Abstract
Background and Purpose
Epidemiologic studies of intracerebral hemorrhage (ICH) have consistently demonstrated variation in incidence, location, age at presentation, and outcomes among non-Hispanic white, black, and Hispanic populations. We report here the design and methods for this large, prospective, multi-center case-control study of ICH.
Methods
The ERICH study is a multi-center, prospective case-control study of ICH. Cases are identified by hot-pursuit and enrolled using standard phenotype and risk factor information and include neuroimaging and blood sample collection. Controls are centrally identified by random digit dialing to match cases by age (+/−5 years), race, ethnicity, gender and metropolitan region.
Results
As of March 22, 2013, 1,655 cases of ICH had been recruited into the study which is 101.5% of the target for that date and 851 controls had been recruited which is 67.2% of the target for that date (1,267 controls) for a total of 2,506 subjects which is 86.5% of the target for that date (2,897 subjects). Of the 1,655 cases enrolled, 1,640 cases had the case interview entered into the database of which 628 (38%) were non-Hispanic black, 458 (28%) were non-Hispanic white and 554 (34%) were Hispanic. Of the 1,197 cases with imaging submitted, 876 (73.2%) had a 24 hour follow-up CT available In addition to CT imaging, 607 cases have had MRI evaluation.
Conclusion
The ERICH study is a large, case-control study of ICH with particular emphasis on recruitment of minority populations for the identification of genetic and epidemiologic risk factors for ICH and outcomes after ICH.
Keywords: Stroke, Intracerebral Hemorrhage, Risk Factors, Hypertension, Minorities, Genetics, Genomics
Introduction
Hemorrhagic stroke occurs in ~100,000 people in the U.S. each year, of which 40% to 50% die within 30 days.1 Despite comprising less than 20% of all strokes, hemorrhagic stroke accounts for 50% of the mortality associated with stroke and 30% of stroke-related costs.2 Hemorrhagic stroke is a heterogeneous disease with intracerebral hemorrhage (ICH), accounting for two-thirds of all the cases. In ICH, half of the mortality occurs within the first two days after stroke, and there are no proven effective treatments. Thus, prevention and prediction are of paramount importance for reducing the healthcare burden related to ICH.
The word “ethnicity” is used in this paper to capture the combined and interacting concepts of race and culture. According to the US 2010 Census, 12.9% of Americans self-report as black and 12.5% as Hispanic.3 Ethnic differences in ICH risk are striking. Among blacks, the risk of ICH is double that of whites.4 The annual incidence rates per 100,000 for ICH has been reported to be 48.9 for blacks compared with 26.6 for whites.5 Hispanics in the United States have twice the risk of ICH of non-Hispanic whites (OR=2.6; 95% CI 1.4–6.1).6
Our long-term goal is to identify the genetic variation that affects risk of ICH in a multi-ethnic population. In addition, we will determine differences in the distribution of risk factors and imaging characteristics based on race and ethnicity. To address these goals, we are performing a prospective, multi-center case-control study of ICH.
Methods and Research Design
The ERICH Study Group
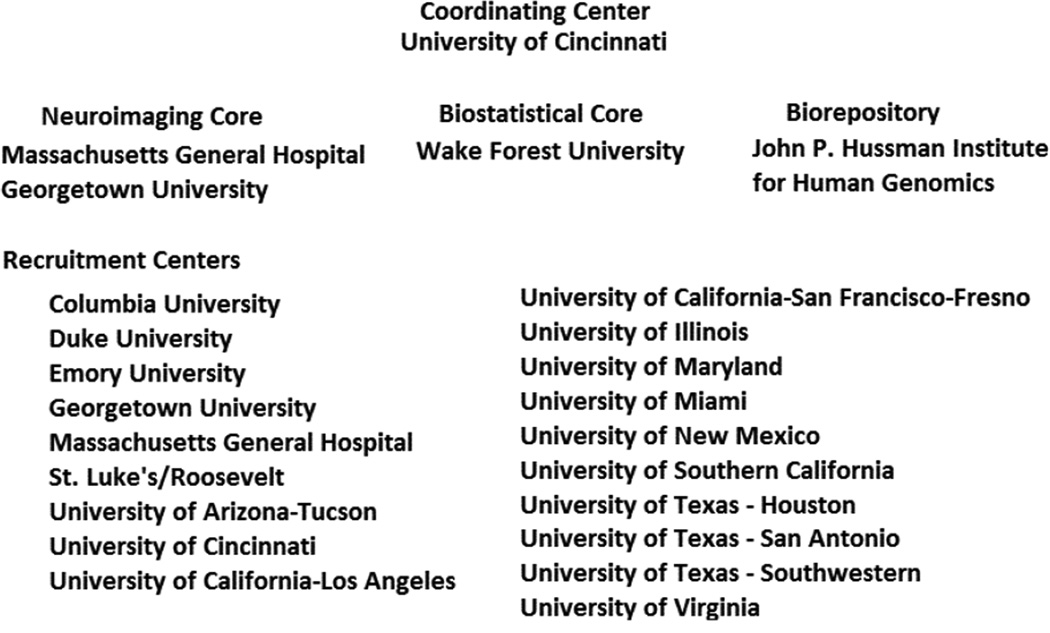
The ERICH Study consists of a coordinating center (University of Cincinnati), neuroimaging cores (Massachusetts General Hospital, Georgetown University), a sample biorepository (John P. Hussman Institute for Human Genomics at the University of Miami), a biostatistical core (Wake Forest University), and 19 clinical recruitment centers that encompass 42 recruitment sites (Figure 1). The goals of the study are to recruit 1,000 non-Hispanic white, 1,000 non-Hispanic black and 1,000 Hispanic ICH cases and 3,000 demographically-matched controls from the same population as the cases. Our scientific aims are to:
Identify differences in risk factor distribution by ethnicity and location of ICH.
Determine differences in the presence of microbleeds and other MR imaging characteristics by ethnicity of ICH.
Determine differences in outcomes of ICH by ethnicity.
Determine the level of population admixture between centers and ethnicity.
Figure 1.
Study Organization
Definition of Phenotype
The primary phenotype is a spontaneous ICH. All cases must meet the following eligibility criteria:
Age 18 years or greater
Resident for at least 6 months within 75 miles of the recruiting center (100 miles for population centers with less than 1 million)
Be of non-Hispanic white, non-Hispanic black, or Hispanic race-ethnicity by self-report.
Diagnosis of spontaneous ICH (see definition)
Ability of the patient or legal representative to provide informed consent.
Intracerebral hemorrhage is defined as (adapted from the Classification of Cerebrovascular Disease III-1989) a spontaneous, non-traumatic, abrupt onset of severe headache, altered level of consciousness, and/or focal neurologic deficit that is associated with a focal collection of blood within the brain parenchyma seen on neuroimaging or at autopsy and is not due to hemorrhagic conversion of a cerebral infarction.7,8 This includes warfarin-associated ICH as well as peripartum ICH. Exclusions include malignancies that lead to coagulopathy, dural venous sinus thrombosis-associated hemorrhage, hemorrhages due to vascular malformations, aneurysms, tumors or hemorrhagic conversion of recent ischemic stroke. To determine a patient’s eligibility as a study case, study neurologists review the patient’s clinical presentation and neuroimaging.
Case Identification and Screening
Our study uses hot-pursuit to enroll subjects to limit survival bias. Each recruitment center reviews admission logs, emergency room logs, and neurology/neurosurgery/neurosurgical ICU admission logs for potential ICH cases. Included in screening is review of ‘hemorrhagic infarcts,’ ‘traumatic hemorrhages,’ ‘stroke’ and ‘CVA,’ ‘subarachnoid hemorrhage,’ ‘focal weakness’, ‘unresponsive’, ‘found down’ and ‘mental status change’ as some cases of true ICH may be misclassified. Site investigators review all potential cases including those with a questionable diagnosis.
Ongoing training and quality checks are in place. For example, each month, a challenging phenotype is presented at the study conference call for both coordinators and principal investigators. The phenotype challenging cases include examples of hemorrhagic infarction, hemorrhage into tumor or traumatic ICH. In addition, once a year per center, five consecutive cases of ICH that were excluded from the study are reviewed for verification of ineligibility. In addition, neuroimaging core review includes verification of case status and discrepancies are reviewed.
Control Identification
To minimize biases in control recruitment, two random digit dialing (RDD) centers are utilized for the study. The Institute for Policy Research (IPR) has been performing RDD since the 1970s and has previously identified >2,000 controls for the Genetic and Environmental Risk Factors for Hemorrhagic Stroke study. The Survey Research Center at the University of Alabama – Birmingham has been the call center for the REasons for Geographic And Racial Differences in Stroke (REGARDS) study.9 The control identification centers use RDD methods to identify ICH-free controls to be matched by age (+/− 5 years), sex, race-ethnicity, and metropolitan area. Certain demographics (e.g., very elderly minority populations) may require the utilization of “friend controls” in which a randomly-selected control is asked if he or she has a non-biologically-related friend who meets a specific demographic for recruitment.
Subject Enrollment and Interview
By design, the ERICH study is a hot-pursuit study with recruitment of often critically ill ICH cases with special emphasis of minority populations into a genetic epidemiology study without an intervention. The study addressed general recruitment barriers and incorporated strategies that address minority recruitment barriers such as cultural competence, cultural perceptions, education on prior unethical treatment of minority populations, and training by those with particular experience in recruitment of minority populations for research studies (Table).
Table.
Recruitment Barriers and Strategies
| Barrier | Strategies |
|---|---|
| Early Mortality |
|
| Minority Recruitment |
|
| Genetic Research |
|
| Severity of Condition |
|
Each recruiting center must have personnel certified for biologic sample handling/shipping. Frequently, remaining clinically drawn blood is available in moribund cases of ICH. If families are unable to decide on participation but have not refused to participate, study personnel request permission to have their clinically drawn blood held/not destroyed to give families additional time to consider enrollment into the study. If at a later date the family agrees to participate and the patient has expired, clinically drawn blood is used and the sample is labeled for quality assurance review.
Institutional Review Board approval at each participating center is required prior to initiation of study enrollment. Informed consent is obtained from each enrolled subject or legally authorized representative. A Spanish language consent form is available for Spanish-only speaking subjects or those that prefer the Spanish consent. Prior to interview of a patient, capacity is screened using a consent comprehension questionnaire. If the patient fails, a proxy/legally authorized representative is contacted for enrollment with priority to guardian/power of attorney, spouse, adult children, parents and siblings, in that order. Once informed consent has been obtained, screening for dementia is performed using the Telephone Interview Cognitive Screen (TICS).10 The TICS is a validated dementia screen that can be used in subjects with motor impairment. A score of 21 or higher is required to provide interview data. Cases or proxies are interviewed using a standardized case report form. In addition, subjects undergo three blood pressure readings using an appropriate size cuff. Weight, height and waist-hip ratio are also recorded.
Chart Abstraction and Follow-up
In addition to the personal interview, chart abstraction is performed for each case to provide additional clinical data including blood pressure treatment, surgical interventions, complications and laboratory testing, including coagulation parameters. Discharge status and outcome are recorded. Each surviving case also is contacted for 3-, 6- and 12-month follow-ups, which include evaluation of modified Rankin scale,11 Barthel Index,12 medication use, EuroQol EQ-5D,13 and Health State Scale.14
Neuroimaging
Central to the diagnosis and prognosis in ICH, is neuroimaging review. Diagnostic challenges include traumatic hematoma, infarction with hemorrhagic transformation, hemorrhage into a tumor or hemorrhage from a venous sinus thrombosis, and intracranial aneurysms. Volume of ICH as well as the presence of intraventricular hemorrhage is critical to determining outcomes related to ICH. We perform centralized neuroimaging review in a standardized fashion. All centers obtain baseline and follow-up neuroimaging CT/MRI de-identified and in digital format.
Because the ordering of MRI may be physician dependent, an MRI is sought on every fifth case to avoid bias. If an MRI is not ordered for clinical care and is safe to perform, a study MRI is requested. If the fifth consecutive case is unable to have an MRI performed, subsequent cases are selected for MRI until one is completed. Neuroimaging studies are stored on DICOM software, de-identified on site, and uploaded electronically to the neuroimaging repository.
All MRIs are performed on 1.5–3.0 T scanners with the following required sequences: gradient echo, diffusion weighted imaging (DWI) with apparent diffusion coefficient (ADC) map, and fluid attenuated inversion recovery (FLAIR). Analyses include ICH volume, location, number of microbleeds by location, presence and severity of small vessel disease and presence, and volume of intraventricular hemorrhage. White matter hyperintensities are analyzed by a visual rating scale and by segmentation using computer-assisted volumetric methods. Intraparenchymal and intraventricular volumes are measured by planimetric analysis using Alice software (Parexel Corporation, Waltham, MA) which has an excellent inter-rater reliability.15 At quarterly neuroimaging review meetings, CT and MRI reading progress is reviewed and a committee adjudicates discrepancies.
Biorepository
The biorepository for the ERICH study provides a standardized blood collection kit with a unique ERICH study ID number for each subject. This ID number allows the local PI to retain identifying information with the consent form, and it protects the subject’s privacy as the sample is processed. The blood draw consists of two 6ml EDTA tubes. The biorepository records receipt of the sample into the laboratory information management system (LIMS). Plasma, to a maximum of 1ml, is isolated from each tube (beginning in 2013). DNA is extracted and analyzed for quantity and quality, and then stored for genetic studies. In the rare case that the quantity or quality of the DNA sample is unacceptable, the second EDTA blood tube will be extracted. Otherwise, this second tube is stored at −80°C.
Statistical Methods
Numerous hypotheses may be explored through the ERICH study regarding risk, treatment and outcome. The study utilizes a Data Access Committee to review queries for data analysis for design, appropriateness, scientific merit and impact on study resources.
The goal of matching in epidemiologic studies is to ensure that the controls are comparable to the cases with respect to the matching variables. In genetic studies, this strategy can reduce confounding introduced by age-specific penetrance or sex-specific effects and population stratification. In general, the analysis of a 1:N matched case-control design with covariates can be analyzed using proportional hazards modeling16 or linear mixed models for logistic regression,17 which reduces to a conditional logistic regression for a 1:1 matched-pair design. Preliminary data suggests that significant differences in risk factors exist by ethnicity. Therefore, we will formally test for these differences in the ICH regression models by including an ethnicity-by-risk factor interaction variable. If multiple interactions are anticipated or observed, data may be more appropriately modeled by stratifying by ethnicity and they may be meta-analytically combined to test for interactions using the test for homogeneity of the effect size.
We will perform a case-only analysis as a complement to the case-control analysis. To test for interactions between two variables that are independent, a case-only analysis can be more powerful than a classic case-control design.18 We will corroborate the results of a case-only analysis with the case-control analysis. We will apply generalized linear models to test for differences in the presence of microbleeds among blacks and Hispanics after adjusting for all relevant covariates such as age, hypertension, and alcohol usage. We will consider logistic regression modeling of presence vs. absence of microbleeds, Poisson regression to model the count of microbleeds, and multiple linear regression for other quantitative variables. These analyses may be stratified by ethnicity or jointly analyzed adjusting for the individual admixture proportion estimates.
Finally, we will test for differences among ethnicities in the location of ICH. These logistic models will use the white cases of ICH as the baseline comparision group in their formal tests.
Estimating individual admixture proportions
Mating among long-separated European, African, and Amerindian populations during colonization of the New World originated admixed populations in the US. Blacks and Hispanic Americans (HA) are two such admixed populations. The genome of an admixed individual is made up of consecutive blocks of markers inherited from each of the ancestral populations. Numerous ancestry informative markers (AIMs) have been identified for estimating ancestry proportions in blacks19 and HA.20 For example, Halder et al selected markers which were informative enough to distinguish between the four main ancestral groups represented in the US: Europeans, Africans, Indigenous Americans, and Asians.21 We will use existing software such as Structure22 and Admixmap23, 24 to compute individual admixture proportion. Structure implements a hidden Markov model to infer the underlying ancestry at the chromosomal region. Admixmap uses a combination of Bayesian and frequentist statistical methods to compute similar estimates. The two methods are expected to provide comparable results, thereby providing a check of the estimation procedures in these data.
To determine the number of ancestral populations in the admixed population of interest, we will be using the likelihood ratio test implemented. The admixture estimates will serve two purposes: (1) since they are more homogeneous genetically than self-reported ethnicity (SRE), they can be used instead of SRE in all analyses where the objective is to determine racial/ethnic differences in ICH and related outcomes; (2) they will be used in the candidate gene analysis. We note here that principal component analysis (PCA) can also be applied to these AIMs to provide similar control variables.
Modifications to the Study Protocol
In Year 2 of the study, control enrollment was identified to be lagging behind target. Recruiters with an exceptionally high rate of recruitment were interviewed for suggestions which along with a review of the process were codified to require that each control have contact information sent to the recruiting center within 24 hours of identification and a minimum number of calls to be made within the first 72 hours and in the following weeks. To facilitate the communication of control information and tracking of reasons for refusal, a web-based interface was developed. The institution of the web-based control calling page was initiated in 7/2012 and rolled out to all centers in 8/2012 and has led to a 31.3% increase in the rate of recruitment of controls from 5/2012 to 4/2013.
It was also identified that Hispanic control enrollment was lagging behind white and black control enrollment. A review found that Hispanic controls, were enrolling at the same or higher rate as white or black controls identified but fewer controls were being identified. RDD methods were adjusted to focus calling in targeted zip codes.
Modifications to the case report form were made to remove redundancy as well as to add variables suggested by the investigators. Case report form versions are dated to identify when changes occurred.
Study Update
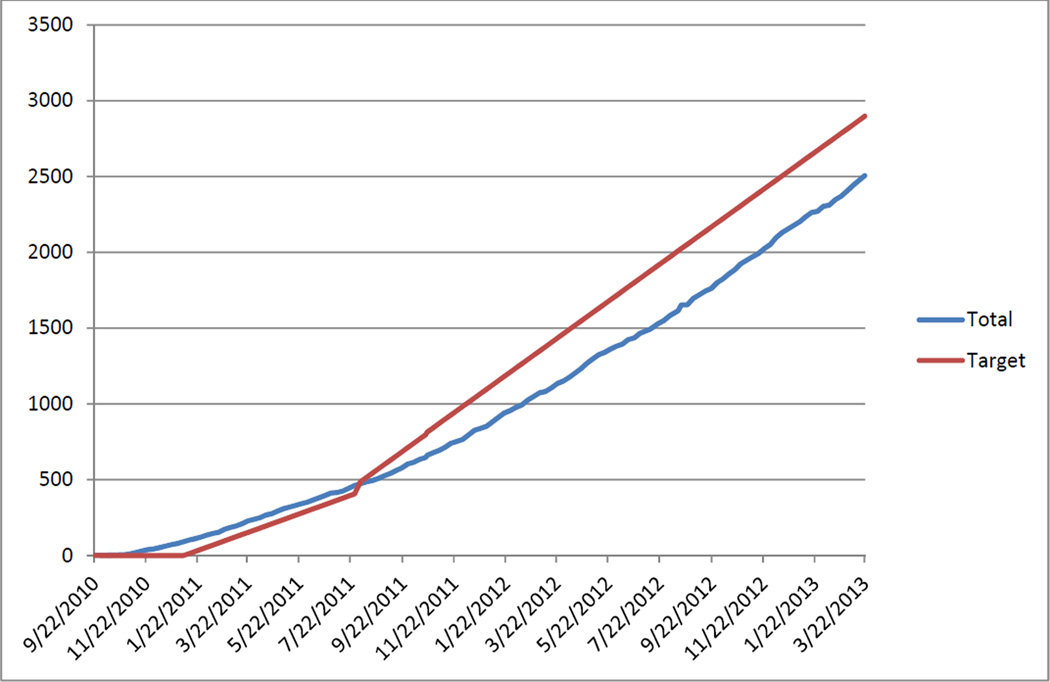
The study began on August 1, 2010, and recruitment officially began for cases on January 1, 2011 with a goal of reaching 3,000 cases by February 1, 2015 (Figure 2). Control enrollment officially began on July 1, 2011, with a goal of reaching 3,000 controls by August 1, 2015. As of March 22, 2013, 1,655 cases of ICH had been recruited into the study which is 101.5% of the target for that date (1,630 cases), and 851 controls had been recruited which is 67.2% of the target for that date (1,267 controls) for a total of 2,506 subjects which is 86.5% of the target for that date (2,897 subjects).
Figure 2.
Recruitment – Total Recruitment vs. Targeted Recruitment
Of the 1,655 cases enrolled, 1,640 cases had the case interview entered into the database of which 628 (38%) were non-Hispanic black, 458 (28%) were non-Hispanic white and 554 (34%) were Hispanic. Thus, the study has been able to achieve excellent recruitment of cases with particular emphasis among minority patients.
Of 2,217 cases that had undergone DNA extraction, average DNA yield of 143.82ug was obtained with only 23 (1.0%) of the samples having less than 10ug of DNA. Genotyping for Apolipoprotein E alleles is complete on 1,269 cases.
In addition, 1,197 baseline CTs had been received at the neuroimaging repository of which 959 have undergone analysis for volume and location of ICH. Of the 1,197 cases with imaging submitted, 876 (73.2%) had a 24 hour follow-up CT available of which 612 (70%) have undergone analysis of which 158 demonstrated expansion of 6ml or 33% or greater volume from baseline scan. In addition to CT imaging, 607 cases have had MRI submitted with the required sequences (DWI/ADC, FLAIR and GRE) and have undergone analysis for volume, location, microbleeds, and DWI evaluation.
Discussion
Blacks and Hispanics are disproportionately affected by ICH in the US, and tend to have a younger age of ICH onset than white populations. However, white populations have a higher proportion of lobar ICH, a higher mortality rate and a higher volume of ICH compared to blacks and non-Hispanic whites. To explore the underlying biologic mechanisms for these differences, we have designed our study to recruit a large case-control study of ICH with a particular emphasis on minority recruitment. Selection of centers was based, in part, on the distribution of minority populations throughout the US.
This study design permits the recruitment of large numbers of minority ICH populations in a prospective fashion. Specific challenges exist for recruitment within the study, specifically the recruitment of minorities, the high early mortality rate of ICH, participation in a genetic epidemiology study without a treatment agent, and no direct benefit to participants. Heavy emphasis was placed on initial training, and education continues for recruitment personnel regarding these issues.
A prospective cohort study would have advantages in study design, providing knowledge of risk factors prior to the onset of ICH. However, with an annual incidence rate of 20 to 40 per 100,000, a cohort study of roughly 30,000 individuals would need to be followed for 250 years to achieve a sample size of 3,000 subjects, assuming stable incidence rates.
A population-based study within specific regions would allow for maximum external validity of the findings of the study. However, the largest city in the US (New York City with 8,244,910 persons per 2011 census) would need to have every hospital/facility in the city prospectively followed for potential cases of ICH for two years to identify and enroll 3,000 cases of ICH (assuming 50% enrollment rates), of which approximately 750 would be black and 750 would be Hispanic. Both of these study designs would therefore be cost-prohibitive and impractical to perform. Gathering cases of ICH with a genetic sample available from disparate stroke studies throughout the United States would similarly fail to yield significant sample sizes and may have led to biases in case ascertainment, control ascertainment, and phenotype definitions.
Thus, ERICH was designed as a multi-center, prospectively recruited case-control study. Cases are identified using uniform methodology, case definitions and phenotype definitions, and controls are identified using a central random digit dialing method. Imaging is centrally analyzed to minimize biases in determination of location of ICH, volume measurements, and presence/absence of intraventricular hemorrhage. Biologic samples are centrally housed and processed using uniform methods.
In summary, the ERICH study is the largest, prospective case-control study of ICH with a particular emphasis on minority populations to examine environmental and genetic risk factors for risk of ICH, volume of ICH and outcomes after ICH. Since initiating the study, recruitment has progressed and we anticipate timely completion of enrollment. At the completion of the study, a large phenotype, neuroimaging and biologic sample database of ICH among white, black, and Hispanic populations along with demographically and geographically matched controls will be available for further study.
Acknowledgements
Robert Brown, M.D.
Misty Wethington, B.S.
Anastasia Veshkevich
Laura Russell, B.S.
Laurie Russell
Faisal Mukarram
Norma Castillo
Naraida Balajonda, M.D.
Sandra Brown, RN BSN
Clare Binley
Katrina Van De Bruinhorst
Gina Norato
Gilda Avila
Omowunmi Olaleye, B.S, M.S
Janet Li
Eve Ostrowski, MS, MPH, MLS
Carlos A. Conde
Maureen Hillmann RN
Theresa Wussow
Cameron Dell
Sharion L. Smith, RN, MSN
Sources of Funding
This study is supported by a grant from the National Institute of Neurological Disorders and Stroke (NINDS: U-01-NS069763).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
There is no institutional conflict of interest regarding this paper. The author conflicts of interest are as follows.
Daniel Woo – NIH
Matthew Flaherty – Boehringer-Ingelheim consultant/advisory board;
Michael L James - Grant funding from Baxter, Hospira, NIH, American Heart Association. Consultant for Cephalogics, Hospira; Kevin Sheth – AMA Clinical Research Award;
Anne Leonard – Consultant with American Heart Association
Jennifer Osborne- NIH;
Charles J Moomaw – NIH
Bradford Worrall - NIH and editorship for the Journal Neurology;
Ralph Sacco - NIH;
Latisha Ali - NIH
Eric Rademacher – NIH;
Nicole R. Gonzales – NIH
This report does not represent the official view of the National Institute of Neurological Disorders and Stroke (NINDS), the National Institutes of Health (NIH), or any part of the US Federal Government. No official support or endorsement of this article by the NINDS or NIH is intended or should be inferred.
References
- 1.Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, et al. Stroke incidence is decreasing in whites but not in blacks: A population-based estimate of temporal trends in stroke incidence from the greater cincinnati/northern kentucky stroke study. Stroke; a journal of cerebral circulation. 2010;41:1326–1331. doi: 10.1161/STROKEAHA.109.575043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the united states. Stroke; a journal of cerebral circulation. 1996;27:1459–1466. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 3.United states census 2010; it's in our hands. 2013 http://www.census.gov/2010census/
- 4.Broderick J, Brott T, Tomsick T, Huster G, Miller R. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. The New England journal of medicine. 1992;326:733–736. doi: 10.1056/NEJM199203123261103. [DOI] [PubMed] [Google Scholar]
- 5.Flaherty ML, Woo D, Haverbusch M, Sekar P, Khoury J, Sauerbeck L, et al. Racial variations in location and risk of intracerebral hemorrhage. Stroke. 2005;36:934–937. doi: 10.1161/01.STR.0000160756.72109.95. [DOI] [PubMed] [Google Scholar]
- 6.Labovitz DL, Halim A, Boden-Albala B, Hauser WA, Sacco RL. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and hispanics. Neurology. 2005;65:518–522. doi: 10.1212/01.wnl.0000172915.71933.00. [DOI] [PubMed] [Google Scholar]
- 7.Broderick J, Phillips S, Whisnant J, O'Fallon W, Bergstralh E. Incidence rates of stroke in the eighties: The end of the decline in stroke? Stroke. 1989;20:577–582. doi: 10.1161/01.str.20.5.577. [DOI] [PubMed] [Google Scholar]
- 8.Broderick J, Brott T, Tomsick T, Miller R, Huster G. Intracerebral hemorrhage is more than twice as common as subarachnoid hemorrhage. Journal of neurosurgery. 1993;78:188–191. doi: 10.3171/jns.1993.78.2.0188. [DOI] [PubMed] [Google Scholar]
- 9.Wadley VG, McClure LA, Howard VJ, Unverzagt FW, Go RC, Moy CS, et al. Cognitive status, stroke symptom reports, and modifiable risk factors among individuals with no diagnosis of stroke or transient ischemic attack in the reasons for geographic and racial differences in stroke (regards) study. Stroke; a journal of cerebral circulation. 2007;38:1143–1147. doi: 10.1161/01.STR.0000259676.75552.38. [DOI] [PubMed] [Google Scholar]
- 10.Buckwalter JG, Crooks VC, Petitti DB. A preliminary psychometric analysis of a computer-assisted administration of the telephone interview of cognitive status-modified. Journal of clinical and experimental neuropsychology. 2002;24:168–175. doi: 10.1076/jcen.24.2.168.994. [DOI] [PubMed] [Google Scholar]
- 11.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke; a journal of cerebral circulation. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 12.Mahoney F, Barthel D. Functional evaluation: The Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 13.Dorman PJ, Waddell F, Slattery J, Dennis M, Sandercock P. Is the euroqol a valid measure of health-related quality of life after stroke? Stroke; a journal of cerebral circulation. 1997;28:1876–1882. doi: 10.1161/01.str.28.10.1876. [DOI] [PubMed] [Google Scholar]
- 14.Pinto EB, Maso I, Vilela RN, Santos LC, Oliveira-Filho J. Validation of the euroqol quality of life questionnaire on stroke victims. Arquivos de neuro-psiquiatria. 2011;69:320–323. doi: 10.1590/s0004-282x2011000300010. [DOI] [PubMed] [Google Scholar]
- 15.Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059–1064. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- 16.Breslow N, Day N. The analysis of case-control studies. Lyon: IARC Scientific Publications No. 32; 1980. [PubMed] [Google Scholar]
- 17.Agresti A. Categorical data analysis. 2002 [Google Scholar]
- 18.Piegorsch WW, Weinberg CR, Taylor JA. Non-hierarchical logistic models and case-only designs for assessing susceptibility in population-based case-control studies. Stat Med. 1994;13:153–162. doi: 10.1002/sim.4780130206. [DOI] [PubMed] [Google Scholar]
- 19.Smith MW, Patterson N, Lautenberger JA, Truelove AL, McDonald GJ, Waliszewska A, et al. A high-density admixture map for disease gene discovery in african americans. Am J Hum Genet. 2004;74:1001–1013. doi: 10.1086/420856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian C, Hinds DA, Shigeta R, Adler SG, Lee A, Pahl MV, et al. A genomewide single-nucleotide-polymorphism panel for mexican american admixture mapping. Am J Hum Genet. 2007;80:1014–1023. doi: 10.1086/513522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T. A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: Utility and applications. Hum Mutat. 2008;29:648–658. doi: 10.1002/humu.20695. [DOI] [PubMed] [Google Scholar]
- 22.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoggart CJ, Parra EJ, Shriver MD, Bonilla C, Kittles RA, Clayton DG, et al. Control of confounding of genetic associations in stratified populations. Am J Hum Genet. 2003;72:1492–1504. doi: 10.1086/375613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoggart CJ, Shriver MD, Kittles RA, Clayton DG, McKeigue PM. Design and analysis of admixture mapping studies. Am J Hum Genet. 2004;74:965–978. doi: 10.1086/420855. [DOI] [PMC free article] [PubMed] [Google Scholar]