Abstract
Background:
Survivors of neonatal infections are at risk of neurodevelopmental impairment (NDI), a burden not previously systematically quantified and yet important for program priority setting. Systematic reviews and meta-analyses were undertaken and applied in a three-step compartmental model to estimate NDI cases after severe neonatal bacterial infection in South Asia, sub-Saharan Africa, and Latin America in neonates of >32 wk gestation (or >1,500 g).
Methods:
We estimated cases of sepsis, meningitis, pneumonia, or no severe bacterial infection from among estimated cases of possible severe bacterial infection ((pSBI) step 1). We applied respective case fatality risks ((CFRs) step 2) and the NDI risk among survivors (step 3). For neonatal tetanus, incidence estimates were based on the estimated deaths, CFRs, and risk of subsequent NDI.
Results:
For 2010, we estimated 1.7 million (uncertainty range: 1.1–2.4 million) cases of neonatal sepsis, 200,000 (21,000–350,000) cases of meningitis, 510,000 cases (150,000–930,000) of pneumonia, and 79,000 cases (70,000–930,000) of tetanus in neonates >32 wk gestation (or >1,500 g). Among the survivors, we estimated moderate to severe NDI after neonatal meningitis in 23% (95% confidence interval: 19–26%) of survivors, 18,000 (2,700–35,000) cases, and after neonatal tetanus in 16% (6–27%), 4,700 cases (1,700–8,900).
Conclusion:
Data are lacking for impairment after neonatal sepsis and pneumonia, especially among those of >32 wk gestation. Improved recognition and treatment of pSBI will reduce neonatal mortality. Lack of follow-up data for survivors of severe bacterial infections, particularly sepsis, was striking. Given the high incidence of sepsis, even minor NDI would be of major public health importance. Prevention of neonatal infection, improved case management, and support for children with NDI are all important strategies, currently receiving limited policy attention.
In 2011, neonatal deaths accounted for 34% (1.1 million) of all child deaths under 5 y in sub-Saharan Africa (sSA), 52% (1.2 million) in South Asia (SA), and 53% (107,000) in Latin America and the Caribbean (LA) (by UNICEF region) (1). Severe bacterial infections have been estimated to account directly for approximately one-third of neonatal deaths in these regions (2). An estimated three-quarters of a million deaths per year worldwide are attributed to neonatal severe bacterial infection—more than child malaria deaths (0.6 million) and about four times those resulting from HIV in children (2).
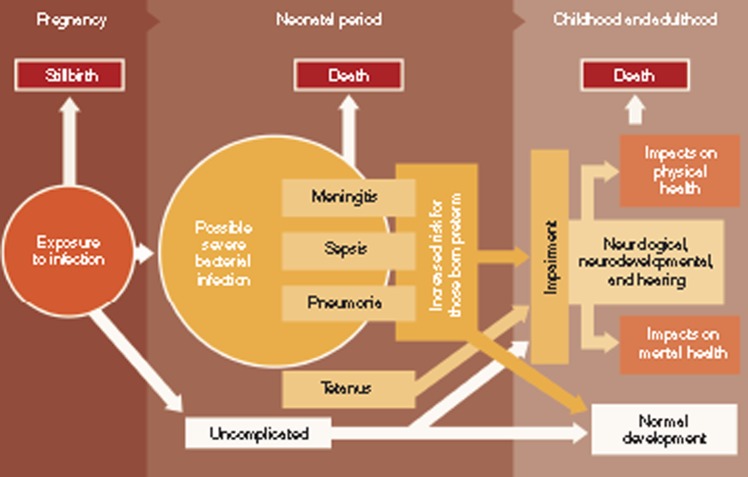
Diagnosis of severe bacterial infection in neonates is challenging in any setting. In resource-poor settings, where expertise and infrastructure are limited, diagnosis depends on simplified diagnostic algorithms with a focus on sensitivity rather than specificity, to allow early empiric antibiotic treatment of neonates with possible severe bacterial infection (pSBI) to prevent neonatal deaths. However, the burden of severe bacterial infection in neonates is not limited to mortality, and for those neonates who are treated and survive, the consequences of neonatal infection (defined in Table 1) may be long-term neurodevelopmental impairment (NDI) and disability, with excess mortality after the neonatal period (Figure 1). Little attention has been paid to the long-term morbidity associated with neonatal infections, particularly in resource-poor settings (3). In resource-rich settings, contemporary work focuses on the strong association of infection in extremely low-birth-weight and preterm neonates and NDI, and there has been surprisingly little recent work addressing long-term outcomes after severe bacterial infections, in term or moderately preterm neonates (4).
Table 1. Definitions for bacterial infection in neonates (see article 1 for further details).

Figure 1.
Disease schema for severe bacterial infection in neonates.
Describing the burden of impairment after severe bacterial infection in neonates is an essential step to support rational individual care and public health policy. The Global Burden of Disease (GBD) exercise estimated impairment outcomes in 2010 for major neonatal conditions, including preterm birth, intrapartum-related neonatal encephalopathy, and neonatal infection (5). However, there were limitations to the GBD estimates; incidence of neonatal sepsis was back calculated from the estimated number of neonatal sepsis deaths using case fatality risks (CFRs); years lived with disability were determined from the acute period of neonatal infection only (1 wk), and impairment after neonatal meningitis and tetanus were modeled using risk after infection in children of all ages. Estimates of impairment after meningitis were based on a recent systematic review, which excluded studies focusing on neonates (6). Outcomes for neonatal meningitis are likely to be different from outcomes after childhood meningitis due to differences in etiology and the effect on the developing brain. The review reported moderate to severe impairment in 12.8% and mild impairment in 8.6% of survivors of childhood meningitis; for survivors of neonatal meningitis, impairment is likely to be higher (6).
In this article, we present systematic reviews, meta-analyses, and the first regional estimates of impairment after neonatal meningitis and neonatal tetanus in SA, sSA, and LA, regions with high disease burden (7). We also discuss the programmatic implications and important data gaps.
Methods
This article is part of a supplement including estimates for four other neonatal conditions. The general methods are described elsewhere (8). Here, we detail specific methods for estimates of impairment after neonatal infections.
Definitions
Case definitions of pSBI and specific diagnoses of neonatal sepsis, meningitis, pneumonia, and tetanus are given in Table 1. The definition of pSBI is based on the most recent WHO multisite study of severe illness in young infants. This study reported that the presence of any one of the seven clinical signs and symptoms predicted severe illness requiring hospitalization in neonates aged 0–7 days, using physician judgment as gold standard, with a sensitivity of 85% and specificity of 75% (9). For sepsis, meningitis, and pneumonia, the primary clinical diagnosis, based on physician judgment and microbiological testing, was used, as far as possible according to the criteria in Table 1.
NDI was classified as moderate/severe (cognitive and/or motor and/or vision and/or hearing impairment) and mild (cognitive and/or motor and/or vision and/or hearing impairment). Detailed definitions within these criteria and comparison with GBD definitions are presented elsewhere (8).
sSA includes GBD regions sSA central, sSA east, sSA west, and sSA southern. SA includes GBD regions Asia south and Asia southeast. LA includes GBD regions LA central, LA Andean, LA southern, LA tropical, and the Caribbean (7).
Data Searches and Inputs
Systematic literature reviews were undertaken for each of the estimation parameters required by applying the general search strategy described elsewhere (8). Parameters required were as follows: incidence of pSBI, proportions of pSBI cases by specific infection diagnosis (sepsis, meningitis, or pneumonia), neonatal CFRs specific to each infectious clinical diagnosis (sepsis, meningitis, pneumonia, and tetanus), and proportions of survivors with long-term impairment outcomes.
Search terms used included multiple variants of terms covering the following areas “meningitis” or “sepsis” or “tetanus” or “pneumonia” or “infection” and “newborn/infant” and “outcome/impairment” and used medical subject headings (MeSH) terms when available (see Supplementary Information online section I for full details of search terms). Snowball searching was used to identify further studies of interest by screening the reference lists of retrieved studies.
Inclusion criteria were as follows: for incidence of pSBI, we included recent (median data collection year 2000 or later) studies, with a population denominator and diagnosis of pSBI consistent with our case definition. For searches to apportion specific diagnoses (sepsis, meningitis, and pneumonia), we only included studies focusing on neonates with pSBI diagnosis based on the seven clinical signs and symptoms indicative of pSBI in the second WHO young infant study (9). For neonatal CFRs, we included studies reporting deaths associated with infection, based as far as possible on our case definitions (Table 1), from low-income countries from 1980 onwards. For impairment outcomes after severe neonatal bacterial infection, to maximize the data available, we included studies worldwide reporting impairment outcomes after specific neonatal infection diagnoses, based as far as possible on our case definitions, from 1964 (the start of a computerized database of medical literature by the US National Library of Medicine) to February 2013. Exclusion criteria were as follows: we excluded studies focusing mostly on very preterm neonates (<32 wk gestation or <1,500 g) from our analyses because they are included in estimates of impairment following preterm birth in this supplement (10).
Modeling Approach for Impairment After Severe Bacterial Infection in Neonates
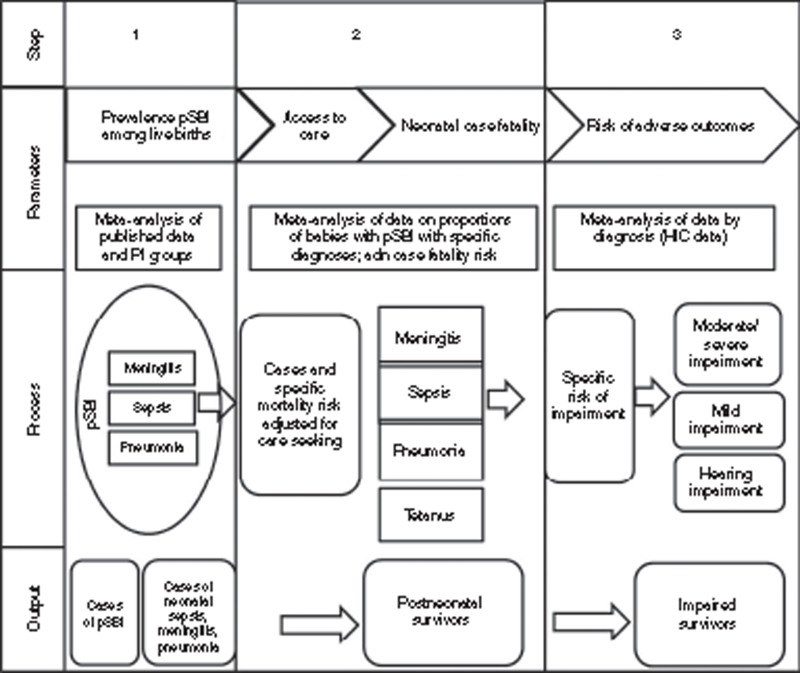
A three-step model to estimate impairment specific to neonatal sepsis, meningitis, and pneumonia was developed (Figure 2), similar to the generic model described elsewhere (8). We calculated a quantitative assessment of the uncertainty surrounding these estimates using a statistical approach based on the compartmental model, taking 1,000 random draws at each step, assuming a normal distribution with mean equal to the point estimate of the parameter and SD equal to the estimated standard error of the parameter. We summed the data at the worldwide or regional level for each draw and present the 2.5th and 97.5th centiles of the resulting distributions as the uncertainty range.
Figure 2.
Parameters required and three-step modeling process for the estimation of the burden of neonatal infection. HIC, high-income country; pSBI, possible severe bacterial infection.
Compartmental model steps 1–3.
A compartmental model was constructed to estimate steps 1–3. In step 1, estimated incidence of pSBI in neonates >32 wk gestation (or >1,500 g) (Seale et al., unpublished data) was used to estimate the proportion of neonates with pSBI who would be diagnosed with a specific clinical infection syndrome, e.g., meningitis, pneumonia, or sepsis (Figure 2). In step 2, to calculate the number of survivors among those estimated to seek care, CFRs were estimated based on facility data (using a pooled estimate). For those cases who did not seek care, high CFRs were assumed (95%). To estimate the number of impaired survivors (step 3), we used the estimated number of survivors from step 2 and pooled estimates of the risk of impairment associated with particular clinical infection syndromes.
For cases of neonatal meningitis, we undertook a sensitivity analysis, using facility-based data to estimate incidence risk of neonatal meningitis where the number of facility live births was the denominator. We applied this incidence risk, adjusting for care seeking, to live births in 2010 to provide an alternative estimate of total meningitis cases in 2010.
Modeling Approach for Impairment After Neonatal Tetanus
Estimation of cases of neonatal tetanus was restricted by the limited availability of incidence data and the lack of concurrent tetanus toxoid vaccination data, especially given the recent scale up to high coverage of this vaccination in women of reproductive age (11,12). Given the lack of reliable incidence data, we back calculated the incidence of tetanus from the estimated number of deaths, using estimated CFRs for neonatal tetanus (see Supplementary Information online for pooled estimate of CFR). This approach has limitations, but as the numbers of deaths and cases are relatively small at the global level, it has less of an impact than using this approach for a more common condition such as neonatal sepsis. This process included an adjustment for estimates of neonatal tetanus deaths to apply them to the GBD regions used here because the published estimates of neonatal tetanus deaths apply to slightly different WHO-defined regions (2).
Estimation of DALYs
DALYs are calculated as the sum of years of life lost due to mortality and years lived with disability (prevalence of a condition × disability weight).
Estimation of DALYs and years lived with disability associated with neonatal meningitis, sepsis, pneumonia, and tetanus, as undertaken in estimates described elsewhere in this supplement (8), and in line with GBD estimates, were not completed for this study due to limitations in parameters and estimates, as described in the discussion.
Results
Input Parameters and Estimation Outputs
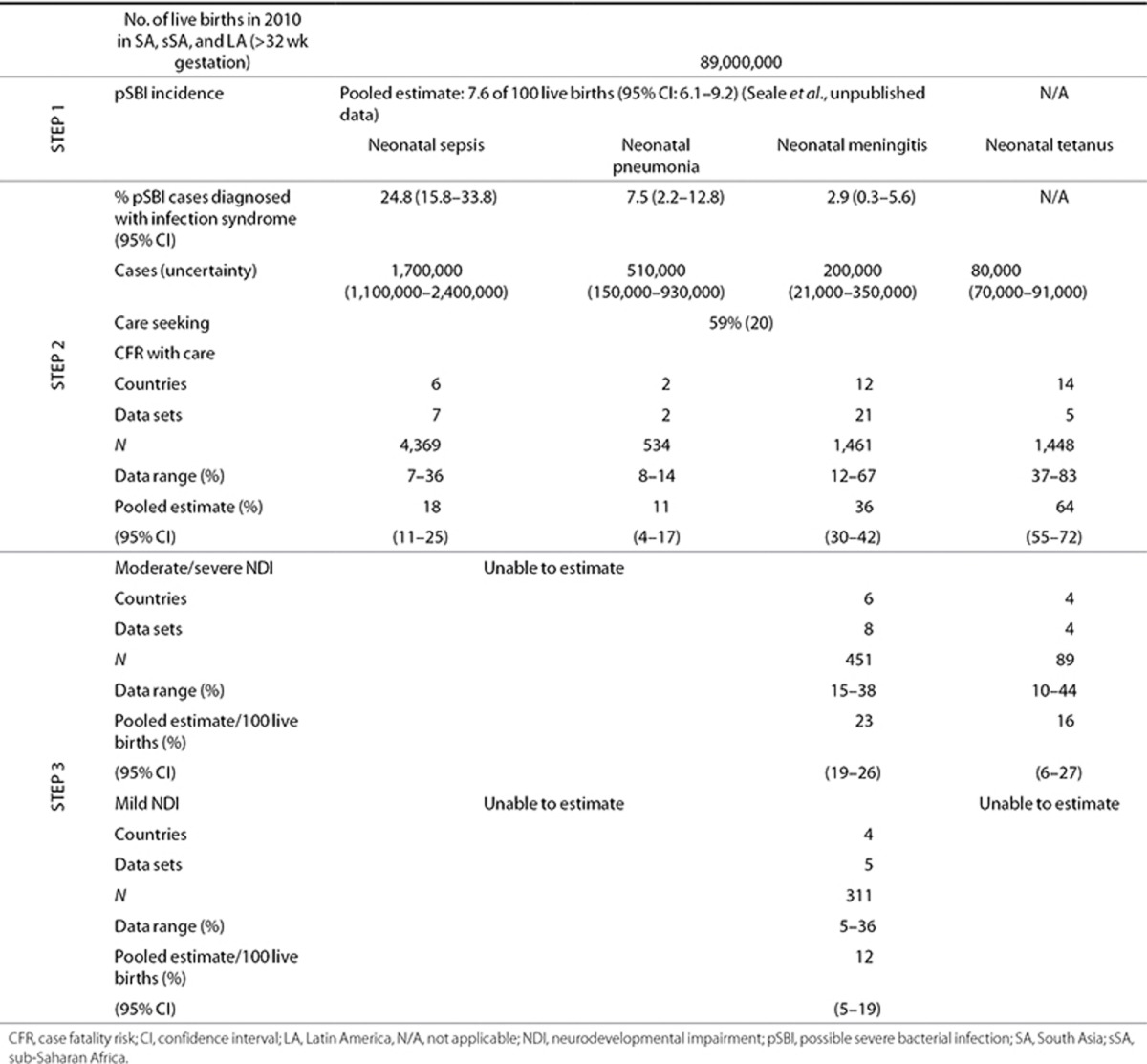
The steps of the compartmental model (Figure 2) were applied in sequence for the number of live births (>32 wk gestation or >1,500 g) in SA, sSA, and LA for the year 2010. The parameters and calculations at each step are detailed with a short summary of the results. A summary of the parameter inputs are shown in Table 2 and detailed in the Supplementary Information online.
Table 2. Summary of input data for estimation parameters in terms of incidence, case fatality rates, and impairment risk for survivors for SA, sSA, and LA.
Step 1: Incidence of Specific Infection Diagnoses
Incidence of pSBI. Incidence of pSBI was determined from a meta-analysis, as described elsewhere (Seale et al., unpublished data). There were an estimated 7.6 pSBI cases per 100 live births (95% confidence interval (CI): 6.1–9.2) and a consistently higher risk for male babies (risk ratio 1.12 (95% CI: 1.06–1.08)) (Seale et al., unpublished data). This estimate does not take into account the estimated sensitivity of pSBI diagnosis (85%) (9). We were unable to include sex differences in further steps of the model due to the paucity of data by sex on specific diagnoses.
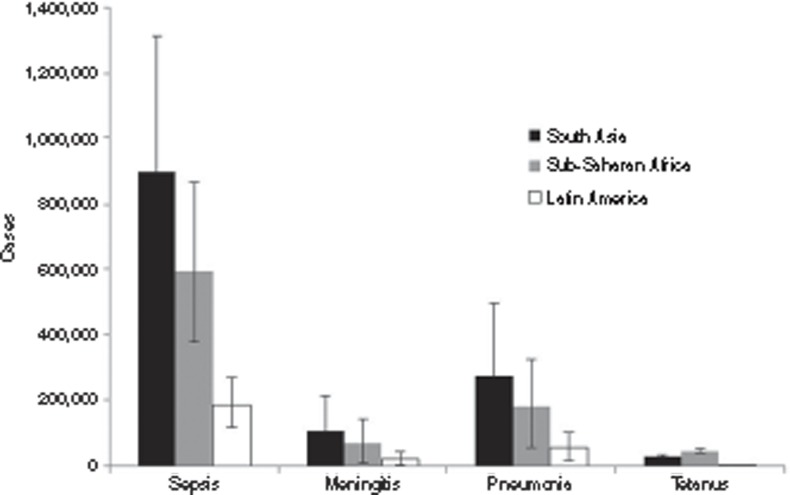
Proportion of pSBI cases with diagnosis of neonatal meningitis, sepsis, or pneumonia. To apportion the envelope of pSBI cases to infection syndromes (meningitis, sepsis, or pneumonia), site-specific data from the second multicountry WHO Young Infant Study of Neonatal Sepsis (9) were used (Bolivia (13), Ghana (14), India (15,16), and South Africa (17). Meta-analyses (see Supplementary Information online) resulted in the following estimates: neonatal sepsis accounted for 25% (95% CI: 16–34%) of pSBI cases, five data sets, N = 532; neonatal meningitis accounted for 3% (95% CI: 0.3–6%), two data sets, N = 217; and neonatal pneumonia accounted for 8% (95% CI: 2–13%), five data sets, N = 532 (Table 2). Estimated numbers of cases of neonatal sepsis, meningitis, and pneumonia by region are shown in Figure 3.
Figure 3.
Estimated cases, with uncertainty of neonatal sepsis, meningitis, pneumonia, and tetanus in South Asia, sub-Saharan Africa, and Latin America, respectively.
The sensitivity analysis of incidence of neonatal meningitis estimated from facility-based data was based on eight data sets (see Supplementary Information online). Based on these data, we estimated one case (95% CI: 1–2) of neonatal meningitis per 1,000 live births, and after adjusting for care seeking, we estimated 150,000 (uncertainty range: 78,000–230,000) cases of neonatal meningitis in SA, sSA, and LA. This is comparable with our estimate based on pSBI and proportionate split of neonatal meningitis at 200,000 cases (21,000–350,000), although both estimates have considerable uncertainty.
Estimate of cases of neonatal tetanus. Cases of neonatal tetanus were estimated based on estimated tetanus deaths by WHO region (2) adjusted for GBD regions defined here, and back calculated using the pooled estimate of CFR given in step 2.
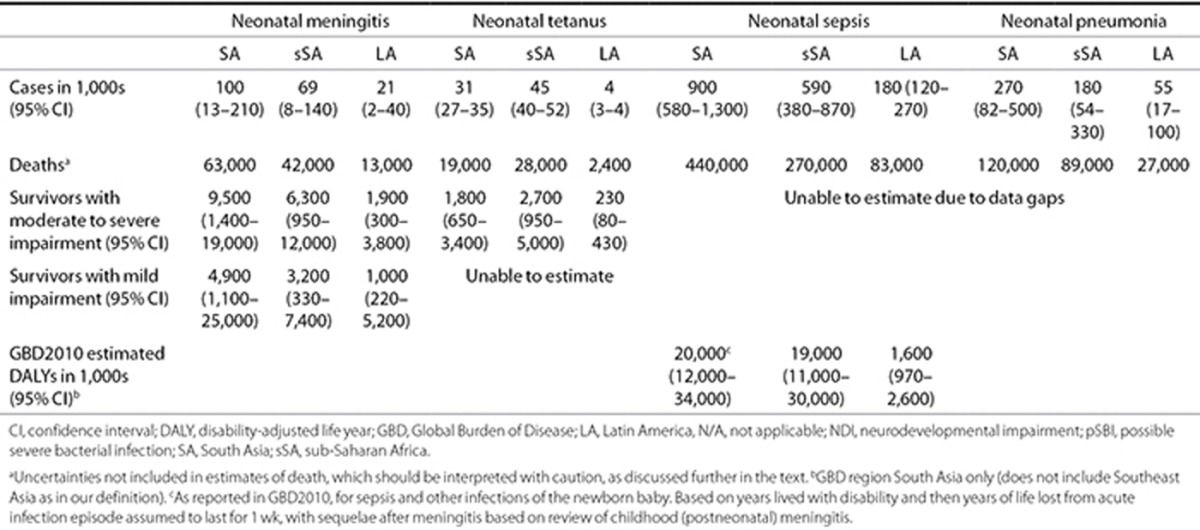
Estimates of neonatal sepsis, meningitis, pneumonia, and tetanus. We estimated 6.8 million (uncertainty range: 5.4–8.1 million) cases of pSBI in 2010 in neonates >32 wk gestation (or >1,500 g), which included 1.7 million (1.1– 2.4 million) cases of neonatal sepsis, 200,000 cases (21,000–350,000) of neonatal meningitis, and 510,000 cases (150,000–930,000) of neonatal pneumonia. There were an estimated 79,000 cases (70,000–91,000) of neonatal tetanus. These are given by region in Table 3.
Table 3. Regional burden of neonatal infection and levels of impairment in survivors of neonatal meningitis and neonatal tetanus.
Step 2: Calculation of the Number of Postneonatal Survivors
CFRs for neonatal meningitis, neonatal sepsis, neonatal pneumonia, and neonatal tetanus in those who seek care. CFR estimates for neonatal sepsis were obtained from 7 data sets with 1,268 cases, for neonatal meningitis from 21 data sets with 1,642 cases, and for neonatal pneumonia from 2 data sets with 534 cases. For neonatal tetanus, they were obtained from 5 data sets with 1,448 cases and from searches included and used in the GBD estimates (18). Estimated CFRs (Table 2) were as follows: neonatal sepsis 18% (95% CI: 11–25%), neonatal meningitis 36% (95% CI: 30–42%), neonatal pneumonia 11% (95% CI: 4–17%), and neonatal tetanus 64% (95% CI: 55–72%) (18). Details of the meta-analyses are included in the Supplementary Information online.
Coverage and quality of care. Data on care-seeking behaviors in low- and middle-income countries were recently assessed and reported in a systematic review (19). We were unable to identify any further data. The published median (59% seek care) was used in our model for all regions (19). We did not calculate estimates of uncertainty around deaths in those who did not seek care using this median value, with a large range in the data inputs (10–100%). We estimated that 95% of cases of neonatal sepsis, meningitis, or pneumonia would die if they did not seek care, so only a small proportion (5%) of meningitis survivors who did not seek care are included in impairment estimates (step 3).
Hence in SA, sSA, and LA, applying the above CFRs to the incidence estimates, in those who seek care, there were 140,000 deaths (uncertainty range: 90,000–300,000) from neonatal sepsis, 42,000 (5,000–97,000) deaths from neonatal meningitis, and 42,000 (6,000–68,000) deaths from neonatal pneumonia in neonates >32 wk gestation (or >1,500 g). In those who did not seek care, we estimated 650,000 deaths from neonatal sepsis, 76,000 deaths from neonatal meningitis, and 200,000 deaths from neonatal pneumonia in neonates >32 wk gestation (or >1,500 g). In total, therefore, with this CFR method, we estimated 800,000 deaths from neonatal sepsis, 120,000 from neonatal meningitis, and 240,000 from neonatal pneumonia, totaling just more than 1.0 million deaths in neonates >32 wk gestation (or >1,500 g).
Estimates of the number of postneonatal survivors. Of those who sought care, we estimated 840,000 survivors (510,000–1.2 million) of neonatal sepsis, 74,000 (8,600–150,000) survivors of neonatal meningitis, and 260,000 (80,000–490,000) survivors of neonatal pneumonia. Of those who did not seek care, we estimated only 34,000 survivors of neonatal sepsis, 4,000 survivors of neonatal meningitis, and 10,000 survivors of neonatal pneumonia.
In total, we estimated 880,000 survivors (540,000–1.2 million) of neonatal sepsis, 78,000 survivors (11,000–150,000) of neonatal meningitis, and 270,000 survivors (89,000–500,000) of neonatal pneumonia.
We applied estimates from previously published work (2) to GBD regions defined here, estimating a total of 50,000 neonatal tetanus deaths. Of these, there were an estimated 29,000 survivors (20,000–41,000). Estimates by region are presented in Table 3.
Step 3: Calculation of the Number of Impaired Survivors
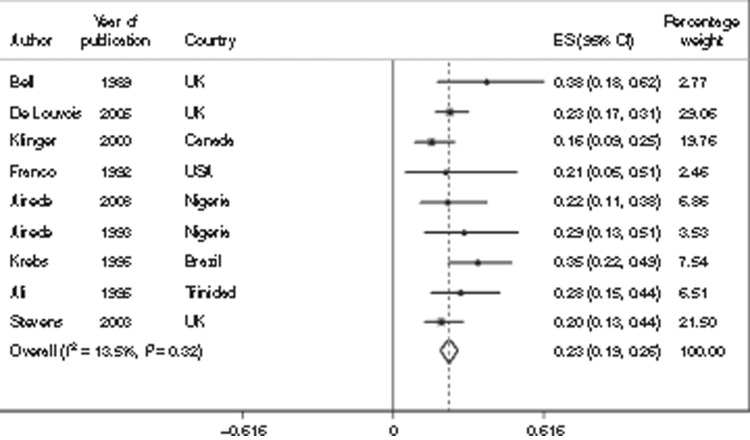
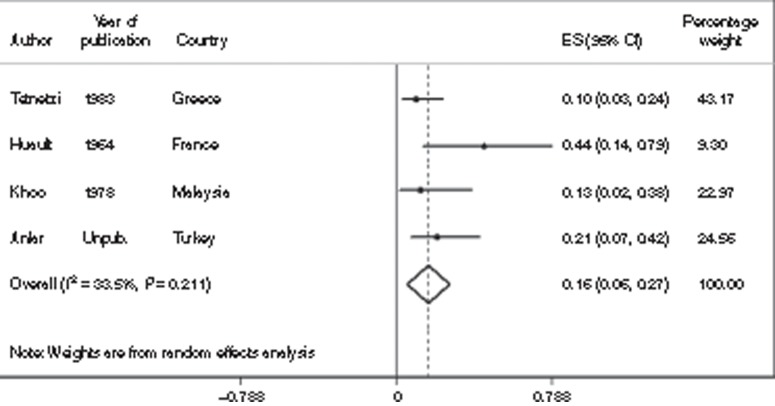
Impairment risk. There are very few data on impairment risk in survivors of neonatal bacterial infections. Estimates of impairment following neonatal meningitis were mainly identified from studies in high-income settings. We used eight studies, which included 451 survivors of neonatal meningitis and estimated that 23% (95% CI: 19–26%) were moderately to severely impaired (Figure 4). For mild impairment, we used five studies (311 survivors), resulting in an estimate of 12% (95% CI: 5–19%). Specifically for hearing impairment, we identified 11 studies (442 survivors), resulting in an estimate of 7% (95% CI: 3–12%); these cases were included in the combined categories of impairment; for details, see Supplementary Information online.
Figure 4.
Meta-analysis of eight studies (N = 451) reporting the incidence of any moderate/severe impairment (motor, cognitive, hearing, or vision) outcomes after neonatal meningitis. CI, confidence interval; ES, estimate.
Estimates of impairment after neonatal tetanus came from 4 studies with 89 survivors, of whom 16% (95% CI: 6–27%) had moderate to severe impairment (Figure 5). Data were even more limited for mild impairment after neonatal tetanus, with widely differing estimates. One study reported 2 of 43 survivors with mild impairment, reviewed 6 mo to 4 y after recovery (20) and another identified 5 of 10 survivors (50%) with mild impairment (21). There was one further study from Kenya, which reported 33 neonatal survivors, 2 of whom died after the neonatal period and 10 of whom moved away (22). One of those who died had a history compatible with severe NDI, but none of those who were able to be followed up had moderate to severe impairment, and data on mild impairment outcomes were not comparable with our definitions and thus not included in the meta-analysis.
Figure 5.
Meta-analysis of four studies (N = 89) reporting the incidence of any moderate/severe impairment (motor, cognitive, hearing, or vision) outcomes after neonatal tetanus. CI, confidence interval; ES, estimate.
We were unable to identify sufficient data on impairment after neonatal sepsis and neonatal pneumonia to estimate the burden from these severe bacterial infections.
Estimates of the number of impaired survivors of neonatal meningitis and neonatal tetanus. After neonatal meningitis (in neonates >32 wk gestation (or >1,500 g)) in 2010, there were an estimated 18,000 (uncertainty range: 2,700–35,000) moderately to severely impaired survivors and 9,200 (2,000–47,000) mildly impaired survivors. Of those with impairment, 5,600 (1,200–29,000) survivors had hearing impairment. After neonatal tetanus infection, there were an estimated 4,700 (1,700–8,900) neonatal survivors with moderate to severe impairment. Estimates by region are presented in Table 3.
Discussion
The estimated 6.7 million (uncertainty range: 5.4–8.1 million) cases of pSBI per year in neonates >32 wk gestation (or >1,500 g) are associated with a major burden on the health system and on health workers, with a need for antibiotic therapy, even though many do not have a final diagnosis of invasive bacterial infection (Seale et al., unpublished data). Our estimates of cases of true invasive bacterial disease are lower but still indicate a major burden from neonatal sepsis, meningitis, and pneumonia in SA, sSA, and LA. These are the first estimates of severe bacterial infections derived from the estimated incidence of pSBI and align with recent estimates of severe pneumonia in young children, which suggested 3 million cases worldwide (23). Although no separate estimates of neonatal infections were provided in the pneumonia study, the incidence risk of admission for “severe pneumonia” (defined using WHO case criteria) was noted to be three times higher for neonates compared with that for all infants (0–59 mo). The burden of neonatal infections in non-preterm babies in SA, sSA, and LA is very high compared with that in babies in resource-rich regions, where the numbers of sepsis and neonatal meningitis cases are much lower but still constitute a considerable burden of disease (24).
There are limitations to these estimates and considerable uncertainty, particularly in apportioning of pSBI to the three infection syndromes (sepsis, pneumonia, and meningitis), because this split is based on just one multicountry study (9), with only two studies reporting meningitis outcomes. The young infant algorithm, which is also only 85% sensitive for pSBI, was based on those admissions, and admissions included other diagnoses such as hyperbilirubinemia, diarrhea, neonatal encephalopathy, and, in some cases, preterm birth (9). However, the estimate of neonatal meningitis cases using facility-based data, and adjusting for care seeking, was comparable with that based on apportioning of the incidence of pSBI. The estimates for each region are based on the number of live births in 2010 (>32 wk gestation or >1,500 g) and on application of the same parameters to each region. The data did not allow region-specific incidence risks and so may be missing important regional differences in pSBI incidence and in proportions of neonatal sepsis, meningitis, and pneumonia, in addition to missing differing care-seeking behavior, treatment rates, and subsequent outcomes.
The mortality burden from severe bacterial infection in neonates is well recognized (25). However, the total neonatal deaths estimated herein (1.2 million) exceed those presented by the Child Health Epidemiology Reference Group, which total 400,000 neonatal deaths due to pneumonia and sepsis/meningitis in sSA and SA and 720,000 worldwide (1,2). The Institute for Health Metrics Evaluation gives a similar total of 750,000 for neonatal infection deaths (26). The methods used by both the Child Health Epidemiology Reference Group (2) and the Institute for Health Metrics Evaluation (26) examine mortality data and assign a proportion of deaths to a specific cause are based on many more input data and are likely more robust. The estimates here may be higher because of assumptions in care-seeking behavior (that 41% did not seek care, based on a published meta-analysis (19)) and the high CFR (95%) applied to those with sepsis, meningitis, or pneumonia who did not seek care. The published estimate that 41% do not seek care is based on limited data (22 studies, 17 of which were from SA (19)) and was applied in our model for all regions. For those who do seek care, CFRs are well known to be problematic in burden estimation work, because they are rarely population based, and tend to miss the mildest cases, resulting in an overestimate of mortality (27). Importantly, the Child Health Epidemiology Reference Group and the Institute for Health Metrics Evaluation estimates differ in the proportional split of deaths attributed to pneumonia vs. sepsis/meningitis, with more deaths attributed to pneumonia in the Child Health Epidemiology Reference Group, underlining the greater uncertainty in attributed syndrome-specific diagnoses based primarily on clinical symptoms that usually overlap in neonates.
The calculation of disability-adjusted life years (DALYs) requires estimates of survivors of the specific condition (e.g., neonatal sepsis), the excess mortality in postneonatal survivors of the condition, and a disability weight for the condition. Neonatal survivors of meningitis, sepsis, pneumonia, and tetanus may have increased rates of mortality in infancy, childhood, and later adult life, associated with disability. Due to the limitations and uncertainty in our estimates of neonatal survivors and expected excess mortality, apart from the lack of disability weights for the specific neonatal infections in the recent GBD2010 estimates, DALYs were not able to be calculated here. The DALYs presented in Table 3 are therefore those published in the GBD2010 estimates (5).
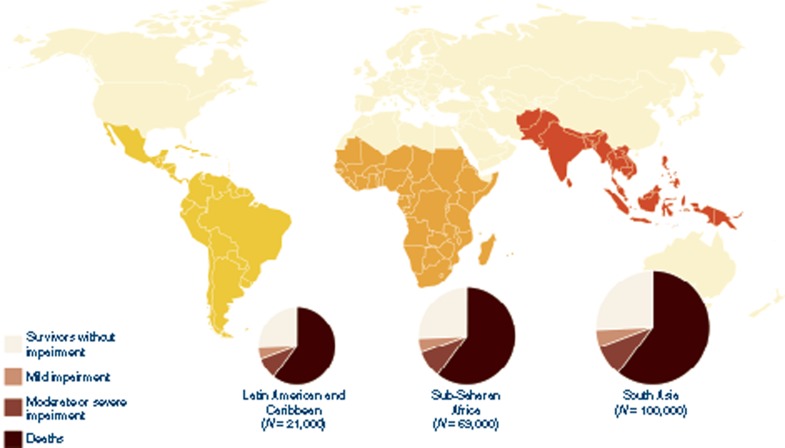
Estimates for NDI are limited by lack of data on impairment after neonatal sepsis and neonatal pneumonia. However, the burden of NDI from neonatal meningitis is high, with a third of all survivors impaired (Figure 6). Our estimate of the risk of moderate to severe NDI (23%; 95% CI: 19–26%) is higher than the previously published estimate of NDI after childhood meningitis (12.8%; 95% CI: 7.2–21.1%) (6). This is to be expected because etiology varies with age and infection during brain development increases the likelihood of NDI after neonatal meningitis, compared with the same for childhood meningitis. Although the risk of impairment from neonatal infection is strongly associated with decreasing gestational age, impairment outcomes after Group B Streptococcus meningitis still show a high (19%) burden of severe impairment in term infants in resource-rich countries in a recent study (1998–2006) from two hospitals in the United States (28).
Figure 6.
Regional burden of neonates with meningitis showing mortality, impairment, and the proportion with impairment-free survival. Note that impairment outcomes following neonatal sepsis could not be estimated due to lack of data. Outcomes following neonatal tetanus are shown in Table 2.
In terms of neonatal tetanus, although our estimate of 4,700 cases (uncertainty range: 1,700–8,900) of moderate to severe NDI is a comparatively small number, and progress has been made in reducing neonatal tetanus incidence, it must be remembered that this is an entirely preventable disease. In this case, therefore, the challenge is to improve prevention, through immunization, to prevent all these cases (11).
Data Gaps and Improving the Data
The data here follow the “inverse care law,” with the heaviest burden areas having the least data (summarized in Table 4) (25). NDI data after neonatal sepsis and neonatal pneumonia are lacking, in babies of >32 wk gestation (Table 4). We cannot, therefore, estimate NDI outcomes in the 880,000 (540,000 to 1.2 million) survivors of neonatal sepsis and 270,000 (89,000–500,000) survivors of pneumonia. With these data gaps for the more common severe bacterial infections, we are left “stumbling in the dark” (29).
Table 4. Research in the context of the burden of neonatal infections.

From resource-poor settings, the only article identified on neurodevelopmental outcomes after neonatal sepsis was from Kenya, which followed up 24 infants previously admitted with neonatal sepsis. It was reported that there were more motor difficulties in cases compared with community controls, in particular 4 of 24 children were unable to stand at 18–32 mo (30). From resource-rich settings, there are historical data from Sweden, where neurodevelopmental outcomes after 320 cases of invasive neonatal bacterial infection are described. Of the 110 who survived neonatal sepsis, 20 had sequelae (5%) (31). Less direct, but more recent, evidence comes from a case–control study from the United States on the impact of maternal infection and inflammation on neonates of >32 wk gestation. Forty-six children with unexplained cerebral palsy were compared with term controls, and 22% of the cases were exposed to maternal infection compared with 2.9% of controls (odds ratio: 9.3; 95% CI: 3.7–23.0) (32). This is supported by another study of antenatal and intrapartum risk factors for seizures in term newborns where maternal fever and infection were associated with increased risk (33). The ORACLE study, a multisite randomized controlled trial evaluating the use of co-amoxiclav and/or erythromycin on preterm rupture of membranes and spontaneous preterm labor, supports this finding. Seven-year follow-up data from this factorial trial indicated that the proportion of children with cerebral palsy born after 32 wk gestation was higher in the spontaneous preterm labor arm (73%) and the preterm rupture of membrane arm (30%) compared with that in the controls (34). There are more data in preterm neonates. In resource-rich settings, brain imaging is used in high-risk neonates to detect white matter injury, which helps to predict likely impairment outcomes. In the United States, in a study of 133 neonates (<34 wk gestation), 13% (6 infants) of infants who had any episode of sepsis had evidence of white matter injury compared with 4.7% (4 infants) who did not have a documented episode of postnatal sepsis (odds ratio: 3.2; 95% CI: 0.8–11.8) (35). This is supported by other studies, including outcomes after late-onset sepsis (36), and a study from Sweden following up extremely preterm infants; sepsis was found to independently increase the risk of cerebral palsy (odds ratio: 3.23; 95% CI: 1.23–8.48) and NDI (odds ratio: 1.69; 95% CI: 0.96–2.98) (37).
For neonatal meningitis, which accounts for the minority of severe bacterial infection cases, as illustrated in Figure 7, the available data go through a cascade of diminishing availability, from pSBI incidence, the split into various infection syndromes, CFRs, and NDI risk. Available published studies of NDI after neonatal meningitis are old (1970–1998) and from high-income countries, with the exception of two from Nigeria. For neonatal tetanus, data on NDI outcomes were only able to be included from four publications (1964–1981) from Europe and Asia.
Figure 7.
Summary of outcomes in terms of deaths and disability for neonates with sepsis, meningitis, or pneumonia born in South Asia, sub-Saharan Africa, and Latin America in 2010. aUncertainties not included in estimates of death, which should be interpreted with caution, and include adjustment for care-seeking behavior, as discussed in the text.
These data gaps, and the lack of investment in this subject to date, are in contrast to the large, long-term cohorts following up HIV-exposed and HIV-infected infants (38), actually a much rarer occurrence and now accounting for ~140,000 child deaths annually (2). Although data are limited, we suggest that there are sufficient data here to indicate that determining the impact of neonatal sepsis at all gestations on long-term impairment is essential, particularly for low-resource settings where the burden is the highest.
Programmatic Implications
Despite the data limitations, it is clear that the burden attributable to neonatal infections justifies greater strategic investment in prevention, improved case management, and better care of impaired survivors.
Prevention of neonatal infection is likely the most cost-effective intervention available not only for facility births and sick and preterm babies in hospitals but also for 50 million home births (39). Effective strategies to prevent severe neonatal infection in resource-poor settings are available. Clean delivery and chlorhexidine cord cleansing at and following delivery have been shown to reduce neonatal mortality in randomized controlled trials in Pakistan and Bangladesh (40,41). Breastfeeding is important to initiate early (42) and is of low cost, yet it has received less global attention in recent years. Reducing exposure to infection in high-risk groups (preterm and small-for-gestational-age neonates) through the use of Kangaroo Mother Care (43) is increasingly used in resource-rich neonatal intensive care units and in low-income settings to reduce mortality and infections, in addition to improving breastfeeding, weight gain, and neurological outcomes (44). Emollients (aquaphor ointment or sunflower seed oil) decreased neonatal mortality by 28 and 32% in a randomized controlled trial in Bangladesh (45). In the longer term, maternal immunization may be an effective strategy to prevent neonatal sepsis, to be added to the existing program for neonatal tetanus.
Improved case management through better supportive care, along with effective antibiotic treatment regimens, would reduce mortality and morbidity (46). Increasing care coverage and bringing management closer to home through community outreach interventions, as shown in a trial of home-based care in India (47) and health facility care in Pakistan (48), to cover “hard to reach” sectors of the population, would help this. Trials are under way to evaluate simplified antibiotic therapy at home or first-level facilities in Bangladesh, Pakistan, and several African countries, with results expected soon (49). There are few studies examining the effect of early treatment with oral antibiotics for local infections (skin, umbilicus, eye, and mouth) on the incidence of severe bacterial conditions.
Other case management strategies, important and applicable to both resource-poor and resource-rich settings, include infection control, targeted antibiotic policies, and strict antibiotic stewardship. Increasing antibiotic resistance and hospital-acquired infection is a concern worldwide (50,51). Participation in infection surveillance networks has reduced hospital-acquired infections in resource-rich settings (52), together with bundles of care to reduce infections associated with central venous access (53,54). A number of other interventions aimed at reducing morbidity and mortality from hospital-acquired sepsis in resource-rich regions have been studied with a focus on extremely preterm babies. Antifungal prophylaxis has shown benefit (55,56), but there are concerns about side effects and increased resistance, particularly in countries with lower incidence of Candida. Other strategies include use of oral bovine lactoferrin supplementation, which reduced the risk of late-onset sepsis in a single study (57); results of further studies are awaited. Several interventions, including intravenous immunoglobulins, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor (58), and glutamine supplementation, have not proven effective (55,59).
Improving diagnostics would support improved case management in resource-poor settings, in addition to increasing knowledge of etiological agents to inform interventions, but data are limited (60). Innovation for diagnostics, especially point-of-care tests, for neonatal sepsis is challenging (61). Development of a rapid diagnostic test would represent a great advance, as seen for malaria diagnosis (62,63). Wider use of pulse oximetry may improve the specificity of diagnosis of severe illness but is yet to be tested in low-income settings (64).
Finally, more programmatic focus must be put on identification and care of survivors with disability. Looking after a disabled child can place a high burden on both families (65) and the children concerned. The children and families are stigmatized, and their educational and social opportunities are often limited.
Conclusion
Severe bacterial infection in neonates is a leading cause of neonatal mortality in low-income countries, despite affordable and feasible prevention and treatment interventions. Impairment in survivors likely represents a significant burden of disease that, to date, has not been fully counted or addressed.
Acknowledgments
We thank the neonatal estimations team, listed below. We are grateful to Chris Rowland for assistance with the figures.
Neonatal infections estimation team (in alphabetical order):
Dwi Agustian, Fernando Althabe, Eduardo Azziz-Baumgartner, Rajiv Bahl, Abdullah Baqui, Jose M. Belizan, James A. Berkley, Zulfiqar Bhutta, Robert Black, Shobha Broor, Hannah Blencowe, Nigel Bruce, Pierre M. Buekens, Harry Campbell, Waldemar A. Carlo, Elwyn Chomba, Anthony Costello, Simon N. Cousens, Richard J. Derman, Mukesh Dherani, Shams El-Arifeen, Fabian Esamai, Hammad Ganatra, Ana Garcés, Bradford D. Gessner, Christopher Gill, Robert Goldenberg, Shivaprasad S. Goudar, K. Michael Hambidge, Davidson Hamer, Nellie I. Hansen, Patricia L. Hibberd, Suhir Khanal, Betty Kirkwood, Patrick Kosgei, Marion Koso-Thomas, Joy E. Lawn, Edward A. Liechty, Alexander A. Manu, Elizabeth M. McClure, Dipak Mitra, Neema Mturi, Luke C. Mullany, Harish Nair, Charles R. Newton, Francois Nosten, Sharma Parveen, Omrana Pasha, Archana Patel, Shamim A. Qazi, Naomi Saville, Anna C. Seale, Katherine Semrau, Sajid Soofi, Shiyam Sunder, Eric A.F. Simões, Barbara J. Stoll, Sana Syed, James M. Tielsch, Claudia Turner, Stefania Vergnano, Tinoco Yeny, and Anita K. Zaidi.
Supplementary Material
References
- UNICEF. Levels and trends in child mortality. ( http://apromiserenewed.org/files/UNICEF_2012_child_mortality_for_web_0904.pdf .) Accessed 10 February 2013.
- Liu L, Johnson HL, Cousens S, et al. Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379:445–52. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Adams-Chapman I, et al. National Institute of Child Health and Human Development Neonatal Research Network Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–65. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:317–28. doi: 10.1016/S1473-3099(10)70048-7. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Ezzati M, Flaxman AD, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–6. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Vos T, Lee AC, et al. Estimates of neonatal morbidities and disabilities at regional and global levels for 2010: introduction, methods overview, and relevant findings from the Global Burden of Disease study Pediatr Res 2013. this issue). [DOI] [PMC free article] [PubMed]
- Young Infants Clinical Signs Study Group. Clinical signs that predict severe illness in children under age 2 months: a multicentre study. Lancet. 2008;371:135–42. doi: 10.1016/S0140-6736(08)60106-3. [DOI] [PubMed] [Google Scholar]
- Lawn JE, Blencowe H, Darmstadt GL, Bhutta ZA.Beyond newborn survival: the world you are born into determines your risk of disability-free survival Pediatr Res 2013. this issue). [DOI] [PMC free article] [PubMed]
- Roper MH, Vandelaer JH, Gasse FL. Maternal and neonatal tetanus. Lancet. 2007;370:1947–59. doi: 10.1016/S0140-6736(07)61261-6. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Lawn J, Vandelaer J, Roper M, Cousens S. Tetanus toxoid immunization to reduce mortality from neonatal tetanus. Int J Epidemiol. 2010;39 Suppl 1:i102–9. doi: 10.1093/ije/dyq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzi E, Bartos AE, Carlin J, Weber MW, Darmstadt GL. Bolivia Clinical Signs Study Group Clinical signs predicting severe illness in young infants (<60 days) in Bolivia. J Trop Pediatr. 2010;56:307–16. doi: 10.1093/tropej/fmq005. [DOI] [PubMed] [Google Scholar]
- Yeboah-Antwi K, Addo-Yobo E, Adu-Sarkodie Y, et al. Clinico-epidemiological profile and predictors of severe illness in young infants (0-59 days) in Ghana. Ann Trop Paediatr. 2008;28:35–43. doi: 10.1179/146532808X270653. [DOI] [PubMed] [Google Scholar]
- Deorari AK, Chellani H, Carlin JB, et al. Clinicoepidemiological profile and predictors of severe illness in young infants (< 60 days) reporting to a hospital in North India. Indian Pediatr. 2007;44:739–48. [PubMed] [Google Scholar]
- Narang A, Kumar P, Narang R, et al. Clinico-epidemiological profile and validation of symptoms and signs of severe illness in young infants (< 60 days) reporting to a district hospital. Indian Pediatr. 2007;44:751–9. [PubMed] [Google Scholar]
- Jeena PM, Adhikari M, Carlin JB, Qazi S, Weber MW, Hamer DH. Clinical profile and predictors of severe illness in young South African infants (<60 days). S Afr Med J. 2008;98:883–8. [PubMed] [Google Scholar]
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert HK, Lee AC, Chandran A, Rudan I, Baqui AH. Care seeking for neonatal illness in low- and middle-income countries: a systematic review. PLoS Med. 2012;9:e1001183. doi: 10.1371/journal.pmed.1001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimpour R. Follow-up studies in tetanus neonatorum. Vienna; Verlag Wiener Medizinischen Akademie; Proceedings of the Thirteenth International Congress of Pediatrics. 1971;vol. 6:402–5. [Google Scholar]
- Vaidya A, Saville N, Shrestha BP, Costello AM, Manandhar DS, Osrin D. Effects of antenatal multiple micronutrient supplementation on children's weight and size at 2 years of age in Nepal: follow-up of a double-blind randomised controlled trial. Lancet. 2008;371:492–9. doi: 10.1016/S0140-6736(08)60172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow JL, Mung'Ala-Odera V, Gona J, Newton CR. Brain damage after neonatal tetanus in a rural Kenyan hospital. Trop Med Int Health. 2001;6:305–8. doi: 10.1046/j.1365-3156.2001.00705.x. [DOI] [PubMed] [Google Scholar]
- Nair H, Simoes EA, Rudan I, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381:1380–90. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Sánchez PJ, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127:817–26. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn JE, Cousens S, Zupan J. Lancet Neonatal Survival Steering Team 4 million neonatal deaths: when? Where? Why. Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005;83:171–7. [PMC free article] [PubMed] [Google Scholar]
- Libster R, Edwards KM, Levent F, et al. Long-term outcomes of group B streptococcal meningitis. Pediatrics. 2012;130:e8–15. doi: 10.1542/peds.2011-3453. [DOI] [PubMed] [Google Scholar]
- Horton R. Stumbling around in the dark. Lancet. 2005;365:1983. [PubMed] [Google Scholar]
- Gordon AL, English M, Tumaini Dzombo J, Karisa M, Newton CR. Neurological and developmental outcome of neonatal jaundice and sepsis in rural Kenya. Trop Med Int Health. 2005;10:1114–20. doi: 10.1111/j.1365-3156.2005.01496.x. [DOI] [PubMed] [Google Scholar]
- Bennet R, Bergdahl S, Eriksson M, Zetterström R. The outcome of neonatal septicemia during fifteen years. Acta Paediatr Scand. 1989;78:40–3. doi: 10.1111/j.1651-2227.1989.tb10884.x. [DOI] [PubMed] [Google Scholar]
- Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278:207–11. [PubMed] [Google Scholar]
- Glass HC, Pham TN, Danielsen B, Towner D, Glidden D, Wu YW. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998–2002. J Pediatr. 2009;154:24–8e1. doi: 10.1016/j.jpeds.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow N, Pike K, Bower E, et al. Characteristics of children with cerebral palsy in the ORACLE children study. Dev Med Child Neurol. 2012;54:640–6. doi: 10.1111/j.1469-8749.2012.04274.x. [DOI] [PubMed] [Google Scholar]
- Glass HC, Bonifacio SL, Chau V, et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics. 2008;122:299–305. doi: 10.1542/peds.2007-2184. [DOI] [PubMed] [Google Scholar]
- van der Ree M, Tanis JC, Van Braeckel KN, Bos AF, Roze E. Functional impairments at school age of preterm born children with late-onset sepsis. Early Hum Dev. 2011;87:821–6. doi: 10.1016/j.earlhumdev.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Schlapbach LJ, Aebischer M, Adams M, et al. Swiss Neonatal Network and Follow-Up Group Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics. 2011;128:e348–57. doi: 10.1542/peds.2010-3338. [DOI] [PubMed] [Google Scholar]
- The Alpha network. London School of Hygiene and Tropical Medicine. ( http://www.lshtm.ac.uk/eph/dph/research/alpha/ .) Accessed 5 February 2012.
- Darmstadt GL, Lee AC, Cousens S, et al. 60 Million non-facility births: who can deliver in community settings to reduce intrapartum-related deaths. Int J Gynaecol Obstet. 2009;107 Suppl 1:S89–112. doi: 10.1016/j.ijgo.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soofi S, Cousens S, Imdad A, Bhutto N, Ali N, Bhutta ZA. Topical application of chlorhexidine to neonatal umbilical cords for prevention of omphalitis and neonatal mortality in a rural district of Pakistan: a community-based, cluster-randomised trial. Lancet. 2012;379:1029–36. doi: 10.1016/S0140-6736(11)61877-1. [DOI] [PubMed] [Google Scholar]
- Arifeen SE, Mullany LC, Shah R, et al. The effect of cord cleansing with chlorhexidine on neonatal mortality in rural Bangladesh: a community-based, cluster-randomised trial. Lancet. 2012;379:1022–8. doi: 10.1016/S0140-6736(11)61848-5. [DOI] [PubMed] [Google Scholar]
- Edmond KM, Zandoh C, Quigley MA, Amenga-Etego S, Owusu-Agyei S, Kirkwood BR. Delayed breastfeeding initiation increases risk of neonatal mortality. Pediatrics. 2006;117:e380–6. doi: 10.1542/peds.2005-1496. [DOI] [PubMed] [Google Scholar]
- Lawn JE, Mwansa-Kambafwile J, Horta BL, Barros FC, Cousens S. ‘Kangaroo mother care' to prevent neonatal deaths due to preterm birth complications. Int J Epidemiol. 2010;39 Suppl 1:i144–54. doi: 10.1093/ije/dyq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Agudelo A, Belizan JM, Diaz-Rossello J. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2011. p. CD002771. [DOI] [PubMed]
- Darmstadt GL, Saha SK, Ahmed AS, et al. Effect of skin barrier therapy on neonatal mortality rates in preterm infants in Bangladesh: a randomized, controlled, clinical trial. Pediatrics. 2008;121:522–9. doi: 10.1542/peds.2007-0213. [DOI] [PubMed] [Google Scholar]
- Seale AC, Berkley JA. Managing severe infection in infancy in resource poor settings. Early Hum Dev. 2012;88:957–60. doi: 10.1016/j.earlhumdev.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang AT, Bang RA, Baitule SB, Reddy MH, Deshmukh MD. Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet. 1999;354:1955–61. doi: 10.1016/S0140-6736(99)03046-9. [DOI] [PubMed] [Google Scholar]
- Zaidi AK, Tikmani SS, Warraich HJ, et al. Community-based treatment of serious bacterial infections in newborns and young infants: a randomized controlled trial assessing three antibiotic regimens. Pediatr Infect Dis J. 2012;31:667–72. doi: 10.1097/INF.0b013e318256f86c. [DOI] [PubMed] [Google Scholar]
- Zaidi AK, Baqui AH, Qazi SA, et al. Scientific rationale for study design of community-based simplified antibiotic therapy trials in newborns and young infants with clinically diagnosed severe infections or fast breathing in South Asia and sub-Saharan Africa. Pediatr Infect Dis J. 2013;32 9 Suppl 1:S7–11. doi: 10.1097/INF.0b013e31829ff5fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie L, Armiento R, Subhi R, Kelly J, Clifford V, Duke T. Community-acquired neonatal and infant sepsis in developing countries: efficacy of WHO's currently recommended antibiotics–systematic review and meta-analysis. Arch Dis Child. 2013;98:146–54. doi: 10.1136/archdischild-2012-302033. [DOI] [PubMed] [Google Scholar]
- Aiken AM, Mturi N, Njuguna P, et al. Kilifi Bacteraemia Surveillance Group Risk and causes of paediatric hospital-acquired bacteraemia in Kilifi District Hospital, Kenya: a prospective cohort study. Lancet. 2011;378:2021–7. doi: 10.1016/S0140-6736(11)61622-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery-Taylor S, Emery F, Anthony M. Eighteen months of “matching Michigan” at a UK neonatal intensive care unit. Pediatr Infect Dis J. 2013;32:565–7. doi: 10.1097/INF.0b013e3182868389. [DOI] [PubMed] [Google Scholar]
- Schwab F, Geffers C, Bärwolff S, Rüden H, Gastmeier P. Reducing neonatal nosocomial bloodstream infections through participation in a national surveillance system. J Hosp Infect. 2007;65:319–25. doi: 10.1016/j.jhin.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Schulman J, Stricof R, Stevens TP, et al. New York State Regional Perinatal Care Centers Statewide NICU central-line-associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127:436–44. doi: 10.1542/peds.2010-2873. [DOI] [PubMed] [Google Scholar]
- Manzoni P, Stolfi I, Pugni L, et al. Italian Task Force for the Study and Prevention of Neonatal Fungal Infections; Italian Society of Neonatology A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med. 2007;356:2483–95. doi: 10.1056/NEJMoa065733. [DOI] [PubMed] [Google Scholar]
- Kaufman DA. Fluconazole prophylaxis: can we eliminate invasive Candida infections in the neonatal ICU. Curr Opin Pediatr. 2008;20:332–40. doi: 10.1097/MOP.0b013e3282f79c48. [DOI] [PubMed] [Google Scholar]
- Manzoni P, Rinaldi M, Cattani S, et al. Italian Task Force for the Study and Prevention of Neonatal Fungal Infections, Italian Society of Neonatology Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302:1421–8. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- Marlow N, Morris T, Brocklehurst P, et al. A randomised trial of granulocyte-macrophage colony-stimulating factor for neonatal sepsis: outcomes at 2 years. Arch Dis Child Fetal Neonatal Ed. 2013;98:F46–53. doi: 10.1136/fetalneonatal-2011-301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Wolkowiez M, Benjamin DK, Jr, Capparelli E. Immunotherapy in neonatal sepsis: advances in treatment and prophylaxis. Curr Opin Pediatr. 2009;21:177–81. doi: 10.1097/MOP.0b013e32832925e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale AC, Mwaniki M, Newton CR, Berkley JA. Maternal and early onset neonatal bacterial sepsis: burden and strategies for prevention in sub-Saharan Africa. Lancet Infect Dis. 2009;9:428–38. doi: 10.1016/S1473-3099(09)70172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa C, Panero A, Osborn JF, Simonetti AF, Pacifico L. Diagnosis of neonatal sepsis: a clinical and laboratory challenge. Clin Chem. 2004;50:279–87. doi: 10.1373/clinchem.2003.025171. [DOI] [PubMed] [Google Scholar]
- Rafael ME, Taylor T, Magill A, Lim YW, Girosi F, Allan R. Reducing the burden of childhood malaria in Africa: the role of improved. Nature. 2006;444 Suppl 1:39–48. doi: 10.1038/nature05445. [DOI] [PubMed] [Google Scholar]
- Wilson ML. Malaria rapid diagnostic tests. Clin Infect Dis. 2012;54:1637–41. doi: 10.1093/cid/cis228. [DOI] [PubMed] [Google Scholar]
- Crede S, Lawn J, Woods D, Wyatt J. Pulse oximetry screening for critical congenital heart defects. Lancet. 2012;380:1305; author reply 1306. doi: 10.1016/S0140-6736(12)61753-X. [DOI] [PubMed] [Google Scholar]
- Gona JK, Mung'ala-Odera V, Newton CR, Hartley S. Caring for children with disabilities in Kilifi, Kenya: what is the carer's experience. Child Care Health Dev. 2011;37:175–83. doi: 10.1111/j.1365-2214.2010.01124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens S, Blencowe H, Stanton C, et al. National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. Lancet. 2011;377:1319–30. doi: 10.1016/S0140-6736(10)62310-0. [DOI] [PubMed] [Google Scholar]
- Berkley JA, Mwangi I, Ngetsa CJ, et al. Diagnosis of acute bacterial meningitis in children at a district hospital in sub-Saharan Africa. Lancet. 2001;357:1753–7. doi: 10.1016/S0140-6736(00)04897-2. [DOI] [PubMed] [Google Scholar]
- Cherian T, Mulholland EK, Carlin JB, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005;83:353–9. [PMC free article] [PubMed] [Google Scholar]
- Pan-American Health Organisation. Neonatal tetanus elimination: field guide 2005. ( http://new.paho.org/hq/dmdocuments/2011/FieldGuide_NeonatalTetanus_2ndEd_e.pdf .) Accessed 26 August 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.