI elevation was noted 6 days prior to clinical recognition of neurologic injury.
DISCUSSION
ECMO is a potentially life-saving measure for patients with refractory cardiorespiratory failure. It is, however, associated with significant neurologic morbidity and mortality including cerebral hemorrhage and cerebral ischemia (1–4). The pathophysiology for neurologic injury in these patients is likely multifactorial and includes: profound hypoxia and shock prior to initiation of ECMO support, alterations in the direction of cerebral blood flow following neck vessel cannulation (15–17), the nonpulsatile flow of venoarterial ECMO (18, 19), cerebral venous hypertension due to internal jugular vein occlusion (20), prolonged heparinization, and cerebral emboli (21). Little is known about how the initiation and continuation of ECMO support affects CBFV in children or the relationship of altered CBFV to neurologic insult.
We present the first evaluation of CBFV in pediatric patients on ECMO and provide the first report of changes in TCD measurements that predict the clinical development of severe, clinically evident ANI in this population. In children not suffering ANI, flow velocities were typically well below age and gender normal values while on ECMO support and increased significantly following decannulation. These findings are in agreement with previously published reports involving CBFV measurements in neonates requiring ECMO support. In a case report of three newborn infants undergoing venoarterial ECMO, the author reports an 18% to 50% reduction in MCA peak and mean flow velocities compared with pre-ECMO values in two of three infants (22). Additionally, in six infants undergoing venoarterial ECMO, Weber (20) noted a significant reduction in right-sided mean MCA flow velocity below pre-ECMO values at initiation of ECMO. This reduction persisted when re-evaluated 8 hours later. Fukuda et al (23) evaluated cerebral blood flow velocities in 14 infants on venoarterial ECMO and 19 infants on venovenous ECMO. These patients had a reduction from baseline values in bilateral MCA flow velocities during the 12 hours following cannulation for both venoarterial and venovenous ECMO, although the reductions were much more dramatic in the venoarterial group of patients. In contrast to those studies, another study involving ten infants on venoarterial ECMO reported a 50% increase in bilateral MCA CBFV compared with expected values 60 minutes following initiation of ECMO support (15).
Reduced CBFV while on ECMO support as seen in most of these studies and verified by our study is likely due to several mechanisms. Patients requiring ECMO support are critically ill and are often deeply sedated and paralyzed. These treatments reduce cerebral metabolic demand and due to flow-metabolism coupling result in decreased cerebral blood flow (24). Following ligation and cannulation of the internal jugular vein, patients on both venovenous and venoarterial ECMO experience increased cerebral venous congestion which may contribute to decreased CBFV in these patients (25, 26). Lastly, patients requiring venoarterial ECMO often have significantly reduced cardiac function (both ejection fraction and cardiac output) with cerebral blood flow then becoming primarily pump dependent (23). Pump flow is characterized by decreased systolic upstroke, lack of dichrotic notch, and continuous diastolic flow (22). These changes would contribute to the reduced Vs and Vm measurements seen in our study. In the study reporting increased bilateral MCA CBFV while on ECMO, circuit flow rate in their cohort of patients ranged between 165 and 210 mL/kg/ minute during the TCD examinations (15). This flow rate is much higher than the mean flow rate of our patients (≈ 80 mL/kg/minute) and of the patients in the other studies cited here (≈ 80–120 mL/kg/minute) and likely contributed to their findings.
In this study, four children suffered clinically evident acute cerebral hemorrhage while undergoing ECMO therapy. CBFVs were significantly different in these children compared with the CBFVs in the children not suffering clinically apparent ANI. These four patients had markedly elevated Vs, Vd, and Vm to supranormal values for 2–6 days before clinical recognition of hemorrhage. LR for all measurements in all four patients was < 3, which is consistent with hyperemia as the etiology of increased CBFVs.
The explanation for these findings is not clear. Profound hypoxia, hypotension, and shock are common prior to initiation of ECMO support and could have resulted in cerebral ischemia in these patients. Brain tissue hypoxia and acidosis results in cerebral vasodilation and increased cerebral flow (24). This phenomenon is known as reactive hyperemia and would give rise to elevated CBFV patterns. Additionally, following an ischemic insult, small cerebral arterioles are often no longer capable of participating in normal cerebral metabolic flow-coupling (14, 27). As cerebral metabolism and cerebral blood flow become uncoupled, a state of relative hyperemia exists given the level of sedation and paralysis most patients on ECMO receive. Reactive and relative hyperemia over time may increase the risk of intracranial hemorrhage, especially in patients with previous ischemic damage of cerebral blood vessels who are undergoing full systemic anticoagulation. Additionally, in animal models of cardiopulmonary bypass, perivascular astrocyte cell injury in response to cannulation and circuit flow has been demonstrated (27, 28). Injured astrocytes may release vasoactive hormones that result in cerebral vasodilation and increased cerebral blood flow (28, 29). As cardiopulmonary bypass and ECMO are similar treatment modalities, it is possible that these pathophysiologic changes could have contributed to the elevated CBFV measurements seen in this study in some patients.
Several investigators note impaired autoregulation in patients undergoing cardiopulmonary bypass (30). If autoregulation is impaired in some children undergoing ECMO support, elevations in cerebral perfusion pressure would lead to hyperemia and elevated CBFV measurements. However, there were no significant differences in MABP or circuit flow between the children who had increased CBFVs and those who did not, making this possibility less likely. Of note, the significant difference in PaCO2 between groups did not likely contribute to our findings. PaCO2 is a potent cerebral vasodilator, so decreased levels should result in decreased cerebral blood flow and measured CBFVs, whereas we observed the opposite in our cohort.
In patients suffering severe, clinically apparent ANI, bilateral PIs were significantly higher than in those patients who did not suffer such injury. As PI is a calculated value relying on both Vs and Vd, either increased Vs or decreased Vd can result in an increased PI. In children suffering cerebral hemorrhage, increased Vs due to hyperemia was likely the primary contributor to PI elevations. In the child suffering diffuse cerebral ischemia, severe edema was noted on head CT. Cerebral edema with increased ICP leads to decreased diastolic cerebral blood flow (6, 7). Therefore, decreased Vd values due to increased ICP was likely the main cause of PI elevations in this patient.
There are several limitations of this study that should be noted. First, we are limited by our small sample size. Additionally, as this is an exploratory pilot study, we evaluated and analyzed three different populations of children (venovenous ECMO, venoarterial ECMO with neck cannulation, and venoarterial ECMO with chest cannulation) together. Future studies should be designed to evaluate these patient groups separately in order to better evaluate the effects of location of cannulation on CBFV in each group. Also, we cannot determine causality between altered CBFV measurements and the occurrence of ANI. Large, multi-centered trials need to be undertaken to validate the findings of our study and to determine relationships between measured CBFV and cerebral complications while on ECMO therapy. In addition, routine cerebral imaging was not performed on all study participants. It is therefore possible that some patients may have had radiologic evidence of neurologic injury not evident on clinical examination. Future studies should focus on protocolized neuroimaging in order to better delineate the sensitivity of TCD in detecting neurologic injury in children while on ECMO.
It should also be noted that the CBFVs reported in this article were compared with normative data drawn from only two papers (10, 11). Limited normative data are a weakness of the field in general and future studies evaluating these values need to be undertaken. Also, TCD measures CBFV rather than absolute cerebral blood flow, making it difficult to ensure that the increased CBFV measured in our patients suffering cerebral hemorrhage actually represented increased cerebral blood flow. However, the flow in a vessel is dependent on the radius of the vessel and on the flow velocity in that vessel. Several studies have demonstrated that the diameter of the MCA remains unchanged over time, thus suggesting observed changes in CBFV are a reflection of change in absolute cerebral blood flow (31, 32).
There is currently no laboratory or radiologic investigation that is able to predict which patients on ECMO therapy will develop neurologic complications. TCD is a noninvasive, portable tool that can easily be used at a patient’s bedside to evaluate CBFV. This study noted clear differences in measured CBFVs in children who ultimately suffered severe, clinically evident ANI while on ECMO support compared with those who did not. These changes were noted for days prior to any clinical recognition of cerebral injury. Based on the results of this investigation, a multi-centered trial evaluating the effectiveness of CBFV measurements as a means to predict the patients at risk of developing cerebral complications on ECMO should be undertaken. Additionally, causality between increased CBFV measurements and the occurrence of ANI should be examined. If our findings are confirmed in a larger cohort of children on ECMO, we speculate that TCD measurements may have a role, through identification of high-risk patients, in the design of subsequent interventional trials aimed at reducing ANI in this population.
CONCLUSIONS
In children not suffering clinically evident ANI, CBFV is lower than expected based on age and gender normal values while on ECMO support and increases after decannulation. However, children who developed cerebral hemorrhage had higher than normal CBFV in the days prior to clinical recognition of bleeding. CBFV measurement may represent a portable, noninvasive way to predict patients at risk for cerebral complications on ECMO and deserves further study.
Footnotes
The authors have not disclosed any potential conflicts of interest.
REFERENCES
- 1.Conrad SA, Rycus PT, Dalton H. Extracorporeal Life Support Registry Report 2004. ASAIO J. 2005;51:4–10. doi: 10.1097/01.mat.0000151922.67540.e9. [DOI] [PubMed] [Google Scholar]
- 2.Hervey-Jumper SL, Annich GM, Yancon AR, et al. Neurological com plications of extracorporeal membrane oxygenation in children. J Neurosurg Pediatr. 2011;7:338–344. doi: 10.3171/2011.1.PEDS10443. [DOI] [PubMed] [Google Scholar]
- 3.Hanekamp MN, Mazer P, van der Cammen-van Zijp MH, et al. Follow-up of newborns treated with extracorporeal membrane oxygenation: a nationwide evaluation at 5 years of age. Crit Care. 2006;10:R127. doi: 10.1186/cc5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nijhuis-van der Sanden MW, van der Cammen-van Zijp MH, Janssen AJ, et al. Motor performance in five-year-old extracorporeal membrane oxygenation survivors: A population-based study. Crit Care. 2009;13:R47. doi: 10.1186/cc7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blankenberg FG, Loh NN, Bracci P, et al. Sonography, CT, and MR imaging: a prospective comparison of neonates with suspected intracranial ischemia and hemorrhage. AJNR Am J Neuroradiol. 2000;21:213–218. [PMC free article] [PubMed] [Google Scholar]
- 6.Hassler W, Steinmetz H, Gawlowski J. Transcranial Doppler ultrasonography in raised intracranial pressure and in intracranial circulatory arrest. J Neurosurg. 1988;68:745–751. [PubMed] [Google Scholar]
- 7.Klingelhöfer J, Conrad B, Benecke R, et al. Evaluation of intracranial pressure from transcranial Doppler studies in cerebral disease. J Neurol. 1988;235:159–162. doi: 10.1007/BF00314307. [DOI] [PubMed] [Google Scholar]
- 8.Martí-Fàbregas J, Belvís R, Guàrdia E, et al. Relationship between transcranial Doppler and CT data in acute intracerebral hemorrhage. AJNR Am J Neuroradiol. 2005;26:113–118. [PMC free article] [PubMed] [Google Scholar]
- 9.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 10.Bode H, Wais U. Age dependence of flow velocities in basal cerebral arteries. Arch Dis Child. 1988;63:606–611. doi: 10.1136/adc.63.6.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vavilala MS, Kincaid MS, Muangman SL, et al. Gender differences in cerebral blood flow velocity and autoregulation between the anterior and posterior circulations in healthy children. Pediatr Res. 2005;58:574–578. doi: 10.1203/01.PDR.0000179405.30737.0F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindegaard KF, Nornes H, Bakke SJ, et al. Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements. Acta Neurochir (Wien) 1989;100:12–24. doi: 10.1007/BF01405268. [DOI] [PubMed] [Google Scholar]
- 13.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 14.Leffler CW, Beasley DG, Busija DW. Cerebral ischemia alters cerebral microvascular reactivity in newborn pigs. Am J Physiol. 1989;257:266–271. doi: 10.1152/ajpheart.1989.257.1.H266. [DOI] [PubMed] [Google Scholar]
- 15.Van Heijst A, Liem D, Hopman J, et al. Oxygenation and hemodynamics in left and right cerebral hemispheres during induction of veno-arterial extracorporeal membrane oxygenation. J Pediatr. 2004;144:223–228. doi: 10.1016/j.jpeds.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Hunter CJ, Blood AB, Bishai JM, et al. Cerebral blood flow and oxygenation during venoarterial and venovenous extracorporeal membrane oxygenation in the newborn lamb. Pediatr Crit Care Med. 2004;5:475–481. doi: 10.1097/01.pcc.0000130992.73123.bc. [DOI] [PubMed] [Google Scholar]
- 17.Lohrer RM, Bejar RF, Simko AJ, et al. Internal carotid artery blood flow velocities before, during, and after extracorporeal membrane oxygen ation. Am J Dis Child. 1992;146:201–207. doi: 10.1001/archpedi.1992.02160140067024. [DOI] [PubMed] [Google Scholar]
- 18.Short BL, Walker LK, Bender KS, et al. Impairment of cerebral autoregulation during extracorporeal membrane oxygenation in newborn lambs. Pediatr Res. 1993;33:289–294. doi: 10.1203/00006450-199303000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Taylor GA, Martin GR, Short BL. Cardiac determinants of cerebral blood flow during extracorporeal membrane oxygenation. Invest Radiol. 1989;24:511–516. doi: 10.1097/00004424-198907000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Weber TR, Kountzman B. The effects of venous occlusion on cerebral blood flow characteristics during ECMO. J Pediatr Surg. 1996;31:1124–1127. doi: 10.1016/s0022-3468(96)90100-1. [DOI] [PubMed] [Google Scholar]
- 21.Zanatta P, Forti A, Bosco E, et al. Microembolic signals and strategy to prevent gas embolism during extracorporeal membrane oxygenation. J Cardiothorac Surg. 2010;5:5. doi: 10.1186/1749-8090-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raju TN, Kim SY, Meller JL, et al. Circle of Willis blood velocity and flow direction after common carotid artery ligation for neonatal extra-corporeal membrane oxygenation. Pediatrics. 1989;83:343–347. [PubMed] [Google Scholar]
- 23.Fukuda S, Aoyama M, Yamada Y, et al. Comparison of venoarterial versus venovenous access in the cerebral circulation of newborns undergoing extracorporeal membrane oxygenation. Pediatr Surg Int. 1999;15:78–84. doi: 10.1007/s003830050521. [DOI] [PubMed] [Google Scholar]
- 24.Lassen NA, Christensen MS. Physiology of cerebral blood flow. Br J Anaesth. 1976;48:719–734. doi: 10.1093/bja/48.8.719. [DOI] [PubMed] [Google Scholar]
- 25.Taylor GA, Walker LK. Intracranial venous system in newborns treated with extracorporeal membrane oxygenation: Doppler US evaluation after ligation of the right jugular vein. Radiology. 1992;183:453–456. doi: 10.1148/radiology.183.2.1561349. [DOI] [PubMed] [Google Scholar]
- 26.O’Connor TA, Haney BM, Grist GE, et al. Decreased incidence of intracranial hemorrhage using cephalic jugular venous drainage during neonatal extracorporeal membrane oxygenation. J Pediatr Surg. 1993;28:1332–1335. doi: 10.1016/s0022-3468(05)80323-9. [DOI] [PubMed] [Google Scholar]
- 27.Abdul-Khaliq H, Uhlig R, Böttcher W, et al. Factors influencing the change in cerebral hemodynamics in pediatric patients during and after corrective cardiac surgery of congenital heart diseases by means of full-flow cardiopulmonary bypass. Perfusion. 2002;17:179–185. doi: 10.1191/0267659102pf563oa. [DOI] [PubMed] [Google Scholar]
- 28.Harder DR, Alkayed NJ, Lange AR, et al. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke. 1998;29:229–234. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- 29.Chopp M, Welch KM, Tidwell CD, et al. Effect of mild hyperthermia on recovery of metabolic function after global cerebral ischemia in cats. Stroke. 1988;19:1521–1525. doi: 10.1161/01.str.19.12.1521. [DOI] [PubMed] [Google Scholar]
- 30.Polito A, Ricci Z, Di Chiara L, et al. Cerebral blood flow during cardiopulmonary bypass in pediatric cardiac surgery: the role of transcranial Doppler–a systematic review of the literature. Cardiovasc Ultrasound. 2006;4:47. doi: 10.1186/1476-7120-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huber P, Handa J. Effect of contrast material, hypercapnia, hyperventilation, hypertonic glucose and papaverine on the diameter of cere bral arteries: angiographic determination in man. Invest Radiol. 1967;l2:17–32. doi: 10.1097/00004424-196701000-00016. [DOI] [PubMed] [Google Scholar]
- 32.van der Linden J, Priddy R, Ekroth R, et al. Cerebral perfusion and metabolism during profound hypothermia in children. A study of middle cerebral artery ultrasonic variables and cerebral extraction of oxygen. J Thorac Cardiovasc Surg. 1991;102:103–114. [PubMed] [Google Scholar]