Abstract
Cell death within cell populations is a stochastic process where cell-to-cell variation in temporal progression through the various stages of cell death arises from asynchrony of subtle fluctuations in the signaling pathways. Most cell death assays rely on detection of the specific marker of cell demise at the end-point of cell culturing. Such an approach cannot account for the asynchrony and the stochastic nature of cell response to the death-inducing signal There is a need therefore for rapid and high-throughput bioassays capable to continuously track viability of individual cells from the time of encountering a stress signal up to final stages of their demise. In this context, a new anthracycline derivative DRAQ7 is gaining increasing interest as an easy to use marker capable of long-term monitoring of cell death in real-time. This novel probe neither penetrates the plasma membrane of living cells nor does it affect cells susceptibility to the death inducing agents. However when the membrane integrity is compromised DRAQ7 enters cells undergoing demise and binds readily to nuclear DNA to report cell death. Here, we provide three sets of protocols for viability assays using DRAQ7 probe. The first protocol describes the innovative use of single color DRAQ7 real-time assay to dynamically track cell viability. The second protocol outlines a simplified end-point DRAQ7 staining approach. The final protocol highlights the real-time and multiparametric apoptosis assay utilizing DRAQ7 dye concurrently with tetramethylrhodamine methyl ester (TMRM), the mitochondrial trans-membrane electrochemical potential (ΔΨm) sensing probe.
INTRODUCTION
The quest for simplified cell viability assays that exploit the powerful multiparametric and high throughput capabilities of modern flow cytometry is still ongoing (Wlodkowic et al., 2010; Wlodkowic et al., 2008; Wlodkowic et al., 2011b; Zhao et al., 2010). Most contemporary cell viability assays are, however, still performed using an “end-point” approach that reveals the frequency of live versus dead cells only at the time of their harvesting (Akagi et al., 2013; Zhao et al., 2010). The end-point approach cannot access the stochastic character and asynchrony of cell death occurring in response to the death-inducing signal (Darzynkiewicz et al., 2001) The ability to non-invasively and continuously track cell viability over an extended period of time in a real-time scenario can provide a kinetic fingerprint of drug action and thus vastly enhance analytical capabilities probing responses of individual cells (Akagi et al., 2013; Akagi et al., 2012; Khoshmanesh et al., 2011; Wlodkowic et al., 2010; Zhao et al., 2010).
In this context, we outline development of innovative real-time cell viability protocols that employs the anthracycline derivative DRAQ7 (Akagi et al., 2013; Akagi et al., 2012). The novel probe does not penetrate the plasma membrane of living cells. However, once the membrane integrity is compromised, DRAQ7 binds readily to nuclear DNA with high affinity and reports cell death by strong far-red fluorescence. The spectral properties of the molecule provide a detection window in the far-red (>660nm) (identical to the cell permeant dye DRAQ5). The far-red fluorescent spectrum of DRAQ7 exhibits convenient spectral properties that allow for multiplexing with makers such as GFP, FITC and PE, Cy3 (Akagi et al., 2013; Akagi et al., 2012). Every protocol highlights a simple, one step assay for rapid assessment of viable versus dead cell subpopulations. Protocols presented below have been extensively tested on selected human hematopoietic cell lines using flow cytometry.
Basic Protocol 1: KINETIC ANALYSIS OF CELL VIABILITY USING DRAQ7 PROBE
The following protocol describes the application of plasma membrane integrity marker DRAQ7 (Ex/Em 488/>660 nm or 633–647/>660 nm) for real-time tracking of cell viability. The assay allows for rapid and sensitive discrimination between live and late apoptotic/necrotic subpopulations based on differential DRAQ7 staining profiles that pertain to uptake of DRAQ7 by dead and dying cells (Akagi et al., 2013). Convenient spectral characteristics of the DRAQ7 probe facilitate implementation of additional markers (e.g. immunophenotyping markers) for multicolor flow cytometry. Importantly, protocols presented below deliver single-step, time saving assays when applied to suspension culture of hematopoietic cells. Neither extensive pipetting nor washing steps are implemented and analysis is performed in a complete cell culture medium to facilitate preservation of apoptosing populations in an intact state. This is of importance since the cells undergoing apoptosis are more fragile and often are lost during centrifugation, repeated pipetting or after other mechanical stress (Darzynkiewicz et al., 2001).
Real-time DRAQ7 staining
Materials
30 μM DRAQ7 stock solution (store protected from light at +4ºC)
Cell suspension in appropriate culture medium
Cell culture vessels (as appropriate)
12x75 mm polystyrene FACS tubes or 1.5 ml Eppendorf tubes (as appropriate)
CAUTION: DRAQ7 probe is a DNA binding molecule and thus can be considered as potential carcinogen. Always use gloves when handling DRAQ7 solutions.
-
Dispense/seed cell suspension in growth medium to an appropriate vessel such as microtiter plate or cell culture flask
Dispense volumes that will allow collection of a number required cell samples during the course of the experiment. E.g. If your sampling volume in 100 μl and you require 10 time points dispense at least 1100 μl of cell suspension.
Add 10 μl of DRAQ7 stock solution to every 1000 μl of cell suspension (final concentration 3 μM of DRAQ7)
Gently mix the cell suspension with DRAQ7 to assure uniform dye distribution
Grow cells in the presence of DRAQ7 for up to 5 days at +37ºC in humidified atmosphere supplemented with appropriate level of CO2 (5% of CO2 is suitable for most human cell lines and primary tissue samples)
-
Periodically sample cell culture by acquiring cell suspensions for the flow cytometric analysis.
Cell volumes should at least match the minimum sampling volumes of the flow cytometer (100–150 μl on average would suffice).
-
Analyze on a flow cytometer without any washing steps with 488 nm or 633–647 nm excitation lines and with emissions collected at >660 nm
DRAQ7 is non-toxic to human cell lines for up to 5 days and cells can be grown in the presence of DRAQ7 (up to 20 μM) without any noticeable disturbances in cell proliferation, cell cycle, viability and reaction to pharmacological agents such as e.g. anti-cancer drugs or apoptosis stimulators. DRAQ7 can be effectively exited by both 488 nm and 633–647 nm lasers. The emission spectra are >665 nm to infra-red >800 nm (Emλmax 678 nm/694 nm intercalated into dsDNA). There is only a minimal spectral overlap between standard channels such as FITC/GFP at 530 nm and PE/Cy3 at 575–610 nm. Small to none adjustments in compensation between channels might be required but do not constitute any significant problem. Adjust the logarithmic amplification scale to distinguish between viable cells (DRAQ7− events), and late apoptotic/necrotic cells with compromised plasma membranes (DRAQ7+ events) as presented in the Fig. 1. In every cell system there might be a need to optimize PMT voltage to achieve maximal resolution between bright and low/negative DRAQ7 events.
Figure 1.
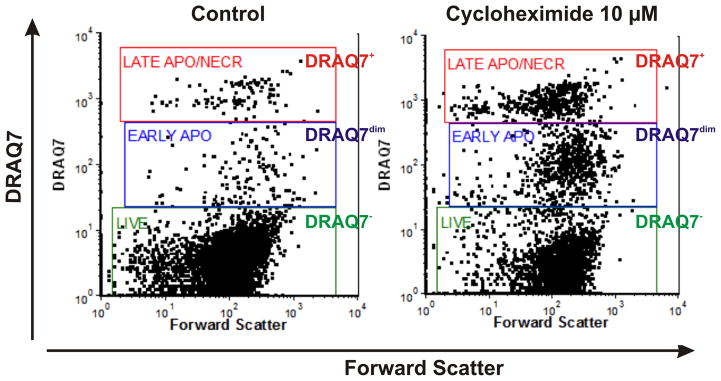
Assessment of live, early apoptotic and late apoptotic/necrotic cells based on stainability with plasma membrane permeability marker DRAQ7. Analysis was based on real-time labeling of THP-1 cells with 3 μM of DRAQ7. Bivariate dot plots DRAQ7 vs. forward scatter (FS) enable discrimination of live (DRAQ7−; green gate), early apoptotic (DRAQ7dim; blue gate) and late apoptotic/necrotic (DRAQ7+; red gate) subpopulations. DRAQ7 probe was excited using 633 nm laser and logarithmically amplified fluorescence signals were collected using 660 nm long-pass filter.
ALTERNATE PROTOCOL 1
The following protocol describes the application of plasma membrane integrity marker DRAQ7 (Ex/Em 488/>660 nm or 633–647/>660 nm) for “end-point” analysis of cell viability. In this assay the staining is performed at the time of cell harvesting in a complete growth medium. As such the assay does not require cell washing o centrifugation. While not proving the unique kinetic tracking capabilities (see Basic Protocol) the assay is still rapid and sensitive alternative to conventional viability markers such as propidium iodide (PI) or 7-aminoactimomycin D (7-AAD). However, the convenient spectral characteristics of DRAQ7 allow for lucid multiplexing with FITC or PE offering substantial analytical advantages over older generations of markers. Discrimination between live and late apoptotic/necrotic subpopulations based on differential DRAQ7 staining profiles that pertain to uptake of DRAQ7 by dead and dying cells (Figure 1; See Basic Protocol).
End-point DRAQ7 staining
Additional Materials (also see Basic Protocol)
CAUTION: DRAQ7 probe is a DNA binding molecule and thus can be considered as potential carcinogen. Always use gloves when handling DRAQ7 solutions.
Collect an aliquot of cell sample in complete cell culture medium to a micro centrifuge tube. Cell volumes should at least match the minimum sampling volumes of the flow cytometer (100–150 μl on average would suffice).
Add 10 μl of DRAQ7 stock solution to every 1000 μl of cell suspension (final concentration 3 μM of DRAQ7)
Gently mix the cell suspension with DRAQ7 to assure uniform dye distribution
Incubate 2 minutes at RT or +37ºC
-
Analyze on a flow cytometer without any washing steps with 488 nm or 633–647 nm excitation lines and with emissions collected at >660 nm
The end-point protocol follows the same principles as the real-time assay (Basic Protocol) and might be preferred option in some applications that do not require kinetic analysis of viability. Moreover, the end-point protocol can be applied to optimize staining vs. cell density conditions and perform inter assays calibration tests.
Alternate Protocol 2: REAL-TIME MULTIPARAMETRIC ASSESSMENT OF CELL DEATH USING DRAQ7 AND MITOCHONDRIAL MEMBRANE POTENTIAL (ΔΨm,) SENSITIVE PROBE TMRM
This protocol describes a novel multiparametric assay for simultaneous detection of early apoptotic events, such as mitochondrial membrane depolarization, by TMRM and plasma membrane permeability by DRAQ7. Both probes are inert to many human cell lines at concentrations not exceeding 500 nM (TMRM) and 20 μM (DRAQ7) when the cells are grown in continuous presence of the probes for up to 5 days (Akagi et al., 2013). This offers a unique capability to perform real-time monitoring of mitochondrial membrane inner membrane potential without any additional staining and washing steps. Spectral characteristics of depicted probes permit also implementation of additional markers such as GFP/FITC and/or probes excited by UV/violet excitation lines. Importantly this is a straightforward one-step protocol that does not require any buffers or washing/centrifugation steps.
Two color real-time assay with DRAQ7 and TMRM
Additional Materials (also see Basic Protocol)
1 mM TMRM stock solution in DMSO (store protected from light at −20ºC)
10 μM TMRM working solution in appropriate cell culture medium
CAUTION: Although there are no reports on TMRM toxicity, appropriate precautions should always be applied when handling TMRM solutions.
-
Dispense/seed cell suspension in growth medium to an appropriate vessel such as microtiter plate or cell culture flask.
Dispense volumes that will allow collection of a number required cell samples during the course of the experiment. E.g. If your sampling volume in 100 μl and you require 10 time points dispense at least 1100 μl of cell suspension.
Add 10 μl of DRAQ7 stock solution to every 1000 μl of cell suspension (final concentration 3 μM of DRAQ7)
Add 15 μl of TMRM working solution to every 1000 μl of cell suspension (final concentration 150 nM of TMRM)
Gently mix the cell suspension to assure uniform dye distribution
Grow cells in the presence of both probes for up to 5 days at 37ºC in humidified atmosphere supplemented with appropriate level of CO2 Incubation at 37 ºC is necessary to allow adequate cell loading with TMRM probe.
-
Periodically sample cell culture by acquiring cell suspensions for the flow cytometric analysis.
Cell volumes should at least match the minimum sampling volumes of the flow cytometer (100–150 μl on average should suffice).
-
Analyze on a flow cytometer without any washing steps with 488 nm and 633–647 nm excitation lines and with emissions collected at 575 nm for TMRM and >660 nm for DRAQ7
Both fluorochromes are easily excited by 488 nm lasers. There is minimal spectral overlap between adjacent channels and some small compensation adjustments may be required if both dyes are to be excited by 488 nm line. DRAQ7 fluorescence can be conveniently detected using a standard allophycocyanin (APC) 660 nm band-pass filter eliminating any need for spectral compensation when excited by 633 nm line. Adjust the logarithmic amplification scale to distinguish between viable cells (bright TMRM+/DRAQ7− events), apoptotic cells (TMRMlow/DRAQ7− events) and late apoptotic/necrotic cells with compromised plasma membranes (TMRMlow/DRAQ7+ events) as seen in Fig. 2. In some cell systems there might be a need to optimize concentration of TMRM and DRAQ7 probes as well as PMT voltage to achieve maximal resolution between bright, dim/low and low/negative events.
Figure 2.
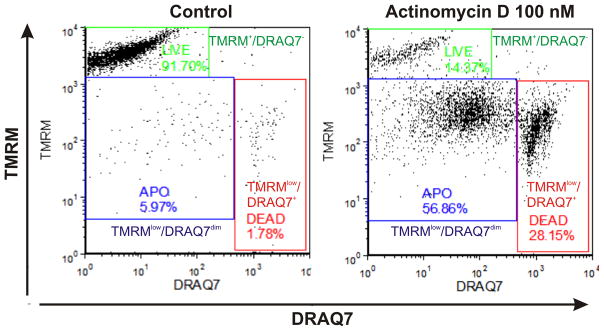
Discrimination of viable, apoptotic and late apoptotic/necrotic cells based on Δψm marker tetramethylrhodamine methyl ester (TMRM) and plasma membrane permeability marker DRAQ7. Analysis was based on real-time labeling of THP-1 cells with 3 μM of DRAQ7 and 150 nm of TMRM. Bivariate dot plots DRAQ7 vs. TMRM enable discrimination of live (TMRM+/DRAQ7−; green gate), early apoptotic (TMRMlow/DRAQ7dim; blue gate) and late apoptotic/necrotic (TMRMlow/DRAQ7+; red gate) subpopulations. DRAQ7 probe was excited using 633 nm laser and logarithmically amplified fluorescence signals were collected using 660 nm long-pass filter. TMRM was excited using 488 nm laser and logarithmically amplified fluorescence signals were collected using 575 nm long-pass filter. Debris signals were excluded electronically by setting the proper low threshold.
REAGENTS AND SOLUTIONS
Use deionized water in all recipes and protocols. For common stock solutions refer to APPENDIX 2A; for suppliers, see SUPLIERS APPENDIX
TMRM (tetramethylrhodamine methyl ester) working solution, 10 μM
Prepare fresh before cell staining by adding 10 μl of 1 mM TMRM stock solution in DMSO to 990 μl of appropriate cell culture medium. Scale up by multiplying if more solution is needed. Protect from direct light and use at the same day.
COMMENTARY
Background Information
To date, diverse methods have been introduced that allow implementation of life-cell assays using e.g. a variety of DNA selective probes (such as Hoechst 33342, DRAQ5), plasma membrane permeability markers (such as PI, 7-AAD, YO-PRO 1), phosphatidylserine (PS) exposure markers (such as Annexin A5, monoclonal antibodies against PS), probes assessing mitochondrial membrane potential (such as JC1, DiOC6(3), TMRM, TMRE) or cell permeable fluorescently labeled inhibitors of caspases (FLICA) (Skommer et al., 2010; Wlodkowic et al., 2010; Wlodkowic et al., 2011b) (see also UNITS 7.18, 7.19, 7.25, 9.2 and 9.14). Most contemporary assays are, however, still performed using an “end-point” approach that reveals the frequency of live versus dead cells only at the time of their harvesting (Skommer et al., 2010; Wlodkowic et al., 2010; Wlodkowic et al., 2011b). As mentioned, the endpoint approach cannot account for the asynchrony and stochastic character of cell response to the inducer of cell death. It also cannot account for differences in the “time-windows” through which particular apoptotic events can be detected (Darzynkiewicz et al., 2001). The ability to non-invasively and continuously track cell viability over an extended period of time in a real-time scenario can provide a kinetic fingerprint of drug action and thus vastly enhances analytical capabilities of probing individual cells response to the drug (Akagi et al., 2013; Akagi et al., 2012; Khoshmanesh et al., 2011; Skommer et al., 2011; Wlodkowic et al., 2011a). We have recently proposed that an ideal approach to monitor cell viability would require the development of non-invasive fluorescent markers that: (i) enable population monitoring and cell tracking over an extended period of time; (ii) do not by themselves modify the viability of the cell system, particularly the structural and bio-physiological properties of the cells; (iii) enable multi-parameter analysis in combination with other markers; and (iv) are transferable to high-throughput formats and automation (Akagi et al., 2013; Khoshmanesh et al., 2011; Skommer et al., 2011; Wlodkowic et al., 2011a).
Protocols outlined here provide new evidence that such real-time labeling procedure can provide multi-parameter and kinetic fingerprints of anti-cancer drug action when employing DRAQ7 probe. Importantly, a substantial reduction of sample processing steps and avoidance of washing protocols achieved with such a kinetic protocol is important for the preservation and retrieval of fragile cell subpopulations (Akagi et al., 2013; Akagi et al., 2012).
DRAQ7 characteristics
The anthracycline derivative DRAQ7 being a far-red fluorescent (emission above >660nm) DNA dye exhibits convenient spectral properties that allow for multiplexing with makers such as GFP, FITC and Cy3. The probe does not penetrate plasma membrane of living cells, however once the membrane integrity is compromised, it readily binds to nuclear DNA and thus reports cell death. Our data indicated that the growth, cell cycle distribution and proliferation of several tumor cell lines were unaffected by the continuous presence of DRAQ7 for up to 3 days of culture (Au- up to what DRAQ7 concentration? ZD). Also, unlike other DNA supravital probes (Zhao et al., 2009) continuous incubation with DRAQ7 led to no evidence of either DNA damage, replication stress or induction of cell senescence. Moreover, recently published data show lack of any noticeable influence on cell responses to- or on interaction with- cytotoxic agents during growth in the presence of DRAQ7, allowing thereby a direct adaptation of this probe for the automated and real-time pharmacological profiling of anti-cancer drugs (Akagi et al., 2013; Akagi et al., 2012).
Critical Parameters and Troubleshooting
Using DRAQ7 probe in real-time modality
It is advisable to consider preliminary dose and time-course optimization prior to commencing experiments with DRAQ7 probe. To achieve maximum resolution a dose range of 0.35 – 10 μM DRAQ7 can be tested to assess its impact on cell viability and stainability of particular cell line (Akagi et al., 2013). In most cases the staining with 3 μM of DRAQ7 achieves the fluorescence levels comparable to to that at the end-point staining with 1 μg/ml of propidium iodide (PI) (Akagi et al., 2013). Even at concentrations of up to 20 μM DRAQ7 proved to be non-toxic to several both cell lines tested (Akagi et al., 2013). Moreover, no dysfunctions in the mitochondrial function were observed as assessed by the multiparameter labeling with ΔΨm sensitive probe TMRM. Likewise,, neither cell proliferation nor cell cycle distribution were affected for up to 72 hours growth in the presence of 3–5 μM DRAQ7. Cells grown continuously in the presence of DRAQ7 were found to lack any evidence of DNA damage signaling such as an increase in phosphorylation of H2AX on Ser139 (expression of γH2AX) and activation of ATM through its phosphorylation on Ser1981 (ATM-S1981P) (Akagi et al., 2013).
Using DRAQ7 for bi-color analysis together with TMRM probe
When used with Δψm marker TMRM the 10–15 min incubation at 37 ºC is critical to allow adequate cell loading with TMRM. It should be noted however that unlike another widely used mitochondrial probe rhodamine 123 (Darzynkiewicz et al., 1981), the prolonged exposure to which leads to cell arrest in early portion of G1 (Darzynkiewicz et al., 1982), cells can be grown for extended periods of time with TMRM (if not exceeding 500 nM) with no significant effect on cell cycle and viability. This procedure results in reproducible data and clear discrimination of populations of distinctive subpopulations (see Fig. 2).
Both fluorochromes are easily excited by 488 nm lasers. There is minimal spectral overlap between adjacent channels and some small compensation adjustments may be required if both dyes are to be excited by a single 488 nm line. DRAQ7 fluorescence can be conveniently detected using a standard allophycocyanin (APC) 660 nm band-pass filters eliminating any need for spectral compensation when excited by 633 nm line.
Anticipated results
Real-time DRAQ7 staining
Live cells with intact plasma membrane integrity do not stain with DRAQ7 whereas dying cells fluorescence brightly when DRAQ7 enters nucleus and binds to DNA. Early apoptotic cells can exhibit intermediate levels of DRAQ7 staining. The discrimination of live, apoptotic and late apoptotic/necrotic cells by SYTO probes can be re-confirmed after back-gating each subpopulation onto bivariate FSC vs. SSC dot plots. Light scatter properties (forward- versus side- scatter) characteristic for apoptosis provide supportive information (Darzynkiewicz et al., 1997; Wlodkowic et al., 2010). It should be noted, however, that the latter are considered to be unspecific, may vary considerably depending on specific cell type and death inducer, and should be analyzed in parallel with another, more specific marker of apoptosis (Darzynkiewicz et al., 1997).
Using DRAQ7 for bi-color analysis together with TMRM probe
Viable cells stain brightly with TMRM probe and exclude DRAQ7 (bright TMRM+/DRAQ7− events). Early apoptotic cells exhibit rapid loss of TMRM staining (deemed cells with Δψm breach) and usually exclude DRAQ7 (TMRMlow/DRAQ7− events). Some apoptotic cells may exhibit intermediate levels of stainability with DRAQ7 due to increased plasma membrane permeability. Finally, late apoptotic/necrotic cells feature progressive loss of TMRM fluorescence and always stain brightly with DRAQ7 (TMRMlow/DRAQ7+ events) as seen in Fig. 2 (Akagi et al., 2013).
Time Considerations
Real-time DRAQ7 staining
The protocol is very rapid and does not require any centrifugation or washing steps. Staining of one sample with DRAQ7 and collection does not take more that 15 seconds per each step. Thus overall time depends on the number of samples required for further analysis but usually takes less than 10 min for average 24-well culture plate. Instrument setup together with compensation should not exceed 5 min in total. Subsequent data analysis will require additional 5–10 min.
Using DRAQ7 for bi-color analysis together with TMRM probe
The protocol is very rapid and does not require any centrifugation or washing steps. Staining of one sample with DRAQ7 and TMRM does not take more that 20 seconds per each step. Collection does not take more that 15 seconds per each step. Thus overall time depends on the number of samples required for further analysis but usually takes less than 15 min for average 24-well culture plate. Instrument setup together with compensation should not exceed 5 min in total. Because bicolor assay provides multiparameter parameter data, subsequent analysis time will depend heavily on the gating strategies employed by an investigator.
Supplementary Material
Literature Cited
- Akagi J, Kordon M, Zhao H, Matuszek A, Dobrucki J, Errington R, Smith PJ, Takeda K, Darzynkiewicz Z, Wlodkowic D. Real-time cell viability assays using a new anthracycline derivative DRAQ7. Cytom Part A. 2013;83A:227–234. doi: 10.1002/cyto.a.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi J, Takeda K, Fujimura Y, Matuszek A, Khoshmanesh K, Wlodkowic D. Microflow cytometry in studies of programmed tumor cell death. Sensors and Actuators, B: Chemical. 2012 in press. http://dx.doi.org/10.1016/j.snb.2012.10.124.
- Darzynkiewicz Z, Bedner E, Traganos F. Difficulties and pitfalls in analysis of apoptosis. Methods Cell Biol. 2001;63:527–559. doi: 10.1016/s0091-679x(01)63028-0. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology. Analysis of apoptosis accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- Darzynkiewicz Z, Staiano-Coico L, Melamed MR. Increased mitochondrial uptake of rhodamine 123 during lymphocyte stimulation. Proc Natl Acad Sci USA. 1981;78:2383–2387. doi: 10.1073/pnas.78.4.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Traganos F, Staiano-Coico L, Kapuscinski J, Melamed MR. Interactions of rhodamine 123 with living cells studied by flow cytometry. Cancer Res. 1982;42:799–806. [PubMed] [Google Scholar]
- Khoshmanesh K, Akagi J, Nahavandi S, Skommer J, Baratchi S, Cooper JM, Kalantar-Zadeh K, Williams DE, Wlodkowic D. Dynamic analysis of drug-induced cytotoxicity using chip-based dielectrophoretic cell immobilization technology. Anal Chem. 2011;83:2133–2144. doi: 10.1021/ac1029456. [DOI] [PubMed] [Google Scholar]
- Skommer J, Darzynkiewicz Z, Wlodkowic D. Cell death goes LIVE: technological advances in real-time tracking of cell death. Cell Cycle. 2010;9:2330–2341. doi: 10.4161/cc.9.12.11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skommer J, Raychaudhuri S, Wlodkowic D. Timing is everything: stochastic origins of cell-to-cell variability in cancer cell death. Front Biosci. 2011;16:307–314. doi: 10.2741/3689. [DOI] [PubMed] [Google Scholar]
- Wlodkowic D, Faley S, Darzynkiewicz Z, Cooper JM. Real-time cytotoxicity assays. Methods Mol Biol. 2011a;731:285–291. doi: 10.1007/978-1-61779-080-5_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodkowic D, Skommer J, Darzynkiewicz Z. Cytometry in cell necrobiology revisited. Recent advances and new vistas. Cytometry A. 2010;77:591–606. doi: 10.1002/cyto.a.20889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodkowic D, Skommer J, Hillier C, Darzynkiewicz Z. Multiparameter detection of apoptosis using red-excitable SYTO probes. Cytometry A. 2008;73:563–569. doi: 10.1002/cyto.a.20564. [DOI] [PubMed] [Google Scholar]
- Wlodkowic D, Telford W, Skommer J, Darzynkiewicz Z. Apoptosis and beyond: cytometry in studies of programmed cell death. Methods Cell Biol. 2011b;103:55–98. doi: 10.1016/B978-0-12-385493-3.00004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Oczos J, Janowski P, Trembecka D, Dobrucki J, Darzynkiewicz Z, Wlodkowic D. Rationale for the real-time and dynamic cell death assays using propidium iodide. Cytometry A. 2010;77:399–405. doi: 10.1002/cyto.a.20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Traganos F, Dobrucki J, Wlodkowic D, Darzynkiewicz Z. Induction of DNA damage response by the supravital probes of nucleic acids. Cytometry A. 2009;75A:510–519. doi: 10.1002/cyto.a.20727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


