Abstract
Studies of human genetic disorders and mouse models reveal the important roles of matriptase in hair growth. Here, we investigate matriptase expression and zymogen activation in hair follicles. We show: 1) layer-dependent distribution patterns, with much higher matriptase expression in cells of the outer root sheath and matrix cells of the hair bulb than in cells of the inner root sheath; 2) cycle-dependent expression patterns, with matriptase expressed in the anagen and catagen phases of the hair lifecycle, but not in the telogen phase; 3) reduced expression of the matriptase inhibitor, HAI-1, in the catagen phase, suggesting increased proteolytic activity in this phase; and 4) definitive matriptase zymogen activation patterns, with the highest matriptase activation observed in matrix cells and outer root sheath cells in the isthmus/bulge region. In sebaceous glands, matriptase is highly expressed in basal and ductal cells, with much lower expression in the differentiated, lipid-filled cells of the interior. We also show that matriptase potently activates hepatocyte growth factor (HGF) in vitro, and that the HGF receptor, c-Met, is co-expressed in those cells that express activated matriptase. Our observations suggest that the matriptase-HGF-c-MET pathway has the potential to be engaged, primarily in proliferative cells rather than terminally differentiated epithelial cells of the human pilosebaceous unit.
Keywords: matriptase, serine protease, zymogen activation, HAI-1, Kunitz-type protease inhibitor, hair follicles
Introduction
Many lines of evidence indicate that the type 2 transmembrane serine protease matriptase is important for hair follicle growth and cycling. Firstly, two genetic disorders linked to mutations in the matriptase gene—autosomal recessive ichthyosis with hypotrichosis (ARIH, OMIM 610765), and ichthyosis, follicular atrophoderma, hypotrichosis and hypohidrosis (IFAH, OMIM 602400)—are associated with defects in hair distribution and structure (Alef et al., 2009; Avrahami et al., 2008; Basel-Vanagaite et al., 2007). Apart from having characteristic icthyotic skin, ARIH and IFAH patients also exhibit a receding frontal hairline with slow growing hair that appears curly, brittle, dry, and lusterless. The second line of evidence is that matriptase can activate several growth factors through its potent trypsin-like proteolytic activity (Bhatt et al., 2007; Lee et al., 2000; Takeuchi et al., 2000; Ustach et al., 2010); some of these growth factors, such as hepatocyte growth factor (HGF) and its membrane receptor c-Met, have been shown to be important in the regulation of hair follicle growth and morphogenesis (Lindner et al., 2000). Thus, matriptase might exert an effect on hair follicles via such downstream targets. Thirdly, matriptase can also activate other serine proteases, including the urokinase-type plasminogen activator (uPA) (Chen et al., 2010b; Lee et al., 2000; Netzel-Arnett et al., 2006; Takeuchi et al., 2000). Activation of the uPA/plasmin system by matriptase could impact extracellular matrix (ECM) remodeling processes, which are known to modulate processes important in normal hair biology, such as involution and downward growth (Gao et al., 2010; Jarrousse et al., 2001; Paus et al., 1994; Weinberg et al., 1990). And lastly, mice with targeted deletion of the matriptase gene exhibit hypoplasia and dysgenesis of the hair follicles, which results in the absence of erupted vibrissal hair shafts and vibrissal hair cannels at birth (List et al., 2002). Together, these findings provide strong evidence for a role of matriptase in hair biology.
Matriptase is synthesized as a zymogen with very weak intrinsic proteolytic activity (Benaud et al., 2001; Inouye et al., 2009). The matriptase zymogen undergoes autoactivation to gain its potent trypsin-like activity (Oberst et al., 2003; Xu et al., 2012). Zymogen activation is tightly controlled in the normal human epidermis and is elevated in several distinct skin diseases that are commonly associated with inflammation (Chen et al., 2011). Matriptase activity is regulated by multiple serine protease inhibitors, among which HGF Activator Inhibitor (HAI)-1 is the predominant inhibitor found in epithelial cells (Xu et al., 2012). Although present at very low levels, activated matriptase has been detected in complexes with three serpin-type inhibitors, including antithrombin in human milk, and the results indicate that epithelial cells could, at least in part, be the source of the milk-derived matriptase-serpin complexes (Chou et al., 2011; Tseng et al., 2008). The role of antithrombin in the control of matriptase activity appears to be augmented in human keratinocytes relative to other epithelial systems. In keratinocytes, the levels of antithrombin bound to the cell surface is inversely correlated with the levels of active matriptase shed to the extracellular milieu (Chen et al., 2013b). In the mouse, HAI-2 has been reported to be involved in the control of matriptase (Szabo et al., 2008). It remains to be determined whether HAI-2 is a relevant matriptase inhibitor in humans, because endogenous matriptase-HAI-2 complexes have not as yet been detected in human body fluids or human cells. In addition to its function in the suppression of matriptase proteolytic activity, HAI-1 plays an important chaperone-like role that is necessary for intracellular trafficking of matriptase to the cell surface (Oberst et al., 2005) and is involved in matriptase zymogen activation (Oberst et al., 2003). Thus, in addition to the absolute level of matriptase expression, the ratio between matriptase and HAI-1 expression levels and the state of zymogen activation are important parameters to fully assess matriptase activity and function.
In human skin, matriptase contributes to epidermal homeostasis primarily via its role in the proliferation and early differentiation of the keratinocytes of the basal and spinous layers, but it does not have a role in the late stage differentiation of the granular layer (Chen et al., 2013a). Because the pilosebaceous unit of the skin shares a similar scheme for the maintenance of cellular homeostasis through control of cell proliferation and differentiation, as in the epidermis, we wondered if matriptase might play a similar role in this system. In the current study, we explore the hypothesis that matriptase is primarily involved in the proliferation and early differentiation of keratinocytes in the pilosebaceous unit of human skin by using immunohistochemistry to examine the expression and activation state of matriptase in human hair follicles at differing phases of their growth cycle, as well as in sebaceous glands. We also examined the expression of HAI-1 and the c-Met in hair follicles to investigate the regulatory and functional aspects of matriptase in the pilosebaceous unit.
Material and Methods
Reagents
Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Kirkegaard & Perry Laboratories (Gaithersburg, MD). Western Lightning Chemiluminescence Reagent Plus was purchased from PerkinElmer Life Sciences (Waltham, MA). Nitrocellulose membrane was purchased from Pall Corp. (Pensacola, FL). Active matriptase was generated and purified from human keratinocyte HaCaT cells, as described in our previous study (Chen et al., 2013a), and pro-HGF was prepared as described previously (Kataoka et al., 2000).
Antibodies
The monoclonal antibodies (mAbs), including M24, M32, M69, and M19, were generated using matriptase-HAI-1 complex as an immunogen, as described previously (Lin et al., 1999). The total matriptase mAbs M24 and M32 recognize both latent and activated forms of human matriptase. M69 mAb is specific for activated matriptase and does not recognize latent matriptase, and M19 mAb specifically recognizes human HAI-1 (Chen et al., 2010b; Lin et al., 1997; Lin et al., 1999; Tseng et al., 2010). HGF protein was detected using a goat polyclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) that recognizes the beta subunit of HGF.
Immunohistochemistry
Immunohistochemical staining was performed as previously described (Chen et al., 2011; Chen et al., 2010a). Tissue sections of frozen human skin were fixed with formalin and stained using the total matriptase mAb M32 and/or M24, activated matriptase mAb M69, or HAI-1 mAb M19, and followed by the secondary antibody (EnVision+ Dual Link System Peroxidase) (Dako, Glostrup, Denmark). DAB (3,3’-diaminobenzidine) was used for the detection of positive staining. Cell nuclei were counterstained with hematoxylin. The staining results were scored by an experienced pathologist (HSL). Images were captured using an Olympus AH2 Vanox Microscope System (Olympus, Melville, NY). The skin tissue sections were obtained with written informed consent from Tri-Service General Hospital, National Defense Medical Center under the IRB 099-05-019, approved by TSGHIRB. The specificity of these mAbs and their applications in immunohistochemical staining can be found in our previous studies (Benaud et al., 2001; Chen et al., 2011; Lin et al., 1999; Tseng et al., 2010; Wang et al., 2009). Mouse IgG was used as a negative control and the well-characterized staining at intercellular junctions and the distribution profiles in the epidermal layers (Chen et al., 2013a) were used as criteria for the quality of the immunohistochemical staining of the hair follicles.
Activation of Pro-HGF
Five ng of pro-HGF was incubated with increasing amounts of active matriptase in a 96-well plate at 37C for 30 min. The samples were then assayed by immunoblot analyses to detect cleavage products of pro-HGF using the HGF beta subunit polyclonal antibody.
Results
Matriptase Is Expressed at High Levels on the Cell Surface of Outer Root Sheath Keratinocytes and Matrix Cells in Human Anagen Hair Follicles
The hair follicle is a regenerating mini-organ that cycles through the following four phases: growth (anagen), regression (catagen), rest (telogen), and shedding (exogen). Based on its cycling characteristics, a hair follicle can be divided into two structural components: 1) the permanent, superficial structure, which includes the infundibulum and the isthmus; and 2) the transient cycling component, which includes the hair bulb and stem. In humans, hair follicles grow asynchronously, with about 85% of the follicles in the anagen-phase.
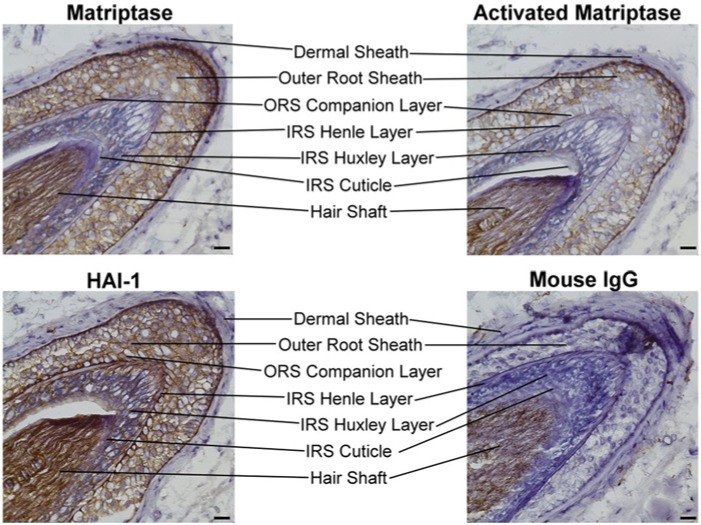
We immunostained anagen-phase hair follicles with monoclonal antibodies (mAbs) specific for total matriptase or HAI-1 to examine the expression profile of each protein. A total of 115 human skin specimens were examined from which 56 hair follicles were identified. Fifty-one were at anagen, three at catagen, and two at telogen phases. The staining of matriptase and HAI-1 were consistent among these specimens. A cross-section of a representative hair follicle stained with each mAb is shown in Figure 1. The outer root sheath (ORS) and the inner root sheath (IRS) layers are indicated.
Figure 1.
Expression of matriptase and HAI-1 in anagen-phase human hair follicles: cross-sectional analyses. Cross sections of anagen-phase human hair follicles were immunostained with the matriptase-specific mAb M32 (Matriptase), the HAI-1-specific mAb M19 (HAI-1) or the activated matriptase-specific mAb M69 (Activated Matriptase). Sections were stained with a non-specific mouse IgG antibody as a negative control (Mouse IgG). Representative examples of the staining observed are presented. The different cell layers of the hair follicle are as indicated. Nuclei of cells were counterstained blue with hematoxylin. Scale bar: 25 µm.
An inspection of Figure 1 revealed that matriptase is highly expressed on the surface of the cells in the ORS. ORS cells provide a protective encasement for the growing hair shaft and are likely derived from the downward migration of bulge stem cells (Panteleyev et al., 2001). In contrast, matriptase expression was much lower in the IRS. The IRS arises from the peripheral portion of undifferentiated matrix cells and contains three concentric cellular layers: the Henle layer, the Huxley layer, and the IRS cuticle. These three layers undergo terminal differentiation via temporally and spatially distinct processes along the longitudinal axis of growth (Ito, 1988). Both the Henle layer and IRS cuticle appeared negative for matriptase expression, with only the Huxley layer staining lightly positive for matriptase. It should be noted that, although the hair shaft stained positive for matriptase, it also stained positive for each of the other antibodies we tested, including the control mouse IgG (Fig. 1). This observation suggests that the staining of the hair shaft results from non-specific binding of the antibodies to the hair shaft. Consequently, the expression of matriptase (or HAI-1) within the hair shaft could not be defined with our assay.
The expression of the cognate inhibitor of matriptase, HAI-1, was detected at high levels on the surface of the cells of both the ORS and IRS (Fig. 1). This expression pattern is in contrast to the expression of matriptase, which was found primarily in cells of the ORS, but not the IRS. This differential expression pattern between matriptase and HAI-1 suggests a functional decoupling between the protease and the protease inhibitor in the more differentiated keratinocytes of the IRS, reminiscent of our observations of matriptase and HAI-1 expression in the human epidermis (Chen et al., 2011; Chen et al., 2013a).
Matriptase and HAI-1 expression patterns were examined throughout the hair follicle by examining longitudinal sections of immunostained hair follicles (Fig. 2). High levels of matriptase and HAI-1 expression were observed in the entire hair follicle, extending from the junctions that connect with the epidermis to the hair bulb at which the ORS tapers and ends. The superficial part of the infundibulum is lined by epidermis with well-developed stratum corneum and stratum granulosum. Matriptase was detected in the keratinocytes of the layers linked to the dermal sheath but not in the central layers linked to the hair shaft. This observation is consistent with our previously reported results that revealed high levels of matriptase expression in the less-differentiated keratinocytes of the basal and spinous layers, with reduced and ultimately no expression in the more-differentiated granular and cornified cells of the epidermis (Chen et al., 2013a). Matriptase expression was also detected at high levels in the stem and hair bulb of the hair follicle, albeit with slightly reduced expression in the lower isthmus and the upper region of the stem. At higher magnification (Fig. 2), matriptase expression was observed on the surface of the matrix cells of the hair bulb. In contrast, the stromal cells in the dermal papilla (DP) were matriptase-negative. The cells immediately above the matrix cells, likely from the cortex and medulla, were also negative for matriptase. HAI-1 expression paralleled matriptase expression, with strong staining of the epithelial cells and no staining of the stromal cells of the DP. However, unlike matriptase, HAI-1 expression was also observed in the more-differentiated cells present in the central area of the infundibulum as well as the cells in the cortex and medulla directly above the matrix cells.
Figure 2.
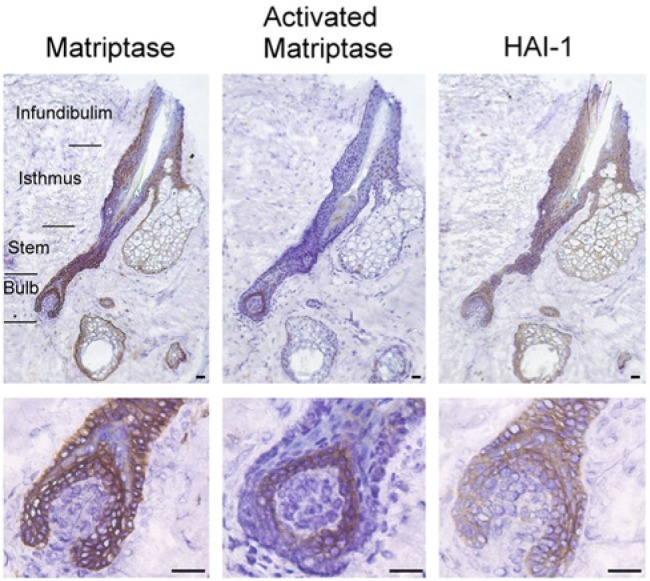
Expression of matriptase and HAI-1 in anagen-phase human hair follicles: longitudinal section analyses. Longitudinal tissue sections of anagen-phase human hair follicles were immunostained with the matriptase-specific mAb M32 (Matriptase), the HAI-1-specific mAb M19 (HAI-1) or the activated matriptase-specific mAb M69 (Activated Matriptase). Sections were also stained with a non-specific mouse IgG antibody as a negative control (not shown). Representative examples of the staining observed are presented. The different regions of the hair follicle are as indicated, with the bulb region shown at higher magnification in the lower panels. Nuclei of cells were counterstained blue with hematoxylin. Scale bar: 50 µm.
The studies described above reveal that matriptase expression appears to be higher in less-differentiated cells (such as the highly proliferative matrix cells and ORS keratinocytes) than in the more-differentiated cells (such as cells in the IRS, cortex, medulla, and the terminally differentiated cells in upper portion of the infundibulum). The distribution profile of HAI-1 expression in hair follicles is essentially identical to that of matriptase, with the exception of staining in: 1) the IRS and 2) the cells directly above the matrix cells. In these two types of late-stage differentiated cells of the hair follicle, HAI-1 is expressed at high levels whereas matriptase expression is very low or absent. This expression pattern echoes the expression pattern of HAI-1 in epidermal granular keratinocytes, which are also at a late stage of differentiation and are matriptase-negative (Chen et al., 2013a).
Matriptase Is Activated in Matrix Cells of the Hair Bulb and in the ORS of the Isthmus
We have previously described the generation and characterization of monoclonal antibodies capable of distinguishing activated matriptase from matriptase zymogen (Benaud et al., 2001). Using these activated matriptase-specific mAbs, we examined the zymogen activation status of matriptase in hair follicles. Among the 56 hair follicles identified in the tissues examined, activated matriptase was detected in only 36 samples. Moreover, the level of activated matriptase detected in the positive samples was generally quite low. The absence or low levels of detectable activated matriptase in the face of the high levels of “total” (zymogen plus activated) matriptase expression is consistent with our previous observations that the activation of matriptase is under very tight control in normal tissues (Chen et al., 2013a; Wang et al., 2009).
An inspection of Figure 2 reveals that the highest levels of activated matriptase present in the cells of the hair follicle were found in the matrix cells of the hair bulb. This observation suggests that matriptase may play an important role in matrix cell functions, which include proliferation and differentiation into the cells of the IRS, the hair fiber, and the hair cuticle. Furthermore, because total matriptase levels were very low or absent in the IRS, the expression and function of matriptase appears to be tightly regulated and confined predominantly to the early stages of hair growth in the matrix cells.
Activated matriptase was also detected in portions of the ORS (Fig. 1). The ORS consists of multiple layers of cells, with only the innermost layer of the ORS, also known as the companion layer, being in intimate contact with the Henle layer of the IRS. The ORS companion layer does not undergo the typical trichilemmal keratinization as observed for the rest of the ORS. Whereas the presence of activated matriptase was readily detected in the cells on or near the surface of the ORS, it was undetectable in the innermost ORS companion layer adjacent to the IRS. These results suggest that matriptase activity is differentially regulated, even within the ORS keratinocytes.
Matriptase activation was also detected in some areas of the upper portion of hair follicles on the surface of the ORS keratinocytes. In Figure 3, images of two representative hair follicles in the same tissue section are presented. Although total matriptase expression was observed throughout the hair follicles, activated matriptase was detected primarily in the isthmus region. Matriptase activation was low or undetectable in infundibulum and stem regions. At higher magnification (Fig. 3), activated matriptase was seen on the surface of the isthmus cells. The isthmus harbors the bulge region, a specialized compartment of the ORS that forms a niche for epithelial and neurectodermal stem cells. Our observations suggest that the active form of matriptase may play a role in the cellular functions of these stem cells.
Figure 3.
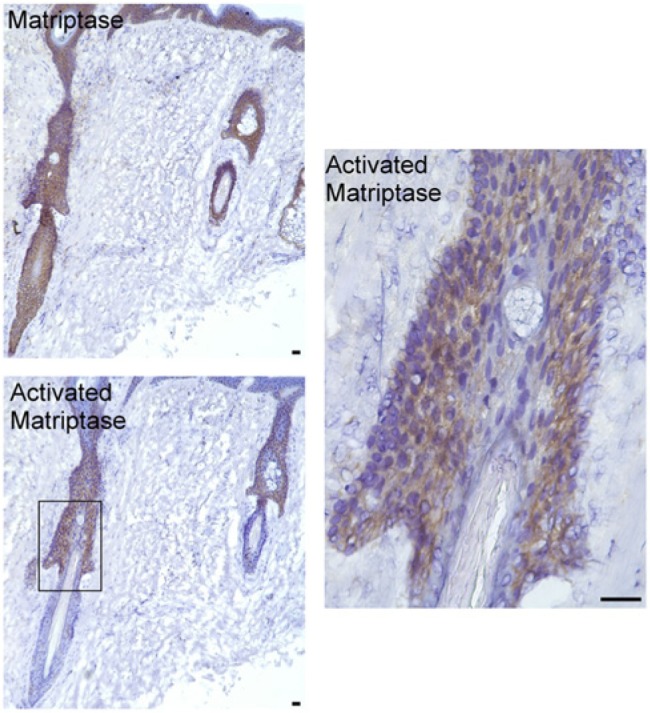
Activated matriptase is present in the ORS keratinocytes of the isthmus. Tissue sections of the upper surface portion of an anagen-phase human hair follicle were immunostained with the matriptase-specific mAb M32 (Matriptase) or the activated matriptase-specific mAb M69 (Activated Matriptase). Sections were also stained with a non-specific mouse IgG antibody as a negative control (not shown). The isthmus region of the hair follicles within the rectangle is shown at higher magnification (right panel). Nuclei of cells were counterstained blue with hematoxylin. Scale bar: 50 µm.
Reduced HAI-1 Expression in the ORS of Catagen Hair Follicles
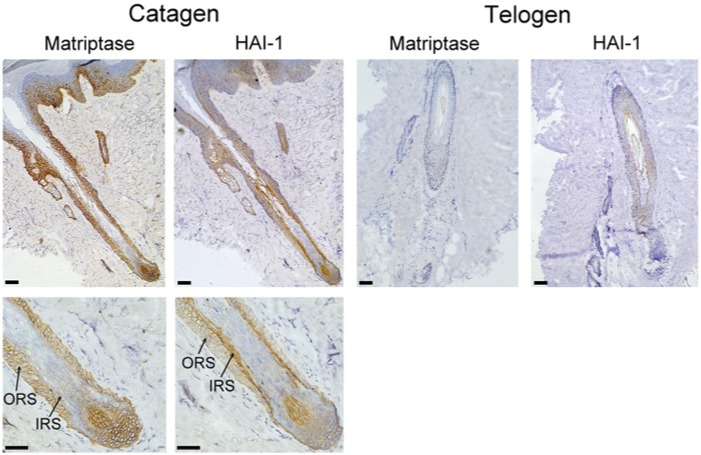
Catagen is a short phase in the life cycle of the hair follicle in which the parts of the follicle involved in hair growth dismantle and the follicle regresses. Matriptase and HAI-1 expression levels in catagen-phase hair follicles were examined using immunohistochemistry, and a representative image is shown in Figure 4. Matriptase was detected in the ORS of both the permanent and the regressing parts of the catagen-phase hair follicle, whereas the IRS was mostly negative for matriptase, similar to that which was observed in anagen-phase hair follicles (Fig. 2). The most significant difference between the anagen- and catagen-phase hair follicles is the reduced expression of HAI-1 in the ORS of the catagen phase relative to the anagen-phase. The reduced expression of the inhibitor HAI-1 in the catagen-phase hair follicles may provide the regressing hair follicles with enhanced proteolytic activity, which may promote its regression.
Figure 4.
Matriptase and HAI-1 expression in catagen- and telogen-phase hair follicles. Tissue sections of catagen- and telogen-phase human hair follicles were immunostained for total matriptase (Matriptase) or HAI-1 (HAI-1). Sections were also stained with a non-specific mouse IgG antibody as a negative control (not shown). Lower panels show the recessive regions of the hair follicle at higher magnification. The outer root sheath (ORS) and the inner root sheath (IRS) are indicated. Nuclei of cells were counterstained blue with hematoxylin. Scale bar: 100 µm.
Loss of Matriptase and HAI-1 in the Telogen Hair Follicles
In the telogen phase of the hair follicle, the follicle continues to regress until it reaches about half of its original size. The dermal papilla is then reduced to a cluster of dermal fibroblast as a small ball of cells underneath the epithelial sac. Neither matriptase nor HAI-1 was detected at any significant levels in telogen-phase hair follicles (Fig. 4), suggesting that matriptase and HAI-1 may not have much of role at this stage. Collectively, the distribution profile of matriptase and HAI-1 suggests that the protease/protease inhibitor system is dynamically regulated in the cycling of hair follicle with its role mainly in the growth (anagen) and regression (catagen) phases and not in the resting (telogen) phase.
Matriptase in Sebaceous Glands
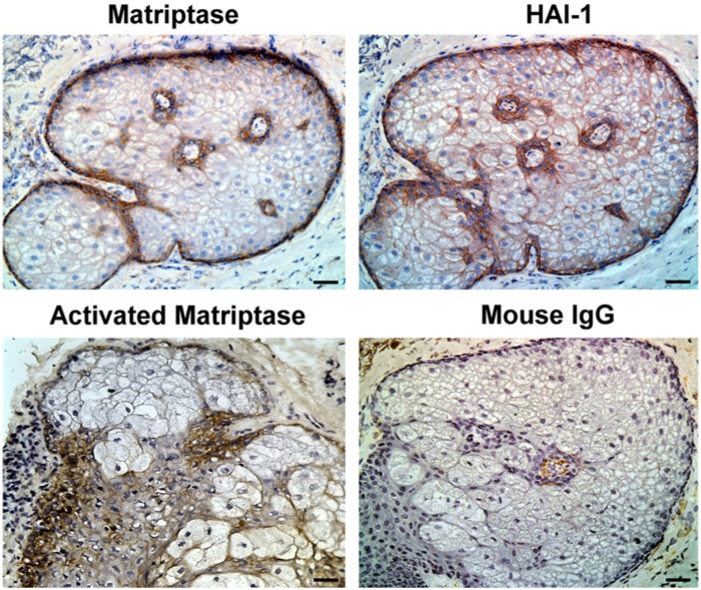
Sebaceous glands are an outgrowth from the ORS of the hair follicles. We used our immunohistochemistry assay to investigate the expression of matriptase and HAI-1 in sebaceous gland. The results, shown in Figure 5, reveal that both matriptase and HAI-1 were strongly expressed in the cells surrounding the sebaceous duct and the basal cells. The basal cells consist of a single layer of flattened cells along the basement membrane at the outer edge of the gland, which are mitotically active. As they divide, the resulting daughter cells move into the interior of the gland and fill with lipid droplets. Matriptase was also detected in these spongy fat cells, though at much lower levels than in the basal and ductal cells. In contrast, HAI-1 expression was clearly detected in the fat cells. Activated matriptase was detectable both in the basal and ductal cells, a distribution similar to that seen in the hair bulb, in which only the mitotically active matrix cells exhibited high levels of activated matriptase.
Figure 5.
Matriptase and HAI-1 expression in human sebaceous glands. Tissue sections of human sebaceous glands were immunostained with the matriptase-specific mAb M32 (Matriptase), the HAI-1-specific mAb M19 (HAI-1), the activated matriptase-specific mAb M69 (Activated Matriptase), or with the control mouse IgG antibody (Mouse IgG). Nuclei of cells were counterstained blue with hematoxylin. Representative examples of the images obtained are presented. Scale bar: 50 µm.
The Matriptase-HGF-c-Met Pathway in Hair Follicles
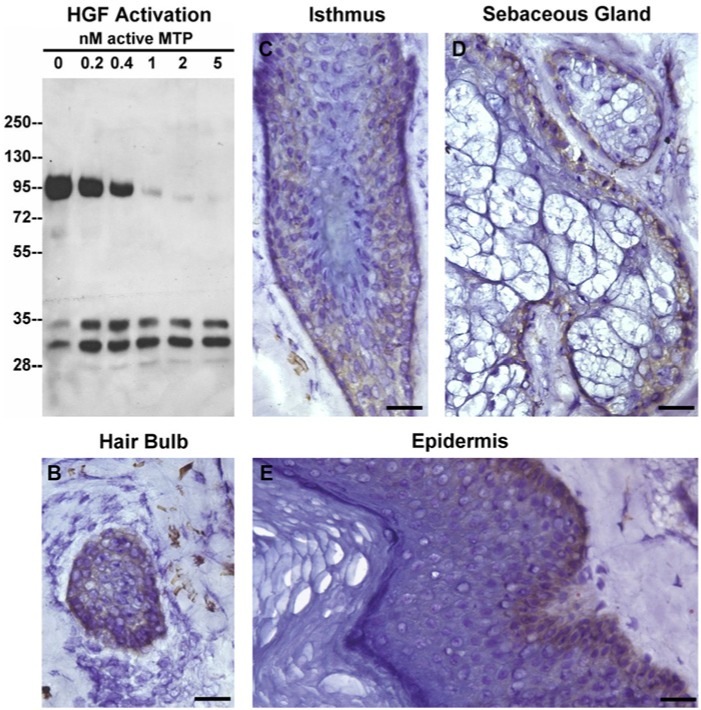
Previous studies have shown that HGF plays an essential role in hair follicle function (Lindner et al., 2000). HGF is synthesized as an inactive pro-form by the stromal cells in the DP. In Figure 6A, we show that the single-chain pro-HGF protein was rapidly converted into the two-chain active form of the protein by nanomolar concentrations of keratinocyte-derived active matriptase in a dose-dependent manner. HGF acts through interactions with its cell surface receptor, c-Met. In Figure 6B and 6C, we show that the c-Met receptor was expressed on the surface of ORS keratinocytes of the isthmus as well as on the surface of matrix cells in the hair bulb, both cell types that exhibited the highest levels of activated matriptase. This observation, coupled with our work demonstrating that matriptase is a potent activator of HGF, supports the hypothesis that the matriptase-HGF-c-Met pathway plays a role in hair follicle growth and function. Furthermore, we observed that c-Met was expressed in the basal cells of sebaceous glands and of the epidermis (Fig. 6D and 6E), both regions in which activated matriptase is present. These observations suggest that the matriptase-HGF-c-Met pathway is likely a common mechanism that regulates the proliferation and differentiation of keratinocytes in various tissue components of the human skin.
Figure 6.
Probing the matriptase-HGF-cMET pathway in human skin. A. Pro-HGF was incubated with increasing amount of active matriptase, as indicated, for 30 min at 37C. The resultant samples were probed for cleavage of HGF by immunoblot analyses using an antibody specific for the beta subunit of HGF. Tissue sections of the hair bulb (panel B) and isthmus of human hair follicles (panel C), sebaceous gland (panel D) and epidermis (panel E) immunostained for the HGF receptor, c-Met. Mouse IgG antibody was used as a negative control (data not shown). Nuclei of cells were counterstained blue with hematoxylin. Scale bar: 50 µm.
Discussion
Although the three major tissue components of the skin, namely the epidermis, the hair follicle and the sebaceous gland, have evolved distinct morphological and functional characteristics, all three components still share a common requirement for continuous tissue regeneration. The basic regeneration scheme is composed of 1) basal cells that can either proliferate (to replenish cell populations) or differentiate, and 2) differentiated cells that execute the biological functions. Our studies here and published elsewhere reveal that matriptase is expressed at high levels in the proliferative cells, including the basal cells of epidermis and sebaceous glands and the matrix cells in hair follicles. In contrast, matriptase is expressed at low or undetectable levels in the differentiated cells, which include the cells in the IRS, the fat cells in sebaceous glands, and the granular and cornified cells in the epidermis. This distribution profile of matriptase expression suggests that the protease plays a role primarily in proliferative cells, rather than in differentiated cells, in the epidermis and pilosebaceous unit. Furthermore, our studies reveal that the activated form of matriptase is primarily detected in the matrix and basal cells, suggesting that matriptase proteolytic activity is likely to be actively involved in the cellular processes of these proliferative and differentiating cells. This hypothesis is further bolstered by our studies in which shRNA-induced suppression of matriptase expression in human keratinocytes decreased both cell proliferation and the expression of differentiation markers (Chen et al., 2013a).
We find that matriptase is widely expressed in the ORS but with activated matriptase being frequently detected in the isthmus/bulge area. The ORS in the isthmus/bulge area is the primary source of skin stem cells that migrate to form the basal cells of epidermis and sebaceous gland and the matrix cells of hair follicles. Our observation that the cells in this area express high levels of matriptase and are frequently positive for activated matriptase suggests that matriptase may play a role in the maintenance of the stem cell niche or in the biology of the stem cells. As a potent and tightly regulated membrane-associated serine protease, matriptase could initiate a proteolytic cascade and thereby modulate the function of growth factors (such as HGF) in the close vicinity of their cognate membrane receptors (such as c-MET).
Matriptase is synthesized as a zymogen and must undergo autoactivation via a cleavage event at the canonical activation motif to gain its full enzymatic activity. Matriptase autoactivation is rapidly followed by the HAI-1-mediated inhibition of the nascent active matriptase. An uncommon feature of matriptase activation and inhibition is that the short-lived active matriptase is still able to effectively activate its downstream substrate, prostasin. Thus, the three events, matriptase autoactivation, activation of prostasin by active matriptase, and inhibition of active matriptase by HAI-1 take place at almost the same time (Chen et al., 2010b). The activated matriptase detected in the ORS cells, the matrix cells, and the sebaceous basal and ductal cells by the mAb M69 is likely activated matriptase in complex with HAI-1. Although free active matriptase possesses potent gelatinolytic activity, activated matriptase in complex with HAI-1 exhibits almost no activity (Benaud et al., 2001). As a result, the activated matriptase that is found in the hair follicles and epidermis is not likely to be the source of the gelatinolytic activity detected in human skin. This is consistent with the finding that the skin gelatinolytic activity is localized to mast cells, as detected by in situ zymography (Krejci-Papa and Paus, 1998).
HGF is a mesenchymal cell-derived, multifunctional growth factor, which is produced by the dermal papilla fibroblasts and can stimulate growth of the hair (Jindo et al., 1995; Shimaoka et al., 1995). The pro-mitogenic activity of HGF is mediated through engagement with the membrane receptor c-MET, which is expressed in the neighboring hair bulb keratinocytes (Lindner et al., 2000). HGF is secreted as an inactive precursor and converted into the active form by proteases. Several proteases, including HGF activator (HGFA), have been detected at the mRNA level in anagen hair follicles of rats (Yamazaki et al., 1999). HGFA can activate HGF with similar potency to that of matriptase (Kirchhofer et al., 2003). HGFA is synthesized as a zymogen and converted into an active protease by other active proteases. In response to tissue injury, HGFA is activated, most likely by thrombin; however, it remains unclear whether anagen hair follicles contain active thrombin and what mechanism is responsible for the zymogen activation of HGFA. In contrast, matriptase can undergo autoactivation to become an active enzyme (Oberst et al., 2003; Xu et al., 2012). More importantly, although active matriptase is rapidly inactivated by HAI-1 in most epithelial cells, in human keratinocytes, a proportion of the active matriptase can be shed into the extracellular milieu (Chen, et al 2013) where it might liberate and activate HGF from the extracellular matrix where the growth factor is commonly deposited (Schuppan et al., 1998).
It has been previously reported that mouse hair follicles express matriptase in the IRS, cortex, medulla, and the matrix cells, but not the ORS (List et al., 2006; Szabo et al., 2008). These studies also reported the detection of matriptase in fat cells, but not in basal cells, of mouse sebaceous glands. Similarly, in mouse epidermis, matriptase has been reported to be expressed by suprabasal keratinocytes with the highest expression in the outermost layer of keratinocytes, but not in the basal cells (Netzel-Arnett et al., 2006). Therefore, unlike human matriptase, mouse matriptase is primarily expressed in differentiated cells and thus is likely to be involved in the regulation of later stages of differentiation, with the exception of the matrix cells, which are highly proliferative. This consistent physiological difference in matriptase expression in the epidermis and pilosebaceous unit of humans versus rodents provides a possible explanation for the significant phenotypic differences between matriptase knockout mice and IFAH patients whose ST14 gene (matriptase) is deleted. Severe skin defects result in homogenous neonatal death within 48 hours of birth in matriptase knockout mice (List et al., 2002). In contrast, IFAH patients have a much less severe, non-life threatening clinical presentation, and live into adulthood (Alef et al., 2009).
In conclusion, matriptase is expressed in a cycle-dependent and cell layer-selective manner in human hair follicles and sebaceous glands, with matriptase playing a role primarily in proliferative cells and its expression negatively regulated by terminal differentiation. It is possible that the potent pericellular proteolytic activity of matriptase is involved in the activation and processing of diverse substrates important for epithelial-mesenchymal interactions that regulate cell proliferation and differentiation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.-Y.L is an inventor on US patents #6,077,938 and #6,677,377 and M.D.J and C.-Y.L are inventors on US patent #7,355,015.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Cancer Institute (NCI) Grants RO1 CA 123223 (to M. Johnson and C.-Y. Lin), and Taiwan Department of Defense Grant D101-14-4 (to J.-K. Wang).
References
- Alef T, Torres S, Hausser I, Metze D, Tursen U, Lestringant GG, Hennies HC. 2009. Ichthyosis, follicular atrophoderma, and hypotrichosis caused by mutations in ST14 is associated with impaired profilaggrin processing. J Invest Dermatol. 129:862–869 [DOI] [PubMed] [Google Scholar]
- Avrahami L, Maas S, Pasmanik-Chor M, Rainshtein L, Magal N, Smitt J, van Marle J, Shohat M, Basel-Vanagaite L. 2008. Autosomal recessive ichthyosis with hypotrichosis syndrome: further delineation of the phenotype. Clin Genet. 74:47–53 [DOI] [PubMed] [Google Scholar]
- Basel-Vanagaite L, Attia R., Ishida-Yamamoto A, Rainshtein L, Ben AD, Lurie R, Pasmanik-Chor M, Indelman M, Zvulunov A, Saban S, Magal N, Shohat M. 2007. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. Am J Hum Genet. 80:467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaud C, Dickson RB, Lin CY. 2001. Regulation of the activity of matriptase on epithelial cell surfaces by a blood-derived factor. Eur J Biochem. 268:1439–1447 [DOI] [PubMed] [Google Scholar]
- Bhatt AS, Welm A, Farady CJ, Vasquez M, Wilson K, Craik CS. 2007. Coordinate expression and functional profiling identify an extracellular proteolytic signaling pathway. Proc Natl Acad Sci U S A 104:5771–5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Wu BY, Tsao PI, Chen CY, Wu MH, Chan YL, Lee HS, Johnson MD, Eckert RL, Chen YW, Chou F, Wang JK, Lin CY. 2011. Increased matriptase zymogen activation in inflammatory skin disorders. Am J Physiol Cell Physiol. 300:C406–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Lee MS, Lucht A, Chou FP, Huang W, Havighurst TC, Kim K, Wang JK, Antalis TM, Johnson MD, Lin CY. 2010a. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am J Pathol. 176: 2986–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Wang JK, Chou FP, Chen CY, Rorke EA, Chen LM, Chai KX, Eckert RL, Johnson MD, Lin CY. 2010b. Regulation of the matriptase-prostasin cell surface proteolytic cascade by hepatocyte growth factor activator inhibitor-1 (HAI-1) during epidermal differentiation. J Biol Chem. 285:31755–31762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Wang JK, Chou FP, Wu BY, Hsiao HC, Chiu H, Xu Z, Baksh AN, Shi G, Kaul M, Barndt R, Shanmugam VK, Johnson MD, Lin CY. 2013a. Matriptase regulates proliferation and early, but not terminal, differentiation of human keratinocytes. J. Invest Dermatol. 10.1038/jid.2013.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Xu Z, Baksh AN, Wang JK, Chen CY, Swanson R, Olson ST, Kataoka H, Johnson MD, Lin CY. 2013b. Antithrombin Regulates Matriptase Activity Involved in Plasmin Generation, Syndecan Shedding, and HGF Activation in Keratinocytes. PLoS One. 8:e62826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Fu G, Huang G, Lian X, Yu J, Yang T. 2010. Relationship between urokinase plasminogen activator receptor (uPAR) and the invasion of human prenatal hair follicle. Arch. Dermatol. Res. 302:409–418 [DOI] [PubMed] [Google Scholar]
- Inouye K, Yasumoto M, Tsuzuki S, Mochida S, Fushiki T. 2009. The Optimal Activity of a Pseudozymogen Form of Recombinant Matriptase under the Mildly Acidic pH and Low Ionic Strength Conditions. J Biochem. 147:485–492 [DOI] [PubMed] [Google Scholar]
- Ito M. 1988. Electron microscopic study on cell differentiation in anagen hair follicles in mice. J Invest Dermatol. 90:65–72 [DOI] [PubMed] [Google Scholar]
- Jarrousse F, Boisnic S, Branchet MC, Beranger JY, Godeau G, Breton L, Bernard BA, Mahé YF. 2001. Identification of clustered cells in human hair follicle responsible for MMP-9 gelatinolytic activity: consequences for the regulation of hair growth. Int J Dermatol. 40, 385–392 [DOI] [PubMed] [Google Scholar]
- Jindo T, Tsuboi R, Imai R, Takamori K, Rubin JS, Ogawa H. 1995. The effect of hepatocyte growth factor/scatter factor on human hair follicle growth. J Dermatol Sci. 10:229–232 [DOI] [PubMed] [Google Scholar]
- Kataoka H, Shimomura T, Kawaguchi T, Hamasuna R, Itoh H, Kitamura N, Miyazawa K, Koono M. 2000. Hepatocyte growth factor activator inhibitor type 1 is a specific cell surface binding protein of hepatocyte growth factor activator (HGFA) and regulates HGFA activity in the pericellular microenvironment. J Biol Chem. 275:40453–40462 [DOI] [PubMed] [Google Scholar]
- Kirchhofer D, Peek M, Li W, Stamos J, Eigenbrot C, Kadkhodayan S, Elliott JM, Corpuz RT, Lazarus RA, Moran P. 2003. Tissue expression, protease specificity, and Kunitz domain functions of hepatocyte growth factor activator inhibitor-1B (HAI-1B), a new splice variant of HAI-1. J Biol Chem. 278:36341–36349 [DOI] [PubMed] [Google Scholar]
- Krejci-Papa NC, Paus R. 1998. A novel in-situ-zymography technique localizes gelatinolytic activity in human skin to mast cells. Exp Dermatol. 7:321–326 [DOI] [PubMed] [Google Scholar]
- Lee SL, Dickson RB, Lin CY. 2000. Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem. 275:36720–36725 [DOI] [PubMed] [Google Scholar]
- Lin CY, Anders J, Johnson M, Dickson RB. 1999. Purification and characterization of a complex containing matriptase and a Kunitz-type serine protease inhibitor from human milk. J Biol Chem. 274:18237–18242 [DOI] [PubMed] [Google Scholar]
- Lin CY, Wang JK, Torri J, Dou L, Sang QA, Dickson RB. 1997. Characterization of a novel, membrane-bound, 80-kDa matrix-degrading protease from human breast cancer cells. Monoclonal antibody production, isolation, and localization. J Biol Chem. 272:9147–9152 [PubMed] [Google Scholar]
- Lindner G, Menrad A, Gherardi E, Merlino G, Welker P, Handjiski B, Roloff B, Paus R. 2000. Involvement of hepatocyte growth factor/scatter factor and met receptor signaling in hair follicle morphogenesis and cycling. FASEB J. 14:319–332 [DOI] [PubMed] [Google Scholar]
- List K, Haudenschild CC, Szabo R, Chen W, Wahl SM, Swaim W, Engelholm LH, Behrendt N, Bugge TH. 2002. Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis. Oncogene 21:3765–3779 [DOI] [PubMed] [Google Scholar]
- List K, Szabo R, Molinolo A, Nielsen BS, Bugge TH. 2006. Delineation of matriptase protein expression by enzymatic gene trapping suggests diverging roles in barrier function, hair formation, and squamous cell carcinogenesis. Am J Pathol. 168:1513–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzel-Arnett S, Currie BM, Szabo R, Lin CY, Chen LM, Chai KX, Antalis TM, Bugge TH, List K. 2006. Evidence for a matriptase-prostasin proteolytic cascade regulating terminal epidermal differentiation. J Biol Chem. 281:32941–32945 [DOI] [PubMed] [Google Scholar]
- Oberst MD, Williams CA, Dickson RB, Johnson MD, Lin CY. 2003. The activation of matriptase requires its noncatalytic domains, serine protease domain, and its cognate inhibitor. J Biol Chem. 278:26773–26779 [DOI] [PubMed] [Google Scholar]
- Panteleyev AA, Jahoda CA, Christiano AM. 2001. Hair follicle predetermination. J Cell Sci. 114:3419–3431 [DOI] [PubMed] [Google Scholar]
- Paus R, Krejci-Papa N, Li L, Czarnetzki BM, Hoffman RM. 1994. Correlation of proteolytic activities of organ cultured intact mouse skin with defined hair cycle stages. J Dermatol Sci. 7:202–209 [DOI] [PubMed] [Google Scholar]
- Schuppan D, Schmid M, Somasundaram R, Ackermann R, Ruehl M, Nakamura T, Riecken EO. 1998. Collagens in the liver extracellular matrix bind hepatocyte growth factor. Gastroenterology. 114:139–152 [DOI] [PubMed] [Google Scholar]
- Shimaoka S, Tsuboi R, Jindo T, Imai R, Takamori K, Rubin JS, Ogawa H. 1995. Hepatocyte growth factor/scatter factor expressed in follicular papilla cells stimulates human hair growth in vitro. J. Cell Physiol. 165:333–338 [DOI] [PubMed] [Google Scholar]
- Szabo R, Hobson JP, List K, Molinolo A, Lin CY, Bugge TH. 2008. Potent inhibition and global co-localization implicate the transmembrane kunitz-type serine protease inhibitor hai-2 in the regulation of epithelial matriptase activity. J Biol Chem. 283:29495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Harris JL, Huang W, Yan KW, Coughlin SR, Craik CS. 2000. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem. 275:26333–26342 [DOI] [PubMed] [Google Scholar]
- Tseng IC, Chou FP, Su SF, Oberst M, Madayiputhiya N, Lee MS, Wang JK, Sloane DE, Johnson M, Lin CY. Purification from human milk of matriptase complexes with secreted serpins: mechanism for inhibition of matriptase other than HAI-1. Am.J.Physiol Cell Physiol. 2008;295:C423–C431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng IC, Xu H, Chou FP, Li G, Vazzano AP, Kao JP, Johnson MD, Lin CY. 2010. Matriptase activation, an early cellular response to acidosis. J Biol Chem. 285:3261–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustach CV, Huang W, Conley-LaComb MK, Lin CY, Che M, Abrams J, Kim HR. 2010. A novel signaling axis of matriptase/PDGF-D/ss-PDGFR in human prostate cancer. Cancer Res. 70:9631–9640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JK, Lee MS, Tseng IC, Chou FP, Chen YW, Fulton A, Lee HS, Chen CJ, Johnson MD, Lin CY. 2009. Polarized epithelial cells secrete matriptase as a consequence of zymogen activation and HAI-1-mediated inhibition. Am J Physiol Cell Physiol. 297:C459–C470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg WC, Brown PD, Stetler-Stevenson WG, Yuspa SH. 1990. Growth factors specifically alter hair follicle cell proliferation and collagenolytic activity alone or in combination. Differentiation. 45:168–178 [DOI] [PubMed] [Google Scholar]
- Xu H, Xu Z, Tseng IC, Chou FP, Chen YW, Wang JK, Johnson MD, Kataoka H, Lin CY. 2012. Mechanisms for the control of matriptase activity in the absence of sufficient HAI-1. Am J Physiol Cell Physiol. 302:C453–C462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Tsuboi R, Lee YR, Ishidoh K, Mitsui S, Ogawa H. 1999. Hair cycle-dependent expression of hepatocyte growth factor (HGF) activator, other proteinases, and proteinase inhibitors correlates with the expression of HGF in rat hair follicles. J Investig Dermatol Symp Proc. 4:312–315 [DOI] [PubMed] [Google Scholar]