Abstract
Objectives
To determine whether the effects of weight loss on arterial function are differentially modified by insulin status.
Background
Clinical studies suggest that plasma insulin levels may predict the extent of cardiovascular benefit achieved with weight loss in obese individuals, but mechanisms are unknown.
Methods
We prospectively followed 208 overweight or obese patients (BMI ≥25 kg/m2) receiving medical/dietary (48%) or bariatric surgical (52%) weight loss treatment during a median period of 11.7 months (inter-quartile range 4.6–13 months). We measured plasma metabolic parameters and vascular endothelial function using ultrasound at baseline and following weight loss intervention, and stratified analyses by median plasma insulin levels.
Results
Subjects age 45±1 yr, BMI 45±9 kg/m2 experienced 14±14% weight loss during the study period. In individuals with higher baseline plasma insulin levels (above median >12 uIU/ml, n=99), ≥10% weight loss (compared to <10%) significantly improved brachial artery macro-vascular flow-mediated vasodilation and micro-vascular reactive hyperemia (P <0.05 for all). In contrast, vascular function did not change significantly in the lower insulin group (≤12 uIU/mL, n=109) despite similar degree of weight loss. In analyses using a 5% weight loss cut-point, only micro-vascular responses improved in the higher insulin group (P=0.02).
Conclusions
Insulin status is an important determinant of the positive effect of weight reduction on vascular function with hyperinsulinemic patients deriving the greatest benefit. Integrated improvement in both micro- and macro-vascular function was associated with ≥10% weight loss. Reversal of insulin resistance and endothelial dysfunction may represent key therapeutic targets for cardiovascular risk reduction in obesity.
Keywords: hyperinsulinemia, weight loss, vasculature, obesity, bariatric surger
Introduction
Obesity has emerged as one of the most critical health care problems in the US and worldwide with nearly 70% of the US population currently overweight or obese (1). Of major concern are data showing disproportionate surges in categories of severe obesity (body mass index [BMI] ≥ 40 kg/m2) which tripled during the 1990s (2). Nearly a third of adults (1) and 17% of children (3) in the US are now obese with 65 million additional cases estimated by 2030 (4). While obesity confers serious health concerns and increased all-cause mortality, the vast majority of deaths are due to cardiovascular causes such as ischemic heart disease and stroke (5,6). Thus, there is pressing need to elucidate potential mechanisms that link excess adiposity to cardiovascular risk and identify groups most likely to benefit from targeted treatment.
While obesity prevention is likely to provide the most optimal public health solution, there is obvious interest in promoting weight loss as a therapeutic strategy to reverse obesity-related cardiometabolic risk (5). Nevertheless, few clinical studies have explicitly examined the relationship between intentional weight loss and cardiovascular mortality. The most convincing data emerged recently from the Swedish Obese Subjects (SOS) study showing reduced long-term cardiovascular mortality following bariatric surgery, largely owing to decreased myocardial infarction risk (7). Although specific mechanisms for cardiovascular benefit remain unknown, plasma insulin appeared to emerge as a primary determinant of cardiac events, providing evidence that insulin resistance may contribute significantly to the pathogenesis of vascular disease in obesity (7).
The vascular endothelium plays a key role in the regulation of arterial tone, blood flow, inflammation, and thrombosis (8,9). Endothelial phenotype serves as a barometer of overall vascular health, displays impairment in insulin resistant states, and severity of both micro- and macro-vascular dysfunction independently predict future cardiovascular events (8,10–14). The purpose of the study was to determine whether the effects of weight loss on arterial function are differentially modified by insulin status in overweight and obese individuals undergoing weight reduction intervention.
Methods
Subjects
Overweight adult men and women (age ≥18 years) with BMI ≥25 kg/m2 seeking weight loss treatment at the Boston Medical Center Nutrition and Weight Management Clinic (n = 208) were prospectively followed. This hospital-based weight loss intervention program utilizes a comprehensive approach for obesity management using behavioral, dietary, medical, and/or surgical treatments that are individualized based on clinical and patient decisions. Interventions comply with established National Heart, Lung, and Blood Institute clinical guidelines (15) and included medical therapy with dietary/lifestyle modification (n = 100, 48%) and bariatric surgery (n = 108, 52%). Low-carbohydrate Atkins-type or Mediterranean diets were not specifically prescribed. Nearly all bariatric surgical procedures comprised of Roux-en-Y gastric bypass operation (n = 106) and two patients underwent laparoscopic adjustable gastric banding. The analyses represent data from subjects collected to date from an ongoing prospective cohort study designed to examine vascular responses to weight loss. Subjects with unstable medical conditions such as recent coronary syndromes (within 6 months), congestive heart failure, systemic infection, acute illness, malignancy or pregnancy were excluded. The study was approved by Boston Medical Center Institutional Review Board and all subjects gave written informed consent.
Vascular function studies
Each subject underwent a forearm brachial artery ultrasound vascular study twice, performed at baseline prior to lifestyle and/or surgical intervention, and follow-up after a median of 11.7 (inter-quartile range 4.6–13.0) months. Vascular studies were performed during a fasting state in a quiet, temperature-controlled room under resting conditions by trained sonographers (16). Brachial vasomotor responses were examined using a noninvasive, standardized method of ultrasound using a Toshiba Powervision 6000 system (Toshiba Medical USA, Tustin, CA). Brachial artery two-dimensional diameter (mm) images and pulse Doppler flow velocity (cm/s) were measured at the antecubital crease. Brachial flow-mediated dilation (FMD) following a 5-minute cuff occlusion in an upper arm position served as a measure of endothelium-dependent macro-vascular function, expressed as percent change in brachial diameter before and 60 seconds after cuff occlusion. Brachial artery reactive hyperemia expressed as the percent change in hyperemic forearm blood flow increase after cuff occlusion served as the index measure of endothelium-dependent micro-vascular function (10,12,17,18).
Clinical and metabolic measures
Clinical characteristics including blood pressure, height, weight, and BMI were recorded on the same day as the vascular studies. Weight was measured using a calibrated scale (Ohaus, Pine Brook, NJ). Biochemical analyses including lipids, glucose, insulin, homeostasis model assessment of insulin resistance (HOMA), and glycosylated hemoglobin (HbA1c) were quantified from blood samples collected in a fasting state during each visit. Medications were recorded for all visits including anti-hypertensive, hypoglycemic, and lipid-lowering regimens.
Statistical analyses
Analyses were completed using SAS for Windows, version 9.1 (SAS Institute Inc., Cary, NC, USA). Data are presented as mean ± SD, or median with inter-quartile range, or proportions (%), unless otherwise indicated. The primary outcome variables were FMD (%) and hyperemic flow increase (%). Absolute change was calculated as the numerical value difference between baseline and follow-up. Histograms and normal probability plots were used to determine whether continuous variables were normally distributed or skewed. Baseline triglycerides, glucose, insulin, and HOMA were non-normally distributed and analyzed following natural log transformation. Informed by data from the SOS study (7), our a priori hypothesis was that the effects of weight loss on vascular function would differ by plasma insulin, and as such we stratified our analyses by baseline insulin level. Participants were dichotomized into two groups based on median baseline insulin concentration, where the lower insulin group was defined as ≤12 uIU/mL (n = 109) and higher insulin group >12 uIU/mL (n = 99). In separate models, analysis of covariance (ANCOVA) (proc GLM in SAS) was used to examine the effect of 5% or 10% weight loss cut-points, based on expert target recommendations for weight reduction in the management of obesity (15), on changes in vascular and clinical parameters stratified by insulin status. Baseline age and BMI were included as covariates in all models. In addition, models were adjusted for baseline values for the outcome of interest. For example, in analyses examining the effect of 10% weight loss on change in FMD, baseline FMD was included as a covariate. The exposures were treated as dichotomous variables (e.g., ≥10% weight loss or not) and outcomes and covariates as continuous ones. We included interaction terms in models to formally test for effect modification of the association between weight loss and vascular function by baseline insulin status. Medication changes were analyzed across insulin strata and weight loss categories. All group differences were examined using the Student’s t test, Chi-square test or Fisher’s exact test as appropriate and within weight loss group changes by paired t tests. Partial Pearson’s correlation adjusted for baseline age and BMI were used to examine the association between changes in clinical data and vascular function parameters. For all analyses, P value <0.05 was considered statistically significant.
Results
Study population
A total of 208 patients (mean age 45 ± 11 y; 82% female; BMI 45 ± 9 kg/m2) were enrolled. Nearly all participants were obese with BMI > 30 kg/m2 (n = 205, 99%). Participants were 55% Caucasian, 26% African-American, and 17% Hispanic reflecting demographics of the general population seeking weight loss treatment in our urban tertiary center. Approximately half (52%, n = 109) of the participants underwent bariatric surgery while 48% (n = 99) received lifestyle intervention alone. For the entire group, median follow-up period was 11.7 months and mean weight loss 14 ± 14%.
Baseline clinical data stratified by median plasma insulin concentration are displayed in table 1. Subjects in the higher insulin group (>12 uIU/mL) had higher BMI, triglycerides, HOMA, and lower HDL-cholesterol values (P < 0.05). Prevalence of diabetes mellitus, coronary artery disease (CAD), hypertension and medications used to treat these conditions were also higher in this group. Micro-vascular function as measured by brachial artery hyperemic flow (reactive hyperemia) was lower in the higher insulin group (P < 0.05), while brachial FMD was comparable between insulin strata.
Table 1.
Baseline clinical characteristics stratified by plasma insulin level
| Lower insulin ≤ 12 uIU/mL n = 109 |
Higher insulin > 12 uIU/mL n = 99 |
P Value | |
|---|---|---|---|
| Age, yr | 44.8 ± 11 | 44.9 ± 11 | 0.92 |
| Female, % | 85 | 78 | 0.16 |
| Race/Ethnicity, % | 0.49 | ||
| Caucasian | 52 | 58 | |
| African-American | 30 | 21 | |
| Hispanic | 16 | 19 | |
| Other | 2 | 2 | |
| Weight, kg | 115 ± 26 | 131 ± 29 | <0.0001 |
| BMI, kg/m2 | 42 ± 8 | 47 ± 9 | <0.0001 |
| SBP, mm Hg | 130 ± 15 | 129 ± 14 | 0.81 |
| DBP, mm Hg | 74 ± 10 | 72 ± 10 | 0.11 |
| Total Cholesterol, mg/dL | 191 ± 36 | 186 ± 38 | 0.37 |
| Triglycerides, mg/dL | 89 (67 – 146) | 130 (89 –171) | <0.001 |
| LDL-C, mg/dL | 117 ± 31 | 112 ± 33 | 0.24 |
| HDL-C, mg/dL | 50 ± 13 | 45 ± 9 | <0.001 |
| Glucose, mg/dL | 92 (87 – 100) | 100 (91 – 116) | 0.05 |
| Insulin, uIU/mL | 8 (6 – 10) | 19 (14 –26) | <0.0001 |
| HOMA | 2.0 (1.4 – 2.4) | 4.6 (3.5 – 6.9) | <0.0001 |
| HbA1c, % | 6.0 ± 1.2 | 6.5 ± 1.5 | 0.01 |
| Vascular function parameter | |||
| Hyperemic flow increase, % | 779 ± 400 | 666 ± 395 | 0.04 |
| FMD, % | 9.2 ± 4.5 | 8.4 ± 4.8 | 0.23 |
| Co-morbidity | |||
| Hypercholesterolemia, % | 34 | 42 | 0.21 |
| Diabetes mellitus, %* | 21 | 43 | <0.001 |
| Coronary artery disease, % | 4 | 15 | 0.01 |
| Hypertension, % | 39 | 57 | 0.01 |
| Medical therapy | |||
| Anti-hypertensive, % | 43 | 59 | 0.03 |
| Lipid-lowering, % | 19 | 30 | 0.06 |
| Hypoglycemic, %† | 17 | 32 | 0.01 |
| Insulin, % | 3 | 8 | 0.12 |
Diabetes mellitus included a medical diagnosis of type 1 or 2 diabetes. One participant in the lower insulin group was diagnosed with type 1 diabetes.
Excludes insulin use.
Weight loss and clinical parameters
As shown in table 2, ≥ 10% weight change was associated with significant decline in BMI, insulin, HOMA, HbA1c, and triglycerides in both higher and lower baseline insulin categories (P < 0.05 for all). A threshold cut-point of 5% weight loss induced similar but less pronounced metabolic changes (data not shown). While individuals with higher plasma insulin achieved improvements in metabolic variables that were directionally similar to the lower insulin group, incremental decrease in insulin and HOMA among those with ≥10% weight loss was over 4 times greater in the higher insulin group (−14.2 uIU/mL and −4.3, respectively) as compared to the lower insulin group (−3.0 uIU/mL and −0.82, respectively) despite comparable degree of weight change. The proportion of patients who underwent surgical intervention was similar in lower compared to higher insulin categories (47% vs. 58%, P = 0.12). In individuals who lost ≥ 10% weight (n = 112), weight change was similar between lower and higher insulin strata (24.0 ± 10.0 % vs. 25.8 ± 10.1 %, respectively, P = 0.34). However, the majority of subjects achieved ≥10% weight loss as a result of bariatric surgical intervention, which did not differ between higher and lower insulin groups (87% vs. 88%, P = 0.89).
Table 2.
Effect of 10% weight loss on clinical parameters stratified by insulin level
| Lower insulin ≤ 12 uIU/mL
|
Higher insulin > 12 uIU/mL
|
|||||
|---|---|---|---|---|---|---|
| Weight loss < 10%
|
Weight loss ≥ 10%
|
P value | Weight loss < 10%
|
Weight loss ≥ 10%
|
P value | |
| n = 59 | n = 50 | n = 37 | n = 62 | |||
| BMI, kg/m2 | −1.4 (0.5) | −10.3 (0.6) | <0.0001 | −0.7 (0.7) | −12.4 (0.5) | <0.0001 |
| SBP, mm Hg | 0 (2.1) | −8.4 (2.4) | 0.01 | −3.2 (2.5) | 3 (1.9) | 0.06 |
| DBP, mm Hg | −0.8 (1.2) | −1.7 (1.4) | 0.65 | 0.2 (1.5) | 2.8 (1.1) | 0.17 |
| Total cholesterol, mg/dL | −6.2 (4) | −13.9 (4.2) | 0.20 | −5.4 (5.2) | −13.7 (3.8) | 0.21 |
| Triglycerides, mg/dL | −6.8 (5.9) | −31.7 (6.2) | 0.01 | −7.3 (10.0) | −35.8 (7.4) | 0.03 |
| LDL-C, mg/dL | −4.3 (3.3) | −12 (3.5) | 0.12 | −4.2 (4.3) | −10 (3.1) | 0.28 |
| HDL-C, mg/dL | 0 (1.5) | 3.9 (1.6) | 0.1 | 0.2 (1.9) | 3.6 (1.4) | 0.16 |
| Glucose, mg/dL | −1.7 (2.7) | −7.3 (2.9) | 0.18 | 13.2 (5.4) | −13.5 (4.1) | <0.001 |
| Insulin, uIU/mL | 1.9 (0.9) | −3 (0.9) | <0.001 | −2.9 (1.8) | −14.2 (1.4) | <0.0001 |
| HOMA | 0.34 (0.21) | −0.82 (0.22) | <0.001 | 0.10 (0.95) | −4.33 (0.74) | <0.001 |
| HbA1c, % | −0.14 (0.07) | −0.51 (0.07) | <0.001 | −0.07 (0.15) | −0.95 (0.11) | <0.0001 |
Values are mean (SE) adjusted for baseline values for age, BMI, and the outcome of interest. ANCOVA was used to determine the difference in Δ clinical parameters between dichotomous weight loss groups.
Weight loss and vascular function
As shown in figure 1, in models adjusted for baseline age, BMI, and vascular function, ≥10% weight change significantly improved hyperemic blood flow (micro-vascular function) and flow-mediated dilation (macro-vascular function) in subjects with higher baseline insulin (both P < 0.05), while 5% weight change was only associated with improved micro-vascular responses (P = 0.02) (figure 2). Although a trend for increased reactive hyperemia was observed (P = 0.08), similar degrees of weight loss did not significantly alter micro- or macro-vascular responses in patients with lower baseline insulin levels. The inclusion of change in hyperemic flow increase as a covariate in models predicting change in FMD did not alter these relationships, suggesting that improvement in FMD in the higher insulin strata was not exclusively mediated by changes in flow. Results for ≥10% weight loss and vascular improvement were similar after excluding subjects using insulin clinically (n = 11), and adjustment for changes in medications, blood pressure, lipids, glucose, HOMA, and HbA1c in ANCOVA models, thus demonstrating that these parameters did not confound the findings. In formal tests of interaction, the association between 10% weight loss and change in FMD significantly differed by baseline insulin status (P=0.04) and there was a suggestion of effect modification with change in hyperemic flow increase set as the dependent variable (P=0.1).
Figure 1. Effect of ≥10% weight loss on vascular function.
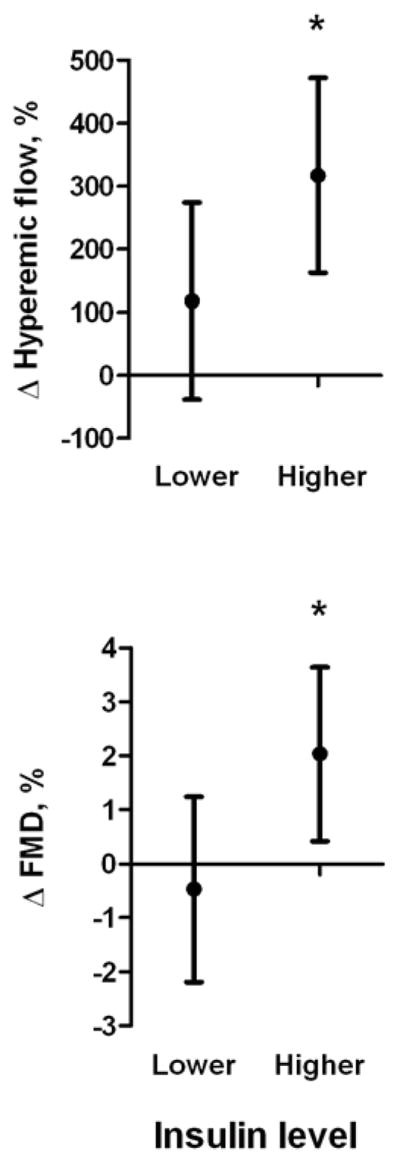
Brachial artery flow-mediated vasodilation and hyperemic flow improved significantly in the baseline higher insulin but not lower insulin group. Values represent mean (95% CI) difference in Δ vascular function between ≥10% vs. <10% weight loss, adjusted for baseline age, BMI, and vascular function. *P<0.05.
Figure 2. Effect of ≥5% weight loss on vascular function.
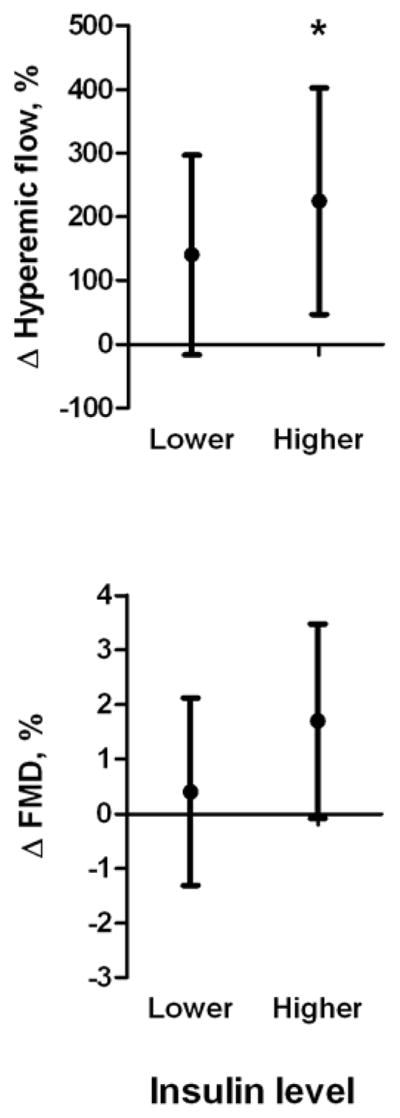
Brachial artery hyperemic flow improved significantly only in the baseline higher insulin group. Values represent mean (95% CI) difference in Δ vascular function between ≥5% vs. <5% weight loss, adjusted for baseline age, BMI, and vascular function. *P<0.05.
As metabolic improvements occurred with weight loss, individuals discontinued medications for the treatment of hypertension, dyslipidemia, and glucose intolerance as displayed in table 3. A ≥10% weight reduction was associated with greater abatement in medication use compared to <10% weight change. There was a strong trend for reduced hypoglycemic drug use following weight loss in the higher versus lower insulin group (P = 0.05), otherwise there were no significant differences in discontinuation of anti-hypertensive or lipid lowering treatment across insulin strata.
Table 3.
Medication discontinuation following weight loss across treatment classes stratified by insulin status.
| Weight loss | Lower insulin ≤ 12 uIU/mL
|
||
|---|---|---|---|
| < 10%
|
≥ 10%
|
P value | |
| n = 59 | n = 50 | ||
| Anti-hypertensive | 10 (6) | 26 (13) | 0.03 |
| Lipid-lowering | 0 (0) | 20 (10) | 0.0002 |
| Hypoglycemic | 2 (1) | 20 (10) | 0.002 |
|
|
|||
| Higher insulin > 12 uIU/mL
|
|||
| n = 37 | n = 62 | P value | |
|
| |||
| Anti-hypertensive | 3 (1) | 37 (23) | <0.0001 |
| Lipid-lowering | 3 (1) | 21 (13) | 0.01 |
| Hypoglycemic | 0 (0) | 36 (22) | <0.0001 |
Values represent % discontinuation (n).
Metabolic and vascular effects stratified by weight loss
Individuals who achieved ≥10% weight loss group (n=112) had significantly improved total cholesterol, LDL-C, triglyceride, HDL-C, glucose, insulin, and HOMA (P<0.05 vs. baseline). Compared to participants with <10% weight loss, there were more favorable changes in triglycerides (P=0.002), HDL-C (P=0.04), glucose (P=0.003), insulin (P<0.0001), HOMA (P<0.0001), and HbA1c (P<0.0001), and trend toward improved LDL-C (P=0.06) and total cholesterol (P=0.09) levels (data not shown). Subjects with ≥10% weight loss exhibited 206% (95% CI: 99, 313; P=0.0002) improvement in hyperemic flow increase compared to < 10% weight decline, but no group difference in change in FMD was detected (P=0.25). In analyses adjusted for age and BMI, changes in micro-vascular function correlated negatively with change in insulin (r = −0.19, P = 0.01), HOMA (r = −0.16, P = 0.03), and LDL-C (r = −0.19, P = 0.01), and macro-vascular function correlated positively with HDL-C (r = 0.15, P = 0.04).
Discussion
In a cohort of obese patients undergoing weight reduction therapy, baseline insulin status was a key determinant of the positive effects of weight loss on vascular function, with higher risk hyperinsulinemic patients deriving the greatest benefit. A modest 5% decrease in body weight improved cardiovascular risk factors and micro-vascular function in subjects with higher insulin levels, while weight reduction of ≥ 10% reversed metabolic dysfunction to a greater extent and improved both micro- and macro-vascular vasodilator responses. Given the key role of vascular homeostasis in mitigating cardiovascular risk, our findings suggest that reversal of insulin resistance and endothelial dysfunction may be mechanistically intertwined and represent important therapeutic targets in obesity.
Few studies have examined the relationship between intentional weight loss and cardiovascular mortality. A 12-year observational study in overweight women reported that weight change with lifestyle modification reduced mortality with greatest impact in high co-morbidity subsets (19). Similarly, retrospective analysis of bariatric surgical patients showed improved cardiovascular survival during a 7.1-year follow-up period (20). The strongest evidence to date comes from the prospective Swedish Obese Subjects (SOS) study which recently reported 15-year follow-up data demonstrating nearly 30% reduction in long-term risk of myocardial infarction and stroke with bariatric weight loss (7,21). Surprisingly, while neither BMI nor improvement in many traditional risk factors were linked to cardiovascular benefit, secondary analyses identified baseline plasma insulin levels as the primary determinant of cardiovascular risk. Our results build upon this observation, as the notion that insulin status stratifies vascular improvement was evident from our study, prompting recognition that reduced cardiovascular events following weight loss may be tied to mechanisms of improved insulin sensitivity and arterial homeostasis.
While prior small studies showed that vascular function improves with weight decline, differential associations by insulin status have not been specifically explored (22–25). Circulating asymmetric dimethylarginine levels, an endogenous endothelial nitric oxide synthase (eNOS) inhibitor, decline preferentially in insulin resistant subjects following weight loss (26). We similarly observed that vascular benefits of weight reduction were primarily manifest in hyperinsulinemic patients. From a clinical perspective, several important points can be emphasized from our study. First, baseline differences in insulin status did not appear to modulate extent of weight loss achieved. Second, it was evident that not all obese individuals gained equal metabolic or vascular benefit from weight reduction. Third, vascular improvement did not appear to be confounded by medication changes that were similar across insulin strata. Fourth, our data provide a potential mechanism for the SOS study findings that confine the effectiveness of weight loss in reducing cardiac events to high insulin categories. Lastly, our results also suggest that ≥10% weight change may be required for integrated improvement in micro- and macro-vascular functions, which further supports NHLBI recommendations targeting 10% weight reduction for medical benefits (15).
Elevated circulating insulin levels likely reflect a pathophysiological state of systemic insulin resistance that is associated with vascular endothelial dysfunction, arterial inflammation, oxidative stress, and accelerated atherosclerosis (27–31). The magnitude of insulin resistance varies widely among obese individuals, and higher insulin concentrations are linked to dysfunctional vascular phenotypes in both coronary and peripheral circulations (32,33). Animal and human data show that pathologic abnormalities involve defects in vascular insulin signaling such as the IRS-1/phosphoinositide 3-kinase/Akt pathway associated with impaired eNOS activity and nitric oxide bioaction, and expression of proinflammatory, prothrombotic, and vasoconstrictive mediators that support a proatherogenic vascular phenotype (30). These abnormalities manifest, in part, as defects in both micro- and macro-vascular endothelial function that have been prospectively and independently linked to adverse cardiovascular events such myocardial infarction and stroke (10,12,34,35).
While our results expand our understanding of the complex relationship between obesity, insulin resistance, arterial disease, further questions remain. Evidence is mounting that bariatric surgery is more effective than conventional treatment for durable weight loss and diabetes remission (36,37). As expected, bariatric intervention was more effective in achieving rapid and more extensive weight loss during the follow-up period (~ 1 year) compared to conventional therapy in our cohort. While our study was not designed to compare weight intervention methods, the proportion of individuals treated surgically was the same across insulin strata, suggesting that vascular benefits were not a consequence of differential operative intervention. In that regard, recent findings from the Look AHEAD (Action for Health in Diabetes) study reported that while intensive lifestyle modification through diet and exercise induced 6% weight loss at 9.6 years with positive health benefits, cardiovascular events were not lowered (38). In addition, several dietary intervention studies where mean weight loss was <10% reported no significant macro-vascular improvement (39,40). This adds further debate not only to the search for optimal weight loss strategy, but also regarding the minimal amount of weight change required to elicit comprehensive health benefits.
As the most important health risk of obesity is the development of cardiovascular disease, the clinical implication that insulin resistance and vascular dysfunction drive, in part, the association between obesity and cardiovascular risk raises issues regarding treatment strategies. As such, pharmacological therapies for insulin sensitization have displayed variable cardiovascular effects (41–43). Enhanced insulin sensitivity achieved by weight loss likely involves additional complex beneficial mechanisms mediated by modulation of fat tissue phenotypes, reduction of adipose-derived inflammatory cytokines, fatty acid mobilization, and hormonal shifts (23,44–46). In our cohort, changes in micro-vascular function were more sensitive to weight modification, and insulin resistance correlated more closely with micro-vascular responses than conduit vessel function. How specific therapeutic strategies intertwine with favorable modification of vascular phenotypes is clinically important and warrants further investigation.
The present study has several limitations. First, the study was not randomized, although interventions were based on clinical decisions that likely reflect quotidian practice. Despite its observational nature, the prospective design maintained temporal relationship between alterations in metabolic parameters and vascular function during the follow-up period. Second, the study did not examine cardiac outcome data which limits generalization regarding absolute risk. We recognize that forearm hyperemia represents a complex physiological response that is only partly endothelium-dependent. However, our endpoints of arterial function as surrogates of cardiovascular risk have been prospectively validated in multiple outcomes studies (11). Third, we used an arbitrary cutoff (median split) to categorize our participants into higher and lower baseline insulin categories. Insulin values of our sample of severely obese participants are elevated compared to normal levels reported in community based studies (47). Additional studies with large sample sizes and conducted in obese populations are needed to explore alternative plasma insulin cutoffs that may maximize the differential relationships observed in our study. Lastly, sustainability of relatively short-term (~1 year) vascular changes in our cohort has not been examined in the long-term and requires continued investigation.
In conclusion, our results suggest that reduction of cardiovascular risk in obesity may vary as a function of reversing insulin resistance and vascular endothelial dysfunction. While we emphasize that intentional weight loss of any kind averts a multitude of health risks associated with excess adiposity, our data suggest that attempts to reduce cardiovascular risk by weight reduction should be particularly emphasized in obese hyperinsulinemic individuals.
Acknowledgments
Funding sources: Dr. Gokce is supported by National Institutes of Health (NIH) grants HL081587, HL1145675, and HL084213. Dr. Farb is supported by an American Heart Association Postdoctoral Fellowship grant 12POST11780028. Dr. Hamburg is supported by NIH grants HL109790 and HL102299. Dr. Vita is supported by NIH grants HL081587, HL083801, HL083269, HL75795, and K12 HL083781. Dr. LaValley is supported by NIH grant HL081587 and HL1145675. Dr. Apovian is supported by NIH grants HL081587, HL1145675, HL084213 and P30DK046200.
Abbreviations
- BMI
Body Mass Index
- CAD
Coronary Artery Disease
- eNOS
Endothelial Nitric Oxide Synthase
- FMD
Flow-Mediated Dilation
- HOMA
Homeostasis Model Assessment
Footnotes
Conflict of Interest: The authors have no conflicts of interest with the current manuscript.
Disclosures: Dr. Apovian has participated on the advisory boards for Amylin Pharmaceuticals, Orexigen Therapeutics, Merck & Co., Inc., Johnson & Johnson, Arena Pharmaceuticals, Nutrisystem, Inc., Zafgen Inc., and Sanofi-Aventis US LLC. She has received research funding from Eli Lilly & Co., Amylin Pharmaceuticals, Pfizer, Inc., Sanofi-Aventis US LLC, Orexigen Therapeutics, MetaProteomics LLC, and the Dr. Robert C. and Veronica Atkins Foundation. None of these sources funded the current study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Freedman DS, Khan LK, Serdula MK, Galuska DA, Dietz WH. Trends and correlates of class 3 obesity in the United States from 1990 through 2000. JAMA. 2002;288:1758–61. doi: 10.1001/jama.288.14.1758. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–25. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 5.Cornier MA, Marshall JA, Hill JO, Maahs DM, Eckel RH. Prevention of overweight/obesity as a strategy to optimize cardiovascular health. Circulation. 2011;124:840–50. doi: 10.1161/CIRCULATIONAHA.110.968461. [DOI] [PubMed] [Google Scholar]
- 6.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 8.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 9.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 10.Anderson TJ, Charbonneau F, Title LM, et al. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123:163–9. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 11.Gokce N. Clinical assessment of endothelial function: ready for prime time? Circ Cardiovasc Imaging. 2011;4:348–50. doi: 10.1161/CIRCIMAGING.111.966218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang AL, Silver AE, Shvenke E, et al. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27:2113–9. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamburg NM, Larson MG, Vita JA, et al. Metabolic syndrome, insulin resistance, and brachial artery vasodilator function in Framingham Offspring participants without clinical evidence of cardiovascular disease. Am J Cardiol. 2008;101:82–8. doi: 10.1016/j.amjcard.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6 (Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 16.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 17.Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol. 1996;270:H1435–40. doi: 10.1152/ajpheart.1996.270.4.H1435. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GF, Parise H, Vita JA, et al. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–9. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 19.Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in never-smoking overweight US white women aged 40–64 years. Am J Epidemiol. 1995;141:1128–41. doi: 10.1093/oxfordjournals.aje.a117386. [DOI] [PubMed] [Google Scholar]
- 20.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 21.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 22.Hamdy O, Ledbury S, Mullooly C, et al. Lifestyle modification improves endothelial function in obese subjects with the insulin resistance syndrome. Diabetes Care. 2003;26:2119–2125. doi: 10.2337/diacare.26.7.2119. [DOI] [PubMed] [Google Scholar]
- 23.Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 24.Saleh MH, Bertolami MC, Assef JE, et al. Improvement of atherosclerotic markers in non-diabetic patients after bariatric surgery. Obes Surg. 2012;22:1701–7. doi: 10.1007/s11695-012-0706-0. [DOI] [PubMed] [Google Scholar]
- 25.Buscemi S, Cosentino L, Rosafio G, et al. Effects of hypocaloric diets with different glycemic indexes on endothelial function and glycemic variability in overweight and in obese adult patients at increased cardiovascular risk. Clin Nutr. 2013;32:346–52. doi: 10.1016/j.clnu.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin T, Stuhlinger M, Lamendola C, et al. Plasma asymmetric dimethylarginine concentrations are elevated in obese insulin-resistant women and fall with weight loss. J Clin Endocrinol Metab. 2006;91:1896–900. doi: 10.1210/jc.2005-1441. [DOI] [PubMed] [Google Scholar]
- 27.Arcaro G, Cretti A, Balzano S, et al. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation. 2002;105:576–82. doi: 10.1161/hc0502.103333. [DOI] [PubMed] [Google Scholar]
- 28.Pande RL, Perlstein TS, Beckman JA, Creager MA. Association of insulin resistance and inflammation with peripheral arterial disease: the National Health and Nutrition Examination Survey, 1999 to 2004. Circulation. 2008;118:33–41. doi: 10.1161/CIRCULATIONAHA.107.721878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–10. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laakso M. How good a marker is insulin level for insulin resistance? Am J Epidemiol. 1993;137:959–65. doi: 10.1093/oxfordjournals.aje.a116768. [DOI] [PubMed] [Google Scholar]
- 32.Ardigo D, Franzini L, Valtuena S, Monti LD, Reaven GM, Zavaroni I. Relation of plasma insulin levels to forearm flow-mediated dilatation in healthy volunteers. Am J Cardiol. 2006;97:1250–4. doi: 10.1016/j.amjcard.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 33.Shinozaki K, Hirayama A, Nishio Y, et al. Coronary endothelial dysfunction in the insulin-resistant state is linked to abnormal pteridine metabolism and vascular oxidative stress. J Am Coll Cardiol. 2001;38:1821–8. doi: 10.1016/s0735-1097(01)01659-x. [DOI] [PubMed] [Google Scholar]
- 34.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–72. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 35.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 36.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–76. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–85. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 38.The Look AHEAD Research Group. Cardiovascular Effects of Intensive Lifestyle Intervention in Type 2 Diabetes. N Engl J Med. 2013;369:145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fayh AP, Lopes AL, da Silva AM, Reischak-Oliveira A, Friedman R. Effects of 5 % weight loss through diet or diet plus exercise on cardiovascular parameters of obese: a randomized clinical trial. Eur J Nutr. 2012 Oct 7; doi: 10.1007/s00394-012-0450-1. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Mohler ER, 3rd, Sibley AA, Stein R, et al. Endothelial function and weight loss: comparison of low-carbohydrate and low-fat diets. Obesity (Silver Spring) 2013;21:504–9. doi: 10.1002/oby.20055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 42.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 43.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 44.Laferrere B. Do we really know why diabetes remits after gastric bypass surgery? Endocrine. 2011;40:162–7. doi: 10.1007/s12020-011-9514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H, Wang Y, Zhang J, Potter BJ, Sowers JR, Zhang C. Bariatric surgery reduces visceral adipose inflammation and improves endothelial function in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2011;31:2063–9. doi: 10.1161/ATVBAHA.111.225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 47.Khan AM, Cheng S, Magnusson M, et al. Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community-based studies. J Clin Endocrinol Metab. 2011;96:3242–9. doi: 10.1210/jc.2011-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]


