Abstract
Background
Identifying risk factors for lymphedema in patients treated for breast cancer has become increasingly important given the current lack of standardization surrounding diagnosis and treatment. Reports on the association of body mass index (BMI) and weight change with lymphedema risk are conflicting. We sought to examine the impact of pre-operative BMI and post-treatment weight change on the incidence of lymphedema.
Methods
From 2005-2011, 787 newly-diagnosed breast cancer patients underwent prospective arm volume measurements with a Perometer pre- and post-operatively. BMI was calculated from same-day weight and height measurements. Lymphedema was defined as a relative volume change (RVC) of ≥10%. Univariate and multivariate Cox proportional hazards models were used to evaluate the association between lymphedema risk and pre-operative BMI, weight change, and other demographic and treatment factors.
Results
By multivariate analysis, a pre-operative BMI ≥30 was significantly associated with an increased risk of lymphedema compared to a pre-operative BMI <25 and 25-<30 (p = 0.001 and p = 0.012, respectively). Patients with a pre-operative BMI 25-<30 were not at an increased risk of lymphedema compared to patients with a pre-operative BMI<25 (p= 0.409). Furthermore, large post-operative fluctuations in weight, regardless of whether they reflected weight gain or loss (i.e. 10 pounds gained/lost per month), resulted in a significantly increased risk of lymphedema (HR: 1.97, p = <0.0001).
Conclusions
Pre-operative BMI of ≥30 is an independent risk factor for lymphedema, whereas a BMI of 25-<30 is not. Large post-operative weight fluctuations also increase risk of lymphedema. Patients with a pre-operative BMI≥30 and those who experience large weight fluctuations during and after treatment for breast cancer should be considered at higher-risk for lymphedema. Close monitoring or early intervention to ensure optimal treatment of the condition may be appropriate for these patients.
Keywords: Lymphedema, Body Mass Index, Weight Fluctuation, Breast Cancer
INTRODUCTION
As survival from breast cancer increases, management of long-term treatment complications, especially those that impact quality of life, has gained greater significance. Lymphedema is a chronic condition characterized by the accumulation of protein-rich fluid in the interstitial tissues of the arm, breast, or chest wall. The swelling that is characteristic of lymphedema has the potential to compromise a patient's physical and psychological well-being [1-4]. Breast cancer survivors who have undergone surgical lymph node removal and/or radiation therapy may be at a lifelong risk of developing lymphedema [5]. It is estimated that approximately 1 in 5 breast cancer patients will develop lymphedema [6]. Therefore, lymphedema remains an important complication of breast cancer treatment.
The most commonly-cited risk factor for lymphedema following breast cancer treatment is axillary lymph node dissection (ALND) [6, 7-10, 11]. Other treatment-related risk factors have been suggested, including mastectomy [2,6,9,12], extent of axillary surgery [6,13-16], number of positive lymph nodes [8,9,17,18], chemotherapy [8,10,11,14], and nodal radiation [9,14,19-21]. In addition, elevated body mass index (BMI) has frequently been reported as a risk factor for lymphedema, yet results are conflicting [7,8,11,13,15,22-31]. Some studies suggest that only obesity (BMI ≥30) is associated with an increased risk of lymphedema, yet others report that being overweight (BMI 25-<30) may also increase lymphedema risk. Likewise, reports on the association between post-operative weight change and lymphedema risk are also inconsistent, with weight gain only occasionally identified as a risk factor for lymphedema. Furthermore, most studies investigating the association of BMI and weight gain with lymphedema risk are retrospective, limited by small sample sizes, lack of pre-operative assessment, and/or varying methods of defining, measuring, and quantifying lymphedema.
Understanding the impact of BMI and weight change on lymphedema risk is important given that these are potentially modifiable risk factors. We sought to evaluate the impact of pre-operative BMI and post-operative weight change on the risk of lymphedema among patients treated for breast cancer.
MATERIALS & METHODS
Design and Participants
From 2005-2011 and with Partners Healthcare Institutional Review Board approval, patients undergoing treatment for primary breast cancer at our institution underwent prospective lymphedema screening via Perometer arm volume measurements. All patients included in this analysis underwent a pre-operative Perometer measurement and subsequent post-operative measurements corresponding with oncology follow-up visits. BMI was calculated from same-day weight and height measurements corresponding with Perometer arm volume measurements, and patient demographic and treatment data were collected via medical record review. Patients who underwent bilateral breast surgery were excluded from the analysis, and measurements occurring after a patient was diagnosed with metastatic disease were also excluded.
Lymphedema
Lymphedema was quantified according to the Relative Volume Change (RVC) equation, which calculates relative change in arm volume compared to the pre-operative assessment and accounts for change in size of the contralateral arm as a control [32]. Briefly, RVC = [(A2U1)/(U2A1) – 1], where A1 and A2 are arm volumes on the side treated for breast cancer at pre-operative assessment and post-operative follow-up, and U1 and U2 are arm volumes on the contralateral side at the corresponding time points. The RVC equation accounts for change in volume of the contralateral arm, and thereby accounts for factors unrelated to lymphedema which could cause changes in arm size, such as weight gain. The RVC equation has been previously demonstrated to reduce the likelihood of a false positive diagnosis of lymphedema due to fluctuations in arm size related to body size or habitus [33].
Lymphedema was defined as an RVC≥10% based on consensus within the literature [9,34], occurring >3 months post-operative. The time from surgery to initial development of lymphedema (RVC ≥10%) was calculated. Patients who never developed lymphedema were considered censored at the time of most recent follow-up visit. The Kaplan-Meier method was used to estimate and plot the cumulative incidence of lymphedema during follow-up.
BMI, Weight Change, and Lymphedema Risk
Univariate and multivariate Cox proportional hazards models were used to assess the association between lymphedema and pre-operative BMI, categorized as <25 (“normal/underweight”), 25-<30 (“overweight”) and ≥30 (“obese”), as well as demographic and treatment factors. Time-dependent covariates were used for adjuvant chemotherapy and radiation therapy, such that patients were included in the “untreated” category until initiation of a given treatment, and included in the “treated” category thereafter.
Several time-dependent variables were constructed to evaluate the association between post-operative change in weight and risk of lymphedema. These included the percent (%) change in weight relative to pre-operative weight, the weight fluctuation relative to the previous measurement, the absolute fluctuation in weight relative to the previous measurement (example below), and the cumulative absolute fluctuation in weight from pre-operative measurement (example below). Each of the four weight change variables were calculated at each follow-up weight measurement and the final three were calculated as rates of change (lbs/month) to account for the variability in time between post-operative measurements. Table 1 provides examples of how these weight change variables were calculated using a fictional patient measured at 1-month intervals from pre-operative. Two-way interactions among significant variables were evaluated by adding interaction terms to the multivariate model.
“Absolute fluctuation” refers to the absolute value of the rate of change in weight from the previous measurement, such that a 5-pound gain in weight is equivalent to a 5-pound loss over time periods of the same length.
“Cumulative absolute fluctuation” reflects the sum of the absolute fluctuations in weight up to a given measurement; for example, if a patient gains 3 pounds per month between pre-operative and post-operative measurement 1 and then loses 2 pounds per month between post-operative measurements 1 and 2, the cumulative absolute fluctuation in weight at visit 2 is 5 pounds per month. In other words, cumulative absolute weight fluctuation takes into account fluctuations in weight over time that reflect both weight gain and weight loss, not weight gain alone or weight loss alone.
Table 1.
Example of how the four weight change variables “Percent Change in Weight From Pre-Operative,” “Weight Fluctuation from Previous Visit,” “Absolute Weight Fluctuation from Previous Visit,” and “Cumulative Absolute Weight Fluctuation from Pre-Operative” were calculated for a fictional patient measured at 1-month intervals from pre-operative.
| Variable | Weight (lbs) | |||
|---|---|---|---|---|
| Pre-op | Month 1 | Month 2 | Month 3 | |
| 140 | 145 | 150 | 148 | |
| Percent change in weight from pre-op (%) | - | 3.6 | 7.1 | 5.7 |
| Weight fluctuation from previous visit (lbs/mo) | - | 5.0 | 5.0 | −2.0 |
| Absolute weight fluctuation from previous visit (lbs/mo) | - | 5.0 | 5.0 | 2.0 |
| Cumulative absolute weight fluctuation from pre-op (lbs/mo) | - | 5.0 | 10 | 12 |
RESULTS
Patient Characteristics
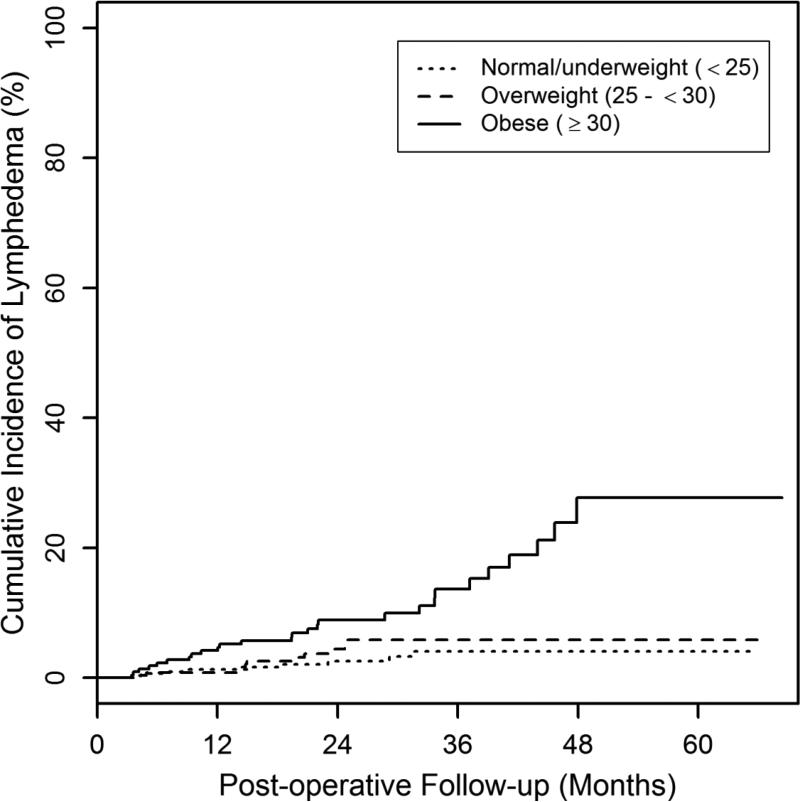
787 patients met eligibility criteria. Patient demographic and treatment characteristics are listed in Table 2. The median post-operative follow-up was 27 months (range: 6-68), and the median age at diagnosis was 56 (range: 27–89). The median pre-operative BMI was 26 kg/m2 (range: 17-50). At the pre-operative measurement, 40% (311/787) of patients had a BMI<25, 33% (258/787) had a BMI 25-<30 and 28% (218/787) had a BMI≥30. The cumulative incidence of lymphedema was 5% (95% CI: 3.5%-6.9%) at 2 years post-operative. The cumulative incidence of lymphedema at 2 years post-operative for patients with a pre-operative BMI<25 was 2.6% (95% CI: 1.2%-5.5%), 25-<30 was 4.4% 2.3%-8.4%), and ≥30 was 8.9% (5.6%-14.1%) (Figure 1).
Table 2.
Univariate analysis of patient demographic and treatment characteristics (N=787).
| Characteristic | Number (Percent) or Median (Range) | HR | CL (Lower–Upper) | P-value |
|---|---|---|---|---|
| Age at Diagnosis (yr) | 56 (27–89) | 1.00 | 0.979–1.03 | 0.712 |
| Pre-operative BMI (kg/m2) | 26 (17–50) | |||
| <25 | 311 (40) | - | - | - |
| 25−<30 | 258 (33) | 1.49 | 0.618–3.60 | 0.374 |
| ≥30 | 218 (28) | 4.46 | 2.10–9.48 | 0.0001a |
| Breast Surgery | ||||
| Lumpectomy | 597 (76) | - | - | - |
| Mastectomy | 190 (24) | 2.35 | 1.31–4.21 | 0.004 |
| Axillary Surgery | ||||
| None | 106 (13) | - | - | - |
| SLNB | 505 (64) | 0.724 | 0.199–2.63 | 0.624 |
| ALND | 176 (22) | 10.2 | 5.04–20.6 | <0.0001b |
| Total # positive LNs | 0 (0–39) | 1.08 | 1.04–1.12 | <0.0001 |
| Neoadjuvant Chemotherapy | ||||
| No | 722 (92) | - | - | - |
| Yes | 65 (8) | 3.78 | 1.92–7.44 | 0.0001 |
| Adjuvant Chemotherapy | ||||
| No | 494 (63) | - | - | - |
| Yes | 293 (37) | 3.59 | 1.94–6.63 | <0.0001 |
| Hormone Therapy | ||||
| No | 237 (30) | - | - | - |
| Yes | 550 (70) | 1.23 | 0.638–2.37 | 0.536 |
| Radiation Therapy | ||||
| None | 140 (18) | - | - | - |
| Breast/chest wall only | 507 (64) | 0.328 | 0.131–0.826 | 0.018 |
| Breast/chest wall + RLNR | 140 (18) | 4.50 | 2.09–9.68 | 0.0001 |
| Weight Change | ||||
| % change from pre-op | 0.3 (−29–37) | 0.985 | 0.943–1.03 | 0.508 |
| Fluctuation from previous visit (lbs/mo) | 0 (−33–38) | 0.895 | 0.751–1.07 | 0.216 |
| Absolute fluctuation from previous visit (lbs/mo) | 0.6 (0–38) | 1.13 | 1.03–1.23 | 0.007 |
| Cumulative absolute fluctuation from pre-op (lbs/mo) | 3.1 (0–40) | 1.10 | 1.07–1.13 | <0.0001 |
HR hazard ratio, CL confidence limit, BMI body mass index, SLNB sentinel lymph node biopsy, ALND axillary lymph node dissection, LN lymph node, RLNR regional lymph node radiation
Age and # positive LNs analyzed as a continuous variables. HRs reported per 1-unit increase in the variables.
Fig.1.
Cumulative incidence of lymphedema by BMI category. The cumulative incidence of lymphedema for women at 2 years post-operative with a pre-operative BMI<25, 25-<30, or ≥30 was 2.6%, 4.4%, and 8.9%, respectively
Analysis of post-operative weight change revealed a median increase in weight from pre-operative of 0.3% (range: −28.5–36.6%), with a median weight change from previous measurement of 0 lbs/month (range: −32.6–37.5 lbs/month) and a median absolute fluctuation of 0.6 pounds gained or lost per month from previous measurement (range: 0–37.5 lbs/month). The median cumulative absolute weight fluctuation was 3.1 pounds gained or lost per month from pre-operative measurement (range: 0–39.9 lbs/month).
BMI, Weight Change, and Risk of Developing Lymphedema
Univariate results for association of demographic and treatment factors with risk of lymphedema are shown in Table 2. Patients with a pre-operative BMI≥30 had a 4.5-fold increased risk of lymphedema compared to BMI<25 (p = 0.0001), and a 3.0-fold increased risk compared to BMI 25-<30 (p=0.002). Pre-operative BMI 25-<30 was not significantly associated with increased risk of lymphedema as compared to BMI<25 (p=0.374).
By univariate analysis, absolute weight fluctuation from previous measurement was significantly associated with an increased risk of lymphedema; for each pound gained or lost per month from previous measurement, there was a 1.13-fold increased risk of developing lymphedema (p = 0.007). The cumulative absolute weight fluctuation from pre-operative was also significantly associated with increased risk of lymphedema; each pound gained or lost per month from pre-operative incurred a 1.01-fold increased risk of lymphedema (p = <0.0001). Percent change in weight from pre-operative was not significantly associated with lymphedema risk (p = 0.508), nor was the weight fluctuation from previous measurement (p = 0.216). Univariate values for other factors are listed in Table 2.
By multivariate analysis, an increased risk of lymphedema was significantly associated with the following factors: pre-operative BMI≥30 compared to pre-operative BMI<25 and BMI 25-<30 (HR: 3.58, p = 0.0011 and HR: 2.46, p = 0.012, respectively), regional lymph node radiation (RLNR) (HR: 2.67, p = 0.011), and ALND (HR: 4.47, p = 0.0003). Cumulative absolute fluctuation in weight from pre-operative was also significantly associated with an increased risk of lymphedema (HR: 1.07 for every 1 lb/month change in weight, p < 0.0001) (Table 3). This means that a cumulative weight fluctuation of 10 pounds gained/lost per month would result in a 1.97-fold increased risk of lymphedema. Furthermore, a cumulative weight fluctuation of 13.3 pounds gained/lost per month would result in a 2.46-fold increased risk of lymphedema, which is equivalent to the increase in risk associated with pre-operative BMI≥30 compared to pre-operative BMI 25-<30. A pre-operative BMI 25-<30 (p = 0.409) was not significantly associated with increased lymphedema risk compared to pre-operative BMI<25 (p = 0.409).
Table 3.
Multivariate results for association of demographic and treatment factors with risk of lymphedema development (RVC≥10%).
| Variable | HR | Lower CL | Upper CL | P-value |
|---|---|---|---|---|
| ALND (vs. SLNB) | 4.47 | 1.98 | 10.1 | 0.0003 |
| BMI≥30 (vs. 25−<30) | 2.46 | 1.22 | 4.99 | 0.012 |
| BMI≥30 (vs. <25) | 3.58 | 1.66 | 7.70 | 0.001 |
| RLNR (vs. breast/chest wall only/none) | 2.67 | 1.25 | 5.70 | 0.011 |
| Cumulative absolute fluctuation in weight from pre-op (1 lb/mo change in weight) | 1.07 | 1.04 | 1.11 | <0.001 |
ALND axillary lymph node dissection, SLNB sentinel lymph node biopsy, BMI body mass index, RLNR regional lymph node radiation
DISCUSSION
Our data suggest that a pre-operative BMI≥30 significantly increases the risk of lymphedema, whereas a pre-operative BMI of 25-<30 does not significantly increase risk. We also found that cumulative absolute weight fluctuation from pre-operative increases the risk of lymphedema. Specifically, large post-operative weight fluctuations (as a gain or loss), are associated with increased risk of lymphedema. Patients with a pre-operative BMI≥30 or those who experience significant absolute weight fluctuations (i.e. a combination of both weight loss and weight gain over time, but not each independently) should be closely monitored for lymphedema.
In this study, a pre-operative BMI≥30 was significantly associated with an increased risk of developing lymphedema compared to a pre-operative BMI<25 or 25-<30. This finding supports the findings of Ridner et al, who investigated the impact of BMI and obesity on the development of lymphedema in 138 patients utilizing perometer arm volume measurements and symptom assessment pre-operatively and up to 30 months post-operatively. They found that a pre-treatment BMI≥30 significantly increased the risk of developing lymphedema by 3.6-fold compared to a pre-treatment BMI<30 [23]. Similarly, we found that a pre-operative BMI≥30 significantly increased the risk of developing lymphedema by 3.6-fold compared to a pre-operative BMI<25 and by 2.5-fold compared to a pre-operative BMI of 25-<30. Since weight loss prior to breast cancer treatment is not feasible, we suggest that these high-risk patients be carefully monitored for the development of lymphedema. Arm measurements prior to and after breast cancer treatment may be especially critical in those patients with a pre-operative BMI≥30. In addition, education regarding lymphedema risk should begin pre-operatively and continue post-operatively with appropriate instruction concerning potential risk reduction and management strategies.
Many studies that have reported on the association between BMI and lymphedema risk are limited by inaccurate lymphedema assessment techniques and timing (Table 4). In this way, the use of Perometry and pre-operative assessment of lymphedema provide strength to our study. The Perometer is known to ensure accurate and reliable data for quantifying lymphedema as a volume change between arms [35]. Obtaining pre-operative arm volume measurements is also critical to allow for the normal asymmetry which may exist between arms to be considered when assessing post-operative changes [5,36]. Although Ahmed et al., Norman et al, and Meeske et al. identified BMI≥30 as a risk factor for lymphedema among cohorts of 1287, 631, and 494 patients, respectively, they measured lymphedema by self-report and they did not incorporate pre-operative lymphedema assessment [8,11,13]. Kwan et al. revealed a similar finding among a cohort study of 997 patients, but lymphedema diagnosis was based upon medical record review and BMI data were also collected via self-report [24]. Other studies identifying a significant association between BMI and lymphedema risk have utilized water displacement or circumferential arm measurements to measure lymphedema [7,15,25-29,31], but these methods are subject to greater error and are more time-consuming than Perometry.
Table 4.
Previous studies reporting on the association of BMI and/or weight gain with lymphedema development vary in sample size, follow-up time, lymphedema assessment method, the use of pre-operative lymphedema assessment, lymphedema incidence, and findings.
| Author, Year | Sample Size | Follow-Up (mo) | LE assessment method | Pre-op LE assessment | LE incidence (%) | BMI (kg/m2) | Weight Gain |
|---|---|---|---|---|---|---|---|
| Ridner, 201123 | 138 | Max: 30 | Perometry (≥200ml or ≥10% difference) & symptoms | Yes | 19.6 | ≥30 | No |
| Ahmed, 20118 | 1287 | Mean: 97 | Self-report via questionnaire | No | 8 | ≥30 | N/A |
| Kwan, 201024 | 997 | Mean: 20.9 | Electronic medical record review | N/A | 13.3 | ≥30 | N/A |
| Goldberg, 201015 | 600 | Median : 60 | Circumferential measurement (>2cm difference) | Yes | 5 | 32 | No |
| Norman, 201011 | 631 | Max: 60 | Self-report via questionnaire | No | 37.7 | ≥30 | N/A |
| Helyer, 201025 | 137 | Median : 20 | Water displacement (≥200ml difference) & symptoms | Yes | 11.7 | > 30 | N/A |
| Swenson, 200926 | 188 | Median : 46 | Circumferential measurement (>2cm difference) & symptoms | No | N/A | ≥25 | N/A |
| Meeske, 200913 | 494 | Mean: 50 | Self-report via interview | No | 24 | ≥30 | N/A |
| Cormier, 200922 | 269 | Median : 29 | Perometry (≥5% change)& symptoms | Yes (for 159 patients) | 61.3 | No | Yes |
| McLaughlin, 20087 | 936 | Median : 60 | Circumferential measurement (>2cm difference) | Yes | 9 | 29 | No |
| Vignes, 200727 | 807 | N/A | Circumferential measurement | No | N/A | 27.1 | N/A |
| Clark, 200529 | 188 | 36 | Circumferential measurement (≥20% difference or ≥5% change from baseline) | Yes | 20.7 | ≥26 | N/A |
| Petrek, 200130 | 263 | 240 | Circumferential self-measurement (>1.27cm or ≤1.27cm difference with symptoms) | No | 49 | No | Yes |
| Werner, 199131 | 282 | Median : 37 | Circumferential self-measurement (≥2.5cm difference) | No | 19.5 | ≥27.3 | N/A |
LE lymphedema, BMI body mass index
It is also important to note that, to our knowledge, this is the largest cohort study (N=787) investigating the association between BMI and lymphedema risk using a lymphedema diagnosis based on relative arm volume change criterion (i.e. the RVC equation). Relative arm volume change can be used to accurately quantify lymphedema independent of body size. This is in contrast to the use of absolute arm size change, in which specificity depends strongly on patient body size. Recently, Ancukiewicz et al. conducted a study in which the authors evaluated the effect of an absolute change in arm size of 200 mL or 2 cm compared with relative arm volume change as criteria for defining lymphedema [33]. They found that temporal changes in the absolute volume of the unaffected arm were correlated with body size measures such as pre-operative arm volume, BMI and weight, whereas relative arm volume changes were not. Thus, when lymphedema diagnosis is based on absolute criteria, the specificity depends on body size, increasing the chances of a false positive lymphedema diagnosis – crossing at random a threshold of specific size, for example, 2 cm or 200 mL – for larger patients. Conversely, when using relative arm volume change, the magnitude of random variation does not depend on patient body size. Many of the studies listed in Table 4 used absolute criteria (200 ml or 2 cm) to diagnose lymphedema, which may have necessarily correlated with patient BMI. Thus, high BMI could result in spurious associations with lymphedema because of false positives related to the use of absolute arm size changes and the fact that arm size fluctuations unrelated to lymphedema have higher magnitude in such patients.
In our series, a pre-operative BMI of 25-<30 was not significantly associated with an increased risk of lymphedema. Previous reports on the association of BMI 25-<30 with lymphedema risk (Table 4) are both conflicting and confounded by the limitations described above. A few prior studies have reported that a BMI≥25 is significantly associated with an increased risk of developing lymphedema. In Swenson et al.'s case-control study of 188 patients, the authors utilized circumferential tape measurement and symptom assessment to assess patients at only one post-operative time point, did not perform pre-operative assessments, and utilized self-reported BMI data [26]. Incorporation of pre-operative arm volume measurements and contralateral arm measurements in lymphedema quantification is essential to account for any normal asymmetry between arms and changes in arm volume which may be caused by factors unrelated to lymphedema, such as weight gain. Likewise, Soran et al.'s case-control study of 156 women suggests that a BMI≥25 increases lymphedema risk, yet their study is retrospective in nature and uses a relatively small sample size [28]. Furthermore, Clark et al.'s finding that a BMI≥26 increases lymphedema risk is similarly limited by the use of circumferential tape measurements and 25.1% loss to follow-up resulting in a relatively small sample size of 188 patients [29]. In light of these limitations and lack of evidence from more recent studies supporting BMI≥25 as a risk factor for lymphedema, our finding that a pre-operative BMI 25-<30 does not increase lymphedema risk may be significant; however further research is warranted.
In this study, we found that large post-operative fluctuations in weight (a combination of increases and decreases, and not weight gain or weight loss alone) significantly increased a patient's risk of developing lymphedema. Specifically, a cumulative absolute weight fluctuation of 10 pounds gained/lost per month resulted in a 1.97-fold increased risk of lymphedema. Perhaps more illustrative is the fact that a cumulative absolute weight fluctuation of 13.3 pounds gained/lost per month resulted in a 2.46-fold increased risk of lymphedema, which is equivalent to the increase in risk associated with pre-operative BMI≥30 compared to pre-operative BMI 25-<30. To our knowledge, this is the first study to report on the association of cumulative absolute weight fluctuation with lymphedema risk. Other studies have reported on weight gain alone as a risk factor for lymphedema. Cormier et al. and Petrek et al. proposed that post-treatment weight gain is a risk factor for lymphedema [22,30]. Conversely, studies by Goldberg et al. and McLaughlin et al. suggest that weight gain does not predict lymphedema development [7,15] (Table 4). Weight gain is a common issue for many women during breast cancer treatment and has been linked to poorer breast cancer prognosis, serious co-morbid conditions such as diabetes and cardiovascular disease, functional decline, and poorer health and overall quality of life [22,37,38-42]. Among these treatment-associated side effects, the potential association between weight gain and increased lymphedema risk provides another reason for the importance of weight management during and after breast cancer treatment as a means to potentially reduce risk.
In addition to weight gain, weight loss alone was not significantly associated with increased lymphedema risk in our study. This finding is consistent with those of the current literature. In fact, the work of Shaw et al. suggests that “weight loss achieved by dietary advice to reduce energy intake can reduce breast cancer-related lymphedema significantly” [43]. This conclusion was the result of a randomized-controlled 12-week intervention trial in which 21 women were randomized either to receive dietary advice for weight reduction or to receive a booklet on general healthy eating. They found a significant reduction in lymphedema, body weight, and BMI among the weight-reduction group at the end of the intervention period. Importantly, lymphedema was measured via circumferential arm measurements with the use of absolute arm volume change, suggesting that weight loss necessarily contributed to a change in arm volume. Our findings suggest that a 10 pound cumulative absolute fluctuation in weight (i.e. a combination of both weight gain and loss) per month increases the risk of developing lymphedema almost 2.0-fold. Although the etiology is unclear, we hypothesize that dramatic weight loss may leave the outlying skin elongated and overstretched as the area underneath it had been previously occupied by adipose tissue. This overly extensible soft tissue state may impact lymphatic drainage, as the superficial lymphatic structures may not have regained enough contractility to transport the lymphatic load effectively.
Our study is limited in that we did not include any correlative symptom or quality of life data to assess lymphedema in conjunction with objective Perometer measurements. In addition, lymphedema and BMI assessments were not conducted at the same time points among all patients; rather, patients were evaluated every 3-8 months after surgery depending upon when their next visit to the clinic was. As a result of this non-standard measurement schedule, women who experienced lymphedema symptoms tended to be measured more often, increasing the likelihood for observing an RVC>10% in these women compared to those who were not symptomatic but had an elevated RVC.
In our series, a pre-operative BMI≥30 was associated with an increased risk of developing lymphedema, whereas a pre-operative BMI of 25-<30 was not. Large post-operative weight fluctuation (as a combination of weight gain and weight loss, not each individually) relative to pre-operative may also be predictive of increased lymphedema risk (i.e. a cumulative weight fluctuation of 10 pounds gained/lost per month increases the risk of developing lymphedema nearly 2.0-fold). Patients with a pre-operative BMI≥30 and those who experience significant post-operative weight fluctuations should be closely monitored for lymphedema given their increased risk. Lymphedema monitoring and education should be initiated prior to breast cancer treatment and continue post-operatively with the incorporation of appropriate risk reduction and management strategies.
ACKNOWLEDGEMENTS
The project described was supported by Award Number R01CA139118 (AGT), Award Number P50CA089393 (AGT) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women's Health Study. J Clin Oncol. 2008;26(35):5689–5696. doi: 10.1200/JCO.2008.16.4731. doi:JCO.2008.16.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. J Clin Oncol. 2008;26(21):3536–3542. doi: 10.1200/JCO.2007.14.4899. doi:26/21/3536. [DOI] [PubMed] [Google Scholar]
- 3.Jager G, Doller W, Roth R. Quality-of-life and body image impairments in patients with lymphedema. Lymphology. 2006;39(4):193–200. [PubMed] [Google Scholar]
- 4.Chachaj A, Malyszczak K, Pyszel K, Lukas J, Tarkowski R, Pudelko M, Andrzejak R, Szuba A. Physical and psychological impairments of women with upper limb lymphedema following breast cancer treatment. Psychooncology. 2009;19(3):299–305. doi: 10.1002/pon.1573. doi:10.1002/pon.1573. [DOI] [PubMed] [Google Scholar]
- 5.Armer JM, Stewart BR. Post-breast cancer lymphedema: incidence increases from 12 to 30 to 60 months. Lymphology. 2010;43(3):118–127. [PMC free article] [PubMed] [Google Scholar]
- 6.Disipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013 doi: 10.1016/S1470-2045(13)70076-7. doi:S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, Hurley KE, Riedel ER, Van Zee KJ. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26(32):5213–5219. doi: 10.1200/JCO.2008.16.3725. doi:JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed RL, Schmitz KH, Prizment AE, Folsom AR. Risk factors for lymphedema in breast cancer survivors, the Iowa Women's Health Study. Breast Cancer Res Treat. 2011;130(3):981–991. doi: 10.1007/s10549-011-1667-z. doi:10.1007/s10549-011-1667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16(7):1959–1972. doi: 10.1245/s10434-009-0452-2. doi:10.1245/s10434-009-0452-2. [DOI] [PubMed] [Google Scholar]
- 10.Shih YC, Xu Y, Cormier JN, Giordano S, Ridner SH, Buchholz TA, Perkins GH, Elting LS. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009;27(12):2007–2014. doi: 10.1200/JCO.2008.18.3517. doi:JCO.2008.18.3517. [DOI] [PubMed] [Google Scholar]
- 11.Norman SA, Localio AR, Kallan MJ, Weber AL, Torpey HA, Potashnik SL, Miller LT, Fox KR, DeMichele A, Solin LJ. Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2734–2746. doi: 10.1158/1055-9965.EPI-09-1245. doi:1055-9965.EPI-09-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JH, Lee WH, Chung HS. Incidence and risk factors of breast cancer lymphoedema. J Clin Nurs. 2008;17(11):1450–1459. doi: 10.1111/j.1365-2702.2007.02187.x. doi:JCN2187. [DOI] [PubMed] [Google Scholar]
- 13.Meeske KA, Sullivan-Halley J, Smith AW, McTiernan A, Baumgartner KB, Harlan LC, Bernstein L. Risk factors for arm lymphedema following breast cancer diagnosis in Black women and White women. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-008-9940-5. doi:10.1007/s10549-008-9940-5. [DOI] [PubMed] [Google Scholar]
- 14.Hayes SB, Freedman GM, Li T, Anderson PR, Ross E. Does axillary boost increase lymphedema compared with supraclavicular radiation alone after breast conservation? Int J Radiat Oncol Biol Phys. 2008;72(5):1449–1455. doi: 10.1016/j.ijrobp.2008.02.080. doi:S0360-3016(08)03264-1. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg JI, Wiechmann LI, Riedel ER, Morrow M, Van Zee KJ. Morbidity of sentinel node biopsy in breast cancer: the relationship between the number of excised lymph nodes and lymphedema. Ann Surg Oncol. 2010;17(12):3278–3286. doi: 10.1245/s10434-010-1155-4. doi:10.1245/s10434-010-1155-4. [DOI] [PubMed] [Google Scholar]
- 16.Paskett ED, Naughton MJ, McCoy TP, Case LD, Abbott JM. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2007;16(4):775–782. doi: 10.1158/1055-9965.EPI-06-0168. doi:16/4/775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Querci della Rovere G, Ahmad I, Singh P, Ashley S, Daniels IR, Mortimer P. An audit of the incidence of arm lymphoedema after prophylactic level I/II axillary dissection without division of the pectoralis minor muscle. Ann R Coll Surg Engl. 2003;85(3):158–161. doi: 10.1308/003588403321661299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crosby MA, Card A, Liu J, Lindstrom WA, Chang DW. Immediate breast reconstruction and lymphedema incidence. Plast Reconstr Surg. 2012;129(5):789e–795e. doi: 10.1097/PRS.0b013e31824a2ab1. doi:10.1097/PRS.0b013e31824a2ab1. [DOI] [PubMed] [Google Scholar]
- 19.Herd-Smith A, Russo A, Muraca MG, Del Turco MR, Cardona G. Prognostic factors for lymphedema after primary treatment of breast carcinoma. Cancer. 2001;92(7):1783–1787. doi: 10.1002/1097-0142(20011001)92:7<1783::aid-cncr1694>3.0.co;2-g. doi:10.1002/1097-0142(20011001)92:7<1783. [DOI] [PubMed] [Google Scholar]
- 20.Graham P, Jagavkar R, Browne L, Millar E. Supraclavicular radiotherapy must be limited laterally by the coracoid to avoid significant adjuvant breast nodal radiotherapy lymphoedema risk. Australas Radiol. 2006;50(6):578–582. doi: 10.1111/j.1440-1673.2006.01658.x. doi:ARA1658. [DOI] [PubMed] [Google Scholar]
- 21.Coen JJ, Taghian AG, Kachnic LA, Assaad SI, Powell SN. Risk of lymphedema after regional nodal irradiation with breast conservation therapy. Int J Radiat Oncol Biol Phys. 2003;55(5):1209–1215. doi: 10.1016/s0360-3016(02)04273-6. doi:S0360301602042736. [DOI] [PubMed] [Google Scholar]
- 22.Cormier JN, Xing Y, Zaniletti I, Askew RL, Stewart BR, Armer JM. Minimal limb volume change has a significant impact on breast cancer survivors. Lymphology. 2009;42(4):161–175. [PMC free article] [PubMed] [Google Scholar]
- 23.Ridner SH, Dietrich MS, Stewart BR, Armer JM. Body mass index and breast cancer treatment-related lymphedema. Support Care Cancer. 2011;19(6):853–857. doi: 10.1007/s00520-011-1089-9. doi:10.1007/s00520-011-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwan ML, Darbinian J, Schmitz KH, Citron R, Partee P, Kutner SE, Kushi LH. Risk factors for lymphedema in a prospective breast cancer survivorship study: the Pathways Study. Arch Surg. 2010;145(11):1055–1063. doi: 10.1001/archsurg.2010.231. doi:145/11/1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helyer LK, Varnic M, Le LW, Leong W, McCready D. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J. 2010;16(1):48–54. doi: 10.1111/j.1524-4741.2009.00855.x. doi:TBJ855. [DOI] [PubMed] [Google Scholar]
- 26.Swenson KK, Nissen MJ, Leach JW, Post-White J. Case-control study to evaluate predictors of lymphedema after breast cancer surgery. Oncol Nurs Forum. 2009;36(2):185–193. doi: 10.1188/09.ONF.185-193. doi:HQ16X6622407521W. [DOI] [PubMed] [Google Scholar]
- 27.Vignes S, Arrault M, Dupuy A. Factors associated with increased breast cancer-related lymphedema volume. Acta Oncol. 2007;46(8):1138–1142. doi: 10.1080/02841860701403020. doi:778575677. [DOI] [PubMed] [Google Scholar]
- 28.Soran A, D'Angelo G, Begovic M, Ardic F, Harlak A, Samuel Wieand H, Vogel VG, Johnson RR. Breast cancer-related lymphedema--what are the significant predictors and how they affect the severity of lymphedema? Breast J. 2006;12(6):536–543. doi: 10.1111/j.1524-4741.2006.00342.x. doi:TBJ342. [DOI] [PubMed] [Google Scholar]
- 29.Clark B, Sitzia J, Harlow W. Incidence and Risk of Arm Oedema Following Treatment for Breast Cancer: A Three-Year Follow-up Study. Q J Med. 2005;98:343–348. doi: 10.1093/qjmed/hci053. [DOI] [PubMed] [Google Scholar]
- 30.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92(6):1368–1377. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. doi:10.1002/1097-0142(20010915)92:6<1368. [DOI] [PubMed] [Google Scholar]
- 31.Werner RS, McCormick B, Petrek J, Cox L, Cirrincione C, Gray JR, Yahalom J. Arm edema in conservatively managed breast cancer: obesity is a major predictive factor. Radiology. 1991;180(1):177–184. doi: 10.1148/radiology.180.1.2052688. [DOI] [PubMed] [Google Scholar]
- 32.Ancukiewicz M, Russell TA, Otoole J, Specht M, Singer M, Kelada A, Murphy CD, Pogachar J, Gioioso V, Patel M, Skolny M, Smith BL, Taghian AG. Standardized method for quantification of developing lymphedema in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79(5):1436–1443. doi: 10.1016/j.ijrobp.2010.01.001. doi:S0360-3016(10)00018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ancukiewicz M, Miller CL, Skolny MN, O'Toole J, Warren LE, Jammallo LS, Specht MC, Taghian AG. Comparison of relative versus absolute arm size change as criteria for quantifying breast cancer-related lymphedema: the flaws in current studies and need for universal methodology. Breast Cancer Res Treat. 2012;135(1):145–152. doi: 10.1007/s10549-012-2111-8. doi:10.1007/s10549-012-2111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. 2005;3(4):208–217. doi: 10.1089/lrb.2005.3.208. doi:10.1089/lrb.2005.3.208. [DOI] [PubMed] [Google Scholar]
- 35.Stanton AW, Northfield JW, Holroyd B, Mortimer PS, Levick JR. Validation of an optoelectronic limb volumeter (Perometer). Lymphology. 1997;30(2):77–97. [PubMed] [Google Scholar]
- 36.Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112(12):2809–2819. doi: 10.1002/cncr.23494. doi:10.1002/cncr.23494. [DOI] [PubMed] [Google Scholar]
- 37.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23(7):1370–1378. doi: 10.1200/JCO.2005.01.079. doi:JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 38.Hoskin PJ, Ashley S, Yarnold JR. Weight gain after primary surgery for breast cancer--effect of tamoxifen. Breast Cancer Res Treat. 1992;22(2):129–132. doi: 10.1007/BF01833342. [DOI] [PubMed] [Google Scholar]
- 39.Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, Blackwell K, Rimer BK. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19(9):2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 40.Huntington MO. Weight gain in patients receiving adjuvant chemotherapy for carcinoma of the breast. Cancer. 1985;56(3):472–474. doi: 10.1002/1097-0142(19850801)56:3<472::aid-cncr2820560310>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. doi:10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 42.Enger SM, Greif JM, Polikoff J, Press M. Body weight correlates with mortality in early-stage breast cancer. Arch Surg. 2004;139(9):954–958. doi: 10.1001/archsurg.139.9.954. discussion 958-960. doi:10.1001/archsurg.139.9.954. [DOI] [PubMed] [Google Scholar]
- 43.Shaw C, Mortimer P, Judd PA. A randomized controlled trial of weight reduction as a treatment for breast cancer-related lymphedema. Cancer. 2007;110(8):1868–1874. doi: 10.1002/cncr.22994. doi:10.1002/cncr.22994. [DOI] [PubMed] [Google Scholar]