Abstract
A growing body of literature has investigated the association between maternal anorexia nervosa and pregnancy outcomes. Infant low birth weight is associated with a number of neurodevelopmental and physical sequelae; however, consistent results on its association with maternal anorexia nervosa are scant. Therefore, a systematic review and meta-analysis of the existing literature were undertaken. PubMed, Embase, and PsychInfo were searched for studies comparing the mean birth weight of babies delivered by mothers with (a history of) anorexia nervosa against those of healthy mothers. Studies were excluded from the meta-analysis if not presenting data from an unexposed comparison group and if using multiple eating disorders as exposure without presenting individual results. Fourteen studies were included in the systematic review and 9 in the meta-analysis, undertaken between 1999 and 2012 in Denmark, the Netherlands, New Zealand, Norway, Sweden, and the United Kingdom. Birth weights were standardized by dividing the difference in mean birth weight by the pooled standard deviation (equivalent to Cohen's d). Results showed a standardized mean difference of −0.19 kg (95% confidence interval: −0.25, −0.15; P = 0.01) in the birth weight of children of mothers with anorexia nervosa, and some bias in favor of papers presenting lower birth weight results for exposed mothers was detected. However, the small power of the analysis due to the small number of available studies and, thus, chance could partially account for this result. Our results confirm that maternal anorexia nervosa predicts lower birth weight and, despite some limitations, they have important clinical implications for prevention of adverse child outcomes.
Keywords: anorexia nervosa, eating disorders, low birth weight, perinatal outcomes, pregnancy complications
INTRODUCTION
Anorexia nervosa, as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (1), is a disorder characterized by the inability to maintain a normal weight (body mass index, ≤17.5), intense fear of fatness, undue influence of body weight and shape on self-evaluation, and, in women, amenorrhea (absence of menstrual periods at least 3 months consecutively) (1).
Anorexia nervosa has a low prevalence in the general population (between 0.3% and 2.2%, among women of reproductive age (2)); however, it has considerable long-term and potentially intergenerational effects (3). In the last 10 years, a growing literature has focused on a number of perinatal outcomes in women with eating disorders (3). Intrauterine growth restriction, miscarriages (4, 5), cesarean sections (5–7), perinatal mortality (8), prematurity, small- and large-for-gestational-age births (7, 9), and low birth weight (5, 7, 9–16) have been associated with maternal eating disorders.
Infant low birth weight is of relevance given its association with several neurodevelopmental and physical sequelae at later stages of the child's life. Depression (17), attention problems (18), schizophrenia (19, 20), and eating disorders themselves (21) have been associated with low birth weight, while diabetes, high blood pressure (22), obesity (23), cardiovascular disease (metabolic syndrome) (24), cerebral palsy, and visual and hearing impairments are among the physical conditions associated with low birth weight (20).
Among women with eating disorders, the risk of delivering low birth weight babies is higher in mothers with active or recovered anorexia nervosa (3, 5, 8, 12–14, 16, 20, 25, 26). Studies investigating the impact of maternal prepregnancy body mass index on perinatal outcomes have identified low maternal prepregnancy weight as a risk factor for the delivery of low birth weight babies (27, 28). Therefore, one of the hypothesized risk mechanisms in mothers with active anorexia nervosa is that low birth weight might be a result of low maternal prepregnancy weight (3). However, because differences in birth weight have been identified also in women recovered from anorexia nervosa (N. Micali, Institute of Child Health, University College London, unpublished manuscript) (5, 10, 12, 13, 16, 26), it is possible that the latter might retain a lower prepregnancy body mass index compared with healthy mothers and that this might influence child birth weight. Despite trends suggesting lower birth weight in children of women with anorexia nervosa, literature findings are inconsistent. While some studies do not find differences in birth weight, those finding differences vary in terms of the estimates and strength of the association with exposure to maternal anorexia nervosa. Studies presenting outcome data for both exposed (anorexia nervosa) and unexposed mothers are scant and use different study designs, populations, and diagnostic tools. These differences are likely to contribute to different results and to lead to either over- or underestimating effect sizes.
We conducted a systematic review of the literature and a meta-analysis in order to quantify the effect of maternal anorexia nervosa (active or past) on birth weight. The hypothesis driving these analyses is that full-term children of anorexia nervosa mothers weigh less at birth than those of unexposed mothers.
MATERIALS AND METHODS
A systematic literature review was conducted to assess the state of literature exploring the association of maternal anorexia nervosa with child birth weight and to evaluate the hypothesis that children of anorexia nervosa mothers weigh less than those of healthy mothers. Prior to conducting a systematic review, we developed a protocol that included our research question, search strategy, inclusion and exclusion criteria, code book for the coding of the papers included in our meta-analysis, and a data analysis strategy.
Search procedure
In this review, guidelines for meta-analysis from the Meta-analysis of Observational Studies in Epidemiology (known as “MOOSE” guidelines) (29) were followed. Papers investigating the association between (history of) maternal anorexia nervosa and child's birth weight were searched by using 3 electronic databases (PubMed, Embase, and PsychInfo) and the terms “anorexia nervosa” and “eating disorders” for the exposure and “pregnancy outcome(s)”, “birth weight”, and “(infant) low birth weight” for the outcome. This search was undertaken by 2 researchers (F. S., N. M.) at different time points. Each exposure term was combined with each of the outcome terms.
Papers were assessed through titles and abstracts to evaluate suitability for inclusion. In order to identify additional papers, we hand-searched bibliographies of the articles meeting inclusion criteria, read abstracts of studies presented at relevant conferences, and contacted lead experts to enquire about studies in preparation.
Inclusion/exclusion criteria
Studies were included if they compared the mean birth weight of babies delivered by mothers with (past/active) anorexia nervosa with that of babies delivered by mothers without (past/active) anorexia nervosa. Studies looking at anorexia nervosa but also comparing other eating disorders against an unexposed group were included if it was possible to obtain individual estimates by eating disorder type.
Studies not presenting data from an unexposed comparison group were excluded.
Standard deviations were calculated when studies reported only standard errors for the presented means. Published studies not presenting either standard deviation or standard error were included if corresponding authors were able to provide the missing information.
Data analysis
Stata, release 12, statistical software (StataCorp LP, College Station, Texas) was used to perform analyses with user-contributed commands: metan (30), metainf (31), and metabias (32). Data were extracted and coded by 2 researchers (F. S., N. M.). The quality of studies included was not assessed with a score, as the literature suggests that “ad hoc scoring” in observational studies might not represent the actual quality of the papers given the absence of a validated scale to measure it (29). Blinding of authors, appropriate selection of exposed and unexposed participants, reporting of results, and exposure and outcome measurement were coded and considered instead.
Differences in mean birth weight were standardized by dividing the difference in mean offspring birth weight in the exposed and the unexposed by the pooled standard deviation (equivalent to Cohen's d). A random-effects model, assuming that variation of effect sizes is not due solely to sampling error but also to variability between studies, was used. Confidence intervals around the random-effects pooled estimates are usually wider than confidence intervals in fixed effects, but the former are generally more realistic in psychiatric studies because of the range of clinical or methodological diversity likely to be contained in the individual studies (33). Homogeneity between studies was assessed by using the I2 [(Q − (df)/Q)] statistic because of the small sample size. This statistic represents the percentage of total variation (range, 0%–100%) among the studies due to heterogeneity rather than chance, and it is independent of sample size (34). Values of 25%, 50%, and 75% are suggested to represent low, moderate, and high heterogeneity (34). Studies were excluded one at a time from the meta-analysis to investigate the influence of each upon the combined estimate.
Publication bias was assessed visually by using a funnel plot, a plot of the standard error of the effect size (an expression of sampling uncertainty, negatively correlated with sample size) against the effect size of each study.
RESULTS
Systematic review
Selection of studies
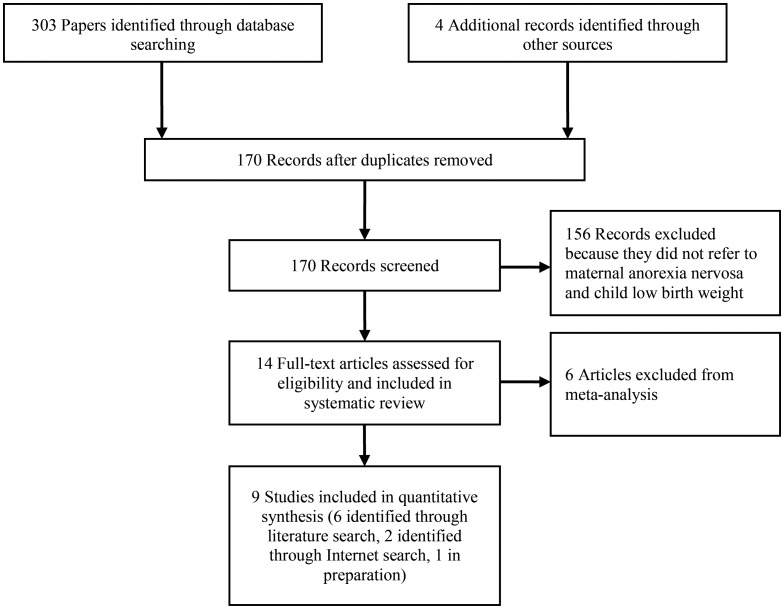
A total of 303 studies were identified by using the above-mentioned search terms, and 4 were found through manual searches. After removing duplicates, we screened 170 papers for inclusion; 14 dealt with pregnancy outcomes of mothers with anorexia nervosa (5, 7, 9–16, 26, 35, 36) and were therefore kept for inclusion in the systematic review. Of these, 10 (5, 7, 9–13, 15, 16, 36) were found through literature searches, 2 articles (N. Micali, Institute of Child Health, University College London, unpublished manuscript) (35) had been submitted for publication at the time the search was conducted, and 2 (14, 26) were found through Internet searches with Google Scholar (Google, Inc., Mountain View, California), as shown in Figure 1.
Figure 1.
Flowchart of literature search for meta-analysis.
Characteristics of included studies
Fourteen studies, undertaken between 1987 and 2012, were included in this systematic review. Three studies were based in Sweden (12, 13, 16), 2 in New Zealand (5, 15), 1 in Norway (11), 2 in the United Kingdom (25, 26), 1 in Israel (7), 1 in Canada (14), 2 in Denmark (N. Micali, Institute of Child Health, University College London, unpublished manuscript) (9), 1 in the United States (36), and 1 in the Netherlands (35).
Assessment of exposure and outcome
Three studies used clinical samples (5, 14, 36); 4 obtained data on exposed women from patient registers (therefore women treated for anorexia nervosa) (7, 9, 12, 26), with diagnoses made by clinicians according to criteria from the Diagnostic and Statistical Manual of Mental Disorders (7) or the International Classification of Diseases (9, 12, 26). One study (15) used a clinical sample diagnosed with criteria from the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, to identify exposed mothers and a register-based sample to select unexposed mothers. Three studies (11, 13, 16) used a community-based sample with anorexia nervosa diagnosed with a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, clinical diagnosis; and, finally, 3 used a self-report diagnosis by way of a questionnaire or questions about whether the subjects had ever had anorexia nervosa (N. Micali, Institute of Child Health, University College London, unpublished manuscript) (25, 35).
The outcome of interest was child birth weight. Ten studies (5, 9–16, 35) presented mean birth weight measured in grams. The studies by Eagles et al. (26) and Pasternak et al. (7) presented low birth weight as a binary variable (<2,500/≥2,500 g). Finally, the paper by Franko et al. (36) presented mean birth weight and standard deviation in pounds.
Low birth weight in children of anorexia nervosa mothers
Nine studies investigated the association between maternal anorexia nervosa and child birth weight. The excluded papers either presented data for both anorexia nervosa and other eating disorder mothers together (7, 9, 15) or did not present data from a healthy comparison group. Stewart et al. (14) presented the mean birth weight of children of active anorexia nervosa mothers, comparing them with children of recovered anorexia nervosa mothers, whereas Franko et al. (36) compared birth weights of infants of anorexia nervosa mothers with those of mothers with bulimia nervosa. Both studies found that children of anorexia nervosa mothers had lower birth weight than their respective comparison groups.
The 3 papers by Pasternak et al. (7), Sollid et al. (9), and Waugh and Bulik (15) grouped anorexia nervosa with other eating disorders, allowing investigation of only the overall effect of maternal eating disorders: Pasternak et al. included women with bulimia nervosa and eating disorders not otherwise specified, whereas the remaining 2 papers investigated only bulimia nervosa. All found that children of mothers with eating disorders weighed less than those of healthy mothers: Sollid et al. (9) found a 137-g difference, while Waugh and Bulik (15) found a 524-g difference. As previously mentioned, Pasternak et al. (7) categorized low birth weight as a binary variable and found that mothers with a history of an eating disorder were nearly 3 times more likely to deliver low birth weight babies (odds ratio = 2.8, 95% confidence interval (CI): 1.4, 5.5; P = 0.003). Waugh and Bulik (15) used a small sample (n = 20) and did not adjust for potential confounding factors; this could partially explain the large difference in birth weight found in their study. Sollid et al. (9) reported a smaller difference in birth weight (101 g) once adjustment was made for gestational age. The results by Pasternak et al. (7) are also adjusted for parity and maternal age; however, crude differences were not presented in the paper and, hence, it is not possible to gauge the extent of the effect of those factors.
Of the studies included in this meta-analysis, 2 (11, 33) found no differences in birth weight. All other studies found that babies of anorexia nervosa mothers had lower birth weight compared with unexposed ones, with differences ranging between 52 and 306 g (Table 1). The 4 studies with the smallest sample size recorded the greatest birth weight differences: Koubaa et al. (13) (n = 91) found a difference of 306 g, Bulik et al. (5) (n = 164) of 198 g, Wentz et al. (16) (n = 58) of 206 g, and Eagles et al. (26) (the biggest study of the 3 in terms of sample size) (n = 804) of 228 g (Table 1).
Table 1.
Characteristics of Studies Included in Meta-analysis of the Association of Maternal Anorexia Nervosa With Child Birth Weight and Published Between 1999 and 2012
| Author, Year (Reference No.) | No. | Anorexia Nervosa Mothers |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Sample Selection | Diagnostic System | Mean Birth Weight, g (SD) | Mean Age, years (SD) | Mean BMIa (SD) | ||||
| Bulik, 1999 (5) | 164 | 66 | Clinical sample | DSM-III-R | 3,139 (617) | 32.4 (8) | 20.1 (2.1) | ||
| Koubaa, 2005 (13) | 91 | 24 | Community based (antenatal clinics) | DSM IV | 3,210 (533) | NR | 19.3 (2.9) | ||
| Ekeus, 2006 (12) | 2,006,475 | 2,059 | Register based | ICD-8/ICD-9 | 3,436.05 (562.78) | NRb | NRb | ||
| Micali, 2007 (10) | 11,973 | 167 | Community based | Self-reported | 3,343.02 (450.13) | 28.9 (5.2) | 21.5 (3.2) | ||
| Bulik, 2009 (11) | 35,929 | 35 | Community based | DSM-IV | 3,577.2 (529.9) | 26.6 (NR) | 18.2 (NR) | ||
| Wentz, 2009 (16) | 58 | 27 | Community based (schools) | DSM-IV | 3,347.9 (548.4) | NR | NR | ||
| Eagles, 2012 (26) | 804 | 134 | Register based | ICD-7/ICD-10 | 3,061.36 (638.36) | 26.77 (4.86) | 22.2 (2.69) | ||
| Micali, 2012 (35) | 3,927 | 134 | Community based | Self-reported | 3,439.89 (443.64) | 30.6 (5) | 22.2 (3.5) | ||
| Micali, unpublished manuscriptc | 82,457 | 1,656 | Community based | Self-reported | 3,500.02 (469.36) | 28.59 (4.9) | 21.62 (3.34) | ||
| Non–Anorexia Nervosa Mothers |
SMD | Multivariable Adjustment |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Sample Selection | Mean Birth Weight, g (SD) | Mean Age, years (SD) | Mean BMI (SD) | Gestational Age | Gender | |||
| Bulik, 1999 (5) | 164 | 98 | Register based | 3,337 (535) | 35.5 (6.2) | 25.6 (6.4) | −0.348 | No | No |
| Koubaa, 2005 (13) | 91 | 67 | Community based (antenatal clinics) | 3,516 (515) | 30 (3.7) | 22.3 (2.8) | −0.59 | No | No |
| Ekeus, 2006 (12) | 2,006,475 | 2,004,416 | Register based | 3,511.8 (585.76) | NRb | NRb | −0.129 | No | No |
| Micali, 2007 (10) | 11,973 | 10,409 | Community based | 3,395.89 (449.75) | 28.2 (4.8) | 22.9 (3.8) | −0.187 | Yes | Yes |
| Bulik, 2009 (11) | 35,929 | 33,742 | Community based | 3,609 (551.07) | 29.9 (NR) | 24.0 (NR) | −0.102 | No | No |
| Wentz, 2009 (16) | 58 | 31 | Community based (schools) | 3,553.8 (600.7) | NR | NR | −0.357 | No | No |
| Eagles, 2012 (26) | 804 | 6,701 | Register based | 3,289.8 (579.27) | 26.87 (4.86) | 24.6 (4.38) | −0.387 | No | No |
| Micali, 2012 (35) | 3,927 | 3,793 | Community based | 3,435.02 (441.27) | 30.4 (4.9) | 23.4 (4) | 0.01 | Yes | Yes |
| Micali, unpublished manuscriptc | 82,457 | 80,801 | Community based | 3,584.71 (469.305) | 29.3 (4.26) | 23.57 (4.2) | −0.185 | Yes | Yes |
Abbreviations: BMI, body mass index; DSM, Diagnostic and Statistical Manual of Mental Disorders; ICD-7, International Classification of Diseases, Seventh Revision (ICD-8, ICD-9, and ICD-10 defined similarly); NR, not reported; SD, standard deviation; SMD, standardized mean difference.
a BMI: weight (kg)/height (m)2.
b Data obtained from authors refer to primiparas + multiparas, whereas data from article only refer to primiparas. Article data include age and body mass index but refer to primiparas sample only and are therefore not reported in the table.
c N. Micali, Institute of Child Health, University College London, unpublished manuscript.
Of these 4 studies, those by Bulik et al. (5) and Eagles et al. (26) included women identified through patient lists or registers, whereas Wentz et al. (16) included women identified from a community screening for eating disorders in schools, and Koubaa et al. (13) included women identified in antenatal clinics. Exposed women from patient registers or clinical samples are more likely to be the most severe cases, and this can explain larger differences in birth weight. Studies using a register or clinical population sample mostly show lower birth weights in the children of exposed women.
Most studies did not present mean birth weights adjusted for gestational age and gender (Table 1). The 3 studies by Micali et al. (10, 25, 35) adjusting for gestational age and gender report contrasting results: 2 found a difference in birth weight between the offspring of exposed and unexposed mothers (10, 35), whereas 1 did not find any (35). Chance could account for the latter finding, as the study was powered to detect differences, and maternal body mass index did not seem to differ from those of the other papers.
The remaining studies using unadjusted values found bigger differences apart from the 2 studies by Ekeus et al. (12) (75.8 g) and Bulik et al. (11) (32 g). In the paper by Ekeus et al. (12), despite reporting crude estimates, the small difference could be attributed to the large sample size (n = 2,006,475) and, therefore, increased power to detect real differences. The small number of exposed women (n = 37) compared with unexposed women (n = 33,742) and the consequent low power to detect any associations could explain the nonsignificant difference found in the paper by Bulik et al. (11). This paper was also the only one that included a sample composed solely of women with current anorexia nervosa.
In all studies that reported gestational weight gain and maternal prepregnancy body mass index (N. Micali, Institute of Child Health, University College London, unpublished manuscript) (5, 10, 11, 13, 26, 35), the latter was lower in the exposed group than it was in the unexposed one (Table 1). Only 2 studies (11, 13) reported gestational weight gain in the mother as well as prepregnancy body mass index. In the study by Bulik et al. (11) (finding a nonsignificant mean birth weight difference of 32 g), exposed mothers had a mean prepregnancy body mass index of 18.2 (standard error (SE), 0.1) and a mean gestational weight gain of 17.8 kg (SE, 0.97) compared with unexposed ones, who had a prepregnancy body mass index of 24 (SE, 0.02) and a mean gestational weight gain of 14.9 kg (SE, 0.03). The authors explain that the higher weight gain in pregnancy by anorexia nervosa mothers could be explained by their need to reach a healthy weight given their lower body mass index at the start of pregnancy. In the study by Koubaa et al. (13), finding a mean birth weight difference of 306 g, anorexia nervosa mothers had a prepregnancy body mass index of 19.3 (standard deviation (SD), 2.9) and a gestational weight gain of 11.3 kg (SD, 3.9), whereas healthy mothers had a prepregnancy body mass index of 22.3 (SD, 2.8) and a gestational weight gain of 12.1 kg (SD, 2.6). Given the smaller difference in the study by Bulik et al. than that found by Koubaa et al., it is possible to hypothesize that low gestational weight gain in women with low prepregnancy body mass index could interact in the delivery of low birth weight babies, whereas weight recovery could partially offset this mechanism.
Meta-analysis
Among the articles identified, 4 (7, 9, 15, 26) were excluded from the meta-analysis because they grouped different eating disorders together without presenting data on child birth weight for the anorexia nervosa group individually. One was excluded (14) because it compared mothers with active anorexia nervosa with those with recovered anorexia nervosa, thus not providing data from a truly unexposed comparison group. Nine papers were included in the meta-analysis.
Two studies (12, 33) did not report standard deviations for the mean birth weight presented in the article. Therefore, the authors were contacted, and data were obtained from the original data analyses and data quality was unaffected. If the data presented in the paper were no longer retrievable, data currently available to the authors were used. The paper by Eagles et al. (26), reporting birth weight as a binary variable, was included in the meta-analysis, and the authors were contacted to obtain the mean birth weight instead.
In all studies but 1 (13), which nevertheless collected exposure and outcome data through medical records, data had previously been collected as either part of prospective studies (N. Micali, Institute of Child Health, University College London, unpublished manuscript) (11, 16, 25, 35), register-based studies (12, 26), or a clinic-based study (5) and, therefore, blinding of authors was not necessary to ensure unbiased results. As explained above, all studies used clinically validated measures to assess anorexia nervosa exposure, and data on outcome (birth weight) were collected through medical records. The quality of studies included therefore seemed consistent.
In total, 2,126,927 individuals were included in the meta-analysis, of whom 2,122,735 were unexposed women, and 4,028 were mothers with anorexia nervosa (past/active).
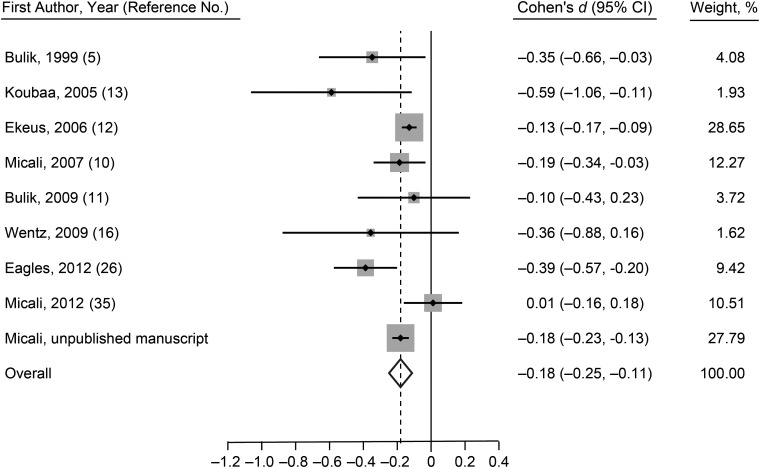
The meta-analysis showed a standardized mean difference of −0.19 kg (95% CI: −0.25, −0.15; P = 0.01), as shown in Figure 2. Good evidence of heterogeneity between the studies was found (χ² = 18.79, P = 0.016; I² = 57.4%). A random-effect model was fit based on a priori assumptions. Fitting a fixed-effects model resulted in identical results.
Figure 2.
Meta-analysis of the association of maternal anorexia nervosa with infant birth weight for selected studies published between 1999 and 2012. N. Micali, first author of the unpublished manuscript, is affiliated with the Institute of Child Health, University College London. CI, confidence interval.
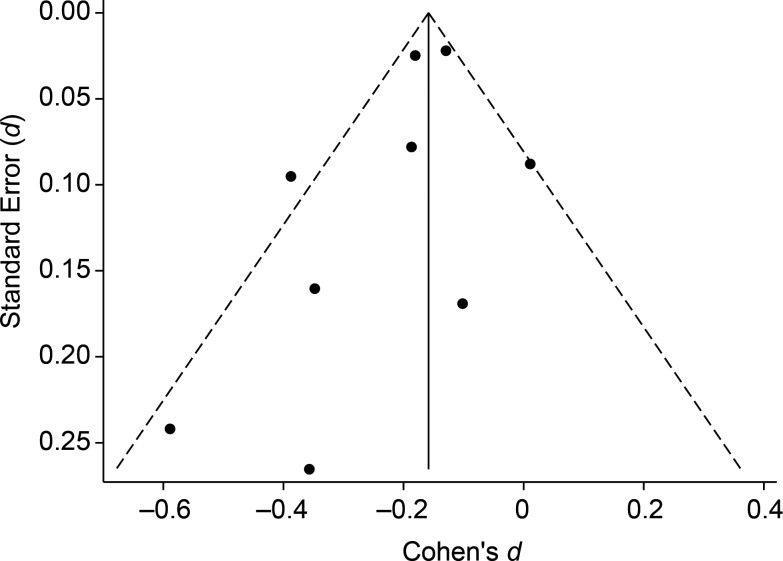
The funnel plot in Figure 3 shows some asymmetry, suggesting possible publication bias, as studies with larger standard errors and small effects seem to be underrepresented. However, given the small number of studies included, it is difficult to conclusively assess publication bias as there may be other possible explanations, including that of chance.
Figure 3.
Funnel plot of selected studies published between 1999 and 2012 and included in the meta-analysis.
Excluding studies 1 at a time from the analysis caused the pooled effect (standardized mean difference: −0.18 kg, 95% CI: −0.25, −0.11) estimate to change slightly (range, from −0.21 to −0.16), without altering the overall significance of the result.
DISCUSSION
The literature on the associations of maternal eating disorders with birth weight has expanded in the last decade. Despite earlier studies focusing on small clinical samples, larger population-based samples have recently increased our knowledge on the topic. Overall, the literature suggests that children of mothers with anorexia nervosa are at increased risk of low birth weight; however, inconsistencies are present in the literature. Differences across studies are likely to reflect sample sizes, with smaller studies showing larger differences, as well as the nature of the sample (clinical or patient register vs. general population), suggesting that the severity of the illness could affect the estimated difference in birth weight.
This meta-analysis offered a quantitative analysis of the effects of maternal lifetime anorexia nervosa on infants’ birth weight. The results obtained show that children of women with (past/active) anorexia nervosa weigh less than those of unexposed women, although the standardized mean difference was small (about 200 g).
Three studies presented the adjusted mean birth weight for gestational age and gender of the child (known to provide a better estimate of birth weight (37)), and 6 reported unadjusted means. The 3 studies reporting adjusted values found comparatively smaller mean differences (standardized mean difference of 0.011 kg (35), -0.185 kg (N. Micali, Institute of Child Health, University College London, unpublished manuscript), and -0.187 kg (10)). Although it was not possible to conduct a subgroup analysis because of the small size of the 3 studies and the consequent lack of power of the analyses, it is possible to hypothesize that adjustment of mean values could reduce the effect size of the results.
This meta-analysis has several strengths. First, this is the first meta-analysis summarizing the existing quantitative literature in the field, and therefore its findings can be useful in deriving hypotheses for future studies. Second, the majority of studies included relied on community-based or register-based samples rather than clinical samples; therefore, selection bias in the original studies is likely to be small. Moreover, if an association of maternal anorexia nervosa is found in community-based samples (which are more likely to include less severe cases of anorexia nervosa), our result is likely to be an underestimation of effect. Finally, the majority of women included in the meta-analysis had a lifetime anorexia nervosa diagnosis (rather than an active disorder), so it is likely that our results might underestimate the effect of active anorexia nervosa in pregnancy, although the only study that included mothers with active anorexia nervosa did not find differences in birth weight.
Although an association of maternal anorexia nervosa with birth weight is evident from our results, these have to be interpreted in light of some limitations. First, because case identification of the exposed women varies across study settings, inconsistencies might exist in case definitions, which could bias estimates. However, diagnostic criteria for anorexia nervosa have not (or minimally) changed in the last 2 decades, so misclassification should be minimal.
Second, the paucity of studies conducted in this area focusing on anorexia nervosa specifically made the assessment of publication bias difficult. We could not gauge whether negative findings appear to have been underreported in peer-reviewed journals. Third, and for the same reason, we could not perform subgroup analyses (for instance, testing for a difference in estimates by setting, parity, and so on). Therefore, further reasons for the differences in the results were not explored.
On the basis of the findings of this meta-analysis, it is possible to speculate, in line with the study hypotheses, that low maternal prepregnancy body mass index could be a risk factor for the delivery of low birth weight babies in mothers with active or past anorexia nervosa. As in all of the studies included in the meta-analysis, the mean maternal body mass index was lower in the exposed than in the unexposed group. None of the studies stratified their results by body mass index categories. Thus, it was not possible to perform subgroup analyses investigating the role of prepregnancy body mass index. Similarly, only 2 studies (11, 13) reported maternal gestational weight gain, with contrasting findings; therefore, it is not possible to infer any conclusions on its association with low birth weight. Future studies should be sure to collect and present information on both prepregnancy body mass index and gestational weight gain in order to better explore the association of these 2 factors with birth weight and other perinatal outcomes in mothers with anorexia nervosa.
Maternal anorexia nervosa is associated with lower birth weight and, as such, the public health and policy implications of this finding are important to prevent adverse child short- and long-term outcomes. Health professionals in antenatal care and mental health settings should be aware of the highlighted risk. More research is needed to explore mediators and risk mechanisms for the association between maternal anorexia nervosa and infant birth weight.
ACKNOWLEDGMENTS
Author affiliations: Brain and Behavioural Science Unit, Institute of Child Health, University College London, London, United Kingdom (Francesca Solmi, Nadia Micali); Medical Research Council and Integrative Epidemiology Unit, University of Bristol, Bristol, United Kingdom (Hannah Sallis); Department of Biostatistics, Institute of Psychiatry, King's College London, London, United Kingdom (Daniel Stahl); and Eating Disorders Department, Institute of Psychiatry, King's College London, London, United Kingdom (Janet Treasure).
This work was supported by a National Institute of Health Research clinician scientist award to N. M. (DHCS/08/08/012).
The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
Conflict of interest: none declared.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Van Hoeken D, Seidell JC, Hoek HW. Epidemiology. In: Treasure J, Schmidt U, Van Furth EF, editors. Handbook of Eating Disorders. 2nd ed. Chicester, United Kingdom: Wiley; 2003. pp. 11–34. [Google Scholar]
- 3.Micali N, Treasure J. Biological effects of a maternal ED on pregnancy and foetal development: a review. Eur Eat Disord Rev. 2009;17(6):448–454. doi: 10.1002/erv.963. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell JE, Seim HC, Glotter D, et al. A retrospective study of pregnancy in bulimia-nervosa. Int J Eat Disord. 1991;10(2):209–214. [Google Scholar]
- 5.Bulik CM, Sullivan PF, Fear JL, et al. Fertility and reproduction in women with anorexia nervosa: a controlled study. J Clin Psychiatry. 1999;60(2):130–137. doi: 10.4088/jcp.v60n0212. [DOI] [PubMed] [Google Scholar]
- 6.Keel PK, Dorer DJ, Eddy KT, et al. Predictors of mortality in eating disorders. Arch Gen Psychiatry. 2003;60(2):179–183. doi: 10.1001/archpsyc.60.2.179. [DOI] [PubMed] [Google Scholar]
- 7.Pasternak Y, Weintraub AY, Shoham-Vardi I, et al. Obstetric and perinatal outcomes in women with eating disorders. J Womens Health (Larchmt) 2012;21(1):61–65. doi: 10.1089/jwh.2011.2907. [DOI] [PubMed] [Google Scholar]
- 8.Brinch M, Isager T, Tolstrup K. Anorexia nervosa and motherhood: reproduction pattern and mothering behavior of 50 women. Acta Psychiatr Scand. 1988;77(5):611–617. doi: 10.1111/j.1600-0447.1988.tb05175.x. [DOI] [PubMed] [Google Scholar]
- 9.Sollid CP, Wisborg K, Hjort J, et al. Eating disorder that was diagnosed before pregnancy and pregnancy outcome. Am J Obstet Gynecol. 2004;190(1):206–210. doi: 10.1016/s0002-9378(03)00900-1. [DOI] [PubMed] [Google Scholar]
- 10.Micali N, Simonoff E, Treasure J. Risk of major adverse perinatal outcomes in women with eating disorders. Br J Psychiatry. 2007;190:255–259. doi: 10.1192/bjp.bp.106.020768. [DOI] [PubMed] [Google Scholar]
- 11.Bulik CM, Von Holle A, Siega-Riz AM, et al. Birth outcomes in women with eating disorders in the Norwegian mother and child cohort study (MoBa) Int J Eat Disord. 2009;42(1):9–18. doi: 10.1002/eat.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekeus C, Lindberg L, Lindblad F, et al. Birth outcomes and pregnancy complications in women with a history of anorexia nervosa. BJOG. 2006;113(8):925–929. doi: 10.1111/j.1471-0528.2006.01012.x. [DOI] [PubMed] [Google Scholar]
- 13.Koubaa S, Hällström T, Lindholm C, et al. Pregnancy and neonatal outcomes in women with eating disorders. Obstet Gynecol. 2005;105(2):255–260. doi: 10.1097/01.AOG.0000148265.90984.c3. [DOI] [PubMed] [Google Scholar]
- 14.Stewart DE, Raskin J, Garfinkel PE, et al. Anorexia nervosa, bulimia, and pregnancy. Am J Obstet Gynecol. 1987;157(5):1194–1198. doi: 10.1016/s0002-9378(87)80293-4. [DOI] [PubMed] [Google Scholar]
- 15.Waugh E, Bulik CM. Offspring of women with eating disorders. Int J Eat Disord. 1999;25(2):123–133. doi: 10.1002/(sici)1098-108x(199903)25:2<123::aid-eat1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 16.Wentz E, Gillberg IC, Anckarsater H, et al. Reproduction and offspring status 18 years after teenage-onset anorexia nervosa—a controlled community-based study. Int J Eat Disord. 2009;42(6):483–491. doi: 10.1002/eat.20664. [DOI] [PubMed] [Google Scholar]
- 17.Costello EJ, Worthman C, Erkanli A, et al. Prediction from low birth weight to female adolescent depression: a test of competing hypotheses. Arch Gen Psychiatry. 2007;64(3):338–344. doi: 10.1001/archpsyc.64.3.338. [DOI] [PubMed] [Google Scholar]
- 18.Bohnert KM, Breslau N. Stability of psychiatric outcomes of low birth weight: a longitudinal investigation. Arch Gen Psychiatry. 2008;65(9):1080–1086. doi: 10.1001/archpsyc.65.9.1080. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson E, Stalberg G, Lichtenstein P, et al. Fetal growth restriction and schizophrenia: a Swedish twin study. Twin Res Hum Genet. 2005;8(4):402–408. doi: 10.1375/1832427054936727. [DOI] [PubMed] [Google Scholar]
- 20.Sydsjö G. Long-term consequences of non-optimal birth characteristics. Am J Reprod Immunol. 2011;66(suppl 1):81–87. doi: 10.1111/j.1600-0897.2011.01035.x. [DOI] [PubMed] [Google Scholar]
- 21.Cnattingius S, Hultman CM, Dahl M, et al. Very preterm birth, birth trauma, and the risk of anorexia nervosa among girls. Arch Gen Psychiatry. 1999;56(7):634–638. doi: 10.1001/archpsyc.56.7.634. [DOI] [PubMed] [Google Scholar]
- 22.Roseboom TJ, Van der Meulen JH, Ravelli AC, et al. Blood pressure in adults after prenatal exposure to famine. J Hypertens. 1999;17(3):325–330. doi: 10.1097/00004872-199917030-00004. [DOI] [PubMed] [Google Scholar]
- 23.Ravelli AC, Van Der Meulen JH, Osmond C, et al. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70(5):811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 24.Roseboom TJ, Van der Meulen JH, Osmond C, et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart. 2000;84(6):595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Micali N, Treasure J, Simonoff E. Eating disorders symptoms in pregnancy: a longitudinal study of women with recent and past eating disorders and obesity. J Psychosom Res. 2007;63(3):297–303. doi: 10.1016/j.jpsychores.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Eagles JM, Lee AJ, Amalraj Raja E, et al. Pregnancy outcomes of women with and without a history of anorexia nervosa. Psychol Med. 2012;42(12):2651–2660. doi: 10.1017/S0033291712000414. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe HM, Zador IE, Gross TL, et al. The clinical utility of maternal body mass index in pregnancy. Am J Obstet Gynecol. 1991;164(5 Pt 1):1306–1310. doi: 10.1016/0002-9378(91)90705-v. [DOI] [PubMed] [Google Scholar]
- 28.Cnattingius S, Bergstrom R, Lipworth L, et al. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338(3):147–152. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 29.Stroup DF. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 30.Bradburn MJ, Deeks JJ, Altman DG. Sbe24: metan—an alternative meta-analysis command. Stata Tech Bull. 1998;44:1–15. [Google Scholar]
- 31.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999;47:15–17. [Google Scholar]
- 32.Steichen T. Sbe19: tests for publication bias in meta-analysis. Stata Tech Bull. 1998;41:9–15. [Google Scholar]
- 33.Everitt B. Modern Medical Statistics. London, United Kingdom: Arnold Publishers; 2003. [Google Scholar]
- 34.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Micali N, De Stavola B, Dos-Santos-Silva I, et al. Perinatal outcomes and gestational weight gain in women with eating disorders: a population-based cohort study. BJOG. 2012;119(12):1493–1502. doi: 10.1111/j.1471-0528.2012.03467.x. [DOI] [PubMed] [Google Scholar]
- 36.Franko DL, Blais MA, Becker AE, et al. Pregnancy complications and neonatal outcomes in women with eating disorders. Am J Psychiatry. 2001;158(9):1461–1466. doi: 10.1176/appi.ajp.158.9.1461. [DOI] [PubMed] [Google Scholar]
- 37.Sciscione AC, Gorman R, Callan NA. Adjustment of birth weight standards for maternal and infant characteristics improves the prediction of outcome in the small-for-gestational-age infant. Am J Obstet Gynecol. 1996;175(3 Pt 1):544–547. doi: 10.1053/ob.1996.v175.a73600. [DOI] [PubMed] [Google Scholar]