Abstract
Proteolysis of the extracellular matrix influences vascular growth. We examined the expression of ADAMTS-1, -4, and -5 metalloproteinases and their proteoglycan substrates versican, decorin, and biglycan as human umbilical vein endothelial cells (HUVECs) formed tubes within type I collagen gels in vitro. Tubulogenic and control HUVEC cultures expressed low levels of ADAMTS-1 and -5 mRNAs, but ADAMTS-4 mRNA was relatively abundant and was significantly elevated (as was ADAMTS-4 protein) in tubulogenic cultures versus controls. Immunocytochemistry revealed ADAMTS-4 in f-actin- and cortactin-positive podosome-like puncta in single cells and mature tubes. Tubulogenic and control cultures expressed low levels of versican and decorin mRNAs; however, peak levels of biglycan mRNA were 400- and 16,000-fold that of versican and decorin, respectively. Biglycan mRNA was highest at 3 hr, declined steadily through day 7 and, at 12 hr and beyond, was significantly lower in tubulogenic cultures than in controls. Western blots of extracellular matrix from tubulogenic cultures contained bands corresponding to biglycan and its cleavage products. By immunocytochemistry, biglycan was found in the pericellular matrix surrounding endothelial tubes and in cell-associated puncta that co-localized with ADAMTS-4 and cortactin. Collectively, our results suggest that ADAMTS-4 and its substrate biglycan are involved in tubulogenesis by endothelial cells.
Keywords: angiogenesis, collagen gel, extracellular matrix, human umbilical vein endothelial cell, matrix metalloproteinases, podosomes, tubulogenesis
Introduction
The growth of new blood vessels from pre-existing vasculature (angiogenesis) is characteristic of the normal development of tissues and organs, the menstrual cycle, inflammation, and wound healing, and pathologies such as diabetes, arthritis, and cancer. Vascular growth and regression are regulated by a variety of processes. Among these are interactions between sprouting endothelial cells and their surrounding extracellular matrix (ECM) that are regulated, in part, by matrix metalloproteinases (MMPs) (Handsley and Edwards 2005). Closely related to the MMPs is the recently discovered family of ADAMTS (A Disintegrin And Metalloproteinase with ThromboSpondin motifs) ECM metalloproteinases, which is represented by 19 genes in humans (Apte 2009; Porter et al. 2005; Rocks et al. 2008). A few studies have implicated specific ADAMTS members in angiogenesis, as shown by the upregulation of ADAMTS-4 in gene expression arrays of tubulogenic endothelial cell cultures (Kahn et al. 2000) and inhibition of vascular development in vivo by ADAMTS-1 and -8 (Vazquez et al. 1999).
Like their MMP relatives, ADAMTS members act on a variety of ECM substrates. Prominent among these are proteoglycans, such as aggrecan (Porter et al. 2005) – a major structural component of cartilage (Roughley 2001). Among the proteoglycan substrates for ADAMTS members are molecules that are implicated in angiogenesis, such as versican (Cattaruzza et al. 2002; Fu et al. 2011; Koyama et al. 2007) – a substrate for ADAMTS-1, -4, and -5 (Sandy et al. 2001), decorin (Fiedler et al. 2008; Järveläinen et al. 1992; Schönherr et al. 2004) – a substrate for ADAMTS-4 (Kashiwagi et al. 2004), and biglycan (Kaji et al. 2000; Schönherr et al. 2004) – a substrate for ADAMTS-4 and -5 (Melching et al. 2006). Although these and other studies have established an enzyme/substrate relationship between ADAMTS-1, -4, and -5 and the proteoglycans versican, decorin, and biglycan, the relationship between these two groups of molecules in the setting of vascular morphogenesis has not been fully investigated. In particular, it is not known whether this enzyme/substrate relationship is confined to specific membrane microdomains during capillary tube formation by sprouting endothelial cells. Accordingly, the present study utilizes an established model of capillary tube formation in vitro in 3-dimensional (3D) collagen gels to examine the relationship between stages of vascular morphogenesis and the expression patterns of ADAMTS-1, -4, and -5 and their proteoglycan substrates versican, decorin, and biglycan.
Materials and Methods
Routine Cell Culture
For routine cell culture, human umbilical vein endothelial cells (HUVECs) (Cascade Biologics, Portland, OR) were grown in plastic culture flasks at 37C/5% CO2 in complete EGM-MV2 medium (Lonza, Basel, Switzerland) supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin. Cells were used for experiments at passage 4 or less.
Assay of HUVEC Tubulogenesis in 3D Collagen Gels
HUVECs were cultured in 3D collagen gels according to an established method (Davis and Camarillo 1996), with modifications as follows. Collagen gels (2.5 mg/ml) were prepared from 1 volume of rat tail type I collagen stock (BD Biosciences, Bedford, MA), 1/9 volume of 10-strength Medium 199 (Sigma-Aldrich, St. Louis, MO), 1% fetal bovine serum (FBS) (final concentration) and EGM-MV2 added q.s. The EGM-MV2 used for collagen gel preparation and cell culture had the manufacturer’s proprietary basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) omitted (Koike et al. 2003). Collagen solutions, containing 1 × 106 HUVECs/ml, were applied in 150 µl volumes to woven nylon rings (Taylor et al. 2006; Vernon and Sage 1999) (12.6 mm outer diameter, 7.9 mm inner diameter) (Sefar America, Inc., Monterey Park, CA), and polymerized for 30 min in a 37C/5% CO2 incubator. The collagen gel/ring assemblies were cultured in 24-well plates in EGM-MV2/1% FBS. To induce tube formation, the cultures were supplemented with 30 ng/ml of recombinant human bFGF (PeproTech, Inc., Rocky Hill, NJ), 30 ng/ml of recombinant human VEGF165 (PeproTech), and 100 ng/ml of phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich) (Koike et al. 2003). Non-tubulogenic (control) cultures of HUVECs were prepared using the same 3D collagen gel/ring assemblies; however, the cultures received reduced quantities of bFGF and VEGF (2 ng/ml each) and no PMA. All cultures were maintained at 37C in a CO2 incubator for up to 7 days with a change of medium every 48 hr. For routine assessment of tube formation, cultures were fixed with 1% neutral-buffered formalin (NBF) and stained with 1% crystal violet (Koike et al. 2003).
Isolation of RNA from HUVECs Cultured in 3D Collagen Gels
Each collagen gel/ring assembly, with HUVECs, was washed briefly with phosphate-buffered saline (PBS), pH 7.4, then vortexed in a 2 ml centrifuge tube in 800 µl of Solution D (Chomczynski and Sacchi 1987) until the collagen gel dissolved. Subsequently, the nylon rings were removed and 80 µl of 2 M Na acetate (pH 4.0), 800 µl of H2O-saturated phenol, and 160 µl of chloroform:isoamyl alcohol (49:1 vol:vol) were added to each tube, shaken for 10 sec, and cooled on ice for 15 min. The emulsions were centrifuged (14,000 × g) at 4C for 15 min and the upper (aqueous) phases transferred to new, RNase-free tubes. An equal volume of isopropanol was added to each tube, mixed, and chilled to −20C for at least 3 hr. The precipitates were pelleted, redissolved, and precipitated twice more with Solution D and isopropanol. The final pellets were washed 3 times in 800 µl of 75% ethanol at −20C, pelleted at 4C, and dissolved into 30 µl of RNase-free water for storage and further evaluation.
Analysis of HUVEC mRNA
Total RNA from HUVEC cultures, isolated as described above, was analyzed by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). cDNA was prepared from 1 µg of total RNA, reverse transcribed in a 40 µl reaction mixture with random primers using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Relative quantitation of gene expression was performed using TaqMan® Gene Expression Assays (Applied Biosystems) with the following assay numbers: Hs00199608_m1 (ADAMTS-1), Hs00192708_m1 (ADAMTS-4), Hs00199841_m1 and Hs01095523_m1 (ADAMTS-5), Hs00171642_m1 (versican), Hs00156076_m1 (biglycan), and Hs00370384_m1 (decorin). To conduct the assays, 40 ng of cDNA was amplified in 1-strength TaqMan Fast Universal PCR mix with 250 nM of TaqMan probe using the Fast program for 50 cycles on an ABI 7900HT PCR System (Applied Biosystems). Samples were assayed in duplicate. mRNA levels were normalized to eukaryotic 18S rRNA. Estimated copy numbers were generated from a standard curve created from a reference cDNA template and TaqMan probe (Shih and Smith 2005). Final values of mRNA expression were averaged from three different experiments, each consisting of triplicate experimental and control HUVEC cultures.
Western Blot Analysis of ADAMTS-4
ADAMTS-4 levels in conditioned media and cell lysates from HUVEC cultures were measured by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting as follows. Conditioned media were collected from culture wells and the collagen gels containing the HUVECs were removed from the nylon rings, transferred to 15 ml tubes, and centrifuged at 1,500 ×g at 4C for 5 min to compress the gels. Medium expressed from each of the centrifuged gels was combined with the corresponding sample of conditioned medium. The pelleted collagen gel/HUVEC material (cell lysate) was dissolved in urea buffer (8 M urea/50 mM Tris-HCl/0.25 M NaCl/2% Triton X-100, pH 7.5) supplemented with the protease inhibitors 6-aminohexanoic acid (100 mM), N-ethylmalemide (5 mM), phenylmethylsulfonyl fluoride (1 mM) (all from Sigma-Aldrich), and benzamidine HCl (5 mM) (Eastman Kodak, Rochester, NY). Protein concentrations of the conditioned media and cell lysates were determined using a Bradford Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL). Cell lysates and conditioned media (50 µg and 100 µg total protein, respectively) were precipitated with ethanol, dried, reconstituted in 3-strength Laemmli sample buffer, resolved by SDS-PAGE, and transferred to nitrocellulose membranes. The membranes were blocked for 1 hr in Tris-buffered saline/0.05% Tween-20 (TBS-T) with 2% bovine serum albumin (BSA) and exposed overnight at 4C to polyclonal rabbit anti-human ADAMTS-4 antibody (PA1-1749A, Thermo Fisher Scientific). Bound antibodies were detected with an alkaline phosphatase-goat anti-rabbit IgG secondary antibody (Jackson ImmunoResearch, West Grove, PA) and chemiluminescence (Tropix®-CSPD®, Applied Biosystems).
Immunofluorescence (IF) Labeling of HUVECs
For ADAMTS-4 IF labeling of HUVECs in 3D collagen gels, the samples were fixed 30 min in NBF, immersed 30 min in 100% ice-cold methanol, and blocked for 2 hr in 5% normal goat serum (NGS). Blocked specimens were incubated overnight in a 1:200 dilution of a polyclonal rabbit anti-human ADAMTS-4 antibody (ab84792, Abcam, Cambridge, MA), washed for 1 hr in PBS, exposed for 3 hr to a 1:800 dilution of Alexa-Fluor®-conjugated goat anti-rabbit IgG secondary antibody (Invitrogen, Grand Island, NY), and washed overnight in PBS. Nuclei were counterstained with TO-PRO®-3 iodide (Invitrogen). Cellular filamentous (f)-actin of selected samples was labeled with Alexa-Fluor-conjugated phalloidin (Invitrogen). For double-label IF of ADAMTS-4 and biglycan, 3D gels containing HUVECs were fixed for 30 min in NBF, immersed for 30 min in 100% ice-cold methanol, treated with 0.2 U/ml chondroitinase ABC lyase (Seikagaku, Tokyo, Japan) for 1 hr at 37C, and blocked for 2 hr in 5% NGS. After blocking, the specimens were incubated overnight in 1:200 dilutions of rabbit anti-human ADAMTS-4 antibody (see above) and a mouse monoclonal antibody to human biglycan (ab54855, Abcam), then washed for 1 hr in PBS, exposed for 3 hr to Alexa-Fluor-conjugated secondary antibodies, and washed overnight in PBS. Nuclei were counterstained with TO-PRO-3 iodide. For some experiments, cultures labeled for ADAMTS-4 (ab84792) or biglycan (using a rabbit polyclonal antibody to human biglycan; ab49701, Abcam) were double-labeled with a mouse monoclonal antibody to cortactin (ab33333, Abcam). Digital images of all IF-labeled specimens were obtained with a Leica TCS-SP5 II confocal microscope (Leica Microsystems, Wetzlar, Germany).
Western Blot Analysis of Proteoglycans
Levels of proteoglycans (decorin, versican, and biglycan) in conditioned media and cell lysates from HUVEC cultures were measured by SDS-PAGE and western blotting in a manner similar to that described above for analysis of ADAMTS-4. To concentrate the proteoglycans, samples were applied to DEAE Sepharose anion exchange mini-columns, washed, and then eluted by three column volumes of 8 M urea/3M NaCl buffer. The eluates were precipitated with ethanol, dried, and digested for 3 hr at 37C in 0.05 U/ml of chondroitin ABC lyase (Northstar BioProducts™, Associates of Cape Cod, Inc., East Falmouth, MA) in a buffer of 0.3 M Tris-HCl/18 mM sodium acetate, pH 8.0. Digested samples were resolved by SDS-PAGE, transferred to nitrocellulose membranes, blocked for 1 hr in TBS-T/2% BSA, and then exposed overnight at 4C to rabbit polyclonal antibodies against human biglycan (LF-51) (Fisher et al. 1995) and human decorin (LF-30) (Corsi et al. 2001; Fisher et al. 1995), and a mouse monoclonal antibody against human versican (2-B-1) (Northstar Bioproducts) (Chan et al. 2010). Bound antibodies were detected with appropriate alkaline phosphatase-conjugated secondary antibodies and chemiluminescence (Tropix-CSPD).
Results
HUVECs Undergo Tubulogenesis in 3D Collagen Gels
For study of vascular growth in vitro, we chose a model in which HUVECs are dispersed within a 3D, native type I collagen gel and exposed to angiogenic factors in the presence of low serum (Davis and Camarillo 1996). Under these conditions, the cells organize within 48 hr into a network of thin-walled tubes that closely resemble native capillaries. These tubes also undergo secondary sprout formation, which is characteristic of angiogenesis. In this model, PMA has been shown to be the most important factor for induction of tube formation, via the activation of protein kinase C (Taylor et al. 2006).
The development of capillary-like structures in tubulogenic HUVEC cultures proceeded as follows: within 6 hr after dispersion in the collagen gel, cells began to extend filopodia (Fig. 1A). By 24 hr, cells began to elongate and interact with one another (Fig. 1B). After 3 days, the cells had organized into networks of multicellular cords, many of which contained large, coalescing vacuoles typical of tube formation (Fig. 1C). After 5 days, multicellular tubes with thin walls and wide lumens predominated (Fig. 1D) and were maintained through day 7 of culture. In contrast, the control HUVEC cultures (also maintained in 3D collagen gels, but without PMA) showed no evidence of tube formation over 7 days (Fig. 1E–H).
Figure 1.
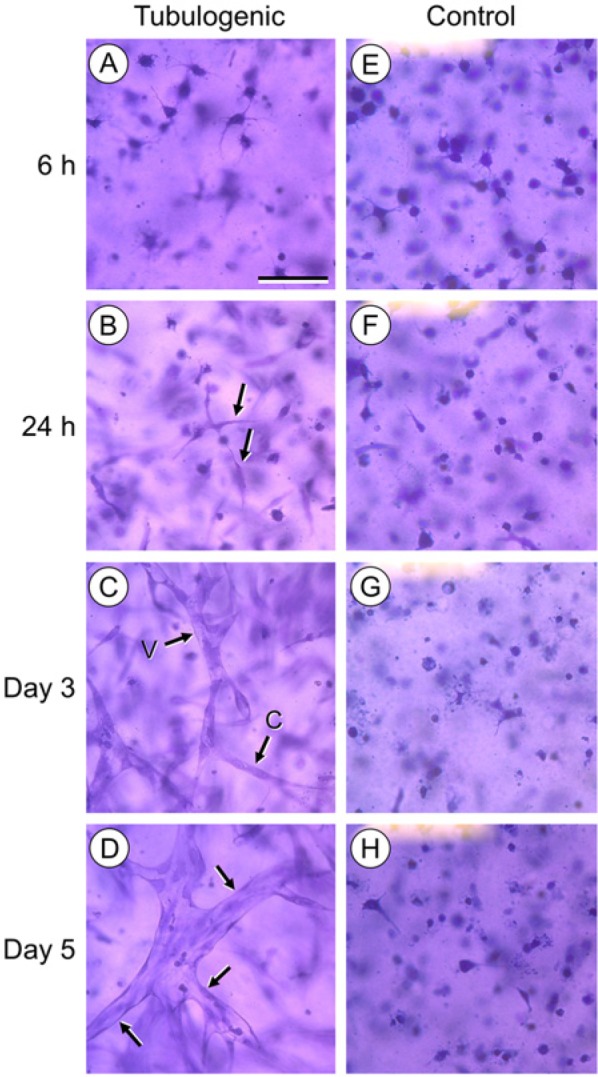
Tubulogenesis of HUVECs in 3D collagen gels. A–D represent tubulogenic cultures. (A) At 6 hr, cells extend filopodia into the surrounding collagen. (B) At 24 hr, cells elongate and interact (arrows). (C) At 3 days, multicellular cords (arrow “C”) and developing tubes with large, coalescing vacuoles (arrow “V”) are present. (D) At 5 days, cultures contain fully-developed tubes (arrows) with patent lumens and thin walls. Corresponding control HUVEC cultures (E–H), where the cells were maintained in 3D collagen gels, but without PMA, showed no evidence of tube formation over 7 days. Cultures A–H are stained with crystal violet and imaged by transmitted light at equal magnification. In A, scale bar = 50 µm.
Tubulogenic HUVECs Upregulate ADAMTS mRNAs
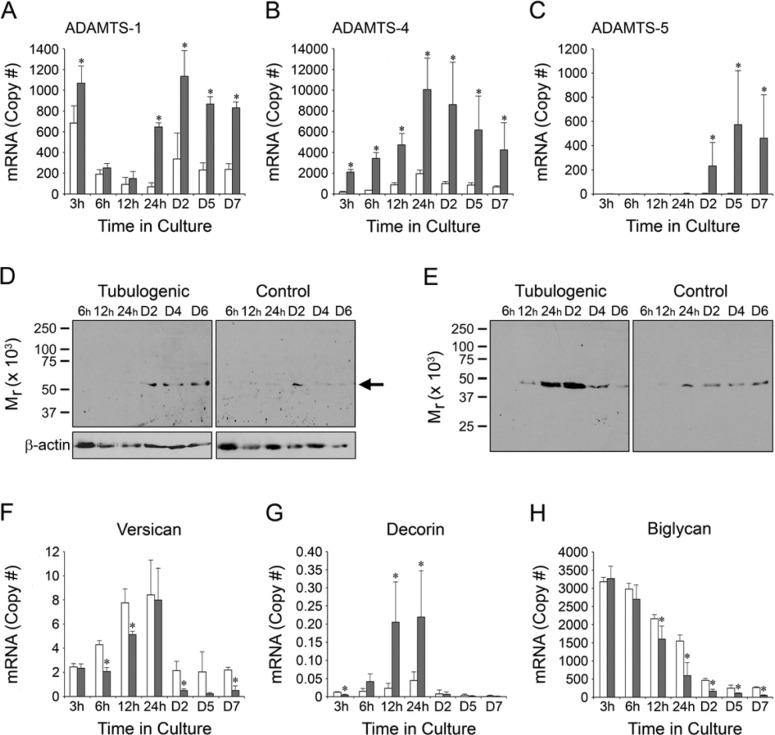
We examined the relationship between the time-course of HUVEC tubulogenesis in vitro and the expression of ADAMTS-1, -4, and -5 mRNAs (Fig. 2A–C). In tubulogenic HUVEC cultures in collagen gels, ADAMTS-1 mRNA (Fig. 2A) was upregulated significantly within 3 hr relative to control, non-tubulogenic HUVECs cultured in collagen gels in the absence of PMA; however, this initial increase was followed by a steady decline in ADAMTS-1 mRNA in both tubulogenic and control cultures by 6–12 hr. Of note, however, ADAMTS-1 mRNA in the tubulogenic cultures increased sharply at 24 hr, which was not observed in the control cultures. The sharp increase in ADAMTS-1 mRNA in the tubulogenic cultures coincided with the beginning of cell-cell interactions and vacuole formation. ADAMTS-1 mRNA levels in the tubulogenic cultures were further increased at 48 hr (when the cultures received fresh medium) and remained significantly elevated, relative to the controls, as tube formation proceeded through day 7 of culture.
Figure 2.
Time-course of expression of ADAMTS mRNA/protein and proteoglycan mRNA during HUVEC tubulogenesis. In graphs A–C and F–H, mRNA expression by tubulogenic and control cultures in 3D collagen gels is indicated by gray and white bars, respectively. Copy numbers of all mRNAs are calculated per 105 18S rRNA. On x-axes and at the top of gel lanes, the designation “D” refers to “day” and “h” refers to “hours.” (A) ADAMTS-1 mRNA in tubulogenic cultures diminishes from 3–12 hr, but increases sharply at 24 hr and remains elevated thereafter. The mRNA expression pattern in controls is similar, but is generally lower and does not increase at 24 hr. (B) ADAMTS-4 mRNA in tubulogenic cultures peaks at 24 hr and decreases thereafter; controls are significantly lower at all time points. (C) ADAMTS-5 mRNA is detectable only at days 2–7 in tubulogenic cultures and is undetectable in controls. (D, E) Western blots of ADAMTS-4 in cell lysates (D) and conditioned media (E). (D) An immunolabeled band of Mr 53–56 kDa is present in tubulogenic cell lysates at days 2 through 6, but in control cell lysates at day 2 only (the position of the band in the representative blots is shown by the arrow at right). Loading (β-actin) controls for the cell lysates are also shown. (E) An immunolabeled band of Mr 47 kDa appears in conditioned media from tubulogenic cultures at 12 hr, peaks strongly at 24–48 hr, and diminishes at days 4 and 6. This band is present in conditioned media from control cultures at 24 hr through day 6, but it does not peak strongly at 24–48 hr. (F–H) Expression of proteoglycan mRNAs. (F) Versican mRNA peaks at 24 hr, but drops sharply thereafter on days 2–7. Levels are similar between tubulogenic and control cultures. (G) In tubulogenic cultures, decorin mRNA increases markedly at 12–24 hr, but is very low before and after these time points. Decorin mRNA is uniformly low in control cultures. (H) In tubulogenic and control cultures, biglycan mRNA levels are highest at 3 hr and, thereafter, decline steadily through day 7. At 12 hr and beyond, mRNA levels are significantly lower in tubulogenic cultures versus controls. Biglycan mRNA is expressed in high abundance relative to versican and decorin mRNAs. For A–C and F–H, error bars = standard deviation. *p < 0.05 tubulogenic cultures versus controls.
The expression pattern of ADAMTS-4 mRNA in tubulogenic HUVEC cultures (Fig. 2B) was distinctly different from that of ADAMTS-1 mRNA. Levels of ADAMTS-4 mRNA increased steadily from the onset of culture, peaking at 24 hr at the beginning of tube formation and declining steadily thereafter through day 7. At peak expression, the copy number of ADAMTS-4 mRNA in the tubulogenic cultures was approximately 10-fold higher than that of ADAMTS-1 mRNA. Expression of ADAMTS-4 mRNA in control cultures also peaked at 24 hr, but with significantly lower levels in comparison to tubulogenic cultures (Fig. 2B).
Compared to ADAMTS-1, and -4 mRNAs, the expression level of ADAMTS-5 mRNA in tubulogenic cultures was distinctly different, being undetectable from 3–24 hr of culture, but appearing at days 2 through 7 (Fig. 2C). At peak expression, however, the abundance of ADAMTS-5 mRNA was very low in comparison to ADAMTS-4 mRNA. ADAMTS-5 mRNA was not detectable in the control cultures.
ADAMTS-4 Accumulates in Cell Lysates and Conditioned Media of Tubulogenic HUVEC Cultures
The robust upregulation and high abundance of ADAMTS-4 mRNA in the tubulogenic HUVEC cultures, and its peak during the onset of tubulogenesis, led us to focus on ADAMTS-4 in subsequent experiments. We examined the expression of ADAMTS-4 protein in western blots of cell lysates and conditioned media from HUVEC cultures by using a specific antibody that recognizes the catalytic domain of ADAMTS-4. The Mr of ADAMTS-4 in cell lysates (53–56 kDa) (Fig. 2D) and conditioned media (47 kDa) (Fig. 2E) was consistently lower than the Mr of 70 kDa for full-length ADAMTS-4, as previously reported (Kashiwagi et al. 2004); however, these authors and others (Corps et al. 2008; Flannery et al. 2002; Powell et al. 2007) also reported forms of ADAMTS-4 of Mr 53 kDa, 45 kDa, and 37–40 kDa in western blots of conditioned media from cultured cells of human or porcine origin, which was attributed to post-secretory processing of the full-length form. In cell lysates of tubulogenic HUVEC cultures (Fig. 2D), ADAMTS-4 was detectable at culture day 2 and beyond, whereas, in conditioned media from these cultures (Fig. 2E), ADAMTS-4 appeared at low levels at 12 hr and peaked strongly at 24–48 hr, diminishing thereafter on culture days 4 and 6. In contrast, ADAMTS-4 in cell lysates from control HUVECs cultured in collagen gels in the absence of PMA was detectable at culture day 2 only (Fig. 2D). In conditioned media from the controls (Fig. 2E), ADAMTS-4 was present from 24 hr onward, but not at the elevated levels at 24–48 hr observed in conditioned media from the tubulogenic cultures.
Expression of Proteoglycan mRNAs by Cultured HUVECs Changes over Time
Subsequent experiments focused on the ADAMTS proteoglycan substrates versican, decorin, and biglycan. We examined the expression of versican, decorin, and biglycan core protein mRNAs by tubulogenic HUVECs (Fig. 2F–H). Versican mRNA (Fig. 2F) increased from 3–24 hr, but dropped sharply thereafter on days 2–7. Levels were expressed at relatively low copy numbers and were similar between tubulogenic cultures and control HUVECs cultured in collagen gels in the absence of PMA. The pattern of decorin mRNA expression (Fig. 2G) was similar to that of versican, but with a more pronounced upregulation at 12–24 hr and a significant difference between tubulogenic and control cultures at these two time points. Peak levels of decorin mRNA (at 24 hr) were only 2.5% those of versican. In contrast to versican and decorin mRNAs, mRNA for biglycan (Fig. 2H) was expressed in relatively high abundance (peak levels were 400- and 16,000-fold those of versican and decorin, respectively) and in a distinctly different pattern (peak levels occurred at 3 hr and, thereafter, declined steadily through day 7). Moreover, from 12 hr through day 7, levels of biglycan mRNA were modestly, but significantly, lower in tubulogenic cultures versus controls.
ADAMTS-4 Expression by HUVECs is Localized Spatially
Following our analyses of expression of ADAMTS-4 mRNA and protein by HUVECs, we used IF and confocal microscopy to examine the spatial distribution of ADAMTS-4 as the cells underwent tubulogenesis in 3D collagen gels. Multicellular tubes expressed ADAMTS-4 strongly as oval-shaped puncta of various sizes (Fig. 3A). These puncta were also expressed by individual cells not incorporated into tubes (Fig. 3B). In some instances, the ADAMTS-4–positive puncta had a ring or rosette shape (Fig. 3B) similar to that reported for podosomes or invadopodia (Aga et al. 2008; Murphy and Courtneidge 2011). In larger puncta, a central region positive for f-actin could be clearly discerned (Fig. 3B). An f-actin–rich center (core) is a typical feature of podosome-like structures (Linder and Kopp, 2005; Murphy and Courtneidge, 2011).
Figure 3.
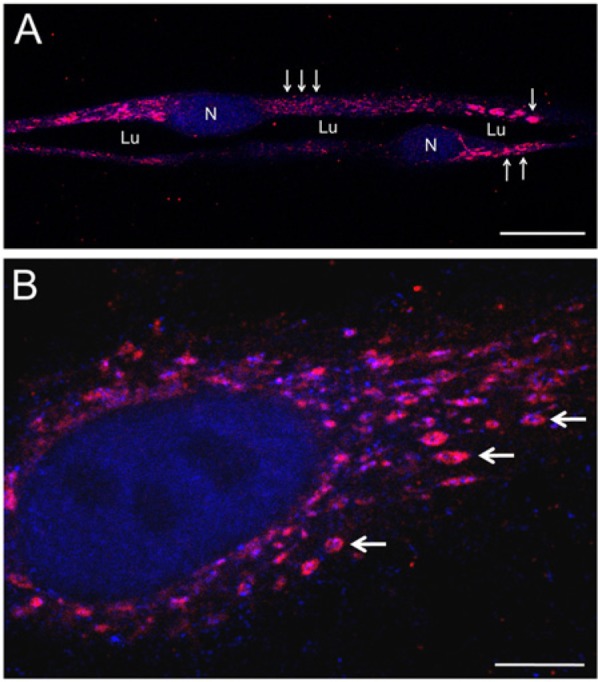
Expression of ADAMTS-4 by HUVECs. (A) Portion of a multicellular tube formed by HUVECs after 4 days of culture in a 3D collagen gel. ADAMTS-4 is expressed as puncta (red) of large (single arrow), intermediate (double arrows), and small (triple arrows) sizes. Lumen (Lu) of the tube and TO-PRO-3-labeled (blue) cell nuclei (N) are indicated. (B) A single HUVEC cultured 48 hr in a 3D collagen gel expresses ADAMTS-4 in an array of oval-shaped puncta (red). Cytoplasmic f-actin (labeled with phalloidin, blue) is found at the centers (cores) of the ADAMTS-4-positive puncta (e.g., arrows). The cell nucleus is labeled with TO-PRO-3 (blue). A and B are confocal images. Scale bars in A and B are 20 µm and 5 µm, respectively.
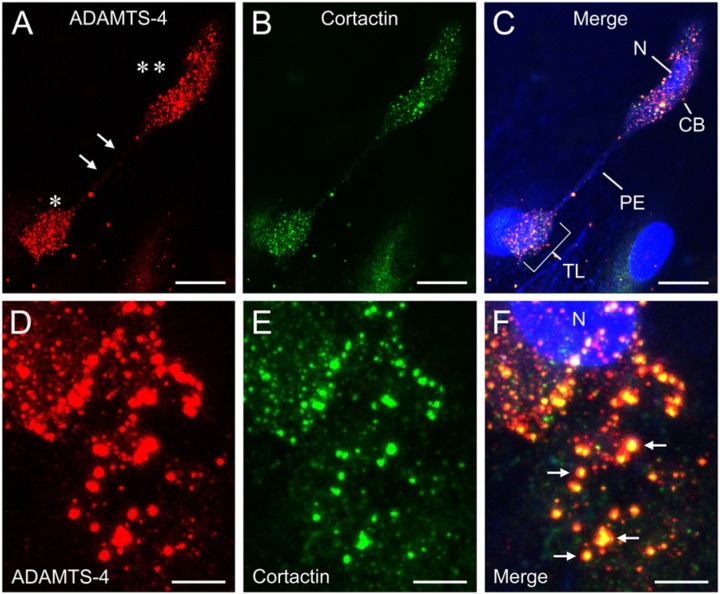
In subsequent experiments, we cultured HUVECs in 3D collagen gels and double-labeled the cells for ADAMTS-4 and cortactin, an actin-organizing protein that, like f-actin, is found in the cores of podosomes (Aga et al. 2008; Murphy and Courtneidge 2011). We found a high degree of co-localization of ADAMTS-4 and cortactin in the puncta (Fig. 4). Interestingly, we found that highly-elongated cells with well-developed, spade-shaped terminal lamellipodia expressed both proteins strongly on the cell body and lamellipodium, but did not express them on the narrow connecting stalk (Fig. 4A–C). Within individual puncta, cortactin was concentrated at the core of the punctum, whereas ADAMTS-4 occupied the core region and also a larger peripheral zone (Fig. 4D–F). A portion of ADAMTS-4 staining in puncta was located deeper in the cytoplasm than the ADAMTS-4 and cortactin staining in the core region (Fig. 5).
Figure 4.
Expression of ADAMTS-4 and cortactin by HUVECs cultured for 4 days in 3D collagen gels. (A) A highly-elongated cell stained for ADAMTS-4 (red) shows intense, punctate labeling over the cell body (double asterisk) and spade-shaped terminal lamellipodium (single asterisk). The stalk-like pseudopodial extension (arrows) between the cell body and lamellipodium is unlabeled. (B) The same cell shown in panel A also exhibits strong, punctate staining for cortactin (green), which is co-localized with ADAMTS-4. The merged image (C) includes phalloidin and TO-PRO-3 stains for the actin cytoskeleton and nucleus, respectively (both blue). CB, cell body; N, nucleus; PE, pseudopodial extension; TL, terminal lamellipodium. Scale bars in A–C = 30 µm. (D–F) Detail of a cell stained for ADAMTS-4 (D, red) and cortactin (E, green). Merged image (F) shows extensive co-localization of the two molecules (e.g., arrows), with cortactin (yellow) concentrated in the center (core) of each circular punctum and ADAMTS-4 present in the core region and in a peripheral zone (red) that lacks cortactin. Nucleus (N) is labeled with TO-PRO-3 (blue). Scale bars in D–F = 3 µm. A–F are confocal images.
Figure 5.
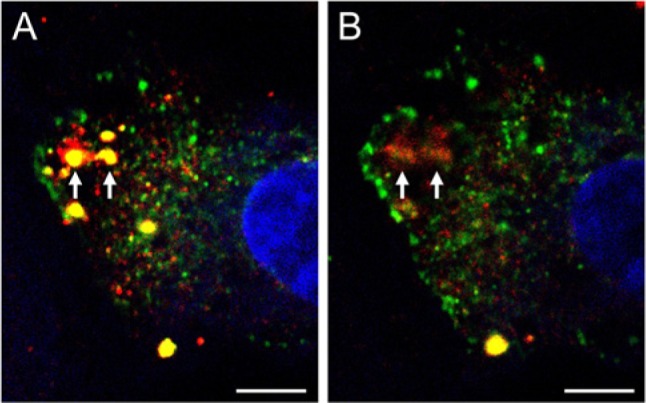
Expression of ADAMTS-4 and cortactin at different depths within a cell. HUVECs were cultured for 4 days in 3D collagen and then stained for ADAMTS-4 (red) and cortactin (green). A single HUVEC is shown, imaged by confocal microscopy. (A) An image representing a single z-plane shows ADAMTS-4 and cortactin co-localized (yellow) in the core regions of two adjacent puncta (arrows). ADAMTS-4 is also seen peripheral to the core regions, particularly in the left-hand punctum. (B) A z-plane deeper in the cytoplasm reveals an absence of cortactin staining in the two puncta, but the retention of ADAMTS-4 staining in peripheral and core areas (arrows). The cell nucleus is stained with TO-PRO-3 (blue). Scale bars in A and B are 5 µm.
ADAMTS-4 and Biglycan are Co-localized in Tubulogenic HUVEC Cultures
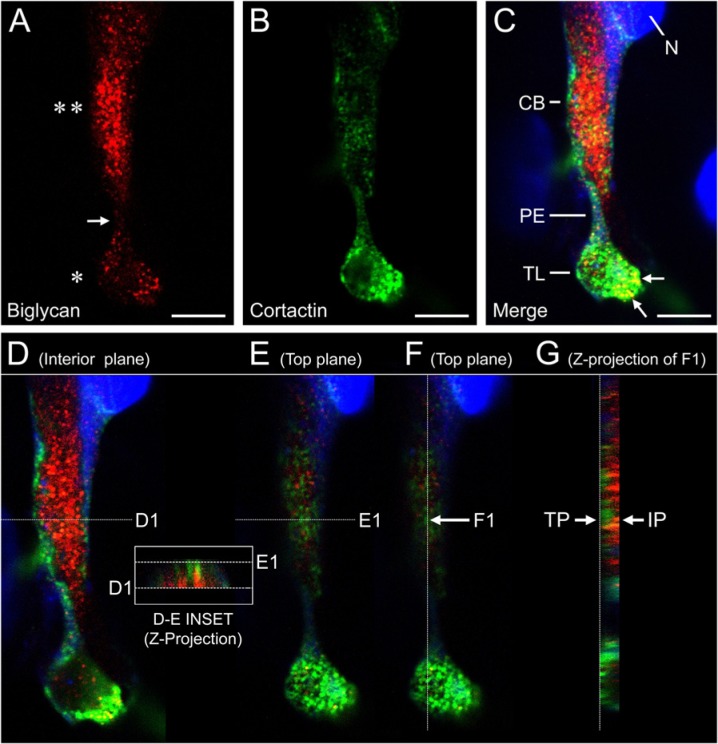
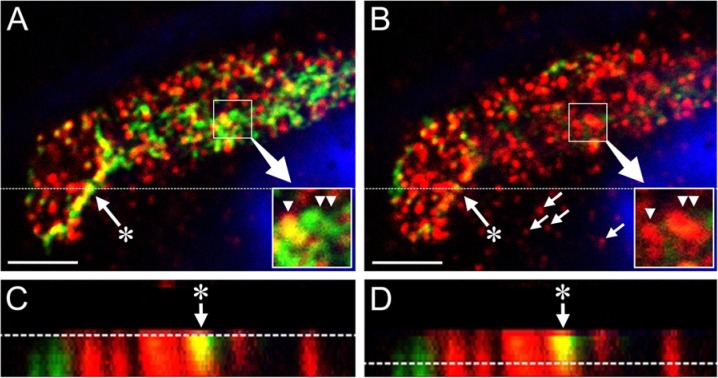
Levels of biglycan mRNA in the HUVEC cultures were several orders of magnitude higher than levels of decorin or versican mRNA. Accordingly, we selected biglycan as the subject for IF studies of the relationship between ADAMTS-4 and a potential proteoglycan substrate. Individual cells in early-stage (24-hr) tubulogenic cultures expressed ADAMTS-4 as punctate, perinuclear staining, with biglycan appearing as a dense pericellular coat extending from the cell surface into the surrounding collagen gel (Fig. 6A). In later-stage cultures, confocal images transecting mature tubes showed that ADAMTS-4 was expressed in the cytoplasm in perinuclear areas and in puncta that co-localized with biglycan (Fig. 6B). Biglycan was also present as a thin, pericellular layer on the abluminal sides of the tubes (Fig. 6B). In single cells, ADAMTS-4 and biglycan were associated in adjacent or co-localized puncta (Fig. 7). Punctate areas of biglycan and cortactin staining were also associated and, in some cases, were co-localized (Fig. 8A–C, Fig. 9), although the degree of co-localization of these two molecules was substantially less than that of ADAMTS-4 and cortactin. Where biglycan was not co-localized with cortactin, the biglycan tended to be distributed more deeply in the cell cytoplasm than cortactin (the latter molecule being relatively near to the cell surface) (Fig. 8D–G, Fig. 9). Interestingly, some puncta that contained cortactin (or co-mingled cortactin and biglycan) were located directly above correspondingly-shaped puncta that expressed biglycan only (Fig. 9A, B insets).
Figure 6.
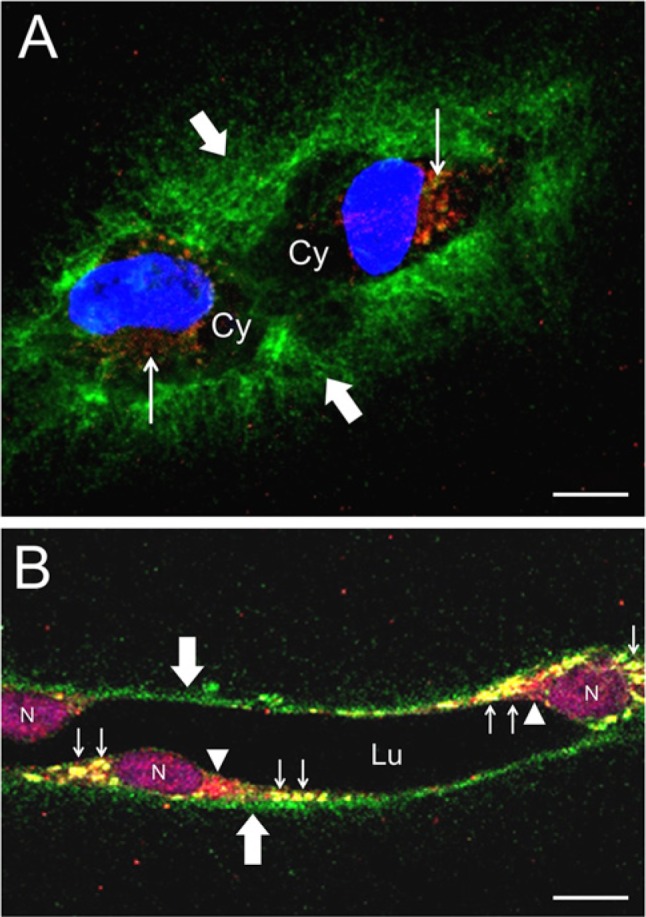
Expression of ADAMTS-4 and biglycan by tubulogenic HUVECs. (A) Two cells labeled after 24 hr of culture in a 3D collagen gel. ADAMTS-4 (red, narrow arrows) resides in punctate areas near the nuclei (blue). Areas of unstained cell cytoplasm (Cy) are indicated. Biglycan (green, broad arrows) is distributed in a dense, pericellular coat. (B) Portion of a multicellular tube formed after 4 days of culture. ADAMTS-4 (red, arrowheads) is localized adjacent to cell nuclei (N) (purple, TO-PRO-3). Biglycan (green, broad arrows) appears as a thin, pericellular layer on the abluminal side of the tube. ADAMTS-4 and biglycan are co-localized (yellow, narrow arrows) in large, oval-shaped puncta. Lumen of the tube (Lu) is indicated. A and B are confocal images. Scale bars in A and B are 10 µm and 20 µm, respectively.
Figure 7.
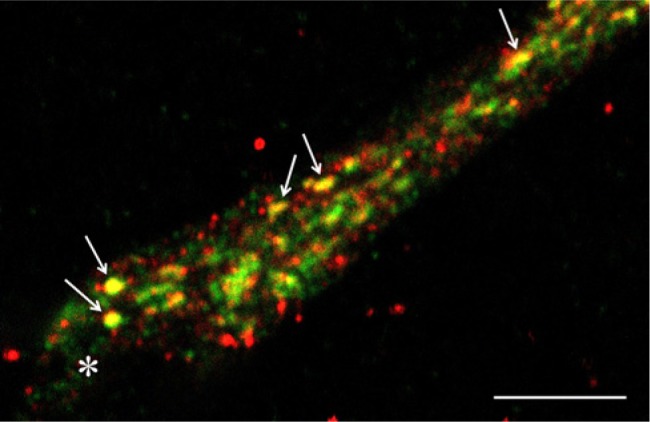
Close association between ADAMTS-4 and biglycan in a single cell. Pseudopod of a single HUVEC cultured for 4 days in a 3D collagen gel under tubulogenic conditions exhibits adjacent, punctate areas of ADAMTS-4 (red) and biglycan (green). Some puncta appear yellow (e.g., arrows), indicating a co-localization of ADAMTS-4 and biglycan. The tip region of the pseudopod is indicated by an asterisk. Confocal image. Scale bar is 5 µm.
Figure 8.
Expression of biglycan and cortactin on a HUVEC cultured for 4 days in a 3D collagen gel: confocal z-stack analysis. (A) A cell stained for biglycan (red) shows intense, punctate labeling over the cell body (double asterisk) and broad terminal lamellipodium (single asterisk). The narrow pseudopodial extension (arrow) between the cell body and lamellipodium is minimally labeled. (B) The same cell shown in panel A also exhibits punctate staining for cortactin (green), which is particularly intense on the lamellipodium. The merged image (C) includes TO-PRO-3 stain for the nucleus (blue). CB, cell body; N, nucleus; PE, pseudopodial extension; TL, terminal lamellipodium. Biglycan and cortactin are co-localized on the lamellipodium (yellow puncta, some are indicated by arrows). Scale bars in A–C = 30 µm. (D–G) Z-stack rendering of the merged image shown in panel C. A single z-plane through the cell interior (D) shows robust staining for biglycan in the cell body (red), but little staining in the central portion of the lamellipodium. A single z-plane near the cell surface (E) shows strong staining for cortactin in both cell body and lamellipodium (green), but little staining for biglycan. A cross-sectional projection of the z-stack sampled at planes D-1/E-1 is shown in the inset. Note that the biglycan staining (red) is interior to the cortactin staining (green). (F) A z-plane near the cell surface. (G) A cross-sectional projection of the z-stack sampled at plane F1 shows cortactin staining of the cell body (green) enriched in the top plane (TP) near the cell surface and biglycan staining enriched in the underlying interior plane (IP).
Figure 9.
Spatial relationship between biglycan and cortactin in individual puncta. A–D are confocal images of a pseudopodial extension of a single HUVEC cultured for 4 days in a 3D collagen gel. (A) A single z-plane image taken near the cell surface shows dense, punctate staining for biglycan (red) and cortactin (green) with some co-localization of the two molecules (yellow). The nucleus (blue, TO-PRO-3) is that of an adjacent cell. Inset (lower right) shows an enlargement of puncta of co-localized biglycan and cortactin (yellow, single arrowhead) and cortactin only (green, double arrowheads). (B) A z-plane deeper in the cytoplasm, relative to panel A, reveals biglycan staining with little evidence of cortactin (biglycan-positive puncta not associated with the cell process, of which four are indicated by small arrows, belong to the adjacent cell). Inset shows biglycan-positive puncta of the cell process (red, single and double arrowheads) that are located directly beneath (and have a similar shape to) the puncta shown in the inset of panel A. Panels C and D show the cross-sectional projection of the z-stack sampled at the plane indicated by the dotted line in panels A and B. The dotted lines in panels C and D represent the z-planes depicted in panels A and B, respectively. A prominent cortactin/biglycan punctum (arrow with asterisk) is identified in panels A–D. Note that the cortactin within this punctum (A–D, yellow) narrows with increasing depth in the cell, but the area of biglycan does not (B–D, red). Scale bars in A and B = 5 µm.
Accumulation of Proteoglycans in 3D HUVEC Cultures
We analyzed the accumulation and processing of decorin, biglycan, and versican in our 3D HUVEC cultures by SDS-PAGE–western blotting, using antibodies to decorin, biglycan, and the 70 kDa fragment of versican known to be generated by ADAMTS -1, -4, and -5 (Fu et al. 2011; Sandy et al. 2001). Neither control nor tubulogenic cultures, which were maintained for 6 hr, 24 hr, or 2 days, exhibited immunoreactive protein bands corresponding to decorin or versican in conditioned media or cell lysates (data not shown). Biglycan was not detectable in conditioned media, but was detectable in cell lysates of both control and tubulogenic cultures as a band of Mr 45 kDa after chondroitinase treatment, which corresponds to the reported molecular weight for the biglycan core protein (Scott et al. 2006). Relative levels of biglycan were lowest in 6-hr cultures, higher in 24-hr cultures, and highest in 2-day cultures (Fig. 10). Of note, a prominent band of Mr 37 kDa was present in 24-hr tubulogenic cultures, with very faint bands of ~ 30 kDa and 23 kDa also visible. In 2-day tubulogenic cultures, the 37 kDa band was not present; however, the faint 30 kDa and 23 kDa bands persisted. None of the control cultures expressed bands below Mr 45 kDa (Fig. 10).
Figure 10.
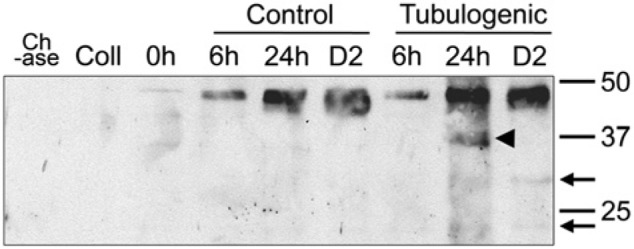
Western blot analysis of biglycan in cell lysates of HUVECs cultured in 3D collagen gels. HUVECs grown under routine culture conditions (i.e., on cell culture plastic in complete EGM-MV2 medium) did not express biglycan in cell lysate (lane marked “0h”). In contrast, tubulogenic cultures maintained in 3D collagen gels (and control, non-tubulogenic cultures grown in 3D collagen gels without PMA) expressed increasing quantities of biglycan (in the form of Mr 45 kDa core protein after chondroitinase ABC digestion) in cell lysates collected after 6 hr, 24 hr, and 2 days of culture (labeled on the figure as 6h, 24h, and D2, respectively). A prominent band of Mr 37 kDa was present in 24-hr tubulogenic cultures (arrowhead) with very faint bands of ~ 30 kDa and 23 kDa also visible. In 2-day tubulogenic cultures, the 37 kDa band was not present; however, the faint 30 kDa and 23 kDa bands persisted (narrow arrows). Neither the chondroitinase ABC preparation used for deglycosylation of the biglycan (lane marked “Ch-ase”) nor gelled collagen that lacked cells (lane marked “Coll”) contained detectable levels of biglycan.
Discussion
Angiogenesis is a complex, multistep process involving local detachment of pericytes, loosening of endothelial cell junctions, degradation of basement membrane matrix, and formation of nascent sprouts that invade the surrounding interstitial ECM (Carmeliet and Jain 2011; Davis and Senger 2005; Pettersson et al. 2000). Subsequent stages of angiogenesis involve increases in the length of the individual sprouts, the formation of lumens, and the anastomosis of adjacent sprouts to form vascular loops and networks – a process that is orchestrated by a program of spatially- and temporally-controlled expression of endothelial gene products.
The process of vascular growth is difficult to monitor and to manipulate in vivo. Therefore, investigators have devised various developmental models in which endothelial cells form multicellular cords or tube-like structures in vitro that resemble capillary sprouts or networks in vivo (Bahramsoltani et al. 2009; Hetheridge et al. 2011; Ucuzian and Greisler 2007; Vernon and Sage 1995, 1999). Behaviors related to capillary morphogenesis are elicited more rapidly and among a greater variety of endothelial cells in culture systems where the cells are placed into contact with 3D ECM (Aplin et al. 2008; Davis et al. 2007; Davis and Senger 2005; Holderfield and Hughes 2008; Koh et al. 2008; Koike et al. 2003; Nakatsu and Hughes 2008). For the present study, we utilized a previously developed culture system (Davis and Camarillo 1996) in which HUVECs, cultured in 3D collagen gels under serum-free (or low serum) conditions in the presence of PMA, form thin-walled multicellular tubes with wide, patent lumens that can undergo secondary, angiogenesis-like sprouting. Compared with other 3D ECM-based models of vascular morphogenesis in vitro, the 3D HUVEC model generates tubular structures that are the closest morphologically to native capillaries. As such, this model is one of the best for evaluating the biochemical processes mediating capillary morphogenesis.
For our studies, we placed both the experimental and control groups in the same 3D collagen gel environment, with the only variable being the application of a tubulogenic stimulus to the experimental group. In this way, we could examine the expression of ADAMTS proteases and their proteoglycan substrates strictly in the context of tubulogenesis. Similarities in gene expression over time between the control and tubulogenic cultures likely represented a general response of HUVECs to the malleable 3D collagen gel environment as the cells changed shape and interacted with the collagen fibrils. Where significant differences in mRNA expression occurred between tubulogenic versus control cultures (e.g., the consistent up-regulation of ADAMTS-4 mRNA in the tubulogenic cultures), such differences were likely to be directly associated in some way with the formation of multicellular tubes.
Using the HUVEC model of tubulogenesis, we observed differential expression over time of mRNAs for ADAMTS-1, -4, and -5 and their potential proteoglycan substrates versican, decorin, and biglycan. Of these molecules, ADAMTS-4 and biglycan were predominant: their mRNAs were expressed at levels from one to several orders of magnitude higher than the mRNAs corresponding to the other ADAMTS members and proteoglycans. Expression of ADAMTS-4 mRNA was significantly higher in tubulogenic cultures than it was in control cultures and peaked at 24 hr, the time when tube formation was beginning. In correlation with this mRNA expression, the quantities of ADAMTS-4 protein secreted into the culture medium by tubulogenic cultures peaked at 24–48 hr and were significantly greater than the quantities secreted by control cultures. The molecular weight of the secreted protein (47 kDa) indicated processing from the full-length (70 kDa) form. Future studies should address the significance of this processing in the regulation of ADAMTS-4 activity and influence on angiogenesis.
In contrast to the expression of ADAMTS-4, the expression of biglycan mRNA in both tubulogenic and control cultures was high at the beginning of culture and steadily diminished over time, with expression beyond 12 hr modestly lower in tubulogenic cultures compared to control cultures. From 6–48 hr, similar levels of biglycan accumulated in the cell lysates of tubulogenic and control cultures; however, lower molecular weight forms of biglycan (indicative of proteolytic processing) were observed only in tubulogenic cultures. Interestingly, although the biglycan mRNA levels of control and tubulogenic cultures dropped over 6-fold and 20-fold (respectively) between 3 hr and 2 days of culture, levels of biglycan protein increased substantially, suggesting that biglycan expression may be regulated post-transcriptionally. There is precedent for both positive and negative post-transcriptional regulation of biglycan in non-endothelial cell culture systems (Templeton and Fan 1996; Ungefroren and Krull 1996).
A common feature of 3D ECM systems of vascular development in vitro is that the endothelial cells need to create a physical space within the 3D ECM via cell surface-associated proteolysis during morphogenesis (Davis et al. 2007; Iruela-Arispe and Davis 2009). Many studies have indicated that endothelial cells are highly proteolytic during neovascularization (Arroyo and Iruela-Arispe 2010; Davis et al. 2002; Ghajar et al. 2008; Pepper 2001; Roy et al. 2006; van Hinsbergh et al. 2006; van Hinsbergh and Koolwijk 2008). Correspondingly, tubulogenesis by endothelial cells is blocked by inhibition of proteases with chemical inhibitors such as GM6001 (Davis et al. 2002; Davis and Saunders 2006; Koike et al. 2002) and also by tissue inhibitors of metalloproteinases (TIMPs), such as TIMP-2 and TIMP-3 (Koike et al. 2003; Saunders et al. 2006). Although soluble MMPs (e.g., MMP-1 and -9) are induced in endothelial cells during tubulogenesis, their role in vascular invasion and morphogenesis remains unclear (Davis and Senger 2005). In contrast, membrane-associated MMPs (e.g., MT1-MMP) appear to be required for endothelial cells to migrate, sprout, and form tubes (Chun et al. 2004; Davis and Senger 2005; Koike et al. 2002; Lafleur et al. 2002; Saunders et al. 2006). In native vasculature, breakdown of the basement membrane is critical prior to invasion of the surrounding interstitial ECM of fibrin or collagen. For example, VEGF-A–induced angiogenesis in the mouse is initiated by degradation of venular basement membrane components such as collagen IV and laminin co-incident with an increase in the expression of the cathepsin family of proteases and a decrease in cysteine protease inhibitors (Chang et al. 2009).
Although considerable information supports a role for MMP- and cathepsin-mediated proteolysis in angiogenesis, less is known about the contribution of ADAMTS proteases to this process. The ADAMTS family members are related to the MMPs in family M12 of the degradome (Rawlings et al. 2010). ADAMTS-1 and -8 have anti-angiogenic activity in mouse corneal pocket and chick chorioallantoic membrane angiogenesis assays (Vazquez et al. 1999). ADAMTS-1 is thought to inhibit angiogenesis by two distinct mechanisms: 1) by binding to VEGF165 and preventing it from binding to its receptor (Luque et al. 2003) and 2) by releasing anti-angiogenic peptides from TSP-1 and -2 (Lee et al. 2006). Because ADAMTS-4 is closely related to ADAMTS-1 in the ADAMTS phylogenetic tree, it makes sense that ADAMTS-4 might have similar anti-angiogenic activity. Indeed, ADAMTS-4 can bind to VEGF and interfere with VEGF receptor phosphorylation, thereby affecting endothelial cell migration (Hsu et al. 2012). However, there is evidence that ADAMTS members may contribute to vascular growth, as indicated by the upregulation of ADAMTS-1 in VEGF-induced angiogenesis in mouse skin (Fu et al. 2011) and the upregulation of the ADAMTS-4 gene (found by a gene screening analysis) during HUVEC tubulogenesis in vitro (Kahn et al. 2000). Although these studies suggested an involvement of ADAMTS members in angiogenesis, it was not clear whether specific ADAMTS members might be differentially expressed or have preferred substrates. We found that ADAMTS-4 mRNA was expressed in tubulogenic HUVECs at a level at least 10-fold higher than mRNAs for ADAMTS-1 and -5, which suggests a predominant role for ADAMTS-4 over other ADAMTS members. Moreover, the elevated expression of ADAMTS-4 mRNA and protein relative to non-tubulogenic controls implicates this protease in some aspect of tube formation.
In the tubulogenic cultures, expression of ADAMTS-4 mRNA and protein in conditioned media was low at the onset of culture, peaked when tubes began to form, and declined steadily thereafter, a finding that suggests a role for ADAMTS-4 in an early phase of the tubulogenic process. Of interest was our observation that the other ADAMTS members had patterns of temporal expression that differed from that of ADAMTS-4. ADAMTS-1 mRNA was expressed in a biphasic fashion – initially high when the cells were introduced into collagen gels, diminishing prior to the onset of tube formation, increasing when tubes began to form, and remaining high thereafter. Interestingly, ADAMTS-5 mRNA was not expressed at all until tubulogenesis was underway at day 2 of culture. Such results underscore the acknowledged importance of temporal control of gene expression during angiogenesis and suggest that each ADAMTS member may carry out a unique function during the course of the tubulogenic program.
A number of studies have implicated proteoglycans in angiogenesis (reviewed in Järveläinen and Wight, 2002) with two prominent proteoglycans being decorin and biglycan (reviewed in Kinsella et al., 2004). Although endothelial cells synthesize both biglycan and decorin (Calabrese et al. 2011; Järveläinen and Wight 2002; Järveläinen et al. 1991; Kaji et al. 2004; Kinsella et al. 1997; Nelimarkka et al. 1997), we found that HUVEC cultures produced far more (up to 16,000-fold) biglycan mRNA than decorin mRNA. Biglycan has been found on the surface of a number of cell types, including endothelial cells (Bianco et al. 1990), and has been localized to the tips and edges of lamellopodia of endothelial cells undergoing migration on glass substrata (Kinsella et al. 1997). Although our IF results suggested a cytoplasmic distribution of biglycan in HUVECs, we also observed biglycan staining that surrounded and extended some distance from the surfaces of individual cells and multicellular tubes. These results suggest that the presence, around cells, of a supportive 3D lattice of ECM (e.g., the collagen gels used here) can provide space for accumulation of an appreciable quantity of pericellular ECM, which may be relevant to tubulogenesis in vitro and to vascular growth and morphogenesis in vivo in wound matrix and interstitial ECM.
Our observation that fragments of biglycan appeared during HUVEC tube formation in vitro is of interest. Although it is not clear that ADAMTS-4 was responsible for the generation of the biglycan fragments, there is precedence that ADAMTS-4 can degrade biglycan in other systems (Gendron et al. 2007; Melching et al. 2006). Proteolytic fragments of ECM molecules with biological activities affecting angiogenesis have been known for a number of years (Arroyo and Iruela-Arispe 2010; Bellon et al. 2004). In this context, earlier studies have shown that smaller fragments of biglycan were generated as endothelial cells were stimulated to migrate following exposure of the cells to the pro-angiogenic cytokine, bFGF (Kinsella et al. 1997). Interestingly, a cleavage site in the 5th leucine-rich repeat (LRR) of biglycan is conserved in all species studied to date and is not present in decorin. Even though a function for the biglycan fragment generated by cleavage at this site has not been identified, it is known that LRRs mediate protein-protein interactions (Kobe and Deisenhofer 1994). Consequently, cleavage within the LRR region of the biglycan core protein might impair functions of biglycan that are mediated via interactions with other proteins.
Our finding that ADAMTS-4 and biglycan are co-localized to discrete cell-associated puncta supports the notion of an enzyme/substrate relationship between these two molecules. Some of these structures have the appearance of podosomes or invadopodia, which have been observed in several other cell types, including macrophages, dendritic cells, vascular smooth muscle cells, and endothelial cells (Aga et al. 2008; Gu et al. 2007; Huang et al. 2003; Linder 2007; McNiven et al. 2000; Ochoa et al. 2000; Wang et al. 2009). In two-dimensional (2D) cell cultures, podosomes are manifested as single puncta or rosette-shaped clusters found on the “ventral” cell surface (i.e., the surface facing the substrate) comprised of a core of cytoplasmic f-actin associated with a variety of signaling and cytoskeletal proteins, which include cortactin (Linder 2007; Linder and Kopp 2005; Murphy and Courtneidge 2011). In this context, we observe that puncta of ADAMTS-4 associated with HUVECs cultured in 3D collagen frequently have f-actin/cortactin cores. Podosomes are involved in cell migration and ECM degradation (Murphy and Courtneidge 2011) and contain a variety of proteases, including MMP-2, MMP-9, MT1-MMP, the ADAM family of sheddases, cathepsins, serine proteases, and urokinase plasminogen activator receptor (uPAR) (Linder 2007; Stylli et al. 2008). Of note, ADAMTS-4 has been shown to co-localize with cortactin in podosomes on cells of the trabecular meshwork of the human eye (Keller et al. 2009). Previous studies (Tatin et al. 2006) have shown that short-term PMA treatment of HUVECs cultured on 2D plastic or glass substrata induced the appearance of podosomes that exhibited proteolytic activity involving MT1-MMP-mediated activation of MMP-2. Our observations suggest that podosomes are also generated by HUVECs cultured in 3D collagen gels, not only by single cells, but also by cells comprising multicellular tubes. Interestingly, we find not only that both ADAMTS-4 and its substrate biglycan co-localize with podosomal cortactin cores, but also that both ADAMTS-4 and biglycan appear to cluster in the cytoplasm underlying the cortactin, suggesting that the podosome might act as an organizing center for ADAMTS-4 and biglycan. In this context, it is noteworthy that ADAMTS-4 and versican are found in podosomes of ocular trabecular meshwork cells (Aga et al. 2008; Keller et al. 2009). The presence of podosomes on HUVECs undergoing tubulogenesis coupled with the expression, in podosomes, of ADAMTS-4 and its proteoglycan substrate biglycan point to an expanded role for these dynamic cell surface structures in vascular morphogenesis.
In conclusion we observed in a model of HUVEC tubulogenesis in 3D collagen gels, the differential expression of mRNAs for ADAMTS-1, -4, and -5 and the proteoglycans versican, decorin, and biglycan, with ADAMTS-4 and biglycan predominant. Expression of ADAMTS-4 mRNA and secreted protein peaked at the onset of tubulogenesis and was significantly greater than in control cultures. Biglycan was expressed at similar levels by tubulogenic and control cultures, but appeared in lower molecular weight forms only in tubulogenic cultures, indicative of proteolytic processing. An enzyme/substrate relationship between ADAMTS-4 and biglycan was suggested by their co-association in puncta on single HUVECs and HUVEC tubes that formed in 3D collagen gels. Both ADAMTS-4 and biglycan were associated with cortactin-positive podosome-like structures.
Acknowledgments
We wish to thank Susan Potter-Perigo, Ph.D. for her critical reading of the manuscript and Virginia M. Green, Ph.D. for her editorial assistance.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL064387 (T.N.W.) and by DOD U.S. Army Medical Research Acquisition Activity (USAMRAA) Award W81XWH-07-01-0246 (T.N.W., R.B.V.).
References
- Aga M, Bradley JM, Keller KE, Kelley MJ, Acott TS. (2008). Specialized podosome- or invadopodia-like structures (PILS) for focal trabecular meshwork extracellular matrix turnover. Invest Ophthalmol Vis Sci 49:5353-5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin AC, Fogel E, Zorzi P, Nicosia RF. (2008). The aortic ring model of angiogenesis. Methods Enzymol 443:119-136 [DOI] [PubMed] [Google Scholar]
- Apte SS. (2009) A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem 284:31493-31497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo AG, Iruela-Arispe ML. (2010). Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res 86:226-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahramsoltani M, Plendl J, Janczyk P, Custodis P, Kaessmeyer S. (2009). Quantitation of angiogenesis and antiangiogenesis in vivo, ex vivo and in vitro - an overview. ALTEX 26:95-107 [DOI] [PubMed] [Google Scholar]
- Bellon G, Martiny L, Robinet A. (2004). Matrix metalloproteinases and matrikines in angiogenesis. Crit Rev Oncol Hematol 49:203-220 [DOI] [PubMed] [Google Scholar]
- Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. (1990). Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem 38:1549-1563 [DOI] [PubMed] [Google Scholar]
- Calabrese GC, Gazzaniga S, Oberkersch R, Wainstok R. (2011). Decorin and biglycan expression: its relation with endothelial heterogeneity. Histol Histopathol 26:481-490 [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. (2011). Molecular mechanisms and clinical applications of angiogenesis. Nature 473:298-307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaruzza S, Schiappacassi M, Ljungberg-Rose A, Spessotto P, Perissinotto D, Morgelin M, Mucignat MT, Colombatti A, Perris R. (2002). Distribution of PG-M/versican variants in human tissues and de novo expression of isoform V3 upon endothelial cell activation, migration, and neoangiogenesis in vitro. J Biol Chem 277:47626-47635 [DOI] [PubMed] [Google Scholar]
- Chan CK, Rolle MW, Potter-Perigo S, Braun KR, Van Biber BP, Laflamme MA, Murry CE, Wight TN. (2010). Differentiation of cardiomyocytes from human embryonic stem cells is accompanied by changes in the extracellular matrix production of versican and hyaluronan. J Cell Biochem 111:585-596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SH, Kanasaki K, Gocheva V, Blum G, Harper J, Moses MA, Shih SC, Nagy JA, Joyce J, Bogyo M, Kalluri R, Dvorak HF. (2009). VEGF-A induces angiogenesis by perturbing the cathepsin-cysteine protease inhibitor balance in venules, causing basement membrane degradation and mother vessel formation. Cancer Res 69:4537-4544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156-159 [DOI] [PubMed] [Google Scholar]
- Chun TH, Sabeh F, Ota I, Murphy H, McDonagh KT, Holmbeck K, Birkedal-Hansen H, Allen ED, Weiss SJ. (2004). MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol 167:757-767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corps AN, Jones GC, Harrall RL, Curry VA, Hazleman BL, Riley GP. (2008). The regulation of aggrecanase ADAMTS-4 expression in human Achilles tendon and tendon-derived cells. Matrix Biol 27:393-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi A, Riminucci M, Fisher LW, Bianco P. (2001). Achondrogenesis type IB: agenesis of cartilage interterritorial matrix as the link between gene defect and pathological skeletal phenotype. Arch Pathol Lab Med 125:1375-1378 [DOI] [PubMed] [Google Scholar]
- Davis GE, Bayless KJ, Mavila A. (2002). Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat Rec 268:252-275 [DOI] [PubMed] [Google Scholar]
- Davis GE, Camarillo CW. (1996). An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res 224:39-51 [DOI] [PubMed] [Google Scholar]
- Davis GE, Koh W, Stratman AN. (2007). Mechanisms controlling human endothelial lumen formation and tube assembly in three-dimensional extracellular matrices. Birth Defects Res C Embryo Today 81:270-285 [DOI] [PubMed] [Google Scholar]
- Davis GE, Saunders WB. (2006). Molecular balance of capillary tube formation versus regression in wound repair: role of matrix metalloproteinases and their inhibitors. J Investig Dermatol Symp Proc 11:44-56 [DOI] [PubMed] [Google Scholar]
- Davis GE, Senger DR. (2005). Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res 97:1093-1107 [DOI] [PubMed] [Google Scholar]
- Fiedler LR, Schonherr E, Waddington R, Niland S, Seidler DG, Aeschlimann D, Eble JA. (2008). Decorin regulates endothelial cell motility on collagen I through activation of insulin-like growth factor I receptor and modulation of alpha2beta1 integrin activity. J Biol Chem 283:17406-17415 [DOI] [PubMed] [Google Scholar]
- Fisher LW, Stubbs JT, 3rd, Young MF. (1995). Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop Scand Suppl 266:61-65 [PubMed] [Google Scholar]
- Flannery CR, Zeng W, Corcoran C, Collins-Racie LA, Chockalingam PS, Hebert T, Mackie SA, McDonagh T, Crawford TK, Tomkinson KN, LaVallie ER, Morris EA. (2002). Autocatalytic cleavage of ADAMTS-4 (Aggrecanase-1) reveals multiple glycosaminoglycan-binding sites. J Biol Chem 277:42775-42780 [DOI] [PubMed] [Google Scholar]
- Fu Y, Nagy JA, Brown LF, Shih SC, Johnson PY, Chan CK, Dvorak HF, Wight TN. (2011). Proteolytic cleavage of versican and involvement of ADAMTS-1 in VEGF-A/VPF-induced pathological angiogenesis. J Histochem Cytochem 59:463-473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron C, Kashiwagi M, Lim NH, Enghild JJ, Thogersen IB, Hughes C, Caterson B, Nagase H. (2007). Proteolytic activities of human ADAMTS-5: comparative studies with ADAMTS-4. J Biol Chem 282:18294-18306 [DOI] [PubMed] [Google Scholar]
- Ghajar CM, George SC, Putnam AJ. (2008). Matrix metalloproteinase control of capillary morphogenesis. Crit Rev Eukaryot Gene Expr 18:251-278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Kordowska J, Williams GL, Wang CL, Hai CM. (2007). Erk1/2 MAPK and caldesmon differentially regulate podosome dynamics in A7r5 vascular smooth muscle cells. Exp Cell Res 313:849-866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handsley MM, Edwards DR. (2005). Metalloproteinases and their inhibitors in tumor angiogenesis. Int J Cancer 115:849-860 [DOI] [PubMed] [Google Scholar]
- Hetheridge C, Mavria G, Mellor H. (2011). Uses of the in vitro endothelial-fibroblast organotypic co-culture assay in angiogenesis research. Biochem Soc Trans 39:1597-1600 [DOI] [PubMed] [Google Scholar]
- Holderfield MT, Hughes CC. (2008). Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res 102:637-652 [DOI] [PubMed] [Google Scholar]
- Hsu YP, Staton CA, Cross N, Buttle DJ. (2012). Anti-angiogenic properties of ADAMTS-4 in vitro. Int J Exp Pathol 93:70-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Asawa T, Takato T, Sakai R. (2003). Cooperative roles of Fyn and cortactin in cell migration of metastatic murine melanoma. J Biol Chem 278:48367-48376 [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Davis GE. (2009). Cellular and molecular mechanisms of vascular lumen formation. Dev Cell 16:222-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järveläinen H, Wight T. (2002). Vascular proteoglycans. In Garg H, Roughly P, Hales P, eds. Proteoglycans in Lung Disease. New York, Marcel Dekker, 291-321 [Google Scholar]
- Järveläinen HT, Iruela-Arispe ML, Kinsella MG, Sandell LJ, Sage EH, Wight TN. (1992). Expression of decorin by sprouting bovine aortic endothelial cells exhibiting angiogenesis in vitro. Exp Cell Res 203:395-401 [DOI] [PubMed] [Google Scholar]
- Järveläinen HT, Kinsella MG, Wight TN, Sandell LJ. (1991). Differential expression of small chondroitin/dermatan sulfate proteoglycans, PG-I/biglycan and PG-II/decorin, by vascular smooth muscle and endothelial cells in culture. J Biol Chem 266:23274-23281 [PubMed] [Google Scholar]
- Kahn J, Mehraban F, Ingle G, Xin X, Bryant JE, Vehar G, Schoenfeld J, Grimaldi CJ, Peale F, Draksharapu A, Lewin DA, Gerritsen ME. (2000). Gene expression profiling in an in vitro model of angiogenesis. Am J Pathol 156:1887-1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji T, Yamada A, Miyajima S, Yamamoto C, Fujiwara Y, Wight TN, Kinsella MG. (2000). Cell density-dependent regulation of proteoglycan synthesis by transforming growth factor-beta(1) in cultured bovine aortic endothelial cells. J Biol Chem 275:1463-1470 [DOI] [PubMed] [Google Scholar]
- Kaji T, Yamamoto C, Oh-i M, Nishida T, Takigawa M. (2004). Differential regulation of biglycan and decorin synthesis by connective tissue growth factor in cultured vascular endothelial cells. Biochem Biophys Res Commun 322:22-28 [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Enghild JJ, Gendron C, Hughes C, Caterson B, Itoh Y, Nagase H. (2004). Altered proteolytic activities of ADAMTS-4 expressed by C-terminal processing. J Biol Chem 279:10109-10119 [DOI] [PubMed] [Google Scholar]
- Keller KE, Bradley JM, Acott TS. (2009). Differential effects of ADAMTS-1, -4, and -5 in the trabecular meshwork. Invest Ophthalmol Vis Sci 50:5769-5777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella MG, Bressler SL, Wight TN. (2004). The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr 14:203-234 [DOI] [PubMed] [Google Scholar]
- Kinsella MG, Tsoi CK, Järveläinen HT, Wight TN. (1997). Selective expression and processing of biglycan during migration of bovine aortic endothelial cells: the role of endogenous basic fibroblast growth factor. J Biol Chem 272:318-325 [DOI] [PubMed] [Google Scholar]
- Kobe B, Deisenhofer J. (1994). The leucine-rich repeat: a versatile binding motif. Trends Biochem Sci 19:415-421 [DOI] [PubMed] [Google Scholar]
- Koh W, Stratman AN, Sacharidou A, Davis GE. (2008). In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Methods Enzymol 443:83-101 [DOI] [PubMed] [Google Scholar]
- Koike T, Vernon RB, Gooden MD, Sadoun E, Reed MJ. (2003). Inhibited angiogenesis in aging: a role for TIMP-2. J Gerontol A Biol Sci Med Sci 58:B798-805 [DOI] [PubMed] [Google Scholar]
- Koike T, Vernon RB, Hamner MA, Sadoun E, Reed MJ. (2002). MT1-MMP, but not secreted MMPs, influences the migration of human microvascular endothelial cells in 3-dimensional collagen gels. J Cell Biochem 86:748-758 [DOI] [PubMed] [Google Scholar]
- Koyama H, Hibi T, Isogai Z, Yoneda M, Fujimori M, Amano J, Kawakubo M, Kannagi R, Kimata K, Taniguchi S, Itano N. (2007). Hyperproduction of hyaluronan in neu-induced mammary tumor accelerates angiogenesis through stromal cell recruitment: possible involvement of versican/PG-M. Am J Pathol 170:1086-1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafleur MA, Handsley MM, Knauper V, Murphy G, Edwards DR. (2002). Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases (MT-MMPs). J Cell Sci 115:3427-3438 [DOI] [PubMed] [Google Scholar]
- Lee NV, Sato M, Annis DS, Loo JA, Wu L, Mosher DF, Iruela-Arispe ML. (2006). ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. EMBO J 25:5270-5283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S. (2007). The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol 17:107-117 [DOI] [PubMed] [Google Scholar]
- Linder S, Kopp P. (2005). Podosomes at a glance. J Cell Sci 118:2079-2082 [DOI] [PubMed] [Google Scholar]
- Luque A, Carpizo DR, Iruela-Arispe ML. (2003). ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J Biol Chem 278:23656-23665 [DOI] [PubMed] [Google Scholar]
- McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, Wong TW. (2000). Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J Cell Biol 151:187-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melching LI, Fisher WD, Lee ER, Mort JS, Roughley PJ. (2006). The cleavage of biglycan by aggrecanases. Osteoarthritis Cartilage 14:1147-1154 [DOI] [PubMed] [Google Scholar]
- Murphy DA, Courtneidge SA. (2011). The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol 12:413-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu MN, Hughes CC. (2008). An optimized three-dimensional in vitro model for the analysis of angiogenesis. Methods Enzymol 443:65-82 [DOI] [PubMed] [Google Scholar]
- Nelimarkka L, Kainulainen V, Schönherr E, Moisander S, Jortikka M, Lammi M, Elenius K, Jalkanen M, Järveläinen H. (1997). Expression of small extracellular chondroitin/dermatan sulfate proteoglycans is differentially regulated in human endothelial cells. J Biol Chem 272:12730-12737 [DOI] [PubMed] [Google Scholar]
- Ochoa GC, Slepnev VI, Neff L, Ringstad N, Takei K, Daniell L, Kim W, Cao H, McNiven M, Baron R, De Camilli P. (2000). A functional link between dynamin and the actin cytoskeleton at podosomes. J Cell Biol 150:377-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS. (2001). Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol 21:1104-1117 [DOI] [PubMed] [Google Scholar]
- Pettersson A, Nagy JA, Brown LF, Sundberg C, Morgan E, Jungles S, Carter R, Krieger JE, Manseau EJ, Harvey VS, Eckelhoefer IA, Feng D, Dvorak AM, Mulligan RC, Dvorak HF. (2000). Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab Invest 80:99-115 [DOI] [PubMed] [Google Scholar]
- Porter S, Clark IM, Kevorkian L, Edwards DR. (2005). The ADAMTS metalloproteinases. Biochem J 386:15-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AJ, Little CB, Hughes CE. (2007). Low molecular weight isoforms of the aggrecanases are responsible for the cytokine-induced proteolysis of aggrecan in a porcine chondrocyte culture system. Arthritis Rheum 56:3010-3019 [DOI] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ, Bateman A. (2010). MEROPS: the peptidase database. Nucl Acids Res 38:D227-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocks N, Paulissen G, El Hour M, Quesada F, Crahay C, Gueders M, Foidart JM, Noel A, Cataldo D. (2008). Emerging roles of ADAM and ADAMTS metalloproteinases in cancer. Biochimie 90:369-379 [DOI] [PubMed] [Google Scholar]
- Roughley PJ. (2001). Articular cartilage and changes in arthritis: noncollagenous proteins and proteoglycans in the extracellular matrix of cartilage. Arthritis Res 3:342-347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R, Zhang B, Moses MA. (2006). Making the cut: protease-mediated regulation of angiogenesis. Exp Cell Res 312:608-622 [DOI] [PubMed] [Google Scholar]
- Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, Lemire JM, Fischer JW, Wight TN, Clowes AW. (2001). Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem 276:13372-13378 [DOI] [PubMed] [Google Scholar]
- Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. (2006). Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol 175:179-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr E, Sunderkotter C, Schaefer L, Thanos S, Grassel S, Oldberg A, Iozzo RV, Young MF, Kresse H. (2004). Decorin deficiency leads to impaired angiogenesis in injured mouse cornea. J Vasc Res 41:499-508 [DOI] [PubMed] [Google Scholar]
- Scott PG, Dodd CM, Bergmann EM, Sheehan JK, Bishop PN. (2006). Crystal structure of the biglycan dimer and evidence that dimerization is essential for folding and stability of class I small leucine-rich repeat proteoglycans. J Biol Chem 281:13324-13332 [DOI] [PubMed] [Google Scholar]
- Shih SC, Smith LE. (2005). Quantitative multi-gene transcriptional profiling using real-time PCR with a master template. Exp Mol Pathol 79:14-22 [DOI] [PubMed] [Google Scholar]
- Stylli SS, Kaye AH, Lock P. (2008). Invadopodia: at the cutting edge of tumour invasion. J Clin Neurosci 15:725-737 [DOI] [PubMed] [Google Scholar]
- Tatin F, Varon C, Genot E, Moreau V. (2006). A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J Cell Sci 119:769-781 [DOI] [PubMed] [Google Scholar]
- Taylor CJ, Motamed K, Lilly B. (2006). Protein kinase C and downstream signaling pathways in a three-dimensional model of phorbol ester-induced angiogenesis. Angiogenesis 9:39-51 [DOI] [PubMed] [Google Scholar]
- Templeton DM, Fan MY. (1996). Posttranscriptional effects of glucose on proteoglycan expression in mesangial cells. Metabolism 45:1136-1146 [DOI] [PubMed] [Google Scholar]
- Ucuzian AA, Greisler HP. (2007). In vitro models of angiogenesis. World J Surg 31:654-663 [DOI] [PubMed] [Google Scholar]
- Ungefroren H, Krull NB. (1996). Transcriptional regulation of the human biglycan gene. J Biol Chem 271:15787-15795 [DOI] [PubMed] [Google Scholar]
- van Hinsbergh VW, Engelse MA, Quax PH. (2006). Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol 26:716-728 [DOI] [PubMed] [Google Scholar]
- van Hinsbergh VW, Koolwijk P. (2008). Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res 78:203-212 [DOI] [PubMed] [Google Scholar]
- Vazquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M, Iruela-Arispe ML. (1999). METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem 274:23349-23357 [DOI] [PubMed] [Google Scholar]
- Vernon RB, Sage EH. (1999). A novel, quantitative model for study of endothelial cell migration and sprout formation within three-dimensional collagen matrices. Microvasc Res 57:118-133 [DOI] [PubMed] [Google Scholar]
- Vernon RB, Sage EH. (1995). Between molecules and morphology. Extracellular matrix and creation of vascular form. Am J Pathol 147:873-883 [PMC free article] [PubMed] [Google Scholar]
- Wang J, Taba Y, Pang J, Yin G, Yan C, Berk BC. (2009) GIT1 mediates VEGF-induced podosome formation in endothelial cells: critical role for PLCgamma. Arterioscler Thromb Vasc Biol 29:202-208 [DOI] [PMC free article] [PubMed] [Google Scholar]