Abstract
Amyloid β1-42 (Aβ1-42), total tau (t-tau), and phosphorylated tau (p-tau) are the main cerebrospinal fluid (CSF) biomarkers for early diagnosis of Alzheimer’s disease (AD). Detection of AD is critically important in view of the growing number of potential new drugs that may influence the course of the disease in its early phases. However, cut-off levels for these CSF biomarkers have not yet been established. Variability in absolute concentrations of AD biomarkers is high among studies and significant differences were noticed even within the same datasets. Variability in biomarkers levels in these assays may be due to many aspects of operating procedures. Standardization of pre-analytical and analytical procedures in collection, treatment, and storage of CSF samples is crucial because differences in sample handling can drastically influence results. Multicenter studies showed that usage of ELISA kits from different manufacturers also affects outcome. So far only very few studies tested the efficiency of ELISA kits produced by different vendors. In this study, the performance of Innogenetics (Gent, Belgium) and Invitrogen (Camarillo, CA, USA) ELISA kits for t-tau and Aβ1-42 was tested. Passing-Bablok analysis showed significant differences between Invitrogen and Innogenetics ELISA methods, making it impossible to use them interchangeably.
Keywords: Alzheimer’s disease, Amyloid β1-42, Biomarkers, Cerebrospinal fluid, ELISA, Standardization, Tau proteins
1. Introduction
Alzheimer’s disease (AD) is the major primary cause of dementia. Ferri and collaborators estimated that 24 million people suffered from dementia in 2005, with this number reaching 81 million by 2040 [1]. Clinical diagnosis of AD, which is still based on symptomatology, is accurate in only 63 to 90% of dementia cases [2]. A growing number of potential treatments for AD are in different phases of preclinical and clinical research and thus much effort is dedicated to identify reliable biomarkers to enable an accurate diagnosis of AD.
Three main cerebrospinal fluid (CSF) biomarkers of AD, amyloid β1-42 (Aβ1-42), total tau (t-tau), and phosphorylated forms of tau (p-tau) reflect two major neuropathological hallmarks of AD - neurofibrillary tangles and senile plaques [3]. These CSF biomarkers are altered in early stages of AD, even before the occurrence of the first dementia symptoms, and permit to differentiate patients with prodromal AD (i.e. those with mild cognitive impairment, MCI) who often progress to AD, from healthy controls [4,5]. CSF biomarkers are also used for differentiation of AD from other primary causes of dementia, such as vascular dementia, frontotemporal dementia (FTD), and dementia with Lewy bodies [6–10]. Reduction of Aβ1-42 in CSF of AD patients is explained by Aβ1-42 aggregation into senile plaques, increase of t-tau reflects neuronal degeneration, while elevation of p-tau is a consequence of neurofibrillary degeneration and consequent tangles formation in the brain [11–13]. Although numerous studies in which diagnostic accuracy of CSF biomarkers was analyzed have been published, an ideal biomarker (with specificity and sensitivity over 85%) could not yet be defined.
High variability in concentrations of CSF biomarkers is observed among different centers and laboratories [14–16]. Causes of variations could be either due to pre-analytical and analytical factors or differences in ELISA kits from various manufacturers. Pre-analytical procedures refer to selection of research participants, CSF sampling and treatment, sample storage (temperature, tube type), and freeze/thaw cycles [7,17]. Analytical factors that influence results include differences in laboratory procedures among different centers [18]. Variability among ELISA kits from different manufacturers is due to differences in production processes of reagents (e.g., usage of different materials for reagents, preparation of standards, antibody purification, and plate coating). Lot-to-lot variability among assays of the same kit is also an issue. Post-analytical procedures such as curve-fitting type, curve-fitting software, and number of samples analyzed (usually singlets or duplicates) can also affect outcome [16].
There are insufficient data on comparability of ELISA kits developed by different vendors. This study compares the performance of Innogenetics (Gent, Belgium) and Invitrogen (Camarillo, CA, USA) ELISA kits for t-tau and Aβ1-42. Analyses were performed in the Laboratory for Developmental Neuropathology (LDN), Croatian Institute for Brain Research, University of Zagreb Medical School, Zagreb, Croatia, and in the Laboratory for Neurobiochemistry (LNB), Department of Laboratory Diagnostics, University Hospital Centre, Zagreb, Croatia.
2. Materials and methods
2.1. Pre-analytical procedures
All patients with suspected dementia were recruited from the University Hospital Centre, Zagreb, underwent complete blood tests including electrolytes, albumin, thyroid function, levels of vitamin B12, VDRL test for syphilis, Mini-Mental State Examination (MMSE), and neurological examination [19]. After exclusion of patients with secondary causes of dementia, selected 90 patients, upon signing the informed consent, underwent lumbar puncture. Out of these, 55 patients fulfilled NINCDS-ADRDA criteria for probable AD, 33 patients suffered from MCI, while 2 patients fulfilled criteria for FTD and corticobasal degeneration (CBD) [20,21]. Additionally, eight healthy control subjects (HC) with no evidence of dementia, or neurologic and psychiatric symptoms, were included. CSF was taken in the L3/L4 or L4/L5 intervertebral spaces, always between 9 a.m. and 11 a.m., and collected in polypropylene tubes. Leukocyte and erythrocyte cell counts, lactate, glucose, total protein concentration, Treponema Pallidum Hemagglutionation Assay (TPHA), and IgG index were also determined in native CSF. At LNB, CSF samples were centrifuged for 10 minutes at 4,000 g, dispensed into 150 μl aliquots and stored at −80°C. At LDN all pre-analytical procedures were exactly the same except for centrifugation (10 minutes at 2,000 g).
2.2. Analytical procedures
CSF t-tau levels were determined on 58 CSF samples of 36 AD patients, 19 patients with MCI and 3 control subjects by using ELISA kits. Among these 36 AD patients, Aβ1-42 concentrations were determined in CSF samples from 32 patients, plus one AD patient that didn’t have determined levels of t-tau. Four AD patients didn’t have determined Aβ1-42 levels. Concentration of Aβ1-42 was not measured in samples of either MCI patients or HC. Invitrogen ELISA kits were used at LDN (Tau/Total/Human ELISA Kit, Aβ1-42 Human ELISA Kit), while at LNB analyses were performed using Innogenetics ELISA kits (Innotest hTau-Ag, Innotest β-amyloid1-42). In both laboratories, the analyses were done on CSF samples of the same patients, only using different ELISA kits. Additionally, t-tau levels were determined on 39 CSF samples of 18 AD patients, 14 patients with MCI, 2 patients with FTD and CBD, and 5 HC using Innogenetics ELISA kits in both laboratories. ELISA analyses were performed according to the manufacturers’ protocols in both laboratories. At LNB washing was performed manually, while t-tau and Aβ1-42 concentrations were calculated on plate reader using curve-fitting software and 4-parameter algorithm. At LDN plates were washed in an automatic washer. Protein concentrations were determined using the same algorithm in GraphPad Prism 5.0 demo version software (San Diego, CA, USA).
2.3. Statistical analysis
T-tau levels among AD, MCI, and HC were compared using a Kruskal-Wallis test, followed by the Mann-Whitney U test for pairwise comparisons. Concentrations of Aβ1-42 and t-tau obtained by Innogenetics and Invitrogen ELISA kits were compared with the Passing-Bablok method [22]. Levels of proteins measured by both methods in each group (AD, MCI, or HC) were compared using Wilcoxon matched pairs test. Statistical analyses were performed with SPSS 19.0.1 (SPSS Inc., Chicago, IL, USA) and MedCalc 12.4.0.0 (Mariakerke, Belgium). P values less than 0.05 were considered statistically significant.
3. Results
Demographic data of all patient groups including HC are presented in Table 1. T-tau and Aβ1-42 CSF concentrations obtained by both Innogenetics and Invitrogen ELISA kits are summarized in Table 2 and Figure 1, and Table 3 and Figure 2, respectively. Protein levels are expressed as means, medians, and percentile ranges (P25–P75).
Table 1.
Demographic data of groups included in this study with MMSE scores.
| Group | Age Mean ± SD (Range) |
Gender Women vs. Men |
MMSE Mean ± SD (Range) |
|---|---|---|---|
| AD (n = 55) | 73.3 ± 6.5 | 29 vs. 26 | 19.5 ± 4.7 |
| MCI (n = 33) | 67.1 ± 11.3 | 20 vs. 13 | 24.9 ± 3.1 |
| HC (n = 8) | 58.8 ± 19.7 | 5 vs. 3 | 27.8 ± 2.4 |
AD – Alzheimer’s disease, MCI – Mild cognitive impairment, HC – healthy control, MMSE – Mini-Mental State Examination.
Table 2.
Levels of t-tau measured by Innogenetics and Invitrogen ELISA kits in cerebrospinal fluid. Data are presented as mean ± SD and median (25th–75th percentile).
| AD (n = 36) | MCI (n = 19) | HC (n = 3) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (P25–P75) | Mean ± SD | Median (P25–P75) | Mean ± SD | Median (P25–P75) | |
| Innogenetics t-tau | 427.5 ± 350.2 | 325.5 (235.3–504) | 242 ± 146.9 | 222 (100–329) | 101 ± 50.1 | 81 (64–158) |
| Invitrogen t-tau | 335.3 ± 216.9 | 279.2 (179.5–354.8) | 211.2 ± 105.8 | 206 (123.6–290) | 136.5 ± 51.3 | 112 (102–195.4) |
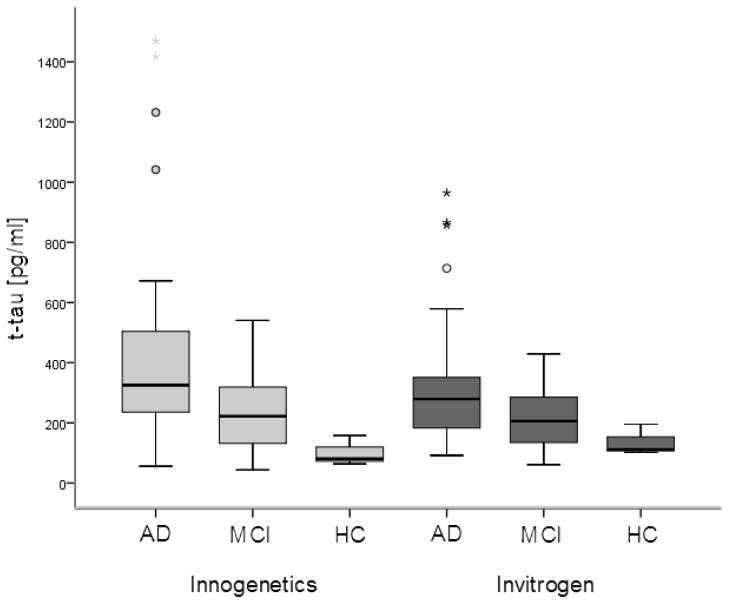
Figure 1.
CSF t-tau levels in AD, MCI and HC subjects measured by Innogenetics and Invitrogen ELISA kit. Boxes represent the median, the 25th and the 75th percentiles, bars indicate the range of data distribution. Circles represent outliers (values more than 1.5 box-length from the 75th/25th percentile). The asterisks represent extreme values (value more than three box-length from the 75th/25th percentile).
Table 3.
Levels of Aβ1-42 measured by Innogenetics and Invitrogen ELISA kits in cerebrospinal fluid. Levels of Aβ1-42 were only determined on samples of AD patients. Data are presented as mean ± SD and median (25th–75th percentile).
| AD (n = 33) | ||
|---|---|---|
| Mean ± SD | Median (P25–P75) | |
| Innogenetics Aβ1-42 | 660.7 ± 298.9 | 591 (432–799) |
| Invitrogen Aβ1-42 | 165.2 ± 114.3 | 140.6 (98.65–204.45) |
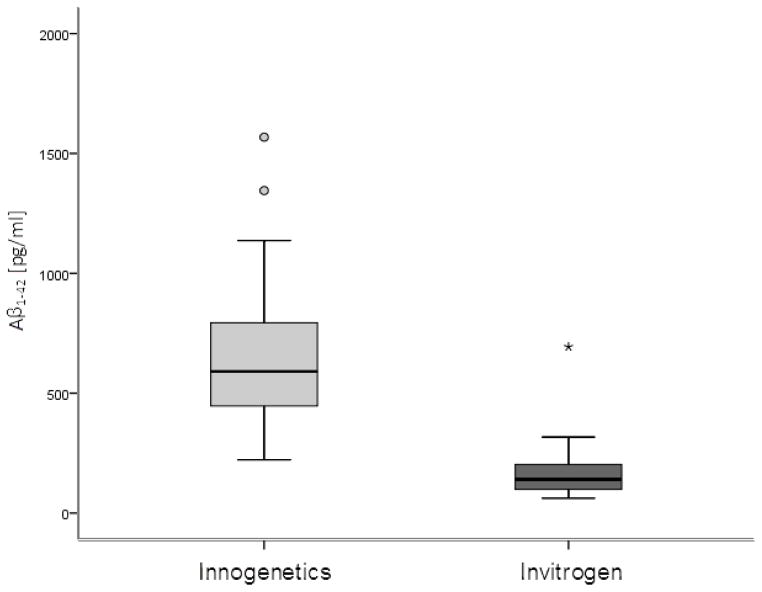
Figure 2.
CSF Aβ1-42 levels in AD patients measured by Innogenetics and Invitrogen ELISA kit. Boxes represent the median, the 25th and the 75th percentiles, bars indicate the range of data distribution. Circles represent outliers, the asterisk represents an extreme datapoint.
There was a significant difference in t-tau levels measured by Innogenetics ELISA among AD, MCI, and HC groups (χ2 = 9.625, df = 2, p = 0.008). T-tau levels were significantly higher in AD patients than in either MCI patients (U = 215.5, Z = −2.239, p = 0.025) or HC (U = 9, Z = −2.372, p = 0.012), but did not differ significantly among MCI and HC (p > 0.05). There was also a significant difference in t-tau levels measured by the Invitrogen kit among all groups (χ2 = 9.1, df = 2, p = 0.011). The difference was again significant between AD and MCI group (U = 209.5, Z = −2.342, p = 0.019), or AD and HC (U = 12.5, Z = −2.187, p = 0.022), while differences in t-tau levels did not reach significance between MCI and HC groups (p > 0.05).
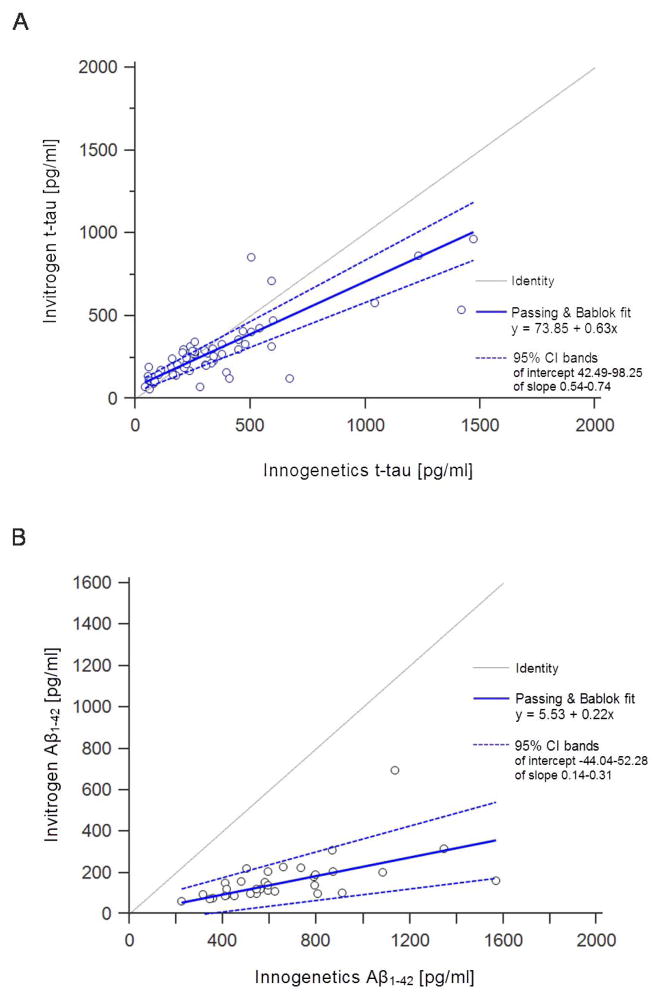
No significant difference in levels of t-tau measured by Invitrogen and Innogenetics ELISA kits was obtained on samples of AD patients (Z = −1.932, p = 0.053), MCI patients (Z = −0.684, p = 0.494) or HC (Z = −1.604, p = 0.109) (Figure 1). Levels of Aβ1-42 measured by the Innogenetics kit were significantly higher than those obtained with the Invitrogen kit (Z = −5.012, p < 0.001) (Figure 2). The results of Passing-Bablok analysis for measurement of agreement between Innogenetics and Invitrogen ELISA kits for t-tau and Aβ1-42 levels are shown in Figure 3A and 3B, respectively. The resulting equation of Passing-Bablok analysis for t-tau was y = 73.85 + 0.63x (95% confidence interval [CI] of intercept 42.49–98.25, and slope 0.54–0.74). Passing- Bablok analysis for Aβ1-42 kits resulted in y = 5.53 + 0.22x (95% CI of intercept −44.04–52.28, and slope 0.14–0.31). Cumulative sum (CUSUM) test for linearity showed no significant deviation from linearity for either t-tau (p = 0.76) or Aβ1-42 (p = 0.93). Figure 4 shows results of Passing-Bablok analysis for t-tau measured by Innogenetics ELISA kit at LDN and LNB. The equation for Passing-Bablok regression was y = 2.54 + 0.99x with 95% CI of intercept −17.89–22.05, and slope 0.93–1.05. Significant deviation from linearity was revealed by a CUSUM linearity test (p = 0.02).
Figure 3.
Results of the Passing-Bablok analysis between Innogenetics and Invitrogen ELISA for (A) t-tau and (B) Aβ1-42. Note that levels of both t-tau and Aβ1-42 measured by the Innogenetics kit were significantly higher than those obtained with the Invitrogen kit.
Figure 4.
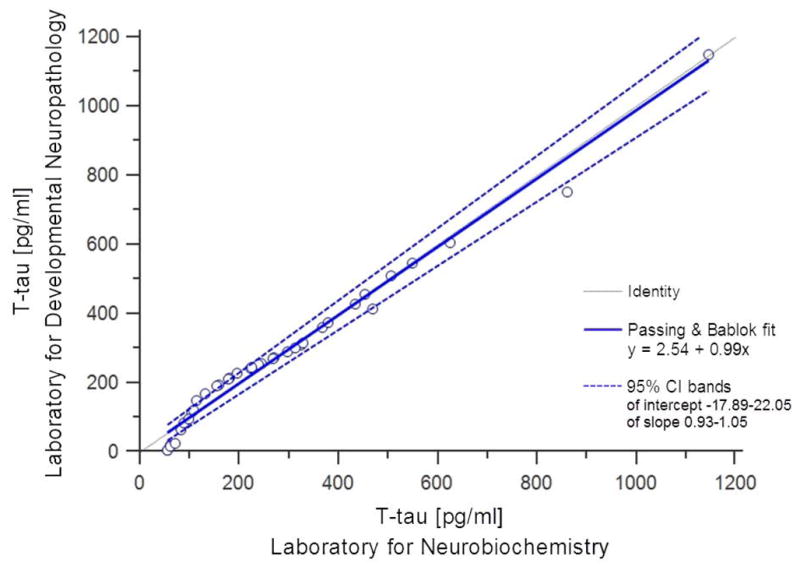
Results of the Passing-Bablok analysis between Innogenetics ELISA for t-tau performed in the Laboratory for Neurobiochemistry (Department of Laboratory Diagnostics, University Hospital Centre, Zagreb, Croatia) and the Laboratory for Developmental Neuropathology (Croatian Institute for Brain Research, University of Zagreb Medical School, Zagreb, Croatia). Note the significant deviation from linearity.
4. Discussion
Our results indicate that there is a difference between Innogenetics and Invitrogen ELISA methods for Aβ1-42 and t-tau. According to Passing and Bablok (1985) if 95% CI for slope has a value of 1 and for intercept a value of 0, then there is no significant difference between two methods [22,23]. Results of Passing-Bablok analysis for comparability of Invitrogen and Innogenetics kits indicate that this assumption cannot be applied for either t-tau (Figure 3A) or Aβ1-42 (Figure 3B). Thus, these two methods for determination of t-tau and Aβ1-42 in CSF cannot be used interchangeably. On the other hand, Passing-Bablok analysis for performance of Innogenetics t-tau ELISA in two different laboratories showed good agreement between this method performed in LDN and LNB (Figure 4). While CUSUM linearity test showed significant deviation from linearity (p = 0.02), this testing of Innogenetics method efficiency in different laboratories should be further tested on larger group of patients.
The cause of the discrepancy between Innogenetics and Invitrogen ELISA kits for t-tau and Aβ1-42 is most probably due to differences in reagents used in the development of these kits. Usage of different materials for reagents or procedures in antibody purification, preparation of standards, and plate coating can cause ELISA kits to perform differently among vendors (CV above 20%; [16,17]). In fact, ELISA kits from the same vendor may have lot-to-lot variability [16,18,24,25].
Pre-analytical procedures (lumbar puncture and sample storage) were same in both laboratories. After lumbar puncture, which was performed by the same physician, samples were aliquoted in polypropylene tubes, stored at −80°C and thawed just once before the analysis. Only centrifugation conditions slightly differed between the laboratories. CSF samples were centrifuged 10 min at 2,000 g and 10 min at 4,000 g at LDN and LNB, respectively. In the study of Bjerke et al. [26] it was reported that centrifugation affects a number of CSF proteins, while Vanderstichele et al. [17] did not confirm this. It is considered that variations in protein levels due to centrifugation are found only in hemorrhagic samples, urging Vanderstichele et al. [17] to recommend centrifugation of hemorrhagic samples only. Although centrifugation speed does not affect levels of proteins it is generally recommended to centrifuge 10 min at 2,000 g at room temperature [27].
Differences in laboratory procedures like manual plate washing or use of a washing machine should not influence the results. In fact, both Invitrogen and Innogenetics ELISA protocols state that this step can be performed either way. Concentrations could also be read out using a plate reader (LNB) or curve-fitting software (LDN) using a 4-parameter algorithm. Possible mistake or variability in laboratory procedures performed by different technicians should be also considered, which in this case would refute the results of Innogenetics ELISA performed in both laboratories (Figure 4). Applying Innogenetics ELISA kit, higher t-tau and Aβ1-42 values were obtained than using the Invitrogen ELISA kit (Table 2). This fact was already noted by Fialova and collaborators [28].
Only a few other studies compared results of ELISA kits from different vendors [5,12,29], and the efficiency of Innogenetics and Invitrogen ELISA for t-tau was compared only by Fialova et al. on CSF samples of 38 patients suffering from multiple sclerosis and other neurological diseases [28]. Good agreement between these two methods was shown in this study, although levels of t-tau measured by the Innogenetics ELISA were higher than those measured by the Invitrogen ELISA. In other studies, the efficiency of MSD multiplex assay and Innogenetics ELISA were determined [29], as well as Innogenetics ELISA and INNO-BIA AlzBio3 using the multiplex xMAP Luminex platform to replace ELISA with a multiparametric method [30–34].
Other data on efficiency of different ELISA methods for CSF biomarkers of AD are available from multicenter studies, although most often the used assay is Innogenetics ELISA [12,15]. In one of the latest studies from the Alzheimer’s Association quality control program of AD biomarkers, 40 laboratories participated. Three different ELISA kits were used (Innogenetics ELISA, INNO-BIA AlzBio3, Meso Scale Discovery), whereas 26 laboratories used the Innogenetics ELISA kit. Biomarkers levels differed among the laboratories with coefficient of variation (CV) ranging from 13% to 36% [16]. Hort and colleagues compared CSF biomarkers measured by Innogenetics ELISA in laboratories from 25 countries [15]. Cut-off levels for Aβ1-42, t-tau and p-tau varied considerably among different countries and even within the same country [15]. Another difficulty in that study was that some laboratories used cut-off levels established by others [35] and not by Receiver Operator Curve (ROC) analysis. Efficiency of INNO-BIA AlzBio3 on the Luminex analytical platform was studied by Lewczuk and collaborators in 12 German geriatric and psychiatric university departments [36]. Results of both t-tau and p-tau measured by Luminex showed high correlation with results obtained by ELISA, whereas in the case of Aβ1-42 correlation was lower [36]. Shaw and coworkers inspected efficiency of this multiparametric method in 7 different centers. Within-laboratory CV was 5.3%, 6.7% and 10.8% for Aβ1-42, t-tau and p-tau181, respectively [37]. Intercenter variability was higher with CV reaching 17.9%, 13.1% and 14.6% for Aβ1-42, t-tau and p-tau181, respectively [37].
External controls were analyzed by Verwey et al. in 20 laboratories to assess inter-center variability. ELISA assays of Biosource, Genetics Company, Innogenetics and one in-house ELISA were used in this study and revealed high variability among laboratories, especially for Aβ1-42 [14]. The goal of multi-center studies is standardization of procedures for determination of AD CSF biomarkers and establishment of cut-off levels. However, despite numerous studies, consensus criteria for cut-off levels are not yet set and thus vary considerably among studies [15,32,35].
Beside early diagnostics of AD, determination of CSF biomarkers is necessary for monitoring of potential treatments. It is generally accepted that immunization using Aβ, the use of β- and γ-secretase inhibitors, and GSK3β inhibitors, which are currently among the most promising disease-modifying therapies, should be applied in the early stages of disease when neurodegeneration is not in an advanced stage [38]. Therefore, future studies are still required to (i) determine cut-off levels for Aβ1-42, t-tau, and p-tau; (ii) standardize pre-analytical procedures across laboratories to account for inter-center variability (for review see [17]; and (iii) further perform detailed comparison of ELISA kits produced by different vendors on higher numbers of subjects and samples.
Acknowledgments
Authors thank to Dr. Lidija Bilić-Zulle, Rijeka University School of Medicine, for helpful advices in statistical analysis. All patients signed standard Patient Consent Form approved by the Ethical Committee of the University Hospital Center Zagreb (approval no. 01-20/53-1/2006 signed on 26th June 2006). This research was also approved by the central Ethical Committee of the University of Zagreb Medical School (case no. 380-59/11-500-77/90, class 641-01/11-02 signed on 19th May 2011). The work is funded by the Croatian Science Foundation grant no. 09/16 “Detection and tracking of biological markers for early therapeutic intervention in sporadic Alzheimer’s disease” to G.Š., by the Croatian Ministry of Science, Education and Sports grant no. 108-1081870-1942 “Phosphorylation of tau proteins during development and Alzheimer’s disease” to G.Š., and in part by NIH grant P50 AG005138 to P.R.H.
Footnotes
The authors declare no conflict of interest.
References
- 1.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings JL, Khachaturian ZS. Definitions and diagnostic criteria. In: Gauthier S, editor. Clinical diagnosis and management of Alzheimer’s disease. 2. Martin Dunitz; London: 2002. pp. 3–13. [Google Scholar]
- 3.Wiltfang J, Esselman H, Maler JM, Bleich S, Huther G, Kornhuber J. Molecular biology of Alzheimer’s dementia and its clinical relevance to early diagnosis and new therapeutic strategies. Gerontology. 2001;47:65–71. doi: 10.1159/000052775. [DOI] [PubMed] [Google Scholar]
- 4.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 5.Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. J Am Med Assoc. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 6.Šimić G, Boban M, Šarac H, Grbić K, Hof PR, Hamann C, et al. CSF tau proteins in evaluation of patients with suspected dementia. Neurodegen Dis. 2007;4:135–136. [Google Scholar]
- 7.Šimić G, Boban M, Hof PR. Cerebrospinal fluid phosphorylated tau proteins as predictors of Alzheimer’s disease in subjects with mild cognitive impairment. Period Biol. 2008;110:27–30. [Google Scholar]
- 8.Boban M, Grbić K, Mladinov M, Hof PR, Süβmair C, Ackl N, et al. Cerebrospinal fluid markers in differential diagnosis of Alzheimer’s disease and vascular dementia. Coll Antropol. 2008;32:31–36. [PubMed] [Google Scholar]
- 9.Boban M, Šarac H, Mimica N, Mladinov M, Süβmair C, Ackl N, et al. CSF tau proteins in differential diagnosis of dementia. Transl Neurosci. 2010;1:43–48. [Google Scholar]
- 10.Spies PE, Slats D, Sjögren JM, Kremer BP, Verhey FR, Rikkert MG, et al. The cerebrospinal fluid amyloid beta42/40 ratio in the differentiation of Alzheimer’s disease from non-Alzheimer’s dementia. Curr Alzheimer Res. 2010;7:470–476. doi: 10.2174/156720510791383796. [DOI] [PubMed] [Google Scholar]
- 11.Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, Johnson-Wood K, Galasko D, et al. Reduction of β-amyloid peptide 42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- 12.Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 13.Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, et al. Decreased beta-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. J Am Med Assoc. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 14.Verwey NA, van der Flier WM, Blennow K, Clark C, Sokolow S, De Deyn PP, et al. A worldwide multicentre comparison of assays for cerebrospinal fluid biomarkers in Alzheimer’s disease. Ann Clin Biochem. 2009;46:235–240. doi: 10.1258/acb.2009.008232. [DOI] [PubMed] [Google Scholar]
- 15.Hort J, Bratos A, Pirttila T, Scheltens P. Use of cerebrospinal fluid biomarkers in diagnosis of dementia across Europe. Eur J Neurol. 2010;17:90–96. doi: 10.1111/j.1468-1331.2009.02753.x. [DOI] [PubMed] [Google Scholar]
- 16.Mattsson N, Andreasson U, Persson S, Arai H, Batish SD, Bernardini S, et al. The Alzheimer’s Association external quality control program for cerebrospinal fluid biomarkers. Alzheimers Dement. 2011;7:386–395. doi: 10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanderstichele H, Bibl M, Engelborghs S, Le Bastard N, Lewczuk P, Molinuevo JL, et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement. 2012;8:65–73. doi: 10.1016/j.jalz.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Teunissen CE, Verwey NA, Kester MI, van Uffelen K, Blankenstein MA. Standardization of assay procedures for analysis of the CSF biomarkers amyloid beta (1-42), tau, and phosphorylated tau in Alzheimer’s disease: report of an international workshop. Int J Alzheimers Dis. 2010 doi: 10.4061/2010/635053. pii: 635053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boban M, Malojčić B, Mimica N, Vuković S, Zrilić I, Hof PR, et al. The reliability and validity of the Mini-Mental State Examination in the elderly Croatian population. Dement Geriatr Cogn Disord. 2012;33:385–392. doi: 10.1159/000339596. [DOI] [PubMed] [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 22.Bablok W, Passing H. Application of statistical procedures in analytical instrument testing. J Automat Chem. 1985;7:74–79. doi: 10.1155/S1463924685000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bilić-Zulle L. Comparison of methods: Passing and Bablok regression. Biochem Med. 2011;21:49–52. doi: 10.11613/bm.2011.010. [DOI] [PubMed] [Google Scholar]
- 24.Mattsson N, Blennow H, Zetterberg H. Inter-laboratory variation in cerebrospinal fluid biomarkers for Alzheimer’s disease: united we stand, divided we fall. Clin Chem Lab Med. 2010;48:603–607. doi: 10.1515/CCLM.2010.131. [DOI] [PubMed] [Google Scholar]
- 25.Verwey NA, Bouwman FH, van der Flier WM, Veerhuis R, Scheltens P, Blankenstein MA. Variability in longitudinal cerebrospinal fluid tau and phosphorylated tau measurements. Clin Chem Lab Med. 2008;46:1300–1304. doi: 10.1515/CCLM.2008.241. [DOI] [PubMed] [Google Scholar]
- 26.Bjerke M, Portelius E, Minthon L, Wallin A, Anckarsäter H, Anckarsäter R, et al. Confounding factors influencing amyloid beta concentration in cerebrospinal fluid. Int J Alzheimers Dis. 2010 doi: 10.4061/2010/986310. pii: 986310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teunissen CE, Petzold A, Bennett JL, Berven FS, Brundin L, Comabella M, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73:1914–1922. doi: 10.1212/WNL.0b013e3181c47cc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fialova L, Bartos A, Svarcova J, Dolezil D, Malbohan I. Stanovení tau proteinu v mozkomíšním moku pacientů s roztroušenou sklerózou dvěma soupravami ELISA [Tau protein determination in cerebrospinal fluid in patients with multiple sclerosis by two ELISA kits] Klin Biochem Metab. 2011;19:113–118. [Google Scholar]
- 29.Regeniter A, Kuhle J, Baumann J, Sollberger M, Herdener M, Kunze U, et al. Biomarkers of dementia: comparison of electrochemiluminescence results and reference ranges with conventional ELISA. Methods. 2012;56:494–499. doi: 10.1016/j.ymeth.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B, et al. Simultaneous measurement of β-amyloid(1–42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51:336–345. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- 31.Reijn TS, Rikkert MO, van Geel WJ, de Jong D, Verbeek MM. Diagnostic accuracy of ELISA and xMAP technology for analysis of amyloid beta (42) and tau proteins. Clin Chem. 2007;53:859–865. doi: 10.1373/clinchem.2006.081679. [DOI] [PubMed] [Google Scholar]
- 32.Fagan AM, Shaw LM, Xiong C, Vanderstichele H, Mintun MA, Trojanowski JQ, et al. Comparison of analytical platforms for cerebrospinal fluid measures of Aβ1-42, total tau and p-tau181 for identifying Alzheimer’s disease amyloid plaque pathology. Arch Neurol. 2011;68:1137–1144. doi: 10.1001/archneurol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L-S, Leung YY, Chang S-K, Leight S, Knapik-Czajka M, Baek Y, et al. Comparison of xMAP and ELISA assays for detecting cerebrospinal fluid biomarkers of Alzheimer’s disease. J Alzheimers Dis. 2012;31:439–445. doi: 10.3233/JAD-2012-120082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Bastard N, Coart E, Vanderstichele H, Vanmechelen E, Martin JJ, Engelborghs S. Comparison of two analytical platforms for the clinical qualification of Alzheimer’s disease biomarkers in pathologically-confirmed dementia. J Alzheimers Dis. 2013;33:117–131. doi: 10.3233/JAD-2012-121246. [DOI] [PubMed] [Google Scholar]
- 35.Sjögren M, Vanderstichele H, Agren H, Zachrisson O, Edsbagge M, Wikkelsø C, et al. Tau and Abeta42 in cerebrospinal fluid from healthy adults 21–93 years of age: establishment of reference values. Clin Chem. 2001;47:1776–1781. [PubMed] [Google Scholar]
- 36.Lewczuk P, Kornhuber J, Vanderstichele H, Vanmechelen E, Esselmann H, Bibl M, et al. Multiplexed quantification of dementia biomarkers in the CSF of patients with early dementias and MCI: a multicenter study. Neurobiol Aging. 2008;29:812–818. doi: 10.1016/j.neurobiolaging.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Shaw LM, Vanderstichele H, Knapik-Czajka M, Figurski M, Coart E, Blennow K, et al. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol. 2011;121:597–609. doi: 10.1007/s00401-011-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw LM, Korecka M, Clark CM, Lee VM-Y, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov. 2007;6:295–303. doi: 10.1038/nrd2176. [DOI] [PubMed] [Google Scholar]