Abstract
Visual recognition memory is subserved by a distributed set of neural circuits, which include structures of the temporal lobe. Conflicting experimental results regarding the role of the hippocampus in nonspatial forms of such memories have been attributed to species, task, and lesion discrepancies. We have overcome obstacles that have prevented a direct evaluation of the role of the hippocampus in this type of memory by developing for rats a nonspatial, picture-based, trial-unique, delayed matching-to-sample task that is a procedural analogue of standard visual recognition memory tasks used in primates. With this task, we demonstrate that rats have a visual memory profile, which is analogous to that in primates and depends on the function of perirhinal cortex. We also find that selective lesions of hippocampus impair delay-dependent visual memory with a profile different from that produced by damage to the perirhinal cortex. These data demonstrate that rats have a visual recognition memory system fundamentally similar to primates that depends on the function of the hippocampus.
Most theories of medial temporal lobe function are in agreement that the hippocampus and perirhinal cortex are important for learning and memory. The specific processes that depend on the hippocampus, however, have been the subject of intense debate: in general, agreement exists that hippocampal function is essential for spatial memory, but wide disagreement occurs on the role of the hippocampus in nonspatial, visual recognition memory, or more generally, in explicit or episodic memory (1-5).
The hallmark of this type of memory is retrieval of specific information from a single event (or episode) (6, 7). Trial-unique, delayed, matching-to-sample (DMTS) and delayed, non-matching-to-sample (DNMTS) tasks, in which the participant must choose between two or more items, one of which is an item seen only once previously, have become the standard for measuring recognition memory in humans and nonhuman primates. Studies in primates have clearly shown that damage to perirhinal cortex disrupts performance on these tasks (8). The role of the hippocampus in this form of memory, on the other hand, cannot be as clearly inferred from existing data. For example, hippocampal damage in humans impairs performance on nonverbal recognition tasks, but the results in nonhuman primates are conflicting as to whether selective hippocampal damage impairs performance on visual recognition memory tasks (9-12).
Visual recognition memory tasks have had limited application in rodents, but in general, recognition memory has been found to be unaffected by hippocampal damage unless the memory task includes a spatial component (13-15). A striking example of the apparent spatial dependence of hippocampal function is found in a study by Dudchenko and colleagues (16), in which memory for places or odors was measured in DNMTS procedures. The memory for places, but not odors, was disrupted by selective damage to the hippocampus. Attempts to measure nonspatial visual recognition memory in rodents have been hampered by the lack of trial-unique tasks that are analogous to the primate forms and yield similar performance. In some reports of rat visual recognition in matching-to-sample (MTS) tasks, memory performance declines to chance in seconds (17). In others, real objects are used (13, 18); thus, nonvisual information, including touch and odor cues, are available to the animals (19). Given the absence of either good performance at long delays or the unequivocal use of vision, it is unlikely that the same memory processes have been engaged in the rat tasks as are engaged in primate tasks.
We designed the present study to characterize the rat's visual DMTS performance and to resolve two outstanding questions on the nature of recognition memory: does the hippocampus (and perirhinal cortex) make an essential contribution to nonspatial visual memory, and does the rat hippocampus make a contribution to visual recognition memory analogous to that already identified in humans? To this end, we developed for rats a trial-unique, visual, DMTS task that is a procedural variant of DMTS tasks used in primates. We show that delay-dependent memory in intact rats has a profile that is similar to primates and depends on the integrity of perirhinal cortex. We then investigated the effects on DMTS performance of bilateral, selective damage to hippocampus. The results reveal that the hippocampus is a necessary component of visual recognition memory circuitry and continuity exists between rodents and humans in the contributions of the medial temporal lobe to this form of memory.
Materials and Methods
The DMTS task developed for these experiments is a modification of the visual water task (20). Rats were first trained to make a simple visual discrimination reliably, and then, in three more stages, were gradually shifted to the DMTS task. Black- and-white pictures were displayed on computer monitors as visual stimuli, and rats were reinforced throughout for swimming toward a correct picture where they could escape from water to a submerged platform. In the final stage, during each trial, rats viewed a single picture in a sample phase selected at random from a large set, and then, in a choice phase, they discriminated the sample from a novel picture, selected at random from the same set, with high accuracy. The left/right position of the correct picture varied randomly to make the task nonspatial. The delay dependence of memory for the sample picture was measured by systematically varying the delay (10 sec to 120 min) between the sample and choice phases. Rats were then assigned at random to one of three surgical groups: bilateral aspiration lesions of perirhinal cortex, bilateral N-methyl-d-aspartate-induced hippocampal lesions, or sham lesion surgery. After postsurgical recovery, DMTS performance was remeasured, and performance before and after the surgery was compared.
Animals. Eighteen young adult, experimentally naïve, female Long-Evans laboratory rats were used as subjects. The animals were bred and raised at the Canadian Centre for Behavioural Neuroscience from stock originally obtained from Charles River Breeding Laboratories. Pairs of rats were housed in hanging Plexiglas cages (45 × 25 × 20 cm) in a room with an ambient temperature of 21°C, 35% relative humidity, 12:12 light/dark cycle (lights on at 7:00 a.m. and lights off at 7:00 p.m.), and where food and water were available ad libitum.
Testing Apparatus. Sample pool. The sample pool was a trapezoidal-shaped (140 cm long × 40 cm wide × 25 cm wide) tank (55 cm high) made of dull-finished stainless steel on all surfaces, except the 40-cm end wall, which was made of clear safety glass. The tank was positioned on a solid table, and tap water (22°C) was added to a depth of 15 cm. A transparent, Plexiglas platform (37 × 13 × 14 cm) was submerged in the pool next to the glass wall. A short faux divider (10 × 40 cm; see Choice pool) made of clear Plexiglas was situated at the midline of the tank 46 cm from the glass wall, and a cover made of sheer-weave vinyl material was draped over the middle three fourths of the pool to reduce extramaze visual information.
Choice pool. The choice pool was made of the same materials as the sample pool, but the glass end was 80 cm wide. A 46-cm-long stainless steel divider (40 cm high) was placed in the tank that extended from the middle of the glass wall into the center of the pool, creating a maze with a stem and two arms. A Plexiglas platform, identical with that above, was submerged in the pool at the end of one of the arms, and a vinyl cover was draped over the tank.
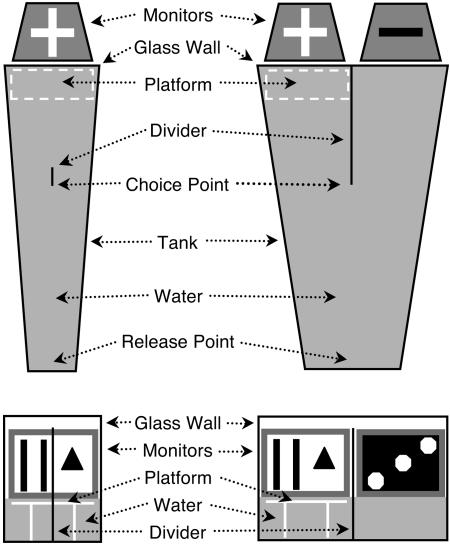
Monitors and computer. A computer monitor (17-inch video graphics array; ViewSonic E70F, Walnut, CA) was faced into the glass end of the sample pool, and two identical monitors were faced into each arm of the choice pool. The bottoms of the screens were situated at water level. The black level and contrast settings of the monitors were equated and the mean luminance of the displays measured from a distance of 46 cm was 43 cd/m2. The monitors were controlled by peripheral component interconnect video cards (Radeon 7000 Mac Edition, ATI Technologies, Markham, ON, Canada) operated over video extension cables by an Apple Macintosh computer (PowerPC G4; 875 MHz). Fig. 1 shows a schematic diagram of the apparatus.
Fig. 1.
Visual water task configured for MTS. (Left) Sample pool. (Right) Choice pool. (Upper) Top view. (Lower) Front view. Sample pool: A sample picture (+) was displayed on a computer monitor that faced into the wide end of a trapezoidal-shaped tank containing water. A platform was hidden under the surface of the water directly below the monitor, and a faux divider was situated 46 cm from the front wall of the tank. Choice pool: The sample image (+) and a novel image (-) were each displayed on monitors facing into the end of a trapezoidal-shaped pool. A 46-cm divider extended into the pool from the front wall of the tank and a platform was hidden under the surface of the water directly below the sample picture.
Stimulus generation and control. More than 100 black-and-white pictures (white on black/black on white; 1,024 × 768 pixels) were drawn in photoshop (Adobe Systems, Mountain View, CA) (see Movie 1, which is published as supporting information on the PNAS web site) and used as visual stimuli. The pictures were displayed on the monitors by a computer program (retrace; CerebralMechanics, Lethbridge, AB, Canada). retrace controlled the presentation of the pictures on the sample and choice displays and the left/right randomization pattern of the pictures on the displays, recorded behavioral responses with the aid of a remote control box, provided control of parameters for individual animals, calculated the behavioral performance of animals, and plotted the data.
DMTS Training and Testing Procedures. Training. Details of the training procedures are presented in Supporting Text, which is published as supporting information on the PNAS web site.
Testing. The effect on MTS accuracy of varying the delay between the sample and choice trials was assessed after the completion of MTS training. Each day, new sample and novel pictures were chosen at random without replacement from the same set of pictures (100 plus pictures in the set) for testing. One delay was assessed over 12 trials each day. Typically, 1-min delay was assessed first, followed the next day by 30 sec, then 10 sec, 2 min, 4 min, 8 min, 16 min, and 120 min. The sequence was then repeated so that 24 trials were assessed at each delay.
In preliminary experiments of this study, we found that a delay of at least 30 sec between the sample and match trials in stage 4 was necessary, because with shorter delays, the side of the faux divider that animals chose to swim to on the sample trial was usually the side they would swim to on the choice trial, regardless of the left/right location of the sample picture. Once animals learned trial-unique, MTS, the minimum 30-sec training delay was no longer necessary.
A probe test was performed to determine whether rats could be detecting the platform hidden below the sample picture rather than remembering the picture itself. In this experiment, MTS accuracy was assessed with 12 trials per day for 2 days by using a 1-min MTS delay. On alternating trials, the platform was placed either below the sample picture or below the novel picture.
Surgery. We investigated the brain substrates of visual recognition memory by measuring DMTS performance in rats with damage to perirhinal cortex or hippocampus. MTS accuracy was first assessed in intact rats at delays between 10 sec and 16 min. Animals were then selected at random for bilateral aspiration lesions of perirhinal cortex, bilateral excitotoxic lesions of the hippocampus, or sham lesion surgery. After 7 days recovery, MTS behavior was reassessed with 24 trials at the same delays, and presurgery performance was compared with postsurgery performance.
Hippocampal lesions. Ten minutes before surgery rats were injected with diazepam (20 mg/kg of body weight i.p.) for its anticonvulsant effect. For surgery, rats were anesthetized with inhaled Isoflurane (induction at 2.5-4.0%, maintenance at 1-2% evaporated in 1-1.5 liters/min O2) and placed in a stereotaxic frame. A topical antibacterial ophthalmic agent (Ventropolycin, Janssen, Toronto) was applied to the eyes and the top of the head was scrubbed with dilute Hibitane and 70% ethanol solutions. A midline incision was made in the scalp and periosteum, and the tissue was retracted to expose the skull. A dental burr was used make five holes in the skull above the hippocampus in each hemisphere. A solution of N-methyl-d-aspartate (3 mg/0.4 ml normal saline) was loaded into polyethylene tubing connected to a 30-gauge cannula and a Hamilton syringe mounted in a syringe pump. The hippocampus was injected with 0.4 μl of the solution at each of 10 sites: 3.1, 4.1, 5.3, and 6.0 mm posterior to bregma, 1.5, 3.0, 3.0, 5.2, and 5.0 mm lateral to bregma, and 3.6, 4.0, 4.0, 7.3, and 7.3 mm ventral to the surface of the skull on both sides of the brain in sequential order. The solution was injected at 0.125 μl/min for 3.5 min, and the cannula was left in place for another 3.5 min after each injection. The incision in the scalp was sutured closed, the animals were injected with an analgesic (0.1 ml of buprenorphine HCl s.c.), and they were allowed to recover on a warm pad. Once animals were showing voluntary movements they were given diazepam (20 mg/kg of body weight i.p.) for its anticonvulsant activity. They were returned to their home cage after ≈3 h of postoperative observation and care. Injections of diazepam continued every 30-60 min for 3 h after surgery. Perirhinal cortex lesions. Rats were prepared for surgery as described above, except that the fascia overlying the muscles on the posterolateral surface of the skull was incised longitudinally along the two parasagittal ridges and, with a blunt spatula, muscle was detached from the skull on each side to expose the bone overlying perirhinal cortex. A trephination was made above the perirhinal cortex, the dura was incised, and the perirhinal cortex was gently aspirated through a small glass pipette under visual guidance. Postsurgical convalescence was the same as above, except diazepam was not administered.
Sham lesion surgery. Sham lesion surgery was identical with that described above, except no holes were made in the skull.
Morphological Assessment of Lesions. Once behavioral testing was completed, all rats were anesthetized and perfused with cold saline followed by buffered 4% paraformaldehyde, and their brains were extracted. The brains were then cryoprotected by storing them in 30% sucrose PBS until sunk; 40-μm coronal sections were then cut through the temporal lobe by using a freezing microtome. The sections were desiccated, stained with cresyl-violet, and mounted on glass slides. The stained sections were digitized and the extent of the lesions was assessed in relation to stereotaxic brain atlas coordinates.
Statistical Analyses. The proportion correct measures were uniformly transformed by using the arcsine transformation before evaluation with a repeated measures ANOVA procedure (spss 11.0) as recommended by refs. 21 and 22. Differences were considered to be statistically significant if the P value was <0.05.
Results
Training. The details of the training results and a video of MTS behavior are presented in Supporting Text and Movie 1.
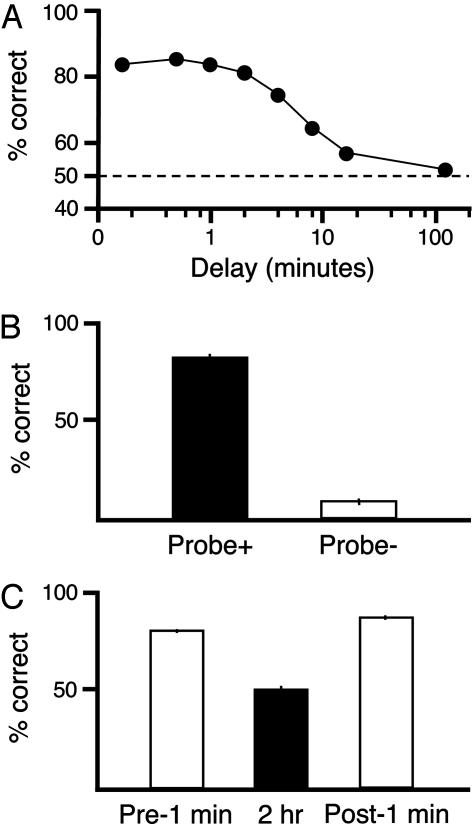
Testing. MTS choice accuracy declined significantly with increasing delay between sample and choice phases [F (6, 66) = 23.1, P < 0.001] as shown in Fig. 2A. At delays of up to 2 min, MTS accuracy was >80%. As the delay was increased, MTS accuracy gradually declined, falling to chance between 16 and 120 min. At the end of delay testing, the rats selected for the different surgery groups showed the same overall levels of MTS accuracy [F (2, 11) = 0.4, P > 0.7] and the groups' choice accuracies declined similarly with delay interval [F (12, 66) = 0.6, P > 0.8]. Accuracy remained above chance (P < 0.5) at all delays up to and including 16 min.
Fig. 2.
MTS performance of intact rats and control tests. (A) MTS accuracy of rats decreased gradually as the delay between the sample and choice phase was increased. Performance of >80% accuracy was maintained with delays up to 2 min. Performance fell with longer delays, but was still above chance at a delay of 16 min. Random performance was seen at a 120-min delay. ±SEMs are smaller than the data point symbols. (B) Probe test. The accuracy of animals during a choice phase with a 1-min delay was 82% when the hidden platform was placed under the sample picture on (Probe +). Accuracy in choosing the sample picture fell to 10% when the hidden platform was placed under the novel picture (Probe -). (C) Long-term stability in delay performance; average choice accuracy with 1-min delay before and after trials completed with 120-min delay. Animals performed with high accuracy (81%) when 1-min delay was tested the first time (Pre1 min), and performance fell to chance (50%) with a 120-min delay was implemented (2 h). When 1-min delay performance was retested on the next day (Post1 min), performance returned to Pre1 min levels (86%).
A hidden platform probe test demonstrated that animals made choices by using the pictures on the monitors and not by direct information from the hidden platform. When the platform was located under the sample picture, MTS accuracy was >80%. However, when the platform was located below the novel picture, on only 10% of the trials did the rat swim to the platform side. The results are illustrated in Fig. 2B and a video of an animal behaving in the platform probe test is presented in Movie 1.
It is possible that the random performance of animals produced by a 120-min delay between the sample and choice phases could cause the rats to forget the rules of the task and bias subsequent performance. This possibility, however, was not the case because the performance of animals with a 1-min delay measured before testing a 120-min delay was not significantly different from performance measured after a 120-min delay (Fig. 2C).
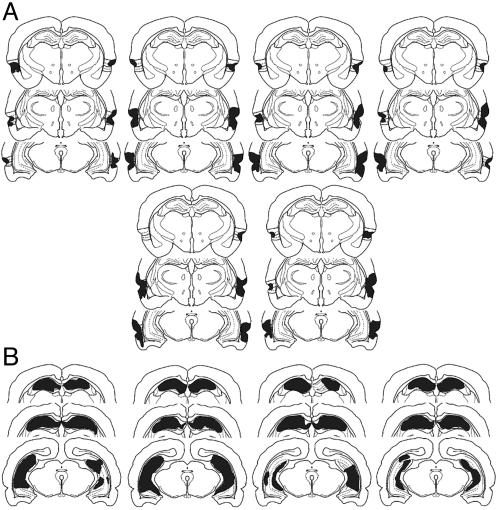
Rats with perirhinal lesions sustained bilateral damage to the perirhinal cortex and inconsistently to the most lateral parts of the lateral entorhinal cortex and suprajacent temporal neocortex (Fig. 3A) but not to the hippocampus or postrhinal cortex. The hippocampal-lesioned rats sustained bilateral damage to >75% of the principal hippocampal subfields, but no damage to adjacent subicular or entorhinal cortices was evident (Fig. 3B). To determine whether more subtle damage might occur in perirhinal cortex after hippocampal N-methyl-d-aspartame injections, we measured bilaterally the thickness of cortex subjacent to the rhinal fissure in three coronal planes separated by ≈250 μm in our hippocampal lesion rats and, with a t test, compared these measurements with those of 10 control rats whose brains were similarly processed histologically. We found that perirhinal cortex thickness did not differ from controls (control = 700.5 μm; hippocampal lesion = 694.3 μm, P = 0.16).
Fig. 3.
Outline of perirhinal cortex and hippocampal lesions. (A) Reconstruction of perirhinal lesions in six animals. Brains were sectioned, stained, and photographed, and the lesions were reconstructed. Three coronal planes of section through perirhinal cortex (from bregma -3.8 mm, -5.3, and -6.72 mm) were chosen for presentation (23) and the reconstructions were superimposed (gray) on line drawings modified from Burwell (24). The size of the lesions varied, with some lesions encroaching on lateral portions of entorhinal cortex and temporal neocortex. All rats, however, sustained bilateral damage to the perirhinal cortex, and none of the lesions involved the hippocampus. (B) Reconstruction of hippocampal lesions in four animals. Brains of animals that sustained hippocampal injections of N-methyl-d-aspartate were sectioned, stained, and photographed, and the lesions were reconstructed. Three coronal planes of section through hippocampus are presented (from bregma -3.8 mm, -5.3, and -6.72 mm) for each animal in the form of line drawings modified from Burwell (24). The extent of hippocampal damage varied (gray), but all lesions covered >75% loss of the principal subfields. In no animal did evidence occur of the lesion encroaching into subicular or entorhinal cortices.
Some animals with hippocampal lesions appeared to be slightly agitated on their first placement into the water after surgery. This tendency, however, did not persist beyond the second day of postsurgical testing. No apparent change occurred in behavior after sham lesion surgery or perirhinal cortex aspirations that might have affected their performance.
We compared the performance of the three groups of rats with operative status as a within-subjects factor. Significant effects of surgery [F (1, 11) = 114.6, P < 0.001] and there was a significant of lesion type [F (2, 11) = 17.5, P < 0.001] occurred. In addition, the effect of surgery significantly interacted with lesion type [F (1, 11) = 43.9, P < 0.001]. To examine this interaction further, we analyzed each lesion group separately comparing pre-with postoperative and the different delay intervals. The Sham lesion control performance was unaffected by surgery, and no significant interaction occurred between surgery and delay interval (F values were <1.0). The perirhinal cortex damage significantly affected performance [F (1, 5) = 705.1, P < 0.001], and there was a significant interaction between surgery and delay interval [F (6, 30) = 5.5, P < 0.001]. Similarly, hippocampal damage significantly affected performance [F (1, 3) = 183.3, P < 0.001], but this effect did not significantly interact with delay interval [F (6, 18) = 0.8, P = 0.55]. For each of the three groups, performance significantly declined with delay interval (P < 0.001).
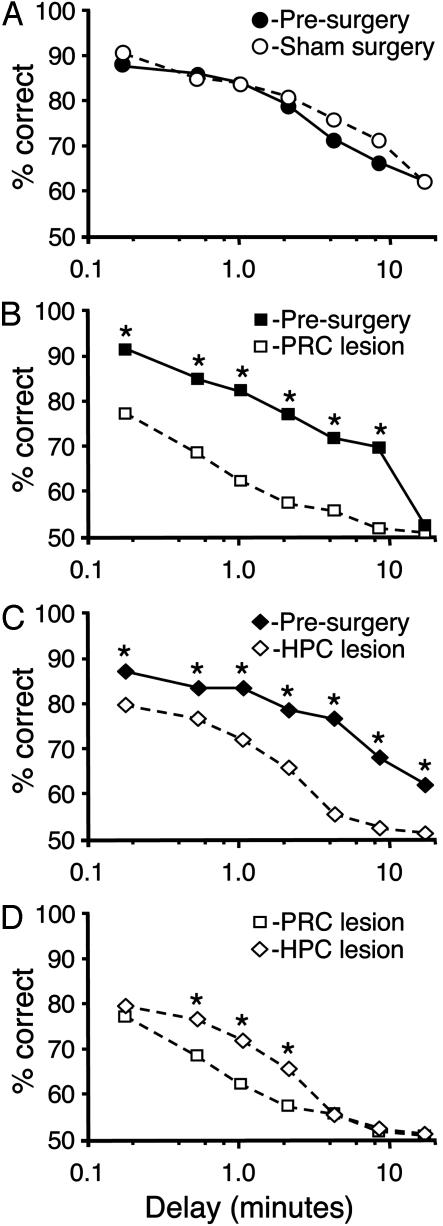
Significant differences occurred in memory performance between the groups [F (2, 11) = 87.8, P < 0.001] after surgery. Damage to both the perirhinal cortex (Fig. 4B) and hippocampus (Fig. 4C) significantly decreased the accuracy of delay-dependent performance compared with sham lesions. The lesion deficits were statistically reliable at each delay interval except the longest tested (16 min; Fisher's least significant difference; all P values were <0.01). Rats with hippocampal damage significantly outperformed those with perirhinal cortex damage at intermediate delay intervals (30, 60, and 120 sec, P values of <0.05; Fig. 4D). The visual memory of sham lesion rats was not significantly affected by surgery at any delay interval (all P values were >0.2; Fig. 4A). Thus, both bilateral perirhinal cortex or hippocampal damage produced reliable deficits in rats with extensive presurgical training, and the memory deficit after hippocampal damage was less pronounced at short delays.
Fig. 4.
Effect of sham surgery, perirhinal cortex removal, and hippocampal lesions on delay-dependent MTS performance. (A) The performance of sham-lesioned animals (n = 5) before (filled circles) and after (open circles) surgery did not differ. (B) Bilateral perirhinal cortex damage dramatically decreased DMTS performance (open squares) compared with prelesion values (filled squares) at all delays except the longest, 16 min. (C) Hippocampal lesions (open diamonds) significantly reduced MTS performance at all delays, compared with prelesion values from the same animals (filled squares). (D) Perirhinal cortex lesions (open squares) resulted in significantly worse performance at delays of 30 sec, 1 min, and 2 min, than did hippocampal lesions (filled diamonds). *, P < 0.05; ±SEMs, which were smaller than the data point symbols.
Discussion
In a one-trial learning task, we demonstrate robust picture-based recognition memory abilities in rats, reminiscent of that found in primates. We anticipate that this task will prove to be useful for testing a form of memory that has so far resisted measurement in nonprimates. Applications of this task can facilitate the use of a wide range of invasive experimental techniques to reveal the neuroanatomical, pharmacological, molecular, and genetic bases of recognition memory that are difficult to evaluate in humans or nonhuman primates. Apart from its use of rodents, our task has several virtues, including being computer-based with a vast array of different, well controlled cues, long-term stable baselines, many tests of memory in daily sessions, accurate retention at relatively long delays, and no need for motivation by using potentially problematic nutrient deprivation, strong aversive stimuli, or novelty-based exploration. In addition, when faced with occasional long delays or other difficulties, the rats also showed no signs that they forgot the procedural elements of the task.
The results validate a pivotal role for the perirhinal cortex in visual recognition memory performance in the rat, as would be predicted from previous work in primates. The substantial deficit in performance in rats with perirhinal lesions, even at the shortest delays, is consistent with the hypothesis that perirhinal circuitry provides a representation of visual information useful for resolving discriminations between similar items, not just for memory (25).
Intact memory at short delays with deficits at longer delay is characteristic of humans with temporal lobe damage, but it has been a matter of some debate whether the same result was obtainable from animal models (22, 26). Experimental work, however, appears to have substantiated this effect in rodents (18, 27, 28): immediate memory (<5 sec) remains intact after temporal lobe damage, whereas delayed memory (1-2 min) is impaired (18). After surgery, our rats were impaired at all delays, including the shortest delay we tested (10 sec). The methodology of our picture-based task in its current form makes testing delays <10 sec difficult, but it is likely that 10 sec is simply not short enough to reveal intact performance after surgery. Another possible explanation is that the lesioned animals may have an enhanced response to the to the transfer procedures between the sample and choice phases of the task. Work is needed on a modification of the task to specifically address these issues.
Of crucial importance to questions regarding the neural substrates of learning and memory, our results reinforce the position that the hippocampus is needed for visual recognition memory, although perirhinal cortex damage produces a more dramatic deficit in performance. The rodent data reported here are consistent with reports of visual recognition memory deficits after damage that includes hippocampus in humans and monkeys (12, 29). Our results also strengthen the argument that deficits after hippocampal damage are not limited to tasks with a spatial memory component and are not necessarily occluded by extensive presurgical training. Furthermore, our results showing hippocampal dependence of memory for pictures in rats stands in marked contrast to the results of Dudchencko et al. (16) showing hippocampal independence of memory for odors. It is now important to determine whether this finding represents a procedural difference, a difference in the hippocampal damage, or a more basic difference in the neural substrates of odor and visual memories.
Across a range of intermediate delay intervals (30-120 sec), rats with hippocampal damage outperformed those with perirhinal damage. This temporal profile suggests that perirhinal and other visual cortical regions can maintain visual memories accurately during intermediate spans and that the effects of perirhinal damage cannot be attributed solely to the disruption of signals going to and coming from the hippocampus. It also indicates that hippocampal deficits may be more difficult to detect in DMTS tasks that do not generate good, long delay performance (10, 11, 30).
In conclusion, we have developed a nonspatial, one-trial visual DMTS task and use it to show that rats have a visual recognition memory system similar to that of humans and monkeys. Using this task, we demonstrate that the hippocampus is an essential part of the neural circuitry for nonspatial recognition memory.
Supplementary Material
Acknowledgments
We thank Sam Lacanilao and Jill Muzylouski for their assistance with histology and Bryan Kolb, Julian Keith, and Derek Hamilton for their helpful comments on the manuscript. This work was supported by a Natural Sciences and Engineering Research Council research grant (to G.T.P.) and National Institutes of Health Grant MH61460 and an Alberta Heritage Foundation for Medical Research Establishment Grant (to R.J.S.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MTS, matching-to-sample; DMTS, delayed MTS; DNMTS, delayed, non-MTS.
References
- 1.Brasted, P. J., Bussey, T. J., Murray, E. A. & Wise, S. P. (2003) Brain 126, 1202-1223. [DOI] [PubMed] [Google Scholar]
- 2.Sutherland, R. J. & Rudy, J. W. (1989) Psychobiology 17, 129-144. [Google Scholar]
- 3.Cohen, N. J. & Eichenbaum, H. (1993) Memory, Amnesia, and the Hippocampal System (MIT Press, Cambridge, MA).
- 4.Squire, L. R. (1992) Psychol. Rev. 99, 195-231. [DOI] [PubMed] [Google Scholar]
- 5.O'Reilly, R. C. & Rudy, J. W. (2001) Psychol. Rev. 108, 311-345. [DOI] [PubMed] [Google Scholar]
- 6.Morris, R. G. (2001) Phil. Trans. R. Soc. London B 356, 1453-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day, M., Langston R. & Morris, R. G. M. (2003) Nature 424, 205-209. [DOI] [PubMed] [Google Scholar]
- 8.Murray, W. A. & Richmond, B. J. (2001) Curr. Opin. Neurobiol. 11, 188-193. [DOI] [PubMed] [Google Scholar]
- 9.Murray, E. A. & Mishkin, M. (1998) J. Neurosci. 18, 6568-6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowalska, D. M., Kusmierek, P., Kosmal, A. & Mishkin, M. (2001) Neuroscience 104, 965-978. [DOI] [PubMed] [Google Scholar]
- 11.Malkova, L. & Mishkin, M. (2003) J. Neurosci. 23, 1956-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zola, S. M., Squire, L. R., Teng, E., Stefanacci, L., Buffalo, E. A. & Clark, R. E. (2000) J. Neurosci. 20, 451-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mumby, D. G. (2001) Behav. Brain Res. 127, 159-181. [DOI] [PubMed] [Google Scholar]
- 14.Nadel, L. (1995) Hippocampus 5, 232-239. [DOI] [PubMed] [Google Scholar]
- 15.Hampson, R. E., Jarrard, L. E. & Deadwyler, S. A. (1999) J. Neurosci. 19, 1492-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudchenko, P. A., Wood, E. R. & Eichenbaum, H. (2000) J. Neurosci. 20, 2964-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews, J. S. & Jansen, J. H. M. (1996) Neurosci. Res. Commun. 18, 115-124. [Google Scholar]
- 18.Clark, R. E., Zola, S. M. & Squire, L R. (2000) J. Neurosci. 20, 8853-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astur, R. S., Klein, R. L., Mumby, D. G., Protz, D. K., Sutherland, R. J. & Martin, G. M. (2002) Neurobiol. Learn. Mem. 78, 186-191. [DOI] [PubMed] [Google Scholar]
- 20.Prusky, G. T., West, P. W. R. & Douglas, R. M. (2000) Vision Res. 40, 2201-2209. [DOI] [PubMed] [Google Scholar]
- 21.Kirk, R. E. (1982) Experimental Design (Wadsworth, Belmont, CA), 2nd Ed.
- 22.Ringo, J. L. (1991) Behav. Brain Res. 42, 123-134. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G. & Watson, C. (1998) The Rat Brain in Stereotaxic Coordinates (Academic, San Diego).
- 24.Burwell, R. D. (2001) J. Comp. Neurol. 437, 17-41. [DOI] [PubMed] [Google Scholar]
- 25.Bussey, T. J., Saksida, L. M. & Murray, E. A. (2003) Eur. J. Neurosci. 17, 649-660. [DOI] [PubMed] [Google Scholar]
- 26.Horel, J. A. (1994) Cortex 30, 269-280. [DOI] [PubMed] [Google Scholar]
- 27.Overman, W. H., Ormsby, G. & Mishkin, M. (1990) Exp. Brain Res. 79, 18-24. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez, P., Zola-Morgan, S. M. & Squire, L. R. (1994) Proc. Natl. Acad. Sci. USA 91, 5637-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duzel, E., Vargha-Khadem, F., Heinze, H. J. & Mishkin, M. (2001) Proc. Natl. Acad. Sci. USA 98, 8101-8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ennaceur, A., Aggleton, J. P. & Fray, P. J., (1997) Neurosci. Res. Commun. 20, 103-111. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.