Abstract
The pronounced effects of the epigenetic diet (ED) and caloric restriction (CR) have on epigenetic gene regulation have been documented in many pre-clinical and clinical studies. Understanding epigenetics is of high importance because of the concept that external factors such as nutrition and diet may possess the ability to alter gene expression without modifying the DNA sequence. The ED introduces bioactive medicinal chemistry compounds such as sulforaphane (SFN), curcumin (CCM), epigallocatechin gallate (EGCG) and resveratrol (RSV) that are thought to aid in extending the human lifespan. CR, although similar to ED in the target of longevity, mildly reduces the total daily calorie intake while concurrently providing all beneficial nutrients. Both CR and ED may act as epigenetic modifiers to slow the aging process through histone modification, DNA methylation, and by modulating microRNA expression. CR and ED have been proposed as two important mechanisms that modulate and potentially slow the progression of age-related diseases such as cardiovascular disease (CVD), cancer, obesity, Alzheimer’s and osteoporosis to name a few. While many investigators have examined CR and ED as separate entities, this review will primarily focus on both as they relate to age-related diseases, their epigenetic effects and their medicinal chemistry.
Keywords: Age related diseases, caloric restriction, dietary polyphenols, epigenetics and epigenetic diet
INTRODUCTION
The term epigenetics was coined by Conrad Waddington in 1942 when he merged the two fields of genetics and epigenesis [1]. Epigenetics is generally defined as heritable changes in gene expression that occur without altering the DNA sequence [2]. Epigenetic modifications including DNA methylation, histone acetylation, and RNA interference may occur through external factors and are widely known for their reversibility. Recently, findings from several epigenetics studies have elucidated several pending mechanistic questions, and have become a common thread for other biomedical research fields. Emerging studies suggest that there may be a correlation between epigenetics, caloric restriction (CR) and organismal longevity [3]. In addition, epigenetic changes have recently been adopted as biomarkers for many age-related diseases [4–6]. Epigenetic modifications may also delay the aging process and impact diverse health benefits by activating numerous intracellular pathways. One leading theory suggests that bioactive phytochemicals including 1-isothiocyanato-4- (methylsulfinyl) butane (sulforaphane/SFN), (2R, 3R)-5,7-dihydroxy-2- (3,4,5-trihydroxyphenyl) - 3-4-dihydro-2H-chromen-3-yl, 3,4,5-trihydroxybenzoate (epigallocatechin gallate/EGCG), 3, 4’, 5 tihydroxystilbene (resveratrol/RSV), and (1E, 6E)-7-bis- (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene (curcumin/CCM) play significant roles as epigenetic modifiers [7, 8]. Moreover, these compounds show great potential in delaying aging-processes and the onset of accelerated degenerative diseases with regular consumption, which may eliminate one or more diseases with long-term intake [7–9]. The aforementioned theory is more commonly referred to as the “Epigenetic Diet” as it introduces bioactive compounds that aid in delaying the onset of aging and age-associated disease processes [10] (Table 1). While CR and ED have been studied individually, the two are similar due to their utilization of similar dietary compounds. Notably, phytochemicals used in the ED are often extracted from fruits and vegetables consumed on a CR diet.
Table 1.
Dietary Phytochemicals, Components of the Epigenetic Diet and Abbreviated Health Effects
| Food | Compound | Structure | Conditions Affected | References |
|---|---|---|---|---|
| Nuts, grapes, dried fruits | Resveratrol (RSV) | 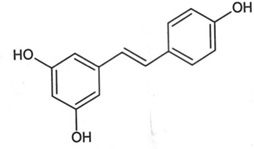 |
Aging and cancer | [16–18, 20, 21, 38–40] |
| Brussels sprouts, broccoli, and kale | Sulforaphane (SFN) | 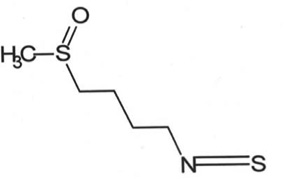 |
Aging and cancer | [61–64, 67–70] |
| Turmeric | Curcumin (CCM) | 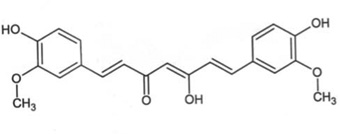 |
Aging and Alzheimer’s disease | [76–78, 85–88] |
| Green tea | Epigallocatechin (EGCG) | 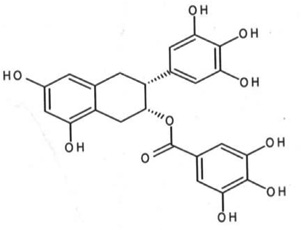 |
Aging and cancer | [7, 12, 28, 71, 72] |
Of all the external environmental factors, nutrition is thought to be one of the most important because it assists in modifying the expression of genes at the transcriptional level [11]. Elucidation of the importance of epigenetics and its many biological roles, such as modification of gene expression, has brought innovation to various fields including aging [12]. In addition, lower occurrences of a number of diseases have been reported through implementing of the ED, which is believed to silence chromatin (also observed in CR) [13].
To date, CR is considered to be one of the most effective and beneficial means of delaying the progression of aging, since it involves reducing the daily caloric intake, while simultaneously providing all essential nutrients [14]. The benefits of CR were initially observed by McCay and colleagues (1935), when an in vivo study conducted on rodents showed that reducing the total amount of calories ultimately prolonged their lifespan [15]. Since then, a number of studies have confirmed McCay’s hypothesis regarding CR and have demonstrated the benefits of a CR diet in a variety of animal models, including yeast [16], worms [17], spiders [18], flies [19], fish [20], mice [21], and rats [22], thereby increasing the probability of developing a method to slow the progression of aging.
CALORIC RESTRICTION’S EFFECTS ON AGING
Aging is often defined as cellular senescence, resulting in the inability of a cell to respond to stress and increased homeostatic imbalance. In addition, aging increases the risk of diseases including but not limited to cancer, Alzheimer’s disease, cardiovascular disease, hypertension, diabetes and others [23]. It is generally accepted that one’s dietary patterns affect disease and health outcomes [24]. According to Buettner et al., genes regulate 25% of longevity, whereas 75% is determined by lifestyle factors such as sleep habits, alcohol beverage consumption, stress levels, exercise, and diet. Furthermore, studies have indicated that vegetarian dietary patterns of the Seventh Day Adventists play a role in extending the life-span of humans, as well as lowering the risk of developing metabolic syndrome, and has been determined to exert beneficial effects on aging [25–27].
While it was once a goal to solely prolong lifespan, it has become increasingly as important to not only extend the lifespan, but to increase the quality of life as well. Since sanitation, vaccines and antibiotics have become readily available, there has been a decline in deaths from age-related diseases, leading to an increased life expectancy of about 30 years [28]. It is suggested that if the trend continues, most children born today are expected to become centenarians. Many studies have accurately elucidated biological aging processes; however, questions remain regarding whether becoming a centenarian is strictly nature or nurture. The increasing average human lifespan over the past century has motivated investigators to focus their efforts on identifying the lifestyle differences for those with average lifespans as compared to centenarians. A vital factor observed between the two is dissimilarities in diets. For instance, the average centenarian diet consists of more plant proteins, whole grains, monounsaturated fats, and low consumption of red meat, sweets and refined grains [29]. A recent study that predicted the one-year mortality rate in elderly hospital patients found that most centenarians were non-obese, had a lower body mass index and were more physically active than the general population [30]. The results were suggestive of CR, due to the adherence to the Mediterranean diet, which consists mostly of high consumption of olive oil, fruits, vegetables, unrefined cereals and fish in combination with a moderate consumption of wine, and low consumption of meat and meat products [30, 31]. Epidemiological studies conducted in Japan found that urban Japanese people consumed more calories and had a higher incidence of cancer than people of rural Okinawa, where CR was observed [32]. Further, Kromphout and colleagues found that the traditional Okinawan diet provides 90% of consumed calories from carbohydrates, albeit, mostly from vegetable and nutritionally dense food consumption [33]. Additionally, compared to more developed countries, Okinawa has 4–5 times the average number of centenarians. Results also show that compared to the United States with a life expectancy of 84.3 and 81.2 years for women and men respectively, Okinawans live to ages 89.1 and 83.5 years for women and men [34].
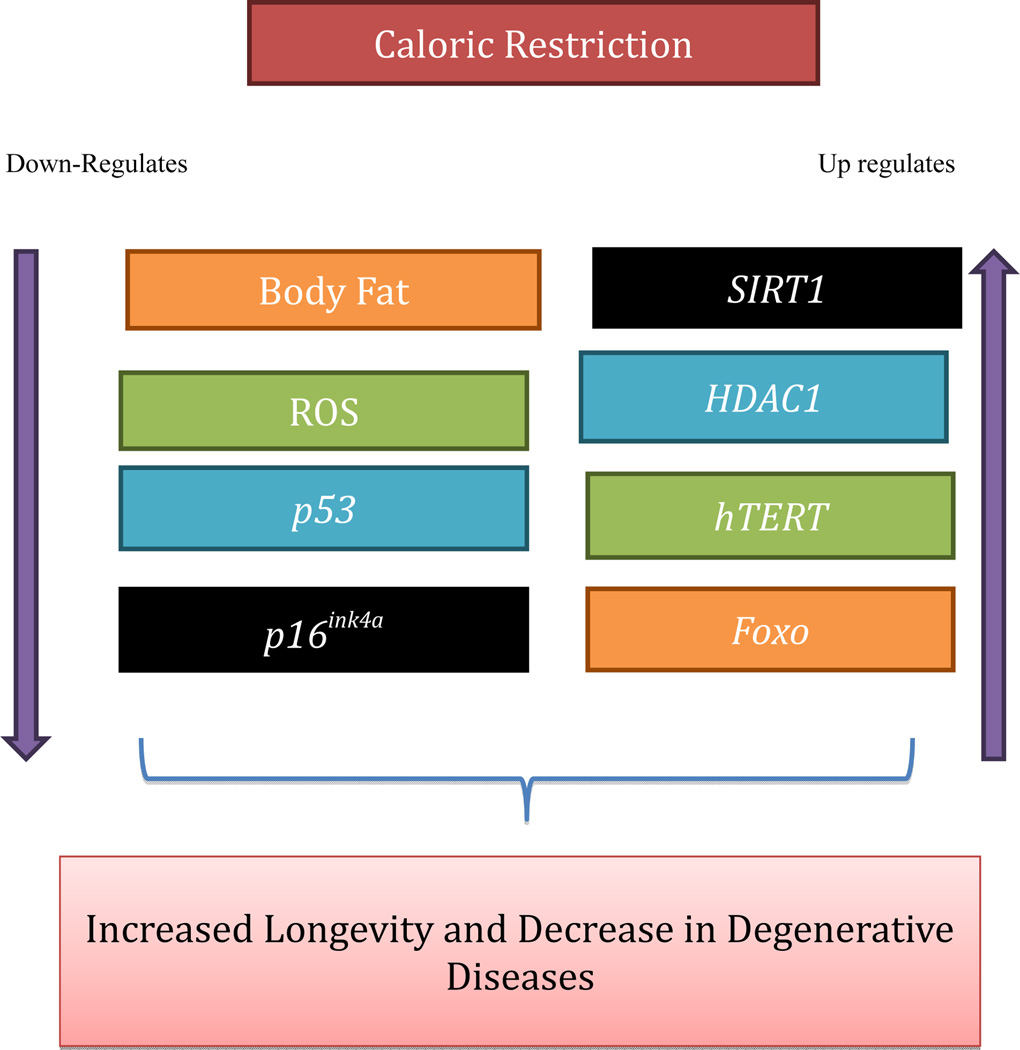
A number of dietary compounds that mimic the effects of CR provide evidence that nutritional factors may continue to extend cellular longevity. In fact, resveratrol (RSV), a dietary polyphenol found in red wine, grapes, nuts, and dried fruits, is among the beneficial compounds found to delay the aging process. It is suggested that RSV acts as a Sirtuin1 (SIRT1) activator and has received considerable attention for its role in aging [35]. SIRT1 is important in several cellular processes such as cell and gene regulation. According to Howitz and colleagues, when comparing silent information regulator 2 (Sir2) and SIRT2 yeast and human enzyme analogs, with SIRT1, significant enzyme activation leading to an increase in human lifespan was seen [36]. In addition to the SIRT1 mechanism, others have attempted to find compounds that would actively imitate the effects of CR because of its beneficial outcome. It was also discovered that not only is CR beneficial for delaying aging, it may be equally beneficial in many forms of cancer as it has consistently been shown to prevent tumors in a number of models [37]. More importantly, CR has the ability to reverse aberrant gene expression, in addition to delaying many age-related diseases (Fig. 1). Barger et al. fed middle age and old mice either an ad libitum (AL or unrestricted calories diet), CR or a diet with low doses of RSV (4.9 mg/day). The results indicated that low doses of RSV prevented gene expression associated with many age-related diseases such as cancer, diabetes, and aging. It was further concluded that low doses of RSV accurately mimics the effects of CR [38].
Fig. (1).
The path to longevity. CR displays the upregulation (indicated by upward arrow) and down-regulation (indicated by downward arrow) of vital intracellular pathways that lead to an increased lifespan (indicated by brace).
CALORIC RESTRICTION AND EPIGENETIC DIET COMPOUNDS HAVE ADVANTAGEOUS EFFECTS ON CARDIOVASCULAR-RELATED DISEASES
Cardiovascular disease (CVD) is the leading cause of death in the United States. It is well understood that long-term consumption of unhealthy food, lack of activity, and poor lifestyle choices lead to an increase in body mass and put individuals at a higher risk for CVD [39]. Studies suggest that CR is exceptionally useful in preventing and reversing cardiovascular disease and other related diseases [40]. More recently, childhood obesity has been implicated in countless cardiovascular incidents.
A clinical study on CR conducted by Fontana et al. involved 18 individuals who practiced unrestricted American diets and 18 individuals who practiced CR for at least 6 years. Results showed that CR lowered low-density lipoproteins (“bad” cholesterol or LDL), triglycerides and diastolic blood pressure, displaying advantageous protective effects against CVD [41]. Several other clinical studies further confirm these findings, including an investigation conducted using 13 men and 30 women where resting heart rates, mental and physical health, anthropometric variables, and blood pressure of patients were monitored. Subjects were then placed on a restricted diet that consisted of several ED compounds including those found in: fruits, vegetables, whole grains, legumes, nuts and seeds for 21 days. Results concluded that there was a significant decrease in total cholesterol, LDL, systolic blood pressure, and an increase in high-density lipoproteins (HDL) in both men and women, which aid in reducing the risk factors for CVD [42].
Studies involving the effects of long-term CR on the myocardium of mice have been assessed and after 30 months, the hearts from AL fed and CR mice were analyzed, revealing that long-term caloric restricted mice hearts were protected from both post ischemic functional deficit as well as infarction, which led to lowering the risk of CVD [43]. Similarly, CR results in protective effects in healthy non-obese individuals. Experts examined CR with and without exercise in 36 healthy individuals. In addition, researchers examined the triacylglycerol levels and blood pressure levels, which displayed levels of significant gradual decreases within CR patients and significant increases in the AL control group. Therefore, these results suggest that CR with or without exercise positively reduces CVD risks in healthy non-obese patients [44]. Moreover, spontaneously hypertensive rats that were fed an AL diet for five weeks, then reduced to 90% CR for two weeks, and 60% CR for three weeks, ultimately resulted in a significant decrease in systolic and diastolic blood pressure at the end of the ten week period [45]. More recently, in as study conducted by Morimoto et al. the dietary phytochemical CCM was shown to prevent heart failure in mice by averting deterioration of systolic function by inducing the myocardial diameter and wall thickness caused by inhibition of HATs, HDACs and p300 activity [46].
CALORIC RESTRICTION AND EPIGENETIC DIET COMPOUNDS ON CANCER
Cancer is the second leading cause of death in the United States [47] and over the past decade, the trend of cancer incidence has increased exponentially. It is well documented that practicing CR may be one of the most prudent way to avoid diseases associated with aging and cancer [48–53]. Rogozina and colleagues tested the effects of CR on mammary tumorigenesis by placing 10 week old female mice in three groups: AL, Intermittent CR (ICR), and Chronic CR (CCR). The AL group was given a diet high in fats and consumed an unrestricted caloric intake. The intermittent group ate ~50% less than the AL diet for three weeks, followed by three weeks of AL, and the chronic CR group were fed ~75% of the AL diet for six weeks. These data portray a dramatic decrease in mammary tumorigenesis as the incidence rate was 71.0%, 35.4%, and 9.1% for AL, CCR, and ICR mice, respectively. Ultimately, the effects of low carbohydrates and Western diets on mice tumorigenesis indicated that tumor prevalence in mice on a western diet was ~50% by the age of 1 year, while caloric restricted mice depicted no observed tumors [54]. It was also noted that of the two groups, only one mouse on the western diet attained a normal lifespan, while more than half of the caloric restricted mice superseded that [55].
Further studies conducted by Shelton et al. tested the effectiveness of CR by placing mice in AL and CR groups and injecting them with malignant glioma cells. After the injection of the tumor cells, the mice were sacrificed and both ipsilateral and contralateral hemispheres were collected to measure the bioluminescence of the tumor cells. These findings indicated that the total percentage of stained cells within the primary tumor as well as the number of blood vessels were significantly lower in the caloric restricted mouse models, demonstrating that CR not only inhibits tumorigenesis but also inhibits tumor metastasis [56]. Considerable evidence has been presented to demonstrate the benefits of CR in monkeys [57–59], dogs [60], and humans [61–63]. Moreover, Colman et al. determined the benefits of CR in non-human primates where 50% of AL and 80% of caloric restricted rhesus monkeys survived past the mean life span [64].
Many believe that the aforementioned ED components are analogous to CR and that dietary phytochemicals can be used to prevent cancer. For example sulforaphane (SFN), an isothiocyanate found in cruciferous vegetables such as broccoli, cauliflower, kale, and Brussels sprouts, has been shown to hold great chemopreventive potential [65, 66]. Although SFN has several epigenetic implications, it is most recognized for its ability to inhibit HDACs. Multiple studies have demonstrated the importance of SFN as a chemopreventive agent due to its ability to induce apoptosis, slow tumor growth, and re-express silenced tumor genes [67–69].
An expanding body of preclinical evidence also suggests that the human telomerase reverse transcriptase (hTERT) gene may be a viable target for cancer diagnosis, due to its upregulation (90%) in cancer cells in comparison to somatic cells. hTERT is regulated by epigenetic modifications at promoter sites such as histone acetylation, histone methylation, and DNA methylation [70, 71]. Histone acetyltransferases (HATs) are enzymes that allow acetyl groups to be transferred to lysine residues in histones, while histone deacetylases (HDACs) remove acetyl groups in order to promote an open chromatin structure. This in turn facilitates the repression or expression of critical genes such as MAD1, CTCF, and hTERT. The hTERT promoter region is hyper-methylated by DNA methyltransferases (DNMTs), namely DNMT1 (which is responsible for maintaining methylation). The demethylation of CpG islands encourages CTCF binding on the hTERT promoter, thereby repressing hTERT. This may offer novel insights on possible targets in human cancer cell lines [72] (Fig. 1). In addition, investigators have found that the aberrant hypermethylation of the hTERT 5’ regulatory region inhibits the binding of the CTCF repressor to hTERT [73]. To further confirm this, Meeran et al. treated MCF-7 and MDA-MB-231 breast cancer cells with low doses of the dietary compound SFN, which significantly inhibited expression of human telomerase reverse transcriptase (hTERT), decreased DNA methyltransferases levels (DNMT1 and DNMT3a), and initiated the process of cellular apoptosis in both breast cancer cell lines. It was also found that SFN allowed transcriptional repressors to bind to the hTERT 5’ regulatory region, thereby decreasing the viability and proliferation of the cancer cells [74] (Table 2).
Table 2.
Epigenetic Diet Compounds and Modifications
| Resveratrol (RSV) | Sulforaphane (SFN) | Curcumin (CCM) | Epigallocatechin Gallate (EGCG) |
|---|---|---|---|
| RSV Down-regulates NF-κB causing cell cycle arrest and apoptosis [104] | SFN Down-regulates hTERT inhibiting telomerase activity [74] | CCM Inhibits HDAC activity [105]and HAT activity [46] | EGCG Inhibits DNMTs [76] |
| RSV Up regulates p53 inducing apoptosis [106] | SFN Induces apoptosis [65, 69] | CCM Inhibits Amyloid beta [93, 94] | EGCG Down-regulates hTERT inhibiting telomerase activity [77] |
| RSV Inhibits HDAC activity [105] | SFN Modulates cyclin D2 [107] | CCM Induce reactive oxygen species (ROS) [108] | EGCG Re-expresses p16 ink4a [109] |
| RSV Up regulates SIRT1 [17, 110] | SFN Induces phase 2 enzymes [65] | CCM Inhibits DNMTs [111] | EGCG Inhibits HDAC activity [105] |
| RSV Down-regulates hTERT inhibiting telomerase activity [112] | SFN Inhibits HDAC activity [68] | CCM Inhibits pro-inflammatory cytokines [113] | EGCG Increases p53 transcriptional activity [114] |
The green tea polyphenol, EGCG, is believed to inhibit cancer through several epigenetic modifications. EGCG is widely consumed in many countries and has become a delaying agent for many diseases. Although the mechanism through which EGCG acts is not thoroughly understood, numerous studies have suggested that hypermethylation of CpG islands is a vital mechanism that silences the expression of an abundance of cancer-related genes. Nandakumar et al. tested the effects of EGCG on A431 (skin) and SCC (squamous) cell carcinoma lines and found that EGCG decreased levels of epigenetic modifiers 5-methylcytosine, methylated H3-Lys 9, DNMT1, DNMT3a, DNMT3b, and HDACs, while increasing levels of acetylated lysine 9 and 14 on histone H3 (H3- Lys 9 and 14) and acetylated lysine 5, 12, and 16 on histone H4. Additionally, EGCG treatments caused a re-expression of the mRNA and proteins of silenced p16ink4a and Cip1/p21 genes [75]. Fang and colleagues further confirmed this mechanism when they performed a similar experiment on KYSE 510 and 150 (human esophageal squamous cell sarcoma) cell lines, which produced similar results [76]. Berletch and others also showed the relationship of EGCG and epigenetics, when MCF-7 (breast) and HL 60 (leukocyte) cells treated with EGCG were shown to down-regulate hTERT, due to paradoxical decreases in the methylation of the hTERT promoter [77].
Studies conducted by Papoutsis et al. treated MCF-7 (breast) cancer cells with RSV to combat the effects of aromatic hydrocarbon receptors (AHR), synonymous for contributing to the etiology of numerous types of malignancies. The authors investigated the effects of AHR with agonist 2,3,7,8 tetrachlorobenzo (p) dioxin (TCDD) on the BRCA-1 gene, which revealed that activation of AHR to the BRCA-1 gene promoter hampers 17βestradiol-dependent stimulation on protein and transcriptional levels. In addition, the treatment of TCDD increased mono-methylated Histone 3 Lysine 9 (H3K9) and DNA methyltransferase 1 (DNMT1), and stimulated the accumulation of DNA strand breaks. It was determined that RSV actively reversed detrimental effects of AHR agonists via epigenetic modifications and in turn the silenced BRCA-1 gene was expressed [78].
CALORIC RESTRICTION AND EPIGENETIC DIET COMPOUNDS MAY AFFECT OBESITY AND INFLAMMATION
Obesity accounts for nearly 300,000 deaths per year in the United States alone [79]. Recent reviews have addressed the need to understand and combat the obesity epidemic and energy balance CR. An increase in energy dense foods and lack of activity appear to be among the leading causes of obesity and related diseases [80]. It has also been suggested that obesity is associated with an immunodeficiency state and chronic inflammation, and that CR and obesity are reciprocal mechanisms capable of regulating immunity and health span, making it a possible target for delaying the aging process [81].
A recent study tested the effects of CR on pulmonary functions and determined markers for oxidative stress, inflammation, and quality of life in caloric restricted overweight asthma patients. In this study, ten patients with a body mass index greater than 30 were maintained for eight weeks, while alternating every other day between an AL and caloric restricted diet (20% less than AL). Questionnaires and blood samples were collected for markers of general health, oxidative stress, and inflammation. Within two weeks of initiation of the diet, the quality of life and levels of antioxidant uric acid increased significantly. Furthermore, inflammation serum tumor necrosis factor-α (TNFα) and brain derived neurotropic factor was decreased. More recently, the effects of CR, weight loss, and exercise on inflammatory biomarkers were examined in nearly 500 obese women. Inflammatory marker- high sensitivity C-reactive protein (hs-CRP), serum amyloid A (SAA), and interleukin-6 (IL-6), leukocyte, and neutrophil levels were measured. The levels in all markers decreased significantly and it was concluded that CR with and without exercise decreases biomarkers of inflammation in overweight asthma patients [82]. To further address this, Schulte et al. monitored 23 obese individuals (BMI 44.1 ± 1.1 kg/m2) and 12 lean individuals (BMI 22.3 ± 0.4 kg/m2) who were treated with an 800 kcal/day for 12 weeks. Principal findings concluded that pro-inflammatory protein genes, wnt5a and sFRP5, were not measurable in serum in lean individuals but were consistently detectable in obese subjects. Concluding that CR constructively affects serum concentrations in obese individuals [83].
More recently, pre-clinical studies performed by Nagao et al. showed the anti-obesity effects of RSV by feeding rats an Otsuka Long-Evans Tokushima fatty (OLETF) diet supplemented with RSV. While OLETF rats suffer from hyperplasia, causing them to become obese with even a normal diet, the results indicated that their fat metabolism significantly decreased in four short weeks due to the adherence of the ED compound [84]. To further confirm these findings, Weisberg and colleagues performed a similar experiment on ob/ob male C57BL/6J mice, where their diet was supplemented with CCM. Their results showed that the addition of CCM in the diets caused a significant decrease in white adipose tissue, hepatomegaly, hepatic nuclear factor-κB activity and markers of hepatic inflammation, while simultaneously increasing adipose tissue adiponectin production. Thereby, ultimately reversing many symptoms commonly associated with obesity [85].
CALORIC RESTRICTION AND EPIGENETIC DIET COMPOUNDS MAY BE EFFECTIVE AGAINST NEURODEGENERATIVE DISEASES
An estimated 5.4 million Americans are currently living with Alzheimer’s disease (AZD). It was originally believed that AZD was the only disease in the top ten causes of death that could not be prevented, cured, or delayed. However, recent discoveries have refuted these beliefs. In a clinical trial fifty elderly normal and overweight individuals were split into three groups: 1) CR 2) relative increase of unsaturated fatty acids and 3) control. Memory was tested before and after intervention and findings supported higher synaptic plasticity and stimulation of neuroprotective pathways in the brain, which resulted in a significant increase in memory scores in the caloric restricted patients but no change in the control or relative increase of unsaturated fatty acid groups [86].
Epidemiological and in vivo studies suggest that tea consumption and neurodegenerative diseases such as dementia, AZD, and Parkinson’s disease (PD) have inverse relationships [87]. Several clinical studies found that two or more cups of green tea (EGCG) per day significantly reduces the risk of PD and results in a lower occurrence of cognitive impairment [88–90]. Additional studies found that several vitamins further support the inverse relationship between bioactive compounds and neurodegenerative diseases. Clinical trials conducted by Wengreen et al. followed 3831 residents who were above the age of 65 for seven years. Each patient was given a questionnaire and cognitive function assessment by following the modified mini mental state examination. Investigators found that high consumption of vitamins A, E, C, and carotene slowed the progression of AZD in elderly patients [91].
Experimental studies suggest that the epigenetic modifying polyphenol curcumin (CCM), found in the Indian spice turmeric, is a beneficial compound in slowing the progression of AZD. Xiong and colleagues treated SH-SY5Y (neuroblastoma) cells with CCM at 0, 1.25, 5.0, and 20 µM for 24, or 5.0µM for 0, 12, 24, and 48 h. The results indicated that CCM decreases the Amyloid Beta (Aβ) (a peptide most commonly associated with AZD) production by inhibiting glycogen synthase kinase 3 beta (GSK-3β) mediated Presenilin 1 (PS1) activation, lowering the occurrence of AZD [92]. These results have been observed both in vivo [93] and in vitro studies, [94] (Table 2). Moreover, epidemiological studies conducted in India where CCM is highly consumed and AZD affects a small percentage of the population, have confirmed these findings [95].
CALORIC RESTRICTION MAY REDUCE BONE DISEASES
Osteoporosis occurs as a result of diminishing bone tissue and decreases in bone density over time. It is a common disease affecting the elderly (mostly women) [96]. Recently, in vitro and in vivo experiments have highlighted the importance of nutrition in gene studies [97]. An in vivo study conducted by Zhang and colleagues fed rats an AIN 93G diet (designed for expecting rodents for growth requirements) with or without blueberries before the onset of puberty, post-natal day 20, and postnatal day 34. Results suggest that early exposure to blueberries inhibited ovariectomy in adult rats by increasing myosin levels that are generally known to be decreased with the disease. It was suggested that the anti-antagonist relationship between blueberries and myosin inhibit osteoblasts from entering senescence by regulating the Runx2 gene and balancing myosin levels [98].
The controversial topic of CR and arthritis has received attention over the past few years due to the misinterpretation of the definition of CR. Although CR is defined as the process of consuming fewer calories without malnutrition, it is often assumed that losing weight is suggestive of lowering disease incidence. Numerous studies evaluate the inverse relationship between osteoarthritis and body mass index in association with dietary restriction [99–101]. Messier et al. tested the effects of intensive dietary restriction with or without exercise or only exercise on 450 patients with osteoarthritis (OA) in one or both knees, with no history of minimal activity (30 min. or less) in the past six months. Results revealed that although there was a decrease in body mass index in patients who practiced exercise only, weight loss unaccompanied by dietary restriction was not suggestive of slowing the progression of OA. However, intensive dietary restriction induced weight loss intervention and showed beneficial data towards the theory of CR [102]. Marshall et al. observed the benefits of weight lost due to CR when they placed 16 canines with OA symptoms, and a body weight of at least 20% above normal, on a CR diet for 18 weeks. The results indicated that the dogs that lost 6.10–8.85% body weight showed vivid improvement in walking and movement [103].
CONCLUSION
In this review, we have discussed the ED and CR and how they affect diseases such as cancer, cardiovascular disease, hypertension, obesity, Parkinson’s disease, Alzheimer’s disease and osteoporosis. Dietary phytochemicals including: SFN, EGCG, vitamins, CCM, and RSV have been indicated in causing epigenetic modifications upon use, thereby, suggesting that they may be useful as ED compounds and capable of influencing disease processes. Results conducted by studies herein suggest that consumption of bioactive compounds might aid in altering the expression of epigenetic modifying genes such as DNMTs and HDACs, in addition to disease-related genes such as hTERT and p16. The epigenetic diet offers some information on gene modifications; however, further investigations are needed in order to determine whether these compounds are effective in altering disease states.
Mounting evidence is suggestive of the benefits of CR in delaying the progression of age-related diseases. Although CR has often been misconstrued as starving oneself to directly target aging, whether CR is detrimental or advantageous in extending human lifespan is still a controversial issue. Perhaps the most beneficial way to target various diseases is to debunk the theory of decreasing one’s weight without the addition of bioactive compounds discussed in this review. While there is insufficient data to conclusively determine that there may be an association between the ED and CR, we believe based on data herein, that there is a direct correlation between the two. However, much work is still needed to determine the epigenetic benefits of utilizing bioactive dietary compounds in combination with CR.
In short, the growing field of epigenetics warrants more exploration of the potential of the ED in inhibiting disease progression, as it remains cutting edge and is not thoroughly understood. This may offer a plausible explanation for the scarcity of recent scientific publications on the ED in association with deleterious diseases. This review will undoubtedly spark new topics of exploration involving epigenetic modifying compounds and their potential to reverse the effects of aging and age-related diseases. This also offers new ideas for future experiments to test the synergistic effects of the two, which may generate novel treatments mimicking the effects of the ED and CR.
ACKNOWLEDGEMENTS
SLM was supported in part by a Bridge to Doctorate fellowship (2006478) program HRD-0-09-25056 at the University of Alabama at Birmingham. Additional support is provided by the National Cancer Institute (CA 129415) and the American Institute for Cancer Research. TMH is supported in part by Institutional Research and Academic Career Development Awards (IRACDA) program 5k126M088010 (Dr. Bryan Noe (PI). The authors would like to thank Natalie Mitchell, Sabita Saldanha, Rishabh Kala and Dr. Yuanyuan Li for helpful comments in preparation of this manuscript.
ABBREVIATIONS
- AHR
Aromatic hydrocarbon receptors
- AL
Ad libitum
- AZD
Alzheimer’s disease
- Aβ
Amyloid beta
- BRCA-1
Breast cancer 1
- CR
Caloric restriction
- CVD
Cardio vascular disease
- CCR
Chronic Caloric Restriction
- CCM
Curcumin
- CTCF
11-zinc finger protein or CCCTC-binding factor
- DNMT 1
DNA methyltransferase 1
- DNMT 3a
DNA methyltransferase 3a
- DNMT 3b
DNA methyltransferase 3b
- EGCG
Epigallocatechin
- ED
Epigenetic Diet
- GSK 3 β
Glycogen synthase kinase 3 beta
- H3K9
Histone 3 Lysine 9
- HAT
Histone acetyl transferase
- HDL
High density proteins
- HS-CRP
High sensitivity c-reactive protein (hs-CRP)
- HDAC
Histone deactetylase
- HTERT
Human telomerase reverse transcriptase
- ICR
Intermittent caloric restriction
- IL-6
Interleukin-6
- LDL
Low density lipoproteins
- MAD-1
Mitotic spindle checkpoint protein
- NF-κB
Nuclear Factor kappa B
- OA
Osteoarthritis
- OLETF
Otsuka Long Evans Tokushima Fatty
- PD
Parkinson’s disease
- PS1
Presenilin 1
- ROS
Reactive Oxygen Species
- RSV
Resveratrol
- Runx 2
Runt-related transcription factor 2
- SAA
Serum amyloid A
- SAM
S-adenosyl-L-methionine
- SAH
S-adenosyl-L-homocysteine
- SFN
Sulforaphane
- SFRP5
Secreted frizzled-related protein 5
- SIR 2
Silent information regulator 2
- SIRT 1
Sirtuin 1
- SIRT 2
Sirtuin 2
- TCDD
2,3,7,8 Tetrachlorobenzo (p) dioxin
- TNF α
Tumor necrosis factor
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Van Speybroeck L. From epigenesis to epigenetics: the case of C. H. Waddington. Ann. N. Y. Acad. Sci. 2002;981:61–81. [PubMed] [Google Scholar]
- 2.Duthie SJ. Epigenetic modifications and human pathologies: cancer and CVD. Proc. Nutr. Soc. 2011;70(1):47–56. doi: 10.1017/S0029665110003952. [DOI] [PubMed] [Google Scholar]
- 3.Vlaming H, van Leeuwen F. Crosstalk between aging and the epigenome. Epigenomics. 2012;4(1):5–7. doi: 10.2217/epi.11.113. [DOI] [PubMed] [Google Scholar]
- 4.Ross SA, Dwyer J, Umar A, Kagan J, Verma M, Van Bemmel DM, Dunn BK. Introduction: diet, epigenetic events and cancer prevention. Nutr. Rev. 2008;66(Suppl 1):S1–S6. doi: 10.1111/j.1753-4887.2008.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulero-Navarro S, Esteller M. Epigenetic biomarkers for human cancer: the time is now. Crit. Rev. Oncol. Hematol. 2008;68(1):1–11. doi: 10.1016/j.critrevonc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Huffman K. The developing, aging neocortex: how genetics and epigenetics influence early developmental patterning and age-related change. Front. Genet. 2012;3:212. doi: 10.3389/fgene.2012.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Daniel M, Tollefsbol TO. Epigenetic regulation of caloric restriction in aging. BMC Med. 2011;9:98. doi: 10.1186/1741-7015-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meeran SM, Ahmed A, Tollefsbol TO. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin. Epigenetics. 2010;1(3–4):101–116. doi: 10.1007/s13148-010-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee IM, Blair SN, Allison DB, Folsom AR, Harris TB, Manson JE, Wing RR. Epidemiologic data on the relationships of caloric intake, energy balance, and weight gain over the life span with longevity and morbidity. J. Gerontol. A. Biol. Sci. Med. Sci. 2001;56(Spec No 1):7–19. doi: 10.1093/gerona/56.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- 10.Hardy TM, Tollefsbol TO. Epigenetic diet: impact on the epigenome and cancer. Epigenomics. 2011;3(4):503–518. doi: 10.2217/epi.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi SW, Friso S. Epigenetics: A New Bridge between Nutrition and Health. Adv. Nutr. 2010;1(1):8–16. doi: 10.3945/an.110.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraga MF, Esteller M. Epigenetics and aging: the targets and the marks. Trends Genet. 2007;23(8):413–418. doi: 10.1016/j.tig.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;71(10):1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Dirks AJ, Leeuwenburgh C. Caloric restriction in humans: potential pitfalls and health concerns. Mech. Ageing Dev. 2006;127(1):1–7. doi: 10.1016/j.mad.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 15.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition. 1989;5(3):155–171. discussion 172. [PubMed] [Google Scholar]
- 16.Mesquita A, Weinberger M, Silva A, Sampaio-Marques B, Almeida B, Leao C, Costa V, Rodrigues F, Burhans WC, Ludovico P. Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc. Natl. Acad. Sci. U. S. A. 2010;107(34):15123–15128. doi: 10.1073/pnas.1004432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell. Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austad SN. Life extension by dietary restriction in the bowl and doily spider, Frontinella pyramitela. Exp. Gerontol. 1989;24(1):83–92. doi: 10.1016/0531-5565(89)90037-5. [DOI] [PubMed] [Google Scholar]
- 19.Kabil H, Kabil O, Banerjee R, Harshman LG, Pletcher SD. Increased transsulfuration mediates longevity and dietary restriction in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2011;108(40):16831–16836. doi: 10.1073/pnas.1102008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 2006;16(3):296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 21.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am. J. Clin. Nutr. 2003;78(3):361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 23.Rakyan VK, Down TA, Maslau S, Andrew T, Yang TP, Beyan H, Whittaker P, McCann OT, Finer S, Valdes AM, Leslie RD, Deloukas P, Spector TD. Human aging-associated DNA hypermethylation occurs preferentially at bivalent chromatin domains. Genome Res. 2010;20(4):434–439. doi: 10.1101/gr.103101.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davinelli S, Willcox DC, Scapagnini G. Extending healthy ageing: nutrient sensitive pathway and centenarian population. Immun. Ageing. 2012;9(1):9. doi: 10.1186/1742-4933-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willett W. Lessons from dietary studies in Adventists and questions for the future. Am. J. Clin. Nutr. 2003;78(3 Suppl):539S–543S. doi: 10.1093/ajcn/78.3.539S. [DOI] [PubMed] [Google Scholar]
- 26.Rizzo NS, Sabate J, Jaceldo-Siegl K, Fraser GE. Vegetarian dietary patterns are associated with a lower risk of metabolic syndrome: the adventist health study 2. Diabetes Care. 2011;34(5):1225–1227. doi: 10.2337/dc10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraser GE. Diet as primordial prevention in Seventh-Day Adventists. Prev. Med. 1999;29(6 Pt 2):S18–S23. doi: 10.1006/pmed.1998.0415. [DOI] [PubMed] [Google Scholar]
- 28.Ten great public health achievements--United States, 1900–1999. MMWR. Morb. Mortal. Wkly. Rep. 1999;48(12):241–243. [PubMed] [Google Scholar]
- 29.Vasto S, Rizzo C, Caruso C. Centenarians and diet: what they eat in the Western part of Sicily. Immun. Ageing. 2012;9(1):10. doi: 10.1186/1742-4933-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sancarlo D, D'Onofrio G, Franceschi M, Scarcelli C, Niro V, Addante F, Copetti M, Ferrucci L, Fontana L, Pilotto A. Validation of a Modified-Multidimensional Prognostic Index (m-MPI) including the Mini Nutritional Assessment Short-Form (MNA-SF) for the prediction of one-year mortality in hospitalized elderly patients. J. Nutr. Health Aging. 2011;15(3):169–173. doi: 10.1007/s12603-010-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giacosa A, Barale R, Bavaresco L, Gatenby P, Gerbi V, Janssens J, Johnston B, Kas K, La Vecchia C, Mainguet P, Morazzoni P, Negri E, Pelucchi C, Pezzotti M, Rondanelli M. Cancer prevention in Europe: the Mediterranean diet as a protective choice. Eur. J. Cancer Prev. 2012;1:90–95. doi: 10.1097/CEJ.0b013e328354d2d7. [DOI] [PubMed] [Google Scholar]
- 32.Willcox BJ, Willcox DC, Todoriki H, Fujiyoshi A, Yano K, He Q, Curb JD, Suzuki M. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world's longest-lived people and its potential impact on morbidity and life span. Ann. N. Y. Acad. Sci. 2007;1114:434–455. doi: 10.1196/annals.1396.037. [DOI] [PubMed] [Google Scholar]
- 33.Kromhout D. Food consumption patterns in the Seven Countries Study. Seven Countries Study Research Group. Ann. Med. 1989;21(3):237–238. doi: 10.3109/07853898909149942. [DOI] [PubMed] [Google Scholar]
- 34.Redman LM, Ravussin E. Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid. Redox Signal. 2011;14(2):275–287. doi: 10.1089/ars.2010.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, Cai G, Fu B, Liu W, Zhuo L, Sun L, Liu F, Chen X. SIRT1 is required for the effects of rapamycin on high glucose-inducing mesangial cells senescence. Mech. Ageing Dev. 2012;133(6):387–400. doi: 10.1016/j.mad.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 37.Cleary MP, Grossmann ME. The manner in which calories are restricted impacts mammary tumor cancer prevention. J. Carcinog. 2011;10:21. doi: 10.4103/1477-3163.85181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3(6):e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorch SM, Sharkey A. Myocardial velocity, strain, and strain rate abnormalities in healthy obese children. J. Cardiometab. Syndr. 2007;2(1):30–34. doi: 10.1111/j.1559-4564.2007.06001.x. [DOI] [PubMed] [Google Scholar]
- 40.Cruzen C, Colman RJ. Effects of caloric restriction on cardiovascular aging in non-human primates and humans. Clin. Geriatr. Med. 2009;25(4):733–743. ix–x. doi: 10.1016/j.cger.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. U. S. A. 2004;101(17):6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bloomer RJ, Kabir MM, Canale RE, Trepanowski JF, Marshall KE, Farney TM, Hammond KG. Effect of a 21 day Daniel Fast on metabolic and cardiovascular disease risk factors in men and women. Lipids Health Dis. 2010;9:94. doi: 10.1186/1476-511X-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edwards AG, Donato AJ, Lesniewski LA, Gioscia RA, Seals DR, Moore RL. Life-long caloric restriction elicits pronounced protection of the aged myocardium: a role for AMPK. Mech. Ageing Dev. 2010;131(11–12):739–742. doi: 10.1016/j.mad.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL, Williamson DA, Smith SR, Ravussin E. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2009;203(1):206–213. doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dolinsky VW, Morton JS, Oka T, Robillard-Frayne I, Bagdan M, Lopaschuk GD, Des Rosiers C, Walsh K, Davidge ST, Dyck JR. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension. 2010;56(3):412–421. doi: 10.1161/HYPERTENSIONAHA.110.154732. [DOI] [PubMed] [Google Scholar]
- 46.Morimoto T, Sunagawa Y, Kawamura T, Takaya T, Wada H, Nagasawa A, Komeda M, Fujita M, Shimatsu A, Kita T, Hasegawa K. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J. Clin. Invest. 2008;118(3):868–878. doi: 10.1172/JCI33160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.American Cancer Society. Cancer with increasing incidence trends. [Accessed May, 23 2012]; http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf. [Google Scholar]
- 48.Calvanese V, Lara E, Kahn A, Fraga MF. The role of epigenetics in aging and age-related diseases. Ageing Res. Rev. 2009;8(4):268–276. doi: 10.1016/j.arr.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- 50.Masoro EJ. Overview of caloric restriction and ageing. Mech. Ageing Dev. 2005;126(9):913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Queen BL, Tollefsbol TO. Polyphenols and aging. Curr. Aging Sci. 2010;3(1):34–42. doi: 10.2174/1874609811003010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weindruch R. The retardation of aging by caloric restriction: studies in rodents and primates. Toxicol. Pathol. 1996;24(6):742–745. doi: 10.1177/019262339602400618. [DOI] [PubMed] [Google Scholar]
- 53.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J. Nutr. 1986;116(4):641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 54.Rogozina OP, Bonorden MJ, Seppanen CN, Grande JP, Cleary MP. Effect of chronic and intermittent calorie restriction on serum adiponectin and leptin and mammary tumorigenesis. Cancer Prev. Res. (Phila) 2011;4(4):568–581. doi: 10.1158/1940-6207.CAPR-10-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho VW, Leung K, Hsu A, Luk B, Lai J, Shen SY, Minchinton AI, Waterhouse D, Bally MB, Lin W, Nelson BH, Sly LM, Krystal G. A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res. 2011;71(13):4484–4493. doi: 10.1158/0008-5472.CAN-10-3973. [DOI] [PubMed] [Google Scholar]
- 56.Shelton LM, Huysentruyt LC, Mukherjee P, Seyfried TN. Calorie restriction as an anti-invasive therapy for malignant brain cancer in the VM mouse. ASN Neuro. 2010;2(3):e00038. doi: 10.1042/AN20100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edwards IJ, Rudel LL, Terry JG, Kemnitz JW, Weindruch R, Cefalu WT. Caloric restriction in rhesus monkeys reduces low density lipoprotein interaction with arterial proteoglycans. J. Gerontol. A. Biol. Sci. Med. Sci. 1998;53(6):B443–B448. doi: 10.1093/gerona/53a.6.b443. [DOI] [PubMed] [Google Scholar]
- 58.Kayo T, Allison DB, Weindruch R, Prolla TA. Influences of aging and caloric restriction on the transcriptional profile of skeletal muscle from rhesus monkeys. Proc. Natl. Acad. Sci. U. S. A. 2001;98(9):5093–5098. doi: 10.1073/pnas.081061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lane MA, Ball SS, Ingram DK, Cutler RG, Engel J, Read V, Roth GS. Diet restriction in rhesus monkeys lowers fasting and glucose-stimulated glucoregulatory end points. Am. J. Physiol. 1995;268(5 Pt 1):E941–E948. doi: 10.1152/ajpendo.1995.268.5.E941. [DOI] [PubMed] [Google Scholar]
- 60.Lawler DF, Larson BT, Ballam JM, Smith GK, Biery DN, Evans RH, Greeley EH, Segre M, Stowe HD, Kealy RD. Diet restriction and ageing in the dog: major observations over two decades. Br. J. Nutr. 2008;99(4):793–805. doi: 10.1017/S0007114507871686. [DOI] [PubMed] [Google Scholar]
- 61.Meydani M, Das S, Band M, Epstein S, Roberts S. The effect of caloric restriction and glycemic load on measures of oxidative stress and antioxidants in humans: results from the CALERIE Trial of Human Caloric Restriction. J. Nutr. Health Aging. 2011;15(6):456–460. doi: 10.1007/s12603-011-0002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buchowski MS, Hongu N, Acra S, Wang L, Warolin J, Roberts LJ. 2nd Effect of modest caloric restriction on oxidative stress in women, a randomized trial. PLoS One. 2012;7(10):e47079. doi: 10.1371/journal.pone.0047079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teeuwisse WM, Widya RL, Paulides M, Lamb HJ, Smit JW, de Roos A, van Buchem MA, Pijl H, van der Grond J. Short-term caloric restriction normalizes hypothalamic neuronal responsiveness to glucose ingestion in patients with type 2 diabetes. Diabetes. 2012;61(12):3255–3259. doi: 10.2337/db11-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol. Cancer Ther. 2007;6(3):1013–1021. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- 66.Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, Singh NK, Chen MS, Stierer T, Garrett-Mayer E, Argani P, Davidson NE, Talalay P, Kensler TW, Visvanathan K. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28(7):1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- 67.Bhamre S, Sahoo D, Tibshirani R, Dill DL, Brooks JD. Temporal changes in gene expression induced by sulforaphane in human prostate cancer cells. Prostate. 2009;69(2):181–190. doi: 10.1002/pros.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dashwood RH, Ho E. Dietary agents as histone deacetylase inhibitors: sulforaphane and structurally related isothiocyanates. Nutr. Rev. 2008;66(Suppl 1):S36–S38. doi: 10.1111/j.1753-4887.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Telang U, Brazeau DA, Morris ME. Comparison of the effects of phenethyl isothiocyanate and sulforaphane on gene expression in breast cancer and normal mammary epithelial cells. Exp. Biol. Med. (Maywood) 2009;234(3):287–295. doi: 10.3181/0808-RM-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008;99(8):1528–1538. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zinn RL, Pruitt K, Eguchi S, Baylin SB, Herman JG. hTERT is expressed in cancer cell lines despite promoter DNA methylation by preservation of unmethylated DNA and active chromatin around the transcription start site. Cancer Res. 2007;67(1):194–201. doi: 10.1158/0008-5472.CAN-06-3396. [DOI] [PubMed] [Google Scholar]
- 72.Choi JH, Min NY, Park J, Kim JH, Park SH, Ko YJ, Kang Y, Moon YJ, Rhee S, Ham SW, Park AJ, Lee KH. TSA-induced DNMT1 down-regulation represses hTERT expression via recruiting CTCF into demethylated core promoter region of hTERT in HCT116. Biochem. Biophys. Res. Commun. 2010;391(1):449–454. doi: 10.1016/j.bbrc.2009.11.078. [DOI] [PubMed] [Google Scholar]
- 73.Renaud S, Loukinov D, Bosman FT, Lobanenkov V, Benhattar J. CTCF binds the proximal exonic region of hTERT and inhibits its transcription. Nucleic. Acids Res. 2005;33(21):6850–6860. doi: 10.1093/nar/gki989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meeran SM, Patel SN, Tollefsbol TO. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS One. 2010;5(7):e11457. doi: 10.1371/journal.pone.0011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nandakumar V, Vaid M, Katiyar SK. (−)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p16INK4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis. 2011;32(4):537–544. doi: 10.1093/carcin/bgq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63(22):7563–7570. [PubMed] [Google Scholar]
- 77.Berletch JB, Liu C, Love WK, Andrews LG, Katiyar SK, Tollefsbol TO. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J. Cell. Biochem. 2008;103(2):509–519. doi: 10.1002/jcb.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papoutsis AJ, Lamore SD, Wondrak GT, Selmin OI, Romagnolo DF. Resveratrol prevents epigenetic silencing of BRCA-1 by the aromatic hydrocarbon receptor in human breast cancer cells. J. Nutr. 2010;140(9):1607–1614. doi: 10.3945/jn.110.123422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282(16):1530–1538. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 80.Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr. Rev. 2006;27(7):750–761. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- 81.Dixit VD. Adipose-immune interactions during obesity and caloric restriction: reciprocal mechanisms regulating immunity and health span. J. Leukoc. Biol. 2008;84(4):882–892. doi: 10.1189/jlb.0108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 2007;42(5):665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schulte DM, Muller N, Neumann K, Oberhauser F, Faust M, Gudelhofer H, Brandt B, Krone W, Laudes M. Pro-inflammatory wnt5a and anti-inflammatory sFRP5 are differentially regulated by nutritional factors in obese human subjects. PLoS One. 2012;7(2):e32437. doi: 10.1371/journal.pone.0032437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nagao K, Jinnouchi T, Kai S, Yanagita T. Effect of dietary resveratrol on the metabolic profile of nutrients in obese OLETF rats. Lipids Health Dis. 2013;12:8. doi: 10.1186/1476-511X-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149(7):3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. Proc. Natl. Acad. Sci. U. S. A. 2009;106(4):1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mandel SA, Amit T, Kalfon L, Reznichenko L, Youdim MB. Targeting multiple neurodegenerative diseases etiologies with multimodal-acting green tea catechins. J. Nutr. 2008;138(8):1578S–1583S. doi: 10.1093/jn/138.8.1578S. [DOI] [PubMed] [Google Scholar]
- 88.Kuriyama S, Hozawa A, Ohmori K, Shimazu T, Matsui T, Ebihara S, Awata S, Nagatomi R, Arai H, Tsuji I. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project 1. Am. J. Clin. Nutr. 2006;83(2):355–361. doi: 10.1093/ajcn/83.2.355. [DOI] [PubMed] [Google Scholar]
- 89.Checkoway H, Powers K, Smith-Weller T, Franklin GM, Longstreth WT, Jr, Swanson PD. Parkinson's disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am. J. Epidemiol. 2002;155(8):732–738. doi: 10.1093/aje/155.8.732. [DOI] [PubMed] [Google Scholar]
- 90.Hu G, Bidel S, Jousilahti P, Antikainen R, Tuomilehto J. Coffee and tea consumption and the risk of Parkinson's disease. Mov. Disord. 2007;22(15):2242–2248. doi: 10.1002/mds.21706. [DOI] [PubMed] [Google Scholar]
- 91.Wengreen HJ, Munger RG, Corcoran CD, Zandi P, Hayden KM, Fotuhi M, Skoog I, Norton MC, Tschanz J, Breitner JC, Welsh-Bohmer KA. Antioxidant intake and cognitive function of elderly men and women: the Cache County Study. J. Nutr. Health Aging. 2007;11(3):230–237. [PubMed] [Google Scholar]
- 92.Xiong Z, Hongmei Z, Lu S, Yu L. Curcumin mediates presenilin-1 activity to reduce beta-amyloid production in a model of Alzheimer's Disease. Pharmacol. Rep. 2011;63(5):1101–1108. doi: 10.1016/s1734-1140(11)70629-6. [DOI] [PubMed] [Google Scholar]
- 93.Wang HM, Zhao YX, Zhang S, Liu GD, Kang WY, Tang HD, Ding JQ, Chen SD. PPARgamma agonist curcumin reduces the amyloid-beta-stimulated inflammatory responses in primary astrocytes. J. Alzheimers Dis. 2010;20(4):1189–1199. doi: 10.3233/JAD-2010-091336. [DOI] [PubMed] [Google Scholar]
- 94.Zhang C, Browne A, Child D, Tanzi RE. Curcumin decreases amyloid-beta peptide levels by attenuating the maturation of amyloid-beta precursor protein. J. Biol. Chem. 2010;285(37):28472–28480. doi: 10.1074/jbc.M110.133520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chandra V, Pandav R, Dodge HH, Johnston JM, Belle SH, DeKosky ST, Ganguli M. Incidence of Alzheimer's disease in a rural community in India: the Indo-US study. Neurology. 2001;57(6):985–989. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- 96.Cooper C. Epidemiology of osteoporotic fracture: looking to the future. Rheumatology (Oxford) 2005;44(Suppl 4):iv36–iv40. doi: 10.1093/rheumatology/kei060. [DOI] [PubMed] [Google Scholar]
- 97.Long JR, Xiong DH, Recker RR, Deng HW. The genetics of osteoporosis. Drugs Today (Barc.) 2005;41(3):205–218. doi: 10.1358/dot.2005.41.3.892525. [DOI] [PubMed] [Google Scholar]
- 98.Zhang J, Lazarenko OP, Blackburn ML, Shankar K, Badger TM, Ronis MJ, Chen JR. Feeding blueberry diets in early life prevent senescence of osteoblasts and bone loss in ovariectomized adult female rats. PLoS One. 2011;6(9):e24486. doi: 10.1371/journal.pone.0024486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spector TD, Hart DJ, Doyle DV. Incidence and progression of osteoarthritis in women with unilateral knee disease in the general population: the effect of obesity. Ann. Rheum. Dis. 1994;53(9):565–568. doi: 10.1136/ard.53.9.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cooper C, Inskip H, Croft P, Campbell L, Smith G, McLaren M, Coggon D. Individual risk factors for hip osteoarthritis: obesity, hip injury, and physical activity. Am. J. Epidemiol. 1998;147(6):516–522. doi: 10.1093/oxfordjournals.aje.a009482. [DOI] [PubMed] [Google Scholar]
- 101.Oliveria SA, Felson DT, Cirillo PA, Reed JI, Walker AM. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, knee. Epidemiology. 1999;10(2):161–166. [PubMed] [Google Scholar]
- 102.Messier SP, Legault C, Mihalko S, Miller GD, Loeser RF, DeVita P, Lyles M, Eckstein F, Hunter DJ, Williamson JD, Nicklas BJ. The Intensive Diet and Exercise for Arthritis (IDEA) trial: design and rationale. BMC Musculoskelet. Disord. 2009;10:93. doi: 10.1186/1471-2474-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marshall WG, Hazewinkel HA, Mullen D, De Meyer G, Baert K, Carmichael S. The effect of weight loss on lameness in obese dogs with osteoarthritis. Vet. Res. Commun. 2010;34(3):241–253. doi: 10.1007/s11259-010-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa, B activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000;164(12):6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 105.Rajendran P, Ho E, Williams DE, Dashwood RH. Dietary phytochemicals, HDAC inhibition, and DNA damage/repair defects in cancer cells. Clin Epigenetics. 2011;3(1):4. doi: 10.1186/1868-7083-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tang HY, Shih A, Cao HJ, Davis FB, Davis PJ, Lin HY. Resveratrol-induced cyclooxygenase-2 facilitates p53-dependent apoptosis in human breast cancer cells. Mol. Cancer Ther. 2006;5(8):2034–2042. doi: 10.1158/1535-7163.MCT-06-0216. [DOI] [PubMed] [Google Scholar]
- 107.Hsu A, Wong CP, Yu Z, Williams DE, Dashwood RH, Ho E. Promoter de-methylation of cyclin D2 by sulforaphane in prostate cancer cells. Clin. Epigenetics. 2011;3:3. doi: 10.1186/1868-7083-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gandhy SU, Kim K, Larsen L, Rosengren RJ, Safe S. Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs. BMC Cancer. 2012;12:564. doi: 10.1186/1471-2407-12-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J. Nutr. 2007;137(1 Suppl):223S–228S. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- 110.Deng CX. SIRT1, is it a tumor promoter or tumor suppressor? Int. J. Biol. Sci. 2009;5(2):147–152. doi: 10.7150/ijbs.5.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011;6(2):93–108. doi: 10.1007/s12263-011-0222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lanzilli G, Fuggetta MP, Tricarico M, Cottarelli A, Serafino A, Falchetti R, Ravagnan G, Turriziani M, Adamo R, Franzese O, Bonmassar E. Resveratrol down-regulates the growth and telomerase activity of breast cancer cells in vitro. Int. J. Oncol. 2006;28(3):641–648. [PubMed] [Google Scholar]
- 113.Soetikno V, Sari FR, Veeraveedu PT, Thandavarayan RA, Harima M, Sukumaran V, Lakshmanan AP, Suzuki K, Kawachi H, Watanabe K. Curcumin ameliorates macrophage infiltration by inhibiting NF-kappaB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr. Metab. (Lond.) 2011;8(1):35. doi: 10.1186/1743-7075-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thakur VS, Gupta K, Gupta S. Green tea polyphenols increase p53 transcriptional activity and acetylation by suppressing class I histone deacetylases. Int. J. Oncol. 2012;41(1):353–361. doi: 10.3892/ijo.2012.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]