Abstract
Malaria parasites are transmitted by mosquitoes, and blocking parasite transmission is critical in reducing or eliminating malaria in endemic regions. Here, we report the pharmacological characterization of a new class of malaria transmission-blocking compounds that acts via the inhibition of Plasmodia CDPK4 enzyme. We demonstrate that these compounds achieved selectivity over mammalian kinases by capitalizing on a small serine gatekeeper residue in the active site of the Plasmodium CDPK4 enzyme. To directly confirm the mechanism of action of these compounds, we generated P. falciparum parasites that express a drug-resistant methionine gatekeeper (S147M) CDPK4 mutant. Mutant parasites showed a shift in exflagellation EC50 relative to the wild-type strains in the presence of compound 1294, providing chemical-genetic evidence that CDPK4 is the target of the compound. Pharmacokinetic analyses suggest that coformulation of this transmission-blocking agent with asexual stage antimalarials such as artemisinin combination therapy (ACT) is a promising option for drug delivery that may reduce transmission of malaria including drug-resistant strains. Ongoing studies include refining the compounds to improve efficacy and toxicological properties for efficient blocking of malaria transmission.
Keywords: Plasmodium falciparum, malaria transmission-blocking, calcium-dependent protein kinase 4, bumped kinase inhibitors
(See the editorial commentary by Durvasula on pages 177–9.)
Continued transmission after malaria therapy is a challenge for malaria control and eradication efforts [1]. Gametocytes, which transmit malaria to the mosquito, remain viable in human circulation for several weeks after drug therapy and allow transmission even after asexual forms are eradicated from the blood stream [2]. Control and eradication efforts require new tools to prevent transmission of malaria parasites, especially given there is increasing mosquito resistance to insecticide-treated bed nets [3]. Plasmodia calcium-dependent protein kinase 4 (CDPK4) is a signaling molecule that is necessary for gametocyte transition into gametes in the mosquito midgut, and its absence prevents male gametocytes from exflagellating and fusing with female gametocytes to form infective zygotes [4, 5]. We previously reported that the PfCDPK4-inhibitor BKI-1 blocks the process of Plasmodium microgamete exflagellation, thereby disrupting malaria transmission [5]. We showed a strong correlation between the ability of inhibitors to inhibit PfCDPK4 enzymatic activity in-vitro and reduced exflagellation in vivo, suggesting that PfCDPK4 is the target responsible for transmission-blocking (exflagellation). Using transgenic P. falciparum parasites, here we demonstrate a chemical-genetic linkage between the activity of the PfCDPK4 enzyme and exflagellation, confirming the important role of PfCDPK4 in parasite transmission. Because blocking transmission requires inhibition of PfCDPK4 in the mosquito midgut [5, 6], a compound must be ingested along with gametocytes to effectively stop malaria transmission. Furthermore, due to the extended presence of viable gametocytes in the mammalian host [7, 8], prolonged drug bioavailability is required for effective transmission-blocking to occur. Therefore, we performed iterative modifications of our lead compound, BKI-1, and obtained a derivative that maintained longer efficacious blood levels with practical dosing intervals. The compound and related derivatives may have significant impact on malaria control and disease containment.
METHODS
Molecular Modeling and Design Strategy
A structural model of PfCDPK4-inhibitor generated on the basis of inhibitor-TgCDPK1 structures (PDB 3sx9 with BKI-1) was used as the initial starting point for synthesis of additional compounds [5]. Inhibitors were docked into this model using the Monte Carlo search procedure of the docking program FLO/QXP [9]. All commercially available R1's and R2's were retrieved from the ZINC [10] database, automatically attached to the scaffold, and docked with the Monte Carlo procedure [9]. The program allows for full ligand flexibility and user controlled protein flexibility. Compounds with favorable predicted potency were selected.
Chemistry
Chemical synthesis of compounds, including BKI-1 and 1294, used in this study was described elsewhere [11, 12]. The purity of all compounds (>98%) was confirmed by reverse-phase HPLC and 1H-NMR.
Mouse and Human Microsome Stability Assay
Mouse, rat, dog (beagle), primate, and human liver-microsome metabolism assays were performed with pooled microsomes (BD Biosciences, San Jose, CA). The reaction mixtures were described elsewhere [13]. Further details can be found in Supplementary Methods.
In-vivo Pharmacokinetics, Absorption, Distribution, Metabolism, and Excretion (PK/ADME)/Toxicity Compound Testing
BKI-1 and compound 1294 were subjected to pharmacokinetic and toxicity studies in mice. These compounds were used in a dose escalation study to define acute toxicity, such as respiratory or neurological abnormalities at 100 mg/kg dose dissolved in 3% ethanol and 7% Tween 80 in saline solution before subsequent PK/ADME testing [13, 14].
Enzyme Activity and Drug Inhibition Assays
A previously described luminescence assay that measures the depletion of ATP in the presence of the peptide-substrate, Syntide-2 (PLARTLSVAGLPGKK) [12, 15], was used to determine the catalytic activity of these enzymes and the inhibitory characteristics of compounds.
P. falciparum Maintenance and Genetic Modification
P. falciparum NF54 wild-type and transgenic lines were maintained in RPMI-1640 supplemented with 50 µM hypoxanthine and 10% A+ heat-inactivated human serum as described elsewhere [16–19]. Further details of this and other methods can be found in Supplementary Methods.
P. falciparum Exflagellation and Transmission Experiments
Cultures of P. falciparum NF54 wild-type, Pfcdpk4 wild-type control, or Pfcdpk4 S147M cultures were started at 0.5%, and the parasites were grown for 15 days with daily media changes. On day 15 the cultures are divided into flasks with or without the addition of 1294 as described elsewhere [5].
Safety Assessment Profile of BKI-1 and 1294
A kinome-wide selectivity profile of BKI-1 and 1294 was determined. Protein kinases in the profiling panel were chosen as representative of different subfamilies of the kinome tree [20]. A Time Resolved Fluorescence Resonance Energy Transfer (TR-FRET) assay, which measures the affinity of a compound via competition with a fluorescent tracer, was used to determine Kis [21]. Compounds were tested in 6 serial dilutions from 10 µM to 0.1 nM to determine IC50 and consequently Ki. Biological profiles were determined in a panel of 24 targets including human ether-a-go-go related gene (hERG), selected by historical liability data, using cell-based and biochemical fluorescent assays [22, 23].
RESULTS AND DISCUSSION
Molecular Modeling and Inhibitor Design
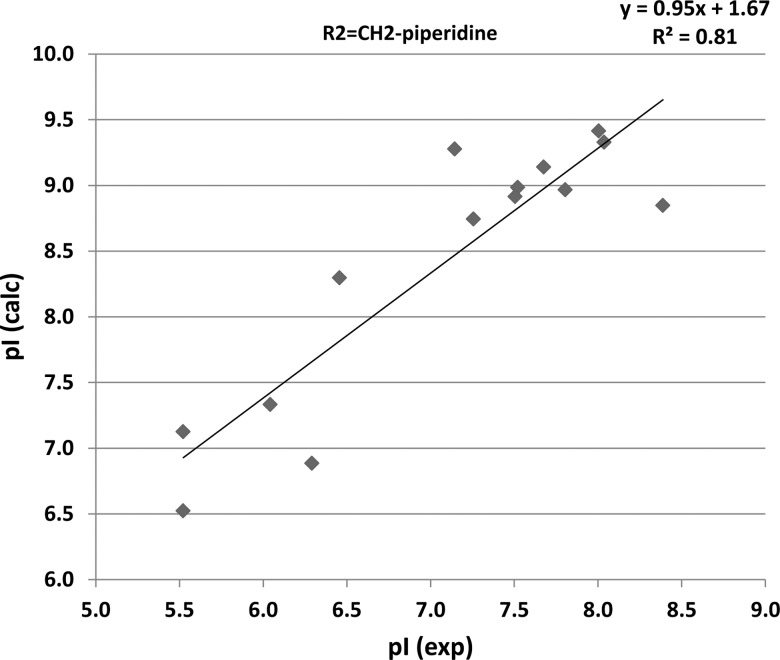
Because our efforts to crystallize PfCDPK4 for molecular structure determinations have been unsuccessful, we used a molecular modeling program—FLO/QXP docking software to dock inhibitors [9]. This allowed us to predict interactions of inhibitor scaffolds within the PfCDPK4 binding pocket and determine characteristics that make them potent and selective [5]. The 4-piperidinemethyl substituent of BKI-1 was predicted to make a hydrogen bond with Glu154, which was earlier observed in crystal structures of TgCDPK1 in complex with BKIs [12]. There were good correlations (Figure 1, R2 = 0.81) between predicted potency of inhibitors (pIs; –log10 [inhibition constant]) and experimentally determined IC50s in the 4-piperidinemethyl R2 series, which validated our binding model for testing the BKI-1 derivatives. Furthermore, the results also lend confidence on modeling and designing analogs that retain (or enhance) potency but have improved pharmacokinetic/absorption, distribution, metabolism, and excretion (PK/ADME) properties.
Figure 1.
Predicted pIs vs experimentally determined IC50s in the 4-piperidinemethyl R2 series The FLO software was used to predict the pI (inhibition of PfCDPK4 or pI [calc]) vs experimentally determined pIs (pI exp) in the methylpiperidine R2 series. There was a correlation of R2 = 0.81, thereby validating the model for this series of compounds. The model was used to select variations that retain potency and vary the PK/ADMET properties of the compounds. The successful modeling efforts that predicted potent PfCDPK4 inhibitors demonstrates how we can select potent derivatives of the pyrazolopyrimidine scaffold that are metabolically-stable for PK/ADMET optimization. Abbreviations: pI, –log10 (inhibition constant) PK, pharmacokinetics, ADMET, absorption, distribution, metabolism, excretion, toxicity.
Modification of BKI-1 for the Prolonged Exposure Required for Effective Transmission-blocking
Although the pharmacokinetic (PK) profile of BKI-1 (a concentration of >1 µM for up to 14 hours after intraperitoneal dosing [5]) was a good starting point for the development of a transmission-blocking therapeutic agent, our aim was to further optimize the PK properties. To predict BKI-1 metabolism, the compound was incubated with liver microsomes, and the primary metabolites were determined using LC-MS. Under these conditions, the most abundant BKI-1 metabolite contained a hydroxyl modification of the piperidine ring, presumably by liver P450 enzymes (data not shown). We predicted that alkylating the secondary amine of the 4-piperidinemethyl group would slow the rate of hydroxylation by P450s. As our inhibitor-binding model predicts that alkylating this position will not disrupt any interactions with the ATP-binding site of PfCDPK4, we generated an N-methylated version of BKI-1, compound 1294. As expected, 1294 displayed a reduced rate of microsomal metabolism compared to BKI-1 (Table 1), while retaining potent PfCDPK4 inhibition. In addition, compound 1294 possesses an 8-fold increase in blood level exposure (area under the curve [AUC]) after single oral dosing compared to BKI-1, probably due to decreased systemic clearance and increased oral bioavailability (Table 2). Blood levels of mice dosed with 40 mg/kg of BKI-1 and 1294 by oral gavage 3 times a day for 4 consecutive days were analyzed by LC-MS to test whether 1294 and/or BKI-1 plasma accumulation would occur with multiple dosing per day over 5 days. The first and fourth troughs, as shown in Table 1, refer to compound levels 17 hours after compound dosing taken at the beginning of day 2 and day 5. The first peak was 1 hour after the first dose. The fourth day peak was 1 hour after the third dose of day 4 (mean ± SD of n = 3). The trough plasma levels of BKI-1 were below the limit of detection, but substantial trough plasma of compound 1294 were seen at the beginning of day 2 (2.0 µM) and day 6 (6.3 µM). This suggests 1294 was cleared more slowly and accumulated during 3-times daily dosing. Furthermore, it seemed likely that a once-a-day dosing regimen with 1294 could lead to 24-hour therapeutic exposure, and indeed 100 mg/kg oral dosing led to 2.7 µM plasma levels at 24 hours after dosing in rats.
Table 1.
In vitro Drug Metabolism and Pharmacokinetics (DMPK) of BKI-1 and 1294 and Blood Levels Accumulation With Repeated Dosing
| Assay Type |
Solubility (µM) |
Plasma Protein Binding |
Compound Stability With Liver Microsomes (NADPH Driven, No Cofactors) t1/2 (min) |
Blood Levels Accumulation With Repeated 40 mg/kg Doses (µM) |
Oral and IV AUC and Bioavailability (From Rat PK Studies) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assay | Buffer pH 6.5 | % cmpd Bound Human Plasma | Mouse | Rat | Dog | Primate | Human | First Peak | First Trough | Fourth Day Peak | Fourth Trough | Oral (10 mg/Kg) | Intravenous (10 mg/Kg) | Bio Availability |
| BKI-1 | 47 | ND | 30 | ND | ND | ND | ND | 0.05 ± 0.08 | 0 ± 0 | 6.6 ± 1.6 | 0 ± 0 | ND | ND | ND |
| 1294 | 82 | 85% | 45 | >60 | 45 | >60 | >60 | 2.1 ± 1.2 | 2.0 ± 1.6 | 8.9 ± 3.4 | 6.3 ± 1.8 | 1512.9 | 1663.5 | 91 |
Table 2.
In vivo Pharmacokinetic Parameters of BKI-1 and 1294 (Mouse)
| Oral (10 mg/kg) |
Oral (100 mg/kg) |
Intraperitoneal [IP] (10 mg/kg) |
Intraperitoneal (100 mg/kg) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Cmax (µM) | tmax (min) | AUC (µM*min) | CL (L/min) | Cmax (µM) | tmax (min) | AUC (µM*min) | CL (L/min) | Urine excretion | Stool excretion | t1/2 (hr) | Cmax (µM) | tmax (min) | AUC (µM*min) | CL (L/min) | t1/2 (hr) | Cmax (µM) | tmax (min) | AUC (µM*min) | CL (L/min) | t1/2 (hr) |
| BKI-1 | 0.2 | 140 | 57 | ND | ND | ND | ND | ND | ND | ND | ND | 1.9 | 30 | 317 | 0.0076 | 1.5 | ND | ND | ND | ND | ND |
| 1294 | 0.8 | 120 | 430 | 0.0112 | 7.19 | 460 | 10 585 | 0.005 | 1% | 0.05% | 13.5 | 3 | 40 | 863 | 0.0056 | 4 | 13.1 | 160 | 13 000 | 0.004 | 14 |
Abbreviations: AUC,area under the curve; ND, no data.
Compound 1294 Blocks Microgametocyte Exflagellation and Malaria Transmission to Mosquitoes
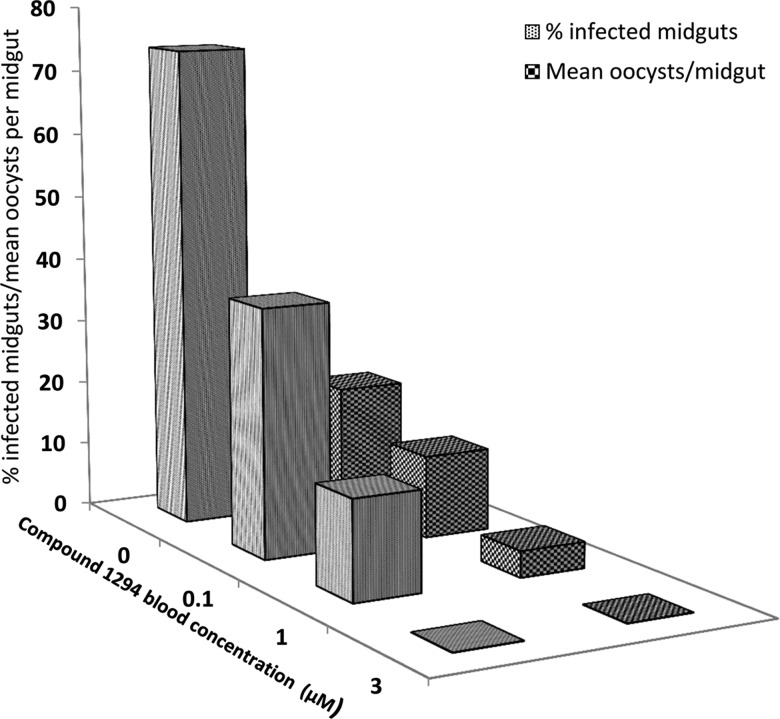
Compound 1294's IC50 of 10 nM against PfCDPK4 enzymatic activity and EC50 of 0.047 µM for blocking P. falciparum gametocyte exflagellation are comparable to that of BKI-1 [5]. The transmission-blocking activity of compound 1294 was confirmed with untransfected, wild-type NF54 P. falciparum gametocytes in human blood supplemented with 0.1, 1, or 3 µM 1294 and fed to Anopheles stephensi mosquitoes (Figure 2). Complete protection of mosquito malaria as indicated by the absence of oocysts was seen at 1294 blood concentration of 3 µM (n = 52). Blood concentrations of 1 µM and 0.1 µM of 1294 resulted in oocyst infectivity of 15% (n = 53) and 38% (n = 50), respectively, which is markedly lower than untreated blood (DMSO control, 74% infected, n = 50). Similarly, the mean oocyst number per infected midgut decreased from 19 in untreated control to 13, 4, and 0 in the 0.1 µM, 1 µM, and 3 µM 1294 treated samples, respectively (Figure 2). Thus, even a blood level of 0.1 µM of 1294 is predicted to have a measureable impact on transmission, but a level of 3 µM is necessary to completely block transmission.
Figure 2.
1294 prevents sexual stage development of Plasmodium falciparum in Anopheles stephensi mosquitoes. Plots show percentage of infected mosquito midguts (gray bars) and the mean number of oocysts per midgut (large checked bars) at varying 1294 concentrations. P. falciparum gametocytes in human blood supplemented with 0, 0.1, 1, or 3 µM of 1294 were fed to A. stephensi mosquitoes. There was substantial reduction of P. falciparum gametocyte stage differentiation to infective zygote in the presence of 1294 as shown by a decreased in number of mosquito midguts infected with oocysts and the mean oocyst number per infected midguts at each blood concentration of 1294 relative to the untreated blood. Sexual stage development in mosquitoes fed with 3 μM of 1294-supplemented blood meal was completely inhibited.
Mechanism of Action of Compound 1294
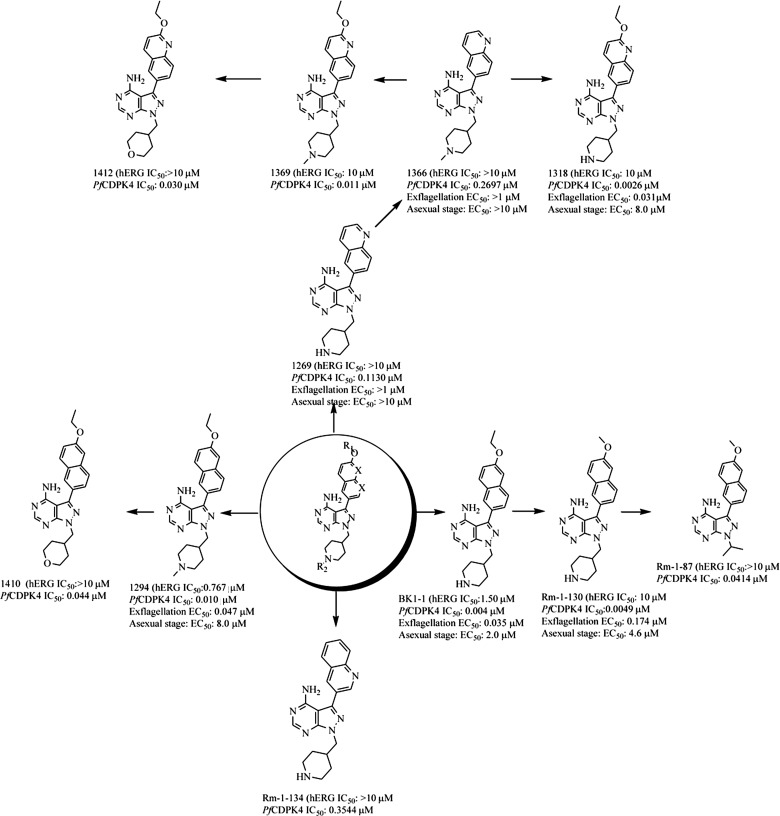
Earlier evidence that BKIs block malaria transmission through the inhibition of PfCDPK4 was based on the strong structure activity relationship (SAR) correlation between inhibition of the in vitro enzymatic activity of PfCDPK4 and the blocking of exflagellation [5]. Further systematic SAR studies validate a correlation between the potency of inhibitors against the enzymatic activity of PfCDPK4 and their ability to block exflagellation (Figure 4). Similarly, there is no significant correlation between PfCDPK4 inhibition and inhibition of asexual stage parasites [5] (Figure 4). To further confirm that the mechanism of action of 1294 in blocking exflagellation and transmission is through PfCDPK4 inhibition, we generated drug-resistant P. falciparum NF54 strains that exogenously express a methionine gatekeeper mutant of PfCDPK4 (PfCDPK4S147M). We predicted that the bulky ethoxynaphthyl R1-group of 1294 would not be accomadated in the constricted ATP-binding site of this PfCDPK4 mutant. Indeed, an enzymatic assay demonstrated that 1294 shows minimal inhibition of PfCDPK4S147M at the highest concentration tested (3 µM; Table 3).
Figure 4.
Compound structures and iterative modifications to obtain hERG inactive molecules. Inhibitors based on the pyrazolopyrimidine scaffold were generated by iterative modifications with the aim of removing hERG activity while retaining PfCDPK4 inhibition. Introduction of a 2-ethoxyquinolin-6-yl R1 group in place of BKI-1 and compound 1294 6-ethoxynaphthalen-2-yl significantly reduced hERG activity in both cases. Similarly, replacing the piperidin-4-ylmethyl or 1-methylpiperidin-4-yl methyl R2 with a nonbasic group, such as a pyran, or isopropyl group, eliminated hERG activity. The IC50s for compounds against PfCDPK4 and hERG have been tested and shown in the figure. Asexual stage EC50 refers to the concentration of drug that inhibits 50% of the replication of P. falciparum in RBCs in human blood cultures. Exflagellation EC50 refers to the concentration of drug that inhibits 50% of the exflagellation of P. falciparum male gametocytes. Abbreviations: hERG, human ether-a-go-go related gene; RBC, red blood cell.
Table 3.
In vitro Efficacy Profile of BKI-1 and 1294
| Enzymatic IC50 (µM) |
Exflaggelation EC50 (µM) |
||||
|---|---|---|---|---|---|
| Assay Type | PfCDPK4 Enzyme | PfCDPK4 S147M Enzyme | WT P. fal. NF54 | NF54WT Control Transfectant | NF54S147M Genetic Mutant |
| Assay | |||||
| BKI-1 | 0.004 | >2 | 0.035 | ND | ND |
| 1294 | 0.010 | >2 | 0.047 | 0.023 | 0.292 |
Abbreviation: ND, no data.
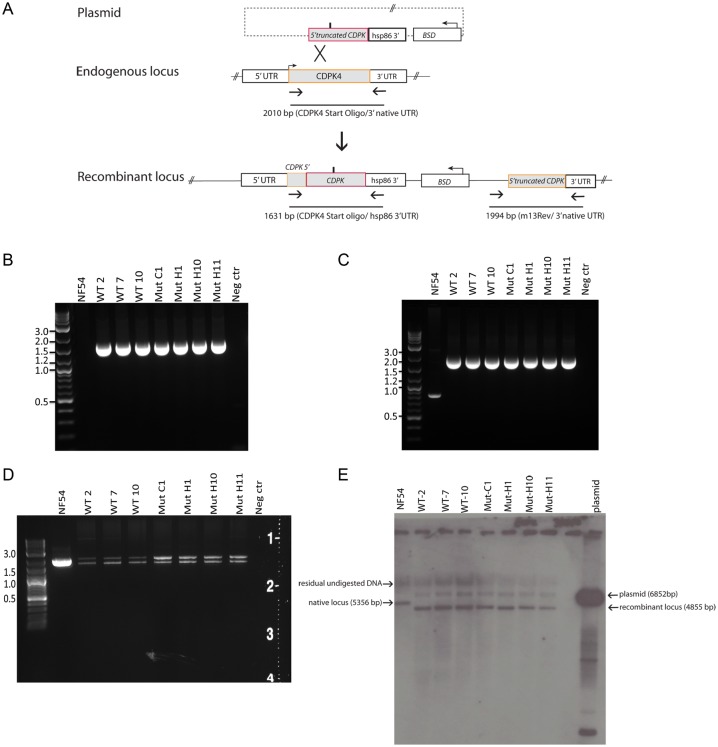
P. falciparum NF54 strains exogenously expressing either S147M or wild-type PfCDPK4 were engineered by allelic exchange, replacing the native 3′ segment of the Pfcdpk4 gene with Pfcdpk4 TCT441ATG (S147M) or a control vector containing the wild-type allele Pfcdpk4 (Pfcdpk4WT; Figure 3A). Both constructs contain a blasticidin selection marker [24]. The resultant strains express either PfCDPK4WT or PfCDPK4S147M gatekeeper mutant under the control of the native Pfcdpk4 promoter with a recombinant hsp86 3′UTR. Pfcdpk4 allelic exchange was confirmed by polymerase chain reaction (PCR; Figure 3B–3D) and Southern blot hybridization (Figure 3E). The amplicons from the coding region (Pfcdpk4 start oligo and either the p863 or 3′ native UTR) were also sequenced and verified to contain the engineered TCT441ATG mutation (S147M construct) or the wild-type allele without detection of any other mutation. From Figure 3D, the Pfcdpk4 Start oligo/3′native UTR PCR gave a unique result producing 2 amplicons (bands). The lower band has the Pfcdpk4 start region (not included in the allelic exchange construct) and the 3′ Pfcdpk4 native UTR with retention of the S147M substitution in the mutant clones, or wild-type allele without the native Pfcdpk4 intron (also not included in the allelic exchange construct). The upper band also has the complete Pfcdpk4WT coding region, 3′ native Pfcdpk4 UTR and the native Pfcdpk4 intron. The presence of further recombination of this locus suggests a strong selective pressure to maintain the wild-type gene with endogenous regulatory elements. Therefore, the recombinant parasites possess a wild-type allele, a recombinant allele with the hsp86 3′ UTR (either wild-type or S147M depending on the parasite) and a nonfunctional allele with a truncation of the 5′ of the coding sequence, as determined by PCR and confirmed by direct sequencing.
Figure 3.
PfCDPK4 TCT441ATG (S147M) allelic exchange and verification strategies. A, Diagram of allelic exchange showing single-crossover event of a truncated wild-type PfCDPK4 or PfCDPK4 coding sequence bearing a TCT441ATG mutation interrupting the endogenous Pfcdpk4 gene. This effectively replaces the endogenous gene with the recombinant locus, producing a full-length Pfcdpk4 with or without the TCT441ATG gatekeeper mutation and a truncated nonfunctional Pfcdpk4 gene downstream of the plasmid integration. Episomal plasmids were selected under BSD pressure. Oligonucleotide sequences for verification of recombination events are shown in Supplementary Table 1. Pfcdpk4 allelic exchange was confirmed by polymerase chain reaction (PCR) using the Pfcdpk4 start oligo (not present in the allelic exchange vector) and p863 oligo, specific to the hsp86 3′ UTR; (B–D) PCR products with an expected sizes using primers listed in Supplementary Table 1. D, Reflects a PCR screen using the oligos Pfcdpk4 start and Pfcdpk4 3′native UTR. Each clone (from multiple independent electroporations) had 2 amplicons: the lower band has the Pfcdpk4 start and 5′ coding region (not included in the allelic exchange construct) and the 3′ native Pfcdpk4 UTR with retention of the methionine mutation in the mutant clones. The upper band also has the complete Pfcdpk4 start and 5′ coding region, 3′ native Pfcdpk4 UTR and the native Pfcdpk4 intron (not present in the allelic exchange construct), the mutant clones lack the engineered methionine mutation in the upper amplicon. E, Southern blot evaluation of the allelic exchange parasites probed with Pfcdpk4 coding sequence. The native Pfcdpk4 locus (5356 bp) is replaced in the recombinant parasites with a band at 4855 bp due to introduction of an XhoI restriction site. Residual episomal plasmid (6852 bp) is also present in the electroporated parasites.
The original intent of the P. falciparum genetic experiments was to express the PfCDPK4S147M allele in trans, as this should be a dominant drug-resistant form, permitting the validation of the molecular target. However, multiple attempts to obtain viable transgenic parasites, either with episomal plasmids or integrated, failed even though the promoter driving expression is restricted to the gametocyte stage, as demonstrated previously [25]. This combined with each of the clones undergoing further genetic recombination after transfection with the allelic exchange constructs suggests that perturbation of the Pfcdpk4 locus, possibly through plasmid integration or use of the hsp86 3′ recombinant UTR, significantly impacts the parasite viability. This drives the selection of parasites with further genetic recombination that at least partially restores an essential function. Regardless, the allelic exchange experiment, although not a clean genetic experiment, is a surrogate for the original experiment of introducing a second copy of the Pfcdpk4 allele permitting the genetic validation of the molecular target of this class of kinase inhibitors.
We performed exflagellation experiments with transfected mutant and wild-type gametocytes [5] to determine if 1294 transmission-blocking activity was a reflection of PfCDPK4 inhibition. Consistent with CDPK4 being the intracellular target of 1294, the PfCDPK4S147M recombinant parasites possess a decreased exflagellation susceptibility, with an EC50 of 0.292 µM, compared to an EC50 of 0.023 µM for PfCDPK4WT control transfected parasites (Table 3). Thus, the shift in the EC50 for the mutant vs wild-type transfectants to block exflagellation was 13.3-fold, which is consistent with 1294 blocking exflagellation through PfCDPK4, although the PfCDPK4S147M enzyme is more than 200-fold less sensitive than PfCDPK4WT. This relative difference in drug resistance may be because PfCDPK4S147M is about 2-fold less active than the wild-type PfCDPK4 enzyme in the in vitro assays, and the activity of PfCDPK4 in the S147M parasites may be even lower when acting upon physiological substrates. In addition, the Pfcdpk4 expression levels may be altered as the recombinant allele carries the hsp86 3′UTR and lacks the native intron. It is also worth mentioning that 1294 is most likely also inhibiting PfCDPK1 at higher concentrations of drug because the IC50 value of this compound for the PfCDPK1 enzyme is 0.117 µM. PfCDPK1 was recently shown to be involved in the malaria parasite mosquito gut invasion process [26]. However, the preponderance of evidence supports that PfCDPK4 is the target of 1294, leading to blocking parasite transmission.
1294 Has Low Toxicity and Good Oral Bioavailability
Signs of toxicity were examined in mice after high-dose administration of 100 mg/kg BKI-1 and 1294 orally twice a day for 5 days. Animals showed no overt signs of toxicity, no weight loss, normal tissue histology, and normal blood metabolic enzymes and complete blood counts after 5 days. Compound 1294 was shown to be drug-like in the mouse-model, with 85% protein binding (Table 1), 50% oral bioavailability (estimated from 10 mg/kg dose AUC, PO vs IP), and long t½ (4–14 hours, depending on dose). Only 1% of 1294 was excreted in urine and <0.1% was excreted in the stool of mice orally dosed with 100 mg/kg, consistent with the hypothesis that 1294 is predominantly cleared by liver metabolism and nearly completely absorbed (Table 2). Comparing the PK of 10 mg/kg and 100 mg/kg dosing of 1294 demonstrates a nonlinear increase in exposure (AUC 430 vs 10 585, respectively) and oral bioavailability (estimating from PO/IP AUC, 50% vs 81%). This suggests that saturation of metabolic clearance of 1294 may increase exposure and oral bioavailability. Compound 1294 oral bioavailability in a rat model was found to be 91% (estimate from PO/IV AUC; Table 1). Administration of multiple doses of 1294 to mice orally over 5 days led to an increased blood accumulation of 1294, compared to BKI-1, as demonstrated by the elevated trough concentration levels (Table 1). Yet, even with accumulation to high blood and serum levels well above concentrations needed to stop transmission, no toxicity was observed in the mice based on analysis of their behavior, body weight, blood chemistries, and tissue histology at the end of the exposure interval. As ACTs are administered 2–3 times daily over 3 days, co-administration of 1294 would lead to a prolonged blood exposure, providing effective transmission-blocking potential. Evaluation of 1294 metabolism in mouse, rat, dog (beagle), primate, and human liver-microsomes in vitro predicts that this compound has a prolonged half-life in rats, primates, and humans, which is consistent with long exposure in humans (Table 1).
1294 Is a Highly Selective Kinase-inhibitor But Has hERG Liability
Selectivity profiling against 80 human kinases revealed that 1294 only detectably inhibited 1 kinase, PRKCN. However, 1294 is 13 times less potent against PRKCN than PfCDPK4. Interestingly, 1294 is more selective than BKI-1 (data not shown). Next, 1294 was profiled against 23 nonkinase targets, including GPCRs and other off target liabilities for potential therapeutics. Although 1294 showed minimal activity against 22 of the 23 targets screened, this compound showed activity against hERG at a concentration similar to that needed to block transmission. Efforts to remove hERG activity by iterative modification of 1294 indicated that replacing the 4-piperidinemethyl R2-group with a nonbasic group, such as pyran, or isopropyl group, eliminated hERG activity (Figure 4). Furthermore, certain derivatives of the ethoxynaphthyl R1-group show reduced hERG activity without reducing the inhibitory effect on PfCDPK4 (Figure 4). Current medicinal chemistry efforts are focused on the development of inhibitors that share the favorable properties of 1294 but lack hERG activity. Nonetheless, based on therapeutic indexes calculated from experimental exflagellation EC50 of 1294 (0.047 µM), BKI-1 (0.035 µM), or 1318 (0.031 µM) and their respective hERG EC50 of 0.767 µM, 1.50 µM, and 10 µM; it is likely a dose regimen can be found in this series with efficacy without cardiovascular risks. Despite the hERG liability of 1294, this inhibitor was used as a proof of concept molecule to explore efficacy and toxicology and to also demonstrate that our transmission-blocking compounds are acting through PfCDPK4.
CONCLUSIONS
There are many drugs for treating the asexual blood stages of malaria but only one drug, primaquine, is currently available for interrupting the transmission of malaria to mosquitoes. Primaquine has safety and tolerability issues, particularly for those with glucose-6-phosphate dehydrogenase (G6PDH) deficiency, resulting in severe and potentially fatal hemolysis after its use [27]. High prevalence of G6PDH deficiency may limit the use of primaquine in malaria-endemic African populations [28]. Novel classes of effective and safe drugs are needed to control malaria by reducing the transmission from humans to mosquitoes and break the cycle of infection. We have developed a series of protein kinase-inhibitors that specifically target plasmodia CDPK4 and can block malaria transmission. Specific inhibitors of CDPK4 can be obtained because CDPK4 differs from human kinases in that it has a very small gatekeeper residue, serine. The small serine gatekeeper residue of CDPK4 exposes an enlarged hydrophobic pocket in the ATP-binding site that is not present in human protein kinases [5]. This hydrophobic pocket can accommodate a large aromatic group displayed from an inhibitor scaffold that mimics adenine. Such “bumped kinase-inhibitors” (BKIs) cannot fit into the ATP-binding site of most human protein kinase and thus provides selectivity for BKIs.
The preclinical lead candidate compound 1294, which is nontoxic in high dose administration to mice, shows efficacy in transmission blocking with significant phenotypic effects at human blood concentration of 0.100 µM and has favorable PK/ADME attributes for prolonged exposure. A compound with characteristics like 1294, co-administration with a 3-day regimen of ACT is predicted to lead to human plasma concentration above transmission-blocking EC90 for several weeks. This compound may provide the basic framework for a future transmission-blocking drug. We therefore sought to confirm the specific biochemical interaction through which 1294 produces its malaria transmission-blocking effect by generating mutant strains exogenously expressing 1294-resistant PfCDPK4. It proved to be difficult to obtain a transfectant P. falciparum line expressing only mutant PfCDPK4 (S147M) for a definitive in-vivo chemical-genetic modification of PfCDPK4. The combined evidence from our attempts suggests that there is selection pressure against a large gatekeeper residue in CDPK4. This observation may mean that resistance to BKIs might be more difficult to achieve through a single codon mutation in the gatekeeper residue of PfCDPK4. It was also observed that the number of exflagellating centers in the mutant clones is significantly lower than the wild type. This may be an indication that even if by some unexplained events, there was a gatekeeper mutant in the natural population, their exflagellation effectiveness may be significantly compromised. This chemical genetic approach nonetheless validates PfCDPK4 as the target of 1294 and supports PfCDPK4 as the target blocked for exflagellation and transmission [6]. 1294 is orally bioavailable, is sufficiently potent, and can maintain a significant level of stability while preventing exflagellation of the male gametocyte in the mosquito. An effective transmission-blocking compound will likely be administered orally in combination with drugs active against asexual stages [8], such as ACT during mass administration for control or eradication campaigns. We propose administering a drug like 1294 with ACT because artemisinin derivatives kill stage I–III gametocytes, and gametocytes are less infectious to mosquitoes at day 7 after ACT treatment relative to other antimalaria such as chloroquine and sulphadoxine-pyrimethamine [29]. An oral adjunctive drug with such exposure seems attainable. The added advantage of co-administration of a drug like 1294 with ACT is a potential reduction in the spread of artemisinin-resistant strains recently reported in parts of Asia and other countries. Transmission of such partially-artemisinin-resistant strains would stop immediately with co-administration of ACT and a drug like 1294, whereas the clearance of such strains asexual stages and probably gametocytes from the bloodstream is clearly delayed [1]. In summary, 1294 is an advance lead candidate due to its excellent absorption, exposure, safety profile, and efficacy in transmission blocking.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors wish to acknowledge with thanks the following scientists for technical support and valuable conversations: Lynn Barrett, Tiffany Silver-Brace, and Jen C. C. Hume.
Financial support. Research reported in this publication was supported by National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under award number R01AI089441, R01AI080625, and NIH grant R01GM086858. Work in the Van Voorhis lab was supported by NIH grants 1 R01 AI089441 and 5 R01 AI080625. Richard Eastman and Xin-zhuan Su were supported by the Divisions of Intramural Research at the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The Maly Lab was supported by NIH grant R01GM086858.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dondorp AM, Yeung S, White L, et al. Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol. 2010;8:272–80. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- 2.Schneider P, Bousema JT, Gouagna LC, et al. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg. 2007;76:470–4. [PubMed] [Google Scholar]
- 3.Trape JF, Tall A, Diagne N, et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect Dis. 2011;11:925–32. doi: 10.1016/S1473-3099(11)70194-3. [DOI] [PubMed] [Google Scholar]
- 4.Kato K, Sudo A, Kobayashi K, Sugi T, Tohya Y, Akashi H. Characterization of Plasmodium falciparum calcium-dependent protein kinase 4. Parasitol Int. 2009;58:394–400. doi: 10.1016/j.parint.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Ojo KK, Pfander C, Mueller NR, et al. Transmission of malaria to mosquitoes blocked by bumped kinase inhibitors. J Clin Invest. 2012;122:2301–5. doi: 10.1172/JCI61822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503–14. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- 7.Bousema T, Okell L, Shekalaghe S, et al. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J. 2010;9:136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doerig C, Billker O, Pratt D, Endicott J. Protein kinases as targets for antimalarial intervention: kinomics, structure-based design, transmission-blockade, and targeting host cell enzymes. Biochim Biophys Acta. 2005;1754:132–50. doi: 10.1016/j.bbapap.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 9.McMartin C, Bohacek RS. QXP: powerful, rapid computer algorithms for structure-based drug design. J Comput Aided Mol Des. 1997;11:333–44. doi: 10.1023/a:1007907728892. [DOI] [PubMed] [Google Scholar]
- 10.Irwin JJ, Shoichet BK. ZINC—A free database of commercially available compounds for virtual screening. J Chem Inf Model. 2005;45:177–82. doi: 10.1021/ci049714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson SM, Murphy RC, Geiger JA, et al. Development of Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) inhibitors with potent anti-toxoplasma activity. J Med Chem. 2012;55:2416–26. doi: 10.1021/jm201713h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy RC, Ojo KK, Larson ET, et al. Discovery of potent and selective inhibitors of CDPK1 from C. parvum and T. gondii. ACS Med Chem Lett. 2010;1:331–5. doi: 10.1021/ml100096t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Voorhis WC, Rivas KL, Bendale P, et al. Efficacy, pharmacokinetics, and metabolism of tetrahydroquinoline inhibitors of Plasmodium falciparum protein farnesyltransferase. Antimicrob Agents Chemother. 2007;51:3659–71. doi: 10.1128/AAC.00246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibata S, Gillespie JR, Ranade RM, et al. Urea-based inhibitors of Trypanosoma brucei methionyl-tRNA synthetase: selectivity and in vivo characterization. J Med Chem. 2012;55:6342–51. doi: 10.1021/jm300303e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ojo KK, Larson ET, Keyloun KR, et al. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat Struct Mol Biol. 2010;17:602–7. doi: 10.1038/nsmb.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabb BS, Triglia T, Waterkeyn JG, Cowman AF. Stable transgene expression in Plasmodium falciparum. Mol Biochem Parasitol. 1997;90:131–44. doi: 10.1016/s0166-6851(97)00143-6. [DOI] [PubMed] [Google Scholar]
- 17.Deitsch K, Driskill C, Wellems T. Transformation of malaria parasites by the spontaneous uptake and expression of DNA from human erythrocytes. Nucleic Acids Res. 2001;29:850–3. doi: 10.1093/nar/29.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eastman RT, Dharia NV, Winzeler EA, Fidock DA. Piperaquine resistance is associated with a copy number variation on chromosome 5 in drug-pressured Plasmodium falciparum parasites. Antimicrob Agents Chemother. 2011;55:3908–16. doi: 10.1128/AAC.01793-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–5. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 20.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The Protein Kinase Complement of the Human Genome. Science. 2002;298:1912–18. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 21.Lebakken CS, Riddle SM, Singh U, et al. Development and Applications of a Broad-Coverage, TR-FRET-Based Kinase Binding Assay Platform. J Biomol Screen. 2009;14:924–35. doi: 10.1177/1087057109339207. [DOI] [PubMed] [Google Scholar]
- 22.Bowes J, Brown AJ, Hamon J, et al. Reducing safety-related drug attrition: the use of in vitro pharmacological profiling. Nat Rev Drug Discov. 2012;11:909–22. doi: 10.1038/nrd3845. [DOI] [PubMed] [Google Scholar]
- 23.Bridal TR, Margulis M, Wang X, Donio M, Sorota S. Comparison of human ether-a-go-go related gene screening assays based on IonWorks Quattro and thallium flux. Assay Drug Dev Technol. 2010;8:755–65. doi: 10.1089/adt.2010.0267. [DOI] [PubMed] [Google Scholar]
- 24.Mamoun CB, Gluzman IY, Goyard S, Beverley SM, Goldberg DE. A set of independent selectable markers for transfection of the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1999;96:8716–20. doi: 10.1073/pnas.96.15.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adjalley SH, Johnston GL, Li T, et al. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc Natl Acad Sci U S A. 2011;108:E1214–23. doi: 10.1073/pnas.1112037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sebastian S, Brochet M, Collins MO, et al. A Plasmodium calcium-dependent protein kinase controls zygote development and transmission by translationally activating repressed mRNAs. Cell Host Microbe. 2012;12:9–19. doi: 10.1016/j.chom.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernando D, Rodrigo C, Rajapakse S. Primaquine in vivax malaria: an update and review on management issues. Malar J. 2011;10:351. doi: 10.1186/1475-2875-10-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg. 2006;75:402–15. [PubMed] [Google Scholar]
- 29.Sutherland CJ, Ord R, Dunyo S, et al. Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of co-artemether. PLoS Med. 2005;2:e92. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.