Abstract
Background
In the human papillomavirus (HPV) era, the best way to assess oropharyngeal squamous carcinomas (SCC) for risk stratification is not clear. Many recommend use of both p16 immunohistochemistry and HPV in situ hybridization (ISH). A significant minority of tumors are p16 positive and HPV ISH negative, the significance of which is unclear.
Methods
Two hundred thirty-nine oropharyngeal SCC were tested by immunohistochemistry for p16 and by ISH for highrisk HPV. For p16 positive, HPV ISH negative cases, PCR was conducted for HPV. The findings were correlated with pathologic and clinical findings.
Results
Of the 239 cases, 187 (78%) were positive for p16. Of these, 139 (74%) were positive for HPV by ISH. Of the remaining 48 cases, 45 had material for PCR. Nineteen were positive for HPV, leaving a group of 26 p16 positive and HPV undetectable SCCs. In the p16 positive cohort, there was no difference in survival between HPV ISH positive and negative cases. Comparing the HPV ISH positive and HPV ISH and PCR negative SCC, there was again no difference in survival. p16 positive, HPV negative SCC still had significantly better survival than p16 negative SCC in univariate and multivariate analysis.
Conclusions
Outcomes for p16 positive, HPV negative oropharyngeal SCC are not significantly different from p16 positive, HPV positive tumors and are significantly better than for p16 negative tumors. These results suggest that p16 immunohistochemistry alone is the best test to use for risk stratification in oropharyngeal SCC.
Keywords: oropharyngeal, squamous cell carcinoma, p16, HPV, in situ hybridization
Oropharyngeal squamous cell carcinoma (SCC) is increasingly recognized as distinct among head and neck SCC because of the common association with human papillomavirus (HPV). On the basis of abundant retrospective and more recent prospective data, HPV-related SCC is now recognized as a unique tumor type, having different demographics, a characteristic molecular profile, and a distinctly better prognosis.2,13,24,29 Epidemiologic studies have shown that HPV-positive SCC more commonly occurs in younger patients of higher socioeconomic status who have higher numbers of sex partners and higher oral sex exposure.9,8,27 In addition, HPV-positive SCC is less strongly associated with alcohol and tobacco use compared with HPV-negative SCC.9 At the molecular level, HPV-positive SCCs less frequently harbor p53 mutations compared with HPV-negative SCC,18 and they almost always overexpress p16, which is uncommon in HPV-negative SCC.16 Finally, these tumors are associated with better patient survival despite a tendency to present with lymph node metastases.2,7,13,19
Although the overall incidence of head and neck SCC has been on the decline, SCC of oropharyngeal sites, in which HPV-positive tumors predominate,32 has been on the rise over the past 30 years. Some have actually classified this as an “epidemic” given its relationship to an infectious agent.21 In light of this and the unique biology of HPV-related SCC, investigation is currently underway to tailor treatment specifically for these tumors. As a result, identification of the HPV status in oropharyngeal SCC has become increasingly important in clinical practice.
The best method for HPV detection remains controversial. Traditional PCR may be too sensitive, potentially amplifying contaminant HPV from the laboratory environment or other specimens, HPV derived from surrounding stroma or adjacent normal epithelium rather than the tumor itself, or from a tumor in which the virus is present but not in sufficient amount or activity to be biologically important in tumorigenesis or clinical behavior. Quantitative PCR methods allow for precise measurement of viral DNA or mRNA in a sample but still do not confirm tumor origin of the virus detected without the use of laser-capture microdissection. These methods are also cumbersome and can be impractical in the clinical setting. In situ hybridization (ISH) for high-risk HPV is more practical in a clinical setting and is quite specific, allowing for direct visualization of virus in tumor cells. However, it is not completely sensitive.15
A frequently recommended strategy is to use a combination of p16 immunohistochemistry followed by ISH for high-risk HPV.2,35 p16 is a tumor suppressor protein that inhibits cyclin-dependent kinase 4A. As such, it is usually absent in head and neck SCC, the gene being mutated or deleted or the expression being abrogated by other mechanisms. In tumors with biologically active HPV, functional inactivation of the retinoblastoma protein (Rb) by HPV E7 protein leads to p16 overexpression because Rb normally represses p16 transcription.2,31 A strong and diffuse pattern of p16 immunostaining is considered a highly sensitive surrogate marker for the identification of HPV-driven tumors. However, it does not guarantee that a tumor is HPV-positive as other pathways may lead to p16 overexpression. In fact, studies have shown that approximately 15% to 20% of p16 positive oropharyngeal SCC cases are HPV ISH negative.31 However, the features of such tumors and their clinical behavior are unknown.
We sought to identify p16 positive, HPV negative SCCs from a large cohort of oropharyngeal SCC patients, to study their clinical and pathologic features, and to specifically compare their outcomes with tumors that are both p16 and HPV positive.
MATERIALS AND METHODS
Cases of oropharyngeal SCC were identified from clinical databases from the divisions of radiation oncology and head and neck surgery at Washington University. The cases were from 1997 to 2008. All patients who received radiation received intensity modulated radiation therapy (IMRT). None of the patients were treated with regard to their p16 or HPV status, as we did not routinely test the tumors in clinical practice for these markers nor were there specific treatments at our hospital based on such testing. Corresponding blocks and slides were retrieved from the files of the Barnes Jewish hospital. Hematoxylin and eosin (H&E) slides were reviewed by one of the researchers (JSL) without knowledge of clinical follow-up or outcome, and the tumors categorized using histologic features, as published earlier,7 into 3 groups. Nonkeratinizing squamous cell carcinoma (NK SCC or Type 3) shows sheets, nests or trabeculae of round to oval and frequently spindled, hyperchromatic cells with indistinct cell borders and lacking prominent nucleoli (Fig. 1). They have only modest amounts of eosinophilic cytoplasm. Comedo-type necrosis and brisk mitotic activity are usually present. There is typically little stromal reaction to the invading tumor. Portions of the tumor can show squamous maturation, characterized by polygonal cells with mature cytoplasm, distinct cell borders, intercellular bridges, and keratin pearls, but these mature areas should constitute less than 10% of the total surface area. Keratinizing-type SCC (K SCC or Type 1) consists entirely of maturing squamous epithelium with no areas with NK SCC or “basal” morphology (Fig. 2). The cells have polygonal shapes with abundant, eosinophilic (keratinizing) cytoplasm, distinct cell borders, and intercellular bridges. The nests are angulated and irregular, and there is frequently marked stromal desmoplasia. Actual keratin formation is common but is not required as long as the cells have the prominent eosinophilic cytoplasm and other features. “Hybrid” SCC (nonkeratinizing with maturation or Type 2) cases have definitive areas with NK SCC morphology but also have squamous maturation in greater than 10% of the tumor (Fig. 3). Other rare histologic types such as basaloid, undifferentiated, and adenosquamous carcinoma were diagnosed based on their published features.3,5,17,33
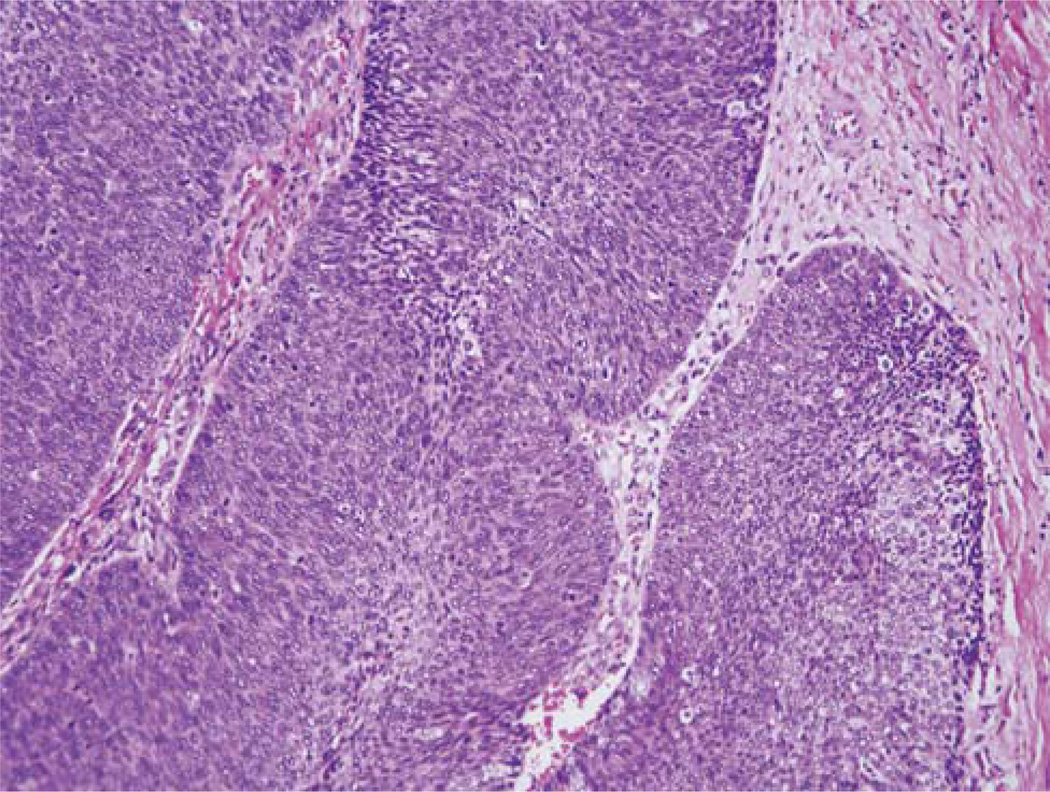
FIGURE 1.
Typical histologic features of a nonkeratinizing (Type 3) SCC with well-circumscribed nests of tumor cells that have round to oval, hyperchromatic nuclei, minimal cytoplasm, and abundant apoptosis and mitotic activity. There is no surrounding stromal reaction. (H&E; 200 × magnification; SCC indicates squamous cell carcinoma).
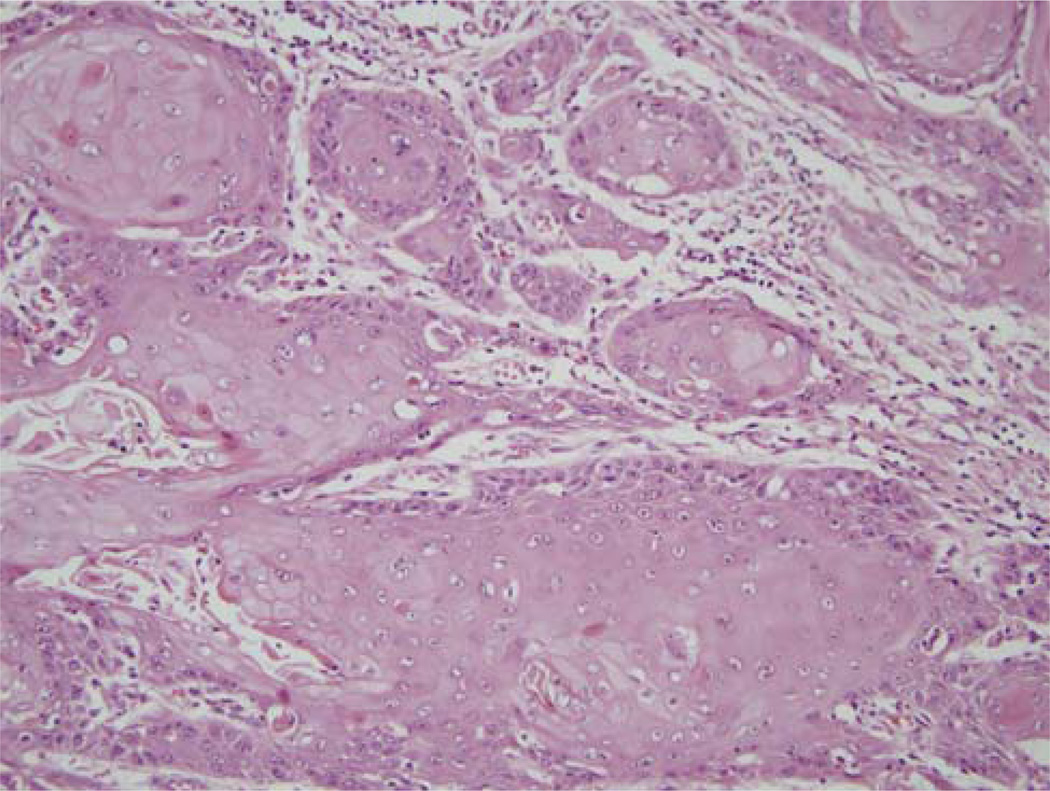
FIGURE 2.
Typical histologic features of a keratinizing-type (Type 1) SCC with angulated nests of tumor cells that have abundant, eosinophilic cytoplasm, well-defined cell borders, and round nuclei with vesicular chromatin and prominent nucleoli. This particular tumor also shows focal, frank keratinization. (H&E; 200 × magnification; SCC indicates squamous cell carcinoma).
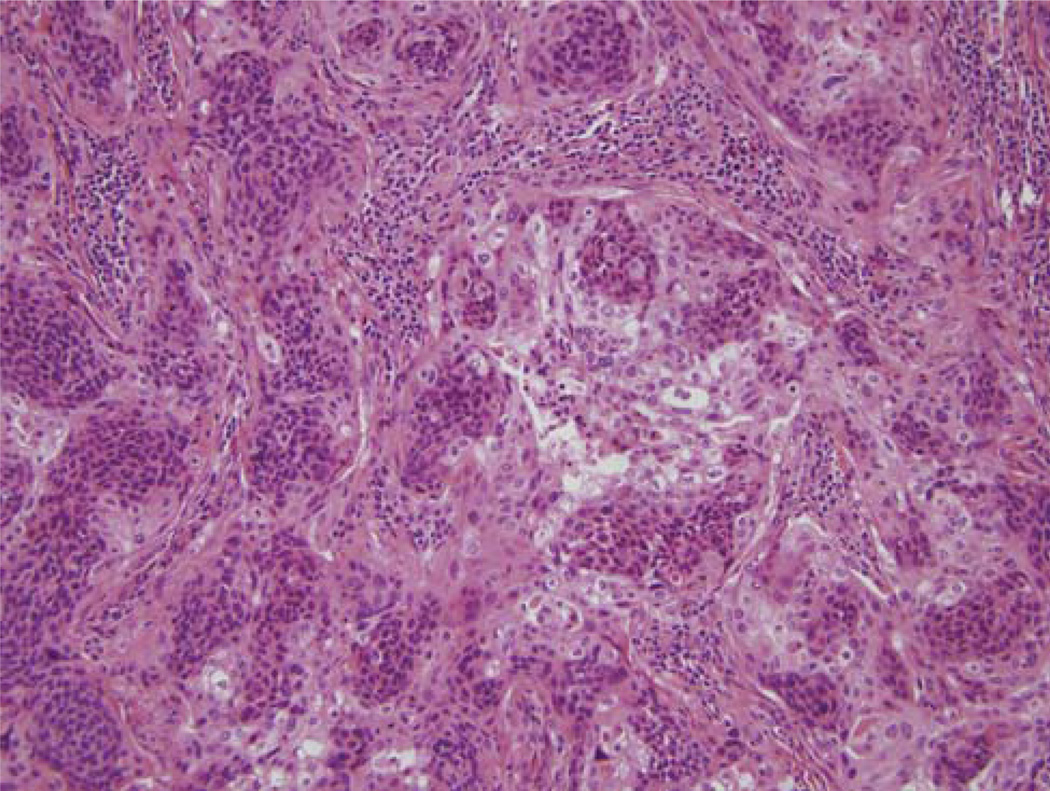
FIGURE 3.
Histologic features of a hybrid (Type 2) SCC that has areas of typical nonkeratinizing SCC with cells with minimal cytoplasm and oval, hyperchromatic nuclei combined with areas (more than 10% of the surface area) in which the tumor cells have abundant, eosinophilic cytoplasm, round nuclei with vesicular chromatin and nucleoli. (H&E; 200 × magnification; SCC indicates squamous cell carcinoma).
Immunohistochemistry
Immunohistochemistry was carried out for p16 on representative 4-µm sections cut from formalin-fixed, paraffin-embedded tissue blocks using a monoclonal antibody to p16 (MTM Laboratories; monoclonal; 1:1 dilution) on a Ventana Benchmark LT automated immunostainer (Ventana Medical Systems, Inc., Tucson AZ) according to standard protocols. Detection involved Ventana’s ultraView Universal DAB Detection Kit that uses a cocktail of enzyme-labeled secondary antibodies that locate the bound primary antibody. The complex is then visualized with hydrogen peroxide substrate and a 3, 3′—diaminobenzidine tetrahydrochloride (DAB) chromogen. No biotin is involved. Antigen retrieval, standard on the machine, used the Ventana CC1, EDTA-Tris, pH 8.0 solution. A known p16 expressing head and neck SCC case was used as the positive control and sections of normal tonsil used for negative controls with each run. Staining was nuclear and cytoplasmic and was graded by a single study researcher (JSL) in a quartile manner for its extent as follows: 0 = negative; 1+ = 1% to 25% of cells positive; 2+ = 26% to 50%; 3+ = 51% to 75%; 4+ = 76% to 100%. For analysis, however, cases were divided binarily into positive (1 to 4+) and negative (0) groups.
In Situ Hybridization for HPV
In Situ hybridization was carried out on formalin fixed, paraffin embedded, 4-µm tissue sections on a Ventana Benchmark LT-automated immunostainer according to the manufacturer’s instructions. This uses the ISH I View Blue Plus Detection Kit (Ventana Medical System, Inc., Tucson, AZ) that is a biotin-streptavidin-based detection system. The Ventana HPV III Family 16, probe B, a cocktail recognizing the high risk HPV (HR HPV) types 16, 18, 31, 33, 35, 45, 51, 52, 56, 58, 59, 68, and 70 was used along with endogenous biotin blocking and counter staining with Ventana Red Counterstain II (Ventana Medical System, Inc., Tucson, AZ). A known HPV-positive head and neck SCC case was used as a positive control and normal tonsil sections as the negative control with each run. Staining was read by a single study researcher (JSL). Positive staining was identified as blue nuclear dots and ranged widely, from strongly and diffusely positive across the entire tumor to extremely focal, with only rare positive cells. The individual nuclear pattern ranged from granular staining across the whole nucleus to single, punctate, single focus staining. These 2 patterns would seem to correspond to episomal versus integrated virus, but as earlier studies have shown, this does not necessarily correlate for this particular assay.15 Any definitive nuclear staining in the tumor cells was considered positive. Cases were classified in a binary manner as either positive or negative.
Polymerase Chain Reaction
Tumor containing areas of paraffin blocks were punched with 1.5-mm sterile, disposable DNA punches (separate punches were used for each case/block). DNA was then prepared using the Purgene DNA purification kit (Qiagen). The tumor samples were deparaffinized using a series of xylene and ethanol washes. Next, they were subjected to a rigorous proteinase K digestion with an incubation time tailored for recovery of DNA. The methodology for DNA purification included RNase treatment, protein precipitation, and DNA precipitation. PCR was then conducted using the INNO-LiPA HPV Genotyping Extra kits.10 A consensus sequence from the L1 region of the HPV genome was amplified using SPF10 primers. For high risk HPV, a 65 base pair fragment was amplified. An additional primer pair for the amplification of the human HLA-DPB1 gene was added to monitor sample quality and extraction. For specimens in which the HPV amplification product was present on initial reaction, they were hybridized to type-specific probes immobilized as parallel lines on membrane strips premade by the manufacturer. After hybridization and stringent washing, streptavidin-conjugated alkaline phosphatase was added. Incubation with BCIP/NBT chromogen yielded a purple precipitate. The results were visually read and compared with the provided interpretation chart to type the HPV.
Statistics
The first date of therapy whether surgery (for surgery alone) or radiation (for surgery with postoperative radiation or for patients undergoing definitive therapy) was considered the start of survival time. Overall survival ended when patient was dead due to any cause. Either death or the date of first recurrence was the endpoint of disease-free survival. If patients died with evidence of persistent or recurrent oropharyngeal SCC, the date of death was used to determine disease-specific survival. The dates and disease status of last known follow-up were also properly recorded and incorporated in specific survival analyses. All the survival times were determined based on Kaplan-Meier estimates. Log-rank tests were used to compare survival intervals upon covariates’ effects. Multivariate analysis using proportional hazard regression modeling was also employed to study multiple variables of interest and their adjusted influences. To examine hypothetic associations in categorical data, χ2 or Fisher tests were used when appropriate. For continuous variables, Student t tests or nonparametric rank tests were used based on distribution normality. We did not make adjustments for multiple comparisons, since the study hypotheses were specifically indicated. All the tests were 2 sided and results were considered significant for P-values less than 0.05. SAS 9.1 was used for major statistical calculations (SAS Institute Inc., Cary, NC).
RESULTS
The entire cohort of 239 patients consisted of 211 (88.3%) men and 28 (11.7%) women (Table 1). There were 208 (87.4%) Whites, 27 (11.3%) African Americans, 2 (0.9%) Asians, and 1 (0.4%) Native American. One hundred sixty-one (70.3%) patients underwent surgery with postoperative IMRT, 57 (24.9%) underwent definitive IMRT, and 11 patients (4.8%) underwent surgery alone. Adjuvant chemotherapy was used for 104 (51.0%) of patients. Histologic types were as follows: Type 1 (K SCC) 54 (23.4%), Type 2 (Hybrid SCC) 50 (21.7%), Type 3 (NK SCC) 126 (54.8%), and 9 others (basaloid, adenosquamous, or undifferentiated).
TABLE 1.
Demographic, Clinical, and Pathologic Characteristics by Group
| Group No. | All (239) | p16+/HPV ISH + (139) |
p16+/HPV ISH − (48) |
p16+/HPV ISH− /HPV PCR−(26) |
p16−/HPV ISH−(47) |
|---|---|---|---|---|---|
| Age (Mean ± S.D.) | 55.9 ± 9.0 | 55.6 ± 9.0 | 55.0 ± 9.5 | 55.7 ± 9.9 | 57.0 ± 10.4 |
| P-value | n/a | P = 0.39 | P = 0.56 | p = 0.61 | n/a |
| Sex (%) | |||||
| Male | 211 (88.3) | 127 (91.4) | 41 (85.4) | 23 (88.5) | 39 (83.0) |
| Female | 28 (11.7) | 12 (8.6) | 7 (14.6) | 3 (11.5) | 8 (17.0) |
| P-value | n/a | P = 0.11 | P = 0.23 | P = 0.53 | n/a |
| Race (%) | |||||
| White | 208 (87.4) | 132 (95.7) | 41 (85.4) | 21 (80.8) | 30 (63.8) |
| AA or other | 30 (12.9) | 6 (4.3) | 7 (14.6) | 5 (19.2) | 17 (36.2) |
| P-value | n/a | P < 0.0001 | P = 0.0155 | P = 0.13 | n/a |
| Smoking (%) | |||||
| Yes (current/former) | 173 (76.5) | 94 (70.1) | 37 (77.1) | 23 (88.5) | 42 (95.4) |
| No (never) | 53 (23.5) | 40 (29.9) | 11 (22.9) | 3 (11.5) | 2 (4.6) |
| n/a | P = 0.0004 | P = 0.0154 | P = 0.35 | n/a | |
| T Stage (%) | |||||
| T1/T2 | 145 (63.6) | 94 (70.7) | 29 (60.4) | 17 (65.4) | 22 (46.8) |
| T3/T4 | 83 (36.4) | 39 (29.3) | 19 (39.6) | 9 (34.6) | 25 (53.2) |
| P-value | n/a | P = 0.0033 | P = 0.18 | P = 0.13 | n/a |
| N Stage (%) | |||||
| N0 | 32 (13.8) | 13 (9.4) | 3 (6.4) | 3 (12.0) | 16 (34.0) |
| N1–3 | 200 (86.2) | 125 (90.6) | 44 (93.6) | 22 (88.0) | 31 (66.0) |
| P-value | n/a | P < 0.0001 | P = 0.0008 | P = 0.0433 | n/a |
| Resection Margins (%) | |||||
| Negative | 145 (85.3) | 98 (85.2) | 29 (90.6) | 15 (83.3) | 18 (78.3) |
| Positive | 25 (14.7) | 17 (14.8) | 3 (9.4) | 3 (16.7) | 5 (21.7) |
| P-value | n/a | P = 0.41 | P = 0.20 | P = 0.68 | n/a |
| IMRT (%) | |||||
| Definitive | 57 (24.9) | 18 (13.4) | 16 (33.3) | 7 (26.9) | 23 (48.9) |
| Postoperative | 161 (70.3) | 109 (81.6) | 32 (66.7) | 19 (73.1) | 20 (42.6) |
| Surgery only | 11 (4.8) | 7 (5.2) | 0 (0) | 0 (0) | 4 (8.5) |
| P-value | n/a | P < 0.0001 | P = 0.0182 | P = 0.0286 | n/a |
| Chemotherapy (%) | |||||
| Yes | 104 (51.0) | 56 (49.1) | 24 (54.5) | 12 (54.5) | 24 (52.2) |
| No | 100 (49.0) | 58 (50.9) | 20 (45.5) | 10 (45.5) | 22 (47.8) |
| P-value | n/a | P = 0.73 | P = 0.82 | P = 0.86 | n/a |
| Histologic Type | |||||
| K SCC (Type 1) | 54 (23.4) | 8 (5.9) | 10 (20.8) | 6 (23.1) | 37 (82.2) |
| Hybrid SCC (Type 2) | 50 (21.7) | 30 (22.2) | 11 (22.9) | 7 (26.9) | 7 (15.6) |
| NK SCC (Type 3) | 126 (54.8) | 97 (71.8) | 27 (56.3) | 13 (50.0) | 1 (2.2) |
| P-value | n/a | P < 0.0001 | P < 0.0001 | P < 0.0001 | n/a |
All P-values are comparing their respective cohort with the p16 negative, HPV ISH negative cohort. Results in bold type are statistically significant (cutoff of P < 0.05).
AA indicates African American; HPV, human papillomavirus; IMRT, intensity modulated radiation therapy; ISH, in-situ hybridization; K, keratinizing; NK, nonkeratinizing; PCR, polymerase chain reaction; SCC, squamous cell carcinoma.
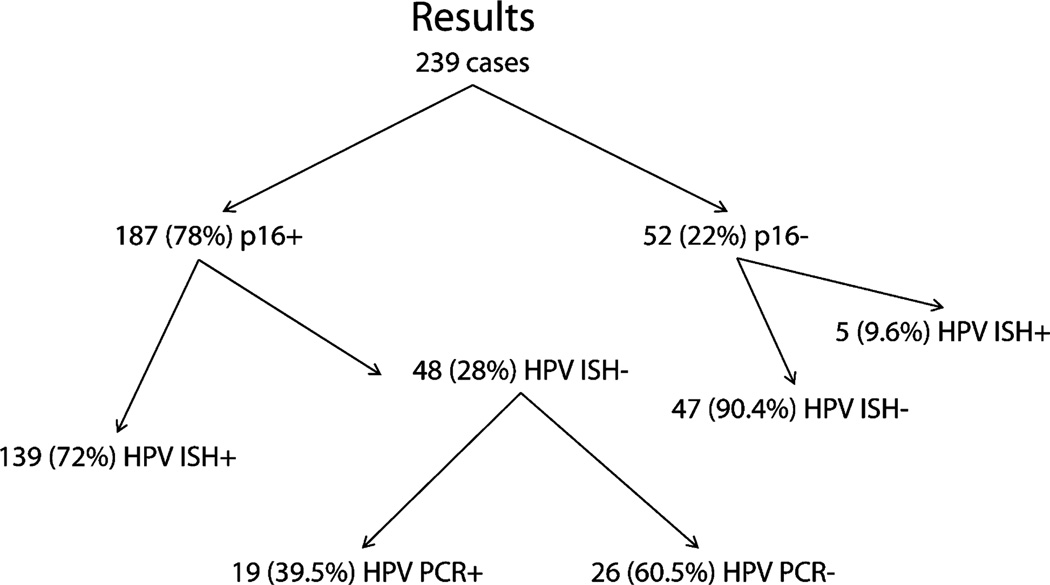
Testing was conducted on all 239 cases (Fig. 4). Fifty-two (22%) were negative for p16 and 187 (78%) were positive. Of the latter, 139 (74%) were positive for high-risk HPV by ISH, leaving a cohort of 48 cases that were p16 positive/HPV ISH negative (Fig. 5). Forty-five of these had material available for PCR, which was positive for high-risk HPV in 19 cases (HPV type 16 in 14 cases, type 33 in 3 cases, combined types 16 and 33 in 1 case, and an undefined type in 1 case). This left 26 p16 positive cases for which HPV was undetectable by either ISH or PCR. There were 52 p16 negative cases, 5 (9%) of which were positive for HPV by ISH. Given the small size of this latter cohort and its uncertain significance, these 5 cases were excluded from further analysis. Of the p16 positive cases, 174 of 189 (92.1%) were 4+, 5 (2.7%) were 3+, 1 (0.6%) was 2+, and 6 (3.2%) were 1+.
FIGURE 4.
Cases segregated by test results. (+ indicates positive; −, negative; HPV, human papillomavirus; ISH, in-situ hybridization; PCR, polymerase chain reaction).
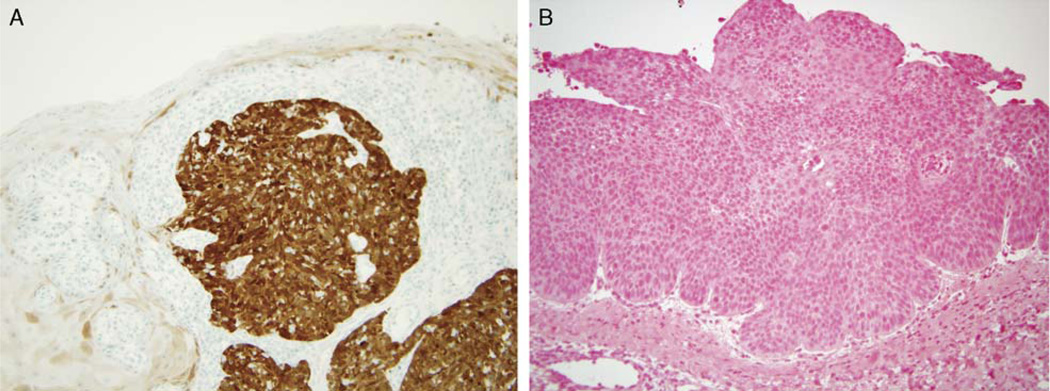
FIGURE 5.
A, p16 IHC showing strong and diffuse nuclear and cytoplasmic expression in the tumor with minimal, patchy staining of normal surface mucosa (200 magnification). B, HPV ISH showing a SCC with no evidence of blue staining and thus no evidence of HPV (200 × magnification) (HPV indicates human papillomavirus; IHC, immunohistochemistry; ISH, in-situ hybridization; SCC, squamous cell carcinoma).
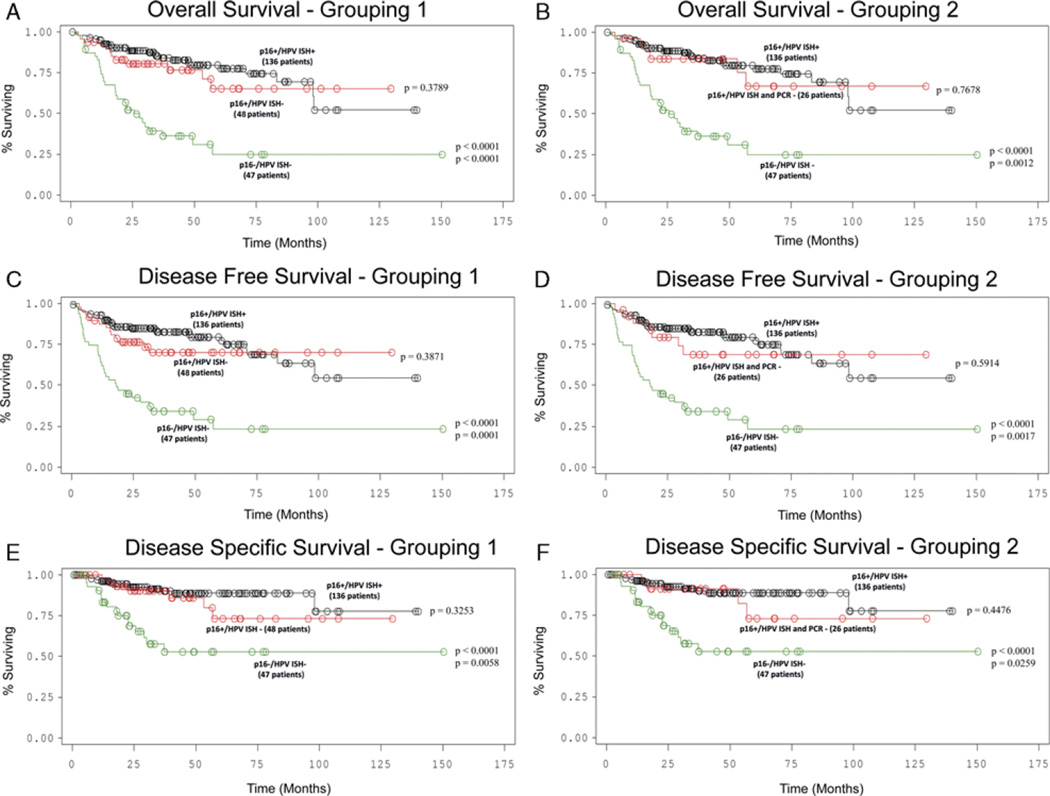
The average clinical follow-up period for all surviving patients was 3.9 years. All three cohorts of p16 positive SCC, whether HPV positive by ISH, HPV negative by ISH alone, or HPV negative by ISH and PCR, showed statistically significant better overall, disease-free, and disease-specific survival than p16 negative, HPV negative SCC (Fig. 6) in univariate analysis. The 2-year disease-specific survival rate for p16 positive, HPV ISH positive SCC was 86.2% (CI 79.0–91.1%) whereas it was only 44.2% (CI 30.2–58.1) for p16 negative, HPV ISH negative SCC. Cases with p16 positivity alone had hazard ratios for death by any cause that were almost identical to p16 positive, HPV ISH positive cases (Table 2).
FIGURE 6.
Survival curves for testing subgroups. A, Overall survival—p16 positive, HPV ISH positive versus p16 positive, ISH negative versus p16 negative, HPV negative SCC. B, Overall survival—p16 positive, HPV ISH positive versus p16 positive, ISH and PCR negative versus p16 negative, HPV negative SCC. C, Disease-free survival—p16 positive, HPV ISH positive versus p16 positive, ISH negative versus p16 negative, HPV negative SCC. D, Disease-free survival—p16 positive, HPV ISH positive versus p16 positive, ISH and PCR negative versus p16 negative, HPV negative SCC. E, Disease-specific survival—p16 positive, HPV ISH positive versus p16 positive, ISH negative versus p16 negative, HPV negative SCC. F, Disease-specific survival—p16 positive, HPV ISH positive versus p16 positive, ISH and PCR negative versus p16 negative, HPV negative SCC. (HPV indicates human papillomavirus; ISH, in-situ hybridization; PCR, polymerase chain reaction; SCC, squamous cell carcinoma).
TABLE 2.
Hazard Ratios for Death From Any Cause by Testing Group
| Group by Testing | Hazard Ratio for Death From Any Cause Compared to p16 Negative SCC (Confidence Intervals) |
|---|---|
| p16 positive/HPV ISH positive (139 patients) | 0.212 (0.125–0.358) |
| p16 positive/HPV ISH negative (48 patients) | 0.289 (0.147–0.556) |
| p16 positive/HPV ISH and PCR negative (26 patients) | 0.257 (0.106–0.622) |
| p16 positive (187 patients) | 0.209 (0.123–0.354) |
| Non-keratinizing morphology (126 patients) | 0.241 (0.142–0.417) |
HPV indicates human papillomavirus; ISH, in-situ hybridization; PCR, polymerase chain reaction; SCC, squamous cell carcinoma.
Other clinical and pathologic features correlated with outcome in univariate analysis among the groups (Table 1). There was a higher percentage of white patients in the p16 positive, HPV positive cohort than either the p16 negative, HPV negative, p16 positive HPV ISH negative, or p16 positive, HPV ISH, and PCR negative cohorts (Table 1). Smoking, when considered in a binary manner as “never smoked” versus “ever smoked,” was different among the groups. The p16 positive, HPV ISH positive and HPV ISH negative cohorts were statistically significantly less likely to have ever smoked (P = 0.0004 and 0.015, respectively). Interestingly, the p16 positive, HPV ISH and PCR negative cohort was not different for smoking status (P = 0.35). In addition, when comparing T1 and T2 versus T3 and T4 tumors, T-stage was higher in the p16 negative, HPV negative cohort than in the 3 different p16 positive cohorts. Nodal stage showed the opposite trend, being higher in the 3 p16 positive cohorts than in the p16 negative, HPV negative cohort. Last, treatment type differed among the groups. p16 negative, HPV negative SCC was more frequently treated with definitive IMRT rather than surgery, compared with the 3 p16 positive cohorts. There were no differences in patient age or use of chemotherapy among the groups, nor were there differences in resection margin positivity rates.
In multivariate analysis (Table 3), we controlled for T-stage (T1 or T2 vs. T3 or T4), lymph node status (metastases present vs. absent), treatment type (definitive radiation therapy versus primary surgery with or without radiation), race (white vs. other), and smoking status (ever versus never smoked). Compared with p16 negative, HPV ISH negative SCC, all 3 p16 positive cohorts retained their statistically significantly better outcomes. Overall, disease-free, and disease-specific survival were statistically significantly better in the p16 positive, HPV ISH positive and in the p16 positive, ISH negative cohorts. For the p16 positive, HPV ISH and PCR negative cohort, overall and disease-free survival were statistically significantly better. Disease-specific survival was better as well, but the strong trend was not statistically significantly better (P = 0.0684).
TABLE 3.
Multivariate Analysis (Compared to p16 Negative, HPV ISH Negative SCC)
| Cohort | Overall Survival |
Disease Free Survival |
Disease Specific Survival |
|---|---|---|---|
| p16+/HPV ISH + | P = 0.0001 | P = 0.0001 | P = 0.0065 |
| p16+/HPV ISH − | P = 0.0006 | P = 0.0006 | P = 0.0061 |
| p16+/HPV ISH and PCR − | P = 0.0149 | P = 0.0157 | P = 0.0684 |
Results in bold type are statistically significant (cutoff of P < 0.05). Statistical analysis controlled for other major variables (see text).
HPV indicates human papilloma virus; ISH, in-situ hybridization; PCR, polymerase chain reaction; SCC, squamous cell carcinoma.
The morphologic features of the tumors correlated strongly with their molecular characteristics (Table 1). The majority of p16 negative, HPV ISH negative SCC were K SCC (Type 1) whereas the majority of p16 positive SCC were NK SCC (Type 3) or hybrid SCC (Type 2). In particular, the correlation of NK SCC (Type 3) morphology with positive p16 staining was very strong, with 124 of 126 cases having NK SCC morphology being positive for p16. All 124 p16 positive NK SCC were strongly positive with 3 or 4+ staining (ie, all greater than 50% or more of the cells positive).
DISCUSSION
The clinical significance of tumor HPV status in oropharyngeal SCC is now well established.24 These tumors have classically been defined by detecting highrisk HPV, and they are genetically less complex, respond well to all standard treatment types whether they be surgery,25 radiation,14 chemotherapy,1 or targeted agents such as cetuximab,6 and have very favorable clinical outcomes.2,25,24 Currently, all patients with oropharyngeal SCC receive essentially the same treatment regardless of tumor biomarkers or other categorization. The next step in this process would be to deintensify or otherwise modify the management of patients with “better prognosis” tumors with the goal of lessening the substantial morbidity associated with cancer treatments while maintaining the same excellent clinical outcomes. Defining which patients are candidates for these alternative treatment regimens is critical. Given the number of possible “tests” to characterize oropharyngeal SCC, such as HPV PCR, HPV ISH, p16 immunohistochemistry, and nonkeratinizing morphology, it is not surprising that there is no consensus among pathologists or clinicians on which test to use. Interestingly, a recent survey of practicing oncologists reports that only 40% of oncologists have special testing done on oropharyngeal SCC cases.30 Where testing is done, the practices and type are widely variable. An informal poll of head and neck pathologists taken by one of the researchers (J.S.L.) in late 2009 revealed that 10 different large academic centers had 10 slightly different approaches (unpublished data).
The focus has largely been on the identification of HPV DNA by PCR.2,13,16,22,24 Although this is certainly logical, it turns out to be quite challenging in practice. In addition to being cumbersome, PCR is also too sensitive and cannot discern biologically meaningful from irrelevant HPV.35 HPV ISH allows one to visualize signal within tumor cell nuclei adding a degree of specificity to the testing, but reactivity can be very focal, background nonspecific staining is common, and the test lacks some degree of sensitivity.15,31 The percentage of biologically active HPV cases that are missed by ISH, either with commercially available assays or by the commonly used “home brew” assays, is not clear as their performance has not been systematically studied. Newer studies specifically analyzing the expression and quantity of HPV oncoproteins E6 and E7 seem logical, as they indicate not only the presence of HPV, but more importantly HPV in its transcriptionally active form.28 Again, though, these assays are cumbersome and may not be of any more utility than other methods. p16, a cell cycle regulatory protein whose normal function is to suppress activity of cyclin-dependent kinases in coordination with retinoblastoma (Rb), has been extensively documented as a surrogate marker of transcriptionally active HPV in oropharyngeal SCC.2,7,12,18,25 Overexpression of p16 has been consistently and repeatedly shown to be associated with better response to therapy and favorable clinical outcome in oropharyngeal SCC.14,25,34 p16 immunostaining is widely available, easy to carry out, and easy to interpret. It is typically either strongly and diffusely positive (with cytoplasmic and nuclear staining) or is completely absent.4,7 In our study, 174 of the 187 (93.0%) p16 positive cases were strongly and diffusely positive, with more than 75% of the cells showing expression. There are different antibody clones for p16, but we and a majority of studies (and critically, several which validate p16 as a biomarker) have used the E6H4 clone (MTM laboratories, Heidelberg, Germany) making it the preferred one for this testing.13,16,25,34 Overexpression of p16 occurs as a result of degradation of Rb by HPV E7 oncoprotein. As Rb normally suppresses p16 transcription, lack of the protein leads to marked overexpression of p16. In addition, as p16 is a tumor suppressor protein with the normal function of inhibiting cyclin-dependent kinases, it is absent or weakly expressed in most non- HPV-related head and neck SCC11,20,23 because the gene is mutated, deleted, or methylated.
The oft-quoted limitation of p16 as the optimal biomarker for oropharyngeal SCC is that it lacks specificity for HPV as p16 can be overexpressed by other mechanisms.2,35 Indeed, a significant minority of oropharyngeal SCC are strongly p16 positive but are HPV negative.7,31 As many as 30% of p16 positive tumors have been reported to be HPV negative so an important and interesting question is “what is the mechanism for the p16 overexpression in the absence of detectable HPV E7 expression?” It is conceivable that HPV has still been involved in the carcinogenic process of these tumors. One could speculate, then, that HPV might have been shed by the tumors as they progress genetically, and that, in such tumors, in which there is now lack of active viral E7 expression, that the Rb gene is deleted or Rb expression is otherwise suppressed in a manner independent of active viral protein expression. Such tumors would still have the favorable “genetic background” of HPV positive ones. Alternatively, one could also speculate that the tumors develop completely independently from HPV, and that they have innate p16 overexpression. As a tumor suppressor gene, it may play a role in their good treatment responsiveness. Last, the current HPV-specific tests may not be recognizing HPV in such tumors. For example, there may be unidentified HPV genotypes that do not contain the consensus DNA sequences detectable by ISH or PCR. Regardless, the clinical significance of p16 expression in the absence of detectable HPV has not been fully explored.
We investigated this issue in our retrospective cohort of 239 oropharyngeal SCC patients with an average of 3.9 years of follow-up for surviving patients. We find that p16 positive, HPV ISH negative SCCs have very favorable clinical outcomes, not statistically different than p16 positive, HPV ISH positive SCCs (Fig. 4). To account for false negatives by ISH, we conducted PCR for HPV on the 45 p16 positive, HPV ISH negative tumors that had material available for this. This identified 19 additional HPV positive cases, indicating the less than ideal sensitivity of the Ventana ISH assay. The remaining, smaller cohort of HPV nondetectable (ISH and PCR negative) patients again showed very favorable clinical outcomes, not statistically different than p16 positive, HPV ISH positive SCC whereas still statistically significantly better than for p16 negative, HPV negative SCC (Fig. 4). The survival differences were still significant in multivariate analysis, even accounting for the differences in T-stage, N-stage, race, and treatment regimen among the cohorts.
p16 positivity identified the largest cohort of “good prognosis” oropharyngeal SCC. Seventy-eight percent of the cases in our cohort were p16 positive. By comparison, 144 (60%) cases were HPV ISH positive. Combining tests, 139 (58%) cases were p16 positive and HPV ISH positive. If we used this “double positivity” standard to triage patients for differing treatments, 28% of the patients in this cohort with “good prognosis” SCC would be excluded. We also showed that simple H&E morphology in oropharyngeal SCC can identify p16 positive tumors with remarkable accuracy; 98% of the NK SCC (Type 3) tumors were strongly p16 positive.
Also, the Ventana INFORM ISH assay for HPV has never been systematically examined for its efficacy in identifying HPV in oropharyngeal SCC. We have not technically done so here, either, but have garnered sufficient data to say that the assay is at best, 88%sensitive for the detection of HPV in p16 positive tumors.
We have shown that p16 positive oropharyngeal SCC has a very favorable prognosis, regardless of the HPV status of the tumor. This argues that HPV-specific tests add little to the clinical information provided by p16. As we are now looking for alternative treatment regimens in “good prognosis” oropharyngeal SCC, it seems that p16 immunoreactivity alone may be the best biomarker for this subset of tumors. This is also supported by data from other investigators. A large international phase III clinical trial evaluating oropharyngeal SCC for p16 and HPV also found that p16 identified a larger cohort of “good prognosis” cancers than HPV testing alone or the combination of p16 and HPV positivity.26
It is important, however, that these tumors in which p16 is overexpressed but without evidence of HPV be further investigated to characterize their molecular phenotype. For if we are to invoke them as “better prognosis” and place them on treatment regimens that deintensify or otherwise modify their treatment, we would really like to know that they have the same genetic background as tumors with identifiable HPV.
The majority of the patients in this study underwent primary surgery followed by IMRT, a minority underwent definitive IMRT, and an even smaller group underwent surgery alone. However, we did show that these differences did not confound the statistically significantly better outcomes for p16 positive tumors in all cohorts relative to p16 negative SCC. Our average follow-up length of 3.9 years for surviving patients is relatively generous and for most of the patients in our cohort, covers the 2-year period within which most head and neck SCC recurs. At this time, however, it is not possible to know about the long-term survival of “good prognosis” oropharyngeal SCC and specifically if the disease, being indolent, will recur much later than typical head and neck SCC. But our disease-specific survival curves plateau quite a bit after 2 to 3 years, suggesting that our follow-up times are adequate to detect disease recurrences for oropharyngeal SCC.
In summary, p16 positive, HPV negative oropharyngeal SCC has survival comparable to p16 positive, HPV positive SCC and statistically significantly better than p16 negative, HPV negative SCC. p16 identifies the largest cohort (~ 80%) of “good prognosis” oropharyngeal SCC. Our data argue that p16 IHC testing alone is sufficient for classifying tumors for treatment stratification and that HPV-specific testing may be unnecessary. Nonkeratinizing morphology by H&E examination is an extremely good surrogate marker of p16 positivity which, with more validation, may provide a simple alternative to IHC testing in a significant number of oropharyngeal SCC patients.
Acknowledgment
The authors thank Jianping Li BS for her excellent work on the immunohistochemistry and in situ hybridization, and Xiaopei Zhu MD for her terrific work running the HPV PCR and typing. They also like to thank John Pfeifer MD, PhD for his assistance with choosing the method for, and guidance in interpreting, the HPV PCR. Mary Madden provided her extensive support in acquiring and organizing the block and slide materials and for entering and collating spreadsheet data, and Walter Clermont provided great help in formatting the figures.
Support of the Biostatistics Core, Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842.
Footnotes
None of the authors has any financial disclosures to report.
REFERENCES
- 1.Adelstein DJ. Concurrent chemoradiotherapy in the management of squamous cell cancer of the oropharynx: current standards and future directions. Int J Radiat Oncol Biol Phys. 2007;69:S37–S39. doi: 10.1016/j.ijrobp.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 2.Adelstein DJ, Ridge JA, Gillison ML, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting; Head Neck; November 9–10, 2008; Washington, D.C. 2009. pp. 1393–1422. [DOI] [PubMed] [Google Scholar]
- 3.Alos L, Castillo M, Nadal A, et al. Adenosquamous carcinoma of the head and neck: criteria for diagnosis in a study of 12 cases. Histopathology. 2004;44:570–579. doi: 10.1111/j.1365-2559.2004.01881.x. [DOI] [PubMed] [Google Scholar]
- 4.Begum S, Cao D, Gillison M, et al. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005;11:5694–5699. doi: 10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 5.Begum S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am J Surg Pathol. 2008;32:1044–1050. doi: 10.1097/PAS.0b013e31816380ec. [DOI] [PubMed] [Google Scholar]
- 6.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 7.Chernock RD, El-Mofty SK, Thorstad WL, et al. HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3:186–194. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Souza G, Agrawal Y, Halpern J, et al. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199:1263–1269. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 10.Dal Bello B, Spinillo A, Alberizzi P, et al. Validation of the SPF10 LiPA human papillomavirus typing assay using formalin-fixed paraffin-embedded cervical biopsy samples. J Clin Microbiol. 2009;47:2175–2180. doi: 10.1128/JCM.00286-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Mofty SK, Lu DW. Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity, in young patients: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2003;27:1463–1470. doi: 10.1097/00000478-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 12.El-Naggar AK, Lai S, Clayman GL, et al. Expression of p16, Rb, and cyclin D1 gene products in oral and laryngeal squamous carcinoma: biological and clinical implications. Hum Pathol. 1999;30:1013–1018. doi: 10.1016/s0046-8177(99)90217-4. [DOI] [PubMed] [Google Scholar]
- 13.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 14.Fischer CA, Zlobec I, Green E, et al. Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int J Cancer. 2010;126:1256–1262. doi: 10.1002/ijc.24842. [DOI] [PubMed] [Google Scholar]
- 15.Guo M, Gong Y, Deavers M, et al. Evaluation of a commercialized in situ hybridization assay for detecting human papillomavirus DNA in tissue specimens from patients with cervical intraepithelial neoplasia and cervical carcinoma. J Clin Microbiol. 2008;46:274–280. doi: 10.1128/JCM.01299-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hafkamp HC, Manni JJ, Haesevoets A, et al. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer. 2008;122:2656–2664. doi: 10.1002/ijc.23458. [DOI] [PubMed] [Google Scholar]
- 17.Klijanienko J, Micheau C, Azli N, et al. Undifferentiated carcinoma of nasopharyngeal type of tonsil. Arch Otolaryngol Head Neck Surg. 1989;115:731–734. doi: 10.1001/archotol.1989.01860300085023. [DOI] [PubMed] [Google Scholar]
- 18.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindquist D, Romanitan M, Hammarstedt L, et al. Human papillomavirus is a favourable prognostic factor in tonsillar cancer and its oncogenic role is supported by the expression of E6 and E7. Mol Oncol. 2007;1:350–355. doi: 10.1016/j.molonc.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakahara Y, Shintani S, Mihara M, et al. High frequency of homozygous deletion and methylation of p16(INK4A) gene in oral squamous cell carcinomas. Cancer Lett. 2001;163:221–228. doi: 10.1016/s0304-3835(00)00699-6. [DOI] [PubMed] [Google Scholar]
- 21.Nasman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 22.Nichols AC, Faquin WC, Westra WH, et al. HPV-16 infection predicts treatment outcome in oropharyngeal squamous cell carcinoma. Otolaryngol Head Neck Surg. 2009;140:228–234. doi: 10.1016/j.otohns.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Ohta S, Uemura H, Matsui Y, et al. Alterations of p16 and p14ARF genes and their 9p21 locus in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:81–91. doi: 10.1016/j.tripleo.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 25.Rich JT, Milov S, Lewis JS, Jr, et al. Transoral laser microsurgery (TLM) ± adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope. 2009;119:1709–1719. doi: 10.1002/lary.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rischin D, Young R, Fisher R, et al. Prognostic significance of HPV and p16 status in patients with oropharyngeal cancer treated on a large international phase III trial. J Clin Oncol. 2009:27. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz SM, Daling JR, Doody DR, et al. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst. 1998;90:1626–1636. doi: 10.1093/jnci/90.21.1626. [DOI] [PubMed] [Google Scholar]
- 28.Shi W, Kato H, Perez-Ordonez B, et al. Comparative prognositc value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27:6213–6221. doi: 10.1200/JCO.2009.23.1670. [DOI] [PubMed] [Google Scholar]
- 29.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 30.Shoushtari AN, Rahimi NP, Schlesinger DJ, et al. Survey on human papillomavirus/p16 screening use in orophary carcinoma patients in the United States. Cancer. 2009;116:514–519. doi: 10.1002/cncr.24752. [DOI] [PubMed] [Google Scholar]
- 31.Smeets SJ, Hesselink AT, Speel EJ, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 32.Syrjanen S. Human papillomavirus (HPV) in head and neck cancer. J Clin Virol. 2005;32(suppl 1):S59–S66. doi: 10.1016/j.jcv.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Wain SL, Kier R, Vollmer RT, et al. Basaloid-squamous carcinoma of the tongue, hypopharynx, and larynx: report of 10 cases. Hum Pathol. 1986;17:1158–1166. doi: 10.1016/s0046-8177(86)80422-1. [DOI] [PubMed] [Google Scholar]
- 34.Weinberger PM, Yu Z, Haffty BG, et al. Prognostic significance of p16 protein levels in oropharyngeal squamous cell cancer. Clin Cancer Res. 2004;10:5684–5691. doi: 10.1158/1078-0432.CCR-04-0448. [DOI] [PubMed] [Google Scholar]
- 35.Westra W. The Changing Face of Head and Neck Cancer in the 21st Century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3:78–81. doi: 10.1007/s12105-009-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]