Abstract
BACKGROUND & AIMS
Central adiposity has been implicated as a risk factor for Barrett’s esophagus (BE) and esophageal adenocarcinoma (EAC), possibly promoting the progression from inflammation to metaplasia and neoplasia. We performed a systematic review and meta-analysis of studies to evaluate the association between central adiposity and erosive esophagitis (EE), BE, and EAC, specifically exploring body mass index (BMI)–independent and gastroesophageal reflux (GERD)–independent effects of central adiposity on the risk of these outcomes.
METHODS
We performed a systematic search of multiple databases through March 2013. Studies were included if they reported effect of central adiposity (visceral adipose tissue area, waist-hip ratio, and/or waist circumference) on the risk of EE, BE, and EAC. Summary adjusted odds ratio (aOR) estimates with 95% confidence intervals (CIs), comparing highest category of adiposity with the lowest category of adiposity, were calculated by using randomeffects model.
RESULTS
Forty relevant articles were identified. Compared with patients with normal body habitus, patients with central adiposity had a higher risk of EE (19 studies; aOR, 1.87; 95% CI, 1.51–2.31) and BE (17 studies; aOR, 1.98; 95% CI, 1.52–2.57). The association between central adiposity and BE persisted after adjusting for BMI (5 studies; aOR, 1.88; 95% CI, 1.20–2.95). Refluxindependent association of central adiposity and BE was observed in studies that used GERD patients as controls or adjusted for GERD symptoms (11 studies; aOR, 2.04; 95% CI, 1.44–2.90). In 6 studies, central adiposity was associated with higher risk of EAC (aOR, 2.51; 95% CI, 1.54–4.06), compared with normal body habitus.
CONCLUSIONS
On the basis of a meta-analysis, central adiposity, independent of BMI, is associated with esophageal inflammation (EE), metaplasia (BE), and neoplasia (EAC). Its effects are mediated by reflux-dependent and reflux-independent mechanisms.
Keywords: Visceral Fat, Body Habitus, Barrett’s Esophagus, Esophageal Cancer
Obesity has been implicated in a spectrum of reflux-related esophageal diseases ranging from esophageal inflammation (erosive esophagitis [EE]) to metaplasia (Barrett’s esophagus [BE]) to neoplasia (esophageal adenocarcinoma [EAC]).1–5 Obesity promotes gastroesophageal reflux disease (GERD) through disruption of the gastroesophageal junction anatomy and physiology, which can lead to EE.6,7 This reflux-induced chronic esophageal inflammation predisposes to BE and a higher risk of progressing to EAC.4 In previous studies, increased body mass index (BMI) was found to be a risk factor for GERD but not for the development of BE in those with GERD.1–3 This suggested that the effect of BMI on BE pathogenesis may be mediated predominantly by promoting reflux.8
Several recent preclinical and observational studies have demonstrated that the pattern of body fat distribution may be more important than overall adiposity in determining the risk of EE, BE, and EAC.4,9–11 Although some studies have shown that central adiposity may have a BMI-independent effect on the risk of these adverse esophageal outcomes,9,12 others have failed to demonstrate this association.13,14 In addition to promoting GERD, metabolically active visceral adipose tissue releases proinflammatory adipocytokines, which may contribute to development of metaplasia and neoplasia.7,11,15 Such a refluxindependent effect of central adiposity on BE and EAC, however, is not consistent among all studies.10,16,17
Therefore, to better understand the relationship between central adiposity and esophageal inflammation, metaplasia and neoplasia, we conducted a systematic review and meta-analysis of all observational studies that investigated the association between central adiposity and risk of these outcomes. Through predetermined subgroup analyses, we sought to understand whether central adiposity has a BMI-independent association with these outcomes, and whether central adiposity has a refluxindependent effect on BE and EAC.
Methods
This systematic review was conducted following guidance provided by the Cochrane Handbook17 and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.18 The process followed a priori established protocol.
Search Strategy
First, a systematic literature search of PubMed (1966 through March 1, 2013), followed by Embase (1988–March 1, 2013) and Web of Science (1993–March 1, 2013) databases, was conducted to identify all relevant articles on the effect of central adiposity on the risk of EE, BE, and EAC. Medical subject heading (MeSH) terms used in the search included a combination of “Obesity”, “Waist Circumference”, “Waist-Hip Ratio”, “Body Fat Distribution”, “Adiposity”, “Abdominal Fat”, “Obesity, Abdominal” AND “Esophagitis”, “Barrett esophagus” OR “esophageal neoplasm”. The title and abstract of studies identified in the search were reviewed by 2 authors independently (S.S., A.N.S.) to exclude studies that did not answer the research question of interest, which was based on prespecified inclusion and exclusion criteria. The full text of the remaining articles was examined to determine whether it contained relevant information. The coefficient of agreement between the 2 reviewers for article selection (κ = 0.84; 95% confidence interval [CI], 0.72–0.94) was excellent. Next, the bibliographies of the selected articles and review articles on the topic were manually searched for additional articles. Third, a manual search of conference proceedings from major gastroenterology meetings (2005–2012) was performed for additional abstracts on the topic. These were not included in the primary analysis, but sensitivity analysis after including these abstracts was performed for each outcome.
Selection Criteria
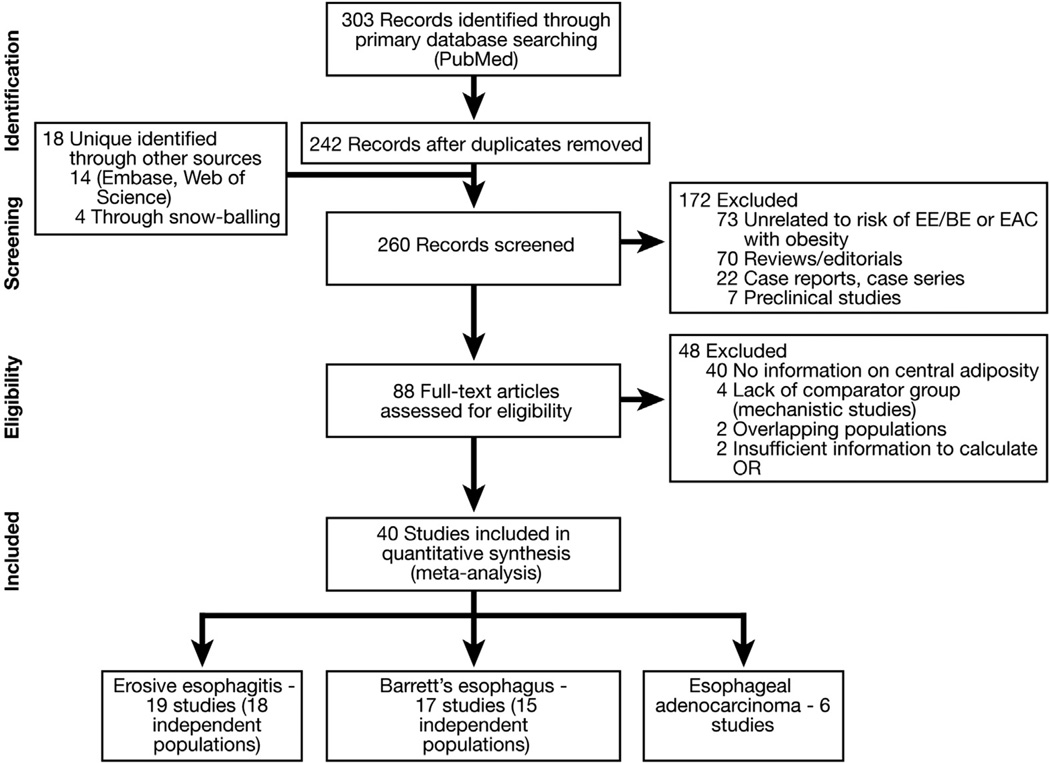
Studies considered in this meta-analysis were observational studies that met the following inclusion criteria: (1) evaluated and defined a measure of central adiposity, visceral adipose tissue area (cm2 ) or volume (cm3 ) as measured using abdominal computed tomography (CT), waist-hip ratio (WHR), and/or waist circumference (WC); (2) reported its association with esophageal disease outcomes (EE defined on upper endoscopy, BE and/or EAC validated by pathology review); and (3) reported a measure of association, relative risk (RR) or odds ratio (OR), or provided data for their calculation. Inclusion was not otherwise restricted by study size, language, or publication type. Figure 1 summarizes the process of study identification, inclusion, and exclusion.
Figure 1.
Flowchart showing study identification and selection process.
Data Abstraction
Data on the following were independently abstracted onto a standardized form by 2 reviewers (S.S., A.N.S.): (1) study characteristics: study design, time period, country, source population, presence or absence of GERD symptoms; (2) exposure assessment: measure of central adiposity (visceral adipose tissue area, WHR, and/or WC), how it was defined, and whether it was reported as a continuous or categorical variable, along with categories (binary divided as median or normal and abnormal, tertiles, quartiles, and reference category), evaluation of doseresponse relationship; (3) primary outcome reported: EE, BE, and/or EAC; and (4) analysis: OR and 95% CIs with and without adjustment for confounding factors, as well as ORs reported after adjustment for BMI and after adjustment for presence of GERD symptoms in each individual study. In addition, for each included study, if the relation between BMI (as a surrogate for overall obesity) and abdominal subcutaneous adipose tissue area (cm2) (measured on CT) and esophageal outcomes was reported as OR, these data were abstracted in the same fashion as above. Conflicts in data abstraction were resolved by consensus, referring back to the original article and in consultation with the principal investigator (P.G.I.). Data on the following confounding risk factors for relevant esophageal outcomes were also abstracted from each study: age, sex, race, BMI, smoking status, alcohol consumption, GERD symptoms, use of proton pump inhibitors or histamine receptor antagonists, presence of hiatal hernia, family history of EAC, caffeine intake, Helicobacter pylori infection, use of putative chemopreventive agents (aspirin, nonsteroidal anti-inflammatory drugs, statins), and for studies reporting EAC as outcome, presence, length, and histology of BE.
Exposure and Outcome Assessment
The primary analysis focused on assessing the relationship between central adiposity and each esophageal disease outcome: EE, BE, or EAC. When multiple measures of central adiposity were reported in the same study, preference was given to OR reported for central adiposity measured by using visceral adipose tissue area, followed by WHR (or waistthigh ratio) and last to WC. When exposure was reported in tertiles or quartiles, the comparison was performed between the highest quartiles and the lowest quartiles (or referent category) for the primary analysis. When results were reported as mean and standard deviations in cases and controls, we transformed this into a binary OR (comparing values above the mean to the referent category that was below the mean) using the Chinn equation (details in Supplementary Material).19 The referent groups for all these outcomes were patients in the lowest category of body habitus (usually normal body habitus).
In addition, to explore the presence of a BMI-independent effect of central adiposity on EE, BE, and EAC, we performed subgroup analysis of studies that provided OR after adjustment for BMI. Likewise, to explore a GERD-independent effect of central adiposity on BE and EAC, we performed subgroup analysis of studies that adjusted for GERD symptoms or studied only patients with GERD. Anticipating potential heterogeneity in the direction and magnitude of effect among the studies, we performed pre-planned subgroup analyses on study-related variables to explore sources of heterogeneity.
Statistical Analysis
We used the random-effects model described by DerSimonian and Laird20 to calculate meta-analytic OR and 95% CI for each outcome. Adjusted ORs (aORs) (for case-control and cross-sectional studies) or RRs (for cohort studies) reported in studies were used for analyses to account for confounding variables. We assessed heterogeneity between study-specific estimates by using 2 methods.21,22 First, the Cochran Q test, which tests the null hypothesis that all studies in a metaanalysis have the same underlying magnitude of effect, was measured. Because this test is underpowered to detect moderate degrees of heterogeneity,23 a P value <.10 was considered suggestive of significant heterogeneity. Second, to estimate the proportion of total variation across studies related to heterogeneity rather than chance, the I2 statistic was calculated. In this, values of <30%, 30%–60%, 60%–75%, and >75% were suggestive of low, moderate, substantial, and considerable heterogeneity, respectively.21,24 Between-study sources of heterogeneity were investigated by using subgroup analyses by stratifying original estimates according to study characteristics, with P < .05 for differences between subgroups (Pinteraction) being considered statistically significant. Publication bias was assessed quantitatively by using Egger regression test (publication bias considered present if P ≤ .10)25 and qualitatively by visual inspection of funnel plots.26 All P values were two-tailed. For all tests (except for heterogeneity and publication bias), a probability level <.05 was considered statistically significant. All calculations and graphs were performed using Comprehensive Meta-Analysis version 2 (Biostat, Englewood, NJ).
Results
From a total of 260 unique studies identified using our search strategy, 40 relevant studies (37 independent populations) were identified. Of these, 19 studies (18 independent populations) reported the association between central adiposity and EE,12,14,27–43 17 studies (15 independent populations) reported the association between central adiposity and BE,9–11,13,16,35,44–54 and 6 studies reported the association between central adiposity and EAC.13,55–59 Lee et al33,34 reported the relationship between central adiposity and EE by using both WHR and CT-measured visceral adipose tissue area (in a subset of patients). Likewise, for one independent population, relation between CT assessment of central adiposity and BE was performed in a subset of patients who underwent anthropometry and was reported in separate studies.53,54 One group reported the association between central adiposity and EE and BE in a single study.35 Corley et al9,56 used 2 separate Kaiser Permanente health checkup cohorts to study the association between central adiposity and BE and EAC. Likewise, the FINBAR study group reported the association between central adiposity and EE, BE, and EAC in 2 separate articles.13,36 Two sets of studies from western Washington10,60 and Kaiser Permanente population9,61 were from overlapping populations, and hence only one from each of these was included.9,10
Characteristics and Quality Assessment of Included Studies
Of the 40 studies, 18 were performed in the Asian population (including 17 studies on risk of EE)12,14,27–34,37–43 and the remainder in the Western population. Eight studies used visceral adipose tissue area as measure of central adiposity (4 each in patients with EE12,28,29,34 and BE16,44,46,53), 23 studies used WHR (7 studies on EE,30,34–37,39,40 11 studies on BE,9–11,13,35,48–52,54 and 5 studies on EAC13,56–59). The characteristics of the included studies for each outcome are shown in Tables 1–3. The overall quality of the included studies was moderate. Supplementary Tables 1, 2, and 3 report details of the quality assessment of included studies.
Table 1.
Characteristics of Included Studies Assessing the Association Between Central Adiposity and EE
| Ascertainment of adiposity |
Controls |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Design | Location | Study setting | Time period | Measure | Categories, highest vs lowest (referent) | EE cases | Definition | N |
| Gunji29 | Cross-sectional | Tokyo, Japan | Voluntary health check-up, hospital-based | 2007–2010 | CT; WC (cm); BMI (kg/m2) | Continuous WC: >85.8 vs <85.8 BMI: >23.8 vs <23.8 |
1831 | Healthy; non-GERD | 8009 |
| Ha30 | Cross-sectional | Seoul, South Korea | Voluntary health check-up, hospital-based | 2004–2006 | WHR; BMI | Binary WHR: >0.9 vs <0.9 BMI: >25 vs <25 |
292 | Patients; GERD | 500 |
| Hsu31 | Cross-sectional | Hualein, Taiwan | Voluntary health check-up, hospital-based | 2007 | WC; BMI | Binary WC: >90 (for men), >80 (for women) vs <90 or <80 BMI: >23.6 vs <23.6 |
131 | Healthy; non-GERD | 612 |
| Kang41 | Cross-sectional | Seoul, South Korea | Voluntary health check-up, hospital-based | 2004–2005 | WC; BMI | Binary WC: ≥90 (for men), ≥80 (for women) vs <90 or <80 BMI: >30 vs <25 |
161 | Healthy; non-GERD | 2281 |
| Kato42 | Cross-sectional | Osaka, Japan | Voluntary health check-up, hospital-based | 2007–2008 | WC; BMI | Continuous WC: >82.9 vs <82.9 BMI: >22.7 vs <22.7 |
235 | Healthy; non-GERD | 2170 |
| Lee43 | Cross-sectional | Daegu, South Korea | Hospital-based | 2008–2010 | WC; BMI | Continuous WC: >84.0 vs <84.0 BMI: >23.6 vs <23.6 |
278 | Patients; non-GERD | 172 |
| Nam37 | Cross-sectional | Goyang, South Korea | Voluntary health check-up, hospital-based | 2001–2006 | WC; BMI | Quartiles WC: ≥ 100.0 vs <80.0 BMI: >30.0 vs <20.0 |
552 | Healthy; non-GERD | 8019 |
| Nam12 | Cross-sectional | Goyang, South Korea | Voluntary health check-up, hospital-based | 2008 | WC, WHR, BMI, CT fat volume | Quartiles WHR: ≥1.0 vs <0.8 WC: ≥ 100.0 vs <80.0 BMI: ≥30.0 vs <20.0 |
495 | Healthy; non-GERD | 3779 |
| Chua27 | C-C | Taipei, Taiwan | Voluntary health check-up, hospital-based | 2004–2006 | WC; BMI | Binary WC: ≥90 (for men), >80 (for women) vs <90 or <80 BMI: ≥25.0 vs <25.0 |
427 | Healthy; non-GERD | 427 |
| Chung28 | C-C | Seoul, South Korea | Voluntary health check-up, hospital-based | 2004–2007 | T; WC; BMI | Quartiles (for CT); binary (for WC) WC: ≥90 (for men), >80 (for women) vs <90 or <80 BMI: ≥25.0 vs <23.0 |
3539 | Healthy; non-GERD | 3539 |
| Lee33,34 | C-C | Seoul, South Korea | Voluntary health check-up, hospital-based | 2003–2005 | CT; WHR; BMI | Tertiles WHR: >1.0 vs <0.8 BMI: >30.0 vs <20.0 |
292 | Healthy; non-GERD | 2896 |
| Mokrowiecka35 | C-C | Lodz, Poland | Hospital-based | 2009–2011 | WHR; WC; BMI | Continuous WHR: >0.65 vs <0.65 WC: >83 vs <83 BMI: >25 vs <25 |
30 | Patients; non-GERD | 30 |
| Mulholland36 | C-C | Belfast, Ireland | Population-based | 2002–2004 | WHR; BMI | Tertiles WHR: Tertiles, NR BMI: >29.0 vs <25.8 |
219 | Healthy; non-GERD | 260 |
| Park38 | C-C | Seoul, South Korea | Voluntary health check-up, hospital-based | 2006 | WC; BMI | Binary WC: ≥90 (for men), ≥80 (for women) vs <90 or <80 BMI: >24.0 vs <24.0 |
1679 | Healthy; non-GERD | 3358 |
| Wu40 | C-C | Shanghai, China | Voluntary health check-up, hospital-based | 2010 | WC; WHR; BMI | Binary WHR: >0.9 (for men), >0.8 (for women) vs ≤0.9 or ≤0.8 WC: ≥90 (for men), ≥80 (for women) vs <90 or <80 BMI: ≥28.0 vs <28.0 |
182 | Healthy; non-GERD | 190 |
| Koo32 | Cohort | Seoul, South Korea | Voluntary health check-up, hospital-based | 2003–2006 | WC; BMI | Tertiles WC: ≥90 vs <80 BMI: ≥25.0 vs <23.0 |
42 | Healthy; non-GERD | 987 |
| Sogabe14 | Cohort | Kagawa, Japan | Hospital-based | 2008–2009 | WC; BMI | Binary WC: ≥90 vs <90 BMI: ≥25.0 vs <25.0 |
55 | Men with metabolic syndrome; non-GERD | 210 |
| Tai39 | Cohort | Kaohsiung, Taiwan | Hospital-based | 2007–2009 | WC; WHR; BMI | Continuous WHR: >0.93 vs <0.93 WC: >120 vs <120 BMI: >42 vs <42 |
84 | Bariatric clinic; non-GERD | 176 |
C-C, case-control
Table 3.
Characteristics of Included Studies Assessing the Association Between Central Adiposity and EAC
| Ascertainment of adiposity |
Controls |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Design | Location | Study setting | Time period | Measure | Categories, highest vs lowest (referent) | EAC cases | Definition | N |
| Anderson13 | C-C | Belfast, Ireland | Population-based | 2002–2004 | WHR; BMI | Tertiles WHR: tertiles, NR BMI: >29.0 vs <25.8 |
227 | Population, non-GERD | 260 |
| Beddy55 | C-C | Dublin, Ireland | Hospital-based | 2005–2008 | CT | Continuous WC, WHR, BMI: not applicable |
110 | Patients, non-GERD | 90 |
| Corley56 | C-C | California | Population-baseda | 1964–2006 | WC; BMI | Quartiles WC: ≥25.0 vs <20.0 BMI: ≥30.0 vs <18.0 |
101 | Population, non-GERD | 2800 |
| MacInnis57 | Cohort | Melbourne, Australia | Population-based | 1990–2004 | WHR; WC; BMI | Tertiles WHR: ≥0.95 (for men) or ≥0.80 (for women) vs <0.9 or <0.75 WC: ≥102 (for men) or ≥88 (for women) vs <94 or <80 BMI: ≥30.0 vs <25.0 |
62b | Population, non-GERD | 55 |
| O’Doherty58 | Cohort | 6 states, USA | Population-based | 1995–2006 | WHR; WC; BMI | Quartiles WHR: ≥1.02 (for men) or ≥0.9 (for women) vs <0.88 or <0.73 WC: ≥110.5 (for men) or >99 (for women) vs <86.4 or <70.6 BMI: ≥35.0 vs 18.5–24.9 |
253 | Population, non-GERD | 218,601 |
| Steffen59 | Cohort | Europec | Population-based | 1992–2007 | WHR; WC; BMI | Quintiles WHR: ≥1.01 (for men) or ≥0.88 (for women) vs <0.86 or <0.71 WC: >107.5 (for men) or >96.0 (for women) vs <82.4 or <67.0 BMI: ≥29.2 (for men) or ≥28.8 (for women) vs <23.4 or <21.7 |
88 | Population, non-GERD | 346,466 |
C-C, case-control
From Kaiser-Permanente Northern California population.
Includes 19 cases of gastric cardia cancers.
Prospective cohort of European Prospective Investigation into Cancer & Nutrition (EPIC).
Erosive Esophagitis
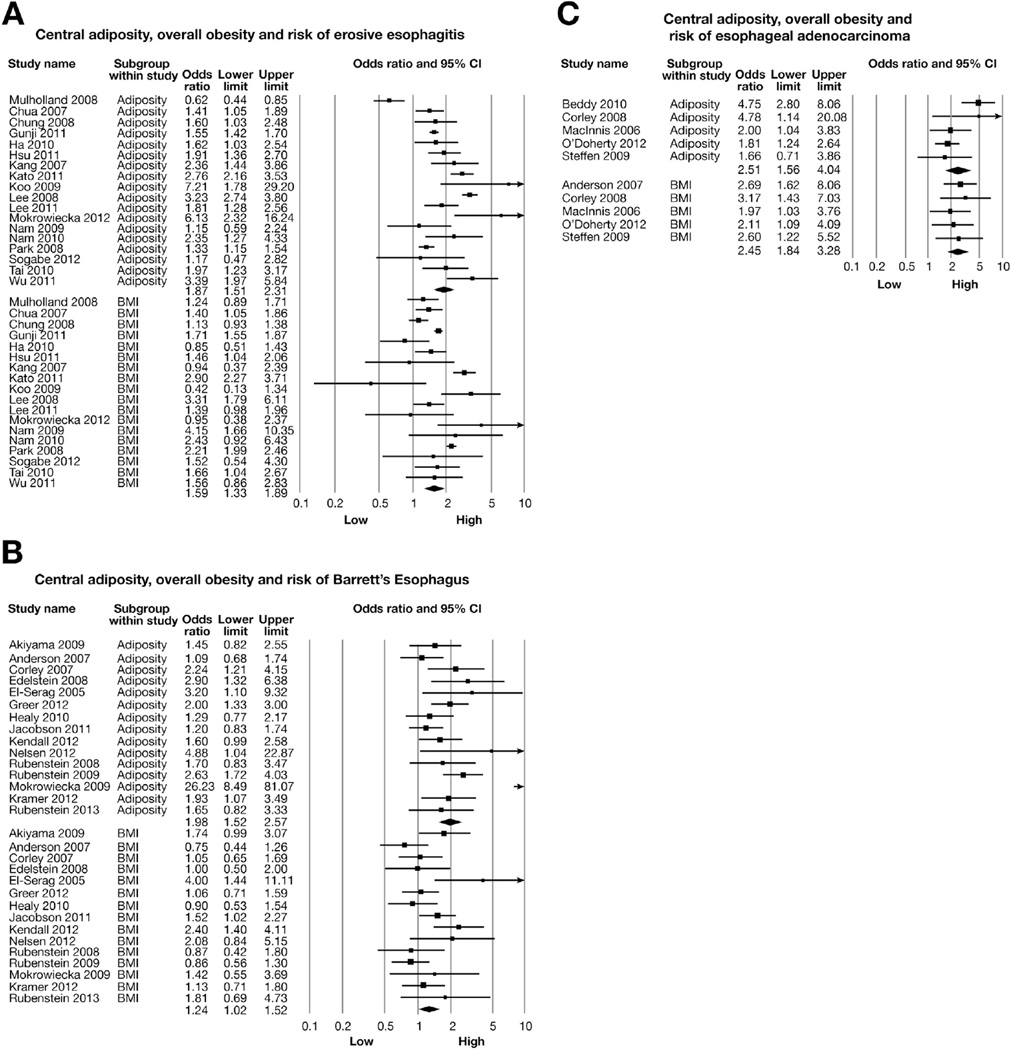
Meta-analysis of 18 independent studies revealed a significantly higher risk of EE with increased central adiposity (highest category of central adiposity) (aOR, 1.87; 95% CI, 1.51–2.31) and with highest category of BMI (aOR, 1.59; 95% CI, 1.33–1.89), compared with lowest category of body habitus and BMI, respectively (Figure 2A). In an analysis restricted to 8 studies that adjusted for overall obesity (BMI),12,14,27,28,30,32,33,37,41 the effect of central adiposity on increased risk of EE persisted (aOR, 1.93; 95% CI, 1.38–2.71). In subgroup analyses, results were stable across study designs, location, and population type, as well as across different measures of central adiposity (Table 4). Each individual measure of central adiposity had an independent significant effect on risk of EE; the association was not significant for subcutaneous adipose tissue area (n = 4 studies; aOR, 1.25; 95% CI, 0.94–1.67). Significant heterogeneity was observed in the overall analysis (Cochran Q, P < .01, I2 = 89%), which was primarily seen in the magnitude of effect and not in the direction of effect and was partly explained by study setting (hospital-based studies reporting higher estimates than population-based studies; P value for difference between groups < .01) (Table 4).
Figure 2.
Central adiposity, overall obesity (BMI), and risk of (A) EE, (B) BE, and (C) EAC. This represents the overall pooled OR by combining categorical OR (for highest category, compared with lowest referent category) with estimated OR from continuous variables.
Table 4.
Subgroup Analysis and Dose-Response Relationship: Central Adiposity and Risk of EE
| Subgroups | Categories | No. of studies | Adjusted OR | 95% CI | P value between groups |
|---|---|---|---|---|---|
| Study-related variables | |||||
| Study location | Asian | 16 | 1.94 | 1.59–2.36 | .97 |
| Western | 2 | 1.85 | 0.20–17.6 | ||
| Study design | Cross-sectional | 8 | 1.90 | 1.54–2.35 | .95 |
| C-C | 7 | 1.86 | 1.15–3.01 | ||
| Cohort | 3 | 2.16 | 1.01–4.60 | ||
| Study setting | Hospital-based | 17 | 2.00 | 1.64–2.44 | <.01a |
| Population-based | 1 | 0.61 | 0.44–0.85 | ||
| Population type | Healthy volunteers | 13 | 1.84 | 1.43–2.37 | .84 |
| Patients | 5 | 1.92 | 1.38–2.68 | ||
| Central adiposity-related variables | |||||
| Adjusted for BMI | Yes | 9 | 1.93 | 1.38–2.71 | .74 |
| No | 9 | 1.80 | 1.38–2.34 | ||
| Reporting adiposityb | Categorical | 13 | 1.76 | 1.28–2.41 | .38 |
| Continuous | 5 | 2.17 | 1.54–3.05 | ||
| Different measures of central adiposity | Visceral adipose tissue area | 4 | 2.08 | 1.28–3.39 | .63 |
| WHR | 7 | 2.04 | 1.13–3.67 | ||
| WC | 14 | 1.66 | 1.43–1.92 | ||
| Dose-response relationship | |||||
| Central adiposity (reported as tertiles/quartiles) | Q2 (vs Q1) | 5 | 1.63 | 0.96–2.77 | .16 |
| Q3/Q4 (vs Q1) | 5 | 2.13 | 1.49–3.04 |
C-C, case-control; Q, quartile.
Statistically significant difference between sub-groups.
Comparison of studies that reported data as categories with studies that reported means and standard deviations of adiposity in cases with EE and controls without EE (unadjusted) and transformed into a binary OR by using a statistical equation.
A trend toward dose-response relationship was observed, with higher levels of central adiposity associated with higher risk of EE (Table 4). Further subgroup analysis that was based on presence or absence of GERD symptoms and genderspecific impact of central adiposity on risk of EE was not possible on the basis of available information. To assess whether any one study had a dominant effect on the metaanalytic OR for risk of EE, each study was excluded, and its effect on the main summary estimate was evaluated. No study dominantly affected the summary estimate or P value for heterogeneity. Inclusion of 2 additional studies published only in the abstract form did not significantly alter the association between central adiposity and EE (aOR, 1.88; 95% CI, 1.54–2.31).62,63
Barrett’s Esophagus
Meta-analysis of 15 independent studies revealed a significantly higher risk of BE with increasing central adiposity (aOR, 1.98; 95% CI, 1.52–2.57), as compared with lowest category of central adiposity. BMI was associated with a borderline significant risk of BE (aOR, 1.24; 95% CI, 1.02–1.52) (Figure 2B). Restricting analysis to studies that adjusted for BMI, the independent effect of central adiposity on increased risk of BE persisted (n = 5 studies; aOR, 1.88; 95% CI, 1.20–2.95).9,13,16,45,52 The results were stable across different measures of central adiposity (visceral adipose tissue area vs WHR vs WC) (Table 5); on the other hand, the association between subcutaneous adipose tissue area and BE was not significant (n = 4 studies; aOR, 1.38; 95% CI, 0.96–1.99). A trend toward dose-response relationship was apparent. The relation between central adiposity was stronger for long-segment BE as compared with shortsegment BE, which was based on pooled analysis of 5 studies.9,10,16,47,53 Significant heterogeneity was observed in the overall analysis (Cochran Q, P < .01, I2 = 66%), which was primarily seen in the magnitude of effect and not in the direction of effect. This was explained by differences in study design (case-control vs cohort, aOR: 2.22 vs 1.27; P value for difference between groups = .05) and method of exposure ascertainment (measured vs selfreported, aOR: 2.08 vs 1.20; P value for difference between groups = .03) (Table 5). No single study markedly affected the overall summary estimate or P value for heterogeneity. There was insufficient information to perform a pooled analysis of effect of central adiposity and risk of dysplasia in patients with BE.
Table 5.
Subgroup Analysis and Dose-Response Relationship: Central Adiposity and Risk of BE
| Subgroups | Categories | No. of studies | Adjusted OR | 95% CI |
P value between groups (Pinteraction) |
|---|---|---|---|---|---|
| Study-related variables | |||||
| Study location | Asian | 1 | 1.45 | 0.82–2.55 | .31 |
| Western | 14 | 2.01 | 1.49–2.72 | ||
| Study design | Cross-sectional | 1 | 1.65 | 0.82–3.33 | .05a |
| C-C | 12 | 2.22 | 1.61–3.06 | ||
| Cohort | 2 | 1.27 | 0.93–1.73 | ||
| Study setting | Hospital-based | 10 | 2.30 | 1.59–3.31 | .10 |
| Population-based | 5 | 1.54 | 1.12–2.11 | ||
| Method of ascertainment | Measured | 14 | 2.08 | 1.58–2.74 | .02a |
| Self-reported | 1 | 1.20 | 0.83–1.74 | ||
| Central adiposity–related variables | |||||
| Adjusted for BMI | Yes | 5 | 1.88 | 1.20–2.95 | .79 |
| No | 10 | 2.03 | 1.45–2.84 | ||
| Reporting adiposityb | Categorical | 9 | 1.56 | 1.25–1.96 | .06 |
| Continuous | 6 | 2.77 | 1.61–4.75 | ||
| Different measures of central adiposity | Visceral adipose tissue area | 4 | 1.78 | 1.19–2.67 | .43 |
| WHR/WTR | 11 | 2.04 | 1.49–2.81 | ||
| WC | 11 | 1.58 | 1.25–1.99 | ||
| Length of BE | |||||
| Long-segment | 5 | 1.96 | 1.39–2.75 | .14 | |
| Short-segment | 5 | 1.34 | 0.93–1.94 | ||
| Dose-response relationship | |||||
| Central adiposity (reported as tertiles/quartiles) | Q2 (vs Q1) | 8 | 1.65 | 1.11–2.44 | .53 |
| Q3/Q4 (vs Q1) | 8 | 1.92 | 1.47–2.50 |
C-C, case-control; Q, quartile; WTR, waist-thigh ratio.
Statistical significance.
Comparison of studies that reported data as categories with studies that reported means and standard deviations of adiposity in cases with BE and controls without BE (unadjusted) and transformed into a binary OR by using a statistical equation.
Reflux-independent Effect of Central Adiposity on Barrett’s Esophagus
When we restricted analysis to studies that used patients with GERD as controls9,11,35,45–47,51 or that adjusted for GERD symptoms,13,50,52,53 the effect of central adiposity on risk of BE persisted (11 studies; aOR, 2.04; 95% CI, 1.44–2.90), whereas no relation was seen between overall obesity and risk of BE (10 studies; aOR, 1.15; 95% CI, 0.89–1.47). Similarly, restricting analysis to studies that compared central adiposity in patients with BE with those with GERD (symptoms and/or endoscopic evidence of EE) but without BE showed that central adiposity was associated with increased risk of BE (7 studies; aOR, 2.51; 95% CI, 1.48–4.25); this effect was again not significant for BMI (7 studies; aOR, 1.23; 95% CI, 0.90–1.66). These results suggest that central adiposity, rather than overall obesity, may have a GERD symptom-independent effect on development of esophageal metaplasia.
Esophageal Adenocarcinoma
Of the 6 studies that reported the association between central adiposity and EAC, in 2 studies,13,55 central adiposity was measured at time of EAC diagnosis, whereas in the other 4 studies, it was measured at least 5 years preceding the diagnosis of EAC. In 1 of the 2 studies,55 the average weight loss since EAC diagnosis was only 1.4%; hence, adiposity assessment was believed to closely reflect pre-diagnosis adiposity; such information on post-diagnosis weight loss was not available for the other study, and it was excluded from further analysis.13
Meta-analysis of these studies revealed a significantly higher risk of EAC with central adiposity (aOR, 2.51; 95% CI, 1.56–4.04) than lowest category of central adiposity (normal body habitus), with substantial heterogeneity (CochranQtest, P = .03, I2 = 62%) (Figure 2C). This relationship was also observed for high BMI and risk of EAC (n = 5 studies; aOR, 2.45; 95% CI, 1.84–3.28). Including one study published only in the abstract form did not alter the relationship between central adiposity and EAC risk (aOR, 2.14; 95% CI, 1.34–3.42).64 Because of the small number of studies, subgroup analysis to understand BMI-independent and GERD-independent effect was not conducted. Data were insufficient to evaluate a dose-response relationship between central adiposity and risk of EAC.
Publication Bias
There was no evidence of significant publication bias observed in the analysis on risk of EE or EAC, both qualitatively by visual inspection of the funnel plot or quantitatively by using the Egger regression test (P value for EE = .72 and for EAC = .67). However, on analysis of the risk of BE with central adiposity, a significant publication bias was observed on visual inspection of the funnel plot (Supplementary Figure 1), as well as with the Egger test (P = .02). Therefore, we performed a sensitivity analysis by using the trim-and-fill method, which conservatively imputes hypothetical negative unpublished studies to mirror the positive studies that cause funnel plot asymmetry.65 The pooled analysis incorporating the hypothetical studies continued to show a statistically significant association between central adiposity and risk of BE (aOR, 1.64; 95% CI, 1.22–2.21).
Discussion
Multiple previous observational studies as well as metaanalyses have noted a strong association between obesity and GERD, BE, and EAC.1–5 In this meta-analysis, we make several key observations. First, we reaffirmed the importance of central adiposity as a key factor in the pathogenesis of EE, BE, and EAC, with consistent results across multiple studies by using multiple different measures of central adiposity (CT assessment of visceral fat [but not subcutaneous fat], measured WHR and WC) and after adjustment for multiple confounders. Second, we observed that central adiposity may have a BMI-independent effect on the risk of EE and BE, further validating the importance of visceral abdominal fat in the pathogenesis of esophageal inflammation and metaplasia. Third, we observed that central adiposity, and not overall obesity, has a GERD-independent effect on the risk of BE. There was insufficient information to assess BMIindependent and GERD-independent effect of central adiposity on EAC. We also observed a trend toward a dose-response relationship between the degree of central adiposity and the risk of EE and BE, further strengthening the possibility of a causative association.
Body fat distribution is thought to play a key role in the pathogenesis of EE, BE, and EAC. Increasing abdominal girth, which is a surrogate for abdominal or visceral fat distribution, can mechanically disrupt the integrity of the gastroesophageal junction barrier and lead to increased esophageal reflux.6,66 Besides direct reflux-induced chronic esophagitis and metaplasia, metabolically active visceral fat may exert systemic as well as paracrine, proinflammatory effects that promote, independently or synergistically, esophageal metaplasia and carcinogenesis.67 These effects may be mediated through proinflammatory cytokines such as tumor necrosis factor-α, interleukin-6,68 and adipokines such as adiponectin11,50 and leptin69,70 released by visceral fat. An adipocytokine-mediated carcinogenic effect of increased visceral fat is also seen with other gastrointestinal malignancies (colon71 and pancreas72). A potential paracrine effect of visceral fat is evident by increased gastroesophageal junction fat area in patients with BE with associated esophagitis and dysplasia, independent of BMI.16 In addition to chronic systemic inflammation, visceral fat also is associated with insulin resistance, and recent studies support the role of the insulin–insulin growth factor-1 axis in promoting esophageal neoplasia.51,73,74 Recent studies have also suggested a higher risk of progression of dysplasia in patients with BE with higher level of central adiposity.16,75 Differential effect of body fat distribution on risk of EAC may also explain the significant sex difference observed in incidence of EAC.4,68 The abdominal fat-predominant apple-shaped body habitus in men versus the predominantly hip and thigh, pear-shaped distribution of fat seen in women may explain the male predominance in the risk of esophageal metaplasia and neoplasia. This gender effect of body fat distribution on risk of EAC could unfortunately not be studied independently in this meta-analysis.
Our review suggests that central adiposity may be associated with BE, independent of GERD. On analysis restricted to studies that accounted for GERD, we observed that the association of central adiposity with BE risk persisted (11 studies; aOR, 2.04; 95% CI, 1.44–2.90), whereas no relation was seen between overall obesity and risk of BE (10 studies; aOR, 1.15; 95% CI, 0.89–1.47). However, the included studies were based on reflux symptoms. It is possible that reflux may still be present in the absence of classical symptoms. Reflux symptoms may be underestimated in patients with EE and particularly BE; we could not be sure the level of reflux injury was similar in these groups. Studies that quantify acid and nonacid reflux are required to further examine the mechanism of obesity-related BE.
The strengths of this analysis include (1) assessment of the association between central adiposity along the spectrum of esophageal inflammation, metaplasia and neoplasia; (2) incorporating the effect of multiple different measures of central adiposity, both collectively and independently, and assessment of a dose-response relationship between central adiposity and esophageal outcomes; (3) subgroup analyses that allowed assessment of BMI-independent and GERD-independent effects of central adiposity; (4) inclusion of all available studies and not restricting analysis on the basis of study design, publication type, or language, and hence being at low risk for selection or publication bias; (5) performance of analyses of maximally adjusted risk estimates reported in the studies to account for the effect of potential confounders; and (6) multiple subgroup analyses to ensure stability of the association and identify potential factors responsible for heterogeneity. Our results are similar to those observed in a recent pooled analysis of 4 studies that showed that WC, and not BMI, was associated with increased risk of BE in both men and women, with a dose-response relationship.76
There were several limitations in our analysis that merit further discussion. First, significant heterogeneity was observed in the overall analysis. However, this heterogeneity was seen primarily in the strength of the association between central adiposity and esophageal outcomes and not in the direction of association. The heterogeneity could be explained by differences in study design, setting, method of exposure ascertainment, and/or differences in reporting central adiposity, as demonstrated through subgroup analyses. Such significant heterogeneity has also been observed in previous meta-analyses assessing the risk of obesity and adverse esophageal diseases.2,3 Second, there was variable adjustment for confounding variables in these studies, especially the effect of BMI and/or GERD. By using prespecified subgroup analysis, however, we were able to estimate the effects of central adiposity after accounting for these key variables. We could not exclude confounding by unmeasured exposures or incomplete control of confounding from measured factors such as diet. Sufficient information was not available to perform subgroup analysis that was based on race. In addition, there was limited information to perform subgroup analysis for EAC. Third, case-control and cross-sectional study designs cannot establish cause and effect. In particular, a temporal association between exposure (central adiposity) and outcome (EE and BE) is not possible to establish, because in most studies, adiposity was assessed at the time of outcome assessment. This is most relevant when studying EAC because cancer can induce weight loss and modify the relation between obesity and cancer through reverse causality. In our analysis, most studies on EAC were cohort studies, and for the one study in which central adiposity was assessed at time of outcome, the extent of cancer-induced weight loss was minimal. Last, a statistically significant publication bias was observed in analysis of central adiposity and risk of BE. However, because of the strong evidence in favor of biological plausibility, the strength of association observed with a potential dose-response relationship, by using multiple different measures of central adiposity, the clinical significance of this publication bias is probably low.
In conclusion, central adiposity has a strong and consistent association with development of esophageal inflammation, metaplasia and neoplasia, independent of BMI. In addition, central adiposity may be more highly associated with a refluxindependent effect on the development of BE and perhaps explains the predominance of EAC in this population. Future studies aimed at understanding the mechanistic effect of obesity on esophageal inflammation and neoplasia should focus on visceral fat rather than overall obesity. The effect of interventions aimed at favorably modifying body fat distribution on the risk of BE and EAC should be studied.
Supplementary Material
Table 2.
Characteristics of Included Studies Assessing the Association Between Central Adiposity and BE
| Ascertainment of adiposity |
Controls |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Design | Location | Study setting | Time period | Measure | Categories, highest vs lowest (referent) |
BE cases | Definition | N |
| Rubenstein52 | Cross-sectional | Michigan | Hospital-based | 2008–2011 | WHR; WC; BMI | Tertiles WHR: >1.024 vs <0.979 WC: >108 vs ≤108 BMI: ≥30.0 vs ≤20.0–25.0 |
70 | Colorectal cancer screenees; non-GERD | 751 |
| Anderson13 | C-C | Belfast, Ireland | Population-based | 2002–2004 | WHR; BMI | Tertiles WHR: Tertiles, NR BMI: >29.0 vs <25.8 |
224 | Healthy; non-GERD | 260 |
| Corley9 | C-C | California | Population-baseda | 2002–2005 | WTR; WC; BMI | Deciles/binary WTR: >1.7 vs <1.7 WC: ≥ 130.0 vs <80.0 BMI: >35.0 vs <25.0 |
329 |
|
317 316 |
| Edelstein10,45 | C-C | Washington | Population-basedb | 1997–2000 | WHR; WTR; WC; BMI | Tertiles WHR: ≥0.9 (for men) or ≥0.85 (for women) vs <0.9 or <0.85 WC: ≥102 (for men) or ≥88 (for women) vs <94 or <79 BMI: ≥30 vs 25 |
97c |
|
211 419 |
| El-Serag46 | C-C | Texas | Hospital-based | 2000–2003 | CT; BMI | Continuous BMI: >30 vs <25 |
36 | Hospitalized patientsd; mixed GERD/non-GERD | 93 |
| Greer51 | C-C | Ohio | Hospital-based | 2005–2009 | WHR; BMI | Continuous WHR: >0.98 vs <0.98 BMI: >30 vs <30 |
135 |
|
182 135 |
| Healy47 | C-C | Dublin, Ireland | Hospital-based | NR | WC; BMI | Binary WC: >94 (for men) or >80 (for women) vs ≤94 or ≤80 BMI: >28.2 vs <28.2 |
118 | Patients; GERD | 113 |
| Kendall49 | C-C | Brisbane, Australia | Population-based | 2003–2009 | WHR; WC; BMI | Tertiles WHR and WC: tertiles, NR BMI: ≥30.0 vs 20.0–25.0 |
237 | Healthy; non-GERD | 247 |
| Kramer53,54 | C-C | Texas | Hospital-based | 2008–2011 | CT; WHR; WC; BMI | Binaryf;tertiles WHR: ≥0.9 (for men) or ≥0.85 (for women) vs <0.9 or <0.85 WC: ≥117.6 vs <99.5 BMI: ≥30.0 vs <25.0 |
237 | Patients; “Endoscopy” and “Colonoscopy” controls | 1500 |
| Mokrowiecka35 | C-C | Lodz, Poland | Hospital-based | 2009–2011 | WHR; WC; BMI | Continuous WHR: >0.65 vs <0.65 WC: >83 vs <83 BMI: >25 vs <25 |
47 | Patients; non-GERD | 30 |
| Nelsen16 | C-C | Minnesota | Hospital-based | 2009 | CT; WC; BMI | Binary WC: ≥97.8 vs <97.8 BMI: ≥30.0 vs <30.0 |
50 | Patients; non-GERD | 50 |
| Rubenstein50 | C-C | Michigan | Hospital-based | NR | WHR; WC; BMI | Continuous NR |
60 | Patients; mixed GERD and non-GERD | 60 |
| Rubenstein11 | C-C | North Carolina | Hospital-based | 2002–2007 | WHR; WC; BMI | Continuous WHR: >0.90 vs <0.90 WC: >93.0 vs <93.0 BMI: >28.5 vs <28.5 |
112 | Patients; GERD | 199 |
| Akiyama44 | Cohort | Yokohama, Japan | Hospital-based | 2003–2009 | CT; BMI | Continuous BMI: >25.0 vs <25.0 |
69 | NAFLD patients; non-GERD | 94 |
| Jacobson48 | Cohort | USA | Population-basede | 1986–2004 | WHR; WC; BMI | Quartiles WHR: ≥0.82 vs ≤0.73 WC: ≥86.4 vs ≤68.6 BMI: >30.0 vs <25.0 |
261 | Healthy women; non-GERD | 15,600 |
C-C case-control; NAFLD, nonalcoholic fatty liver disease; WTR, waist-thigh ratio.
From Kaiser-Permanente Northern California population.
Community gastroenterology clinics.
193 patients with squamous intestinal metaplasia, but only 97 with both endoscopic and histologic diagnosis.
Able to do separate subgroup analysis comparing patients with BE and patients with GERD leading to EE.
Prospective Nurses’ Health Study.
CT assessment of visceral adipose tissue surface area reported as both binary (for GERD + stratified analysis) and tertiles (for GERD-nonadjusted, used in assessing dose-response relationship).
Acknowledgments
Funding
Supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (RC4DK090413) and the American College of Gastroenterology.
Abbreviations used in this paper
- aOR
adjusted odds ratio
- BE
Barrett’s esophagus
- BMI
body mass index
- CI
confidence interval
- CT
computed tomography
- EAC
esophageal adenocarcinoma
- EE
erosive esophagitis
- GERD
gastroesophageal reflux disease
- OR
odds ratio
- RR
relative risk
- WC
waist circumference
- WHR
waist-hip ratio
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2013.05.009.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Cook MB, Greenwood DC, Hardie LJ, et al. A systematic review and meta-analysis of the risk of increasing adiposity on Barrett’s esophagus. Am J Gastroenterol. 2008;103:292–300. doi: 10.1111/j.1572-0241.2007.01621.x. [DOI] [PubMed] [Google Scholar]
- 2.Corley DA, Kubo A. Body mass index and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:2619–2628. doi: 10.1111/j.1572-0241.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- 3.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 4.Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat Rev Gastroenterol Hepatol. 2011;8:340–347. doi: 10.1038/nrgastro.2011.73. [DOI] [PubMed] [Google Scholar]
- 5.Turati F, Tramacere I, La Vecchia C, et al. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol. 2013;24:609–617. doi: 10.1093/annonc/mds244. [DOI] [PubMed] [Google Scholar]
- 6.Pandolfino JE, El-Serag HB, Zhang Q, et al. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology. 2006;130:639–649. doi: 10.1053/j.gastro.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Friedenberg FK, Xanthopoulos M, Foster GD, et al. The association between gastroesophageal reflux disease and obesity. Am J Gastroenterol. 2008;103:2111–2122. doi: 10.1111/j.1572-0241.2008.01946.x. [DOI] [PubMed] [Google Scholar]
- 8.Moayyedi P. Barrett’s esophagus and obesity: the missing part of the puzzle. Am J Gastroenterol. 2008;103:301–303. doi: 10.1111/j.1572-0241.2007.01618.x. [DOI] [PubMed] [Google Scholar]
- 9.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett’s esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. quiz 311. [DOI] [PubMed] [Google Scholar]
- 10.Edelstein ZR, Farrow DC, Bronner MP, et al. Central adiposity and risk of Barrett’s esophagus. Gastroenterology. 2007;133:403–411. doi: 10.1053/j.gastro.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Rubenstein JH, Kao JY, Madanick RD, et al. Association of adiponectin multimers with Barrett’s oesophagus. Gut. 2009;58:1583–1589. doi: 10.1136/gut.2008.171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nam SY, Choi IJ, Ryu KH, et al. Abdominal visceral adipose tissue volume is associated with increased risk of erosive esophagitis in men and women. Gastroenterology. 2010;139:1902–1911. doi: 10.1053/j.gastro.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Anderson LA, Watson RG, Murphy SJ, et al. Risk factors for Barrett’s oesophagus and oesophageal adenocarcinoma: results from the FINBAR study. World J Gastroenterol. 2007;13:1585–1594. doi: 10.3748/wjg.v13.i10.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sogabe M, Okahisa T, Kimura Y, et al. Visceral fat predominance is associated with erosive esophagitis in Japanese men with metabolic syndrome. Eur J Gastroenterol Hepatol. 2012;24:910–916. doi: 10.1097/MEG.0b013e328354a354. [DOI] [PubMed] [Google Scholar]
- 15.Tilg H, Moschen AR. Visceral adipose tissue attacks beyond the liver: esophagogastric junction as a new target. Gastroenterology. 2010;139:1823–1826. doi: 10.1053/j.gastro.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 16.Nelsen EM, Kirihara Y, Takahashi N, et al. Distribution of body fat and its influence on esophageal inflammation and dysplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2012;10:728–734. doi: 10.1016/j.cgh.2012.03.007. quiz e61–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT. Cochrane handbook for systematic reviews of interventions. Oxford, UK: The Cochrane Collaboration; 2011. [updated March 2011] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 19.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19:3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Kanwal F, White D. Systematic reviews and meta-analyses in Clinical Gastroenterology and Hepatology. Clin Gastroenterol Hepatol. 2012;10:1184–1186. doi: 10.1016/j.cgh.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson SG, Pocock SJ. Can meta-analyses be trusted? Lancet. 1991;338:1127–1130. doi: 10.1016/0140-6736(91)91975-z. [DOI] [PubMed] [Google Scholar]
- 24.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7—rating the quality of evidence: inconsistency. J Clin Epidemiol. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Easterbrook PJ, Berlin JA, Gopalan R, et al. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 27.Chua CS, Lin YM, Yu FC, et al. Metabolic risk factors associated with erosive esophagitis. J Gastroenterol Hepatol. 2009;24:1375–1379. doi: 10.1111/j.1440-1746.2009.05858.x. [DOI] [PubMed] [Google Scholar]
- 28.Chung SJ, Kim D, Park MJ, et al. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: a cross-sectiona case-control study of 7078 Koreans undergoing health check-ups. Gut. 2008;57:1360–1365. doi: 10.1136/gut.2007.147090. [DOI] [PubMed] [Google Scholar]
- 29.Gunji T, Sato H, Iijima K, et al. Risk factors for erosive esophagitis: a cross-sectional study of a large number of Japanese males. J Gastroenterol. 2011;46:448–455. doi: 10.1007/s00535-010-0359-5. [DOI] [PubMed] [Google Scholar]
- 30.Ha NR, Lee HL, Lee OY, et al. Differences in clinical characteristics between patients with non-erosive reflux disease and erosive esophagitis in Korea. J Korean Med Sci. 2010;25:1318–1322. doi: 10.3346/jkms.2010.25.9.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu CS, Wang PC, Chen JH, et al. Increasing insulin resistance is associated with increased severity and prevalence of gastrooesophageal reflux disease. Aliment Pharmacol Ther. 2011;34:994–1004. doi: 10.1111/j.1365-2036.2011.04817.x. [DOI] [PubMed] [Google Scholar]
- 32.Koo JS, Lee SW, Park SM, et al. Abdominal obesity as a risk factor for the development of erosive esophagitis in subjects with a normal esophago-gastric junction. Gut Liver. 2009;3:276–284. doi: 10.5009/gnl.2009.3.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HL, Eun CS, Lee OY, et al. Association between GERD-related erosive esophagitis and obesity. J Clin Gastroenterol. 2008;42:672–675. doi: 10.1097/MCG.0b013e31806daf64. [DOI] [PubMed] [Google Scholar]
- 34.Lee HL, Eun CS, Lee OY, et al. Association between erosive esophagitis and visceral fat accumulation quantified by abdominal CTscan. J Clin Gastroenterol. 2009;43:240–243. doi: 10.1097/MCG.0b013e318167b88a. [DOI] [PubMed] [Google Scholar]
- 35.Mokrowiecka A, Daniel P, Jasinska A, et al. Serum adiponectin, resistin, leptin concentration and central adiposity parameters in Barrett’s esophagus patients with and without intestinal metaplasia in comparison to healthy controls and patients with GERD. Hep-atogastroenterology. 2012;59:2395–2399. doi: 10.5754/hge12587. [DOI] [PubMed] [Google Scholar]
- 36.Mulholland HG, Cantwell MM, Anderson LA, et al. Glycemic index, carbohydrate and fiber intakes and risk of reflux esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma. Cancer Causes Control. 2009;20:279–288. doi: 10.1007/s10552-008-9242-6. [DOI] [PubMed] [Google Scholar]
- 37.Nam SY, Choi IJ, Nam BH, et al. Obesity and weight gain as risk factors for erosive oesophagitis in men. Aliment Pharmacol Ther. 2009;29:1042–1052. doi: 10.1111/j.1365-2036.2009.03965.x. [DOI] [PubMed] [Google Scholar]
- 38.Park JH, Park DI, Kim HJ, et al. Metabolic syndrome is associated with erosive esophagitis. World J Gastroenterol. 2008;14:5442–5447. doi: 10.3748/wjg.14.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tai CM, Lee YC, Tu HP, et al. The relationship between visceral adiposity and the risk of erosive esophagitis in severely obese Chinese patients. Obesity (Silver Spring) 2010;18:2165–2169. doi: 10.1038/oby.2010.143. [DOI] [PubMed] [Google Scholar]
- 40.Wu P, Ma L, Dai GX, et al. The association of metabolic syndrome with reflux esophagitis: a case-control study. Neurogastroenterol Motil. 2011;23:989–994. doi: 10.1111/j.1365-2982.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- 41.Kang MS, Park DI, Oh SY, et al. Abdominal obesity is an independent risk factor for erosive esophagitis in a Korean population. J Gastroenterol Hepatol. 2007;22:1656–1661. doi: 10.1111/j.1440-1746.2006.04518.x. [DOI] [PubMed] [Google Scholar]
- 42.Kato M, Watabe K, Hamasaki T, et al. Association of low serum adiponectin levels with erosive esophagitis in men: an analysis of 2405 subjects undergoing physical check-ups. J Gastroenterol. 2011;46:1361–1367. doi: 10.1007/s00535-011-0453-3. [DOI] [PubMed] [Google Scholar]
- 43.Lee SJ, Jung MK, Kim SK, et al. [Clinical characteristics of gastroesophageal reflux disease with esophageal injury in Korean: focusing on risk factors] Korean J Gastroenterol. 2011;57:281–287. doi: 10.4166/kjg.2011.57.5.281. [DOI] [PubMed] [Google Scholar]
- 44.Akiyama T, Yoneda M, Inamori M, et al. Visceral obesity and the risk of Barrett’s esophagus in Japanese patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2009;9:56. doi: 10.1186/1471-230X-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edelstein ZR, Bronner MP, Rosen SN, et al. Risk factors for Barrett’s esophagus among patients with gastroesophageal reflux disease: a community clinic-based case-control study. Am J Gastroenterol. 2009;104:834–842. doi: 10.1038/ajg.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Serag HB, Kvapil P, Hacken-Bitar J, et al. Abdominal obesity and the risk of Barrett’s esophagus. Am J Gastroenterol. 2005;100:2151–2156. doi: 10.1111/j.1572-0241.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 47.Healy LA, Ryan AM, Pidgeon G, et al. Lack of differential pattern in central adiposity and metabolic syndrome in Barrett’s esophagus and gastroesophageal reflux disease. Dis Esophagus. 2010;23:386–391. doi: 10.1111/j.1442-2050.2010.01052.x. [DOI] [PubMed] [Google Scholar]
- 48.Jacobson BC, Chan AT, Giovannucci EL, et al. Body mass index and Barrett’s oesophagus in women. Gut. 2009;58:1460–1466. doi: 10.1136/gut.2008.174508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kendall BJ, Macdonald GA, Hayward NK, et al. The risk of Barrett’s esophagus associated with abdominal obesity in males and females. Int J Cancer. 2013;132:2192–2199. doi: 10.1002/ijc.27887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubenstein JH, Dahlkemper A, Kao JY, et al. A pilot study of the association of low plasma adiponectin and Barrett’s esophagus. Am J Gastroenterol. 2008;103:1358–1364. doi: 10.1111/j.1572-0241.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 51.Greer KB, Thompson CL, Brenner L, et al. Association of insulin and insulin-like growth factors with Barrett’s oesophagus. Gut. 2012;61:665–672. doi: 10.1136/gutjnl-2011-300641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubenstein JH, Morgenstern H, Appelman H, et al. Prediction of Barrett’s esophagus among men. Am J Gastroenterol. 2013;108:353–362. doi: 10.1038/ajg.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Serag HB, Hashmi A, Garcia J, et al. Visceral abdominal obesity measured by CT scan is associated with an increased risk of Barrett’s oesophagus: a case-control study. Gut. 2013 Feb 13; doi: 10.1136/gutjnl-2012-304189. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kramer JR, Fischbach LA, Richardson P, et al. Waist-to-hip ratio, but not body mass index, is associated with an increased risk of Barrett’s esophagus in white men. Clin Gastroenterol Hepatol. 2013;11:373–381. doi: 10.1016/j.cgh.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beddy P, Howard J, McMahon C, et al. Association of visceral adiposity with oesophageal and junctional adenocarcinomas. Br J Surg. 2010;97:1028–1034. doi: 10.1002/bjs.7100. [DOI] [PubMed] [Google Scholar]
- 56.Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:352–358. doi: 10.1158/1055-9965.EPI-07-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacInnis RJ, English DR, Hopper JL, et al. Body size and composition and the risk of gastric and oesophageal adenocarcinoma. Int J Cancer. 2006;118:2628–2631. doi: 10.1002/ijc.21638. [DOI] [PubMed] [Google Scholar]
- 58.O’Doherty MG, Freedman ND, Hollenbeck AR, et al. A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH-AARP Diet and Health Study. Gut. 2012;61:1261–1268. doi: 10.1136/gutjnl-2011-300551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steffen A, Schulze MB, Pischon T, et al. Anthropometry and esophageal cancer risk in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2009;18:2079–2089. doi: 10.1158/1055-9965.EPI-09-0265. [DOI] [PubMed] [Google Scholar]
- 60.Thompson OM, Beresford SA, Kirk EA, et al. Serum leptin and adiponectin levels and risk of Barrett’s esophagus and intestinal metaplasia of the gastroesophageal junction. Obesity (Silver Spring) 2010;18:2204–2211. doi: 10.1038/oby.2009.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kubo A, Levin TR, Block G, et al. Cigarette smoking and the risk of Barrett’s esophagus. Cancer Causes Control. 2009;20:303–311. doi: 10.1007/s10552-008-9244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leggett C, Dunagan KT, Katzka DA, et al. Influence of central obesity on esophageal injury: a population based study. Gastroenterology. 2012;142 S-759. [Google Scholar]
- 63.Rubenstein JH, Chey WD, Murray JA, et al. Acid reflux, erosive esophagitis, and Barrett’s esophagus are associated with different measures of abdominal obesity in men. Gastroenterology. 2012;142 S-754. [Google Scholar]
- 64.Cooper SC, Podmore LK, Nightingale P, et al. The effect of smoking, body mass index, and waist circumference on the development of oesophageal adenocarcinoma: results from MOSES (Midlands Oesophageal Adenocarcinoma Epidemiology Study) Gastroenterology. 2009;136:A456. [Google Scholar]
- 65.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 66.El-Serag HB, Ergun GA, Pandolfino J, et al. Obesity increases oesophageal acid exposure. Gut. 2007;56:749–755. doi: 10.1136/gut.2006.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryan AM, Duong M, Healy L, et al. Obesity, metabolic syndrome and esophageal adenocarcinoma: epidemiology, etiology and new targets. Cancer Epidemiol. 2011;35:309–319. doi: 10.1016/j.canep.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Kendall BJ, Macdonald GA, Hayward NK, et al. Leptin and the risk of Barrett’s oesophagus. Gut. 2008;57:448–454. doi: 10.1136/gut.2007.131243. [DOI] [PubMed] [Google Scholar]
- 70.Francois F, Roper J, Goodman AJ, et al. The association of gastric leptin with oesophageal inflammation and metaplasia. Gut. 2008;57:16–24. doi: 10.1136/gut.2007.131672. [DOI] [PubMed] [Google Scholar]
- 71.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a metaanalysis of prospective studies. Am J Clin Nutr. 2007;86:556–565. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 72.Aune D, Greenwood DC, Chan DS, et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:843–852. doi: 10.1093/annonc/mdr398. [DOI] [PubMed] [Google Scholar]
- 73.Doyle SL, Donohoe CL, Finn SP, et al. IGF-1 and its receptor in esophageal cancer: association with adenocarcinoma and visceral obesity. Am J Gastroenterol. 2012;107:196–204. doi: 10.1038/ajg.2011.417. [DOI] [PubMed] [Google Scholar]
- 74.McElholm AR, McKnight AJ, Patterson CC, et al. A population-based study of IGF axis polymorphisms and the esophageal inflammation, metaplasia, adenocarcinoma sequence. Gastroenterology. 2010;139:204–212. doi: 10.1053/j.gastro.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 75.Hardikar S, Onstad L, Blount PL, et al. The role of tobacco, alcohol, and obesity in neoplastic progression to esophageal adenocarcinoma: a prospective study of Barrett’s esophagus. PLoS One. 2013;8:e52192. doi: 10.1371/journal.pone.0052192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kubo A, Cook MB, Shaheen NJ, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s oesophagus: a pooled analysis from the international BEACON consortium. Gut. 2013 Jan 26; doi: 10.1136/gutjnl-2012-303753. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.