Abstract
Background:
Intrapartum hypoxic events (“birth asphyxia”) may result in stillbirth, neonatal or postneonatal mortality, and impairment. Systematic morbidity estimates for the burden of impairment outcomes are currently limited. Neonatal encephalopathy (NE) following an intrapartum hypoxic event is a strong predictor of long-term impairment.
Methods:
Linear regression modeling was conducted on data identified through systematic reviews to estimate NE incidence and time trends for 184 countries. Meta-analyses were undertaken to estimate the risk of NE by sex of the newborn, neonatal case fatality rate, and impairment risk. A compartmental model estimated postneonatal survivors of NE, depending on access to care, and then the proportion of survivors with impairment. Separate modeling for the Global Burden of Disease 2010 (GBD2010) study estimated disability adjusted life years (DALYs), years of life with disability (YLDs), and years of life lost (YLLs) attributed to intrapartum-related events.
Results:
In 2010, 1.15 million babies (uncertainty range: 0.89–1.60 million; 8.5 cases per 1,000 live births) were estimated to have developed NE associated with intrapartum events, with 96% born in low- and middle-income countries, as compared with 1.60 million in 1990 (11.7 cases per 1,000 live births). An estimated 287,000 (181,000–440,000) neonates with NE died in 2010; 233,000 (163,000–342,000) survived with moderate or severe neurodevelopmental impairment; and 181,000 (82,000–319,000) had mild impairment. In GBD2010, intrapartum-related conditions comprised 50.2 million DALYs (2.4% of total) and 6.1 million YLDs.
Conclusion:
Intrapartum-related conditions are a large global burden, mostly due to high mortality in low-income countries. Universal coverage of obstetric care and neonatal resuscitation would prevent most of these deaths and disabilities. Rates of impairment are highest in middle-income countries where neonatal intensive care was more recently introduced, but quality may be poor. In settings without neonatal intensive care, the impairment rate is low due to high mortality, which is relevant for the scale-up of basic neonatal resuscitation.
Over the last decade, with increasing focus on Millennium Development Goal (MDG) 4 for child survival, more scientific rigor has been applied to mortality estimation. Intrapartum-related hypoxic events, previously referred to as “birth asphyxia,” remain a major cause of global mortality, contributing to almost one-quarter of the world's 3 million neonatal deaths and almost half of 2.6 million third-trimester stillbirths (1,2). In previous Global Burden of Disease (GBD) estimates, “birth asphyxia” accounted for one of the highest numbers of disability adjusted life years (DALYs) for any single condition (3). However, estimates of the morbidity and impairment for this condition are constrained by several specific factors beyond the usual data gaps in low- and middle-income countries (LMICs), including shifting case definitions and terminology surrounding “birth asphyxia” (Table 1), difficulty in measuring impairment/disability, and the complexity of attributing causation to intrapartum insults, particularly when comorbidity such as infection or moderate preterm birth exists (4).
Table 1. Paradigm shifts in the language of “birth asphyxia”.

Early estimates of intrapartum-related neonatal mortality varied considerably, reflecting the different case definitions, data uncertainties, and changing estimation methodologies (Table 2) (4). The first systematic national estimates of intrapartum-related mortality were generated by Lawn et al. with the Child Health Epidemiology Reference Group (CHERG) using both single-cause and multi-cause regression methods and resulted in similar global totals of 904,000 (uncertainty range (UR): 650,000–1,170,000) and 910,000 (600,000–1,080,000; Table 2) for the year 2000 (1,5). In the most recent Child Health Epidemiology Reference Group analysis (CHERG) using multi-cause modeling for 2010, there were an estimated 717,000 (610,000–876,000) intrapartum-related neonatal deaths (6). The mortality estimates undertaken by the Institute of Health Metrics and Evaluation and used by GBD for 2010 (GBD2010) are generated using similar inputs but different methods (ensemble modeling) and estimated 638,000 intrapartum-related deaths (517,000–798,000) in 1990 and 510,000 deaths (400,000–620,000) in 2010 (7). In addition, there have now been two published systematic estimates of intrapartum stillbirths; the most recent in The Lancet stillbirth series estimated 1.2 million stillbirths (0.66–1.48) in 2009 (1,8). In total, there are ~2 million deaths (maternal, fetal, and neonatal) annually related to intrapartum complications, and they occur almost entirely in LMICs (1,2).
Table 2. Previous estimates of intrapartum-related outcomes (“birth asphyxia”).
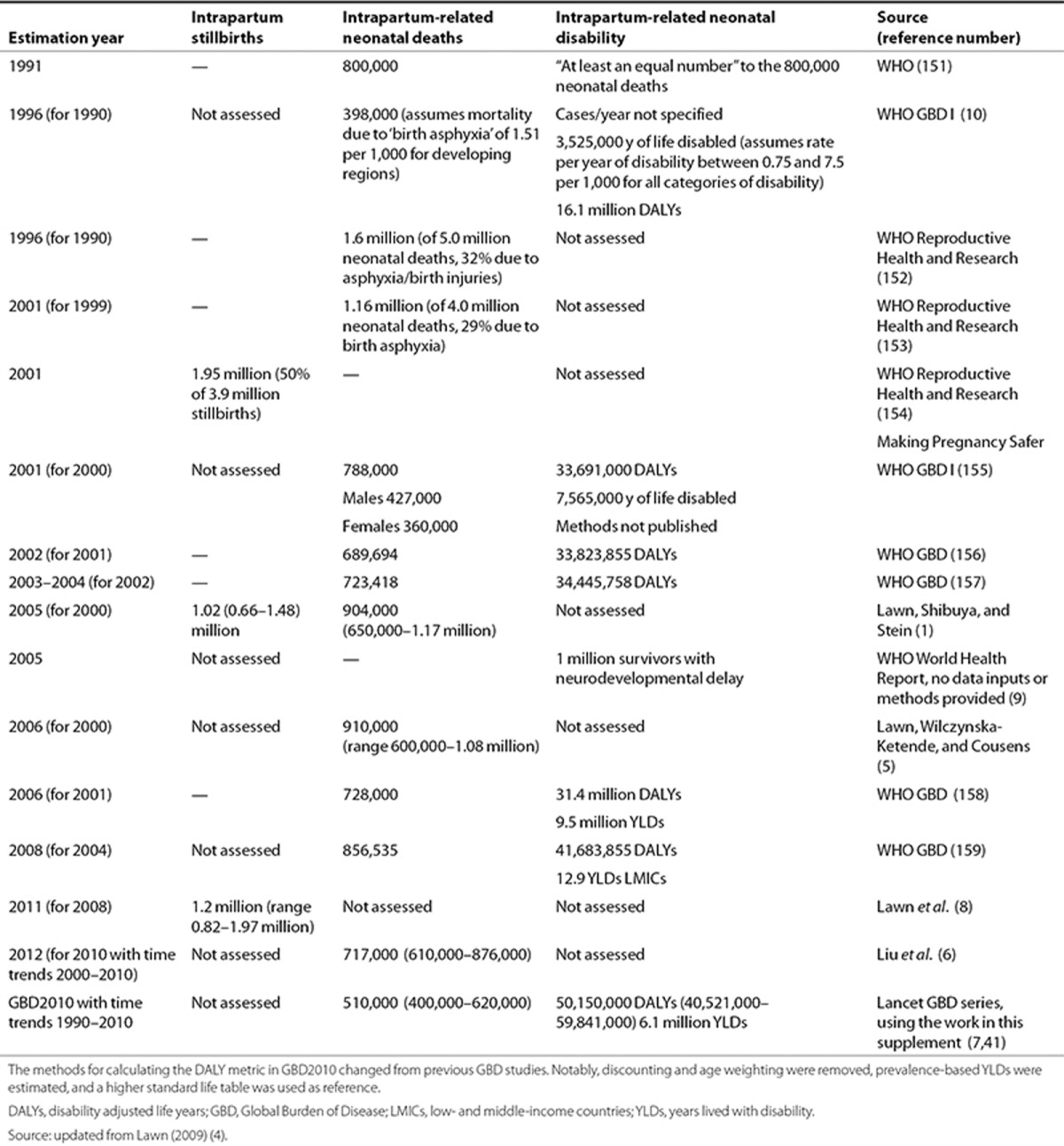
However, there are no systematic estimates with detailed methods for intrapartum-related impairment outcomes, which also carry a heavy burden and lifelong consequences for families. Such outcomes are increasingly important to measure and address as mortality begins to decline. The post–MDG dialogue emphasizes health and sustainable development as well as survival. The World Health Organization (WHO) World Health Report 2005 estimated that annually as many as 1 million survivors of “birth asphyxia” may develop cerebral palsy, learning difficulties or other disabilities (9), although detailed methods were not published. GBD DALYs consist of years of life lost (YLLs) to premature mortality and years of life with disability (YLDs). The first GBD estimated that “birth asphyxia” was responsible for 16.9 million DALYs (3.5 million YLDs and 13.4 YLLs) in 1990 (3,10). The GBD2008 update (for the year 2004) used different methods and estimated that “birth asphyxia” was responsible for 42 million DALYs (12.9 million YLDs in LMICs), which was double that due to diabetes and almost three-quarters of the burden due to HIV/AIDS (58 million DALYs), although no methods were published regarding the “asphyxia” estimates (Table 2).
The GBD2010 Expert Team for Intrapartum-related Neonatal Conditions overlaps with the author group on this paper. After the inputs were finalized for GBD2010, further data have been published and became available for analyses, including the coverage of care assumptions used throughout this supplement. The current analyses are in the spirit of continually improving the input data. Inputs or methods deviating from GBD2010 will be noted (11).
The overall objective of this work was to estimate the burden of neonatal impairment related to intrapartum-related hypoxic events. Neonatal encephalopathy (NE) following intrapartum events is a strong predictor of long-term impairment. In this manuscript, we detail the modeling process, input parameters, and estimation outputs for the following three-step compartmental model:
Incidence of NE related to intrapartum events at the national, regional, and global level for the year 2010, with time trends since 1990,
Neonatal case fatality rate (CFR) in order to estimate a cohort of affected survivors,
Risk of long term impairment in survivors, including both moderate–severe and mild neurodevelopmental impairment (NDI).
In our parallel analyses for GBD2010, the following additional parameters were estimated: prevalence of impairment due to NE by age, YLDs, YLLs, and DALYs.
Methods
Definitions and Measurement
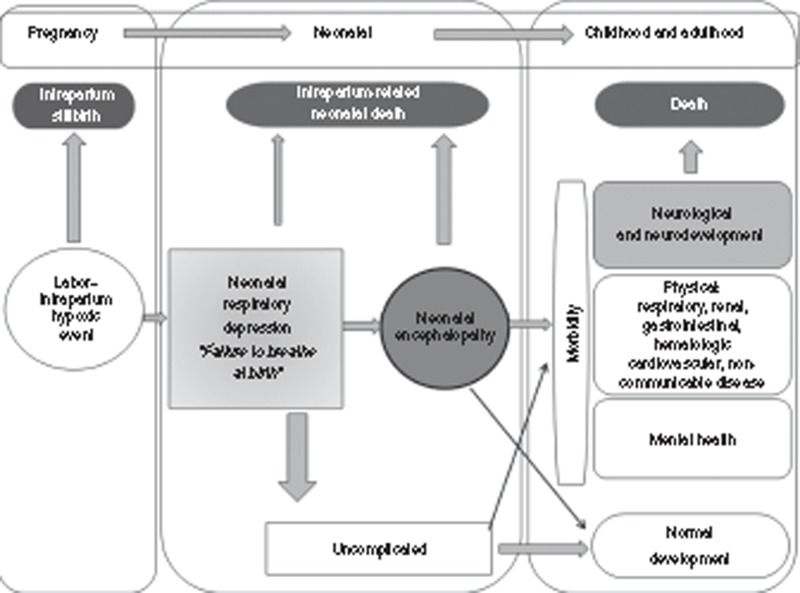
Figure 1 illustrates the pathways and adverse outcomes related to intrapartum complications, and Table 3 defines the epidemiologic terminology used in this paper. The language used for this condition and related outcomes has changed over time as a result of clarification of the epidemiology as well as concerns over litigation (Table 1).
Figure 1.
Global burden of disease schematic for intrapartum-related events “birth asphyxia.” See Table 4 for clinical definitions pertaining to intrapartum-related events.
Table 3. Definitions of clinical outcomes pertaining to intrapartum-related events.

“Birth asphyxia” was broadly defined by the WHO as “failing to initiate or maintain regular breathing at birth” (12). This clinical diagnosis is commonly referred to as neonatal respiratory depression and indicated by low Apgar scores, which were intended to be sensitive and nonspecific (Figure 1). “Asphyxia” was commonly assumed to result from insults during pregnancy and childbirth, with associated hypoxia and metabolic acidosis (4). However, neonatal respiratory depression may have other etiologies such as preterm birth, infection, maternal analgesia, congenital malformations, or metabolic disorders and is not predictive of long-term outcomes. In low-resource settings with poor intrapartum care and low access to neonatal resuscitation, some of these nonbreathing babies will die immediately in the delivery room or within a few hours of life.
For those infants suffering a severe hypoxic event who do not die immediately, some infants will develop NE—a “disturbance of neurological function in the earliest days of life (onset: 1–3 d of life) in the term infant manifested by difficulty initiating and maintaining respiration, depression of tone or reflexes, abnormal level of consciousness, and often by seizures” (13). Staging of NE was first defined by Sarnat and Sarnat (14) using clinical and electroencephalogram criteria, with classification into mild, moderate, and severe stages (Table 4). Subsequently several other clinical classifications have been used (14,15,16,17,18,19) (Supplementary Information online). NE is strongly predictive of long-term impairment (20,21) and clearer to diagnose than neonatal respiratory depression, and impairment after an intrapartum hypoxic insult is uncommon in the absence of NE. For those reasons, our estimates use NE as the starting point to estimate long-term impairment associated with intrapartum hypoxic events, rather than neonatal respiratory depression or low Apgar score (Figure 1).
Table 4. Original sarnat staging of NE(14).

The terms “post-asphyxial encephalopathy (PAE)” and “hypoxic ischemic encephalopathy (HIE)” have been frequently used since the 1970s to refer to encephalopathy associated with intrapartum injury. Given the possibility of other causes and difficulty in proving causation, the term NE is now preferred, and several recent consensus statements and an editorial have cautioned against the use of the terms PAE and HIE (13,22,23,24,25,26). HIE may be used when there is very clear evidence of intrapartum insult including low arterial cord blood pH, neuroimagining consistent with hypoxic–ischemic insult, and depressed Apgar score (27), although this is still controversial. Furthermore, the extent to which infectious disease may exacerbate intrapartum events and perinatal brain injury (double hit hypothesis) remains to be fully described (28). Estimates of the contribution of intrapartum events to NE are varied. In studies requiring stringent clinical criteria and neuroimaging (magnetic resonance imaging, electroencephalogram), the contribution of hypoxic events ranges between 50 and 80% (29,30,31) and is presumably the most common cause, even in these high-income settings (27). In an Australian population–based study using a broad clinical definition of NE, the intrapartum contribution was as low as 30% (16). However, in low-resource settings with poor intrapartum care, the proportion of NE attributable to intrapartum-related events is likely to be at the higher end of the range. For example, in a maternity hospital in Nepal, evidence of intrapartum compromise preceded 68% of cases (30). Given the 50 million annual home births and poor quality of obstetric care even in facility births in many LMICs, the majority of NE at the global level is associated with intrapartum complications—which we refer to as “NE related to intrapartum events” in this paper.
We estimated long-term, mild, and moderate-to-severe neurodevelopmental impairment (NDI) NDI following NE, including motor and/or cognitive-intellectual impairments. Impairment categories and definitions were standardized per the CHERG neonatal morbidity expert group and described in detail in the methods paper in this supplement (11) and are defined in Table 3. For intervention trials, we used impairment rates from control arms, given that the interventions (primarily therapeutic hypothermia) are not yet implemented as the standard of care in most regions of the world.
Searches and Data Sources
We systematically reviewed the literature with no date restrictions until November 2009 for the GBD2010 project (Supplementary Information online). The searches were updated in October 2012 and identified new data previously not included in GBD2010. The following databases were searched without language restrictions but limited to “human subjects”: Medline, Popline, Cochrane, EMRO, EMBASE, Web of Science, LILACS (Latin American and Caribbean Health Sciences Literature), and African Index Medicus. The search terms included “neonatal encephalopathy” and “hypoxic ischemic encephalopathy.” Snowball searching added literature referenced in key papers. Furthermore, abstracts from pediatric conference proceedings were searched for relevant data. Extensive efforts were also made to contact investigators and program managers for unpublished data, particularly in low–middle-income settings.
Inclusion/Exclusion Criteria and Data Abstraction
Titles and abstracts were reviewed; studies that reported data on incidence, prevalence, NE staging, case fatality, or impairment/morbidity associated with NE were abstracted. Studies without such data but reporting the number of NE cases were included if the principal investigator could be contacted and precise data regarding the size of the original birth cohort obtained. Studies meeting preliminary inclusion criteria were excluded if:
they did not limit the NE definition to full-term infants (13),
selection of the birth cohort or NE cases was not clearly defined,
substantial selection biases existed or cases were drawn from a specialized subpopulation,
they reported the same data from a previously included cohort, or
median birth year of the study cohort was before 1980.
We did not examine Apgar score as an outcome since Apgar score is an unreliable indicator of mortality and long-term morbidity (32,33). Due to selection bias in intervention trials, data from intervention studies were not included for the incidence or case fatality analysis.
Data were abstracted into a standard form and rechecked by a second investigator. Study characteristics were abstracted with regard to the study identifiers, context, design, and limitations. The following NE-related data were summarized: case definition; evidence of intrapartum-related events/insult; exclusion criteria, incidence (numerator of term-NE cases diagnosed, denominator of total live births in the same period); proportion of NE cases classified as mild (stage 1), moderate (stage 2), and severe (stage 3); neonatal case fatality (overall and by stage); and long-term impairment (overall and by stage). Neonatal mortality levels were defined as NMR level 1: <5 neonatal deaths/1,000 live births; NMR level 2: 5 to <15 neonatal deaths/1,000 live births; and NMR level 3: ≥15 neonatal deaths/1,000 live births (11).
Modeling Approach
A three-step compartment model was applied, including the input parameters and process to estimate firstly NE incidence, secondly the proportion of those with NE who survive the neonatal period, and thirdly the proportion of those survivors of NE who survive with impairment (Figure 2). Analyses were performed using Stata version 12 (StataCorp LP, College Station, TX).
Figure 2.
Compartmental model: parameters required and methods for estimation of the global burden of impairment related to intrapartum-related events and neonatal encephalopathy.
Step 1: estimation of NE incidence by statistical modeling. The NE risk was modeled using linear regression with random effects using the GBD region as the cluster unit. A total of 40 data inputs were included in the modeling, with observations from 26 countries, with median study years ranging from 1980 to 2010. National predictor variables were tested that might plausibly predict the risk of NE. The covariates tested included: biologic/demographic factors (NMR, stillbirth rate, low-birth-weight prevalence, HIV prevalence); health care access (cesarean section rate, skilled birth attendance, DPT3 coverage); and socioeconomic/geographic factors (GDP, urbanization, female literacy). Natural logarithms were used to transform continuous variables and log-odds to proportions to stabilize variance (1,34). Data points were evaluated for leverage by visual inspection of residual vs. fitted plots using Cook's D statistic, and extreme outlying/leverage studies (35,36) were excluded (Cook's D: >4/n).
Dummy variables were tested to control for:
NE definition quality, i.e., “good” (clear case definition consistent with NE and indication of history of intrapartum-related events) or “poor” (poorly defined criteria and/or not restricted to intrapartum-related “birth asphyxia”),
severity, i.e., studies reporting incidence of moderate-to-severe grade only (instead of all grades),
population selection bias, i.e., population-based sample vs. data presenting selection bias (facility-based, tertiary care hospital, or neonatal intensive care unit cohort), and
study decade.
Models were tested with individual predictors, and additional potential models were developed using automatic forward and backward stepwise selection (significance level of P < 0.10). Models were compared with respect to adjusted R2, Akaike's Information Criterion, and out-of-model prediction accuracy. Prediction accuracy was calculated using cross validation methods similar to the approach described by Liu et al. (37). Study data were randomly divided into five subsamples. One subsample was withheld while modeling was conducted on the remaining 80% of the data. A standardized error term was calculated as (observed(ln NE) − predicted(ln NE))2 on the excluded 20%. This was repeated with each successive subsample withheld, and a summary measure of the prediction error was generated for the entire data set. The final model selected was that which had the lowest overall prediction error based on the standardized error term and estimated by the following equation:
 |
where i is country; j is source; t is median year of study; Def is a set of NE case definition dummy variables; X is set of dummy variables for population selection/bias; Y is set of predictor variables representing socioeconomic factors and health care utilization for country (i) and year (t); ε is normally distributed error term; and η, normally distributed regional random effect.
National incidence of NE was predicted for 184 countries using the final model and multiplied by the number of live births in 2010 to obtain the estimated number of cases of NE (38). For the analysis of time trends, national covariate data were used for NMR (39) and births for the years 1990 and 2000 (38). We generated uncertainty estimates by drawing 1,000 bootstrapped samples from the modeling data set and repeated this for each step of the estimation process. For each bootstrapped sample, we ran the model and generated a new set of predicted national estimates for the prevalence of NE, including random effects at the regional level. For national-level estimates, we took the 2.5 and 97.5 centiles of the 1,000 predictions for our uncertainty bounds. To obtain uncertainty ranges for regional- and global-level estimates, we summed the relevant national estimates derived from each bootstrap sample, again taking the 2.5 and 97.5 centiles of the resulting distributions.
Steps 2–3: estimation of NE-related impairment using a compartmental model. In step 2, we estimated the number of postneonatal survivors and in step 3, the number of these survivors who were estimated to be impaired (Figure 2) for the year 2010. For the purpose of these estimates, and to be consistent with the other estimates in this supplement (11), studies were grouped by NMR level (study or national NMR of the median study year) reflecting similar settings in terms of access to and quality of care. Meta-analysis with random effects was used to calculate pooled proportions according to NMR level.
The following equation was used:
 |
Uncertainty was calculated by taking 1,000 random draws from a normal distribution for the proportion derived from the meta-analysis at each step of the compartmental model.
GBD2010 Estimation of YLDs, YLLs, and DALYs
GBD2010 used a similar approach as the three steps above to estimate the number of impaired NE survivors. The main differences in GBD2010 were (i) estimation of years 1990–2010 (vs. compartmental model 2010 only), (ii) data inputs (GBD2010 literature review completed in 2009 with fewer included studies), and (iii) assumptions in NE incidence modeling as GBD2010 did not include regional random effects or corrections for NE definition variation. After estimating the number of impaired survivors, GBD2010 performed the following further steps to calculate YLDs, YLLs, and DALYs using input data and assumptions as detailed in the results section (7,40,41). A Bayesian meta-regression method DisMod-MR was used to estimate the prevalence of long-term impairments by age, sex, and country (11) over each year during 1990–2010.
YLDs at all ages were calculated as the prevalence of impaired survivors at all ages multiplied by the disability weights derived for the GBD2010 study (11,42). Moderate-to-severe impairment outcomes were modeled together. This combines a range of health states, each with their own disability weight (11,42). An Australian register of 32 children with cerebral palsy following NE was used to apportion the distribution of severity to motor impairment, cognitive impairment, seizure disorder, blindness, and all possible combinations of these (43). The final disability weight used for moderate-to-severe NDI for high-income regions was 0.38 (uncertainty range: 0.29–0.49) and 0.42 (uncertainty range: 0.32–0.53) in low- and middle-income regions. The higher disability weights in low- and middle-income regions are because the epilepsy disability weights distinguish those with and without treatment differently.
DALYs at all ages were then calculated as the sum of YLLs and YLDs due to intrapartum complications (11,40). YLLs were calculated for the GBD2010 study using the Cause of Death Ensemble model and standard life expectancy tables (11,41) The total DALYs were calculated as the sum of the YLDs and YLLs attributable to NE (7,44).
Results
Input Parameters and Estimates
The parameters and process of each step (Figure 2) are detailed with a short summary of the results. A summary of the input parameters is shown in Table 5, with study-specific details in the Supplementary Information online, and a summary of regional results in Table 6.
Table 5. Summary of data published on the incidence, grading, neonatal case fatality, and impairment of neonatal encephalopathy by CHERG NMR levels (1980–2013).

Table 6. Number of NE incident cases, neonatal deaths, impaired survivors, YLDs, YLLs, and total DALYs by collapsed GBD regions (rounded to nearest thousand).

A total of 3,701 articles on NE/hypoxic ischemic encephalopathy (HIE) were identified from the databases searched and two unpublished data sets were included (Supplementary Information online). After initial screening of title and/or abstract, there were 132 studies remaining, of which 80 met inclusion criteria. Data were used for NE incidence from 40 studies, sex ratio from 14, neonatal case fatality from 33, and long-term impairment from 45 studies.
Step 1: estimating the incidence of NE
Data inputs. The studies included in the NE incidence analysis are shown in Supplementary Information online. Most incidence data were facility based, with only seven studies reporting incidence from population-based birth registries, all in high-income settings. Few data were from Africa (n = 7 studies) and South Asia (n = 5). Several studies reported NE incidence among full-term births only; these incidence rates were adjusted by dividing the incidence rate by the proportion of term births for the country and year (45). In one case, the HIE mortality rate (HIE deaths/live births) was reported (46), and the CFR was derived using a counterfactual of the national neonatal mortality rate (NMR) for that year. A limitation of the data was the variability of case definitions and inclusion criteria used to classify NE. Definitions of NE varied across studies: Sarnat (14), Fenichel (47), Levene (15), Badawi (16), International Classification of Diseases (ICD) codes, Amiel Tison (48), Ellis (49), Thompson Scoring (17), or other. Case inclusion differed with respect to the requirement for evidence of an “asphyxial” or “intrapartum insult.” The majority of studies (n = 18) required evidence of intrapartum hypoxia with a range of criteria from fetal distress, low Apgar scores with differing cutoffs/time of assessment (1 vs. 5 min), acidosis, meconium, and/or need for neonatal resuscitation (Supplementary Information online).
The median NE incidence for NMR level 1 (NMR <5/1,000 live births) was 1.60/1,000 live births (range: 0.68–3.75/1,000). Most data sets were from Western Europe and North America. There was an observed historical trend of decreasing incidence with increasing year of birth cohort in several high-income settings (Supplementary Information online: Derby, UK (50,51); Edinberg, UK (52); Sweden (53); and Perth, Australia (43,54)). For example, in the United Kingdom, the incidence of “HIE” in a large district health authority (Derby) decreased from 7.7/1,000 for 1976–1980, to 4.6/1,000 for 1984–1988, to 1.9/1,000 for 1992–1996 (51).
Fewer data were available from higher mortality settings. For NMR level 2 (NMR 5-<15), the median incidence of NE was 4.66/1,000 live births (range: 1.44–10.20), whereas in NMR level 3 (NMR ≥15), the median NE incidence was 12.10/1,000 live births (range: 3.64–26.51). It is very likely that these primarily facility-based incidence rates may underestimate NE incidence in high mortality settings in South Asia and Africa, where over half of births may occur outside of hospital facilities. There was only one community-based report of NE incidence from Nepal (55). Time trend data were available from the Indian National Perinatal Database. Incidence of NE did not change markedly between 1995 and 2002 (56) (13.83 vs. 14.25/1,000 births, respectively) (Supplementary Information online).
The severity of NE was consistent across mortality levels (Table 5). The pooled proportion of the distribution of NE grade was: grade 1: 37.8% (31.0–44.6%)l; grade 2: 37.7% (32.9–42.5%); and grade 3: 23.4% (19.9–27.0%). Given that separate meta-analyses were conducted for each grade, the sum of the pooled proportions does not equal 100.
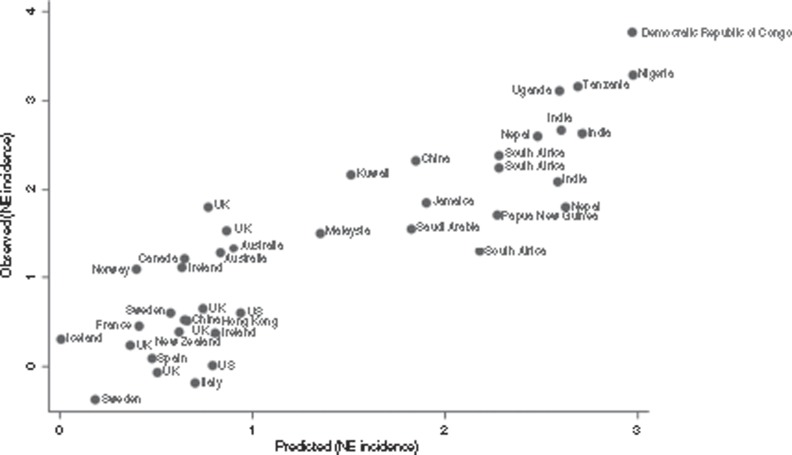
Regression modeling of NE incidence. The regression model shown in Table 7 was used to predict the natural log of the incidence of NE. Although meeting initial inclusion criteria, two data sets (35,36) were excluded due to extreme outlying/leverage (Cook's D: >4/n). The final model chosen used ln(NMR) as the main covariate and had the highest adjusted R2 (0.7457) and lowest prediction error. Dummy variables representing NE definition quality (including evidence of an intrapartum-related hypoxic insult), NE severity, and population selection bias were retained to adjust for these variables. Figure 3 shows a plot of the natural log of the incidence of NE observed in the studies vs. those predicted by the regression model. Residuals were normally distributed (Supplementary Information online).
Table 7. Prediction model for the incidence of intrapartum-related NE.
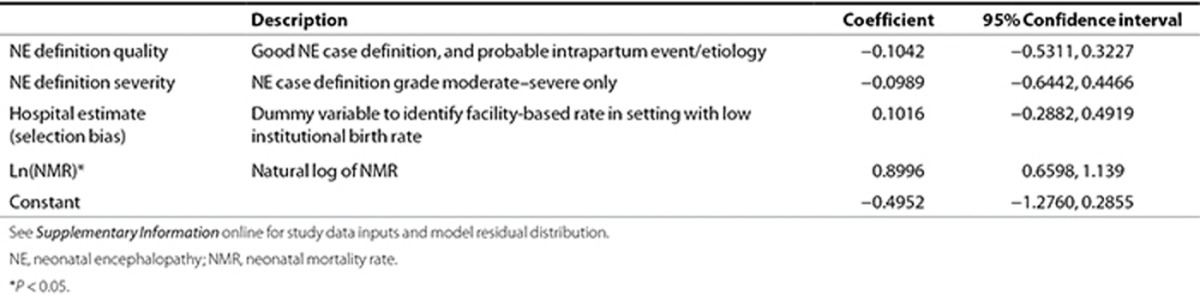
Figure 3.
Modeling estimates of the incidence of intrapartum-related neonatal encephalopathy (NE): predicted values compared to observed values for study data inputs. See Supplementary Information online for details of the study data inputs.
Sex differential among NE cases. Intervention studies were excluded from this analysis due to potential selection/recruitment biases. There was a consistent predominance of male cases across NE birth cohorts in different regions and mortality levels (pooled proportion adjusted for population sex ratio at birth, 58.7% males (55.6–61.8%)) (Supplementary Information online). The adjusted proportion of male cases ranged from 48.5% in the Democratic Republic of Congo (57) to 70.6% in Italy (58). The NE sex risk ratios were adjusted for the live birth sex ratio for the year of the study, then pooled by random-effects meta-analysis. The sex-specific incidence was estimated by the following equation:  where I is incidence, RR is risk of male sex, r is NE incidence among females, and p is proportion of male.
where I is incidence, RR is risk of male sex, r is NE incidence among females, and p is proportion of male.
Although evidence supports increased risk for mortality and long-term impairment for male babies when compared with female babies, data were insufficient to include this in subsequent steps of the model after the sex-specific NE incidence risk.
Estimates of incident cases of NE. Globally, in 2010, there were an estimated 1.15 million (0.89–1.60 million) new cases of NE associated with intrapartum-related events (686,000 male, 466,000 female), an incidence of 8.5/1,000 live births (Figure 4). We estimate that 439,000 were grade 1 (mild), 438,000 were grade 2 (moderate), 273,000 were grade 3 (severe) NE. The majority of cases occurred in South–Southeast Asia (532,000) and sub-Saharan Africa (478,000), which together accounted for more than 85% of incident cases. The highest regional incidence of NE was in sub-Saharan Africa (14.9/1,000 live births), followed by South Asia (10.4/1,000). In high-income regions, the incidence was as low as 1.5/1,000 live births.
Figure 4.
Estimated incident cases of neonatal encephalopathy by region by the sex of the baby in 2010. White, male; black, female.
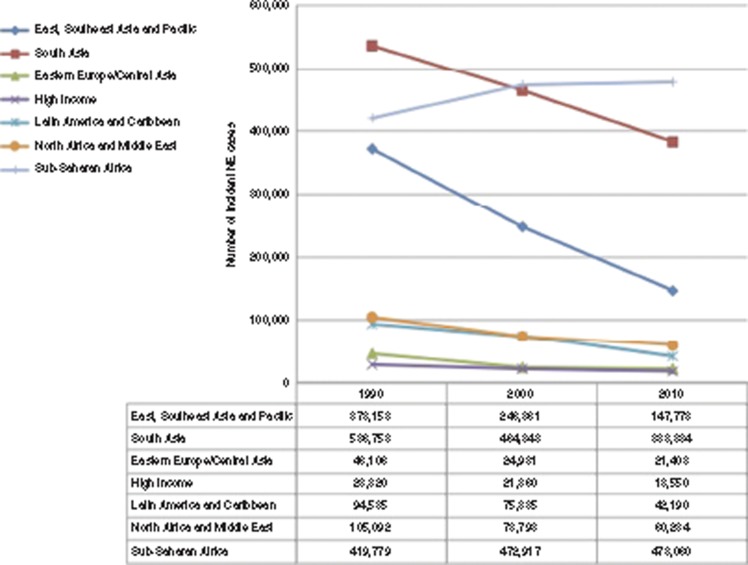
Regional estimates of NE cases in 1990, 2000, and 2010 are shown in Figure 5. There were an estimated 1,604,000 cases of NE born worldwide in 1990 (global incidence: 11.7/1,000 live births), which decreased to 1,380,000 in 2000 (10.6/1,000 live births) and to 1,152,000 in 2010 (8.5/1,000 live births). There were reductions in the incidence of NE in all regions, with steeper drops in South Asia and East–Southeast Asia, and Pacific. In sub-Saharan Africa, although there was a reduction in NE incidence from 18.3/1,000 live births in 1990 to 14.9/1,000 in 2010, there was an estimated overall increase in the number of NE cases due to a substantial increase in the number of live births (23 million in 1990 to 32 million in 2010).
Figure 5.
Estimated time trends in incident neonatal encephalopathy cases by region 1990–2010. Diamond, East, Southeast Asia and Pacific; square, South Asia; triangle, Eastern Europe/Central Asia; X, high income; asterisk, Latin American and Caribbean; circle, North Africa and Middle East; vertical bar, sub-Saharan Africa.
Step 2: Calculation of the Number of Postneonatal Survivors After NE
Case fatality rate (CFR) by care available. A total of 31 studies were included in the NE neonatal case fatality analysis (Supplementary Information online). Two of the NMR level 1 studies were population-based surveillance studies (in Sweden (53,59)); the remaining 12 NMR level 1 studies were all conducted in tertiary care hospitals with neonatal intensive care. The NMR level 2 studies (n = 6) were from teaching hospitals with neonatal intensive care in middle-income countries, or from higher income countries from the mid-1980s. In the highest mortality group (level 3, NMR ≥15), all studies (n = 11) were from tertiary care hospitals, except for one community-based study from Nepal (55), where the neonatal CFR was substantially higher at 49%. Prehospital discharge case fatality was used in cases where neonatal CFR was not otherwise reported. In NMR level 1, the pooled neonatal case fatality was 9.9% (95% confidence interval (CI): 7.9–11.9%), with a substantially higher CFR of 76.8% (95% CI: 61.9–91.7%) among grade 3 NE (Table 5). In NMR level 2, the pooled neonatal CFR was 10.7% (95% CI: 7.6–13.7%) and among grade 3 cases, CFR was 47.9% (95% CI: 36.2–59.5%). In NMR level 3, pooled neonatal CFR for all NE was higher at 27.7% (95% CI: 18.7–36.6%) and notably higher for grade 3 NE at 91.8% (83.4–99.4%). Given the sparser neonatal CFR data by NE grade as well as incidence by NE grade, the pooled estimates for the average all-grade NE CFR were used for our estimates.
Estimates of NE case fatality and postneonatal survivors of NE. CFR from NE depends largely on access to neonatal care to treat complications. Almost all of our data on neonatal case fatality arose from tertiary care hospitals, neonatal intensive care units, or in populations with high-level access to this care, and thus likely underestimate the NE case fatality at a global level. In most LMICs, access to neonatal intensive care is limited for both facility and home births. In countries with <70% facility birth rate, we have estimated that <5% of infants born in facilities have access to neonatal intensive care, whereas for countries with 70–90% facility birth rates, 25–50% of infants may have access to neonatal intensive care (60). Furthermore, 50 million births occur outside of facilities annually (61), and most of these take place without a skilled birth attendant or access to neonatal intensive care unit. Case fatality among babies with NE is expected to be much higher in these settings, as demonstrated by the one community-based study from Nepal where neonatal CFR was nearly 50% (55).
With these caveats and limitations, we estimate that 287,000 (181,000–440,000) infants diagnosed with NE related to intrapartum events died during the first month of life in 2010, leaving 865,000 neonatal survivors (Table 6). These estimates are likely underestimates in LMICs, where access to neonatal intensive care is low. Importantly, these numbers do not account for the intrapartum-related deaths in babies who die shortly after birth and before onset of NE, notably where there is no resuscitation. Those deaths will also appear in mortality registrations as deaths from birth trauma or “birth asphyxia.” Impairment in newborns who survive the first few hours of life is considered to occur in those who develop identifiable NE.
Step 3: Calculation of the Number of Postneonatal Survivors With Impairment
Risk of NDI among NE survivors. The majority of data on morbidity and long-term NDI were from low NMR settings (30/47 data sets on moderate-to-severe NDI and 9/17 on mild NDI) (Supplementary Information online). Given the substantially lower data availability disaggregating impairment rates by three NE grades (<50% of data identified), we pooled data on impairment rates for all grades of NE. Some studies reported follow-up for only NE cases that were graded as combined moderate-to-severe. For comparability between studies, it was assumed that 30% of all NE would have been mild or stage 1, with zero impairment rate, based on our literature review and a recent meta-analysis (20). Thus, for these studies, to account for attenuation of the impairment rate if mild-stage 1 cases had been represented in the data, the impairment rate was reduced by 30%. For intervention studies, particularly therapeutic hypothermia trials, impairment rates were used from the control arm (noncooling).
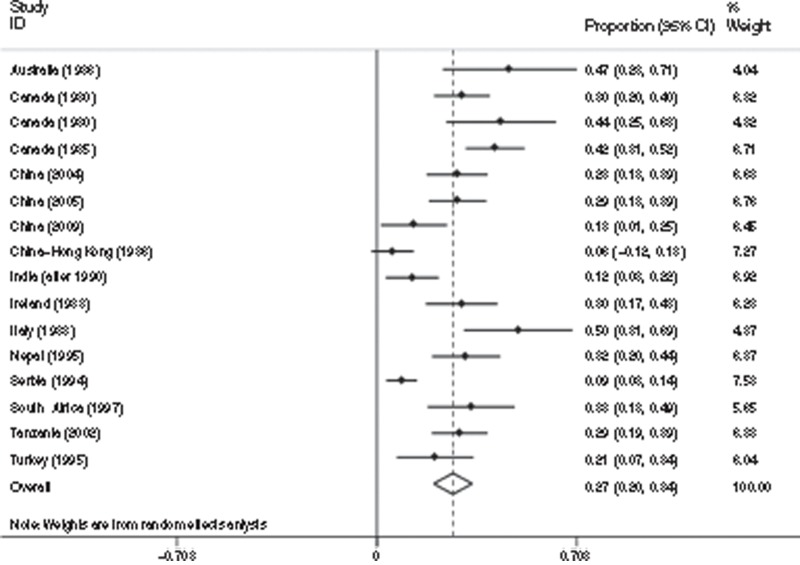
In NMR level 1, there were 29 studies (n = 1,482 NE survivors) included in the meta-analysis, with follow-up ranging from 1 to 10 y (Table 5). A pooled estimate of 26% of survivors of NE developed moderate-to-severe NDI (95% CI: 22–31%) (Figure 6). For mild NDI, eight studies were identified (n = 384 NE survivors) mostly reporting intellectual/cognitive impairment, and pooled estimate was 14% (95% CI: 9–19%) (Figure 7). In NMR level 2–3, there was a total of 16 studies identified (n = 858 NE survivors) with follow-up ranging from 6 mo to 12 y for moderate-to-severe NDI, and 9 studies (n = 533 NE survivors) that assessed mild NDI up to 10 y. A pooled estimate of 27% (95% CI: 20–34%) of infants with NE developed moderate-to-severe NDI (Figure 8), whereas 21% (95% CI: 10–32%) developed mild NDI (Figure 9).
Figure 6.
Meta-analysis of 29 studies (1,482 neonatal encephalopathy (NE) survivors) reporting moderate/severe impairment for infants with NE in countries with neonatal mortality rate <5 per 1,000 (level 1). Study name = country and median year of study. Citations: Australia (1989) (115), Australia (1995) (67), Australia (1996) (116), Canada (1993) (31), France (2000) (117,118), Ireland (2004) (118), Italy (1998) (119), Italy (2001) (120), Italy (2004) (58), Italy (2005) (121), Multi-country (2001) (122), Multi-country (2004) (105), Multi-country (2004) (107), Netherlands (1995) (123), Netherlands (1998) (124), Netherlands (2001) (125), New Zealand (1998) (126), New Zealand (1998) (127), Singapore (1994) (128), Sweden (1985) (59), Sweden (1988) (53), Switzerland (1981) (129), Switzerland (1994) (130), United Kingdom (1994) (51), United Kingdom (1997) (131), United Kingdom (2004) (106), United States (1997) (132), United States (1999) (133), United States (2002) (134).
Figure 7.
Meta-analysis of 16 studies (858 neonatal encephalopathy (NE) survivors) reporting moderate/severe impairment for infants with neonatal encephalopathy from countries with neonatal mortality rate ≥5 per 1,000 (level 2–3). Study name = country and median year of study. Citations: Australia (1986) (135), Canada (1980) (136), Canada (1980) (62), Canada (1985) (19), China (2004) (137), China (2005) (138), China (2009) (139), China–Hong Kong (1986) (140), India (after 1990) (141), Ireland (1983) (142), Italy (1988) (143), Nepal (1995) (68), Serbia (1994) (144), South Africa (1997) (17), Tanzania (2002) (145), Turkey (1995) (146).
Figure 8.
Meta-analysis of eight studies (384 neonatal encephalopathy (NE) survivors) reporting mild cognitive impairment for infants with NE for countries with neonatal mortality rate <5 per 1,000 (level 1). Study name = country and median year of study. Citations: Italy (2005) (121), Netherlands (1995) (123), New Zealand (1998) (126), Sweden (1985) (59), United Kingdom (1997) (131), United Kingdom (2004) (106), United States (1999) (133), United States (2002) (134).
Figure 9.
Meta-analysis of nine studies (533 neonatal encephalopathy (NE) survivors) reporting mild cognitive impairment for infants with neonatal encephalopathy for countries with neonatal mortality rate ≥5 per 1,000 (level 2–3). Australia (1986) (135), Canada (1980) (62), Canada (1985) (19), China (2004) (137), China–Hong Kong (1986) (140), India (after 1990) (141), Nepal (1995) (68), Serbia (1994) (144), South Africa (1997) (17).
Estimates of the number of NE survivors with NDI. Globally in 2010, there were an estimated 413,000 impaired survivors of NE, 233,000 (163,000–342,000) with moderate-to-severe NDI, and 181,000 (82,000–319,000) with mild NDI. The majority of cases of impairment were concentrated in Asia (South, East, Southeast, and Pacific) (108,000 with moderate-to-severe NDI; 84,000 with mild NDI) and sub-Saharan Africa (93,000 cases with moderate-severe NDI; 73,000 with mild NDI). See Figure 10 for regional summary.
GBD2010-Specific Inputs and Results
In order to calculate the prevalence of NE survivors across different age groups beyond the first month of life, additional data on the excess postneonatal mortality risk survivors were required. Mortality among children with NE occurs primarily during the neonatal period, although there is an ongoing excess risk beyond the neonatal period and first year of life. There were three longitudinal NE cohorts from NMR level 1, which reported NE case fatality up to 8 y. In the United Kingdom, Levene et al. (15) followed up a cohort of 126 newborns with NE; neonatal case fatality was 9%, whereas case fatality at 2.5 y was 11%. In Canada, Finer and colleagues and Robertson and colleagues followed a cohort of 226 infants for 8 y; the NE case fatality was 7% at 1.5 y, 12% at 3.5 y, and 13% at 8 y (62,63,64,65). In Australia, Badawi and colleagues (66,67) provided a 5-y follow-up for a birth cohort of 276 NE cases; neonatal case fatality was 9%, 1.5-y case fatality was 12%, and 5-y case fatality was 13%. In the single report from a developing country, case fatality was substantially higher. Ellis et al. (68) followed up 131 newborns with NE identified in the principal maternity hospital in Kathmandu, Nepal, up to 1 y. The neonatal case fatality was 33%, and at 1-y follow-up, it was 44%.
However, the NE-specific studies had cohort follow-up only for a short part of the total life span (up to 8 y) and therefore could not be used as the sole sources of mortality risk for the longer term estimation of YLD for moderate-to-severe impairment in the GBD. Therefore, for the GBD2010 model, the excess mortality estimation also used eight studies that reported on long-term mortality risk in cerebral palsy which had much longer term follow-up (69,70,71,72,73,74,75,76).
Estimates of the number of YLDs and DALYs associated with intrapartum conditions/NE. DisMod-MR estimated 50.2 million DALYs (40.5–59.8) (7,40) worldwide in 2010 for intrapartum-related NE (Table 6), accounting for 2.0% of the GBD. This was a reduction from 1990 when 60.6 million DALYs (50.2–75.0) were estimated (2.4% of GBD). South Asia (21.0 million) and sub-Saharan Africa (15.0 million) had the highest burden in 2010.
For NE disability, there were an estimated 6.1 million (4.5–8.0) YLDs attributed to NE in 2010 (12% of DALYs) as compared with 5.6 million YLDs (4.1–7.2) in 1990. The highest numbers of YLDs were in South Asia (2.1 million) and East Asia/Pacific (1.5 million). YLDs comprised the highest proportion of DALYs in high-income regions (32%) and middle-income regions—East Asia/Pacific (21%) and North Africa/Middle East (21%). Whereas YLDs were a small proportion of total DALYs in sub-Saharan Africa (9%) and South Asia (9%).
Discussion
Our estimates underline the huge global public health burden related to childbirth complications (Figure 11). In 2010, intrapartum-related hypoxic events resulted in 510,000 (44)–717,000 (6) neonatal deaths, 1.15 million new cases of NE and 413,000 impaired survivors. These are the first systematic estimates of impairment after intrapartum complications, including 233,000 children with severe neurodevelopmental outcomes, often cerebral palsy, and the equally important milder impairment outcomes affecting cognitive potential among 21% of NE survivors. Over 95% of the deaths and impairment occurred in LMICs, depriving the poorest countries of development potential. Newborn boys are at increased risk. In addition, an estimated 54% of ~255,000 maternal deaths are intrapartum related (44). Importantly, an estimated 1.2 million intrapartum stillbirths are not counted in GBD or any global metrics (8).
Figure 11.
Summary of deaths and disability outcomes that are intrapartum related based on 125 million births worldwide in 2010. Sources: intrapartum stillbirth estimate (8), neonatal deaths (6). Neonatal encephalopathy (NE) cases, mortality, and impairment outcomes are derived from the estimation process described in this article.
These data show that babies may be born into three very different worlds. In high-income countries, major intrapartum injury has become rare with intrapartum stillbirth rates of under 0.1 per 1,000 births in some Nordic countries and very few neonatal deaths from these complications. We estimated a low NE incidence of 1.6 cases per 1,000 live births in high-income regions (19,000 new cases annually). Although intrapartum mortality has substantially declined, long-term impairment among survivors remains an important issue. The recent International Liaison Committee on Resuscitation and National Institute for Health and Clinical Excellence recommendations (77,78) on therapeutic hypothermia are based on head and body cooling trials demonstrating significantly lower rates of mortality and major impairment among survivors (79).
In contrast, in low-income countries, 50 million home births occur annually without skilled birth care (80), and many facility births occur in settings with poor quality of intrapartum care and poor access to neonatal intensive care (11,81). Intrapartum stillbirth and NMRs are 20–50-fold higher comparing the lowest to highest income countries (2). Without access to neonatal resuscitation, the majority (~60%) of intrapartum “asphyxia” deaths occur shortly after birth, before infants develop NE (Figure 1), and impairment rates are lower than previously estimated. Almost three-quarters (862,000 annually) of all NE cases occur in sub-Saharan Africa (14.9/1,000 live births) and South Asia (10.4/1,000 live births). These estimates likely underestimate the burden in low-income countries, where NE diagnosis requires neonatal assessment by a physician. Furthermore, our estimates of neonatal case fatality among cases of NE should be cautiously interpreted, as these estimates are primarily based on data from tertiary care facilities and may therefore significantly also underestimate NE deaths in settings with high rates of home births and health systems without viable networks of transport and referral to specialty care. In a rural community–based setting in Nepal, 39% of newborns with probable intrapartum-related NE died before they were ever visited by a health worker (55).
Between the “first world” and the “third world” lie a number of middle-income, emerging economies, where neonatal care is improving, and mortality is declining (81), yet our data and the papers in this supplement on preterm outcomes (60) and retinopathy of prematurity (82) also suggest that there is a hidden burden of impairment. In these settings, the inappropriate application of technological interventions, such as induction of labor without adequate intrapartum monitoring or cooling interventions may likely result in higher rates of impaired survivors. YLDs comprise a larger proportion of total DALYs in these settings (21% in East Asia/Pacific and Middle East/North Africa, vs. 9% in sub-Saharan Africa/South Asia). Over half of the global total of impaired survivors were in Latin America/Caribbean, Eastern Europe/Central Asia, Middle East/North Africa, and East/Southeast Asia/Pacific, where NE incidence ranged from 4.0 to 6.2 per 1,000 live births. Urgent focus is required to track these outcomes and assess prevention strategies, notably quality of obstetric and neonatal care, in these settings.
Comparing the various estimates of intrapartum-related neonatal mortality, our CFR-based approach suggests that 287,000 deaths occur among babies with NE annually. However, these results should be cautiously interpreted since CFR-based estimates are more uncertain than multi-cause mortality data approaches. These estimates of NE deaths are likely an underestimate in LMICs. In addition to the aforementioned underdiagnosis issues the case fatality data were based primarily on neonatal intensive care unit cohorts, and access to neonatal intensive care is lower in these settings. However, these are still consistent with estimates of total intrapartum-related neonatal deaths in 2010 (717,000 (UR 610,000–876,000) (6) or 510,000 (UR 400,000–620,000) (44)) (Figure 11) since most intrapartum deaths happen soon after birth before the onset of NE, particularly in low-income countries with poor obstetric care and access to resuscitation. NE only develops at several hours of age since it is a manifestation of brain injury and inflammation.
A consistent male predominance among NE cases was observed in this systematic review, across different regions and levels of care. We adjusted for population-based sex ratios at birth but were not able to account for potential biases in care-seeking for male infants. Male infants may be of larger fetal size and predisposed to obstructed labor. Males may also be more vulnerable to hypoxic ischemic brain injury due to hormonal modulation by testosterone and sex-specific genetic differences in apoptosis (83). Finally, there may be a sex-specific difference in maturation of the ventilatory response to hypoxia.
Although the burden of disease is large, we estimated a steady 0.9% annual reduction in global NE incidence from 1990 to 2010. Rates of decline of global neonatal deaths and intrapartum-related neonatal deaths are slightly higher, ~2% annually from 2000 to 2010. These declines are likely correlated with improved access to intrapartum care and facility birth rates achieved globally over the past two decades. The few available historical data in our review demonstrated similar rates of decline for mortality in several high-income countries since the 1980s (51,53). This fits with the overall picture for the global burden of neonatal conditions, with most of the limited reduction being due to mortality reduction, and inadequate morbidity trend data from the highest burden countries impeding detailed analysis of time trends for impairment (11,84).
The recent literature from therapeutic hypothermia trials increased the data inputs on long-term impairment following NE/HIE for this systematic review. A meta-analysis of six studies by van de Riet et al. (21) reported neurologic disability accompanied by severe cognitive impairment among <0.5% of survivors with grade 1, 30% with grade 2, and 84% of grade 3 NE. Pin et al. (20) conducted a meta-analysis (2008) including 13 studies and reported that 47% (95% CI: 36–57%) of all stages of “post-asphyxial” NE had death, cerebral palsy, or motor/cognitive impairment >2 SD below the norm. Our meta-analysis included substantial new data and showed higher neonatal CFRs in high mortality settings (27.7% in NMR level 3 vs. 9.9% in NMR level 1; including 31 studies and 2,639 NE cases), but similar rates of moderate-to-severe NDI among NE survivors (27% in NMR level 3 vs. 26% NMR level 1; 45 studies and 2,340 NE survivors).
Data Limitations
The most important limitation was the lack of data from the highest mortality settings, the inverse data law (84,85). Furthermore, almost all of the studies identified were from referral-level facilities or neonatal intensive care units. Data selected from these settings may be biased with respect to NE incidence, staging, case fatality, and impairment, as mothers and infants who attend these facilities will not be representative of the general population.
The shifting terminology and definitions of “birth asphyxia” and NE or HIE add specific challenges for comparability (22). We included studies that specified criteria for probable intrapartum-related events, although these definitions also varied widely (requirement of fetal distress, degree of acidosis, timing and cutoff of Apgar scores, requirement of resuscitation, and neuroimaging), and our model adjusted the incidence of studies that reported NE without specifying evidence of an intrapartum insult. The limited data on incidence and outcome separated by NE staging, particularly in higher mortality settings, was another limitation.
The variability in disability assessment was substantial. Differing definitions, domains, timing, and tools are problematic and also affected by postneonatal excess mortality and losses to follow-up (84). Losses to follow-up over 30% were excluded from this analysis.
Data Improvement
As discussed in detail in a separate paper of this supplement (84), there is a need for standardized, well-defined minimum perinatal data sets including main pregnancy outcomes and impairment outcomes. Standardization of impairment outcomes using validated definitions of specific domains would reduce heterogeneity and allow pooling of data. Furthermore, conducting assessment at standardized ages/stages in development and using common, validated assessment tools are critical.
With effective intrapartum interventions increasingly available in LMICs, it is critical to improve measurement of impact and process. Infants born with severe respiratory depression are frequently misclassified as stillbirths, particularly in settings where newborns are not routinely assessed at the time of birth and even within facilities. Classification as stillbirth may be a simple lack of assessment or may be to avoid culpability, circumvent the requirement of a death certificate, or in cases of neonaticide (2). Three studies of neonatal resuscitation (86,87,88) have demonstrated substantial reductions in “fresh stillbirth” rates after training, with reductions ranging from 25 to 45%. A critical component of resuscitation training is newborn assessment, thus the reduction in misclassification of depressed live born babies as stillbirths was probably the major factor leading to overall reductions in stillbirth rates. Nonetheless, two studies showed reductions in stillbirths without increases in early neonatal mortality (86,88), and one showed reductions of both stillbirth and day 1 neonatal mortality (87). These demonstrate that substantial reductions in overall intrapartum-related mortality can be achieved with resuscitation training in LMIC settings. A composite measure of intrapartum mortality has been suggested by United Nations Population Fund that combines intrapartum stillbirth with day 1 neonatal deaths (2) but has yet to be clearly defined and tested.
The measurement of disability becomes increasingly important with declines in intrapartum-related mortality. The first critical step is the consistent use of NE definitions and classification for staging. Furthermore, standardized criteria are required to define “intrapartum-related” events. Even in low-income or community settings, data on certain risk factors strongly associated with intrapartum hypoxic insult could be more routinely collected: for example, conditions such as nonvertex presentation, multiple birth and prolonged/obstructed labor, and maternal pyrexia particularly when associated with signs of dystocia. The development of simplified, validated definitions of NE that could be ascertained at the community level or on verbal autopsy may help to better quantify the burden in low-income settings. A breech presentation baby who develops seizures shortly after birth with persistent poor feeding and abnormal tone maybe diagnosable by a community health worker or even maternal report.
Program Implications
The prevention of intrapartum-related conditions can be approached at three levels:
the primary prevention of intrapartum hypoxic injury during labor and childbirth,
secondary prevention of mortality, morbidity, and long-term impairments among infants who have experienced a hypoxic intrapartum event or develop NE, and
tertiary prevention with improved identification and care of infants with disability.
We have recently reviewed the impact of a wide range of interventions to avert intrapartum-related injury in the International Journal of Gynecology and Obstetrics (80,89,90,91,92).
The primary prevention of intrapartum injury is an important global public health priority to avert mortality and morbidity and is associated with accelerated reduction of maternal mortality (4.2% per year) and a smaller effect on intrapartum neonatal deaths (81). Improving the coverage and the quality of obstetric care and improving access to emergency obstetric care, particularly for high-risk pregnancies, are key priorities. Comprehensive emergency obstetric care is estimated to reduce intrapartum neonatal deaths by 85% (93), and emergency obstetric training has been associated with 50% reduction in rates of HIE (94). Promising and feasible interventions in LMICs include improved intrapartum monitoring by use of partographs and identification and management of childbirth complications including nonvertex presentation, shoulder dystocia, multiple pregnancy, and postterm gestation (89). Furthermore, the early identification and treatment of maternal intra-amniotic infection is promising given the synergistic NE risk with maternal pyrexia/chorioamnionitis and hypoxia (95). For the 50 milllion women giving birth at home, investments in maternal care services must be made to increase access and quality and organize transport systems especially for obstetric and neonatal emergencies.
Among babies who suffer an intrapartum hypoxic insult, basic newborn resuscitation is an effective, feasible secondary prevention intervention and reduces intrapartum-related neonatal mortality by 30% (96). Training programs, like Helping Babies Breathe (97), have been developed, widely implemented, and are being scaled up in ~50 countries (87,97,98). Our data show overall low rates of impairment in low-income settings due to high early mortality, which is relevant for this scale-up, suggesting low impairment risk in contexts with only basic resuscitation and no intensive care. Preliminary data from the First Breath trial found no differences in average Bayley Mental Development Index among infants in the resuscitation vs. control arms (99). However, in middle-income settings, impairment rates are higher, similar to the pattern seen for preterm birth survivors (60). More focus must be placed on measuring impairment-free survival in these settings (84). Quality of care must be emphasized with the wide-scale implementation of technological interventions. High oxygen concentration during resuscitation is associated with increased risk of mortality and impairment and should be avoided (100). Neonatal hyperthermia may exacerbate hypoxic brain injury and increase risk of apnea (101,102), thus preventing overheating under radiant infant warmers is a key intervention in these settings.
For those babies who develop NE, improved care can reduce mortality and impairment outcomes. This is an increasingly important aspect of service provision and planning, especially in middle-income countries (84). Basic supportive neonatal care for babies experiencing intrapartum-hypoxic injury has received little attention (91). Prompt recognition and treatment of hypoglycemia, hyperbilirubinemia, and seizures (103) after potential hypoxic–ischemic insult can limit the extent of cerebral injury (104). Therapeutic hypothermia may significantly reduce disability among survivors of NE (32% reduction in major disability rates at 18 mo (79,105,106,107)) and is currently recommended as standard of care for infants with moderate-to-severe NE in high-income countries (77,78). Initiation within the therapeutic window of 6 h of life is critical (108) and emphasizes the importance of early, rapid diagnosis of NE and timely transport to a facility with the capacity to deliver the intervention. Simpler methods of identification of infants with NE by health workers in first-level facilities and community settings may facilitate early recognition and triage. The development of simpler, low-cost methods to implement hypothermia in LMICs have been piloted and are feasible (109). Some small clinical trials of cooling have been performed in India (110). However, the safe and effective administration of therapeutic hypothermia requires a high level of intensive care support with equipment for blood pressure monitoring, recognition and prompt treatment of infection, regular assessment of blood gases to ensure normocapnia and normoxia (111), and appropriate sedation (112).
Tertiary prevention involves earlier identification and care of children with disabilities, as well as family support. Furthermore, the role of early neurocognitive stimulation interventions should be considered in these infants (113). As part of the Global Network study in rural India, Pakistan, and Zambia, Carlo et al. (114) showed that the implementation of an early developmental intervention by parents with biweekly home visits by trainers resulted in significantly higher Mental Development Index scores on the Bayley Scale at 3 y of age. Intrapartum care and care of affected babies should be addressed in early childhood development strategies that have traditionally focused on infant nutrition. This is further discussed elsewhere in the supplement (84).
Conclusion
These are the first systematic global estimates of NE and subsequent neurodevelopmental impairment (see Table 8 for summary of findings). Our mortality estimate for NE is lower than current global estimates of the total intrapartum-related neonatal deaths, because many nonbreathing babies currently die without resuscitation and prior to manifesting NE. Our impairment estimate is also lower than previous GBD work, mainly because of more data showing a high CFR in cases of NE in the absence of intensive care. Hence, the number of impaired survivors is lower than previously believed, but still substantial in LMICs, where 27% of NE survivors are affected with a moderate-to-severe impairment (233,000 infants) and 21% of NE survivors (181,000 infants) with a mild impairment, underlining the effects on a child's long-term potential for school learning, academic achievement, and economic productivity. Intrapartum-related conditions in live born babies result in 50 million DALYs, 2% of all GBD2010. There are also 1.2 million intrapartum stillbirths not counted in GBD. Universal coverage of obstetric care and neonatal resuscitation would prevent most of this burden. Investment in quality care at birth is critical. Missing data on stillbirths, neonatal deaths, and long-term impairment means that we fail to appreciate the full extent of the burden, undervalue the effect of these investments, and miss the opportunity to achieve our goal of impairment-free survival and improved development and economic potential.
Table 8. Research findings and implications of the burden of intrapartum-related neonatal encephalopathy and impairment.
Statement of Financial Support
This article is published as part of a supplement sponsored by The Bill and Melinda Gates Foundation to the Child Health Epidemiology Reference Group (CHERG) through US Fund for UNICEF and to Save the Children's Saving Newborn Lives program. A.C.L., N.K., H.B., and S.C. were supported through a grant from the Bill & Melinda Gates Foundation through the Child Health Epidemiology Reference Group. A.C.L. was also funded by grants from USAID and National Institutes of Health (NIH). J.E.L. was funded by the Bill & Melinda Gates Foundation through Save the Children's Saving Newborn Lives program.
Disclosure
A.C.L. and N.K. have served as consultants for Save the Children's Saving Newborn Lives program. S.N. has received consulting fees from the American Academy of Pediatrics and Pan American Health Organization, and has received grant support from the Colorado Department of Public Health and Environment (CDPHE). M.E. has served as an advisor for the Nepal Health Sector Strengthening Programme with Options, the consulting arm of Marie Stopes. The other authors declare no conflict of interest.
Figure 10.
Regional burden of intrapartum-related stillbirths, neonatal deaths, and neonatal encephalopathy outcomes for babies affected by intrapartum-related events in 2010.
Acknowledgments
We thank Chaomin Wan, Department of Pediatrics, Clinical Epidemiology Unit, Department Evidence Based Medicine and Clinical Epidemiology, Sichuan University for collecting data to contribute to the study. We also thank the reviewers of the manuscript for the extremely thoughtful and detailed review, and Martin Frenzel and Chris Rowland for assistance with the figures.
Supplementary Material
References
- Lawn J, Shibuya K, Stein C. No cry at birth: global estimates of intrapartum stillbirths and intrapartum-related neonatal deaths. Bull World Health Organ. 2005;83:409–17. [PMC free article] [PubMed] [Google Scholar]
- Lawn JE, Lee AC, Kinney M, et al. Two million intrapartum-related stillbirths and neonatal deaths: where, why, and what can be done. Int J Gynaecol Obstet. 2009;107 Suppl 1:S5–18, S19. doi: 10.1016/j.ijgo.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–42. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- Lawn J.4 Million Neonatal Deaths: An Analysis of Available Cause-of-Death Data and Systematic Country Estimates With a Focus on “Birth Asphyxia.”Doctoral Thesis, University College London, 2009202
- Lawn JE, Wilczynska-Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. Int J Epidemiol. 2006;35:706–18. doi: 10.1093/ije/dyl043. [DOI] [PubMed] [Google Scholar]
- Liu L, Johnson HL, Cousens S, et al. Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Lawn JE, Blencowe H, Pattinson R, et al. Lancet's Stillbirths Series steering committee Stillbirths: Where? When? Why? How to make the data count. Lancet. 2011;377:1448–63. doi: 10.1016/S0140-6736(10)62187-3. [DOI] [PubMed] [Google Scholar]
- World Health Organization . The World Health Report 2005—Make Every Mother and Child Count. Geneva, Switzerland; World Health Organization; 2005. [Google Scholar]
- Shibuya K, Murray CJL.Birth asphyxia. Murray CJL, Lopez AD.eds. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability From Diseases, Injuries and Risk Factors in 1990 and Projected to 2020 Cambridge, MA; Harvard University Press; 1996429–53. [Google Scholar]
- Blencowe H, Vos T, Lee AC, et al. Estimates of neonatal morbidities and disabilities at regional and global levels for 2010: introduction, methods overview, and relevant findings from the Global Burden of Disease study Pediatr Res 2013. this issue). [DOI] [PMC free article] [PubMed]
- World Health Organization . Basic Newborn Resuscitation: A Practical Guide. Geneva, Switzerland; World Health Organization; 1997. [Google Scholar]
- Leviton A, Nelson KB. Problems with definitions and classifications of newborn encephalopathy. Pediatr Neurol. 1992;8:85–90. doi: 10.1016/0887-8994(92)90026-u. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- Levene ML, Kornberg J, Williams TH. The incidence and severity of post-asphyxial encephalopathy in full-term infants. Early Hum Dev. 1985;11:21–6. doi: 10.1016/0378-3782(85)90115-x. [DOI] [PubMed] [Google Scholar]
- Badawi N, Kurinczuk JJ, Keogh JM, et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. 1998;317:1554–8. doi: 10.1136/bmj.317.7172.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86:757–61. doi: 10.1111/j.1651-2227.1997.tb08581.x. [DOI] [PubMed] [Google Scholar]
- Ellis M, Costello A. Birth asphyxia, Apgar score and neonatal encephalopathy. Indian Pediatr. 1997;34:975–8. [PubMed] [Google Scholar]
- Low JA, Galbraith RS, Muir DW, Broekhoven LH, Wilkinson JW, Karchmar EJ. The contribution of fetal-newborn complications to motor and cognitive deficits. Dev Med Child Neurol. 1985;27:578–87. doi: 10.1111/j.1469-8749.1985.tb14129.x. [DOI] [PubMed] [Google Scholar]
- Pin TW, Eldridge B, Galea MP. A review of developmental outcomes of term infants with post-asphyxia neonatal encephalopathy. Eur J Paediatr Neurol. 2009;13:224–34. doi: 10.1016/j.ejpn.2008.05.001. [DOI] [PubMed] [Google Scholar]
- van de Riet JE, Vandenbussche FP, Le Cessie S, Keirse MJ. Newborn assessment and long-term adverse outcome: a systematic review. Am J Obstet Gynecol. 1999;180:1024–9. doi: 10.1016/s0002-9378(99)70676-9. [DOI] [PubMed] [Google Scholar]
- Nelson KB. Defining hypoxic-ischemic birth events. Dev Med Child Neurol. 2003;45:71; author reply 71–2. doi: 10.1111/j.1469-8749.2003.tb00863.x. [DOI] [PubMed] [Google Scholar]
- ACOG Task Force on Neonatal Encephalopathy and Cerebral Palsy . Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. Washington, DC; ACOG; 2003. [Google Scholar]
- Task Force American College of Obstetricians and Gynecologists and the American Academy of Pediatrics Neonatal encephalopathy and cerebral palsy. Defining the pathogenesis and pathophysiology. American College of Obstetrics and Gynecology. 2003.
- Nelson KB, Leviton A. How much of neonatal encephalopathy is due to birth asphyxia. Am J Dis Child. 1991;145:1325–31. doi: 10.1001/archpedi.1991.02160110117034. [DOI] [PubMed] [Google Scholar]
- Dammann O, Ferriero D, Gressens P. Neonatal encephalopathy or hypoxic-ischemic encephalopathy? Appropriate terminology matters. Pediatr Res. 2011;70:1–2. doi: 10.1203/PDR.0b013e318223f38d. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy. Ann Neurol. 2012;72:156–66. doi: 10.1002/ana.23647. [DOI] [PubMed] [Google Scholar]
- Girard S, Kadhim H, Roy M, et al. Role of perinatal inflammation in cerebral palsy. Pediatr Neurol. 2009;40:168–74. doi: 10.1016/j.pediatrneurol.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Cowan F, Rutherford M, Groenendaal F, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361:736–42. doi: 10.1016/S0140-6736(03)12658-X. [DOI] [PubMed] [Google Scholar]
- Ellis M, Manandhar DS, Manandhar N, Wyatt J, Bolam AJ, Costello AM. Stillbirths and neonatal encephalopathy in Kathmandu, Nepal: an estimate of the contribution of birth asphyxia to perinatal mortality in a low-income urban population. Paediatr Perinat Epidemiol. 2000;14:39–52. doi: 10.1046/j.1365-3016.2000.00233.x. [DOI] [PubMed] [Google Scholar]
- Shah DK, Lavery S, Doyle LW, Wong C, McDougall P, Inder TE. Use of 2-channel bedside electroencephalogram monitoring in term-born encephalopathic infants related to cerebral injury defined by magnetic resonance imaging. Pediatrics. 2006;118:47–55. doi: 10.1542/peds.2005-1294. [DOI] [PubMed] [Google Scholar]
- Committee on Fetus and Newborn, American Academy of Pediatrics, Committee on Obstetric Practice, American College of Obstetricians and Gynecologists Use and abuse of the apgar score. Pediatrics. 1996;98:141–2. [PubMed] [Google Scholar]
- Marlow N. Do we need an Apgar score. Arch Dis Child. 1992;67 7 Spec No:765–7. doi: 10.1136/adc.67.7_spec_no.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton C, Lawn JE, Rahman H, Wilczynska-Ketende K, Hill K. Stillbirth rates: delivering estimates in 190 countries. Lancet. 2006;367:1487–94. doi: 10.1016/S0140-6736(06)68586-3. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Kasdorf E, Engel M, Grunebaum A, Perlman JM. Changing pattern of perinatal brain injury in term infants in recent years. Pediatr Neurol. 2012;46:106–10. doi: 10.1016/j.pediatrneurol.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2004;89:F152–5. doi: 10.1136/adc.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hing WC, Yeoh TT, Yeoh SF, Lin RT, Li SC. An evaluation of antimicrobial prophylaxis in paediatric surgery and its financial implication. J Clin Pharm Ther. 2005;30:371–81. doi: 10.1111/j.1365-2710.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- UN Population Division . World Population Prospects, 2010. New York; 2013. [Google Scholar]
- UN Inter-agency Group for Child Mortality Estimation . Child Mortality Estimates. New York; 2010. [Google Scholar]
- Murray CJ, Ezzati M, Flaxman AD, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–6. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–43. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi N, Felix JF, Kurinczuk JJ, et al. Cerebral palsy following term newborn encephalopathy: a population-based study. Dev Med Child Neurol. 2005;47:293–8. doi: 10.1017/s0012162205000575. [DOI] [PubMed] [Google Scholar]
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- Velaphi S, Pattinson R. Avoidable factors and causes of neonatal deaths from perinatal asphyxia-hypoxia in South Africa: national perinatal survey. Ann Trop Paediatr. 2007;27:99–106. doi: 10.1179/146532807X192462. [DOI] [PubMed] [Google Scholar]
- Fenichel GM. Hypoxic-ischemic encephalopathy in the newborn. Arch Neurol. 1983;40:261–6. doi: 10.1001/archneur.1983.04050050029002. [DOI] [PubMed] [Google Scholar]
- Amiel-Tison C, Ellison P. Birth asphyxia in the fullterm newborn: early assessment and outcome. Dev Med Child Neurol. 1986;28:671–82. doi: 10.1111/j.1469-8749.1986.tb03914.x. [DOI] [PubMed] [Google Scholar]
- Ellis M, Shrestha L, Shrestha PS, Manandhar DS, Bolam AJ, L Costello AM. Clinical predictors of outcome following mild and moderate neonatal encephalopathy in term newborns in Kathmandu, Nepal. Acta Paediatr. 2001;90:316–22. [PubMed] [Google Scholar]
- Hull J, Dodd KL. Falling incidence of hypoxic-ischaemic encephalopathy in term infants. Br J Obstet Gynaecol. 1992;99:386–91. doi: 10.1111/j.1471-0528.1992.tb13754.x. [DOI] [PubMed] [Google Scholar]
- Smith J, Wells L, Dodd K. The continuing fall in incidence of hypoxic-ischaemic encephalopathy in term infants. BJOG. 2000;107:461–6. doi: 10.1111/j.1471-0528.2000.tb13262.x. [DOI] [PubMed] [Google Scholar]
- Becher JC, Stenson BJ, Lyon AJ. Is intrapartum asphyxia preventable. BJOG. 2007;114:1442–4. doi: 10.1111/j.1471-0528.2007.01487.x. [DOI] [PubMed] [Google Scholar]
- Thornberg E, Thiringer K, Odeback A, Milsom I. Birth asphyxia: incidence, clinical course and outcome in a Swedish population. Acta Paediatr. 1995;84:927–32. doi: 10.1111/j.1651-2227.1995.tb13794.x. [DOI] [PubMed] [Google Scholar]
- Badawi N, Kurinczuk JJ, Keogh JM, et al. Antepartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. 1998;317:1549–53. doi: 10.1136/bmj.317.7172.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Mullany LC, Tielsch JM, et al. Incidence of and risk factors for neonatal respiratory depression and encephalopathy in rural Sarlahi, Nepal. Pediatrics. 2011;128:e915–24. doi: 10.1542/peds.2010-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul VK, Jain S, Jain P, et al. Morbidity and mortality among outborn neonates at 10 tertiary care institutions in India during the year 2000. J Trop Pediatr. 2004;50:170–4. doi: 10.1093/tropej/50.3.170. [DOI] [PubMed] [Google Scholar]
- Biselele T, Naulaers G, Bunga Muntu P, et al. A descriptive study of perinatal asphyxia at the University Hospital of Kinshasa (Democratic Republic of Congo). J Trop Pediatr. 2013;59:274–9. doi: 10.1093/tropej/fmt011. [DOI] [PubMed] [Google Scholar]
- Ancora G, Soffritti S, Lodi R, et al. A combined a-EEG and MR spectroscopy study in term newborns with hypoxic-ischemic encephalopathy. Brain Dev. 2010;32:835–42. doi: 10.1016/j.braindev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Lindström K, Hallberg B, Blennow M, Wolff K, Fernell E, Westgren M. Moderate neonatal encephalopathy: pre- and perinatal risk factors and long-term outcome. Acta Obstet Gynecol Scand. 2008;87:503–9. doi: 10.1080/00016340801996622. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Lee AC, Cousens S, et al. Preterm birth–associated neurodevelopmental impairment estimates at regional and global levels for 2010 Pediatr Res 2013. this issue). [DOI] [PMC free article] [PubMed]
- March of Dimes, The Partnership for Maternal, Newborn, and Child Health, Save the Children, WHO Howson C, Kinney M, Lawn J. Born Too Soon: The Global Action Report on Preterm Birth. Geneva, Switzerland; World Health Organization; 2012. [Google Scholar]
- Finer NN, Robertson CM, Richards RT, Pinnell LE, Peters KL. Hypoxic-ischemic encephalopathy in term neonates: perinatal factors and outcome. J Pediatr. 1981;98:112–7. doi: 10.1016/s0022-3476(81)80555-0. [DOI] [PubMed] [Google Scholar]
- Robertson CM, Etches PC. Decreased incidence of neurologic disability among neonates at high risk born between 1975 and 1984 in Alberta. CMAJ. 1988;139:225–9. [PMC free article] [PubMed] [Google Scholar]
- Robertson CM, Finer NN. Long-term follow-up of term neonates with perinatal asphyxia. Clin Perinatol. 1993;20:483–500. [PubMed] [Google Scholar]
- Robertson CM, Perlman M. Follow-up of the term infant after hypoxic-ischemic encephalopathy. Paediatr Child Health. 2006;11:278–82. [PMC free article] [PubMed] [Google Scholar]
- Badawi N, Keogh JM, Dixon G, Kurinczuk JJ. Developmental outcomes of newborn encephalopathy in the term infant. Indian J Pediatr. 2001;68:527–30. doi: 10.1007/BF02723247. [DOI] [PubMed] [Google Scholar]
- Dixon G, Badawi N, Kurinczuk JJ, et al. Early developmental outcomes after newborn encephalopathy. Pediatrics. 2002;109:26–33. doi: 10.1542/peds.109.1.26. [DOI] [PubMed] [Google Scholar]
- Ellis M, Manandhar N, Shrestha PS, Shrestha L, Manandhar DS, Costello AM. Outcome at 1 year of neonatal encephalopathy in Kathmandu, Nepal. Dev Med Child Neurol. 1999;41:689–95. doi: 10.1017/s0012162299001413. [DOI] [PubMed] [Google Scholar]
- Crichton JU, Mackinnon M, White CP. The life-expectancy of persons with cerebral palsy. Dev Med Child Neurol. 1995;37:567–76. doi: 10.1111/j.1469-8749.1995.tb12045.x. [DOI] [PubMed] [Google Scholar]
- Khan A, Kinney MV, Hazir T, et al. Newborn survival in Pakistan: a decade of change and future implications. Health Policy Plan. 2012;27 Suppl 3:iii72–87. doi: 10.1093/heapol/czs047. [DOI] [PubMed] [Google Scholar]
- Strauss D, Cable W, Shavelle R. Causes of excess mortality in cerebral palsy. Dev Med Child Neurol. 1999;41:580–5. doi: 10.1017/s001216229900122x. [DOI] [PubMed] [Google Scholar]
- Blair E, Watson L, Badawi N, Stanley FJ. Life expectancy among people with cerebral palsy in Western Australia. Dev Med Child Neurol. 2001;43:508–15. doi: 10.1017/s0012162201000949. [DOI] [PubMed] [Google Scholar]
- Hemming K, Hutton JL, Pharoah PO. Long-term survival for a cohort of adults with cerebral palsy. Dev Med Child Neurol. 2006;48:90–5. doi: 10.1017/S0012162206000211. [DOI] [PubMed] [Google Scholar]
- Westbom L, Bergstrand L, Wagner P, Nordmark E. Survival at 19 years of age in a total population of children and young people with cerebral palsy. Dev Med Child Neurol. 2011;53:808–14. doi: 10.1111/j.1469-8749.2011.04027.x. [DOI] [PubMed] [Google Scholar]
- Evans PM, Evans SJ, Alberman E. Cerebral palsy: why we must plan for survival. Arch Dis Child. 1990;65:1329–33. doi: 10.1136/adc.65.12.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger ER, Allaway NC, Peltin S. Survivorship in cerebral palsy. Am J Public Health Nations Health. 1959;49:343–9. doi: 10.2105/ajph.49.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman J, Davis P, Wyllie J, Kattwinkel J. Therapeutic hypothermia following intrapartum hypoxia-ischemia. An advisory statement from the Neonatal Task Force of the International Liaison Committee on Resuscitation. Resuscitation. 2010;81:3. [Google Scholar]
- National Institute for Clinical Excellence . Therapeutic Hypothermia With Intracorporeal Temperature Monitoring for Hypoxic Perinatal Brain Injury. London; 2010. [Google Scholar]
- Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166:558–66. doi: 10.1001/archpediatrics.2011.1772. [DOI] [PubMed] [Google Scholar]
- Darmstadt GL, Lee AC, Cousens S, et al. 60 Million non-facility births: who can deliver in community settings to reduce intrapartum-related deaths. Int J Gynaecol Obstet. 2009;107 Suppl 1:S89–112. doi: 10.1016/j.ijgo.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn JE, Kinney MV, Black RE, et al. Newborn survival: a multi-country analysis of a decade of change. Health Policy Plan. 2012;27 Suppl 3:iii6–28. doi: 10.1093/heapol/czs053. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C.Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res 2013. this issue). [DOI] [PMC free article] [PubMed]
- Hill CA, Fitch RH. Sex differences in mechanisms and outcome of neonatal hypoxia-ischemia in rodent models: implications for sex-specific neuroprotection in clinical neonatal practice. Neurol Res Int. 2012;2012:867531. doi: 10.1155/2012/867531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H, Vos T, Lee AC, et al. Estimates of neonatal morbidities and disabilities at regional and global levels for 2010: introduction, methods overview, and relevant findings from the Global Burden of Disease study Pediatr Res 2013. this issue). [DOI] [PMC free article] [PubMed]
- Lawn JE, Cousens S, Zupan J. Lancet Neonatal Survival Steering Team 4 million neonatal deaths: When? Where? Why. Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- Goudar SS, Somannavar MS, Clark R, et al. Stillbirth and newborn mortality in India after helping babies breathe training. Pediatrics. 2013;131:e344–52. doi: 10.1542/peds.2012-2112. [DOI] [PubMed] [Google Scholar]
- Msemo G, Massawe A, Mmbando D, et al. Newborn mortality and fresh stillbirth rates in Tanzania after helping babies breathe training. Pediatrics. 2013;131:e353–60. doi: 10.1542/peds.2012-1795. [DOI] [PubMed] [Google Scholar]
- Carlo WA, Goudar SS, Jehan I, et al. First Breath Study Group Newborn-care training and perinatal mortality in developing countries. N Engl J Med. 2010;362:614–23. doi: 10.1056/NEJMsa0806033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeyr GJ, Haws RA, Bergstrom S, et al. Obstetric care in low-resource settings: what, who, and how to overcome challenges to scale up. Int J Gynaecol Obstet. 2009;107 Suppl 1:S21–44, S44–25. doi: 10.1016/j.ijgo.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Lee AC, Lawn JE, Cousens S, et al. Linking families and facilities for care at birth: what works to avert intrapartum-related deaths. Int J Gynaecol Obstet. 2009;107 Suppl 1:S65–85, S86–68. doi: 10.1016/j.ijgo.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall SN, Lee AC, Niermeyer S, et al. Neonatal resuscitation in low-resource settings: what, who, and how to overcome challenges to scale up. Int J Gynaecol Obstet. 2009;107 Suppl 1:S47–62, S63–4. doi: 10.1016/j.ijgo.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattinson R, Kerber K, Waiswa P, et al. Perinatal mortality audit: counting, accountability, and overcoming challenges in scaling up in low- and middle-income countries. Int J Gynaecol Obstet. 2009;107 Suppl 1:S113–121, S121–2. doi: 10.1016/j.ijgo.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Lee AC, Cousens S, Darmstadt GL, et al. Care during labor and birth for the prevention of intrapartum-related neonatal deaths: a systematic review and Delphi estimation of mortality effect. BMC Public Health. 2011;11 Suppl 3:S10. doi: 10.1186/1471-2458-11-S3-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draycott T, Sibanda T, Owen L, et al. Does training in obstetric emergencies improve neonatal outcome. BJOG. 2006;113:177–82. doi: 10.1111/j.1471-0528.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- Cooke R. Chorioamnionitis, maternal fever, and neonatal encephalopathy. Dev Med Child Neurol. 2008;50:9. doi: 10.1111/j.1469-8749.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- Lee AC, Cousens S, Wall SN, et al. Neonatal resuscitation and immediate newborn assessment and stimulation for the prevention of neonatal deaths: a systematic review, meta-analysis and Delphi estimation of mortality effect. BMC Public Health. 2011;11 Suppl 3:S12. doi: 10.1186/1471-2458-11-S3-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics Helping Babies Breathe, 2010. ( http://www.helpingbabiesbreathe.org/ .) Accessed 1 June 2013.
- USAID . Project Brief: Scaling-up of Helping Babies Breathe Initiative to Strengthen Newborn Resuscitation in Bangladesh. Washington, DC; USAID; 2011. [Google Scholar]
- Carlo WA, Goudar SS, Pasha O, et al. Brain Research to Ameliorate Impaired Neurodevelopment-Home-based Intervention Trial Committee; National Institute of Child Health and Human Development Global Network for Women's and Children's Health Research Neurodevelopmental outcomes in infants requiring resuscitation in developing countries. J Pediatr. 2012;160:781–5.e1. doi: 10.1016/j.jpeds.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstad OD, Ramji S, Soll RF, Vento M. Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology. 2008;94:176–82. doi: 10.1159/000143397. [DOI] [PubMed] [Google Scholar]
- Perlstein PH, Edwards NK, Sutherland JM. Apnea in premature infants and incubator-air-temperature changes. N Engl J Med. 1970;282:461–6. doi: 10.1056/NEJM197002262820901. [DOI] [PubMed] [Google Scholar]
- Laptook AR, Shankaran S, Ambalavanan N, et al. Hypothermia Subcommittee of the NICHD Neonatal Research Network Outcome of term infants using apgar scores at 10 minutes following hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:1619–26. doi: 10.1542/peds.2009-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain Injury. J Pediatr. 2009;155:318–23. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Liaison Committee on Resuscitation The International Liaison Committee on Resuscitation (ILCOR) consensus on science with treatment recommendations for pediatric and neonatal patients. Pediatr Bas Adv Life Supp Pediatr. 2006;117:e955–77. doi: 10.1542/peds.2006-0206. [DOI] [PubMed] [Google Scholar]
- Jacobs SE, Morley CJ, Inder TE, et al. Infant Cooling Evaluation Collaboration Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165:692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- Azzopardi DV, Strohm B, Edwards AD, et al. TOBY Study Group Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- Simbruner G, Mittal RA, Rohlmann F, Muche R. neo.nEURO.network Trial Participants Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126:e771–8. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Gunn TR, Gunning MI, Williams CE, Gluckman PD. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics. 1998;102:1098–106. doi: 10.1542/peds.102.5.1098. [DOI] [PubMed] [Google Scholar]
- Robertson NJ, Nakakeeto M, Hagmann C, et al. Therapeutic hypothermia for birth asphyxia in low-resource settings: a pilot randomised controlled trial. Lancet. 2008;372:801–3. doi: 10.1016/S0140-6736(08)61329-X. [DOI] [PubMed] [Google Scholar]
- Bharadwaj SK, Bhat BV. Therapeutic hypothermia using gel packs for term neonates with hypoxic ischaemic encephalopathy in resource-limited settings: a randomized controlled trial. J Trop Pediatr. 2012;58:382–8. doi: 10.1093/tropej/fms005. [DOI] [PubMed] [Google Scholar]
- Klinger G, Beyene J, Shah P, Perlman M. Do hyperoxaemia and hypocapnia add to the risk of brain injury after intrapartum asphyxia. Arch Dis Child Fetal Neonatal Ed. 2005;90:F49–52. doi: 10.1136/adc.2003.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoresen M, Satas S, Løberg EM, et al. Twenty-four hours of mild hypothermia in unsedated newborn pigs starting after a severe global hypoxic-ischemic insult is not neuroprotective. Pediatr Res. 2001;50:405–11. doi: 10.1203/00006450-200109000-00017. [DOI] [PubMed] [Google Scholar]
- Maulik PK, Darmstadt GL. Community-based interventions to optimize early childhood development in low resource settings. J Perinatol. 2009;29:531–42. doi: 10.1038/jp.2009.42. [DOI] [PubMed] [Google Scholar]
- Carlo WA, Goudar SS, Pasha O, et al. Brain Research to Ameliorate Impaired Neurodevelopment-Home-Based Intervention Trial Committee and the National Institute of Child Health and Human Development Global Network for Women's and Children's Health Research Investigators Randomized trial of early developmental intervention on outcomes in children after birth asphyxia in developing countries. J Pediatr. 2013;162:705–712.e3. doi: 10.1016/j.jpeds.2012.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PH, Tudehope DI, Masel JP, et al. Perinatal hypoxic-ischaemic brain injury: prediction of outcome. Dev Med Child Neurol. 1993;35:965–73. doi: 10.1111/j.1469-8749.1993.tb11578.x. [DOI] [PubMed] [Google Scholar]
- Carli G, Reiger I, Evans N. One-year neurodevelopmental outcome after moderate newborn hypoxic ischaemic encephalopathy. J Paediatr Child Health. 2004;40:217–20. doi: 10.1111/j.1440-1754.2004.00341.x. [DOI] [PubMed] [Google Scholar]
- Pierrat V, Haouari N, Liska A, Thomas D, Subtil D, Truffert P. Groupe d'Etudes en Epidémiologie Périnatale Prevalence, causes, and outcome at 2 years of age of newborn encephalopathy: population based study. Arch Dis Child Fetal Neonatal Ed. 2005;90:F257–61. doi: 10.1136/adc.2003.047985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem M, Murray D, Boylan G, Dempsey EM, Ryan CA. Blood carbon dioxide levels and adverse outcome in neonatal hypoxic-ischemic encephalopathy. Am J Perinatol. 2010;27:361–5. doi: 10.1055/s-0029-1243309. [DOI] [PubMed] [Google Scholar]
- Locatelli A, Incerti M, Paterlini G, et al. Antepartum and intrapartum risk factors for neonatal encephalopathy at term. Am J Perinatol. 2010;27:649–54. doi: 10.1055/s-0030-1249761. [DOI] [PubMed] [Google Scholar]
- Pisani F, Orsini M, Braibanti S, Copioli C, Sisti L, Turco EC. Development of epilepsy in newborns with moderate hypoxic-ischemic encephalopathy and neonatal seizures. Brain Dev. 2009;31:64–8. doi: 10.1016/j.braindev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Todeschini A, Guidotti I, et al. General movements in full-term infants with perinatal asphyxia are related to Basal Ganglia and thalamic lesions. J Pediatr. 2011;158:904–11. doi: 10.1016/j.jpeds.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- van Kooij BJ, van Handel M, Nievelstein RA, Groenendaal F, Jongmans MJ, de Vries LS. Serial MRI and neurodevelopmental outcome in 9- to 10-year-old children with neonatal encephalopathy. J Pediatr. 2010;157:221–7.e2. doi: 10.1016/j.jpeds.2010.02.016. [DOI] [PubMed] [Google Scholar]
- L'Abee C, de Vries LS, van der Grond J, Groenendaal F. Early diffusion-weighted MRI and 1H-Magnetic Resonance Spectroscopy in asphyxiated full-term neonates. Biol Neonate. 2005;88:306–12. doi: 10.1159/000087628. [DOI] [PubMed] [Google Scholar]
- van Schie PE, Becher JG, Dallmeijer AJ, Barkhof F, Van Weissenbruch MM, Vermeulen RJ. Motor testing at 1 year improves the prediction of motor and mental outcome at 2 years after perinatal hypoxic-ischaemic encephalopathy. Dev Med Child Neurol. 2010;52:54–9. doi: 10.1111/j.1469-8749.2009.03302.x. [DOI] [PubMed] [Google Scholar]
- Battin MR, Penrice J, Gunn TR, Gunn AJ. Treatment of term infants with head cooling and mild systemic hypothermia (35.0 degrees C and 34.5 degrees C) after perinatal asphyxia. Pediatrics. 2003;111:244–51. doi: 10.1542/peds.111.2.244. [DOI] [PubMed] [Google Scholar]
- West CR, Harding JE, Knight DB, Battin MR. Demographic characteristics and clinical course in infants with moderate or severe neonatal encephalopathy. Aust N Z J Obstet Gynaecol. 2005;45:151–4. doi: 10.1111/j.1479-828X.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- Toh V, Rajadurai VS. Term infants with hypoxic ischaemic encephalopathy: poor neurodevelopmental outcome despite standard neonatal intensive care. J Trop Pediatr. 1999;45:229–32. doi: 10.1093/tropej/45.4.229. [DOI] [PubMed] [Google Scholar]
- Lipp-Zwahlen AE, Deonna T, Micheli JL, Calame A, Chrzanowski R, Cêtre E. Prognostic value of neonatal CT scans in asphyxiated term babies: low density score compared with neonatal neurological signs. Neuropediatrics. 1985;16:209–17. doi: 10.1055/s-2008-1059539. [DOI] [PubMed] [Google Scholar]
- Pfenninger J, Bachmann D, Wagner BP. Survivors with bad outcome after hypoxic-ischaemic encephalopathy: full-term neonates compare unfavourably with children. Swiss Med Wkly. 2001;131:267–72. doi: 10.4414/smw.2001.09717. [DOI] [PubMed] [Google Scholar]
- Barnett A, Mercuri E, Rutherford M, et al. Neurological and perceptual-motor outcome at 5 - 6 years of age in children with neonatal encephalopathy: relationship with neonatal brain MRI. Neuropediatrics. 2002;33:242–8. doi: 10.1055/s-2002-36737. [DOI] [PubMed] [Google Scholar]
- Miller SP, Latal B, Clark H, et al. Clinical signs predict 30-month neurodevelopmental outcome after neonatal encephalopathy. Am J Obstet Gynecol. 2004;190:93–9. doi: 10.1016/s0002-9378(03)00908-6. [DOI] [PubMed] [Google Scholar]
- Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11–7. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Pappas A, McDonald SA, et al. Eunice Kennedy Shriver NICHD Neonatal Research Network Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–92. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo CL, Tudehope DI. Outcome of resuscitated apparently stillborn infants: a ten year review. J Paediatr Child Health. 1994;30:129–33. doi: 10.1111/j.1440-1754.1994.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Muttitt SC, Taylor MJ, Kobayashi JS, MacMillan L, Whyte HE. Serial visual evoked potentials and outcome in term birth asphyxia. Pediatr Neurol. 1991;7:86–90. doi: 10.1016/0887-8994(91)90002-3. [DOI] [PubMed] [Google Scholar]
- Zhou WH, Cheng GQ, Shao XM, et al. China Study Group Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010;157:367–72, 372.e1. doi: 10.1016/j.jpeds.2010.03.030. [DOI] [PubMed] [Google Scholar]
- Zhu C, Kang W, Xu F, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:e218–26. doi: 10.1542/peds.2008-3553. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Pan KL, Zhao XL, Qiang H, Cheng SQ. [Therapeutic effects of erythropoietin on hypoxic-ischemic encephalopathy in neonates]. Zhongguo Dang Dai Er Ke Za Zhi. 2011;13:855–8. [PubMed] [Google Scholar]
- Lam BC, Yeung CY. Perinatal features of birth asphyxia and neurologic outcome. Acta Paediatr Jpn. 1992;34:17–22. doi: 10.1111/j.1442-200x.1992.tb00919.x. [DOI] [PubMed] [Google Scholar]
- George B, Padmam MS, Nair MK, Indira MS, Syamalan K, Padmamohan J. Hypoxic ischemic encephalopathy developmental outcome at 12 years. Indian Pediatr. 2009;46 Suppl:s67–70. [PubMed] [Google Scholar]
- McShane M, Maguire S, McClure G, Halliday H, McC Reid M. Birth asphyxia, encephalopathy and outcome. Ir Med J. 1987;80:421–2. [PubMed] [Google Scholar]
- Prechtl HF, Ferrari F, Cioni G. Predictive value of general movements in asphyxiated fullterm infants. Early Hum Dev. 1993;35:91–120. doi: 10.1016/0378-3782(93)90096-d. [DOI] [PubMed] [Google Scholar]
- Cerovac N, Petrović I, Klein C, Kostic V. Delayed-onset dystonia due to perinatal asyphxia: a propsective study. Mov Disord. 2007;22:2426–9. doi: 10.1002/mds.21747. [DOI] [PubMed] [Google Scholar]
- Mwakyusa SD, Manji KP, Massawe AW. The hypoxic ischaemic encephalopathy score in predicting neurodevelopmental outcomes among infants with birth asphyxia at the Muhimbili National Hospital, Dar-es-Salaam, Tanzania. J Trop Pediatr. 2009;55:8–14. doi: 10.1093/tropej/fmn061. [DOI] [PubMed] [Google Scholar]
- Hallioglu O, Topaloglu AK, Zenciroglu A, Duzovali O, Yilgor E, Saribas S. Denver developmental screening test II for early identification of the infants who will develop major neurological deficit as a sequalea of hypoxic-ischemic encephalopathy. Pediatr Int. 2001;43:400–4. doi: 10.1046/j.1442-200x.2001.01418.x. [DOI] [PubMed] [Google Scholar]
- Lawn JE, Yakoob MY, Haws RA, Soomro T, Darmstadt GL, Bhutta ZA. 3.2 million stillbirths: epidemiology and overview of the evidence review. BMC Pregnancy Childbirth. 2009;9 Suppl 1:S2. doi: 10.1186/1471-2393-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics Use and abuse of the apgar score. Pediatrics. 1996;98:141–2. [PubMed] [Google Scholar]
- Nelson KB, Ellenberg JH. Apgar scores as predictors of chronic neurologic disability. Pediatrics. 1981;68:36–44. [PubMed] [Google Scholar]
- MacLennan A. A template for defining a causal relation between acute intrapartum events and cerebral palsy: international consensus statement. BMJ. 1999;319:1054–9. doi: 10.1136/bmj.319.7216.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Child Health and Development: Health of the Newborn. Geneva, Switzerland; World Health Organization; 1991. [Google Scholar]
- World Health Organization's Maternal Health and Safe Motherhood Programme . Perinatal Mortality: A Listing of Available Information. Geneva, Switzerland; World Health Organization; 1996. [Google Scholar]
- Save the Children . State of the World's Newborn. Washington, DC; Save the Children; 2001. [Google Scholar]
- World Health Organization, International Conferderation of Midwives, International Federation of Gynaecology and Obstetrics World Health Organization Department of Reproductive Health and Research . Making Pregnancy Safer: The Critical Role of the Skilled Attendant. Geneva, Switzerland; World Health Organization; 2004. [Google Scholar]
- World Health Organization . World Health Report 2001. Geneva, Switzerland; World Health Organization; 2001. [Google Scholar]
- World Health Organization . World Health Report 2002. Geneva, Switzerland; World Health Organization; 2002. [Google Scholar]
- World Health Organization . World Health Report 2003. Geneva, Switzerland; World Health Organization; 2003. [Google Scholar]
- Lopez AD, Mathers C, Ezzati M, Jamison DT, Murray CJL. Global Burden of Disease and Risk Factors. Washington, DC; The International Bank for Reconstruction and Development/The World Bank Group; 2006. [PubMed] [Google Scholar]
- World Health Organization . The Global Burden of Disease 2004 Update. Geneva, Switzerland; World Health Organization; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.