Abstract
The active component of the acne drug Accutane is 13-cis-retinoic acid (RA), and it is highly teratogenic for the developing central nervous system. Very little is known, however, regarding the effect of this drug on the adult brain. Regions of the brain that may be susceptible to RA are those that continue to generate new neurons. In the adult mouse, neurogenesis is maintained in the hippocampus and subventricular zone. This report demonstrates that a clinical dose (1 mg/kg/day) of 13-cis-RA in mice significantly reduces cell proliferation in the hippocampus and the subventricular zone, suppresses hippocampal neurogenesis, and severely disrupts capacity to learn a spatial radial maze task. The results demonstrate that the regions of the adult brain where cell proliferation is ongoing are highly sensitive to disruption by a clinical dose of 13-cis-RA.
Accutane, the active component of which is 13-cis-retinoic acid (RA), is the primary treatment for chronic acne. RA induces differentiation and reduces proliferation of stem and progenitor cells, and 13-cis-RA helps control acne by inducing similar events in basal sebocytes (1). These same actions also lead to 13-cis-RA's side effects, and these are directed toward proliferating cells in the adult such as in the skin, gut, and bone (2). However, Accutane has also been reported to modify behavior, sometimes resulting in severe depression with suicidal ideation (2-4). It is surprising that 13-cis-RA might influence the adult brain, an organ in which there is not extensive proliferation. Certainly, this effect has been considered contentious (5), in large part because no mechanism has been described for such an etiology. Cell proliferation, however, is known to occur in restricted regions of the adult brain. New neurons are born and develop in the subgranular zone (SGZ) of the hippocampal formation as well as in the subventricular zone (SVZ) of the forebrain and in its extension via the rostral migratory stream to the olfactory bulb (6). If 13-cis-RA influences the brain, then these proliferating cells would be likely targets. We show, in a mouse model, that 13-cis-RA, at a dose of 1 mg/kg/day, reduces both the rate of hippocampal cell birth and the number of newborn neurons. Because the hippocampus is primarily associated with memory processing, and new neuronal birth has been suggested to be required for this task (7), it would also be predicted that 13-cis-RA would result in a decline in hippocampal-dependent memory. In this study, we demonstrate that, by using a radial arm maze test, there is a severe defect in the ability of 13-cis-RA-injected mice to perform this task, suggesting that long-term exposure to 13-cis-RA can disrupt hippocampal-dependent behavior.
Methods
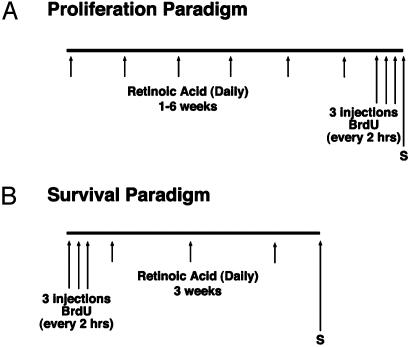
BrdUrd Treatment. Mice were injected with BrdUrd in two types of experimental paradigms, the first to determine the influence of RA on the rate of cell proliferation in the hippocampus and the second as a cell marker to follow cell survival. To follow cell proliferation (Fig. 1A), RA was injected daily i.p. at a concentration of 1 mg/kg for between 1 and 6 weeks with three mice per group. After this time, BrdUrd was injected once every 2 h over a 6-h period to label proliferating cells, and the animals were then killed for immunohistochemical processing. To determine cell survival (Fig. 1B), mice were injected with BrdUrd at the beginning of the experiment to follow the survival of the labeled cells after 3 weeks of daily treatment with 1 mg/kg RA. Again, three mice were used per group. As described below, the sections were immunostained for either BrdUrd or double labeled for both BrdUrd and the neuronal marker Neu-N.
Fig. 1.
Experimental paradigms of BrdUrd labeling. (A) Mice were injected with 1 mg/kg 13-cis-RA or vehicle daily for 1-6 weeks. After this period, the mice were injected with BrdUrd at 50 mg/kg every 2 h over a 6-h period. The brains were then perfused, sectioned, and immunostained, and the number of BrdUrd-labeled cells was counted in every 12th section in the hippocampal formation and SVZ. (B) The mice were injected with BrdUrd at 50 mg/kg every 2 h over a 6-h period, and the mice were allowed to survive for another 3 weeks with continued daily RA injections. The animals were then killed and processed as described above and stained for BrdUrd or double labeled for BrdUrd and the neuronal marker Neu-N.
Immunohistochemistry. Tissues for histology were sectioned with a cryostat or microtome and, for immunohistochemistry, standard procedures were followed by using fluorescent-conjugated secondary antibodies (8, 9). To label for BrdUrd, mice were deeply anesthetized and intracardially perfused, first with physiological saline at room temperature, then with 100 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2). The mice were then sunk in 30% sucrose/phosphate buffer and sectioned coronally with a sledge microtome at 40 μm to be labeled as free-floating sections in meshed wells (Costar) and labeled with BrdUrd primary antibody (1:500) (Accurate Scientific, Westbury, NY) as described (8) by using an anti-rat secondary antibody (Alexafluor 546; Molecular Probes).
To identify neuronal precursors cells, sections were first incubated with Neu-N (1:500) (Chemicon) antibody, followed by incubation with anti-mouse secondary antibody (Alexafluor 488; Molecular Probes). Sections were stabilized in 4% paraformaldehyde/0.1 M phosphate buffer for 15 min and further processed for anti-BrdUrd immunohistochemistry as above. When analyzed by confocal microscopy, cells were considered double-labeled if both chromophores were seen within the same cell in three consecutive 1-μm optical sections in the z axis (10). For each treatment, at least 50 BrdUrd-positive cells in the SGZ were sampled to determine whether they were also Neu-N positive.
A modified unbiased stereological protocol was used that has been reported to successfully quantitate BrdUrd labeling in hippocampus (11-13). BrdUrd-labeled cells were counted in every 12th section at 400×. The average number of sections per brain is eight, and the results were presented as the total number of cells in these eight sections, taking the average of between three and four mice per treatment. During the count, cells in the outermost focal plane were omitted so that no BrdUrd-labeled cells were counted twice, and the area counted within the structure was consistent in each section (11). In all cases, labeled cells were counted by an investigator blinded to the coded slides. In those experiments focusing on the SGZ of the dentate gyrus, a cell was counted if it touched or was within two cell diameters of the SGZ (14).
The Radial Arm Maze. The central part of the radial maze (15, 16) measured 22 cm in diameter. The arms (25 cm long, 6 cm high, 6 cm wide) were enclosed and made of transparent Plexiglas with a dark gray floor. Arm entrances could be blocked by lowering clear Plexiglas guillotine doors. At the end of each arm, some food pellets were deposited behind a perforated Plexiglas wall to prevent the mice from smelling the presence or absence of a food reward. Just in front of this perforated wall, a small food pellet (≈10 mg) was placed behind a small barrier to prevent the animal from seeing whether or not a specific arm still contained a reward or not. The maze was placed on the floor of a mouse room rich in spatial content (size 3.5 × 2.5 m). In addition, several extra maze cues were provided close (5-30 cm) to the arms in a fixed configuration. On the first day of testing, each mouse was subjected to a habituation session in which it was allowed to explore the maze freely for 10 min. Arm doors remained open, and no food was accessible in the maze. Mice were deprived of food but not water. They were maintained at about 85% of their initial body weight throughout the experiment. The habituation trial was followed by five daily training trials, during which all eight arms contained a food reward. Animals were confined between arm visits for 5 s on the central platform by lowering the guillotine doors. An error was noted if the animals failed to eat the food reward or revisit an arm in which the food reward had previously been eaten. In addition, the numbers of different arms visited among the first eight arms sampled (new entries) were noted. Trials ended when animals had found and eaten all eight rewards.
Determination of Plasma RA Concentration. Blood was collected rapidly by heart puncture under dim yellow light into tubes containing sodium heparin. The blood was transferred to microfuge tubes and spun at 14,000 rpm for 5 min (Eppendorf model 5415C). The supernatant was removed and snap frozen on dry ice. Aliquots (100 μl) of plasma sample were extracted with isopropanol-ethanol 2:1 (vol/vol) and RA detected by absorption at 354 nm by using a Spectrasystem UV2000 detector (Thermo Separations Products, Riviera Beach, FL), as described (17).
Assay for RA Bound to Cellular RA Binding Protein (Holo-CRABP). This test was performed as described (18). Briefly, the hippocampus was dissected 20 min after injection of 1 mg/kg trans-RA. The tissue was homogenized, and proteins were separated by isoelectric focusing. The lanes were cut into slices and transferred into 100 μl of L15-CO2 medium containing 2 mM DTT and incubated for 2 h at 37°C, allowing release of the protein-bound RA, which was detected and quantified by using RA reporter cells.
Results
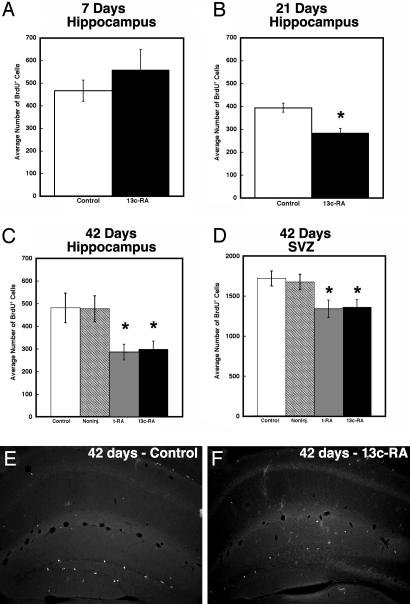
A wide-ranging effect of RA is to inhibit proliferation in dividing cells, and this accounts for its frequent consideration as an anti-cancer agent. RA has been reported to suppress cell proliferation in many neural progenitor cells (19) and hippocampus-derived stem cell clones (20). If 13-cis-RA influences the adult brain, then potential targets would be the neurons that derive from populations of progenitors maintained as a dividing pool in the hippocampus and the SVZ. The reported effects of 13-cis-RA on the brain and behavior do not occur immediately but require longer-term exposure (3). A similar delayed response is also evident for 13-cis-RA's anti-acne effects on the skin (21). We investigated the effects of short- and long-term RA treatment on the proliferation of hippocampal cell precursors comparing the effect of vehicle-injected (50% DMSO, 50% saline) and uninjected controls with all-trans-RA- and 13-cis-RA-treated mice. We examined the influence on cell division as determined by nuclear uptake of BrdUrd and immunohistochemical detection in tissue sections (22) as described in Methods and Fig. 1 A. In humans, Accutane (13-cis-RA) is used orally between 0.5 and 2.0 mg/kg/day over a 4-month treatment period, predominantly in a teenage population in whom the rate of neurogenesis would be predicted to be relatively high (14). To parallel these conditions, our studies were performed on young adult CD-1 mice, an age and strain with relatively high levels of neurogenesis (14, 23). To avoid the trauma of daily oral gavage, 13-cis-RA was injected i.p. at a daily dose of 1 mg/kg. After 7 days of 13-cis-RA exposure, a mild but not statistically significant increase in proliferation in the hippocampus was found (Fig. 2A). However, if 13-cis-RA exposure was extended to 21 days, a significant decrease in proliferation was apparent (Fig. 2B). By 42 days, proliferation had declined by 41% (Fig. 2C). Because of the possibility that the additional anxiety of daily injections could alter the rate of proliferation, as reported in various stress models (ref. 24 and references within), the effects of RA were compared to two controls, one injected with vehicle and the other left uninjected (Fig. 2C). The results for the two controls were identical, and it was clear that proliferation in the hippocampus was unaffected by this injection regime. The two isomers of RA, all-trans-RA and 13-cis-RA, were also compared, and these had identical effects on proliferation (Fig. 2C), suggesting that, as described in other systems (25), they have the same mechanism of action, i.e., activation of RA receptors (RARs). RA is known to influence a wide range of neural precursors (19), and 13-cis-RA would not be expected to show specificity for proliferative cells only in the hippocampus. BrdUrd-positive cells were counted in the SVZ and were also found to be reduced in number after 42-day treatment with either alltrans-RA or 13-cis-RA, although to a lesser extent than in the hippocampus (Fig. 2D).
Fig. 2.
The decline in hippocampal and SVZ cell proliferation after long-term RA treatment. Seven days of exposure to 13-cis-RA resulted in no statistically significant difference in the average number of BrdUrd-positive cells between the experimentally treated and control animals in the hippocampus (A). However, by 21 days of treatment (B), a significant decline was evident, which had reached a reduction of 41% by 42 days (C). This effect was not specificto the hippocampus as indicated by a decline in proliferation in the SVZ (D), although this decline was not to the same degree. The decline in BrdUrd labeling in the hippocampal formation is exemplified in control (E) and 13-cis-RA-treated mice (F) for 42 days. Note that different y axis scales were used between the hippocampus (A-C) and SVZ (D). (A) Control = 467 ± 47, 13-cis-RA = 559 ± 91. (B) Control = 395 ± 20; 13-cis-RA = 285 ± 20, t = 4.59, P = 0.004 compared to control. (C) Control = 481 ± 38, noninjected = 478 ± 33. tRA = 284 ± 21, t = 4.55, P = 0.010 compared to control (vehicle injected). 13-cis-RA = 299 ± 21, t = 4.21, P = 0.014 compared to control (vehicle injected). (D) Control = 1721 ± 54, noninjected = 1,676 ± 55. tRA = 1342 ± 62, t = 4.55, P = 0.010 compared to control (vehicle injected). 13-cis-RA = 1,362 ± 54, t = 4.68, P = 0.009 compared to control (vehicle injected).
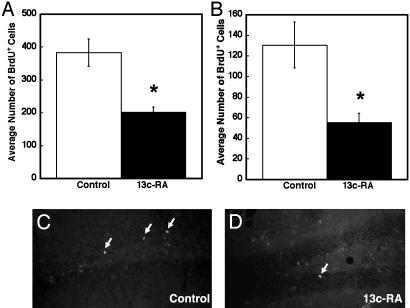
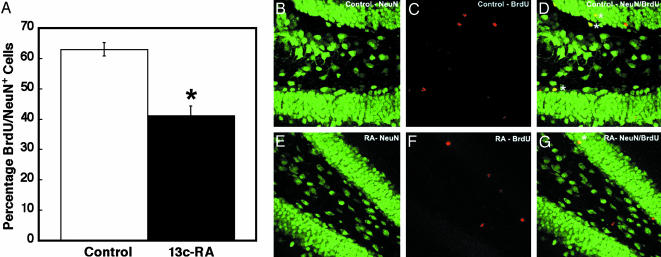
Thus, daily exposure to clinical levels of 13-cis-RA can depress cell proliferation in both the hippocampus and SVZ. To determine whether this influences the rate of new neuronal birth in the hippocampal SGZ, mice were pulsed with BrdUrd and exposed to 13-cis-RA for 21 days, as described in Methods and in Fig. 1B. Cell survival was followed by counting the numbers of BrdUrd-positive cells in the hippocampal formation (Fig. 3A) and in the SGZ (Fig. 3B). In both structures, it was evident that there was a decrease in the number of BrdUrd-positive cells remaining after 13-cis-RA exposure. In the SGZ, the number was reduced by 57% after 21 days. To determine the extent of this decline on precursors destined to become neurons, we used confocal microscopy analysis of BrdUrd-labeled cells in the SGZ to identify the number of BrdUrd-positive cells that were double labeled for the neuronal marker Neu-N (Fig. 4A). The number of neuronal precursors was reduced from 63% to 41%, implying that 13-cis-RA not only depressed rates of proliferation but also decreased the number of these cells that would become neurons.
Fig. 3.
The decline in number of BrdUrd-positive cells remaining in the hippocampus and hippocampal SGZ after 21 days of exposure to 13-cis-RA. If proliferating cells are labeled with BrdUrd and 13-cis-RA treatment is instigated and continued over 21 days, then a significant reduction in the number of BrdUrd-positive cells is evident both throughout the hippocampal formation (A) and SGZ (B), illustrated in representative sections (C and D) with labeled cells marked with arrows. (A) Control = 383 ± 42; 13-cis-RA = 203 ± 15, t = 4.07, P = 0.015. (B) Control = 131 ± 22; 13-cis-RA = 56 ± 9, t = 3.15, P = 0.035.
Fig. 4.
The decline in neurogenesis resulting from 21 days of 13-cis-RA treatment. Neurogenesis, as determined by the number of BrdUrd and Neu-N double-positive cells, is significantly reduced by RA treatment (A), illustrated in representative sections of control Neu-N (B), BrdUrd (C), and Neu-N/BrdUrd merged (D) and RA-treated Neu-N (E), BrdUrd (F), and Neu-N/BrdUrd merged (G). Double-labeled cells are tagged with asterisks. (A) Control = 63 ± 2.2; 13-cis-RA = 41 ± 3, t = 4.79, P = 0.009.
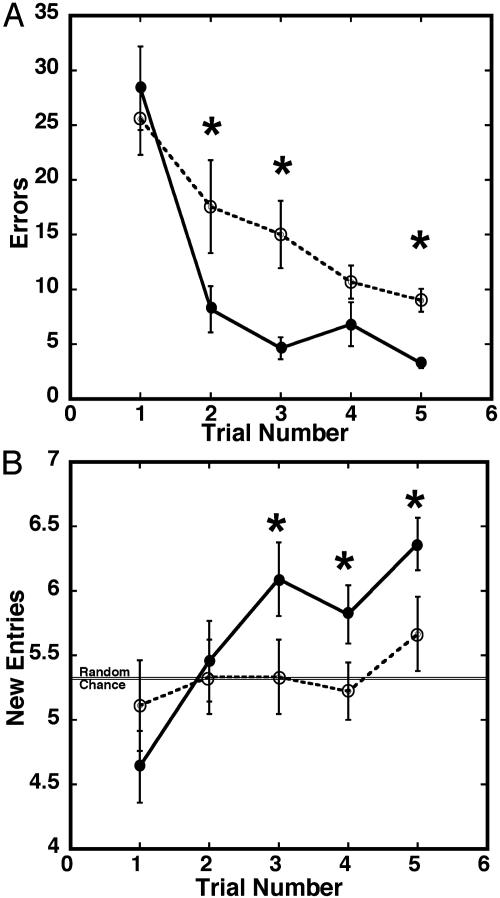
Several reports have suggested that hippocampal neurogenesis is necessary to support memory and learning (7, 26). To test whether 13-cis-RA influences this behavior in mice, animals were injected daily with 13-cis-RA for 28 days and subsequently tested in a spatial radial maze task (16) (Fig. 5). This task was chosen because it is very sensitive to hippocampal damage (27). In the five daily training trials of the spatial radial maze test, an error was noted if animals failed to eat the food reward or revisited an arm in which the food reward had previously been eaten. In addition, the numbers of different arms visited among the first eight arms sampled (i.e., new entries) were noted. The average number of errors was significantly higher for the RA-treated mice than the controls on trials two, three, and five (Fig. 5A), indicating a slower rate of learning after RA treatment. Further, the average number of new entries was significantly lower for the RA-treated mice compared to controls on trials three through five. As described by Olton and Samuelson (28), animals lacking spatial memory that enter arms in a random way will make an average of 5.3 new entries during their first eight arm choices. Our controls exhibited good spatial memory by visiting significantly more different arms as of the third trial, but RA-treated animals never exceeded chance levels. These results indicate a large deficit in spatial learning ability in 13-cis-RA-treated mice.
Fig. 5.
The influence of 13-cis-RA exposure on radial maze performance after 28 days of exposure to 13-cis-RA (RA). This treatment regime dramatically decreases the ability of mice to perform a spatial 8-arm radial maze test (five daily trials) (16) as shown by numbers of errors made (A) and number of new arms visited during the first eight choices (B) (chance level is 5.3). n = 10 per group, and errors bars are mean ± SE. •, control; ○, RA treated. The statistical significance of the errors for each trial in A is as follows: er2: t = 4.21, P < 0.0018; er3: t = 4.62, P < 0.0009; er4: t = 3.40, P < 0.0067; and er5: t = 7.29, P < 0.0001. The significance of the new entries for each trial in B is as follows: ne2: t = 0.49, P = 0.6314; ne3: t = 2.78, P = 0.0195; ne4: t = 2.29, P = 0.0451; and ne5: t = 5.23, P = 0.0004.
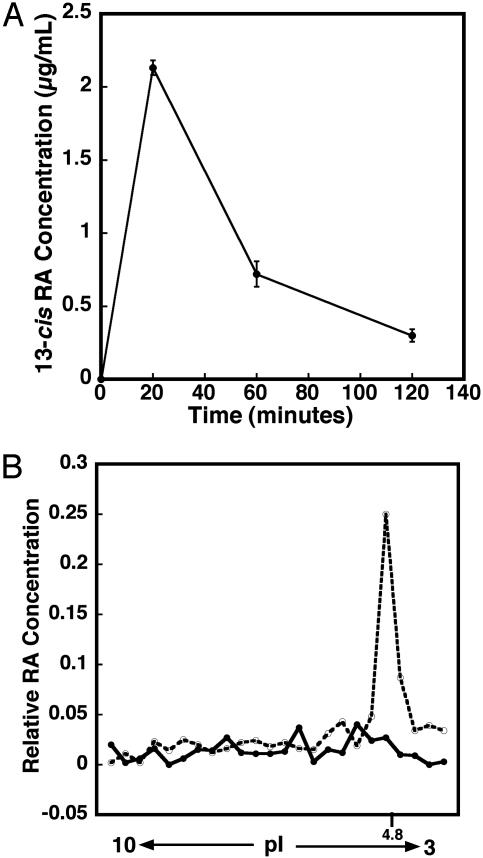
We have assumed that the 13-cis-RA treatments in this study are acting directly on the hippocampus. However, it is not known whether 13-cis-RA directly reaches this region of the brain. To address this question, we initially determined plasma 13-cis-RA concentrations. Because 13-cis-RA is very sensitive to light, heat, and oxygen, the blood samples were collected by heart puncture and snap frozen within 2 min. In the mouse, taking the average of three HPLC analyses (Fig. 6A), plasma levels peaked after 20 min at 2.2 μg/ml and quickly dropped to 0.3 μg/ml after 2 h. In comparison, human plasma levels (29) vary among individuals. Treatment with 0.5 mg/kg results in plasma concentrations up to 0.74 μg/ml, whereas treatment with 4 mg/kg leads to plasma concentrations from 0.828 to 1.95 μg/ml, with the peak ranging in time from 1.5 to 6 h. Thus, although a 1 mg/kg dose in the mouse results in somewhat higher peak concentrations, it reaches a maximal concentration earlier than in the human, leading to an overall exposure to 13-cis-RA that would be comparable to the human.
Fig. 6.
The plasma concentration of 13-cis-RA after a single injection of RA at 1 mg/kg and the entry of RA into the hippocampus to bind to cellular RA-binding protein I (CRABPI). (A) The plasma RA concentration quickly reached 2.2 μg/ml after 20 min and was down to 0.3 μg/ml after 2 h(n = 3). (B) The movement of RA into hippocampal cells could be shown by separating hippocampal CRABPI by isoelectric focusing (which runs at a pI of 4.8) and eluting the RA bound to this. •, hippocampus without RA; ○, hippocampus plus RA.
To demonstrate that RA enters the hippocampus and binds to the cytoplasmic RA binding proteins in the hippocampal cells, mice were injected with all-trans-RA; 6 h later, the hippocampi were removed and homogenized, and proteins were separated by isoelectric focusing. The gel was sliced, and all-trans-RA was eluted from the RA-binding proteins and assayed by using RA reporter cells (18). These reporter cells respond sensitively only to all-trans-RA, and hence this isomer was used for this experiment. The single injection of RA at 1 mg/kg could easily be detected bound to a protein of an isoelectric point identical to cellular retinoic acid binding protein-1 (Fig. 6B, pI 4.8), indicating that systemically applied RA enters hippocampal cells and incorporates into the endogenous pathways of RA signaling.
Discussion
Although 13-cis-RA is known to severely disrupt brain development in both the mouse (30) and the human (31), almost nothing is known about the effects of this drug on the adult brain. The potential influences of 13-cis-RA on the brain are important to identify given the use of 13-cis-RA in the anti-acne drug Accutane. Our results show that cell proliferation and neurogenesis in the adult mouse brain are targets of 13-cis-RA, and this drug leads to a decrease in both proliferation and neuronal production in the hippocampus, which are accompanied by a significant reduction in the ability to perform a learning task.
One likely explanation for the sensitivity of hippocampal neural progenitors to RA is that these cells normally require RA to regulate proliferation and differentiation. The adult mouse hippocampus expresses all of the molecular components necessary for RA signaling, i.e., synthetic enzymes, receptors, and binding proteins. Several RARs are expressed in the adult hippocampus including RARα (32). A source of RA is present in the form of the retinaldehyde dehydrogenase that synthesizes RA (33), whereas the cellular retinol binding protein-1 (CRBP-1), which promotes RA synthesis, is expressed at high levels in the hippocampus and dentate gyrus (34). That RA-regulated gene activation occurs in the hippocampus is indicated from studies with an RA reporter mouse, an in vivo retinoid detection assay that shows high levels of RA signaling in the dentate gyrus (35). In the adult, the hippocampus is the only region of strong reporter response (36), with lesser staining in the SVZ. The focus of RA signaling in the hippocampus would likely render this region the most sensitive to disruption by abnormal RA levels.
It is probable that RA performs multiple functions in the hippocampus, but given that the hippocampus is one of the few CNS regions in which neurogenesis takes place in the adult, and that RA regulates cell proliferation in many neural precursor cell types (20, 37-42), this would likely be a function regulated by RA. Our results demonstrate that long-term exposure to all-trans-RA or 13-cis-RA, at the 1 mg/kg clinical dose, results in a significant decrease in cell proliferation in the hippocampus, as well as in the SVZ. That cell survival is also decreased implies that chronic exposure to RA may interfere with the normal action of RA to promote neuronal birth, just as exposure of the embryo to RA interferes with the normal functions of RA to guide neurogenesis (43). However, the mechanism for this decrease in survival is unknown. The mouse stem cell line P19 (44) or its human equivalent, NT2 (45), exhibits increased apoptosis as a result of RA-induced differentiation, which may result from an insufficient supply of the appropriate growth factors necessary for the maturing cells (19). If chronic exposure of the hippocampus to RA leads to a decrease in proliferation and increase in neuronal differentiation, but absence of a corresponding increase in growth factors does not support the maintenance of these new cells, then a decrease in survival would be predicted. Further experiments to determine the extent of apoptotic cell death, and whether it may be reversed by supplementation with growth factors, are necessary to confirm this.
It is surprising perhaps that 13-cis-RA had a substantial effect on hippocampal neurogenesis. In vitro, this isomer has only a low affinity for the RARs compared to the all-trans isomer (46). However, when applied systemically, its effects are presumed to be mediated by the RARs (25), which is possible because 13-cis-RA can be isomerized to all-trans-RA in vivo (47). In addition, although 13-cis-RA has a relatively low affinity for the RARs, it is quite efficient at transactivation (48). Indeed, 13-cis-RA is slightly more effective than all-trans-RA in transactivation by means of RARα, the only RAR present in the dentate gyrus (32). Our results indicate that 13-cis-RA and all-trans-RA have similar effects on cell proliferation in the hippocampus.
This study focused on a behavioral trait (spatial learning) that reflected a hippocampus-based function in mice. We found that the daily application of 13-cis-RA at a dose of only 1 mg/kg/day severely disrupted learning in the radial maze test. Several studies have shown a correlation between neurogenesis and learning (7, 26), hence a plausible mechanism for this loss in learning performance is the concomitant decline in hippocampal neurogenesis and cell proliferation induced by 13-cis-RA. Other mechanisms for RA-induced changes in learning, however, may exist. For instance, the D2 dopamine receptor is inducible by RA and may be influenced by chronic exposure to RA (49). These receptors are known regulators of neurogenesis (50). RA is also known to induce differentiation of several neuronal progenitors toward a phenotype of GABAA receptor expression (51, 52), and these receptors are essential for hippocampal-mediated cognitive function via regulation of synaptic transmission (53). A third mechanism may be proposed based on the influence of RA on hippocampal long-term potentiation and long-term depression. When RA signaling is disrupted through null mutation of RARβ receptor, long-term potentiation and long-term depression are almost completely lost (54), whereas depletion of vitamin A results in a similar loss in these synaptic processes in CA1 and CA3 (35), together with learning deficits (55). In the case of acute RA treatment, as opposed to deficiency, treatment of aged mice with a low dose of RA (0.15 mg/kg) helps to reverse an age-related deficit in long-term potentiation amplitude and improve the ability of the mice to perform relational learning tasks (56). It is plausible that long-term exposure to RA may interfere with the normal role of RA, which has been proposed to support synaptic effectiveness (35), resulting in a decline in long-term potentiation and long-term depression. Such alternatives will be vital subjects of future research on the long-term effects of RA on hippocampal function.
Acknowledgments
Thanks are given both to Mr. Liam Grant for financial support and Dr. Marjorie Lees for discussion. J.C. is supported by National Institute of Child Health and Human Development Grant HD05515, and P.M. is supported by National Institute of Mental Health Grant MH66037.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RA, retinoic acid; SGZ, subgranular zone; SVZ, subventricular zone; RAR, RA receptor.
References
- 1.Orfanos, C. E. & Zouboulis, C. C. (1998) Dermatology 196, 140-147. [DOI] [PubMed] [Google Scholar]
- 2.Hull, P. R. & Demkiw-Bartel, C. (2000) J. Cutan. Med. Surg. 4, 66-70. [DOI] [PubMed] [Google Scholar]
- 3.Scheinman, P. L., Peck, G. L., Rubinow, D. R., DiGiovanna, J. J., Abangan, D. L. & Ravin, P. D. (1990) J. Am. Acad. Dermatol. 22, 1112-1114. [DOI] [PubMed] [Google Scholar]
- 4.Josefson, D. (1998) Br. Med. J. 316, 723. [DOI] [PubMed] [Google Scholar]
- 5.Jick, S. S., Kremers, H. M. & Vasilakis-Scaramozza, C. (2000) Arch. Dermatol. 136, 1231-1236. [DOI] [PubMed] [Google Scholar]
- 6.Gage, F. H. (2002) J. Neurosci. 22, 612-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shors, T. J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T. & Gould, E. (2001) Nature 410, 372-376. [DOI] [PubMed] [Google Scholar]
- 8.Palmer, T. D., Willhoite, A. R. & Gage, F. H. (2000) J. Comp. Neurol. 425, 479-494. [DOI] [PubMed] [Google Scholar]
- 9.Crandall, J. E., Dibble, C., Butler, D., Pays, L., Ahmad, N., Kostek, C., Puschel, A. W. & Schwarting, G. A. (2000) J. Neurobiol. 45, 195-206. [DOI] [PubMed] [Google Scholar]
- 10.Kornack, D. R. & Rakic, P. (2001) Science 294, 2127-2130. [DOI] [PubMed] [Google Scholar]
- 11.West, M., Slomianka, L. & Gundersen, H. (1991) Anat. Rec. 231, 482-497. [DOI] [PubMed] [Google Scholar]
- 12.Gould, E., Beylin, A., Tanapat, P., Reeves, A. & Shors, T. J. (1999) Nat. Neurosci. 2, 260-265. [DOI] [PubMed] [Google Scholar]
- 13.Malberg, J. E., Eisch, A. J., Nestler, E. J. & Duman, R. S. (2000) J. Neurosci. 20, 9104-9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn, H. G., Dickinson-Anson, H. & Gage, F. H. (1996) J. Neurosci. 16, 2027-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crusio, W. E., Schwegler, H. & Lipp, H. P. (1987) Brain Res. 425, 182-185. [DOI] [PubMed] [Google Scholar]
- 16.Schwegler, H., Crusio, W. E. & Brust, I. (1990) Neuroscience 34, 293-298. [DOI] [PubMed] [Google Scholar]
- 17.McCaffery, P., Evans, J., Koul, O., Volpert, A., Reid, K. & Ullman, M. (2002) J. Lipid Res. 43, 1143-1149. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto, M., Drager, U. C., Ong, D. E. & McCaffery, P. (1998) Eur. J. Biochem. 257, 344-350. [DOI] [PubMed] [Google Scholar]
- 19.McCaffery, P. & Drager, U. C. (2000) Cytokine Growth Factor Rev. 11, 233-249. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi, J., Palmer, T. D. & Gage, F. H. (1999) J. Neurobiol. 38, 65-81. [PubMed] [Google Scholar]
- 21.Peck, G. L. & DiGiovanna, J. J. (1994) in The Retinoids: Biology, Chemistry, and Medicine, eds. Sporn, M. B., Roberts, A. B. & Goodman, D. S. (Raven, New York).
- 22.Miller, M. W. & Nowakowski, R. S. (1988) Brain Res. 457, 44-52. [DOI] [PubMed] [Google Scholar]
- 23.Kempermann, G., Kuhn, H. G. & Gage, F. H. (1997) Proc. Natl. Acad. Sci. USA 94, 10409-10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McEwen, B. S. (2002) Neurobiol. Aging 23, 921-939. [DOI] [PubMed] [Google Scholar]
- 25.Tsukada, M., Schroder, M., Roos, T. C., Chandraratna, R. A., Reichert, U., Merk, H. F., Orfanos, C. E. & Zouboulis, C. C. (2000) J. Invest. Dermatol. 115, 321-327. [DOI] [PubMed] [Google Scholar]
- 26.Kempermann, G., Kuhn, H. G. & Gage, F. H. (1997) Nature 386, 493-495. [DOI] [PubMed] [Google Scholar]
- 27.Ward, M. T., Stoelzel, C. R. & Markus, E. J. (1999) Neurobiol. Aging 20, 373-380. [DOI] [PubMed] [Google Scholar]
- 28.Olton, D. S. & Samuelson, R. J. (1976) J. Exp. Psychol. 2, 97-117. [Google Scholar]
- 29.Kerr, I. G., Lippman, M. E., Jenkins, J. & Myers, C. E. (1982) Cancer Res. 42, 2069-2073. [PubMed] [Google Scholar]
- 30.Adams, J. (1993) Neurotoxicol. Teratol. 15, 193-202. [DOI] [PubMed] [Google Scholar]
- 31.Lammer, E. J. & Armstrong, D. L. (1992) in Retinoids in Normal Development and Teratogenesis, ed. Morris-Kay, G. M. (Oxford Univ. Press, Oxford), pp. 281-295.
- 32.Zetterstrom, R. H., Lindqvist, E., Mata de Urquiza, A., Tomac, A., Eriksson, U., Perlmann, T. & Olson, L. (1999) Eur. J. Neurosci. 11, 407-416. [DOI] [PubMed] [Google Scholar]
- 33.Connor, M. J. & Sidell, N. (1997) Mol. Chem. Neuropathol. 30, 239-252. [DOI] [PubMed] [Google Scholar]
- 34.Zetterstrom, R. H., Simon, A., Giacobini, M. M. J., Ericksson, U. & Olson, L. (1994) Neuroscience 62, 899-918. [DOI] [PubMed] [Google Scholar]
- 35.Misner, D. L., Jacobs, S., Shimizu, Y., de Urquiza, A. M., Solomin, L., Perlmann, T., De Luca, L. M., Stevens, C. F. & Evans, R. M. (2001) Proc. Natl. Acad. Sci. USA 98, 11714-11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner, E., Luo, T. & Drager, U. C. (2002) Cereb. Cortex 12, 1244-1253. [DOI] [PubMed] [Google Scholar]
- 37.Matsuoka, I., Mizuno, N. & Kurihara, K. (1989) Brain Res. 502, 53-60. [DOI] [PubMed] [Google Scholar]
- 38.Andrews, P. W., Nudelman, E., Hakomori, S. I. & Fenderson, B. A. (1990) Differentiation 43, 131-138. [DOI] [PubMed] [Google Scholar]
- 39.Ingraham, C. A. & Maness, P. F. (1990) Dev. Neurosci. 12, 273-285. [DOI] [PubMed] [Google Scholar]
- 40.McBurney, M. W. (1993) Int. J. Dev. Biol. 37, 135-140. [PubMed] [Google Scholar]
- 41.Bain, G., Kitchens, D., Yao, M., Huettner, J. E. & Gottlieb, D. I. (1995) Dev. Biol. 168, 342-357. [DOI] [PubMed] [Google Scholar]
- 42.Hill, D. P. & Robertson, K. A. (1998) Brain Res. Protoc. 2, 183-190. [DOI] [PubMed] [Google Scholar]
- 43.McCaffery, P. J., Adams, J., Maden, M. & Rosa-Molinar, E. (2003) Eur. J. Neurosci. 18, 457-472. [DOI] [PubMed] [Google Scholar]
- 44.Ninomiya, Y., Adams, R., Morriss-Kay, G. M. & Eto, K. (1997) J. Cell. Physiol. 172, 25-35. [DOI] [PubMed] [Google Scholar]
- 45.Guillemain, I., Fontes, G., Privat, A. & Chaudieu, I. (2003) J. Neurosci. Res. 71, 38-45. [DOI] [PubMed] [Google Scholar]
- 46.Allenby, G., Bocquel, M., Saunders, M., Kazmer, S., Speck, J., Rosenberger, M., Lovey, A., Kastner, P., Grippo, J. F., Chambon, P. & Levin, A. A. (1993) Proc. Natl. Acad. Sci. USA 90, 30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kraft, J. C., Kochhar, D. M., Scott, W. J. & Nau, H. (1987) Toxicol. Appl. Pharmacol. 87, 474-482. [DOI] [PubMed] [Google Scholar]
- 48.Idres, N., Marill, J., Flexor, M. A. & Chabot, G. G. (2002) J. Biol. Chem. 277, 31491-31498. [DOI] [PubMed] [Google Scholar]
- 49.Samad, T. A., Krezel, W., Chambon, P. & Borrelli, E. (1997) Proc. Natl. Acad. Sci. USA 94, 14349-14354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohtani, N., Goto, T., Waeber, C. & Bhide, P. G. (2003) J. Neurosci. 23, 2840-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reynolds, J. N., Ryan, P. J., Prasad, A. & Paterno, G. D. (1994) Neurosci. Lett. 165, 129-132. [DOI] [PubMed] [Google Scholar]
- 52.Neelands, T. R., Greenfield, L. J., Jr., Zhang, J., Turner, R. S. & Macdonald, R. L. (1998) J. Neurosci. 18, 4993-5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collinson, N., Kuenzi, F. M., Jarolimek, W., Maubach, K. A., Cothliff, R., Sur, C., Smith, A., Otu, F. M., Howell, O., Atack, J. R., et al. (2002) J. Neurosci. 22, 5572-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiang, M. Y., Misner, D., Kempermann, G., Schikorski, T., Giguere, V., Sucov, H. M., Gage, F. H., Stevens, C. F. & Evans, R. M. (1998) Neuron 21, 1353-1361. [DOI] [PubMed] [Google Scholar]
- 55.Cocco, S., Diaz, G., Stancampiano, R., Diana, A., Carta, M., Curreli, R., Sarais, L. & Fadda, F. (2002) Neuroscience 115, 475-482. [DOI] [PubMed] [Google Scholar]
- 56.Etchamendy, N., Enderlin, V., Marighetto, A., Vouimba, R. M., Pallet, V., Jaffard, R. & Higueret, P. (2001) J. Neurosci. 21, 6423-6429. [DOI] [PMC free article] [PubMed] [Google Scholar]