INTRODUCTION
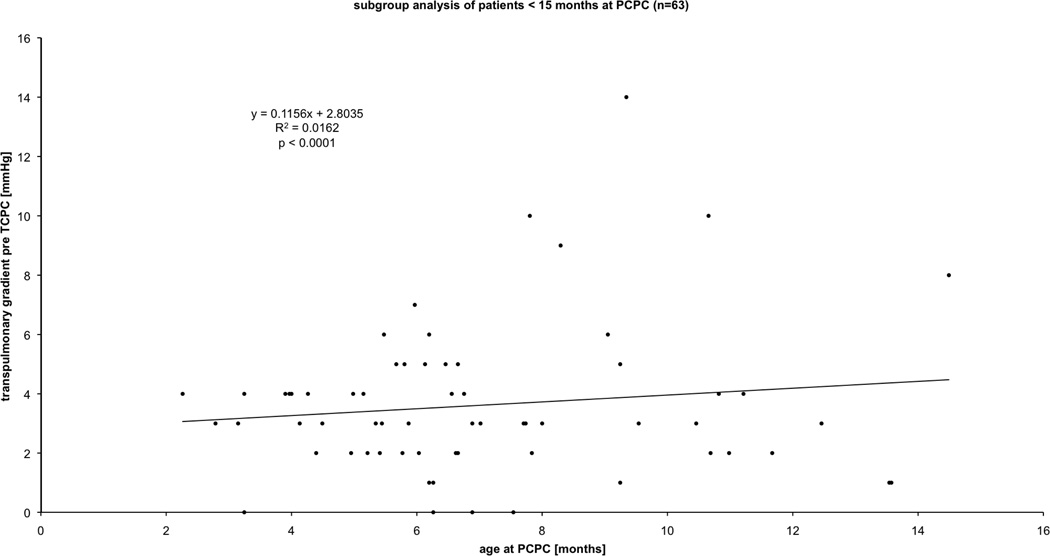
In this article, we review the current state of knowledge about early changes in the pulmonary vasculature resulting from persistent systemic-to-pulmonary arterial shunting in newborn lambs. Data generated in this model system may be important in children with various forms of congenital heart disease (CHD), but perhaps most so in those with single ventricle anomalies. Children born with a functional single ventricle (e.g. hypoplastic left heart syndrome, tricuspid atresia, pulmonary atresia) represent a subgroup of patients with congenital heart disease with the poorest outcome, with 5-year mortality rates up to 50%1. Although functionally single ventricle heart disease comprises many structural variants, a shared physiologic feature is that both systemic and pulmonary circulations are supplied in parallel by a functionally single pumping chamber. Immediately after birth, the pulmonary vasculature of these infants is exposed to abnormal conditions such as increased flow and/or pressure2–4. Over time, if these abnormal forces are not modified, they can lead to progressive functional and morphologic abnormalities in the pulmonary vasculature that are characterized by altered reactivity, increased resistance, and structural alterations (remodelling)3. Clinically, these abnormalities can have an important impact on surgical options and peri-operative outcome. The current approach to neonates with a functional single ventricle is to establish a controlled, low-pressure source pulmonary blood flow that facilitates systemic oxygen delivery sufficient to permit somatic growth and development without excessive ventricular volume loading, typically by means of a surgical systemic-to-pulmonary artery or ventricle-to-pulmonary artery conduit, or by restricting the main or branch pulmonary arteries2. The pulmonary-to-systemic blood flow balance can be difficult to achieve, and the infant runs the gauntlet between pulmonary blood flow that is too low (resulting in chronic hypoxemia and the potential consequences thereof) and pulmonary blood flow that is too high (potentially leading to chronic heart failure, compromised systemic blood flow, and abnormal pulmonary vascular remodeling. Since subsequent stages of surgical palliation (bidirectional or partial cavopulmonary connection, PCPC, followed by Fontan-type surgery or total cavopulmonary connection, TCPC), result in passive pulmonary blood flow in the absence of a pumping ventricle, a normal low pulmonary vascular resistance is critical for maintaining low central venous pressure, overall health, and long-term survival5. The underlying mechanisms by which some patients with single ventricle physiology suffer from increased pulmonary vascular resistance resulting in failed surgical palliation while others do not still remains unclear. We speculate that these differences in outcome are related to early, clinically undetectable, pulmonary vascular abnormalities. For example, age at surgery correlates with increased transpulmonary gradient post-operatively in infants with a functional single ventricle undergoing and surviving PCPC (Figure 1). This may be due to the longer duration over which the pulmonary vasculature was exposed to increased flow. Figure 1 suggests that a relatively large surgical shunt with relatively high pulmonary blood flow (therefore allowing older age at PCPC) may lead to a subtly higher transpulmonary gradient (which suggests higher pulmonary vascular resistance) that may be detrimental in the long-term. This suggestion remains speculative as it is unknown if subtly increased resistance leads to demonstrably worse outcome. However, it is likely that there are subtle differences in signaling pathways and/or gene expression variations between patients. These signaling pathways, acted upon by the increases in pulmonary blood flow, can then lead to subtle differences in pulmonary vascular resistance to dramatically affect the surgical outcome. The underlying pathways in these newborns with single ventricle heart disease are only just beginning to be resolved. In this review we will focus on the work over the last decade that has begun to elucidate the molecular pathways that appear to be important in the initial pulmonary vascular remodelling in newborns with single ventricle heart disease.
Figure 1.
Retrospective analysis of n= 69 consecutive patients younger than 15 months (subgroup of reference 9) with single ventricle physiology who underwent and survived partial cavo-pulmonary connection (PCPC). The X-axis depicts age at PCPC in months. The Y-axis depicts transpulmonary gradient defined as left atrial pressure minus pulmonary artery pressure during heart catheterization before total cavo-pulmonary connection (TCPC) surgery.
METHODS
A lamb model of neonatal increased pulmonary blood flow
Although many animal models have been used to study increased blood flow and/or pressure on the pulmonary vasculature, most models use older or adult animals6. Therefore these models neglect the initial postnatal effects of increased blood flow and/or pressure on the developing pulmonary vasculature during the natural course of postnatal transition from fetal to adult pulmonary blood flow. A model to study the initial onset of pulmonary vascular remodelling associated with single ventricle physiology needs to have a large aorto-pulmonary shunt from birth so that the natural course of postnatal transition from fetal to adult pulmonary blood flow can be considered in the pathophysiology. To our knowledge, the only existing animal model that can be used to study the initial onset of neonatal pulmonary vascular remodelling associated with single ventricle physiology is the neonatal lamb model with an aorto-pulmonary shunt placed in-utero (Figure 2)7. It should be noted that this model does have its limitations as a true mimic of single ventricle physiology. The Shunt model allows studies into the effects of increased pulmonary blood flow and pressure on pulmonary vascular signaling and gene expression. However, it may not take into account the influence of ventricular compliance or function that likely plays a role in the pulmonary vascular remodeling observed neonates with single ventricle physiology. However, it is the best model available for these type of investigations and over the last decade this model has been used extensively to study the early sequential evolution of pulmonary vascular remodeling mimicking the pathophysiological relevant elements of neonatal pulmonary blood flow in single ventricular heart disease. In the following sections we will briefly describe the current knowledge regarding the well-described nitric oxide (NO) and endothelin-1 (ET-1) signaling pathways as well as newly emerging pathways that may have potential for therapeutic intervention.
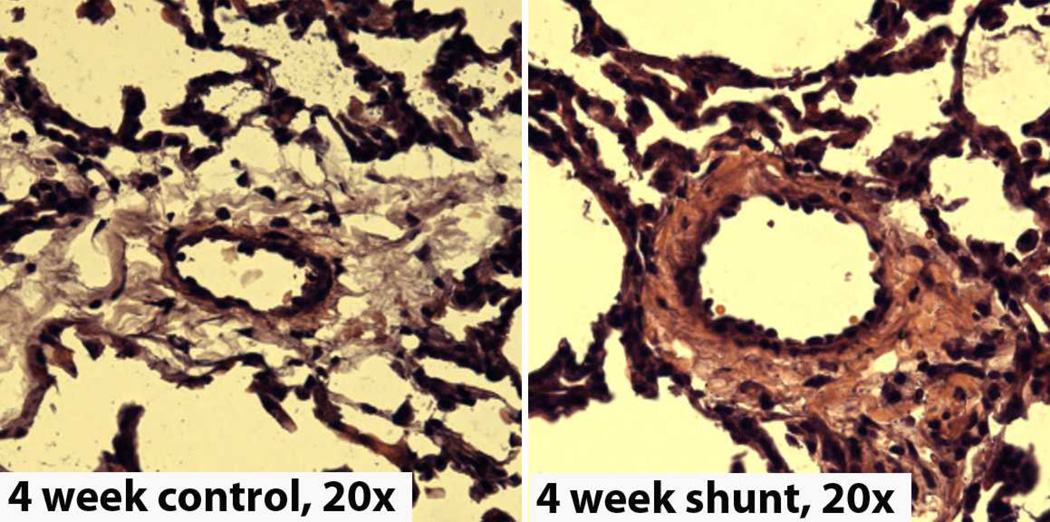
Figure 2.
Representative H & E stains of peripheral lung fixed in 4% paraformaldehyde and sectioned at 7 microns, from 4-week control (left) and Shunt (right) lambs. Note the increase in medial thickness in the Shunt lambs. Magnification=20×.
RESULTS & DISCUSSION
1. Established signaling pathways
A. The NO-cGMP Signaling Cascade
NO production by the vascular endothelium is integral to the maintenance of the low resistance state of the pulmonary vasculature, and dynamic alterations in NO production modulate vascular relaxation and constriction in response to various stimuli. Once formed, NO diffuses into vascular smooth muscle cells where it activates soluble guanylate cyclase, leading to increases in the second messenger, cyclic guanosine monophosphate (cGMP). The resulting activation of cGMP-dependent protein kinase in the pulmonary vascular smooth muscle layer leads to a decrease in intracellular calcium and relaxation of the vessel8. Extensive temporal investigations in the Shunt model over the first two months of life have shown that there is a complex pattern of regulation in the NO-cGMP signalling cascade that occurs both before, and after, overt alterations in endothelial function or vascular remodelling. As shown in Table 1 over the first two months of life there is a progressive loss of NO signalling. Loss of NO signalling is also found in humans with advanced forms of pulmonary vascular remodeling9, 10. Decreases in NO metabolites have also been found in children with CHD and increased PBF11, 12 as well as after cardiopulmonary bypass13, 14. Interestingly a genetic polymorphism (894G>T) has also been identified with CHD15. This polymorphism has been associated with decreased NO generation in humans16, 17. Accordingly, inhalation of NO gas18 and therapies aimed at enhancing NO signalling have already become a common treatment strategy for newborns with increased pulmonary resistance19, 20.
Table 1.
Developmental changes in NO and ET-1 signaling in Shunt lambs
| NO Signaling | 1 week | 2 week | 4 week | 8 week |
| eNOS protein levels | ↔ | ↔ | ↑ | ↔ |
| Relative NOS activity | ↔ | ↓ | ↓↓ | ↓↓↓ |
| Plasma NOx levels | nd | ↑ | ↔ | ↔ |
| Lung Tissue NOx levels | nd | ↑ | ↔ | ↔ |
| Nitrated eNOS | nd | ↑ | ↑↑ | ↑↑↑ |
| Hsp90 protein levels | nd | ↔ | ↔ | ↔ |
| eNOS bound Hsp90 | nd | ↓ | ↓↓ | nd |
| Plasma cGMP levels | ↔ | ↑ | ↑ | ↑ |
| Lung tissue cGMP levels | nd | ↑ | ↑ | ↑ |
| sGC-α protein levels | nd | ↑ | ↑ | ↔ |
| sGC-β protein levels | nd | ↔ | ↑ | ↔ |
| PDE5 protein levels | nd | ↔ | ↑ | ↔ |
| PDE5 activity | nd | nd | ↑ | nd |
| Plasma BNP levels | nd | ↔ | ↑ | ↑ |
| ET signaling | 1 week | 4 week | 8 week | |
| Plasma ET-1 | nd | ↑ | nd | |
| Immunoreactive ET-1 | ↑ | nd | nd | |
| preproET-1 mRNA | ↔ | ↔ | nd | |
| preproET-1 protein levels | ↔ | ↔ | nd | |
| ECE-1 mRNA | nd | ↑ | nd | |
| ECE-1 protein levels | ↑ | ↑ | nd | |
| ETA receptor protein levels | ↔ | ↑ | ↑ | |
| ETB receptor protein levels | ↓ | ↓ | ↔ |
nd=not determined
B. The Endothelin system
The hemodynamic effects of Endothelin-1 (ET-1), are mediated by at least two distinctive receptor populations, ETA and ETB, the densities of which are different depending on the vascular bed studied. ETA receptors are located on vascular smooth muscle cells and mediate vasoconstriction, whereas ETB receptors are located on endothelial cells and mediate vasodilation7, 21, 22. In addition, a second subpopulation of ETB receptors is located on smooth muscle cells and mediate vasoconstriction23. The vasodilating effects of ET-1 are thought to be associated with the release of NO and potassium channel activation22, 24, 25. The vasoconstricting effects of ET-1 are associated with phospholipase activation, the hydrolysis of phosphoinositol to inositol 1,4,5-triphosphate and diacylglycerol, and the subsequent release of Ca2+26. In addition to its vasoactive properties, ET-1 is mitogenic for pulmonary arterial smooth muscle cells via ETA receptor-mediated superoxide generation, and therefore may participate in vascular remodeling27. Patients with advanced forms of pulmonary vascular remodelling have increased lung and circulating ET-1 levels28. Furthermore, pulmonary blood pressure and not flow is associated with net endothelin-1 production in patients with congenital heart disease and normal pulmonary vascular resistance2930. As with the NO-cGMP signaling pathway, Shunt lambs exhibit temporal alterations in the ET-1 cascade. As shown in Table 1, within one week of life there is enhanced ET-1 mediated signaling in Shunt lambs while at 4-weeks of age the enhanced circulating levels of ET-1 leads to increased vasoconstriction through increases in the expression of ETA receptor and decreases in ETB receptor. By two months of age the expression of ETB receptor has returned to control levels but its localization is altered being predominantly on the SMC where it now mediates vasoconstriction. Therapies aimed at decreasing the vasoconstrictor and proliferative effects of ET-1 are being pursued for newborns with increased pulmonary resistance20, 31. However, it should be noted that the fact that there are changes in the expression and localization of the ET receptors in the Shunt lambs during the temporal progression of both the endothelial dysfunction and the vascular remodelling means that the correct receptor antagonist strategy (ETA vs. ETB vs. dual) is still controversial. As Table 1 suggests the best therapeutic option may depend on where in the disease process the patient is.
2. Emerging signaling pathways
Recent studies in Shunt lambs have identified five signalling pathways that may represent therapeutic targets: PPARγ, arginine metabolism, oxidative and nitrosative stress, thrombin, and carnitine. Furthermore, microarray analyses have identified new classes of genes that appear to be associated with early pulmonary vascular remodelling and may prove to be amenable to therapeutic interventions.
A. Peroxisome proliferator-activated receptors (PPAR)
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that belong to the nuclear hormone receptor family. Recent evidence has established a role for altered PPAR signaling in the development of both systemic and pulmonary vascular disease32. Recent experimental studies suggest that the PPAR-γ isoform may have a role in advanced pulmonary hypertension33, 34. PPAR-γ signaling is attenuated in Shunt lambs35. A microarray analysis has been carried out in ovine pulmonary endothelial cells exposed to the PPAR-γ inhibitor, GW9662, to mimic the loss of PPAR-γ signaling. This analysis identified over 100 genes that were either up- or down-regulated (Table 2)35. The upregulated genes are broadly classified into four categories: cell cycle-related genes, angiogenesis-related genes, ubiquitin-related genes, and zinc finger proteins. Furthermore, these in vitro results were confirmed in the Shunt lamb by Western blot analysis35. Therefore, potential targets that may be good candidates for therapeutic intervention in patients with early neonatal pulmonary vascular remodelling associated with single ventricle physiology may soon be identified.
Table 2.
Endothelial cell genes regulated by PPARγ
| Functional classifications | Number of genes up-regulated | Number of genes down-regulated |
|---|---|---|
| Cell cycle related genes | 7 | 0 |
| Angiogenesis related genes | 11 | 1 |
| Ubiquitin related genes | 9 | 0 |
| Zinc finger genes | 17 | 1 |
| unclassified | 56 | 18 |
| total | 100 | 20 |
B. Arginine Metabolism
L-arginine bioavailability plays a key role in the generation of NO in the pulmonary vasculature. L-arginine is actively transported into endothelial cells through the cationic amino acid transporter-1 (CAT-1) where it is then used as a substrate by eNOS to form NO and L-citrulline or metabolized by arginase to form urea and ornithine. Thus, eNOS and arginase are in direct competition for L-arginine. In addition, there resides within the caveolus a recycling pathway for L-arginine, in which the enzymes argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL) recycle L-citrulline back to L-arginine. L-arginine metabolism is disrupted in Shunt lambs through a number of mechanisms36. L-arginine recycling is impaired due to decreased activity of ASS and ASL and L-arginine catabolism is enhanced due to increases in arginase activity36. Although the expression of CAT-1 is increased which would enhance L-arginine levels in the cell, the decrease in L-citrulline recycling combined with increased L-arginine catabolism results in a decrease in relative NO signalling36. Thus, it is likely that the progressive loss of NO signaling in Shunt lambs8 is mediated at least in part by decreases in available L-arginine. This new description of the L-arginine-NO pathway fits well with previous studies in a rat model of hypoxia induced pulmonary hypertension37. Taken together, these studies suggest that treatments strategies to enhance L-arginine bioavailability in neonates with early pulmonary vascular remodelling associated with single ventricle physiology may have clinical utility.
C. Oxidative and nitrosative stress
Excessive production of reactive oxygen species (ROS), outstripping endogenous antioxidant defence mechanisms, has been implicated in many disease processes in of the vasculature38. Decreases in bioavailable NO can be related to increases in oxidative stress. In Shunt lambs, oxidative stress occurs secondary to both increased production of ROS39 and decreases in their scavenging40. The source of the increased superoxide is multi-factorial involving the upregulation of vascular xanthine oxidase and NADPH oxidase as well as an uncoupling of eNOS8, 41, 42. The mechanisms underlying the uncoupling of eNOS, i.e., the consumption of NADPH becomes uncoupled from NO synthesis, are also complex and involve decreases in L-arginine36 as well as alterations in biopterin metabolism39. Superoxide and other ROS have also been shown to have signalling function in vascular cells promoting hypertrophy, proliferation, migration, matrix remodelling and even the formation of new vessels. The activation of NADPH oxidases with subsequent ROS production has been associated with increased proliferation of pulmonary artery smooth muscle cells isolated from both sheep27 and humans43.
Both NO and superoxide possess unpaired electrons, and react rapidly to form the reactive nitrogen species (RNS), peroxynitrite (ONOO−) decreasing NO bioavailability. In addition, ONOO− can exert effects through the nitration of protein tyrosine residues. Tyrosine nitration, a covalent modification that adds a nitro group (-NO2) to the ortho carbon of the phenolic ring, yields 3-nitrotyrosine (3-NT)44. The nitration of tyrosine residues to form 3-NT is widely used as a marker of ONOO− formation and recent studies have shown that there is a temporal increase in protein nitration in the lungs of Shunt lambs8. Further, eNOS itself is susceptible to nitration8 and mass spectroscopy studies have demonstrated that these nitrated residues are in regions of the protein that are important to its function45. Thus, the potential of anti-oxidant therapies to reduce both ROS and RNS formation has been postulated for the treatment of early neonatal pulmonary vascular remodelling associated with single ventricle physiology. However, these studies highlight the limitations of current investigations: the lack of easily available means to identify modified proteins and residues. This is a roadblock in understanding the potential mechanistic contribution of these modifications and it is imperative that there is a greater focus on identifying the individual tyrosine residues targeted by nitration and the effect these nitration events have on the structure-function relationship of the protein. Only when these goals have been met will it be possible to develop directed therapies.
D. Thrombin
Recent studies have elucidated a novel positive feedback loop in which thrombin increase ROS levels via the consecutive activation of protease activated receptor 1 (PAR1), Rac1, and p21-activated kinase (PAK-1)43. This also results in the activation of the transcription factor, NFκB which in turn leads to the activation of hypoxia inducible factor 1α (HIF-1α)46, 47. Under these conditions, HIF-1 target genes such as plasminogen activator inhibitor-1 (PAI-1) or vascular endothelial growth factor are upregulated. The upregulation of PAI-1, which is the primary physiological inhibitor of tissue plasminogen activator and urokinase plasminogen activator, can then lead to reductions in fibrin clearance. In addition, increases in PAI-1 can also promote pulmonary artery SMC proliferation. Together, these anti-fibrinolytic and growth modulating activities have an immediate impact on pulmonary vascular remodelling and PAK-1 levels are elevated in remodelled pulmonary vessels in patients with congenital heart disease and pulmonary vasculopathy43, 48. There appears to be a secondary feed-forward loop activated by HIF-1 leading to further increases the expression of Rac1 and PAK-1 producing enhanced cytoskeletal remodelling and proliferation of pulmonary artery SMC which further contributing to the medial hypertrophy and vascular remodelling43. As part of this feed-forward mechanism, ROS levels are stimulated due to the increased abundance of Rac1 and PAK-1 producing sustained activation of the HIF-1 pathway and increased pulmonary vascular remodelling. This novel pathway may play an important role in early neonatal pulmonary vascular remodelling associated with single ventricle physiology and therapeutic treatments strategies targeting thrombin blockade in these patients may have clinical utility.
E. Carnitine Metabolism and Mitochondrial Dysfunction
L-carnitine is a trimethylated amino acid is involved in the transport of long chain fatty acids across the inner mitochondrial membrane (Figure 3). Carnitine is present in the form of either free carnitine (non-esterified molecule; FC), or acylcarnitines (esterified form; AC). Acylcarnitines are products of the reaction in which acyl moieties are transferred to carnitine from acyl-CoA. This reaction is catalyzed by acyltransferases. Thus, the AC/FC ratio is a measure of acylated carnitines versus the free carnitines. A low AC/FC ratio indicates a healthy mitochondria and a high AC/FC ratio a mitochondria with a reduced capacity for ATP production. Data in Shunt lambs indicate that there is a disruption in carnitine metabolism involving decreased expression of carnitine palmitoyltransferase 1 and 2 as well as carnitine acetyltransferase (CrAT). In addition, RNS appear to be involved in the disruption of carnitine homeostasis as nitrated CrAT is elevated in Shunt lambs while the exposure of purified CrAT to authentic ONOO− decreases its activity49. Together these alterations result in mitochondrial dysfunction as demonstrated by decreased superoxide dismutase (SOD) 2-, increased uncoupling protein-2 (UCP-2)-expression, and an increased lactate/pyruvate ratio49. Decreased expression of SOD2 has also been recently shown in SMC isolated from patients with PA hypertension (PAH)50. It is now becoming apparent that mitochondria play a key role in the development of various cardiovascular diseases including heart disease51, diabetes52, and atherosclerosis53. Studies have shown that mitochondrial targeted antioxidants have a beneficial effect in stroke prone rats54, sepsis induced cardiac dysfunction55, and cardiac ischemia-reperfusion injury56 and there is increasing interest in the potential of mitochondrial targeted antioxidants in treating cardiovascular disease57, 58. It remains to be seen if this is a potential therapeutic strategy for children with single ventricle physiology.
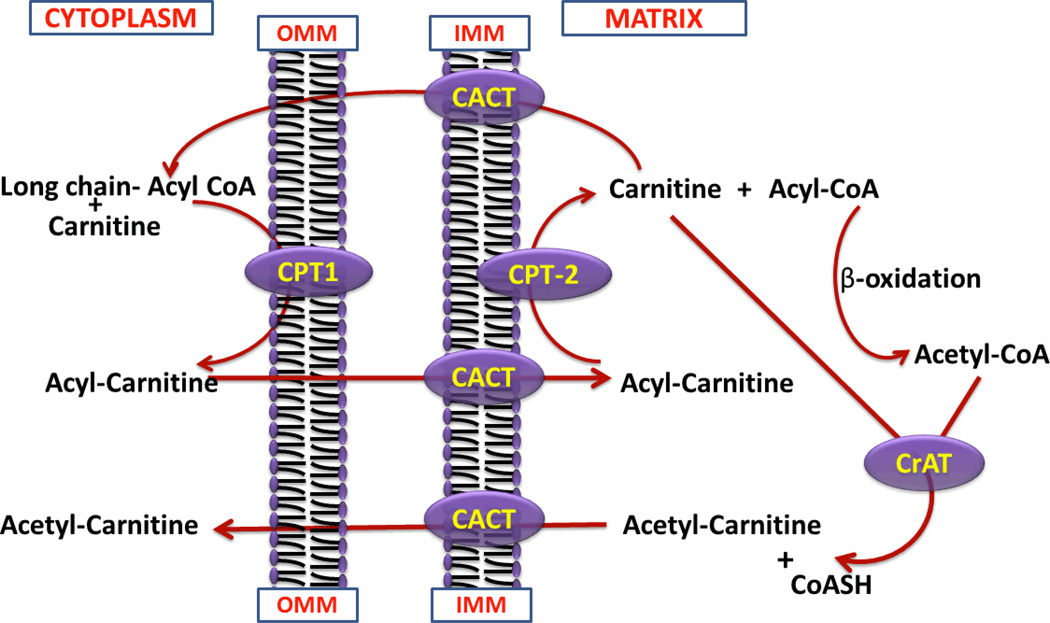
Figure 3.
The Carnitine Shuttle and role of carnitine in the mitochondrial oxidation of fatty acids. Abbreviations: CPT-I, carnitine palmitoyltransferase-1; CPT-II, carnitine palmitoyltransferase-2; CACT, carnitine acylcarnitine translocase; CrAT, carnitine acetyl transferase; OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane.
Mitochondria are the major site of cellular ATP production. ATP can stimulate NO release via the activation of eNOS59. Studies have also demonstrated a key role for the 90kD heat shock protein (Hsp90) in the activation of eNOS60 and the disruption of the Hsp90/eNOS complex adversely affects eNOS activation and induces eNOS uncoupling61. Hsp90 is ATP dependent and there is a temporal decrease in eNOS/Hsp90 interactions in Shunt lambs correlating with a decline in mitochondrial function49. Data indicate that the mitochondrial dysfunction may result from an increase in the nitration of mitochondrial proteins due to a asymmetric dimethylarginine (ADMA) mediated redistribution of eNOS to the mitochondria62. Increased ADMA levels are implicated in the pathogenesis of pulmonary hypertension63, 64 and in a single study of children and young adults with congenital heart disease and increased pulmonary blood flow65. As studies support the view that the ratio between L-arginine and ADMA is a key component in the regulation of endothelial NOS activity and it is possible that L-arginine could be a therapy for children with single ventricle physiology.
F. The identification of new genes involved in early neonatal pulmonary vascular remodeling
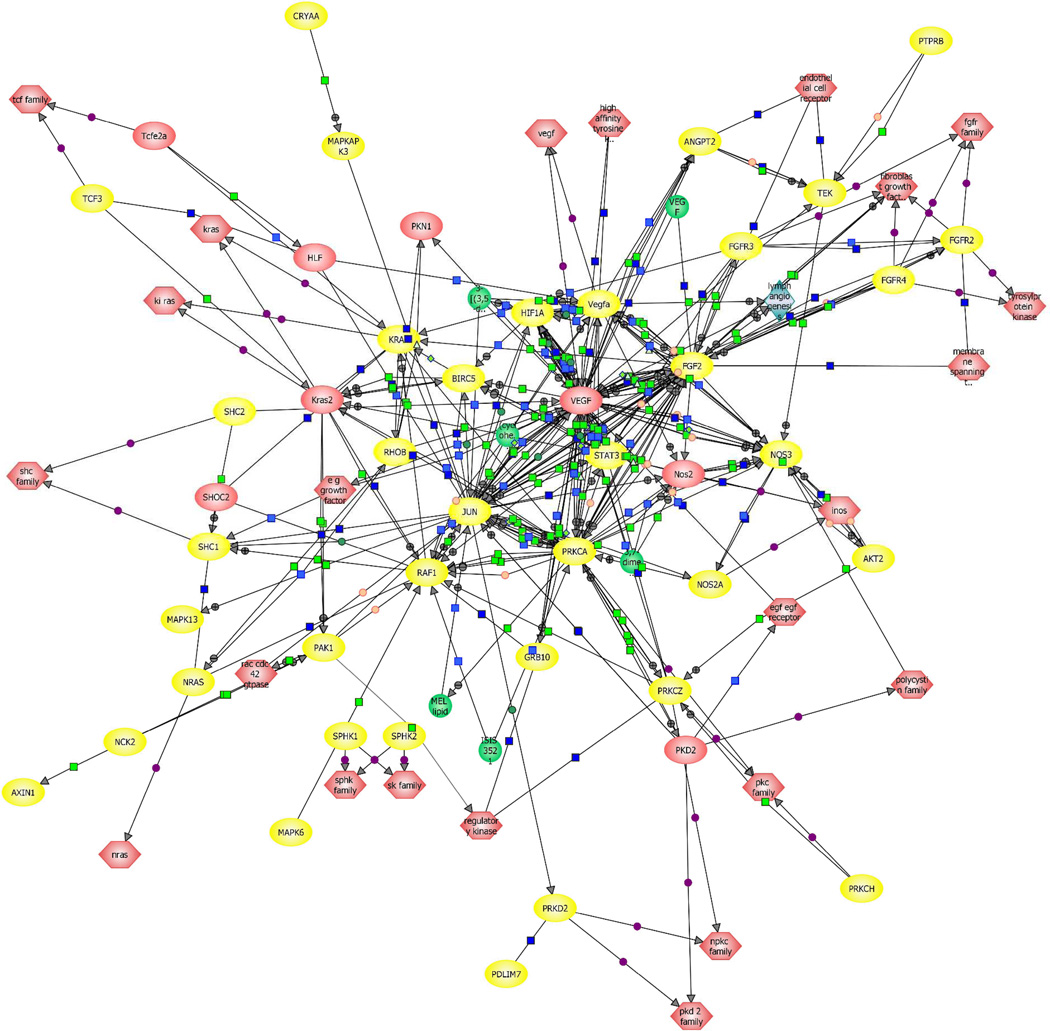
Previous studies have identified a burst of pulmonary angiogenesis in Shunt lambs that peaks at 4-weeks of age and is then lost. However, it is unclear how this angiogenic and apparent anti-angiogenic signaling is regulated. Micro-array analysis has been used to identify gene changes in Shunt lambs at an early time point prior to the angiogenic burst (3 days of age) and at 4-weeks of age to determine if there are differences in gene expression between the two ages and between the age matched control lambs130. The data indicate that there are a large number of gene categories that are regulated between the Shunt lambs and the age-matched controls at both 3 days and 4-weeks of age (Table 3). Further, when only genes directly related to angiogenesis and the extracellular matrix are examined the data indicate that these genes are predominantly up-regulated at 3 days of age and down-regulated at 4-weeks of age (Table 3). However, relevance Network analyses in the 3-day old Shunt lambs reveals just how complex and inter-connected these signalling pathways are (Figure 4). Thus, although these studies will likely lead to novel signalling pathways that can be investigated to produce exciting new results it is unlikely to lead to new therapeutic strategies in the near future.
Table 3.
Developmental Changes in Gene Expression in Shunt lambs Gene functional classification in 3-day Shunts
| Functional classifications | Number of genes up-regulated | Number of genes down-regulated |
| cell cycle related genes | 7 | 0 |
| cell proliferation related genes | 5 | 0 |
| angiogenesis related genes | 6 | 2 |
| extracellular matrix and cell adhesion related genes | 6 | 2 |
| transcription factors | 4 | 0 |
| unclassified | 90 | 15 |
| total | 118 | 19 |
| Gene functional classification in 4-wk Shunts | ||
| Functional classifications | Number of genes up-regulated | Number of genes down-regulated |
| cell proliferation related genes | 8 | 0 |
| extracellular matrix related genes | 3 | 28 |
| angiogenesis related genes | 0 | 6 |
| specific cell signaling pathways | 6 | 0 |
| transcription factors | 6 | 0 |
| unclassified | 132 | 62 |
| total | 155 | 96 |
Figure 4.
Relevance network of genes belonging to angiogenesis related signaling pathways identified using Pathway Architect software. Yellow indicates the genes that are altered between Shunt and Control lambs at 3-days of age.
CONCLUSIONS
In summary, utilizing an animal model allowing us to study the initial onset of neonatal pulmonary vascular remodelling associated with single ventricle physiology, new pathways for further mechanistic studies have been identified. New potential targets for therapeutic intervention have been identified and are now being tested: L-arginine, anti-oxidants, thrombin, and L-carnitine. In the longer term we can hope that the use of newer techniques such as metabolomics, proteomics, and gene profiling may lead to new therapeutic targets to address the initial onset of neonatal pulmonary vascular remodelling associated with single ventricle physiology.
Acknowledgements
Furthermore, The authors want to thank the rest of the members of their respective research groups without whom this review could not have been written.
Funding Sources
This research was generously supported by the Fondation Leducq to allow the formation of a Transatlantic Network. This work was also funded in part by grants HL60190 (to SMB), HL67841 (to SMB), HL084739 (to SMB), R21HD057406 (to SMB), HL61284 (to JRF), HL08 6513 (to PO), all from the National Institutes of Health, an American Heart Association Southeast Affiliates Beginning Grant In Aid Award (09BGIA2310050, to SS), and a Seed Award from the Cardiovascular Discovery Institute of the Medical College of Georgia to (SS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
REFERENCES
- 1.Fixler DE, Nembhard WN, Salemi JL, Ethen MK, Canfield MA. Mortality in first 5 years in infants with functional single ventricle born in Texas, 1996 to 2003. Circulation. 121:644–650. doi: 10.1161/CIRCULATIONAHA.109.881904. [DOI] [PubMed] [Google Scholar]
- 2.Stumper O. Hypoplastic left heart syndrome. Heart. 2010;96:231–236. doi: 10.1136/hrt.2008.159889. [DOI] [PubMed] [Google Scholar]
- 3.Reddy VM, Doff BM, Phillip M, Edwin P, Frank LH. Pulmonary artery growth after bidirectional cavopulmonary shunt: Is there a cause for concern? The Journal of Thoracic and Cardiovascular Surgery. 1996;112:1180–1192. doi: 10.1016/S0022-5223(96)70131-9. [DOI] [PubMed] [Google Scholar]
- 4.Cleuziou J, Schreiber C, Cornelsen JK, Horer J, Eicken A, Lange R. Bidirectional cavopulmonary connection without additional pulmonary blood flow in patients below the age of 6 months. European Journal of Cardio-Thoracic Surgery. 2008;34:556–562. doi: 10.1016/j.ejcts.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 5.Hess J. Long-term problems after cavopulmonary anastomosis: diagnosis and management. Thor Cardiov Surg. 2001;49:98–100. doi: 10.1055/s-2001-11698. [DOI] [PubMed] [Google Scholar]
- 6.Herget J. Animal models of pulmonary hypertension. Eur Respir Rev. 1993;3:559–563. [Google Scholar]
- 7.Reddy VM, Meyrick B, Wong J, Khoor A, Liddicoat JR, Hanley FL, Fineman JR. In utero placement of aortopulmonary shunts: a model of postnatal pulmonary hypertension with increased pulmonary blood flow in lambs. Circulation. 1995;92:1–8. doi: 10.1161/01.cir.92.3.606. [DOI] [PubMed] [Google Scholar]
- 8.Oishi PE, Wiseman DA, Sharma S, Kumar S, Hou Y, Datar SA, Azakie A, Johengen MJ, Harmon C, Fratz S, Fineman JR, Black SM. Progressive dysfunction of nitric oxide synthase in a lamb model of chronically increased pulmonary blood flow: a role for oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2008;295:L756–L766. doi: 10.1152/ajplung.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 10.Archer SL, Djaballah K, Humbert M, Weir KE, Fartoukh M, Dall'ava-Santucci J, Mercier JC, Simonneau G, Dinh-Xuan AT. Nitric oxide deficiency in fenfluramine- and dexfenfluramine-induced pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1998;158:1061–1067. doi: 10.1164/ajrccm.158.4.9802113. [DOI] [PubMed] [Google Scholar]
- 11.Kiettisanpipop P, Lertsapcharorn P, Chotivittayatarakorn P, Poovorawan Y. Plasma levels of nitric oxide in children with congenital heart disease and increased pulmonary blood flow. Journal of the Medical Association of Thailand. 2007;90:2053–2057. [PubMed] [Google Scholar]
- 12.Ikemoto Y, Teraguchi M, Kobayashi Y. Plasma levels of nitrate in congenital heart disease: comparison with healthy children. Pediatric Cardiology. 2002;23:132–136. doi: 10.1007/s00246-001-0036-9. [DOI] [PubMed] [Google Scholar]
- 13.Beghetti M, Silkoff PE, Caramori M, Holtby HM, Slutsky AS, Adatia I. Decreased exhaled nitric oxide may be a marker of cardiopulmonary bypass-induced injury. Annals of Thoracic Surgery. 1998;66:532–534. doi: 10.1016/s0003-4975(98)00447-0. [DOI] [PubMed] [Google Scholar]
- 14.Hiramatsu T, Imai Y, Takanashi Y, Hoshino S, Yashima M, Tanaka SA, Chang D, Nakazawa M. Time course of endothelin-1 and nitrate anion levels after cardiopulmonary bypass in congenital heart defects. Annals of Thoracic Surgery. 1997;63:648–652. doi: 10.1016/s0003-4975(96)01055-7. [DOI] [PubMed] [Google Scholar]
- 15.van Beynum IM, Mooij C, Kapusta L, Heil S, den Heijer M, Blom HJ. Common 894G>T single nucleotide polymorphism in the gene coding for endothelial nitric oxide synthase (eNOS) and risk of congenital heart defects. Clinical Chemistry and Laboratory Medicine. 2008;46:1369–1375. doi: 10.1515/CCLM.2008.271. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey V, Chan SL, Cassidy A, Butler R, Choy A, Fardon T, Struthers A, Lang C. The functional consequence of the Glu298Asp polymorphism of the endothelial nitric oxide synthase gene in young healthy volunteers. Cardiovasc Drug Rev. 2007;25:280–288. doi: 10.1111/j.1527-3466.2007.00017.x. [DOI] [PubMed] [Google Scholar]
- 17.Veldman BA, Spiering W, Doevendans PA, Vervoort G, Kroon AA, de Leeuw PW, Smits P. The Glu298Asp polymorphism of the NOS 3 gene as a determinant of the baseline production of nitric oxide. Journal of Hypertension. 2002;20:2023–2027. doi: 10.1097/00004872-200210000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Bloch KD, Ichinose F, Roberts JD, Zapol WM. Inhaled NO as a therapeutic agent. Cardiovasc Res. 2007;75:339–348. doi: 10.1016/j.cardiores.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huddleston A, Knoderer C, Morris J, Ebenroth E. Sildenafil for the Treatment of Pulmonary Hypertension in Pediatric Patients. Pediatric Cardiology. 2009;30:871–882. doi: 10.1007/s00246-009-9523-1. [DOI] [PubMed] [Google Scholar]
- 20.Tulloh R. Etiology, diagnosis, and pharmacologic treatment of pediatric pulmonary hypertension. Paediatr Drugs. 2009;11:115–128. doi: 10.2165/00148581-200911020-00003. [DOI] [PubMed] [Google Scholar]
- 21.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [see comments.] [DOI] [PubMed] [Google Scholar]
- 22.Shetty SS, Okada T, Webb RL, DelGrande D, Lappe RW. Functionally distinct endothelin B receptors in vascular endothelium and smooth muscle. Biochemical & Biophysical Research Communications. 1993;191:459–464. doi: 10.1006/bbrc.1993.1240. [DOI] [PubMed] [Google Scholar]
- 23.Bradley LM, Czaja JF, Goldstein RE. Circulatory effects of endothelin in newborn piglets. Am J Physiol. 1990;259:H1613–H1617. doi: 10.1152/ajpheart.1990.259.5.H1613. [DOI] [PubMed] [Google Scholar]
- 24.Cassin S, Kristova V, Davis T, Kadowitz P, Gause G. Tone-dependent responses to endothelin in isolated perfused fetal sheep pulmonary circulation in situ. J. Appl. Physiol. 1991;70:1228–1234. doi: 10.1152/jappl.1991.70.3.1228. [DOI] [PubMed] [Google Scholar]
- 25.Hell JW, Yokoyama CT, Wong ST, Warner C, Snutch TP, Catterall WA. Differential phosphorylation of two size forms of the neuronal class C L-type calcium channel alpha 1 subunit. J. Biol. Chem. 1993;268:19451–19457. [PubMed] [Google Scholar]
- 26.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [see comments.] [DOI] [PubMed] [Google Scholar]
- 27.Wedgwood S, Dettman R, Black S. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am. J. Physiol. 2001;281:L1058–L1067. doi: 10.1152/ajplung.2001.281.5.L1058. [DOI] [PubMed] [Google Scholar]
- 28.Giaid A, Michel RP, Stewart DJ, Sheppard M, Corrin B, Hamid Q. Expression of endothelin-1 in lungs of patients with cryptogenic fibrosing alveolitis. Lancet. 1993;341:1550–1554. doi: 10.1016/0140-6736(93)90694-c. [DOI] [PubMed] [Google Scholar]
- 29.Fratz S, Geiger R, Kresse H, Roemer G, Hennig M, Sebening W, Hess J. Pulmonary blood pressure, not flow, is associated with net endothelin-1 production in the lungs of patients with congenital heart disease and normal pulmonary vascular resistance. J Thorac Cardiovasc Surg. 2003;126:1724–1729. doi: 10.1016/s0022-5223(03)00937-1. [DOI] [PubMed] [Google Scholar]
- 30.Gorenflo M, Gross P, Bodey A, Schmitz L, Brockmeier K, Berger F, Bein G, Lange PE. Plasma endothelin-1 in patients with left-to-right shunt. Am Heart J. 1995;130:537–542. doi: 10.1016/0002-8703(95)90363-1. [DOI] [PubMed] [Google Scholar]
- 31.Beghetti M. Current treatment options in children with pulmonary arterial hypertension and experiences with oral bosentan. Eur J Clin Invest. 2006;36:16–24. doi: 10.1111/j.1365-2362.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- 32.Nisbet RE, Sutliff RL, Hart CM. The role of peroxisome proliferator-activated receptors in pulmonary vascular disease. PPAR Res. 2007;2007:18797. doi: 10.1155/2007/18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansmann G, de Jesus Perez VA, Alastalo T-P, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. The Journal of Clinical Investigation. 2008;118:1846–1857. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansmann G, Wagner RA, Schellong S, de Jesus Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary Arterial Hypertension Is Linked to Insulin Resistance and Reversed by Peroxisome Proliferator-Activated Receptor-{gamma} Activation. Circulation. 2007;115:1275–1284. doi: 10.1161/CIRCULATIONAHA.106.663120. [DOI] [PubMed] [Google Scholar]
- 35.Tian J, Smith A, Nechtman J, Podolsky R, Aggarwal S, Snead C, Kumar S, Elgaish M, Oishi P, Goerlach A, Fratz S, Hess J, Catravas JD, Verin AD, Fineman JR, She JX, Black SM. Effect of PPARgamma inhibition on pulmonary endothelial cell gene expression: gene profiling in pulmonary hypertension. Physiol Genomics. 2009;40:48–60. doi: 10.1152/physiolgenomics.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma S, Kumar S, Sud N, Wiseman DA, Tian J, Rehmani I, Datar S, Oishi P, Fratz S, Venema RC, Fineman JR, Black SM. Alterations in lung arginine metabolism in lambs with pulmonary hypertension associated with increased pulmonary blood flow. Vascul Pharmacol. 2009;51:359–364. doi: 10.1016/j.vph.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howell K, Costello CM, Sands M, Dooley I, McLoughlin P. L-Arginine promotes angiogenesis in the chronically hypoxic lung: a novel mechanism ameliorating pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1042–L1050. doi: 10.1152/ajplung.90327.2008. [DOI] [PubMed] [Google Scholar]
- 38.Harrison DG, Gongora MC. Oxidative stress and hypertension. Medical Clinics of North America. 2009;93:621–635. doi: 10.1016/j.mcna.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1069–L1077. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- 40.Sharma S, Grobe AC, Wiseman DA, Kumar S, Englaish M, Najwer I, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Lung antioxidant enzymes are regulated by development and increased pulmonary blood flow. Am J Physiol Lung Cell Mol Physiol. 2007;293:L960–L971. doi: 10.1152/ajplung.00449.2006. [DOI] [PubMed] [Google Scholar]
- 41.Sharma S, Kumar S, Wiseman DA, Kallarackal S, Ponnala S, Elgaish M, Tian J, Fineman JR, Black SM. Perinatal changes in superoxide generation in the ovine lung: Alterations associated with increased pulmonary blood flow. Vascul Pharmacol. 53:38–52. doi: 10.1016/j.vph.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sud N, Sharma S, Wiseman DA, Harmon C, Kumar S, Venema RC, Fineman JR, Black SM. Nitric oxide and superoxide generation from endothelial NOS: modulation by HSP90. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1444–L1453. doi: 10.1152/ajplung.00175.2007. [DOI] [PubMed] [Google Scholar]
- 43.Diebold I, Petry A, Djordjevic T, BelAiba RS, Fineman JR, Black SM, Schreiber C, Fratz S, Hess J, Kietzmann T, Gorlach A. Reciprocal regulation of Rac1 and PAK-1 by HIF-1α: A positive feedback loop promoting pulmonary vascular remodeling. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2009.3013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44.Calabrese V, Cornelius C, Rizzarelli E, Owen JB, Dinkova-Kostova AT, Butterfield DA. Nitric oxide in cell survival: a janus molecule. Antioxid Redox Signal. 2009;11:2717–2739. doi: 10.1089/ars.2009.2721. [DOI] [PubMed] [Google Scholar]
- 45.Zickus M, Fonseca FV, Tummala M, Black SM, Ryzhov V. Identification of the Tyrosine Nitration Sites in Human Endothelial Nitric Oxide Synthase by Liquid Chromatography-Mass Spectrometry. Eur. J. Mass Spectrom. 2008;14:239–247. doi: 10.1255/ejms.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Djordjevic T, Hess J, Herkert O, Gorlach A, BelAiba RS. Rac regulates thrombin-induced tissue factor expression in pulmonary artery smooth muscle cells involving the nuclear factor-kappaB pathway. Antioxid Redox Signal. 2004;6:713–720. doi: 10.1089/1523086041361703. [DOI] [PubMed] [Google Scholar]
- 47.Bonello S, Zahringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Gorlach A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 48.BelAiba RS, Djordjevic T, Bonello S, Artunc F, Lang F, Hess J, Gorlach A. The serum- and glucocorticoid-inducible kinase Sgk-1 is involved in pulmonary vascular remodeling: role in redox-sensitive regulation of tissue factor by thrombin. Circulation Research. 2006;98:828–836. doi: 10.1161/01.RES.0000210539.54861.27. [DOI] [PubMed] [Google Scholar]
- 49.Sharma S, Sud N, Wiseman DA, Carter AL, Kumar S, Hou Y, Rau T, Wilham J, Harmon C, Oishi P, Fineman JR, Black SM. Altered carnitine homeostasis is associated with decreased mitochondrial function and altered nitric oxide signaling in lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294:L46–L56. doi: 10.1152/ajplung.00247.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 121:2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davidson SM. Endothelial mitochondria and heart disease. Cardiovascular Research. doi: 10.1093/cvr/cvq195. [DOI] [PubMed] [Google Scholar]
- 52.Cheng X, Siow RC, Mann GE. Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: a role for mitochondria and the Nrf2-Keap1 defense pathway. Antioxid Redox Signal. doi: 10.1089/ars.2010.3283. [DOI] [PubMed] [Google Scholar]
- 53.Leduc L, Levy E, Bouity-Voubou M, Delvin E. Fetal programming of atherosclerosis: possible role of the mitochondria. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 149:127–130. doi: 10.1016/j.ejogrb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP, Dominiczak AF. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 55.Supinski GS, Murphy MP, Callahan LA. MitoQ administration prevents endotoxin-induced cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1095–R1102. doi: 10.1152/ajpregu.90902.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB Journal. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 57.Rocha M, Esplugues JV, Hernandez-Mijares A, Victor VM. Mitochondrial-targeted antioxidants and oxidative stress: a proteomic prospective study. Current Pharmaceutical Design. 2009;15:3052–3062. doi: 10.2174/138161209789058138. [DOI] [PubMed] [Google Scholar]
- 58.Armstrong JS. Mitochondria-directed therapeutics. Antioxid Redox Signal. 2008;10:575–578. doi: 10.1089/ars.2007.1929. [DOI] [PubMed] [Google Scholar]
- 59.Konduri GG, Mital S. Adenosine and ATP cause nitric oxide-dependent pulmonary vasodilation in fetal lambs. Biol. Neonate. 2000;78:220–229. doi: 10.1159/000014274. [DOI] [PubMed] [Google Scholar]
- 60.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 61.Pritchard K, Stepp D, Konduri G. Inhibition of heatshock protein 90 activity impairs vasorelaxation by increasing superoxide anion generation by endothelial nitric oxide synthase (eNOS) Circulation. 2001 Abstract 500. [Google Scholar]
- 62.Sud N, Wells SM, Sharma S, Wiseman DA, Wilham J, Black SM. Asymmetric dimethylarginine inhibits HSP90 activity in pulmonary arterial endothelial cells: role of mitochondrial dysfunction. Am J Physiol Cell Physiol. 2008;294:C1407–C1418. doi: 10.1152/ajpcell.00384.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arrigoni FI, Vallance P, Haworth SG, Leiper JM. Metabolism of asymmetric dimethylarginines is regulated in the lung developmentally and with pulmonary hypertension induced by hypobaric hypoxia. Circulation. 2003;107:1195–1201. doi: 10.1161/01.cir.0000051466.00227.13. [DOI] [PubMed] [Google Scholar]
- 64.Millatt LJ, Whitley GS, Li D, Leiper JM, Siragy HM, Carey RM, Johns RA. Evidence for dysregulation of dimethylarginine dimethylaminohydrolase I in chronic hypoxia-induced pulmonary hypertension. Circulation. 2003;108:1493–1498. doi: 10.1161/01.CIR.0000089087.25930.FF. [DOI] [PubMed] [Google Scholar]
- 65.Gorenflo M, Zheng C, Pöge A, Bettendorf M, Werle E, Fiehn W, Ulmer H. Metabolites of the L-arginine-NO pathway in patients with left-to-right shunt. Clin Lab. 2001;47:441–447. [PubMed] [Google Scholar]