SUMMARY
Our understanding of the complexity of nervous system cancers has been enhanced through the incorporation of cellular heterogeneity into tumor models, with cellular subsets displaying stem cell characteristics. Advanced cancers such as glioblastoma are organized in a hierarchy with cancer stem cells at the apex. Cancer stem cells are functionally defined by their ability to self-renew and propagate tumors similar to the parental tumors from which they are derived. We will discuss advances in cancer stem cells, including the ability to prospectively isolate and interrogate cancer stem cells, by defining molecular mechanisms responsible for the tumor maintenance and growth. While the field of cancer stem cell biology is relatively young, continued elucidation of the tumor hierarchy holds promise for the development of novel patient therapies.
Practice Points.
Brain tumors contain highly resistant and angiogenic tumor cells called cancer stem cells. Many brain tumors recur in a nodular pattern, suggesting a clonal origin that may be derived from a combination of genetic changes and a selection of cancer stem cells.
Cancer stem cells reside in supportive environments called niches that function to maintain these cells. Targeting the supportive niche may be a useful therapeutic approach as there may be a lower likelihood of resistance. In fact, radiation and chemotherapy may function as niche inhibitors.
Cancer stem cells secrete proangiogenic factors to stimulate tumor growth. Bevacizumab may function in part to block this effect. The blood vessels also serve as part of the cancer stem cell niche.
Cancer stem cells are often invasive in animal models. Recent studies suggest that bevacizumab may switch active pathways in the tumor to stimulate greater invasion. This may explain the changes in tumor growth patterns seen with some bevacizumab-treated patients.
The pathways in cancer stem cells that increase resistance to current therapies may be amenable to pharmacologic inhibition. Clinical trials are testing these approaches.
Immunologic therapies for brain tumors include vaccines and immune modulators. These may be effective in targeting cancer stem cells.
Clinical trials with anticancer stem cell therapies are underway. They often include functional assays that measure characteristics of cancer stem cells.
Personalized or precise medicine approaches may be better informed by the consideration of cancer stem cells.
Brain tumors arise from tissues of the CNS or as a result of metastasis of primary tumors originating in distant organs of the body. They are classified by WHO primarily based on the cell types involved, location and degree of malignancy. Gliomas are the most common primary form of neoplasia in the CNS and account for approximately 80% of malignant brain tumors [1]. Gliomas appear histologically similar to glial cells, which include astrocytes and oligodendrocytes [2]. Low-grade gliomas (grades I and II) are slower growing and less aggressive than their grade III and IV counterparts, which include anaplastic ependymoma, anaplastic oligoastrocytoma, anaplastic astrocytoma, anaplastic oligodendroglioma and glioblastoma multiforme (GBM). In addition to being the most common and well-characterized primary brain tumor in humans, GBM is also the most malignant and lethal.
The Centralized Brain Tumor Registry of the United States estimates 24,620 new cases of malignant brain or CNS tumors will be diagnosed in 2013 [3]. While Richard Nixon declared war on cancer in the 1970s, limited advancement has been made in GBM, with median survival times remaining poor at 12–18 months following diagnosis [4]. Independent prognostic factors for survival include patient age, performance status, number of lesions and resection status [5]. The 5year survival rate is 5%, which is among the lowest of any cancer, with a mean age of presentation of 53 years [6]. Given the high mortality and the challenges associated with treatment, GBM will be the main focus of this article.
Hallmarks & challenges of GBM
Cancers are associated with several defining characteristics, including inhibition of apoptosis, immune suppression and evasion, sustained proliferative signaling, evasion of growth inhibition, invasion and metastasis, immortality, and angiogenesis [7,8]. GBM tumors display these hallmark characteristics and are particularly distinguished by robust vascularization, necrosis, tissue infiltration and resistance to chemotherapy and radiation [9–12]. Vascularization and necrosis separate grade III and IV gliomas, and are viewed as traits of the latter. GBMs display a high level of inter- and intra-tumor heterogeneity, with conserved and individual mutations observed in each case, compounding the difficulty in designing targeted therapies that may be utilized across a broad patient population [13,14]. The Cancer Genome Atlas research effort and independent genomic profiling studies have identified at least four groups of GBM [15,16] that have subsequently been separated into neural, proneural, mesenchymal and classical subtypes based on gene expression [17]. Recently, it has been argued that additional subtypes exist based on global methylation status instead of protein expression and that these new groups are better able to encompass pediatric GBMs, which are now recognized as molecularly distinct from their adult counterparts [14,18,19]. Therefore, molecular characterization and tumor grouping/classification has focused on the goal of tailored therapies, targeting specific abnormalities unique to each GBM tumor subset or individual tumor [17].
Current treatment regimens, which are palliative in nature, involve resection in conjunction with radiation, chemotherapy or other experimental treatments, such as targeted antiangiogenic immunotherapies [20–22]. In many instances tumor recurrence is observed in GBM. With these considerations in mind, a paradigm shift in the way we view and treat GBMs must occur. Tumors cannot be thought of as distinct intact entities residing in normal tissues, but instead as aberrant organs with a high degree of stromal tissue and tumor cell interaction. Furthermore, tumors are no longer thought of as a homogeneous population of cells all possessing equal tumorigenic potential, leading us into an alternative hierarchical view of GBM, with a stem-like population of cells contributing to tumor progression and therapeutic resistance.
Cancer stem cell hypothesis
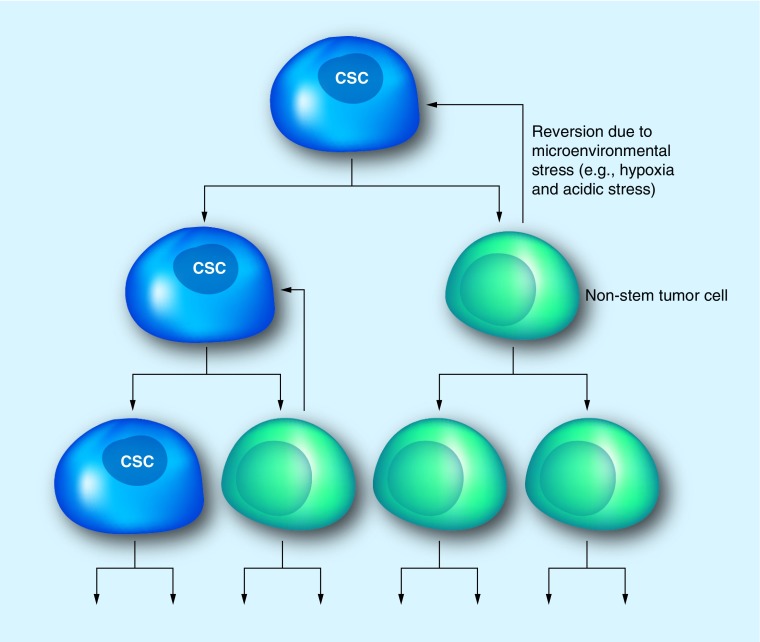
The cancer stem cell (CSC) hypothesis, outlined in Figure 1, posits the existence of a cellular hierarchy within an individual tumor. At the apex, or head of the hierarchy, are CSCs (also referred to as tumor-initiating cells, stem-like tumor cells or tumor-propagating cells) that are able to give rise to the diverse array of cells observed in a tumor. While CSCs express markers associated with the normal stem cell state (i.e., CD133), they are functionally defined by their ability to self-renew, proliferate in a sustained manner and form heterogeneous tumors that recapitulate the cellular diversity observed in parental tumors. However, the CSC hypothesis is not without controversy. Critics of the CSC hypothesis advocate that this theory conflicts with the stochastic model of tumor formation, which argues that all tumor cells possess an equal ability to propagate a tumor, and genetic cues dictate which cells drive tumor progression. Furthermore, confusion has prevailed regarding the term CSC; are these cells dedifferentiated tumor cells or transformed normal stem cells? Tumors contain a population of cells, whether from normal stem cells or dedifferentiated tumor cells, which possess stem-like properties and reside in distinct anatomical regions termed niches. CSCs receive cues from the microenvironment, comprised of somatic and neoplastic cells and the extracellular matrix, and give rise to more-differentiated tumor cell progeny. These progeny may still possess a degree of multipotency, allowing for further selection. In addition, chromosomal instability has been described in CSC populations, highlighting a possible mechanism for selection and tumor evolution by CSCs [23]. Thus, the CSC hypothesis does not exclude stochastic selection or the acquisition of resistance by tumors; instead it may be viewed as a concurrent or complementary model aimed at addressing the complexity of tumorigenesis and therapeutic resistance.
Figure 1. Cancer stem cell hypothesis.
Tumors are hierarchically arranged with CSCs at the apex able to give rise to the heterogeneous populations of cells seen throughout the tumor. These CSCs have the ability to self-renew and demonstrate increased tumorigenic potential over their non-stem counterparts. However, non-stem tumor cells also possess some classical features of CSCs and are able to revert to a more ‘stem-like’ state in response to microenvironmental stress.
CSC: Cancer stem cell.
CSCs in GBM
The first description of an identified CSC population in cancer came in 1997 in leukemia by Bonnet and Dick [24]. Subsequent, studies in GBM [25], breast [26], prostate [27], colon [28] and pancreatic [29] cancers have confirmed the presence of therapeutically resistant progenitor populations able to recapitulate the parental lesions. In the context of GBM, CSCs were first identified in 2002 [30,31] and successive studies have more thoroughly characterized GBM CSCs (GSCs). This subpopulation of tumor cells has been shown to form neurospheres in defined serum-free stem cell media supplanted with growth factors, such as EGF and bFGF, self-renew, express normal neural precursor cell markers, and possess multipotent potential and the ability to reform the parent tumor upon orthotopic implantation. Following this discovery, it was demonstrated that GBM cells cultured under serum-free conditions displayed a high degree of genotypic and phenotypic similarity to the parental tumor [32]. Confirmation of the tumor cell identity of GSCs by FISH analysis demonstrated the presence of conserved chromosomal mutations with the parent tumor suggesting lineage commonality [33]. Identification and isolation of putative CSCs has largely relied on the use of differential cell surface marker expression profiling. Following their identification, extensive efforts were undertaken to characterize enrichment markers for this highly tumorigenic population of cells with the goal of developing selective targeting strategies. Prominin-1, better known as CD133, was identified by flow cytometry in a small fraction of GBM cells. It was subsequently shown to enrich for tumor cells able to form spheres and recapitulate the original tumor, features not traditionally thought to be shared by their CD133- tumor cell counterparts [34,35]. As such, CD133 has become the prototypic marker for GSCs. Numerous other markers have been explored for their utility in identifying and targeting CSCs from various cancers. In GBM integrin α6 [36], CD15 [37], EGFR [38], A2B5 [39], L1CAM [40], CD44 [41] and CXCR4 [42] have all been studied as CSC markers. In breast cancer CD44, ALDH and integrin α6 [43] have been used as CSC markers, while in colon cancer ALDH1, CD44 and CD166 are more commonly used [44]. Marker overlap has been observed across multiple cancers, allowing for the possibility of conserved pathways and the potential for conserved therapies.
While the functions of some of these markers have yet to be fully described in GSCs, others have proven to be promising anti-GBM therapies. CD133 has emerged as being essential to the maintenance and tumorigenic potential of GSCs [45]. Silencing CD133 in GSCs using shRNA knockdown strategies demonstrates that both self-renewal and tumorigenic capacity were ablated, while the inhibitory phenotype was recovered completely following CD133 re-expression [45]. Identifying additional markers could accelerate GBM research as targeting multiple cell surface makers has the potential to increase the therapeutic efficacy of GBM treatment by further identifying which are necessary for GSC maintenance and self-renewal, and designing strategies to prevent the malignant phenotype associated with their expression. Of note, integrin α6 has been found to be highly expressed in the GSC population, localizing with CD133 as well as its coreceptor, integrin β1, and ligand, laminin, in both GBM surgical biopsies and tumorspheres [34]. The study also demonstrates, through the use of shRNA silencing and antibody blockade, that targeting integrin α6 in GSCs inhibits self-renewal, proliferation and tumor formation capacity both in vitro and in vivo [46]. Another promising marker for GSCs is the EGFR, which has been shown to be activated in more than 50% of patients with GBM through a constitutively active mutation [38]. A recent study has suggested that constitutively activated EGFR signaling confers increased tumorigenicity to glioma cells through the acquisition of GSC characteristics and angiogenesis by induction of ID3 and ID3-regulated cytokines via AKT-dependant activation of Smad5 [38]. The importance of EGFR signaling cannot be understated as pharmaceutical inhibitors of EGFR (AF1478 and gefitinib) initially suppress GSC phenotypes, such as proliferation and clonal potential, but lose effectiveness soon after withdrawal of treatment [38]. The identification of such GSC markers is invaluable in the long term in order to increase the amount of available targets for future therapeutic delivery. An interesting possibility for future study would be elucidating whether targeting multiple GSC markers at one time has a synergistic effect on GSC and patient survival. Owing to their innate cellular heterogeneity, GSCs display differential expression of these markers across their population. Using one targeting strategy may ablate a subpopulation of GSCs while leaving another untouched to regrow the tumor. Using multiple markers to target GSCs bypasses this problem and, in combination with current radiation and chemotherapy treatment paradigms, may potentially be a viable clinical solution to GBM. More aggressive therapy may also be possible for extremely malignant tumors by targeting multiple GSC markers, thereby sensitizing tumor cells to treatment with chemotherapeutic agents as well as radiation, leaving them unable to regrow the tumor. In addition, it is important to note that some of these markers are also observed in normal tissues. Thus, it is vital to effectively target the tumor compartment while leaving host tissues intact.
Recently, it has been demonstrated that populations of CD133- GBM cells also possess classical features of CSCs, such as neurosphere formation, multipotency, self-renewal and recapitulation of the original tumor [47]. It has been postulated that this subpopulation of tumor cells may in fact simply express low levels of CD133 and fail to be identified as positive. Alternatively, cell cycle variation in CD133 expression has previously been described [48] and CD133- cells were shown to reside mainly in G0/G1. Thus, care should be taken when utilizing CD133 in targeting GSCs, as quiescent cells may not express CD133 at detectable levels. Furthermore, this highlights the fact that no single surface marker may be definitively utilized as an absolute marker of stemness, whether in normal or neoplastic tissues. Alternative strategies have been proposed for the identification and isolation of not only GSCs, but also CSCs from multiple cancer types as a whole. With regard to GSCs, these alternatives have focused on functional marker-independent strategies, including dye retention [49], migratory capability [50] and drug resistance markers, such as ALDH and ABC transporters [51]. Although attractive, side populations are not informative for human GSCs [52] and drug resistance markers require further investigation.
An inability to reproduce several studies and conflicting reports with regard to the utility of certain cell surface markers have emphasized the fact that GSCs display a high degree of heterogeneity, which may be tied to the cell cycle. Therefore, we should view these elusive cells as dynamic entities in a constant state of flux, changing marker expression profiles and phenotypes in response to internal and external cues. As demonstrated in Figure 1, aside from having self-renewal capacity and the ability to recapitulate tumors, CSCs can differentiate into non-stem tumor cells that still retain a measure of ‘stemness’ and are able to revert back to a CSC state in response to microenvironmental stress. The dynamic movement of CSCs within the tumor hierarchy makes them especially difficult to detect as CSC markers are upregulated or downregulated depending on whether a cell is gaining or losing ‘stemness.’ With regards to external cues, CSCs are not randomly interspersed throughout the tumor mass, but instead are maintained within discrete microenvironmental locations, termed niches. Within GBMs, at least two such anatomical locations have been identified and described in detail: the hypoxic and perivascular niches.
Induction of the CSC phenotype by microenvironmental cues
Oxygen is a universal and vital energy source for cellular metabolism. In the adult brain, oxygen levels vary from 0.55 to 8.0% [53]. GBMs are highly vascularized, requiring vessel recruitment to supply oxygen and nutrients, thereby allowing expansion and metastasis. Upon histologic examination, tumors display areas of robust cellular division surrounding capillaries. Further from vessels, oxygen tension drops as there is limited diffusion into the surrounding stroma, largely due to rapid utilization by respiring tissues [53]. In GBM, oxygen tension is similar to that observed in normal brain tissue, in the range of 0.1–10% [54]. Regions of hypoxia have been identified in GBM ranging from mild (0.5–2.5% oxygen) to moderate (0.1–0.5% oxygen) to severe (≤0.1% oxygen) [55]. Hypoxic regions are typically associated with necrotic areas, as well as at the leading edge of pseudopalisading tumor cells infiltrating normal tissue, and play a vital role in promoting stem marker expression profiles and phenotypes, such as CD133 expression and increased self-renewal and differentiation capacity, via increased levels of hypoxia inducible factors [54,55].
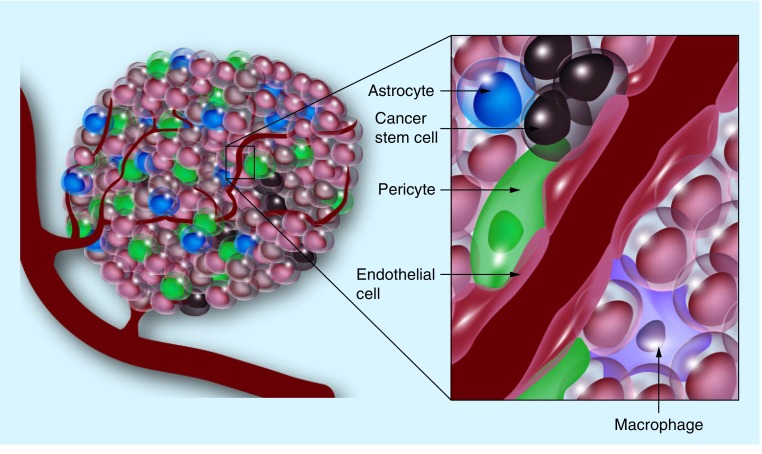
The molecular mechanisms controlling the perivascular niche are better understood than those active in hypoxic regions of GBMs. However, the structural framework of the niche remains largely unknown and the in vivo regulation of GSCs is unclear. Supporting the role of the perivascular niche in GSC maintenance, it has been demonstrated that vascular density in GBM correlates with CSC content [56]. Within the perivascular niche, aside from non-stem GBM cells and GSCs, numerous supporting cell types have been identified, including microvascular endothelial cells, microglia, pericytes and astrocytes. Each of the aforementioned cell types uniquely contribute to the formation and maintenance of the perivascular microenvironment and subsequently GSCs via contribution to the structural framework of the niche (through stabilization of the tumor vasculature or deposition of extracellular matrix), direct interaction with GSCs and/or by the secretion of soluble factors.
Aside from forming the tumor vasculature and delivering oxygen and nutrients to the expanding tumor, GBM-associated endothelial cells have also been shown to secrete soluble factors, such as nitric oxide, which promote self-renewal and proliferation of GBM cells in vivo [57]. Reports have also described vasculogenic mimicry and transdifferentiation of GSCs into cell types resembling vascular smooth muscle cells and endothelial cells [58–60]. Microglia/infiltrating macrophages, originally thought to be a natural host response to cancerous cells, have been shown to be recruited by GSCs [61,62]. Recruited microglia/macrophages are converted to an immunosuppressive phenotype, actively secreting IL-10 and TGF-β1. Induction of inflammation and secretion of matrix metalloproteinases by microglia has also been shown to promote GBM vascularization, proliferation and migration [63,64]. Additionally, pericytes are thought to be vital to vascular integrity, directly associating with and regulating microvascular endothelial cells [65]. It is thought that these cells, mesenchymal in lineage, may be instrumental in the formation and maintenance of the structure of the perivascular niche in GBM [66]. Other cell types, such as perivascular astrocytes, are poorly understood in GBM; however, they are known to regulate normal neural stem cells [67]. Astrocytes are the main producers of extracellular matrix proteins, laminin and collagen, in the brain and extracellular matrix deposition has been shown to regulate the GSC state via interaction between GSC cell surface receptor integrin α6 [36] and laminin [68]. Astrocyte activation of matrix metalloproteinases has also been implicated in tumor invasion [69] and astrocytes have been shown to express SHH, a signaling pathway known to regulate self-renewal and GBM progression [70].
From these studies we gain a better appreciation for the diverse cell types and mechanisms regulating GSCs within the perivascular niche. As demonstrated in Figure 2, it is composed of endothelial cells and pericytes, forming the main body of the tumor vasculature. Around this vessel, astrocytes, in addition to endothelial cells, secrete extracellular matrix proteins, building a scaffold for cell migration and communication. Figure 2 also demonstrates the close physical proximity of the heterogenic cell populations residing in the niche. The interaction of macrophages, pericytes and endothelial cells along with GSCs most likely contribute to the latter's tumorigenicity. GSCs residing in close association with the vasculature modulate tumor growth, contributing to the differentiated tumor bulk and recruitment of microglia/macrophages, further promoting a protumor microenvironment. However, the complexity of the hypoxic niche is not well understood and further investigation into this particular tumor microenvironment is warranted. GSCs and their respective niches present numerous potential targets to more effectively target and treat GBM in the clinical setting.
Figure 2. The tumor perivascular and hypoxic niches.
Tumors are composed of heterogeneous cell populations that interact within the microenvironment. The perivascular niche is composed of macrophages, pericytes and endothelial cells that probably interact with cancer stem cells and may contribute to their tumorigenicity. The structural and extracellular elements of the hypoxic niche are currently poorly understood and, therefore, not shown.
Expression & function of miRNAs in CSCs
miRNAs are endogenous, ssRNA molecules that have been described as highly conserved regulators of gene expression. These molecules act by base pairing with their target mRNAs through perfect or near-perfect complementarity, particularly at the 3´ untranslated regions of the target mRNAs, leading to their translational repression and/or direct cleavage [71]. Several studies have shown that miRNA expression profiles are altered in tumors, including GBM [72], prostate [73] and breast cancer [74], all of which contain a heterogeneous cell population with a self-renewing CSC population at the apex of the hierarchy. There are approximately 500–1000 different mammalian miRNA genes whose products act in a tissue-specific and temporally regulated manner during development [72]. Although the majority of miRNAs and the pathways that they regulate have yet to be characterized, recent reports have suggested that certain miRNAs act as critical regulators of stem cell function, self-renewal and differentiation. However, increasing evidence has linked miRNAs to cancer, where they are implicated in tumor pathogenesis and progression [73].
In the context of GBM, miRNAs have many pro-oncogenic functions as some are overexpressed (e.g., miR-21 [71], miR-26a [75], miR-10b [76] and miR-10a [77]) in GBM versus normal brain tissue, as well as GSCs versus enriched bulk GBM cells. While the exact mechanisms by which these miRNAs function in GBM are unclear, recent evidence has emerged which suggests that miR-26a acts as a negative regulator of the tumor suppressor gene PTEN. In addition, it was shown that PTEN repression by miR-26a increases de novo tumor formation in a mouse model of high-grade glioma [76]. Similar to miR-26a knockdown, it has been reported that miR-21 knockdown disrupts glioma growth in an in vivo glioma model [72], while others have demonstrated similar results with miR-10b inhibition both in vitro and in stem cell-derived orthotopic GBM xenografts [75]. However, in these instances the molecular targets of the miRNAs remain poorly understood and additional work is needed in order to better understand the direct signaling pathways involved.
While some miRNAs are overexpressed in GBM versus normal brain tissue, others, such as miR-7 [78], miR-125b [79], miR-34a [80] and miR-326 [81], are downregulated and function as tumor suppressors. miR-7 has been shown to be a tumor suppressor in GBM by targeting critical cancer pathways, suppressing EGFR expression and inhibiting the Akt pathway by targeting its upstream regulators. Importantly, miR-7 overexpression also led to decreased cell viability and invasiveness of primary GBM cells [78]. Other miRNAs, such as miR-125b, have been implicated in normal neural stem cell commitment and may play many other tissue-specific roles as studies have revealed that there may be hundreds of predicted targets for this miRNA [79]. However, in the context of GBM, it has been shown that miR-125b inhibits the proliferation of CD133+ GSCs through the induction of cell cycle arrest at the G1 phase by directly downregulating E2F2, which plays a central role in regulating G1/S transition [79]. It is important to note that miRNAs are not limited to one particular target or pathway. miR-34a is a transcriptional target of p53 in some cancer cell lines and has been shown to potently inhibit c-Met protein expression and c-Met 3´ untranslated region-reporter activity in glioma and medulloblastoma [80]. Of particular note, miR-34a inhibited both Notch-1 and -2 protein expression in glioma cells, although forced expression of c-Met, Notch-1 and -2 partially rescued the effects of miR-34a on cell death in glioma [80]. In a similar manner to miR-7 and miR-125b, miR-34a overexpression inhibited cell proliferation, cell cycle progression and in vivo glioma xenograft growth, while showing no inhibitory effects on astrocyte survival, and demonstrated the ability to suppress tumor growth by affecting several malignancy end points via downregulation of multiple oncogenes [80]. Interestingly, Notch-1 inhibition also upregulates miR-326, which functions through a feedback loop to inhibit the Notch protein and its activity [81], indicating that miRNAs may function in a synergistic manner to reduce GSC tumorigenicity. Such miRNA feedback loops have been described as a cellular response designed to resist metabolic stress functions in the decision of cancer cells to migrate or proliferate as a result of microenvironmental stress [81,82] and demonstrate the complex epigenetic factors involved in GBM. These studies suggest that miRNAs may be used as biomarkers of tumor progression and therapeutic agents to target GSCs. However, GSCs and normal neural cells share core developmental properties and, in order to ensure optimal therapeutic efficacy, miRNAs should only target GSCs and leave normal neural cells intact. Therefore, identifying miRNAs that are differentially expressed in GSCs and normal neural stem cells becomes essential for the development of optimal miRNA-based therapies for GBM patients.
Clinical significance of the CSC hypothesis in GBM
GBM is a uniformly fatal disease with one of the poorest prognoses among cancer types for which little progress has been made with regards to treatment and quality of life over the past 30 years. This devastating disease is characterized by recurrence following primary resection and resistance to conventional therapies, such as chemotherapy and radiation. GSC resistance and location of stem niches at the infiltrating edges of the tumor may explain resection difficulties and the frequently observed relapses in GBM patients. Current standard treatment for GBM involves a combinatorial approach utilizing radiotherapy in combination with DNA alkylating chemotherapeutic agents, such as temozolomide. Together, these therapies have shown significant effects on patient survival; however, prognosis remains poor with tumor resistance to therapy and lesion recurrence. To address this issue, numerous current clinical trials are underway, exploring targeted therapies for the treatment of GBM.
Selectively ablating the tumor vasculature through targeting of the VEGF signaling pathway has shown utility. Treatment with bevacizumab, a humanized monoclonal antibody against VEGF-A, has also been shown to reduce tumor bulk in preclinical and clinical studies [83,84]. Unfortunately, recent evidence suggests that antiangiogenic targeting may in fact select for more malignant cells with increased tumor cell invasiveness observed [84]. Additional signaling pathways have been explored in the tumor bulk as a whole, irrespective of cell type. These focus on inhibiting cellular survival and include targeting EGFR [85], histone acetylation [86] and the PI3K/mTOR pathway [87]. These treatments have had limited efficacy, with little statistical success and frequent relapse.
Exploiting the CSC hypothesis of GBM progression is a promising new therapeutic possibility. Supporting a role for CSC status in tumor malignancy, neurosphere culture provides a unique model to investigate the role of CSCs in GBM progression and severity [88]. It has been found that both renewable neurosphere formation and tumorigenic capacity are significantly associated with clinical outcome measures. Renewable neurosphere formation in cultured human GBM cells significantly predicted an increased hazard of patient death and more rapid tumor progression for patients with GBM whose cultured tumors had increased in vitro tumorigenic capacity as compared with those whose tumors either did not grow or did not form tumors [88]. The model reflects the severity of the original patient tumor, regardless of grade, age or overall cell proliferation, and while it may preclude direct clinical application, these results exemplify how neurosphere culture serves as a clinically relevant model for the study of malignant glioma [88]. Further supporting a role for GSCs in GBM, CD133 has been implicated as a prognostic factor for patient outcome. Investigators demonstrated that expression of CD133 in >2% of GBM cells from patients was negatively correlated with overall and progression-free survival in patients [89].
Targeting the GSC population of GBMs is conceptually similar to pulling the roots of a weed. If the residual initiating structure remains, the CSC roots, regrowth is anticipated. Several groups have assessed selective targeting of the stem-like populations in GBM. These studies have focused on traditional developmental programs, including SHH [90], Notch [91] and Wnt [92], shown to be integral to the regulation and resistance of GSC populations. Unfortunately, no clinical success over conventional treatment has been achieved at present when selective small molecule inhibitors targeting these pathways have been utilized in GBM [92]. The innate heterogeneity of the tumor bulk prevents any single therapy from eliminating the CSC compartment in GBM, enabling tumor regrowth. Recent work in mouse models has demonstrated that sensitizing GSCs to radiotherapy through Notch-1 or -2 inhibition rendered GSCs more sensitive to radiation at clinically relevant doses [93]. The study used γ-secretase inhibitors, inhibitors of the Notch pathway, to enhance radiation-induced cell death of GSCs while leaving the non-stem glioma cells untouched. The study also suggests that the mechanism by which Notch inhibition sensitizes GSCs is by impairing radiation-induced Akt activation and upregulation of the truncated apoptotic isoform of Mcl-1, thereby inhibiting their ability to proliferate and reducing their clonogenic potential [93]. These studies, both in vitro and in vivo, support the investigation of strategies combining current therapeutic treatment along with specifically targeting the CSC component in GBM.
Conclusion & future perspective
The inter- and intra-tumor cellular heterogeneity observed in GBM highlights the complexity of cancer. CSC populations have been definitively identified in GBM among many other advanced cancers. Furthermore, these populations have shown increased resistance to conventional radiation and chemotherapies, suggesting a mechanism behind GBM relapse and regrowth. Known to reside in distinct anatomical niches, the regulation of GSCs remains unclear with mixed results both in vitro and in vivo. Although the cell types within these microenvironments have been identified, the specific anatomical structure of the hypoxic and perivascular niches remain poorly understood. This devastating disease remains uniformly lethal despite recent advances in our understanding of the fundamental events regulating GBM initiation and progression.
The Cancer Genome Atlas represents one of the most promising efforts towards a more thorough understanding of the complexities of GBM and cancer as a whole. By more thoroughly characterizing genetic abnormalities observed and grouping subclasses of GBM we may allow for more targeted therapeutic intervention. In line with selective targeting, the CSC hypothesis presents us with a new host of possibilities in our quest to effectively combat and possibly cure GBM. While current clinical trials targeting CSC-associated pathways in GBM have been unsuccessful, this should not deter future investigation. The innate biological nature of CSCs makes it difficult to detect and distinguish them from normal neural cells in the brain and the possibility of ablating the normal neural compartment, as well as the surrounding extracellular matrix, puts additional pressure on the need to correctly identify this subpopulation. Combination therapies after resection along with radiation and chemotherapy approaches are most likely to succeed as they target CSCs through multiple mechanisms while preserving as much of the normal architecture of the brain as possible.
We are continuing to uncover the complexity of cancer as well as the transient nature of cellular states. Additional work is required to more thoroughly identify, define and characterize putative stem populations in GBM. The discovery of additional pathways and the finite nature of cellular interactions within CSC niches present diverse options for patient-personalized targeting of GBM. In addition, as a broader therapy, it is attractive to consider the possibility that instead of directly selecting CSCs, we may be able to ablate this population by destroying its reservoir within the tumor, the CSC microenvironment or niche.
Acknowledgements
The authors sincerely apologize to all our colleagues whose important work could not be directly cited due to space limitations.
Footnotes
Financial & competing interests disclosure
Work in the JN Rich laboratory is supported by the James D McDonnell Foundation and the NIH (CA112958, CA116659 and CA154130). Work in the JD Lathia laboratory is supported by the Lerner Research Institute, Voices Against Brain Cancer, the Ohio Cancer Research Associates, NIH K99/R00 Pathway to Independence Award (CA157948), a V Scholar Award from the V Foundation for Cancer Research and Grant #IRG-91-022-18 to the Case Comprehensive Cancer Center from the American Cancer Society. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪ of interest ▪▪ of considerable interest
- 1.Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genet. 2012;205(12):613–621. doi: 10.1016/j.cancergen.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN. Annual Review of Pathology – Mechanisms of Disease. Annual Reviews; CA, USA: 2006. Annual review of pathologymechanisms of disease; pp. 97–117. [Google Scholar]
- 3.Central Brain Tumor Registry of the United States. Central Brain Tumor Registry of the United States analyses of the NPCR and SEER data, 2005-2009. Central Brain Tumor Registry of the United States; IL, USA: 2012. [Google Scholar]
- 4.Grossman SA, Ye X, Piantadosi S, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin. Cancer Res. 2010;16:2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okita Y, Narita Y, Miyakita Y, et al. Pathological findings and prognostic factors in recurrent glioblastomas. Brain Tumor Pathol. 2012;29(4):192–200. doi: 10.1007/s10014-012-0084-2. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence YR, Mishra MV, Werner-Wasik M, et al. Improving prognosis of glioblastoma in the 21st century: who has benefited most? Cancer. 2012;118(17):4228–4234. doi: 10.1002/cncr.26685. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 9.Frosina G. DNA repair and resistance of gliomas to chemotherapy and radiotherapy. Mol. Cancer Res. 2009;7(7):989–999. doi: 10.1158/1541-7786.MCR-09-0030. [DOI] [PubMed] [Google Scholar]
- 10.Kaur B, Cork SM, Sandberg EM, et al. Vasculostatin inhibits intracranial glioma growth and negatively regulates in vivo angiogenesis through a CD36-dependent mechanism. Cancer Res. 2009;69(3):1212–1220. doi: 10.1158/0008-5472.CAN-08-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rong Y, Durden DL, Van Meir EG, Brat DJ. ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J. Neuropathol. Exp. Neurol. 2006;65(6):529–539. doi: 10.1097/00005072-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Claes A, Idema A, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007;114(5):443–458. doi: 10.1007/s00401-007-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nickel GC, Barnholtz-Sloan J, Gould MP, et al. Characterizing mutational heterogeneity in a glioblastoma patient with double recurrence. PLoS One. 2012;7(4):e35262. doi: 10.1371/journal.pone.0035262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Chin L, Meyerson M, Aldape K, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]; ▪▪ First description of different molecular subtypes of glioblastoma multiforme (GBM).
- 17.Verhaak RGW, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1 . Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leibetseder A, Ackerl M, Flechl B, et al. Outcome and molecular characteristics of adolescent and young adult patients with newly diagnosed primary glioblastoma: a study of the Society of Austrian Neurooncology (SANO) Neuro. Oncol. 2013;15(1):112–121. doi: 10.1093/neuonc/nos283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hingtgen S, Figueiredo J-L, Farrar C, et al. Real-time multi-modality imaging of glioblastoma tumor resection and recurrence. J. Neurooncol. 2013;111(2):153–161. doi: 10.1007/s11060-012-1008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eichten A, Adler A, Cooper B, et al. Rapid decrease in tumor perfusion following VEGF blockade predicts long-term tumor growth inhibition in preclinical tumor models. Angiogenesis. 2013;16(2):429–441. doi: 10.1007/s10456-012-9328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melguizo C, Prados J, Gonzalez B, et al. MGMT promoter methylation status and MGMT and CD133 immunohistochemical expression as prognostic markers in glioblastoma patients treated with temozolomide plus radiotherapy. J. Transl Med. 2012;10(1):250. doi: 10.1186/1479-5876-10-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odoux C, Fohrer H, Hoppo T, et al. A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Res. 2008;68(17):6932–6941. doi: 10.1158/0008-5472.CAN-07-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonnet D, Dick J. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]; ▪▪ First report of cancer stem cells (CSCs) in human cancer.
- 25.Yuan X, Curtin J, Xiong Y, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogne. 2004;23(58):9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 26.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 28.Vitiani-Ricci L, Lombardi GD, Pilozzi E, et al. Identification and expansion of human coloncancer-initiating cells. Nature. 2007;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Lee CJ, Simeone D. Identification of human pancreatic cancer stem cells. In: Yu JS, editor. Cancer Stem Cells. Humana Press; NY, USA: 2009. pp. 161–173. [DOI] [PubMed] [Google Scholar]
- 30.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro . Glia. 2002;39(3):193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]; ▪ Highlights the ability to culture GBM tissue as stem cell spheres.
- 31.Katsetos CD, Del Valle L, Geddes JF, et al. Localization of the neuronal class III [beta]tubulin in oligodendrogliomas: comparison with Ki-67 proliferative index and 1p/19q status. J. Neuropathol. Exp. Neurol. 2002;61(4):307–320. doi: 10.1093/jnen/61.4.307. [DOI] [PubMed] [Google Scholar]
- 32.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]; ▪ Highlights the importance of serum-free conditions to mimic in vivo GBM cell biology.
- 33.Wakimoto H, Mohapatra G, Kanai R, et al. Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells. Neuro. Oncol. 2012;14(2):132–144. doi: 10.1093/neuonc/nor195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh KS, Hawkins C, Clarke D I, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]; ▪▪ First description of CSCs in GBM.
- 35.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 36.Lathia JD, Gallagher J, Heddleston JM, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6(5):421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Highlights the importance of niche adhesion in maintaining the CSC state in GBM.
- 37.Son MJ, Woolard K, Nam D-H, Lee J, Fine HA. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4(5):440–452. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin X, Yin J, Kim S-H, et al. EGFR–AKT–Smad signaling promotes formation of glioma stem-like cells and tumor angiogenesis by ID3-driven cytokine induction. Cancer Res. 2011;71(22):7125–7134. doi: 10.1158/0008-5472.CAN-11-1330. [DOI] [PubMed] [Google Scholar]
- 39.Ogden AT, Waziri AE, Lochhead RA, et al. Identification of A2b5+Cd133- tumorinitiating cells in adult human gliomas. Neurosurgery. 2008;62(2):505–514. doi: 10.1227/01.neu.0000316019.28421.95. [DOI] [PubMed] [Google Scholar]
- 40.Bao S, Wu Q, Li Z, et al. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 2008;68(15):6043–6048. doi: 10.1158/0008-5472.CAN-08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anido J, Sáez-Borderías A, Gonzàlez-Juncà A, et al. TGF-β receptor inhibitors target the CD44high/Id1high glioma-initiating cell population in human glioblastoma. Cancer Cell. 2010;18(6):655–668. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 42.Ping Y, Yao X, Jiang J, et al. The chemokine CXCL12 and its receptor CXCR4 promote glioma stem cell-mediated VEGF production and tumour angiogenesis via PI3K/AKT signalling. J. Pathol. 2011;224(3):344–354. doi: 10.1002/path.2908. [DOI] [PubMed] [Google Scholar]
- 43.Guler G, Balci S, Costinean S, et al. Stem cell-related markers in primary breast cancers and associated metastatic lesions. Mod. Pathol. 2012;25(7):949–955. doi: 10.1038/modpathol.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGaughey DM, McCallion AS. Efficient discovery of ASCL1 regulatory sequences through transgene pooling. Genomics. 2010;95(6):363–369. doi: 10.1016/j.ygeno.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brescia P, Ortensi B, Fornasari L, Levi D, Broggi G, Pelicci G. CD133 is essential for glioblastoma stem cell maintenance. Stem Cells. 2013;31(5):857–869. doi: 10.1002/stem.1317. [DOI] [PubMed] [Google Scholar]
- 46.Lathia JD, Gallagher J, Heddleston JM, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6(5):421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gambelli F, Sasdelli F, Manini I, et al. Identification of cancer stem cells from human glioblastomas: growth and differentiation capabilities and CD133/prominin-1 expression. Cell Biol. Int. 2012;36(1):29–38. doi: 10.1042/CBI20110013. [DOI] [PubMed] [Google Scholar]
- 48.Jaksch M, Múnera J, Bajpai R, Terskikh A, Oshima RG. Cell cycle-dependent variation of a CD133 epitope in human embryonic stem cell, colon cancer, and melanoma cell lines. Cancer Res. 2008;68(19):7882–7886. doi: 10.1158/0008-5472.CAN-08-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deleyrolle LP, Harding A, Cato K, et al. Evidence for label-retaining tumour-initiating cells in human glioblastoma. Brain. 2011;134(Pt 5):1331–1343. doi: 10.1093/brain/awr081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamase A, Muraguchi T, Naka K, et al. Identification of tumor-initiating cells in a highly aggressive brain tumor using promoter activity of nucleostemin. Proc. Natl Acad. Sci. USA. 2009;106(40):17163–17168. doi: 10.1073/pnas.0905016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris MA, Yang H, Low BE, et al. Cancer stem cells are enriched in the side population cells in a mouse model of glioma. Cancer Res. 2008;68(24):10051–10059. doi: 10.1158/0008-5472.CAN-08-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Golebiewska A, Bougnaud S, Stieber D, et al. Side population in human glioblastoma is nontumorigenic and characterizes brain endothelial cells. Brain. 2013;136(Pt 5):1462–1475. doi: 10.1093/brain/awt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Persano L, Rampazzo E, Della Puppa A, Pistollato F, Basso G. The three-layer concentric model of glioblastoma: cancer stem cells, microenvironmental regulation, and therapeutic implications. ScientificWorldJournal. 2011;11:1829–1841. doi: 10.1100/2011/736480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCord AM, Jamal M, Shankavarum UT, Lang FF, Camphausen K, Tofilon PJ. Physiologic oxygen concentration enhances the stem-like properties of CD133+ human glioblastoma cells in vitro . Mol. Cancer Res. 2009;7(4):489–497. doi: 10.1158/1541-7786.MCR-08-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bar EE, Lin A, Mahairaki V, Matsui W, Eberhart CG. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am. J. Pathol. 2010;177(3):1491–1502. doi: 10.2353/ajpath.2010.091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]; ▪▪ First description of the perivascular niche in GBM.
- 57.Charles N, Ozawa T, Squatrito M, et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6(2):141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scully S, Francescone R, Faibish M, et al. Transdifferentiation of glioblastoma stem-like cells into mural cells drives vasculogenic mimicry in glioblastomas. J. Neurosci. 2012;32(37):12950–12960. doi: 10.1523/JNEUROSCI.2017-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soda Y, Marumoto T, Friedmann-Morvinski D, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc. Natl Acad. Sci. USA. 2011;108(11):4274–4280. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El Hallani S, Boisselier B, Peglion F, et al. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010;133(4):973–982. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu A, Wei J, Kong L-Y, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12(11):1113–1125. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pollard W J. Trophic marcophages in development and disease. Nat. Rev. Immunol. 2009;9(4):259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. J. Neurosci. Res. 2005;81(3):447–455. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- 64.Du R, Lu KV, Petritsch C, et al. HIF1α induces the recruitment of bone marrowderived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sims DE. Diversity within pericytes. Clin. Exp. Pharmacol. Physiol. 2000;27(10):842–846. doi: 10.1046/j.1440-1681.2000.03343.x. [DOI] [PubMed] [Google Scholar]
- 66.Bababeygy RS, Cheshier HS, Hou CL, Higgins MOD, Weissman LI, Tse CKV. Hematopoietic stem cell-derived pericytic cells in brain tumor angio-architecture. Stem Cells Dev. 2008;17(1):11–18. doi: 10.1089/scd.2007.0117. [DOI] [PubMed] [Google Scholar]
- 67.Környei Z, Szlávik V, Szabó B, Gócza E, Czirók A, Madarász E. Humoral and contact interactions in astroglia/stem cell co-cultures in the course of glia-induced neurogenesis. Glia. 2005;49(3):430–444. doi: 10.1002/glia.20123. [DOI] [PubMed] [Google Scholar]
- 68.Lathia JD, Li M, Hall PE, et al. Laminin alpha 2 enables glioblastoma stem cell growth. Ann. Neurol. 2012;72(5):766–778. doi: 10.1002/ana.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Le DM, Besson A, Fogg DK, et al. Exploitation of astrocytes by glioma cells to facilitate invasiveness: a mechanism involving matrix metalloproteinase-2 and the urokinase-type plasminogen activator–plasmin cascade. J. Neurosci. 2003;23(10):4034–4043. doi: 10.1523/JNEUROSCI.23-10-04034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG–GLI1 signaling regulates human glioma growth, cancer stem cell selfrenewal, and tumorigenicity. Curr. Biol. 2007;17(2):165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conti A, Aguennouz M, Torre D, et al. miR-21 and 221 upregulation and miR-181b downregulation in human grade II–IV astrocytic tumors. J. Neurooncol. 2009;93(3):325–332. doi: 10.1007/s11060-009-9797-4. [DOI] [PubMed] [Google Scholar]
- 72.González-Gómez P, Sánchez P, Mira H. MicroRNAs as regulators of neural stem cell-related pathways in glioblastoma multiforme. Mol. Neurobiol. 2011;44(3):235–249. doi: 10.1007/s12035-011-8196-y. [DOI] [PubMed] [Google Scholar]
- 73.Majid S, Dar AA, Saini S, et al. miR-23b Represses proto-oncogene Src kinase and functions as methylation-silenced tumor suppressor with diagnostic and prognostic significance in prostate cancer. Cancer Res. 2012;72(24):6435–6446. doi: 10.1158/0008-5472.CAN-12-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu H. MicroRNAs in breast cancer initiation and progression. Cell. Mol. Life Sci. 2012;69(21):3587–3599. doi: 10.1007/s00018-012-1128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huse JT, Brennan C, Hambardzumyan D, et al. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo . Genes Dev. 2009;23(11):1327–1337. doi: 10.1101/gad.1777409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lang M-F, Yang S, Zhao C, et al. Genomewide profiling identified a set of miRNAs that are differentially expressed in glioblastoma stem cells and normal neural stem cells. PLoS One. 2012;7(4):e36248. doi: 10.1371/journal.pone.0036248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guessous F, Alvarado-Velez M, Marcinkiewicz L, et al. Oncogenic effects of miR-10b in glioblastoma stem cells. J. Neurooncol. 2013;112(2):153–163. doi: 10.1007/s11060-013-1047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kefas B, Godlewski J, Comeau L, et al. MicroRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res. 2008;68(10):3566–3572. doi: 10.1158/0008-5472.CAN-07-6639. [DOI] [PubMed] [Google Scholar]
- 79.Wu N, Xiao L, Zhao X, et al. miR-125b regulates the proliferation of glioblastoma stem cells by targeting E2F2. FEBS Lett. 2012;586(21):3831–3839. doi: 10.1016/j.febslet.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Guessous F, Zhang Y, et al. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009;69(19):7569–7576. doi: 10.1158/0008-5472.CAN-09-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kefas B, Comeau L, Floyd DH, et al. The neuronal microRNA miR-326 acts in a feedback loop with notch and has therapeutic potential against brain tumors. J. Neurosci. 2009;29(48):15161–15168. doi: 10.1523/JNEUROSCI.4966-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Godlewski J, Nowicki MO, Bronisz A, et al. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol. Cell. 2010;37(5):620–632. doi: 10.1016/j.molcel.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vredenburgh JJ, Desjardins A, Herndon JE, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin. Cancer Res. 2007;13(4):1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 84.Keunen O, Johansson M, Oudin A, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc. Natl Acad. Sci. USA. 2011;108(9):3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van den Bent MJ, Brandes AA, Rampling R, et al. Randomized Phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J. Clin. Oncol. 2009;27(8):1268–1274. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee EQ, Puduvalli VK, Reid JM, et al. Phase I study of vorinostat in combination with temozolomide in patients with high-grade gliomas: North American brain tumor consortium study 0403. Clin. Cancer Res. 2012;18(21):6032–6039. doi: 10.1158/1078-0432.CCR-12-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Workman P, Clarke PA, Raynaud FI, van Montfort RLM. Drugging the PI3 kinome: from chemical tools to drugs in the clinic. Cancer Res. 2010;70(6):2146–2157. doi: 10.1158/0008-5472.CAN-09-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laks DR, Masterman-Smith M, Visnyei K, et al. Neurosphere formation is an independent predictor of clinical outcome in malignant glioma. Stem Cells. 2009;27(4):980–987. doi: 10.1002/stem.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pallini R, Ricci-Vitiani L, Banna GL, et al. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin. Cancer Res. 2008;14(24):8205–8212. doi: 10.1158/1078-0432.CCR-08-0644. [DOI] [PubMed] [Google Scholar]; ▪ Demonstrates the correlation between CSC marker expression and GBM patient survival in bioinformatic datasets.
- 90.Bar EE, Chaudhry A, Lin A, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25(10):2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fan X, Khaki L, Zhu TS, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28(1):5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ohka F, Natsume A, Wakabayashi T. Current trends in targeted therapies for glioblastoma multiforme. Neurol. Res. Int. 2012;2012:878425. doi: 10.1155/2012/878425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang J, Wakeman TP, Lathia JD, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28(1):17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]