Abstract
We illuminate inositol pyrophosphate turnover and cell-signaling activities, by showing that regulation of yeast cyclin-kinase by 1-InsP7 is not conserved for mammalian CDK5, and by kinetically characterizing Ddp1p/DIPP-mediated dephosphorylation of 1-InsP7, 5-InsP7 and InsP8. Each phosphatase exhibited similar Km values for every substrate (range: 35-148 nM). The rank order of kcat values (1-InsP7 > 5-InsP7 = InsP8) was identical for each enzyme, although DIPP1 was 10-60 fold more active than DIPP2α/β and DIPP3α/β. We demonstrate InsP8 dephosphorylation preferentially progresses through 1-InsP7. Conversely, we conclude that the more metabolically and functionally significant steady-state route of InsP8 synthesis proceeds via 5-InsP7.
1. Introduction
Among the many members of the inositol phosphate signaling family, the diphosphoinositol polyphosphates (inositol pyrophosphates;1-InsP7, 5-InsP7 and InsP8) receive particular attention. These “high-energy” signals operate at the interface of cell signaling and metabolic homeostasis [1,2], by phosphorylating proteins [3] (but see [4]), by regulating transcriptional responses to environmental stress [5-7], and by modulating PtdIns(3,4,5)P3-signaling [8,9].
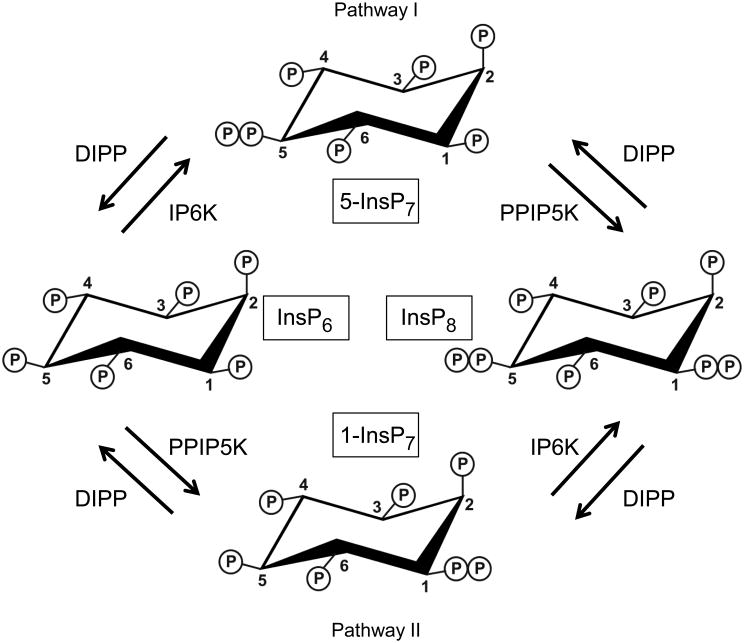
Understanding metabolic regulation of inositol pyrophosphate turnover reveals how this signaling cascade is controlled. Indeed, no metabolic pathways can be modeled accurately without knowledge of the kinetic parameters of the participating enzymes [10]. Inositol pyrophosphate metabolism is complicated by the two kinase pathways from InsP6 to InsP8 (I and II; ref [11] and Fig. 1), and by diphosphoinositol polyphosphate phosphohydrolases (DIPPs). Another confounding factor is that humans express five DIPP isoforms: types 1, 2α, 2β, 3α and 3β [12-15] S. cerevisiae only express one: the diadenosine and diphosphoinositol phosphohydrolase (Ddp1p) [16]. Little is known concerning the Michaelis-Menten kinetic parameters for Ddp1p/DIPPs; no such data are published for 1-InsP7 and InsP8. While some kinetic data have been provided for 5-InsP7, their reliability is now being questioned. For instance, Km values for 5-InsP7 vary from 4 nM for DIPP1 [12] to 4 μM for DIPP3β [15]. Such variation seems inconsistent with the high conservation of DIPP's catalytic domain. Instead, recent improvements in enzymatic [17] and chemical [18,19] methods for synthesizing inositol pyrophosphates have called into question the quality of some early sources of 5-InsP7 (see [18]). Here, using improved methods for the enzymatic synthesis and electrophoretic purification of 1-InsP7, 5-InsP7 and InsP8 [17], we have kinetically characterized the DIPP family.
Figure 1. Metabolic interconversions of InsP6, InsP7and InsP8.
DIPP = Diphosphoinositol polyphosphate phosphohydrolase (Ddp1p in yeast); IP6K = inositol hexakisphosphate kinase (Kcs1p in yeast); PPIP5K = Diphosphoinositol polyphosphate kinase (Vip1p in yeast). Pathways I and II are named as in [11].
2. Methods and Materials
2.1 Materials
GST-DIPP3α and GST-DIPP3β were prepared as described [15]. GST-DIPPs 1, 2α and 2β plasmids [12,13] were subcloned from pQE30 into pGEX6P-1 using BamHI and SalI. Expression plasmids were transformed into E. coli (BL21), and induced at 26°C overnight with 100 μM IPTG. Cells were harvested and sonicated in 20 mM Tris, 150 mM NaCl, 2 mM DTT, 0.1 mM EGTA, pH 7.5, 5 μg/ml leupeptin and 1 μg/ml aprotinin. GST-DIPPs were then purified using a Glutathione Sepharose-4 Fast-Flow column (Amersham Pharmacia Biotech). Protein was stored at −80°C in 10% v/v glycerol. Professor A.G. McLennan kindly provided Ddp1p.
The CDK5RAP1 open-reading frame was PCR-amplified from IMAGE clone 4418659, subcloned into pENTR/TEV/D-TOPO, and further subcloned into pDest606 in E. coli (DH5α). The CDK5RAP1-pDest606 construct was transformed into E. coli DH10Bac cells, yielding CDK5RAP1/bMON14272 bacmid; this was amplified by successive infections of Sf9 cells, then used to infect Sf9 cells for protein expression. After 72 hr., cells were lysed in I-Per (Pierce) plus protease inhibitor cocktail (Roche). CDK5RAP1 was purified using Ni-NTA resin and then an amylose resin column. Protein was stored at −80°C.
InsP6 was obtained from Calbiochem. Enzymatically-prepared inositol pyrophosphates were electrophoretically purified [17,20]. Samples from “blank” gels (i.e. no inositol pyrophosphates), processed in parallel, provided vehicle controls. Purity (by HPLC) of 5-InsP7, 1-InsP7, and InsP8 was, respectively, 86%, 95% and 94%. Such slight decomposition of the pyrophosphate groups does not significantly affect assays of DIPP activity [19]. Assays were corrected for the “zero-time” products.
2.2 Assays
Phosphatase activity was determined at 30°C (Ddp1p) or 37°C (DIPPs) in 20 μl of buffer containing 50 mM KCl, 50 mM HEPES (pH 7.2 with KOH), 4 mM CHAPS, 0.05 mg/ml BSA, 1 mM Na2EDTA, 2 mM MgSO4. [3H]labeled and non-radiolabeled InsP7 or InsP8 [20] were added as indicated (see Figures). After 2-25 min (corresponding to approx. 20% substrate metabolism), reactions were quenched and neutralized [14], loaded onto a HiChrom 4.6 × 125 mm Partisphere SAX column, and eluted (1 ml/min) with one of two protocols generated by mixing Buffer A (1 mM Na2EDTA) with buffer B [A + 1.3 M (NH4)2HPO4, pH 3.85 with H3PO4 ]: protocol 1), 0–5 min, B=0%; 5–10 min, B=0–45%; 10–55 min, B=45-100% (1ml fractions); protocol 2), 0–5 min, B=0%; 5–10 min, B=0–50%; 10–55 min, B=50-100% (0.5ml fractions). [3H] was assayed by liquid scintillation spectrometry. Km and kcat values were obtained by non-linear regression.
CDK5 activity was measured after preincubating (30 min) 50 ng CDK5/p35 (Sigma) and 20 ng CDK5RAP1 in 45 μl reactions containing 20 mM MOPS pH 7.4, 1 mM EGTA, 20 mM MgCl2, 1 mM dithiothreitol, 100 μM (γ-32P)ATP (0.05 μCi) and the appropriate inositol phosphate. Reactions were initiated with 5 μl of 1 mg/ml peptide substrate (BioMol International). After 30 min, 40 μl of each reaction was quenched by spotting onto P81 phospho-cellulose filters (Millipore). These were washed (4×; 0.75% H3PO4) and acetone-rinsed. [32P] was assayed by Cerenkov counting.
3. Results and Discussion
3.1 Ddp1p kinetics
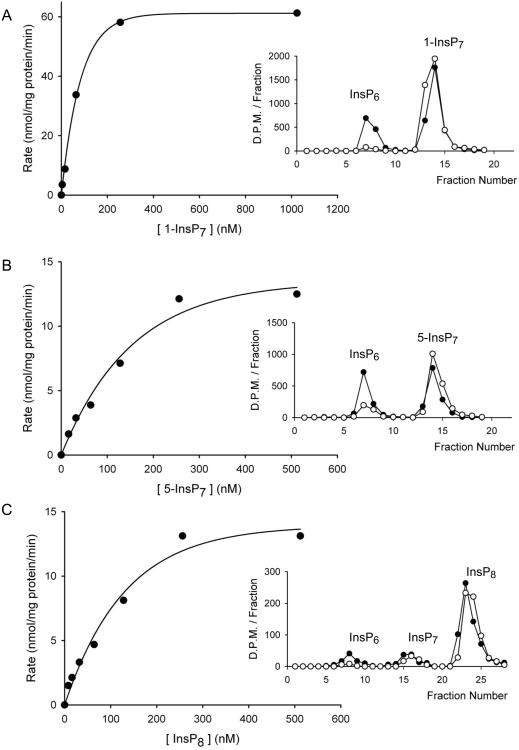
By HPLC analysis of the dephosphorylation of [3H]-radiolabeled substrates, we have kinetically characterized recombinant Ddp1p from S. cerevisiae. Ddp1p hydrolyzed 1-InsP7 and 5-InsP7 to InsP6 (Fig 2A,B). InsP8 was dephosphorylated to InsP6, with relatively little accumulation of InsP7 (Fig. 2C, and see below). Substrate saturation plots (Fig. 2) showed that Km values for each substrate were very similar (93nM, 105 nM, 148 nM; Table 1), but 1-InsP7 was hydrolyzed at a 5 to 6-fold faster rate than 5-InsP7 and InsP8 (Table 1).
Figure 2. Analysis of the catalytic activities of Ddp1p.
HPLC was used to obtain substrate saturation plots for Ddp1p against either A, 1-InsP7, (0-1024 nM incubated with 2 ng Ddp1p for 0-25 min); B, 5-InsP7 (0-512 nM incubated with 8 ng Ddp1p for 0-25 minutes); or C, InsP8, (0-512 nM incubated with 8 ng Ddp1p for 0-25 min). Insets provide illustrative HPLC analyses using protocol 1 (Section 2.2): A, 10 nM 1-InsP7, 20 ng Ddp1p, 10 min (not used in the substrate saturation plot). B 10 nM 5-InsP7, 10 ng Ddp1p, 6 min (not used in the substrate saturation plot). C, 16nM InsP8, 8ng Ddp1p, 4 min.
Table 1. Ddp1p/DIPP kinetic data.
| 1-InsP7 | 5-InsP7 | InsP8 | ||||
|---|---|---|---|---|---|---|
| Km (nM) | 102 × kcat (s-1) | Km (nM) | 102 × kcat (s-1) | Km (nM) | 102 × kcat (s-1) | |
| Ddp1p | 105 | 2.4 ± 0.05 | 93 | 0.4 ± 0.1 | 148 | 0.5 ± 0.1 |
| DIPP1 | 42 | 110 ± 30 | 52 | 13 ± 3 | 85 | 10 ± 2 |
| DIPP2α | 60 | 5 ± 2 | 35 | 0.7 ± 0.2 | 55 | 0.24 ± 0.08 |
| DIPP2β | 70 | 1.7 ± 0.5 | 40 | 0.3 ± 0.09 | 42 | 0.16 ± 0.02 |
| DIPP3α | 104 | 23 ± 2 | 146 | 4 ± 2 | 126 | 2.2 ± 0.4 |
| DIPP3β | 73 | 8 ± 2 | 63 | 0.88 ± 0.07 | 78 | 0.37 ± 0.06 |
Kinetic data were obtained as described in Section 2. Average (n=3-5) substrate affinities were first compiled as -log Km (SEMs were <3% of the mean) and then transformed to Km for the Table.
No previous studies have described Michaelis-Menten kinetic parameters for Ddp1p activity towards either 1-InsP7 or InsP8. There is one earlier study [21] that incubated Ddp1p with 250 μM of either 1-InsP7 or 5-InsP7; in an electrophoretic analysis, only 1-InsP7 was found to be dephosphorylated. That inability to detect 5-InsP7 metabolism may have reflected its assay concentration exceeding the Km value by 3 orders of magnitude (see Table 1). Also, SDS-PAGE mass analysis is less sensitive than is HPLC analysis of [3H]-labeled substrates. So although the preference of Ddp1p for 1-InsP7 over 5-InsP7 was previously known [21], our new kinetic data indicate the relative reaction rates are closer than previously suggested.
3.2 Human DIPP kinetics
We obtained substrate saturation plots (not shown) to derive Km and kcat values for all five human DIPPs (Table 1). DIPP1 exhibited the highest kcat, irrespective of substrate (Table 1). Thus, for example, the relative levels of cellular expression of DIPP1, versus the 20 to 60-fold less active DIPP2 isoforms, could dictate the rapidity of inositol pyrophosphate turnover on a cell-to-cell basis. This in turn could influence the sensitivity with which levels of inositol pyrophosphates respond to appropriate stimuli. This is analogous to how differential expression of cAMP-phosphodiesterases isoforms influence cell-to-cell differences in the sensitivity of agonist-mediated cAMP signaling [22].
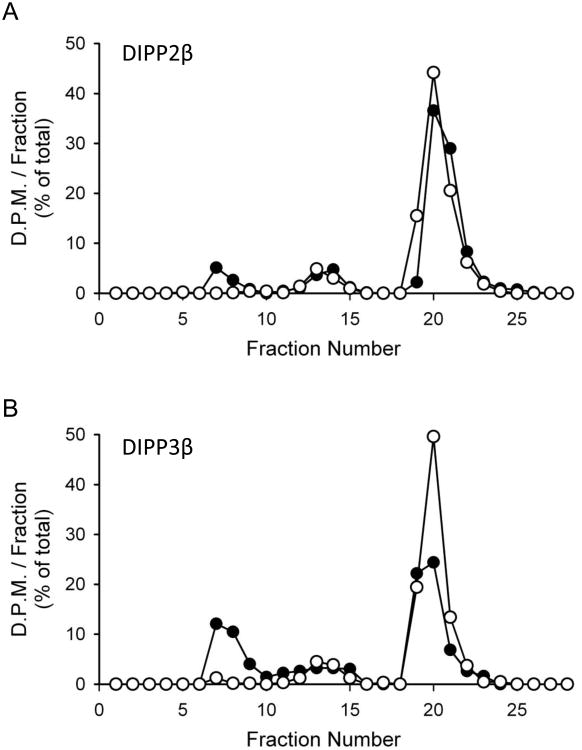
All human DIPPs behaved similarly to Ddp1p in exhibiting higher kcat values for 1-InsP7 compared to 5-InsP7 and InsP8 (Table 1). The Km data only vary over a 4-fold range (35 to 148 nM), across every enzyme and every substrate. The demonstration that rates of InsP7 dephosphorylation equal or exceed those of InsP8 metabolism (Table 1) explains why so little InsP7 accumulated when InsP8 was incubated with DIPPs (Fig. 3); the InsP7 was rapidly converted to InsP6.
Figure 3. HPLC analyses of the hydrolysis of InsP8 by DIPP2β and DIPP3β.
HPLC analyses (protocol 1; Section 2.2) of the metabolism (filled circles) at 37°C of 16 nM InsP8 incubated with (A) 10 ng DIPP2β for 6 min, or (B) 22.5 ng DIPP3β for 2 min. Open circles depict zero-time controls.
Our new kinetic data yield further conclusions: since cellular levels of 5-InsP7 are about 1-2 μM [15,23], these can now be considered sufficient (see Table 1) to be at a saturating concentration for all DIPPs. The kcat values for 5-InsP7 dephosphorylation are 5 to 9-fold lower than those for 1-InsP7 (Table 1). However, the affinities of the two InsP7 isomers for DIPPs are similar (Table 1), so they will compete for the DIPP active sites in direct proportion to their cellular concentration ratios. In mammalian cells, steady-state levels of 1-InsP7 are10 to15-fold lower than those of 5-InsP7 [24]. Thus, in vivo it is unlikely that the steady-state rate of 1-InsP7 dephosphorylation will be significantly greater than that for 5-InsP7. The latter conclusion - which can only be made now that kinetic parameters are available (Table 1) - counters a recent proposal [21] that, in vivo, a preferential DIPP activity towards 1-InsP7 “masks” its rate of kinase-mediated synthesis relative to that for 5-InsP7.
3.3 Inositol pyrophosphates do not regulate the human homologue of the yeast Pho80/81/85 cyclin kinase complex
The debate over the relative functional significance of pathway I and II (see above) has been influenced by the observation [5] that, in yeast, 1-InsP7 reversibly empowers Pho81 to inhibit the Pho80/85 cyclin kinase complex. This remains the only demonstration to date that 1-InsP7 serves a unique biological function. The mammalian homologs of Pho85 (the kinase), Pho80 (the kinase activator) and Pho81 (the kinase inhibitor) are, respectively, CDK5, p35 and CDK5RAP1 (originally: C42) [25,26]. However, unlike Pho81, there is no evidence recombinant CDK5RAP1 requires ancillary factors for inhibiting cyclin kinase activity [27].
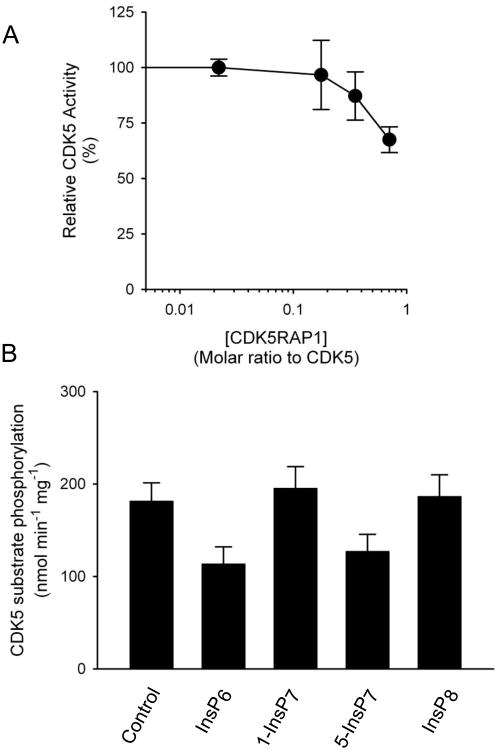
In earlier experiments [6], 1-InsP7 augmented inhibition of Pho85 by Pho81 when the latter was added at a molar ratio with Pho85 that was 20-30 fold lower that which, by itself, inhibited Pho85. In our CDK5 assays, we increased assay sensitivity by having CDK5RAP1 at the threshold level at which its own inhibitory effects can be detected (Fig. 4). We then added 4 μM 1-InsP7, a concentration that is >20-fold higher than is physiological (see above); CDK5 activity was unaffected (Fig. 4B); InsP6, 5-InsP7 and InsP8 were similarly ineffective (i.e. p>0.05 vs controls) (Fig. 4B). These data provide the first evidence that specific regulation of yeast cyclin kinase activity by 1-InsP7 [5] is not conserved in mammals.
Figure 4. Inositol pyrophosphates do not regulate CDK5 activity.
Panel A describes the concentration-dependent inhibition of CDK5 activity by CDK5RAP1, measured as described in Section 2. In panel B the molar ratio of CDK5RAP1:CDK5 was 0.4:1. None of the added inositol phosphates (4 μM) affected CDK5 activity (p>0.05 vs vehicle control; analysis by ANOVA with a Dunnett's post hoc test, n=5-8). Further experiments (not shown) did not uncover “order-of-addition” effects upon CDK5 activity.
3.4 The positional specificity of DIPPs towards InsP8
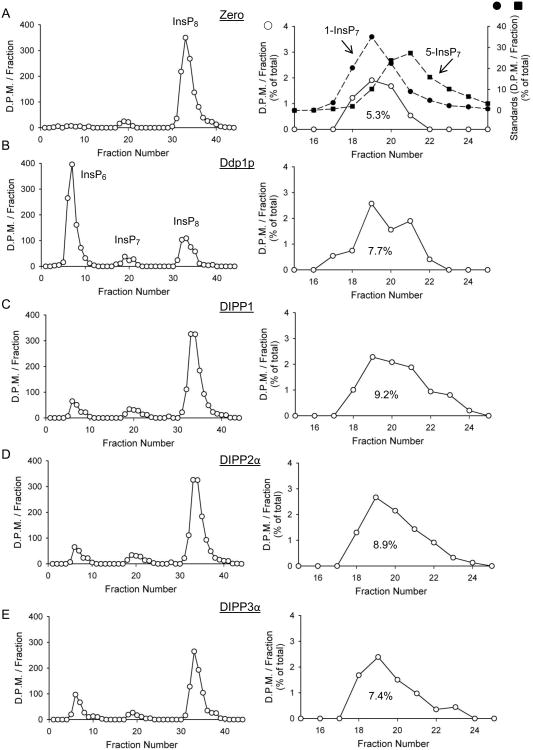
The positional specificity of DIPPs towards the 1- and/or 5-diphosphate groups on InsP8 also influences relative fluxes through the inositol pyrophosphate pathways (Fig. 1). Positional specificity is difficult to quantify, as so little InsP7 accumulates when InsP8 is dephosphorylated by DIPPs (e.g. Fig 3). Furthermore,1-InsP7 and 5-InsP7 are not generally resolved by strong anion-exchange HPLC. However, the columns used in the current study yielded a partial separation, particularly when we collected smaller fractions from a shallower gradient (Fig. 5A).
Figure 5. HPLC analyses of InsP8 dephosphorylation by Ddp1p, DIPP1, DIPP2α and DIPP3α.
Representative HPLC runs (protocol 2; Section 2.2) are shown for reactions containing either no enzyme (A; “zero”), or B, 16 ng Ddp1p, C, 2 ng DIPP1, D, 20ng DIPP2α and E, 10ng DIPP3α, all incubated for 8 min with 16 nM InsP8. The right hand panels in each pair amplify the InsP7 region of the chromatograph (open circles; InsP7 is quantified as percentage of total); filled symbols show elution of 1-InsP7 and 5-InsP7 standards (determined individually).
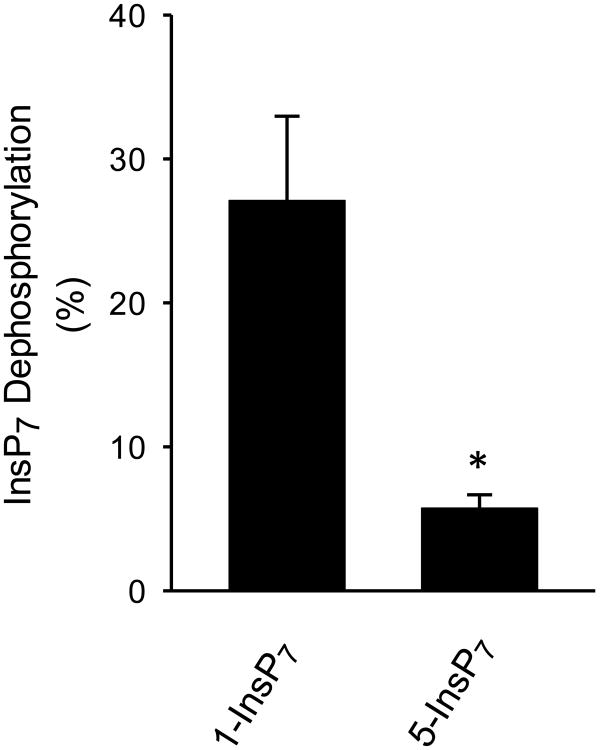
During InsP8 dephosphorylation by Ddp1p, DIPP1, DIPP2α and DIPP3α, the accumulation of new InsP7 product was 45-70% above the level of the (predominantly) 1-InsP7 that was present in the no-enzyme assays (Fig. 5). Both the increase in InsP7 peak height, and the larger degree of peak spreading, were sufficient to indicate accumulation of both 1-InsP7 and 5-InsP7. However, the kcat data in Table 1 allow us to conclude that, in these experiments (Fig. 5), 1-InsP7 that is formed from InsP8 will be degraded faster than the 5-InsP7 that is produced. That is, the rate of the 5-phosphate removal from InsP8 (yielding 1-InsP7) is underestimated relative to the rate of 1-phosphate removal from InsP8 (producing 5-InsP7). This conclusion is confirmed by data in Fig. 6, which show DIPP1 metabolizes 1-InsP7 faster than 5-InsP7 from a 1:1 mixture. Thus, where the data allow us to determine positional specificity (Fig. 5), we conclude Ddp1p/DIPPs preferentially (but not exclusively) remove the 5-phosphate from InsP8. Positional specificity for DIPPs 2β/3β was less clear (Figs 3), but based on sequence conservation and kinetic similarities, it seems likely they also show positional selectivity for the 5-phosphate on InsP8. Thus, the preferential route of InsP8 dephosphorylation is metabolically distinct from the main pathway for InsP8 synthesis. This contrasts with the old idea [28] that DIPPs remove from InsP8 the diphosphate group that is added after 5-InsP7 (now known to be in the 1-position [29]). The latter conclusion arose from a different experimental approach in which commercially-prepared substrates were incubated with liver homogenates. It is unclear why the earlier experiments led to a different conclusion - current concern over the quality of the substrates used (see Section 1) could be relevant here - but the possibility of a separate InsP8 phosphatase is another option that bears some consideration.
Figure 6. Metabolism of a 1:1 mixture of 1-InsP7 and 5-InsP7 by DIPP1.
1-InsP7 plus 5-InsP7 (10 nM each) were together incubated for 10 min with 0.3 ng DIPP1 plus approx 1000 D.P.M. of either 1-[3H]InsP7 (left hand bar) or 5-[3H]InsP7 (right-hand bar). HPLC analysis determined the metabolism of each InsP7. *p=0.02 (t-test; n=3).
3.5 Concluding Comments
Our study increases insight into inositol pyrophosphate turnover and function in vivo by providing the first kinetic characterization of Ddp1p/DIPP-mediated hydrolysis of 1-InsP7, 5-InsP7 and InsP8. Our results lead us to argue against previous suggestions [11,21] (made in the absence of the relevant kinetic data) that DIPPs conceal the importance of pathway II (see Fig. 1) by masking the rate of PPIP5K-driven phosphorylation of InsP6 to1-InsP7. Furthermore, much of the attention given to the functional significance of pathway II arises from evidence that 1-InsP7 inhibits the yeast Pho85 cyclin kinase [5,6]. Our data (Fig. 4) indicate that this mechanism is not conserved in mammals. Indeed, in mammals the 5-InsP7 that is produced by pathway I has functional significance, by competing with PtdIns(3,4,5)P3 for PH domains [8,9]; 1-InsP7 is much less potent [8].
The DIPP/Ddp1 family can also hydrolyze inorganic polyphosphates [21], 5-phosphoribosyl 1-pyrophosphate [30] and nucleotide dimers [16]. However, these particular catalytic activities are unlikely to affect the conclusions reached in this study, as they have alkaline pH optima and are orders of magnitude slower than the rates of inositol pyrophosphate hydrolysis.
Although mammalian cells contain only submicromolar levels of 1-InsP7 (Section 3.2), its concentration in S. cerevisiae has been argued to reach 30 μM in phosphate-starved cells [5]. While others have disputed that claim [21], it has nevertheless been reported that, in ddp1Δ yeast, levels of (presumably) 1-InsP7 were elevated 5-fold upon inhibition of Kcs1p by TNP, or by kcs1 deletion [11]. Those experiments were proposed to reveal the true extent of steady-state 1-InsP7 synthesis, again arguing that Pathway II is underappreciated. However, perhaps in those experiments, Vip1p's phosphorylation of InsP6 was artificially enhanced merely because of the absence of the kinase's preferred (and competing) substrate, 5-InsP7 (see [20,29]).
Highlights.
Understanding inositol pyrophosphate turnover through kinetic study of Ddp1p/DIPPs
kcat values for 1-InsP7 are 5-20 fold higher than those for 5-InsP7 and InsP8
InsP7 does not regulate CDK5, the human homologue of the yeast Pho85 cyclin kinase
Kinetics imply differential expression of DIPP isoforms sets signaling sensitivity
Metabolically and functionally separate routes for InsP8 synthesis and hydrolysis
Acknowledgments
RS Kilari was supported by a PhD studentship from the University of Wolverhampton's Research Institute of Healthcare Science. This research was also supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Szijgyarto Z, Garedew A, Azevedo C, Saiardi A. Influence of inositol pyrophosphates on cellular energy dynamics. Science. 2011;334:802–805. doi: 10.1126/science.1211908. [DOI] [PubMed] [Google Scholar]
- 2.Shears SB. Diphosphoinositol polyphosphates: metabolic messengers? Mol Pharmacol. 2009;76:236–252. doi: 10.1124/mol.109.055897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhandari R, Saiardi A, Ahmadibeni Y, Snowman AM, Resnick AC, Kristiansen TZ, Molina H, Pandey A, Werner JK, Jr, Juluri KR, Xu Y, Prestwich GD, Parang K, Snyder SH. Protein pyrophosphorylation by inositol pyrophosphates is a posttranslational event. Proc Natl Acad Sci U S A. 2007;104:15305–15310. doi: 10.1073/pnas.0707338104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shears SB, Gokhale NA, Wang H, Zaremba A. Diphosphoinositol polyphosphates: what are the mechanisms? Adv Enzyme Regul. 2011;51:13–25. doi: 10.1016/j.advenzreg.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YS, Mulugu S, York JD, O'Shea EK. Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science. 2007;316:109–112. doi: 10.1126/science.1139080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YS, Huang K, Quiocho FA, O'Shea EK. Molecular basis of cyclin-CDK-CKI regulation by reversible binding of an inositol pyrophosphate. Nat Chem Biol. 2008;4:25–32. doi: 10.1038/nchembio.2007.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worley J, Luo X, Capaldi AP. Inositol Pyrophosphates Regulate Cell Growth and the Environmental Stress Response by Activating the HDAC Rpd3L. Cell Rep. 2013;3:1476–1482. doi: 10.1016/j.celrep.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gokhale NA, Zaremba A, Janoshazi AK, Weaver JD, Shears SB. PPIP5K1 Modulates Ligand Competition Between Diphosphoinositol Polyphosphates and PtdIns(3,4,5)P3 for Polyphosphoinositide-Binding Domains. Biochem J. 2013;453:413–426. doi: 10.1042/BJ20121528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty A, Koldobskiy MA, Bello NT, Maxwell M, Potter JJ, Juluri KR, Maag D, Kim S, Huang AS, Dailey MJ, Saleh M, Snowman AM, Moran TH, Mezey E, Snyder SH. Inositol pyrophosphates inhibit akt signaling, thereby regulating insulin sensitivity and weight gain. Cell. 2010;143:897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohwer JM. Kinetic modelling of plant metabolic pathways. J Exp Bot. 2012;63:2275–2292. doi: 10.1093/jxb/ers080. [DOI] [PubMed] [Google Scholar]
- 11.Padmanabhan U, Dollins DE, Fridy PC, York JD, Downes CP. Characterization of a selective inhibitor of inositol hexakisphosphate kinases: Use in defining biological roles and metabolic relationships of inositol pyrophosphates. J Biol Chem. 2009;284:10571–10582. doi: 10.1074/jbc.M900752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safrany ST, Caffrey JJ, Yang X, Bembenek ME, Moyer MB, Burkhart WA, Shears SB. A novel context for the “MutT” module, a guardian of cell integrity, in a diphosphoinositol polyphosphate phosphohydrolase. EMBO J. 1998;17:6599–6607. doi: 10.1093/emboj/17.22.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caffrey JJ, Safrany ST, Yang X, Shears SB. Discovery of Molecular and Catalytic Diversity Among Human Diphosphoinositol Polyphosphate Phosphohydrolases: An Expanding NUDT Family. J Biol Chem. 2000;275:12730–12736. doi: 10.1074/jbc.275.17.12730. [DOI] [PubMed] [Google Scholar]
- 14.Hidaka K, Caffrey JJ, Hua L, Zhang T, Falck JR, Nickel GC, Carrel L, Barnes LD, Shears SB. An Adjacent Pair of Human NUDT Genes on Chromosome X are Preferentially Expressed in Testis and Encode Two New Isoforms of Diphosphoinositol Polyphosphate Phosphohydrolase. J Biol Chem. 2002;277:32730–32738. doi: 10.1074/jbc.M205476200. [DOI] [PubMed] [Google Scholar]
- 15.Leslie NR, McLennan AG, Safrany ST. Cloning and characterization of hAps1 and hAps2, human diadenosine polyphosphate-metabolizing Nudix hydrolases. BMC Biochemistry. 2002;3:20. doi: 10.1186/1471-2091-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safrany ST, Ingram SW, Cartwright JL, Falck JR, McLennan AG, Barnes LD, Shears SB. The diadenosine hexaphosphate hydrolases from Schizosaccharomyces pombe and Saccharomyces cerevisiae are homologues of the human diphosphoinositol polyphosphate phosphohydrolase: Overlapping substrate specificities in a MutT motif. J Biol Chem. 1999;274:21735–21740. doi: 10.1074/jbc.274.31.21735. [DOI] [PubMed] [Google Scholar]
- 17.Loss O, Azevedo C, Szijgyarto Z, Bosch D, Saiardi A. Preparation of quality inositol pyrophosphates. J Vis Exp. 2011:e3027. doi: 10.3791/3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capolicchio S, Thakor DT, Linden A, Jessen HJ. Synthesis of Unsymmetric Diphospho-Inositol Polyphosphates. Angew Chem Int Ed Engl. 2013;52:6912–5916. doi: 10.1002/anie.201301092. [DOI] [PubMed] [Google Scholar]
- 19.Wu M, Dul BE, Trevisan AJ, Fiedler D. Synthesis and characterization of non-hydrolysable diphosphoinositol polyphosphate second messengers. Chem Sci. 2013;4:405–410. doi: 10.1039/C2SC21553E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver JD, Wang H, Shears SB. The kinetic properties of a human PPIP5K reveal that its kinase activities are protected against the consequences of a deteriorating cellular bioenergetic environment. Biosci Rep. 2013;33:228–241. doi: 10.1042/BSR20120115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lonetti A, Szijgyarto Z, Bosch D, Loss O, Azevedo C, Saiardi A. Identification of an evolutionarily conserved family of inorganic polyphosphate endopolyphosphatases. J Biol Chem. 2011;286:31966–31974. doi: 10.1074/jbc.M111.266320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houslay MD. Adaptation in cyclic AMP signalling processes: a central role for cyclic AMP phosphodiesterases. Semin Cell Dev Biol. 1998;9:161–167. doi: 10.1006/scdb.1997.0221. [DOI] [PubMed] [Google Scholar]
- 23.Ingram SW, Safrany ST, Barnes LD. Disruption and overexpression of the Schizosaccharomyces pombe aps1 gene and the effects on growth rate, morphology, and intracellular diadenosine 5′, 5‴-P1, P5-pentaphosphate and diphosphoinositol polyphosphate concentrations. Biochem J. 2003;369:519–528. doi: 10.1042/BJ20020733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin H, Fridy PC, Ribeiro AA, Choi JH, Barma DK, Vogel G, Falck JR, Shears SB, York JD, Mayr GW. Structural analysis and detection of biological inositol pyrophosphates reveals that the VIP/PPIP5K family are 1/3-kinases. J Biol Chem. 2009;284:1863–1872. doi: 10.1074/jbc.M805686200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ching YP, Pang AS, Lam WH, Qi RZ, Wang JH. Identification of a neuronal Cdk5 activator-binding protein as Cdk5 inhibitor. J Biol Chem. 2002;277:15237–15240. doi: 10.1074/jbc.C200032200. [DOI] [PubMed] [Google Scholar]
- 26.Huang D, Patrick G, Moffat J, Tsai LH, Andrews B. Mammalian Cdk5 is a functional homologue of the budding yeast Pho85 cyclin-dependent protein kinase. Proc Natl Acad Sci U S A. 1999;96:14445–14450. doi: 10.1073/pnas.96.25.14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ching YP, Qi Z, Wang JH. Cloning of three novel neuronal Cdk5 activator binding proteins. Gene. 2000;242:285–294. doi: 10.1016/s0378-1119(99)00499-0. [DOI] [PubMed] [Google Scholar]
- 28.Shears SB, Ali N, Craxton A, Bembenek ME. Synthesis and metabolism of bis-diphosphoinositol tetrakisphosphate in vitro and in vivo. J Biol Chem. 1995;270:10489–10497. doi: 10.1074/jbc.270.18.10489. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Falck JR, Hall TM, Shears SB. Structural basis for an inositol pyrophosphate kinase surmounting phosphate crowding. Nat Chem Biol. 2012;8:111–116. doi: 10.1038/nchembio.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher DI, Safrany ST, McLennan AG, Cartwright JL. Nudix hydrolases that degrade dinucleoside and diphosphoinositol polyphosphates also have 5-phosphoribosyl 1-pyrophosphate (PRPP) pyrophosphatase activity that generates the glycolytic activator ribose 1,5-bisphosphate. J Biol Chem. 2002;277:47313–47317. doi: 10.1074/jbc.M209795200. [DOI] [PubMed] [Google Scholar]