Abstract
Human cortical activity has been intensively examined at frequencies ranging from 0.5 Hz to several hundred Hz. Recent studies have, however, reported also infraslow fluctuations in neuronal population activity, magnitude of electroencephalographic oscillations, discrete sleep events, as well as in the occurrence of interictal events. Here we use direct current electroencephalography to demonstrate large-scale infraslow oscillations in the human cortex at frequencies ranging from 0.02 to 0.2 Hz. These oscillations, which are not detectable in conventional electroencephalography because of its limited recording bandwidth (typical lower limit 0.5 Hz), were observed in widespread cortical regions. Notably, the infraslow oscillations were strongly synchronized with faster activities, as well as with the interictal epileptic events and K complexes. Our findings suggest that the infraslow oscillations represent a slow, cyclic modulation of cortical gross excitability, providing also a putative mechanism for the as yet enigmatic aggravation of epileptic activity during sleep.
Keywords: epilepsy, slow cortical oscillations, phase synchrony, direct current electroencephalography, full-band electroencephalography
A large body of literature has characterized functional and clinical correlates of cortical oscillatory activity seen in the human electroencephalography (EEG) at frequencies ranging from 0.5 Hz to several hundred Hz (1). In addition to these relatively fast EEG oscillations, some studies have demonstrated infraslow fluctuations in, for instance, neuronal population activity (2-5), EEG power (5-9), discrete sleep events (arousals, spindles, K complexes) (9-12), as well as in the occurrence of epileptic events (4, 11, 13). These observations have raised the possibility that the human cortex may generate infraslow oscillations (ISOs) underlying such fluctuations. ISOs are not detectable in conventional EEG because of its limited recording bandwidth (typical lower limit, 0.5 Hz; ref. 1).
In the present study, we used direct current (DC)-coupled EEG scalp recordings (14-16) to demonstrate that ISOs are, in fact, a salient, large-scale feature of the human EEG. They were tightly associated with a cyclic modulation of fast EEG activity as well as discrete EEG events such as K complexes and interictal activity. In analogy with recent studies demonstrating that slow delta oscillations (0.5-1 Hz) may modulate brain excitability (17, 18), our data points to a role of ISOs in the control of gross cortical excitability. Our findings also provide a window on the modulation of interictal epileptiform events (IIEs) and on sleep-epilepsy interactions in the human brain.
Methods
Subjects and EEG Recording. We studied 16 subjects (four females; average age, 34.3 years; range, 16-51 years) during overnight (n = 9) or daytime (n = 7) sleep. This study was approved by the Human Subjects Committee of the University of Washington. Twelve subjects were recorded using a custom-made 16-channel DC-coupled EEG amplifier and Ag/AgCl electrodes (refs. 14, 15, and 19; for further details, see Supporting Text and Fig. 4, which are published as supporting information on the PNAS web site). In two subjects, we used a 40-channel DC-coupled amplifier (NuAmps, Neuroscan, El Paso, TX) to analyze the global, spatio-temporal evolution of ISO. EEG was digitized at 250-500 Hz. The recording reference was placed near vertex or left mastoid. Fourteen subjects had epilepsy (eight with focal onset and six with primary generalized epilepsy). Their EEG recordings were performed simultaneously with a clinically indicated EEG examination (15). Two neurologically normal subjects were recorded to confirm that ISOs and their relationships to fast EEG oscillation and K complexes are also seen in nonepileptic subjects. All non-REM sleep was analyzed after dividing it into two categories, non-slow wave sleep (non-SWS; stages 1 and 2) and SWS (stages 3 and 4). Artifact-free epochs (n = 53; 10 subjects; length, 3-10 min) were exported for further analysis after low-pass filtering at 100 Hz (no high-pass filtering). An offline rereferencing to calculated linked mastoids was performed before further time series analysis. (See also Supporting Text.)
Signal Analysis. ISO was extracted from the EEG recordings (see Fig. 1) with pairs of finite impulse response (FIR) low-pass and high-pass filters. The low-pass passband was 0.1 Hz and the stopband was 0.2 Hz for analyses of ISOs vs. faster oscillations. These values were 0.05 Hz and 0.1 Hz for the analysis of ISO phase vs. occurrence of IIEs and K complexes. In all cases, the high-pass passband was 0.02 Hz and the stopband was 0.01 Hz. By performing all analyses also using median filtering before FIR low-pass filtering, we confirmed that the possible monophasic nature of, for example, delta waves did not introduce artefactual ISO deflections. The median filtering was computed in windows having widths of at least two times the delta wave basal width. For details of filtering used to isolate frequency bands above that of ISOs, see supporting information. Comparison of the ISO extracted with above filter parameters with a signal with lower or no high-pass filtering showed that the filtering used to extract ISOs did not impose artificial periodicity to the estimates of ISO phase as a function of time (see Fig. 3, Supporting Text, and Fig. 5, which is published as supporting information on the PNAS web site).
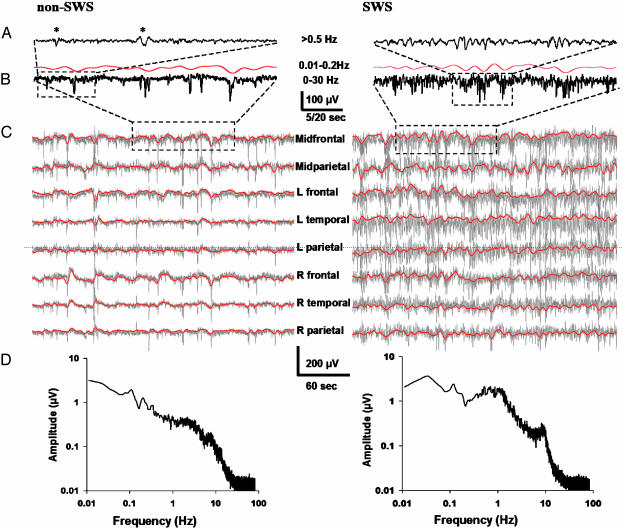
Fig. 1.
Infraslow oscillations during non-SWS (Left) and SWS (Right). (A) High-pass-filtered (>0.5 Hz; 30 s long) epoch during sleep. Asterisks depict K complexes. (B) A prominent infraslow oscillation is readily seen in the 2-min epoch in the full-bandwidth EEG signal (black), as well as with bandpass filtering at 0.01-0.2 Hz (red). (C) Five-minute recordings from various scalp locations demonstrates ISOs in all of them (gray traces at full bandwidth, superimposed red traces with bandpass filtering at 0.01-0.2Hz). (D) Amplitude spectra for non-SWS and SWS, representing averages of three 180-s segments (90-s Hanning window; 60-Hz mains artifact removed) from one representative subject each. In all figures, EEG traces are shown against a calculated linked mastoid reference, and negative deflections are downward.
The correlation between ISOs and faster EEG oscillations was quantified by using two approaches. In the first approach (see Fig. 2 A and B), the amplitude envelopes of frequency bands at 0.5-1, 8-11, 11-15, and 1-100-Hz were compared with the instantaneous levels of ISO. FIR filtering was used to obtain the real part of the band-limited activity, and the Hilbert transform was used to obtain the corresponding imaginary part. The modulus and angle of the thereby obtained complex vector represent the amplitude and phase of that frequency band as a function of time (see Fig. 2A). These amplitude envelope values were sorted sample-by-sample according to the concurrent ISO voltages (i.e., the real values of the ISO-bandpass filtered EEG-signal) and averaged into eight bins corresponding to eight percentiles of ISO voltages (see x axes in Fig. 2B). Note that the ISO voltages were “instantaneous” only with the temporal precision allowed by the used band-pass filtering. Conversely, we also binned and averaged the ISO voltages as a function of the percentiles of amplitude envelope values (data not shown, as the relationship turned out to be monotonic). The histograms were normalized with the respective maximum variations of both variables estimated by binning and averaging the ISO voltages as a function of the percentiles of the ISO voltages, and likewise for the amplitude envelope values. After normalization, the histogram values deviating from zero indicate negative or positive correlation (see y axes in Fig. 2B), and the sum over the absolute values of the histogram X(Y) ranges from 0 to 1 and reflects the dependency of X of Y, and conversely for Y(X). The confidence intervals (±2 SD) were obtained by using an identical procedure for surrogate data (signal X vs. 20 time-shifted signals Y). The angle of the regression line in the histogram was estimated for both the real histograms and the ±2 SD confidence intervals. Statistical significances were assessed with t test by comparing the Spearman's correlation coefficients of the real and surrogate data histograms (ISO vs. >1 Hz; see also Fig. 2B), as well as with binomial statistics to evaluate whether significant findings (based on confidence limits) in individual subjects can be considered statistically significant on the group level. For a second approach, we used a method for the detection of nested oscillations (see Fig. 2 A and C). The amplitude envelopes of the frequency bands at 0.5-1, 1.5-4, 4-8, and 7-18 Hz were obtained after bandpass filtering using the Hilbert transform. These amplitude envelopes were filtered with the filters used for the extraction of ISO, and the phase of the amplitude modulation, as well as the phase of the ISO, were again obtained with the Hilbert transform. The two signals were considered phase locked if their phase difference was not uniformly distributed (see Supporting Text).
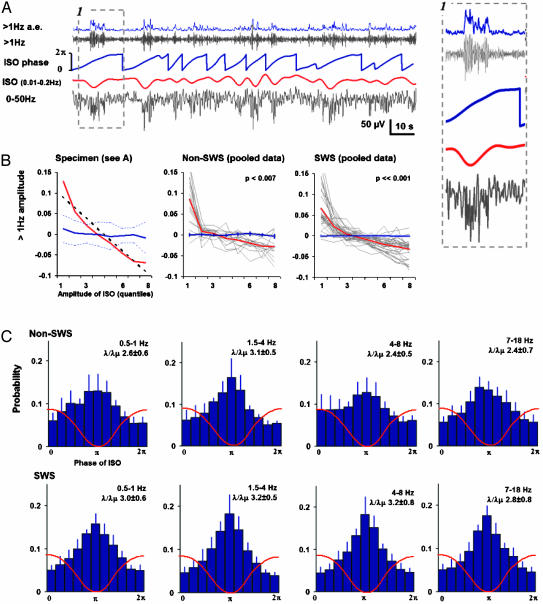
Fig. 2.
Correlation between ISOs and faster EEG oscillations. (A) Extraction of infraslow (red) and faster oscillations using bandpass filtering (upper gray trace shows an example with high-pass filtering at 1 Hz). The amplitude envelope of the faster oscillations (a.e.; blue trace, Top) and the phase of ISO (blue trace, Middle) were obtained with the Hilbert transform. A blown-up figure of one ISO cycle (stippled line box) is demonstrated on the right side of the traces. (B) Correlation histogram for the epoch in A (Left), demonstrating that the instantaneous level of ISO voltage is clearly correlated with the (normalized) amplitude envelope of the >1 Hz band. Red line depicts the correlation histogram, the thick blue stippled line depicts its linear regression, and the solid blue line depicts surrogate data with the thin blue stippled lines representing its ± 2 SD confidence intervals. The two other graphs (Center and Right) show the correlation histograms for all sleep epochs analyzed from non-SWS and SWS (gray lines depict individual epochs, red is their average, and the blue line represents the mean of the surrogates (±SEM). (C) Histograms demonstrating that the amplitudes in all frequency bands (as indicated in each plot) are strongly correlated with the phase (x axis) of ISO during both non-SWS (Upper) and SWS (Lower). A schematic ISO wave is superimposed on the histograms. Values next to λ/λμ are the PLFreal/PLFshuffled,mean ratios (±SEM) averaged over subjects, indicating the statistical significance of the phase locking. (λ/λμ > 2.41 corresponds to P < 0.01, and λ/λμ > 2.94 corresponds to P < 0.001 in individual subjects.)
Phase Locking of Interictal Epileptiform Events and K Complexes to ISO. Periods with visually prominent ISO in midfrontal derivations during non-SWS sleep were identified from recordings. The IIEs (spikes and discharges; n = 238; eight subjects; average spike count per patient was 30, range 11-97) were identified from the simultaneously registered conventional EEG. The K complexes (n = 383; seven subjects) were identified from the same DC-EEG recordings by using a viewing window short enough (10-15 s) to ensure that the reader was unaware of the background ISO. Phase of ISO detected in the EEG signal at the derivation midfrontal vs. linked mastoid was obtained with the Hilbert transform as above (see Fig. 3). The latencies of occurrence of both IIEs and K complexes were then plotted against the phase of ISO and the significance of phase locking was estimated both for each subject and for data pooled over subjects (see Fig. 3 and Supporting Text).
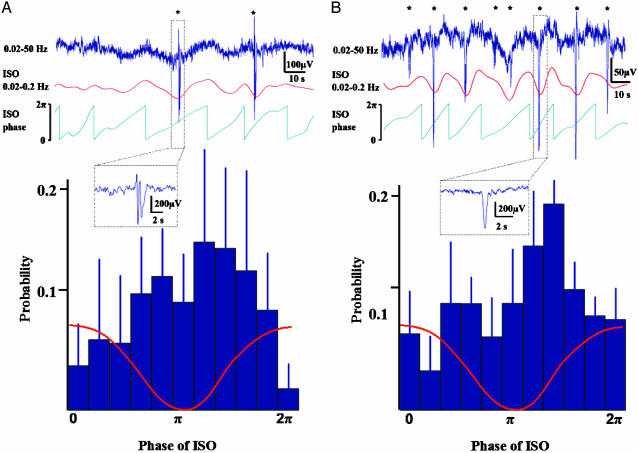
Fig. 3.
Phase locking of interictal events (A) and K complexes (B) to infraslow oscillations. Traces in the upper part demonstrate 2 min epochs of the raw data (blue traces on top) during non-SWS with repeated interictal spikes and K complexes (see the Insets in A and B, respectively), as well as the bandpass-filtered ISO (red) and its phase (green). In the histograms, the occurrence of IIEs (n = 238; six subjects) and K complexes (n = 383; seven subjects) is plotted against the phase of ISO, revealing highly significant locking of both phenomena to the phase of ISO. A schematic ISO wave is superimposed on the histograms.
Results
Visual inspection of the EEG revealed prominent slow oscillations in all electrodes with amplitudes of up to several tens of microvolts and frequencies at ≈0.02-0.2 Hz (Fig. 1 and Fig. 6, which is published as supporting information on the PNAS web site). A closer examination of the spatio-temporal characteristics of ISOs with source modeling showed that the slow waves were seen in widespread cortical areas. Within the accuracy of cortical current reconstruction obtainable from a limited number of scalp signals, it seemed that the apparent movements of ISOs occurred within few seconds between brain areas, without any visually obvious spatial propagation patterns (see Supporting Text, Movie 1, and Fig. 7, which are published as supporting information on the PNAS web site).
A highly significant correlation was found between the instantaneous level of ISO voltage and the amplitude of EEG at higher (1-100 Hz) frequencies (pooled data P < 0.001; Fig. 2 A and B) during both SWS and non-SWS. We also found a robust phase locking between the ISO and the amplitude fluctuations over a wide range of frequencies as illustrated for the bands at 0.5-1 Hz, 1.5-4 Hz, 4-8 Hz, and 7-18 Hz (Fig. 2C). In both analyses, the faster EEG activity had its highest amplitude during the negative deflection of the ISO wave (phase ≈1 π).
The observations above raise the idea that ISOs might reflect fluctuations of gross network excitability, akin to the up- and down-states recently described for oscillations at low delta frequencies (0.5-1 Hz; refs. 17, 18, and 20). Hence, one might expect that, in addition to the faster spontaneous oscillations, also phasic events, including pathophysiological ones, are linked to ISOs. To address this question, we identified 238 IIEs from eight subjects during non-SWS. Strikingly, we found a significant phase locking of IIEs to ISOs in seven of eight subjects (Fig. 3A; P < 0.05 for each subject; three focal and four generalized epilepsies, combined Pbinomial < 6 × 10-9). In six of these seven subjects, the IIEs had a highly preferential occurrence during the negative peak of the ISO (grand average mean phase 1.02 π; in the seventh subject the mean phase was 0.39 π; for data of each individual, see Supporting Text and Fig. 8, which is published as supporting information on the PNAS web site).
If the occurrence of IIEs is modulated by cyclic changes in cortical excitability, a similar modulation of other types of phasic events should take place. To address this hypothesis, we took advantage of the fact that the K complex, which is a frequent phasic EEG event during sleep, has been shown to arise from slow delta frequency waves (21). Notably, however, it is unclear why only some delta waves manifest themselves as K complexes. In the light of the results above, a plausible explanation could be that periodic increases in cortical excitability at the ISO frequency lead to the conversion of brief oscillations (delta waves) into more prolonged ones (K complexes). We thus identified visually 383 K complexes from seven subjects and quantified the relationship between their occurrence and the phase of ISO. Indeed, the K complexes were strongly synchronized with ISOs and, as hypothesized, occurred preferentially during the negative deflection of ISO waves (Fig. 3B; mean phase 1.08 π; P < 0.01 for pooled data; P < 0.05 in five of seven subjects, Pbinomial < 6 × 10-6).
Discussion
A meaningful interpretation of the mechanisms and functions of distinct neuronal events can be achieved only in the context of the ongoing large-scale activity (22-24), taking into account the full bandwidth of cortical oscillation, which include both very fast (25) and infraslow (ref. 16 and this work) frequencies. So far, the lower frequencies have been largely ignored for technical reasons (14, 15, 19, 26). The ISOs described in the present work have properties that are unprecedented in the human EEG literature: a very low frequency of 0.02-0.2 Hz and a slow apparent propagation over cortical hemispheres.
The phase of ISO was robustly correlated with the magnitude of faster EEG oscillations, as well as with the occurrence of IIEs and K complexes, which suggests that ISOs reflect a slow fluctuation in gross cortical excitability. This may explain previous findings in humans (3), monkeys (2), cats (5), and rats (4, 13), which have demonstrated that neuronal population activities fluctuate at ≈0.1 Hz, and that these fluctuations are coherent over long distances (2, 8). The amplitude of faster EEG oscillations (5-7, 9), as well as the occurrence of discrete physiological EEG events (e.g., K complexes or arousals; refs. 9, 10, and 12) and IIEs (4, 11, 13) have all been shown to exhibit fluctuations at 0.025-0.3 Hz.
Although sleep is a major precipitator of epileptic activity, the intervening mechanisms have remained enigmatic (27). Studies on animal models have suggested that the synchronous slow delta oscillations predispose brain to epileptic activity (17, 28). However, clinical studies have demonstrated a high occurrence of IIEs also during non-SWS (27, 29, 30), a state characterized by a low amount of delta frequency oscillations, calling hence for explanations other than those solely related to delta oscillations of SWS. Here, the present finding of a robust phase locking between ISOs and IIEs during non-SWS is of particular clinical interest. It is likely that further work on ISOs will promote the design of novel diagnostic and therapeutic strategies in the treatment of epilepsy.
In view of their global nature, ISOs may provide useful electrophysiological information on the elusive “activity baseline” required in the interpretation of functional MRI data (31). Earlier studies have indicated that the cortical evoked responses (32), as well as cognitive performance (24, 33), oscillate at an infraslow rate. It may thus be that the ISO introduced in our study is not restricted to sleep states, but it may rather reflect a continuous oscillatory behavior of wide range of brain functions during both sleep and awakeness (see also ref. 2).
A recent in vitro (20) study with cortical slices and in vivo study with deafferented cortical slabs (34) demonstrated that deep cortical layers may exhibit self-sustaining infraslow oscillations. However, the large amplitude and spatial extent, as well as the long duration of individual ISO waveforms, suggest that generation of ISO likely involves inputs from subcortical structures (ref. 35; see also ref. 2). In addition, multiple lines of evidence have pointed to a generation of large-scale, slow electric signals by nonneuronal sources, such as the glial cells (17, 36-38) and the blood-brain barrier (see refs. 14 and 39 and references therein). Hence, it is plausible to propose that generation of ISO may involve multiple intracranial structures and mechanisms.
Supplementary Material
Acknowledgments
This study was supported by The Academy of Finland, The Sigrid Juselius Foundation, the Arvo and Lea Ylppö Foundation (Finland), and the Regional Epilepsy Center.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: EEG, electroencephalography; ISO, infraslow oscillation; DC, direct current; IIE, interictal epileptiform event; FIR, finite impulse response; SWS, slow wave sleep.
References
- 1.Niedermeyer, E. & Lopes da Silva, F., eds. (1999) Electroencephalography: Basic Principles, Clinical Applications, and Related Fields (Williams & Wilkins, Baltimore).
- 2.Leopold, D. A., Murayama, Y. & Logothetis, N. I. (2003) Cereb. Cortex 13, 422-433. [DOI] [PubMed] [Google Scholar]
- 3.Staba, R. J., Wilson, C. L., Bragin, A., Fried, I. & Engel, J., Jr. (2002) J. Neurosci. 22, 5694-5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penttonen, M., Nurminen, N., Miettinen, R., Sirvio, J., Henze, D. A., Csicsvari, J. & Buzsaki, G. (1999) Neuroscience 94, 735-743. [DOI] [PubMed] [Google Scholar]
- 5.Steriade, M., Nunez, A. & Amzica, F. (1993) J. Neurosci. 13, 3252-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon, N. R., Manshanden, I. & Lopes da Silva, F. H. (2000) Brain Res. 860, 64-76. [DOI] [PubMed] [Google Scholar]
- 7.Linkenkaer-Hansen, K., Nikouline, V. V., Palva, J. M. & Ilmoniemi, R. J. (2001) J. Neurosci. 21, 1370-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bullock, T. H., McClune, M. C., Achimowicz, J. Z., Iragui-Madoz, V. J., Duckrow, R. B & Spencer, S. S. (1995) Proc. Natl. Acad. Sci. USA 92, 11568-11572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Achermann, P. & Borbely, A. A. (1997) Neuroscience 81, 213-222. [DOI] [PubMed] [Google Scholar]
- 10.Terzano, M. G., Mancia, D., Salati, M. R., Costani, G., Decembrino, A. & Parrino, L. (1985) Sleep 8, 137-145. [DOI] [PubMed] [Google Scholar]
- 11.Parrino, L., Smerieri, A., Spaggiari, M. C. & Terzano, M. G. (2000) Clin. Neurophysiol. 111, Suppl. 2, S39-S46. [DOI] [PubMed] [Google Scholar]
- 12.Amzica, F. & Steriade, M. (1997) Neurology 49, 952-959. [DOI] [PubMed] [Google Scholar]
- 13.Jando, G., Carpi, D., Kandel, A., Urioste, R., Horvath, Z., Pierre, E., Vadi, D., Vadasz, C. & Buzsaki, G. (1995) Neuroscience 64, 301-317. [DOI] [PubMed] [Google Scholar]
- 14.Voipio, J., Tallgren, P., Heinonen, E., Vanhatalo, S. & Kaila, K. (2003) J. Neurophysiol. 89, 2208-2214. [DOI] [PubMed] [Google Scholar]
- 15.Vanhatalo, S., Holmes, M. D., Tallgren, P., Voipio, J., Kaila, K. & Miller, J. W. (2003) Neurology 60, 1098-1102. [DOI] [PubMed] [Google Scholar]
- 16.Vanhatalo, S., Voipio, J. & Kaila, K. (2004) in Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, eds. Niedermeyer, E. & Lopes da Silva, F. (Williams & Wilkins, Baltimore), in press.
- 17.Amzica, F. & Steriade, M. (2000) J. Neurosci. 20, 6648-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massimini, M., Rosanova, M. & Mariotti, M. (2003) J. Neurophysiol. 89, 1205-1213. [DOI] [PubMed] [Google Scholar]
- 19.Vanhatalo, S., Tallgren, P., Andersson, S., Sainio, K., Voipio, J. & Kaila, K. (2002) Clin. Neurophysiol. 113, 1822-1825. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Vives, M. V. & McCormick, D. A. (2000) Nat. Neurosci. 3, 1027-1034. [DOI] [PubMed] [Google Scholar]
- 21.Amzica, F. & Steriade, M. (1998) Neuroscience 82, 671-686. [DOI] [PubMed] [Google Scholar]
- 22.Arieli, A., Sterkin, A., Grinvald, A. & Aertsen, A. (1996) Science 273, 1868-1871. [DOI] [PubMed] [Google Scholar]
- 23.Steriade, M. (2001) J. Neurophysiol. 86, 1-39. [DOI] [PubMed] [Google Scholar]
- 24.Gilden, D. L. (2001) Psychol. Rev. 108, 33-56. [DOI] [PubMed] [Google Scholar]
- 25.Traub, R. D., Jefferys, J. G. R. & Whittington, M. A. (1999) Fast Oscillations in Cortical Circuits (MIT Press, Detroit).
- 26.Lagerlund, T. D. & Gross, R. A. (2003) Neurology 60, 1062-1063. [DOI] [PubMed] [Google Scholar]
- 27.Bazil, C. W., Malow, B. A. & Sammaritano, M. R. (2002) Sleep and Epilepsy: The Clinical Spectrum (Elsevier, Amsterdam).
- 28.Steriade, M. & Contreras, D. (1995) J. Neurosci. 15, 623-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herman, S. T., Walczak, T. S. & Bazil, C. W. (2001) Neurology 56, 1453-1459. [DOI] [PubMed] [Google Scholar]
- 30.Malow, B. A., Lin, X., Kushwaha, R. & Aldrich, M. S. (1998) Epilepsia 39, 1309-1316. [DOI] [PubMed] [Google Scholar]
- 31.Gusnard, D. A. & Raichle, M. E. (2001) Nat. Rev. Neurosci. 2, 685-694. [DOI] [PubMed] [Google Scholar]
- 32.Prince, D. A. (1965) Electroenceph. Clin. Neurophysiol. 19, 139-151. [DOI] [PubMed] [Google Scholar]
- 33.Gilden, D. L., Thornton, T. & Mallon, M. W. (1995) Science 267, 1837-1839. [DOI] [PubMed] [Google Scholar]
- 34.Timofeev, I., Grenier, F., Bazhenov, M, Sejnowski, T. J. & Steriade, M. (2000) Cereb. Cortex 10, 1185-1199. [DOI] [PubMed] [Google Scholar]
- 35.Usher, M., Cohen, J. D., Servan-Schreiber, D., Rajkowski, J. & Aston-Jones, G. (1999) Science 283, 549-554. [DOI] [PubMed] [Google Scholar]
- 36.Laming, P. R., Kimelberg, H., Robinson, S., Salm, A., Hawrylak, N., Muller, C., Roots, B & Ng, K. (2000) Neurosci. Biobehav. Rev. 24, 295-340. [DOI] [PubMed] [Google Scholar]
- 37.Kivi, A., Lehmann, T. N., Kovacs, R., Eilers, A., Jauch, R., Meencke, H. J., von Deimling, A. & Heunemann, U. (2000) Eur. J. Neurosci. 12, 2039-2048. [DOI] [PubMed] [Google Scholar]
- 38.Dietzel, I., Heinemann, U. & Lux, H. D. (1989) Glia 2, 25-44. [DOI] [PubMed] [Google Scholar]
- 39.Vanhatalo, S., Tallgren, P., Becker, C., Holmes, M., Miller, J., Voipio, J. & Kaila, K. (2003) Clin. Neurophysiol. 114, 1744-1754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.