Abstract
The finding that murine and simian cells have differential susceptibility to diphtheria toxin (DTx) led to the development of genetically engineered mouse strains that express the simian or human diphtheria toxin receptor (DTR) under the control of various mouse gene promoters. Injection of DTx into DTR engineered mice allows for rapid and transient depletion of various cell populations. There are several advantages to this approach over global knockout mice, including normal mouse development and temporal control over when cell depletion occurs. As a result, many DTR engineered mouse strains have been developed, resulting in significant insights into the cell biology of various disease states. We used Foxp3DTR mice to attempt local depletion of Foxp3+ cells in the lung in a model of tolerance breakdown. Intratracheal administration of DTx resulted in robust depletion of lung Foxp3+ cells. However, DTx administration was accompanied by significant local inflammation, even in control C57Bl/6 mice. These data suggest that DTx administration to non-transgenic mice is not always an immunologically inert event, and proper controls must be used to assess various DTx-mediated depletion regimens.
Keywords: diphtheria toxin, lung, regulatory T cells, tolerance
1. Introduction
The recent development of targeted cell ablation strategies is yielding rapid advances in our understanding of the role of specific cell types in immune responses. One such approach has taken advantage of differences in diphtheria toxin (DTx) sensitivity between simian and murine cells. Studies of the effect of DTx on various cell types resulted in the discovery that monkey cells were susceptible to DTx-mediated killing, while mouse cells were resistant (1, 2). Sequencing of the two diphtheria toxin receptors (DTRs) showed that several mutations existed between the two, and subsequent mutagenesis studies identified the critical DTR residues for toxin sensitivity (3, 4). This sensitivity was found to be the related to the efficiency of cytoplasmic release of the active DTx-A subunit (5, 6), thus contributing to the mechanism of DTx-mediated killing of monkey cells.
The above findings allowed genetic manipulation of mice to rapidly and transiently target specific cell types for deletion in live animals. Linking cell-type specific mouse gene promoters with the DTx-sensitive human diphtheria toxin receptor, researchers found that DTx administration to mice resulted in deletion of human DTR expressing cells, while cells bearing the mouse DTR were not affected (7). In addition, systemic administration of DTx in control mice was not hepatotoxic. Subsequently, many DTR-based mouse strains have been developed and provided important insights into the roles of specific cell types in immune responses. These mice have been used in dozens of reports to determine the role of various cell types in immunity and disease models. While most published work has reported systemic administration of DTx via intraperitoneal injection in mice (7, 8), mucosal DTx administration has also been used to deplete cells of interest in the lung (9–12).
Despite the utility of DTR engineered mouse strains in mechanistic studies, the depletion of cell types can have unintended consequences (13, 14). For example, Tittel, et. al. showed that systemic administration of DTx to CD11cDTR mice resulted in significant neutrophilia, leading to enhanced bacterial clearance in a mouse pyelonephritis model (15). This effect varied depending on the strain of DTR mice used. Other studies have demonstrated that repeated intraperitoneal DTX administration to CD11cDTR mice results in significant toxicity likely due to a non-hematopoietic radioresistant cell type (16).
There is limited information available about the utility of DTx-mediated immune cell depletion in the respiratory tract. In on-going studies investigating the initiation and breakdown of tolerance in the lung, we examined the role of Foxp3-expressing regulatory T cells (Treg) in these processes. Using the well-established Foxp3DTR mouse model, we administered DTx locally into the lung by intratracheal instillation. Lung DTx instillation resulted in significant local depletion of Foxp3+ cells in Foxp3DTR mice, but was accompanied by substantial DTx-dependent lung inflammation. The DTx-induced inflammation was sufficient to break inhalation tolerance and promote lung inflammation in Foxp3DTR mice following mucosal sensitization and challenge with model allergen. DTx from two different commercial vendors was equally effective in this regard in non-transgenic C57Bl/6 mice. Here we discuss the potential mechanisms for this effect and consider the consequences for using DTx-mediated cell ablation in the lung.
2. Materials and Methods
2.1 Mice and reagents
Male C57Bl/6 mice (6–12 weeks of age) were purchased from NCI. Foxp3DTR mice on the C57Bl/6 background were a gift from Dr. Troy Randall (University of Alabama-Birmingham) and bred in the University of Rochester animal facility. All mice were housed in the University of Rochester animal facility. Animal protocols were approved through the University Committee on Animal Resources and were conducted according to safety guidelines. Diphtheria toxin was purchased from Sigma (#D0564) and Calbiochem (Millipore #322326). Toxin was reconstituted in sterile PBS at 100ug/ml, and aliquots stored at −80°C. Grade V OVA was purchased from Sigma, reconstituted in sterile PBS at 10mg/ml, and aliquots stored at −20°C. Lipopolysaccharide (LPS) was purchased from Sigma (E. Coli strain O55:B5), reconstituted in sterile PBS at 1mg/ml, and aliquots stored at −80°C.
2.2 Allergen sensitization model
On day 0–2, naïve mice were exposed daily to either PBS as control or 100ug Grade V OVA in 30ul via intranasal inoculation to induce inhalation tolerance (17). In order to initiate a mucosal immune response (Figure 2), mice were sensitized day 12–14 daily by oropharyngeal administration of 100ug OVA mixed with 100ng LPS or PBS control in 50ul. One day prior to the first sensitization (day 11), mice were given a single dose of either 50ng DTx or PBS control in 50ul by intra-tracheal (i.t.) inoculation. Twice-daily challenges (1hr. each) were done day 26–28 in an inhalation chamber with 1% Grade V OVA aerosolized via jet nebulizer (Salter) at 10psi. Mice were sacrificed on day 30. Before all mucosal inoculations, mice were first sedated with Avertin (2,2,2-tribromoethanol).
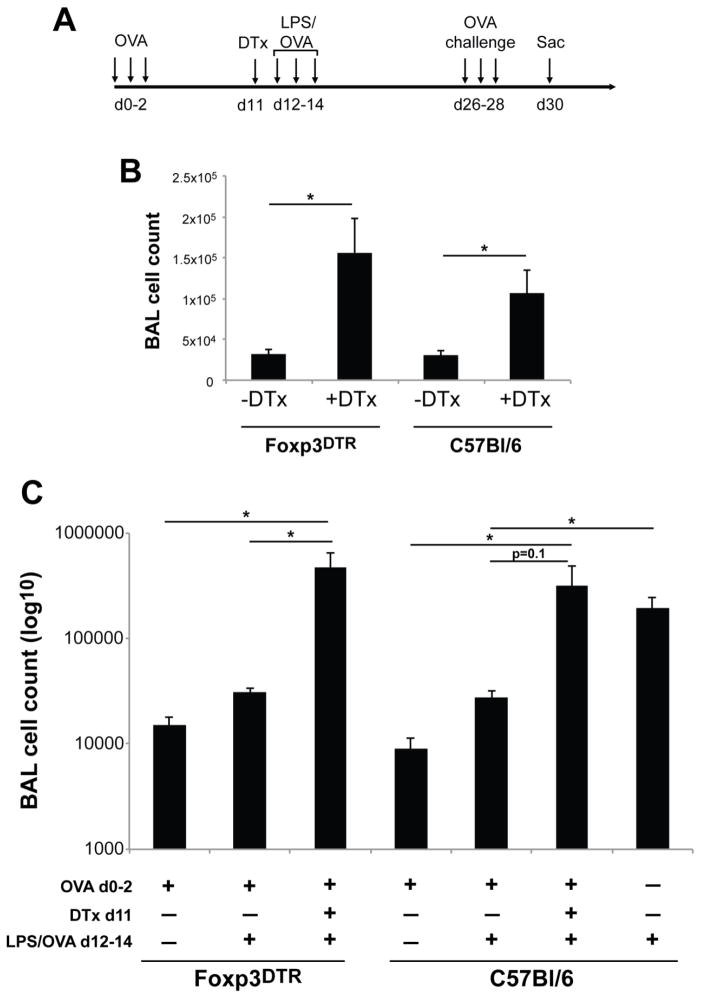
Figure 2.
Intratracheal DTx administration induces pulmonary inflammation in both Foxp3DTR and control C57Bl/6 mice after OVA sensitization and challenge.
A) Mice were tolerized and then received DTx or control treatments as in Figure 1. Following a single DTx treatment, mice were given 100ug OVA and 100ng LPS by o.p. route three consecutive days, followed two weeks later by twice daily OVA aerosol challenges for three consecutive days. Mice were sacrificed two days after the last challenge. B) Foxp3DTR and C57Bl/6 mice were sensitized and challenged as in Figure 2A; DTx from Calbiochem was used to deplete Foxp3+ cells. BAL cell recovery was determined by Trypan blue exclusion on day 30. C) Same experimental setup as Figure 2B, but DTx from Sigma was used and additional control groups were included (see legend at the bottom of the Figure). Data are n=3–5 for each group. * = p<0.05.
2.3 Sample acquisition and analysis
After animals were sacrificed, bronchoalveolar lavage (BAL) was obtained by washing the airways twice with 750ul PBS from a 1ml syringe connected to a Teflon cannula. Mediastinal lymph node (MLN) and spleen were removed and homogenized separately using glass homogenizers. Single cell suspensions were counted by Trypan blue exclusion. After counting, cell populations were identified by surface and intracellular FACS staining. Fluorescent antibodies for CD3-PE, CD4-PerCP/Cy5.5, CD44-Pacific Blue, CD25-PE/Cy5, and Foxp3-APC were purchased from BD, eBioscience, and Biolegend. Cells were surface stained in PBS/BSA for 25 minutes on ice in the dark. After washing, cells were fixed (eBioscience #00-5523-00) for 15 minutes. After one Perm/Wash, intracellular Foxp3 staining was done in Perm/Wash for 30 minutes in the dark. After washing, samples were re-suspended in PBS/BSA for FACS analysis. Fluorescence minus one controls were used to determine positive staining cells. Data was collected on an LSRII flow cytometer (BD) and analyzed using FlowJo (Treestar).
2.4 Statistical analysis
Statistical analyses were performed using Student’s T test. A p value less than 0.05 was considered significant.
3. Results
3.1. Intratracheal administration of DTx results in robust Treg depletion in Foxp3DTR mice but induces inflammation in both wild type and transgenic mice
Most studies to-date using DTR transgenic mice have employed systemic DTx delivery method by intraperitoneal injection to deplete targeted cell types of interest. However, several groups have also successfully adapted the system to deplete cells locally in the lung via i.t. delivery of DTx (9–12). This has primarily been done using the CD11cDTR mouse to deplete CD11c-expressing dendritic cell and macrophage populations. We attempted to adapt this model of local DTx-mediated depletion to the Foxp3DTR mouse for depletion of Foxp3-expressing lung cells in a mouse model of inhalation tolerance.
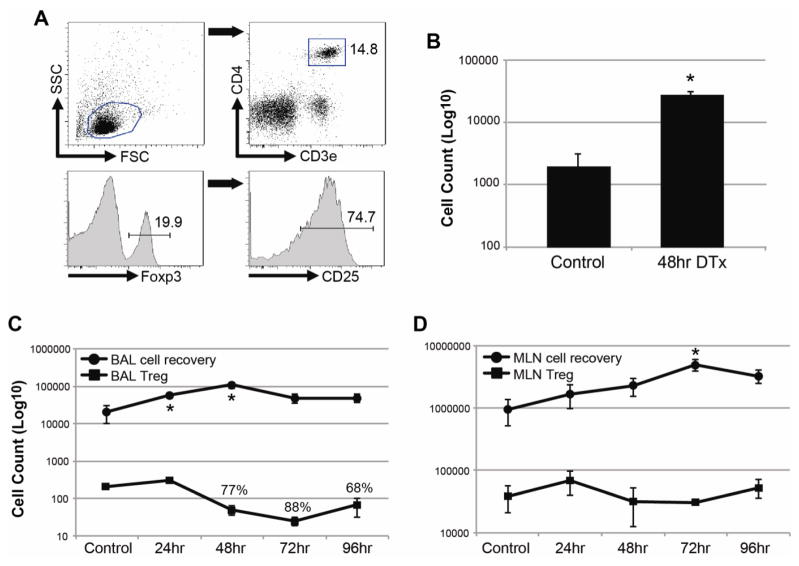
Tolerance was induced in wild type control and Foxp3DTR mice by repeated administration of OVA protein by intranasal inoculation (17). In naive mice, mucosal sensitization using OVA plus LPS (100ng) results in substantial lung inflammation 2 weeks later following aerosol challenge with OVA alone (18). However, in previously tolerized mice this dose of LPS is unable to break tolerance and induce pulmonary inflammation following OVA challenge (Chapman et al., Journal of Immunology, in press). We speculated that Foxp3+ regulatory cells were involved in this process and wanted to deplete these cells locally using Foxp3DTR mice. In preliminary studies, we found that a single 50ng dose of DTx given by i.t. administration was as good as two daily doses of DTx in depleting CD3+CD4+Foxp3+ Tregs from BAL (data not shown). Forty-eight hours after a single DTx administration, most Tregs were depleted from Foxp3DTR mice (89% and 93% depletion from BAL in two independent experiments) but not control C57Bl/6 mice. In fact, control mice had a trend toward higher Treg recovery after DTx administration (data not shown). Depletion of Tregs from the lung tissue of Foxp3DTR mice was significant but not as robust as that seen in the BAL (53% depletion on average).
In order to better understand the kinetics of cell depletion and recovery following DTx administration, we administered a single dose of DTx i.t. to previously tolerized Foxp3DTR mice and analyzed BAL and MLN for overall cellularity and Treg recovery at different time points. A representative flow cytometry gating strategy for Treg identification is shown in Figure 1A. Similar to previous experiments, we found robust depletion of Tregs from BAL after DTx administration, with maximal depletion within 48–72 hours post-DTx (Figure 1C). In contrast, Tregs in the draining MLN were minimally affected by mucosal DTx treatment (Figure 1D). In addition to the observation of Treg depletion, there was a surprising increase in BAL cell recovery that was significant the first two days post-DTx, then declined (Figure 1C). Increased cell recovery was also observed in the MLN, which was statistically significant at 72 hours post-DTx (Figure 1D).
Figure 1.
Depletion of T regulatory cells is accompanied by increased inflammation after intratracheal DTx administration in Foxp3DTR mice.
Foxp3DTR and C57Bl/6 mice were given three consecutive daily doses of 100ug Grade V OVA to induce inhaled tolerance. Ten days after the last dose of OVA, control PBS or DTx were given i.t. to tolerized mice. A) Representative flow cytometry gating strategy for identification of Tregs. After scatter gating, CD3+CD4+ T cells were identified. The gating of Foxp3+ cells is shown in the bottom left panel; CD25 expression on gated CD3+CD4+Foxp3+ cells is shown in the bottom right panel. B) BAL cell recovery in tolerized C57Bl/6 mice that received control PBS or DTx. Data are 48hr post treatment. C–D) Organ cell recovery (circles) and number of CD3+CD4+Foxp3+ cells (squares) in Foxp3DTR mice are reported for BAL (A) and MLN (B) each of the first four days after DTx administration. For organ cell count data, n=3–4 per group per day. For Foxp3+ cell quantification, all are n=3–4 per group per day, but Control group was pooled. Percentages in A) are percent reduction from Control group. * = p<0.05 compared to Control.
The increased BAL cell recovery observed after i.t. DTx could be the result of an inflammatory response induced by DTx itself, or a by-product of Treg cell depletion. Since increased cell recovery occurred before significant Treg depletion (Figure 1C), it seemed unlikely that Treg depletion was the cause. However, we further tested this by giving DTx in the same manner to C57Bl/6 mice and obtained BAL 48 hours later. These mice also had increased cell recovery compared to PBS controls (Figure 1B), further suggesting that DTx administration was responsible for inducing lung inflammation in mice.
3.2. DTx treatment of Foxp3DTR and C57Bl/6 mice results in increased BAL inflammation after OVA challenge
Despite the inflammation observed after DTx administration, Tregs were efficiently depleted by the treatment regimen. This prompted us to test the original hypothesis as to whether Foxp3+ Tregs were important for maintaining tolerance to inhaled OVA. Tolerized mice were depleted of Tregs via one 50ng DTx treatment i.t. After DTx or control treatment, we attempted to sensitize mice using OVA with 100 ng LPS as the inhaled adjuvant, followed two weeks later with OVA aerosol challenge (Fig. 2A). Using this protocol, both Foxp3DTR and wild-type mice that did not receive DTx developed no significant lung inflammation after subsequent allergen challenge (Figure 2 and data not shown), indicating that as expected tolerance was maintained. In contrast, Foxp3DTR mice treated with i.t. DTx one day prior to sensitization developed significant inflammation after subsequent allergen challenge (Figure 2B), suggesting that transient local Treg depletion facilitated the breakdown of tolerance. However, we observed similar results using wild type, C57Bl/6 mice exposed to inhaled DTx one day prior to mucosal sensitization (Figure 2B).
We wondered whether there were impurities or other components in the DTx preparation that could have acted as an “inhaled adjuvant” that caused local tissue inflammation sufficient to overcome the tolerogenic environment. Three observations argue against this possibility. First, the DTx used was commercially purified and verified to have limited contamination by SDS-PAGE. Second, when DTx was applied to RAW macrophages in an IL-6 bioassay (as a sensitive means of detecting endotoxin), DTx concentrations up to 100ug/ml elicited less IL-6 secretion than equivalent amounts of Sigma Grade V OVA (data not shown), the OVA used to induce inhaled tolerance. Third, we compared DTx from a different vendor (Sigma) with the DTx used in the above studies (from Calbiochem). Figure 2C shows that these experiments yielded almost identical results, and indicate that DTx from two different vendors when administered i.t. can break inhalation tolerance in both Foxp3DTR as well as non-transgenic, C57BL/6 mice. In addition, the inflammation in DTx-treated groups was similar to mice that were immunized with LPS/OVA in the absence of tolerance (Figure 2C). These data suggest that inhaled DTx itself acts as an inflammatory agent independent of the presence of simian DTR, and allows for effective mucosal sensitization even in the absence of Treg depletion.
4. Discussion
Engineering of mice with simian DTR has proven to be a powerful approach to studying the role of specific cell types in immune responses and disease processes. However, data presented in this report show that DTx is not immunologically inert in mice when administered topically into the lung. We found using two different commercially available preparations that very low doses of intra-tracheal DTx can break established tolerance and allow for development of lung inflammation following allergen sensitization and challenge even in wild type non-transgenic mice. This suggests that DTx-mediated cell depletion studies will not be useful to investigate mucosal immune responses in the lung using topical application to ensure local cell targeting. Our data contrast with systemic administration, where intraperitoneal DTx administration in wild type mice is often well tolerated. Since very subtle differences in mucosal inflammation during antigen exposure can powerfully influence subsequent adaptive responses, we wonder if potential off-target effects of systemic DTx administration in wild-type mice may have been missed in previous studies focusing solely on systemic immunity.
The mechanism(s) by which inhaled DTx promotes the breakdown of inhalation tolerance in non-transgenic wild type mice is currently not known. One possibility is that contaminants in commercially available DTx preparations cause sufficient mucosal inflammation to act as an “inhaled adjuvant”, but we do not favor this possibility since we did not detect contaminating LPS or cell-active components in two different commercially available DTx preparations. In addition, i.t. administration of 100ng LPS at the same time point that DTx was given was not sufficient to break tolerance to OVA following aerosol challenge (data not shown). Another possibility is that the DTx preparation itself is responsible for causing the observed inflammation. DTx from both Calbiochem and Sigma are reported to contain less than 5% nicked DTx (DTx that has been cleaved into its two product fragments A and B). Mouse cells can express DTR, but mutations in the receptor prevent cytosolic entry of the processed exotoxin into cells. If the commercial DTx contains contaminating nicked DTx, it is feasible that the active fragment A is able to impact mouse cells independent of the need for receptor-dependent cytosolic entry (5). Finally, it is formally possible that lung cells are more susceptible to the effects of nicked DTx than other cell types, but future studies will be needed to explore this and other possibilities.
Our results indicate that inhaled DTx can have effects on immune responses independently of targeted cell ablation, and underscore the importance of using non-transgenic controls. In addition, we suggest that published reports using DTx/DTR dependent cell ablation in the lung may need to be re-evaluated. For example, local depletion of lung dendritic cells using i.t. DTx in CD11cDTR and LangerinDTR mice demonstrated the importance of dendritic cells for adaptive immune response to infection, highlighting the importance of these innate cells in promoting T cell responses (10). However, it is possible that transient inflammation following topical DTx treatment resulted in an underestimate of the importance of CD11c+ (or Langerin+) cells in this model.
There are several factors that influence the outcome of DTx administration in mice. Dose, frequency of administration, route of administration, and the particular strain of DTR mice used are all potentially important variables when designing similar studies. The utility of DTR engineered mice in facilitating the specific depletion of different cell populations is now well established. However, potential side effects of DTx administration require the use of controls to be sure data obtained from depletion studies is not impacted by off-target inflammatory effects of DTx, especially when studying mucosal immune responses in the lung.
Acknowledgments
Funding sources: NIH R01 HL071933, ES01247, and P30 ES001247 to SNG; NIH F32 HL110718-01 and T32 HL66988-09 to TJC
We would like to thank Jason Emo and Sara Hillman for excellent technical support, as well as Dr. Bart Lambrecht for helpful suggestions using CD11cDTR mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Middlebrook JL, Dorland RB. Response of cultured mammalian cells to the exotoxins of pseudomonas aeruginosa and corynebacterium diphtheriae: Differential cytotoxicity. Canadian journal of microbiology. 1977;23:183–189. doi: 10.1139/m77-026. [DOI] [PubMed] [Google Scholar]
- 2.Naglich JG, Metherall JE, Russell DW, Eidels L. Expression cloning of a diphtheria toxin receptor: Identity with a heparin-binding egf-like growth factor precursor. Cell. 1992;69:1051–1061. doi: 10.1016/0092-8674(92)90623-k. [DOI] [PubMed] [Google Scholar]
- 3.Cha JH, Brooke JS, Eidels L. Toxin binding site of the diphtheria toxin receptor: Loss and gain of diphtheria toxin binding of monkey and mouse heparin-binding, epidermal growth factor-like growth factor precursors by reciprocal site-directed mutagenesis. Molecular microbiology. 1998;29:1275–1284. doi: 10.1046/j.1365-2958.1998.01015.x. [DOI] [PubMed] [Google Scholar]
- 4.Hooper KP, Eidels L. Glutamic acid 141 of the diphtheria toxin receptor (hb-egf precursor) is critical for toxin binding and toxin sensitivity. Biochemical and biophysical research communications. 1996;220:675–680. doi: 10.1006/bbrc.1996.0463. [DOI] [PubMed] [Google Scholar]
- 5.Didsbury JR, Moehring JM, Moehring TJ. Binding and uptake of diphtheria toxin by toxin-resistant chinese hamster ovary and mouse cells. Molecular and cellular biology. 1983;3:1283–1294. doi: 10.1128/mcb.3.7.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris RE, Saelinger CB. Diphtheria toxin does not enter resistant cells by receptor-mediated endocytosis. Infection and immunity. 1983;42:812–817. doi: 10.1128/iai.42.2.812-817.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nature biotechnology. 2001;19:746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 8.Kim JM, Rasmussen JP, Rudensky AY. Regulatory t cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 9.Fei M, Bhatia S, Oriss TB, Yarlagadda M, Khare A, Akira S, Saijo S, Iwakura Y, Fallert Junecko BA, Reinhart TA, Foreman O, Ray P, Kolls J, Ray A. Tnf-alpha from inflammatory dendritic cells (dcs) regulates lung il-17a/il-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5360–5365. doi: 10.1073/pnas.1015476108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, Osterhaus AD, Rimmelzwaan GF, Lambrecht BN. Clearance of influenza virus from the lung depends on migratory langerin+cd11b- but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin FJ, Parker D, Harfenist BS, Soong G, Prince A. Participation of cd11c(+) leukocytes in methicillin-resistant staphylococcus aureus clearance from the lung. Infection and immunity. 2011;79:1898–1904. doi: 10.1128/IAI.01299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. In vivo depletion of lung cd11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Blijswijk J, Schraml BU, Sousa CR. Advantages and limitations of mouse models to deplete dendritic cells. European journal of immunology. 2013;43:22–26. doi: 10.1002/eji.201243022. [DOI] [PubMed] [Google Scholar]
- 14.Westermark L, Fahlgren A, Fallman M. Immune response to diphtheria toxin-mediated depletion complicates the use of the cd11c-dtr(tg) model for studies of bacterial gastrointestinal infections. Microbial pathogenesis. 2012;53:154–161. doi: 10.1016/j.micpath.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Tittel AP, Heuser C, Ohliger C, Llanto C, Yona S, Hammerling GJ, Engel DR, Garbi N, Kurts C. Functionally relevant neutrophilia in cd11c diphtheria toxin receptor transgenic mice. Nature methods. 2012;9:385–390. doi: 10.1038/nmeth.1905. [DOI] [PubMed] [Google Scholar]
- 16.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of cd11c+ dendritic cells abrogates priming of cd8+ t cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing il-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 18.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent t helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]