Abstract
Biomarker assessment plays a critical role in the study and prevention of disease. However, variation in biomarkers attributable to the menstrual cycle in premenopausal women may impair understanding the role of certain biomarkers in disease development and progression. Thus, in light of the recently increasing evidence of menstrual cycle variability in multiple cardiometabolic biomarkers, a reexamination of approaches for appropriately studying and diagnosing cardiovascular disease in premenopausal women is warranted. We reviewed studies (from 1934 through 2012) evaluating changes in cardiometabolic biomarkers across phases of the menstrual cycle, including markers of oxidative stress, lipids, insulin sensitivity, and systemic inflammation. Each was observed to vary significantly during the menstrual cycle. For example, nearly twice as many women had elevated cholesterol levels warranting therapy (≥200 mg/dL) during the follicular phase compared with the luteal phase (14.3% vs. 7.9%), with only 3% having consistently high levels during all phases of the cycle. Similarly, nearly twice as many women were classified as being at an elevated risk of cardiovascular disease (high sensitivity C-reactive protein >3 mg/L) during menses compared with other phases (12.3% vs. 7.4%). Menstrual cycle–associated variability in cardiometabolic biomarkers is an important source of variability that should be accounted for in both research and clinical settings.
Keywords: biomarkers, cardiometabolic; inflammation; menstrual cycle; variability
INTRODUCTION
Major advances have been made in the prevention, diagnosis, and treatment of cardiovascular diseases (CVDs) over the past few decades, yet heart disease is still the leading cause of death among women in the United States (1). Several advancements are owed, in part, to the identification and characterization of important biomarkers that aid in the assessment of CVD risk and inform clinical care (2, 3). Specifically, traditional CVD risk scores are recommended for the first-line clinical assessment of CVD risk in asymptomic adults (3), including the Framingham risk score (4), SCORE (5), PROCAM (6), and Reynolds score (7), each of which incorporates several biomarkers (total cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triglycerides, hemoglobin A1c, and/or high sensitivity C-reactive protein (hsCRP)).
Importantly, changes in these biomarkers are often evident long before overt clinical symptoms or a cardiovascular event occurs. Thus, monitoring of such markers during this long latent period permits the opportunity for reversal or delay of further pathology and occurrence of a life-threatening cardiovascular event, as well as characterization of the pathogenesis of the disease in research studies. Besides the routinely monitored clinical biomarkers, many advances in CVD-related research have relied upon several different nonclinical biomarkers related to CVD and its comorbidities (e.g., metabolic syndrome, diabetes), including markers of systemic oxidative stress and inflammation, as well as specific atherosclerotic cholesterol subtypes and surrogate indices of insulin sensitivity.
A continuing challenge to research seeking to elucidate CVD progression, prevention, and treatment, however, is the apparent differences between men and pre- versus postmenopausal women (8). Interestingly, among men and women of the same age (40 years) and metabolic profile (total cholesterol, 240 mg/dL; HDL-C, 42 mg/dL; smoker; systolic blood pressure, 140 mm Hg; and not on medication to treat high blood pressure), the 10-year risk as estimated by the total Framingham point scores was only 8% in women compared with 12% in men (9). Furthermore, though CVD is the leading cause of death in women, the rate of CVD events in premenopausal women is much lower in women than in men, with the sex disparity in CVD narrowing with time, as the incidence of CVD increases with age (10).
The differences in risk between men and pre- and postmenopausal women have led researchers to consider estrogen as a cardioprotective agent in younger, menstruating women. However, pre- and postmenopausal women have markedly different hormonal profiles, of which estrogen is only one component, and several biomarkers of CVD risk factors have been suggested to be associated with such hormones (11–15). It remains unclear whether the protective role of estrogen and other sex hormones is an independent and direct protective effect on CVD, or whether sex hormones indirectly influence CVD through effects on intermediate risk factors, such as the lipid profile (Figure 1). There is some evidence from the Women's Health Initiative and the Heart and Estrogen/progestin Replacement Study trial that would support an indirect effect, as women on hormone therapy were observed to have an improved lipid profile despite an overall increase in CVD events (11, 16). As shown in Figure 1, sex hormones in this case act as a confounder (17), potentially biasing effect estimates of CVD risk factors on CVD and introducing additional variability. As such, increasing variability in effect estimates due to cyclic variability of hormones and CVD risk factors throughout the menstrual cycle could lead to misinterpretation of such CVD risk factors in premenopausal women compared with noncycling, postmenopausal women and men. Furthermore, limited but persuasive evidence regarding the onset of acute cardiovascular events indicates that women are far more likely to experience an acute cardiac event during the follicular, and particularly early follicular or menstrual, phase of the cycle compared with the luteal phase (18, 19), indicating that a biologically meaningful change in cardiovascular physiology occurs across the menstrual cycle.
Figure 1.

Diagram outlining the potential direct effect of sex hormones on cardiovascular disease (CVD) and the potential indirect effect through intermediate CVD risk factors (e.g., lipid profile).
The variability in hormonal profiles and associated changes in cardiovascular and metabolic risk markers in women, coupled with the relatively rare occurrence of CVD events in women compared with men, may contribute to the lack of strong findings relating biomarkers demonstrated to be markers of cardiovascular risk in men to comparable risk in women. Thus, in light of the recently burgeoning body of evidence related to menstrual cycle variability, a reexamination of approaches for appropriately studying and diagnosing CVD risk using biomarkers in women is warranted.
THE ROLE OF VARIANCE IN ASSESSING RISK
The importance of timing for certain biomarker assessments is well established. Diurnal variation, for example, has been a consideration in clinical trials and clinical care for years and is the prominent reason for fasting and morning collection of blood and urine specimens. Timing biomarker measurement within a woman's menstrual cycle may be equally important. Because menstrual cycles vary both between and within individual women, biomarkers affected by this cyclicity are likely to have appreciable variation between and within individual women, in addition to the existing level of variation attributable to usual non–sex-specific sources (e.g., diet, obesity, time of day/season, and so on). Thus, in order to increase consistency among studies and take into account variation attributable to menstrual cyclicity, it may be important for measurement of certain biomarkers to be timed to, or otherwise standardized for, menstrual cycle phase. Understanding the role of endogenous estrogen and other cyclic hormones as potential modulators of biomarkers may facilitate a more accurate comparison of biomarker data across sexes.
Regarding the mechanisms of variability in cardiovascular markers induced by the menstrual cycle, it has been well documented that steroid hormones, such as estrogen, intimately regulate fundamental cardiovascular functions such as blood pressure, blood flow, vasodilatation/vasoconstriction, and vascular inflammation, playing a critical role in the onset of CVD (20–22). Estrogen has also been shown to influence a variety of the biomarkers for the metabolic risk factors of chronic diseases. In particular, biomarkers of oxidative stress, lipoprotein metabolism, inflammation, and glucose metabolism have been shown to be associated with endogenous estrogen levels, as well as with adverse outcomes. Because literature in premenopausal women has demonstrated that circulating sex hormones are not static and fluctuate during a woman's menstrual cycle (23), it is logical that markers of cardiovascular disease would vary as well in premenopausal women.
Although some studies have attempted to understand the effect of menstrual cycle phase on certain cardiometabolic biomarkers (Table 1), few have adequately addressed the issue. The BioCycle Study, funded by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, was a recent longitudinal study of 259 healthy, normally menstruating, premenopausal women (aged 18–44 years) from western New York who were not taking hormonal contraceptives, were not seeking pregnancy, and had no known history of infertility or reproductive disorders. The study was designed to address several of the important limitations affecting prior research, including small sample sizes, few measurements across the cycle, and inadequate timing of assessments to menstrual cycle phase, providing a valuable resource for evaluating biomarker variability across the menstrual cycle in normally cycling, premenopausal women; the study design and recruitment are described in detail elsewhere (24). A significant advantage of the BioCycle Study was the use of fertility monitors to time clinic visits according to biologically relevant events marking the menstrual cycle (i.e., timing of ovulation). Fertility monitors tracked luteinizing hormone (LH) and estrogen metabolites daily in urine and used a built-in algorithm to detect the LH surge and time of predicted ovulation (25). Participant visits occurred up to 8 times throughout the cycle, instead of only 2–3 times as most previous studies had attempted. Therefore, biomarker measures were attained at specific phases of the menstrual cycle with the most hormonal variability, corresponding to menses, early follicular phase, LH/follicle-stimulating hormone surge, predicted time of ovulation, and the early, mid, and late luteal phase. Understanding the impact of endogenous estrogens and other cyclic hormones in relation to variation in circulating biomarkers is a crucial step in assessing cyclic variation in CVD and metabolic risk factors in premenopausal women. Failure to consider menstrual cycle phase may lead to misinterpretation of biomarker data, with important implications from both an epidemiologic and a clinical standpoint. Therefore, the objective of this review was to evaluate whether menstrual cycle variability is an important source of variation in key biomarkers related to cardiometabolic risk assessment in regularly menstruating, premenopausal women.
Table 1.
Summary of Key Studies Reviewed Evaluating Cardiometabolic Biomarkers Across the Menstrual Cycle in Normally Cycling, Premenopausal Women
| First Author, Year (Reference No.) | Biomarker Measured | Sample Size, no. | No. of Measures per Cycle | Determination of Cycle Phase and/or Ovulation | Main Findingsa |
|---|---|---|---|---|---|
| Lipoproteins | |||||
| Barnett, 2004 (32) | TG, TC, HDL-C, LDL-C | 48 | 2 | Days from menses and OPK | TG ↔, TC ↔, HDL-C ↔, LDL-C ↑F |
| Larsen, 1996 (33) | TC, HDL-C, LDL-C | 19 | ∼ 8 (2 per week for 9 weeks) | Days from menses | TC ↑F, HDL-C ↑periOv, LDL-C ↑F |
| Tonolo, 1995 (34) | TG, TC, HDL-C, LDL-C | 16 | Daily | Days from ovulation (serum LH) and menses | TG ↔, TC ↑F, HDL-C ↑periOv, LDL-C ↑preOv |
| Wall, 1994 (35) | TG, TC, HDL-C, LDL-C | 15 | ≥16 (4 per week for 5 weeks) | Hormone patterns (serum E2, P4) between ovulation (serum LH) and menses | TG ↔, TC ↑F and Ov, HDL-C ↑F-Ov, LDL-C ↑F |
| Muesing, 1996 (36) | HDL-C, LDL-C | 12 | 4 (for 3 cycles) | Days from ovulation (serum LH, luteal phase by P4) and menses | HDL-C ↑F, LDL-C ↑F |
| Schijf, 1993 (37) | TG, TC, HDL-C, LDL-C | 56 | 2 | Days from menses and serum hormones | TG ↔, TC ↑F, HDL-C ↔, LDL-C ↑F |
| Mattsson, 1984 (38) | TG, TC, HDL-C, LDL-C | 23 | 4 | Days from menses, serum hormones, and basal body temperature | TG ↑Ov, TC ↔, HDL-C ↑L, LDL-C ↑F |
| Ahumada Hemer, 1985 (39) | TG, TC, HDL-C, LDL-C | 114b | 5 groups/phases | Days from menses and serum hormones | TG ↔; TC ↑F; HDL-C ↔, LDL-C ↑F |
| Haines, 1997 (41) | TG, TC, HDL-C, LDL-C | 47 | 2 | Days from menses | ↔ |
| Lebech, 1989 (42) | TG, TC, HDL-C, LDL-C | 37 | 3 | Days from menses and serum hormones | ↔ |
| Azogui, 1992 (43) | TG, TC, HDL-C, LDL-C | 18 | 3 | Days from menses | ↔ |
| Elhadd, 2003 (44) | TG, TC, HDL-C, LDL-C | 20 | 3 | Days from menses and cycle length | ↔ |
| Kim, 1979 (40) | TG, TC, HDL-C, LDL-C | 14 | ∼ 7 (for 3 cycles) | Samples every 3–5 days; days from menses and basal body temperature | TG ↔; TC ↑F; HDL-C ↔, LDL-C ↑F |
| Woods, 1987 (45) | TG, TC, HDL-C, LDL-C | 15 | 3 | Days from menses, OPK, and luteal P4 | TG highest at Ov; TC ↔, HDL-C ↔, LDL-C ↔ |
| Mumford, 2010 (47) | TG, TC, HDL-C, LDL-C | 259 | 8 (for 2 cycles) | Days from menses, OPK, serum LH, E2, and P4 | TG ↑F; TC ↑F; HDL-C ↑mid cycle; LDL-C ↑F |
| F2-Isoprostanes | |||||
| Nhan, 2005 (54) | Urinary 8-iso-PGF-2α | 8 | Daily | Days from menses and ovulation (serum LH) | ↔ |
| Schisterman, 2010 (55) | F2-isoprostanes, TBARS | 259 | 8 (for 2 cycles) | Days from menses, OPK, serum LH, E2, and P4 | F2-Isoprostanes ↑ preOv and early L; TBARS ↑F |
| C-Reactive Protein | |||||
| Wunder, 2006 (65) | CRP | 36 | ∼ 16 | Days before (F: −12, −11 days) and after (L: +7, +8 days) ovulation (urinary and serum LH) | ↔ |
| Capobianco, 2010 (66) | CRP | 18 | 3 | Days from menses (plus serum LH, E2, and P4) | ↔ |
| Jilma, 1997 (67) | CRP | 18 | 3 | Days from menses and ovulation (OPK; plus serum E2 and P4) | ↑Mid cycle and L |
| Blum, 2005 (68) | hsCRP | 8 | 15 | Days from ovulation (serum LH) | ↑Early F |
| Wander, 2008 (69) | CRP (dried blood spots) | 8 | ∼12 | Days from menses and ovulation (urinary E2 and P4) | ↑Menses |
| Gaskins, 2012 (70) | hsCRP | 259 | 8 (for 2 cycles) | Days from menses, OPK, serum LH, E2, and P4 | ↑Menses and L |
| Insulin Sensitivity | |||||
| Blum, 2005 (68) | HOMA-IR | 8 | 15 | Days from ovulation (serum LH) | IS ↔ |
| Valdes, 1991 (73) | ISFSIVGTT | 8 | 3 | Days from menses with serum E2 | IS ↑F |
| Pulido, 1999 (74) | ISFSIVGTT | 12 | 2 | Days from menses | IS ↑F |
| Yki-Jarvinen, 1984 (75) | ISClamp | 7 | 2 | Days from menses | IS ↔ |
| Bingley, 2008 (76) | ISFSIVGTT | 13 | 2 (1.5 cycles) | Days from menses and ovulation (OPK, basal body temperature, serum P4) | IS ↔ |
| Yeung, 2010 (77) | HOMA-IR | 259 | 8 (for 2 cycles) | Days from menses, OPK, serum LH, E2, and P4 | IS ↑F |
| Uric Acid | |||||
| Mumford, 2013 (83) | Uric acid | 259 | 8 (for 2 cycles) | Days from menses, OPK, serum LH, E2, and P4 | ↑F |
| Pucher, 1934 (84) | Uric acid | 2 | 4 (weekly for 1 year) | Weeks from menses | ↑F |
| Mira, 1984 (85) | Uric acid | 22 | 2 | Days from menses and urinary LH | ↑F |
Abbreviations: CRP, C-reactive protein; E2, estradiol; F, follicular phase of menstrual cycle; FSIVGTT, frequently sampled intravenous glucose tolerance test; HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; hsCRP, high sensitivity C-reactive protein; IS, insulin sensitivity; L, luteal phase of menstrual cycle; LDL-C, low density lipoprotein cholesterol; LH, luteinizing hormone; OPK, ovulation predictor kit; Ov, ovulatory phase of menstrual cycle; P4, progesterone; PGF, prostaglandin F; TBARS, thiobarbituric acid-reactive substances; TC, total cholesterol; TG, triglycerides.
a Symbols denote relative concentration change across the cycle: ↑, highest point; ↓, lowest point; ↔, no change across cycle.
b Cross-sectional.
CARDIOMETABOLIC BIOMARKERS AND THE MENSTRUAL CYCLE
Lipoproteins
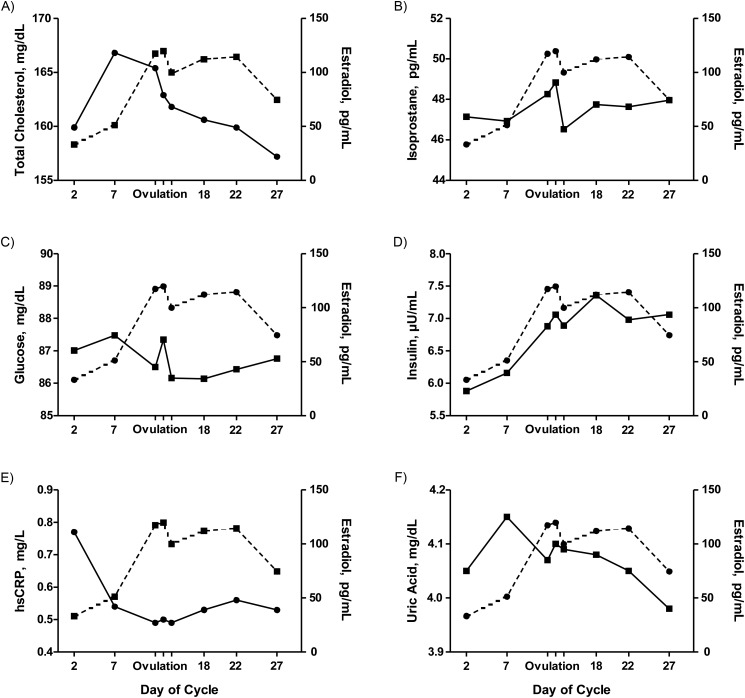
Lipoprotein metabolism has been shown to play a critical role in the development of CVD and has also been associated with circulating levels of estrogen. For example, estrogen has been shown to promote the clearance of chylomicron remnants from circulation, stimulate hepatic lipid production, increase very low density lipoprotein synthesis and the production of HDL-C and LDL-C, and enhance activity of the low density lipoprotein receptor (26–31). However, until recently, data to support a relationship between lipoprotein cholesterol and hormone levels across the menstrual cycle have been inconsistent (Table 1). Numerous studies report lower LDL-C in the luteal compared with follicular phase of the menstrual cycle (32–40), while others report no significant differences in LDL-C across cycle phases (41–45). The majority of such studies, however, did not time blood sampling to ovulation, did not verify cycle phase with measured estradiol or progesterone concentrations, and/or collected only a single “follicular phase” sample that was either during (43) or too close to (45) menses, prior to the mid-to-late follicular LDL-C peak identified in subsequent studies. In the studies designed to evaluate specific phases of the menstrual cycle on the basis of LH peak (to verify ovulation timing) and estradiol and/or progesterone measurements, however, LDL-C was reported to peak in the follicular/pre–ovulatory phase and to decline in the luteal phase (34, 35). In agreement with these latter studies, observations of LDL-C throughout the menstrual cycle among women in the BioCycle Study showed that LDL-C peaked in the mid follicular phase after menses and then declined during the peri–ovulatory phase, continuing to remain low through the luteal phase (46, 47). Collectively, these studies indicate that LDL-C changes approximately 7%–17% across the cycle, which translates to a difference of up to approximately 15 LDL-C points (ng/dL) due to normal variation attributable to the menstrual cycle of premenopausal women. Changes in total cholesterol tended to follow a pattern similar to that of LDL-C, with peak levels observed during the mid follicular phase, declining during the peri–ovulatory phase, and continuing to remain low through the luteal phase. The mean changes in total cholesterol across the cycle varied between 4% and 10%, with the mean intraindividual variability reported to range from 8% to 19% (46, 47).
Regarding HDL-C, a cardioprotective lipoprotein, studies have frequently reported no change between follicular and luteal cycle phases. However, such studies failed to identify rising HDL-C through the follicular phase, followed by a peri–ovulatory peak and decline throughout the luteal phase that were captured only in studies with more frequent sampling protocols and sound characterization of the pre–ovulatory LH surge (34, 35) and confirmed in the BioCycle Study (46, 47). Thus, in combination, evidence indicates that HDL-C rises approximately 7%–9% from menses to ovulation (and falls by a comparable amount throughout the luteal phase), which translates to an approximate difference of up to 5 HDL-C points (ng/dL) attributable to menstrual cycle variation in healthy, premenopausal women.
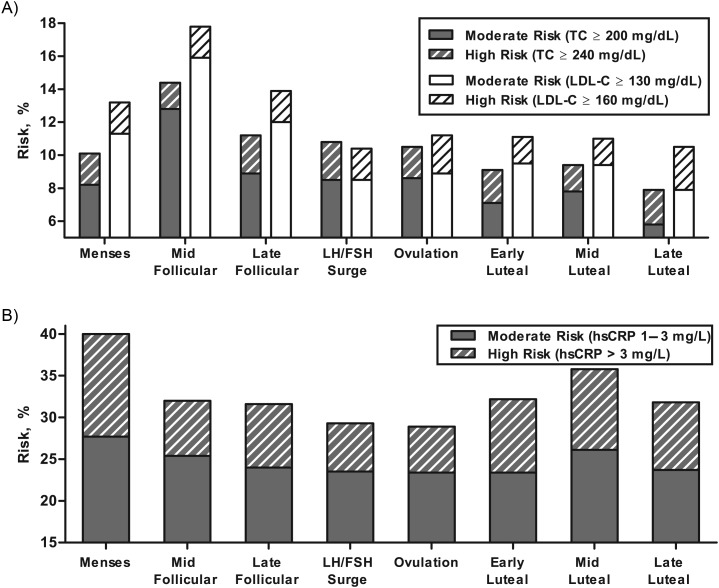
Although the changes observed in mean levels by cycle phase were modest, these differences have potential clinical implications for reproductive-aged women. In fact, women were observed to cross clinical boundaries of acceptable lipoprotein cholesterol levels when tested at different phases of the menstrual cycle. Specifically, fewer women were classified as having high cholesterol when measured during the luteal phase compared with the follicular phase (total cholesterol: 7.9% vs 14.3%; LDL-C: 10.5% vs. 17.8%) (46, 47). On the basis of these findings, the mid follicular phase may be the best phase for measurement to reduce false negatives, if we assume that management of a woman's cholesterol should be based on a level outside the National Cholesterol Education Program guidelines at any point during the cycle (48). Although treatment decisions regarding the lipid profile may still require repeated samples above the recommended level, standardizing the timing of lipid measurements may improve the interpretability of results and consequently reduce the overall number of clinical tests.
F2-isoprostanes
Oxidative stress has been implicated in a wide variety of disease processes. F2-isoprostanes, a group of prostaglandin F2α-like compounds derived from free radical–mediated oxidation of arachidonic acid and higher order polyunsaturated fatty acids, are considered the “gold standard” biological marker of oxidative stress, and they provide an accurate assessment of oxidative stress both in vitro and in vivo (49, 50). To date, there has been little research on the interplay between F2-isoprostanes and endogenous reproductive hormones throughout the menstrual cycle (Table 1), despite both F2-isoprostane and reproductive hormones being prominent biomarkers of disease in postmenopausal women (51–53). One study reported urinary F2-isoprostane throughout a single menstrual cycle in 8 women in response to high versus low dietary soy isoflavone intake (54). Daily averages in F2-isoprostane level varied widely, with a between- and within-subjects coefficient of variation of approximately 40% and 20%–53%, respectively, though no specific pattern across the cycle was identified (54). Among the relatively larger population of women participating in the BioCycle Study (n = 259), F2-isoprostane levels were observed to vary across the menstrual cycle and were significantly and inversely associated with estradiol concentrations after adjustment for age, race, age at menarche, γ-tocopherol, beta-carotene, total cholesterol, and homocysteine by inverse probability weighting (55). Specifically, levels of F2-isoprostanes were highest around the expected time of ovulation and lowest during the follicular phase (55) (Figure 2B). Interestingly, the variability of F2-isoprostanes (standard deviation of measurements) was shown to differ significantly across the menstrual cycle as well. The variability was on average 66% higher during the early follicular phase, compared with the lowest variability observed during the early luteal phase (55). Thiobarbituric acid–reactive substances, a less specific marker of oxidative stress, had similar associations in the same study.
Figure 2.
Biomarkers of cardiometabolic disease risk (solid lines), including total cholesterol (A), F2-isoprostanes (B), glucose (C), insulin (D), high sensitivity C-reactive protein (hsCRP) (E), and uric acid (F), depicted across the menstrual cycle with estradiol (dashed lines). Data reproduced with permission (47, 55, 70, 77, 83).
A prime example of differences in cardiovascular biomarker variability in men compared with premenopausal women was observed in the Coronary Artery Risk Development in Young Adults (CARDIA) Study, which evaluated the relation between F2-isoprostanes and coronary artery calcification (56). The mean level of F2-isoprostanes was 140.4 (standard deviation (SD), 55.6) pmol/L in men (n = 1,302) and 190 (SD, 108.9) pmol/L in women (n = 1,548). Subsequently, the adjusted odds ratios for coronary artery calcification in men versus women were 1.19 (95% confidence interval (CI): 1.01, 1.40) and 1.13 (95% CI: 0.89, 1.44), respectively (56). Although the point estimates are comparable across sexes, the variability in women was larger than that in men, despite the fact that the sample sizes were roughly equivalent, which could have reduced the precision and resulted in different conclusions regarding the strength of the relationship and the value of F2-isoprostanes as a predictive marker of coronary artery calcification in men versus women. Thus, the menstrual cycle as an important source of biomarker variability in women could help to explain the inconsistencies of findings between sexes in previous studies relating specific biomarkers to CVD risk.
However, a different study measuring changes in F2-isoprostanes in response to antioxidant vitamin supplementation, which sampled women specifically between the 7th and 14th days of the menstrual cycle, reported a baseline standard deviation that was similar, if not lower, relative to the mean value in women (mean = 164 (SD, 25) pg/mg creatinine; n = 51) compared with men (mean = 292 (SD, 56) pg/mg creatinine; n = 52) (57). Taken together with data from the BioCycle Study, it is clear that the impact of the menstrual cycle on variability in the measurement of F2-isoprostanes in premenopausal women is striking and has potentially dramatic implications for interpreting outcomes of studies measuring F2-isoprostanes, a gold standard marker of systemic oxidative stress.
C-reactive protein
Vascular inflammation is a crucial basic mechanism through which CVD develops and progresses. Controversial data underscore the complexity of effects on inflammation exerted by estrogen, with both anti- and proinflammatory effects reported (58). Estrogen has been shown to have a variety of potential antiinflammatory roles including the generation of nitric oxide, the regulation of leukocyte recruitment, the scavenging of free radicals, and the promotion of cell survival (59, 60). hsCRP, an acute-phase protein secreted by the liver, is a known sensitive marker for subclinical inflammation (61). Elevated concentrations of hsCRP have recently emerged as a prominent biomarker of chronic disease risk in both pre- and postmenopausal women. In addition, clinical measurement of hsCRP is recognized to be of potential utility as an adjunct measurement to the other major risk factors used in assessing risk for CVD (9). In fact, among healthy women, elevated hsCRP is one of the most significant predictors of cardiovascular disease and heart attack risk (62–64). Established cutpoints may be used to qualify low (<1.0 mg/L), medium (1.0–3.0 mg/L), and high (>3.0 mg/L) relative risk for clinical disease based on clinical hsCRP determination (9).
Previous reports have indicated that there is no menstrual cycle variation in C-reactive protein (65, 66), whereas others reported higher C-reactive protein at midcycle and the luteal phase as compared with the follicular phase (67) or higher C-reactive protein in the early follicular phase followed by a decline to the mid follicular phase (68, 69) (Table 1). However, all of these previous conflicting studies were limited by small (n ≤ 36) sample size. Among participants in the BioCycle Study, levels of hsCRP varied significantly across the menstrual cycle, where hsCRP was highest during menses, decreased during the follicular phase, was lowest on the expected day of ovulation, and increased in the luteal phase (Figure 2E) (70). These results were similar to those in the report of Blum et al. (68), which also utilized a multiple sampling approach with determination of LH surge. Of clinical significance, the largest percentage of women were classified as moderate to elevated risk of CVD when measured during the menses, while the fewest number of women were classified as such on the predicted day of ovulation. In particular, more women were classified as being at elevated risk of cardiovascular disease (hsCRP, >3 mg/L) during the menses compared with other phases (12.3% vs. 7.4%; P < 0.001). Moreover, a 10-fold increase in estradiol was associated with a 24.3% decrease in hsCRP (95% CI: 19.3, 29.0), and a 10-fold increase in luteal progesterone was associated with a 19.4% increase in hsCRP (95% CI: 8.4, 31.5). These results support the hypothesis that endogenous estradiol might have antiinflammatory effects and highlight the need for standardization of hsCRP measurement to menstrual cycle phase in reproductive-aged women.
Insulin sensitivity
Insulin resistance describes an impaired biological response to insulin, primarily prevalent in obese individuals, and has been linked to CVD. Conflicting findings have been reported regarding the effect of menstrual cycle phase and sex hormones on insulin sensitivity, which could have important implications for chronic disease. Furthermore, estradiol has been shown to play a role in the stimulation of glucose uptake in skeletal muscle, alteration of adipocyte number and size, and the regulation of hepatic energy metabolism (71, 72).
A few small, but intensive studies of young, healthy women with normal menstrual cycles have evaluated insulin sensitivity across menstrual cycle phases; however, data are conflicting (Table 1). Insulin sensitivity in 2 studies using modeled insulin sensitivity from a tolbutamide-modified, frequently sampled intravenous glucose tolerance test was shown to be highest in the follicular phase and lowest in the luteal phase (n ≤ 12 each) (73, 74). However, other studies using hyperinsulinemic, clamp-based measures of glucose metabolism (75) and modeled insulin sensitivity from an intravenous glucose tolerance test (76) showed no difference between follicular and luteal phase insulin sensitivity. The latter study closely controlled for environmental factors (e.g., diet on the evening prior to testing), confirmed ovulation and cycle phase timing with the combination of menses and fertility monitors (to determine ovulation), and included surrogate measures of insulin sensitivity (e.g., homeostasis model assessment (HOMA) and quantitative insulin sensitivity check index “QUICKI”) in addition to clamp-measured insulin sensitivity (76). However, like other studies using labor-intensive and relatively invasive gold-standard measures, this latter study included only 13 women. Fasting insulin, glucose, and the homeostasis model of insulin resistance (HOMA-IR) measured in the BioCycle Study rose before ovulation and reached a maximum during the luteal phase, indicating relative insulin resistance during the luteal phase of the cycle (Figure 2C and 2D) (77), in agreement with the studies previously showing lower insulin sensitivity in the luteal phase using the intravenous glucose tolerance test (73, 74). Insulin and HOMA-IR were also positively associated with changes in estradiol and progesterone and were inversely associated with follicle-stimulating hormone and sex hormone–binding globulin (77).
Although the changes in insulin sensitivity across the cycle may not be large enough to be clinically meaningful, these findings do suggest that clinical research studies of insulin sensitivity among premenopausal women could be more optimally conducted by timing visits to menstrual cycle phase to reduce the overall variability in measure of insulin sensitivity, and that assessment of these markers as components of the metabolic syndrome in cycling women may also be more precise if menstrual cycle was taken into account.
Uric acid
Uric acid is associated with CVD mortality risk in men and women, although outcomes in women, particularly premenopausal women, often display greater variation than they do in men (78). Though uric acid is associated with CVD in such studies, it has not consistently been considered an independent risk factor for CVD after controlling for numerous other known risk factors (79). However, uric acid likely contributes to risk of renal injury and hypertension that, in turn, raises risk for heart disease (80), thereby explaining the lack of association observed after adjustment for hypertension-related covariates (e.g., blood pressure, antihypertensive medication use, ventricular hypertrophy) in many studies. Uric acid likely has a variety of direct and indirect effects on renal and cardiovascular health and disease that are incompletely understood (80).
Lower uric acid concentrations in women compared with men (78) have been attributed to the effects of estrogen on lowering serum uric acid concentrations. Notably, premenopausal women rarely experience high enough levels of uric acid to cause health problems, which could be due to the higher levels of circulating endogenous estrogen. However, the authors have noted that the effect size of increasing serum uric acid concentration on increasing CVD risk is greater than that in men (78). Furthermore, postmenopausal women have been shown to have significantly increased levels of uric acid due in part to a drop in estrogen levels. Estrogen may decrease serum levels of uric acid in postmenopausal women; however, the interplay between endogenous reproductive hormones and uric acid levels among regularly menstruating women has not been elucidated. It has been hypothesized that estradiol may affect serum levels of uric acid through mechanisms involving renal clearance, secretion, and reabsorption (81, 82). Thus, prudent evaluation of this serum biomarker and its role in cardiovascular disease pathology and risk prediction is critical.
Among women in the BioCycle Study, mean uric acid levels peaked during the follicular phase, dropped around ovulation, and further declined during the luteal phase (Figure 2F) (83). Specifically, mean uric acid levels decreased by 1.9% (means: 4.21 vs. 4.14 mg/dL; P = 0.09) from the mid follicular phase to around ovulation by 2.4% (means: 4.21 vs. 4.11 mg/dL; P = 0.04) at the mid luteal phase and by 3.9% (means: 4.21 vs. 4.05 mg/dL; P = 0.001) at the late luteal phase. However, the mean change within a woman over the cycle was much greater (30% change; range, 6–139%; mean = 1.1 (SD, 0.5) mg/dL). Only a small number of women were hyperuricemic at any given point in a cycle (uric acid ≥ 6 mg/dL; n = 20 women). Only 10 of these women had high levels on more than one visit. In particular, 7 women had high levels on day 2 (1.4%), 5 during the mid follicular phase (1.0%), 2 during late follicular (0.4%), 3 during the LH/follicle-stimulating hormone peak visit (0.6%), 4 during predicted ovulation (0.8%), 6 during early luteal (1.2%), 6 during mid luteal (1.2%), and 3 during the late luteal phase (0.6%).
Although the mean uric acid changes observed across the cycle were modest (only 2%–4% on average), the within-woman variability was much greater (about 30% on average). Previous studies are limited, although 2 earlier studies (84, 85) assessed the variability of uric acid across the menstrual cycle and observed similar results (Table 1); serum uric acid levels were highest during menstruation and the follicular phase and continuously fell thereafter. Of note, this reported variability across the cycle was observed among healthy women of reproductive age, further emphasizing the importance of considering menstrual cycle variability as greater changes may be observed among other at-risk populations (overweight/obese, women with polycystic ovary syndrome, and so on). These findings suggest that it is important to take menstrual cycle phase into account when measuring uric acid in premenopausal women, and they confirm the hypothesized effects of endogenous estrogen on lowering uric acid. As the relationship between uric acid and cardiovascular disease risk continues to be explored, such factors contributing to variability in uric acid unique to women must be considered.
IMPLICATIONS
Study design
Given that biomarkers of CVD risk have demonstrated cyclic variability across the menstrual cycle among premenopausal women, researchers designing studies with biomarker-based outcomes should account for the menstrual cycle as a source of variability. Researchers should consider menstrual cycle variability in a priori study design not only by menopause status but also by the day or phase of the menstrual cycle at the time of biospecimen sampling. Although the best time to measure biomarkers of CVD risk during a woman's menstrual cycle has yet to be established, measurements should be made at the same time each month for consistent comparisons. Even measurements taken a week or 2 apart may be quite different solely because of changing estrogen levels. Women, physicians, and researchers should take menstrual cycle phase into account when interpreting a woman's biomarker measurements.
Ideally, biospecimen collections should occur on the same day of the cycle across all study subjects timed by using both menses and LH/estrogen monitoring with fertility monitors of blood samples. When such rigorous control for menstrual cycle day is not feasible, the phase of the menstrual cycle could be determined and accounted for by determining the last day of menses onset, use of fertility monitors to identify periovulation, serum measurement of estradiol and progesterone, and/or a combination of these elements. At the very least, in all studies, the day(s) of menses and menstrual cycle history should be recorded and taken into account during data analysis and reporting whenever possible to improve the quality of our understanding of health and disease in young women.
Random measurement of clinically used biomarkers in premenopausal women may lead to misclassification of disease risk (increased false negative or false positive test rates) and/or a misinterpretation of changes over time of a particular marker used to inform medical observation and treatment decisions. In large-scale research studies where timing blood samples according to menstrual cycle phase may not be feasible, alternative strategies need to be developed and tested. For certain biomarkers, it may be possible to develop specific algorithms for menstrual cycle standardized biomarker concentrations, based on concurrent estradiol and/or progesterone concentrations, which improve the interpretability of such measurements in epidemiologic studies of premenopausal women. Others have also championed the development and use of adapted reference ranges for biomarkers in menstruating women (68), a recommendation that, to date, has apparently gone unheeded. Further work to evaluate such methods is needed and could have significant implications for the conduct and interpretation of epidemiologic studies in young women.
Moreover, standardization of biomarker measurement also has potential implications for study power. As previously discussed, coronary heart disease is more prevalent in men (7.8%) than women (4.6%) (86). The difference in overall prevalence alone contributes to decreased power for detecting significant associations between biomarkers and CVD in women. The potential for increased biomarker variability in women would further decrease the power in this setting, underscoring the importance of minimizing and appropriately measuring biomarker variability. Specifically, if we assume equal effect sizes (15 units) and variability (50 units), the power to detect a difference among a group with 5% incidence, compared with a group with 10% incidence of an outcome, is 54% and 81%, respectively. Clearly, the lower incidence of cardiovascular events in women could contribute to an inability to appropriately link changes in biomarkers to changes in CVD risk in research studies inappropriately powered for premenopausal women. Therefore, standardization of biomarkers to reduce variability due to menstrual cycle phase becomes even more important while trying to unmask biomarker relationships to disease.
Clinical practice
The clinical implications of variability introduced due to menstrual cycle fluctuations are considerable. Of note, we evaluated whether women were observed to cross standard clinical cutpoints for the 2 biomarkers considered here with established standards, namely, lipoprotein cholesterol and hsCRP. We found that women did in fact cross clinical boundaries of acceptable lipoprotein cholesterol and hsCRP levels when tested at different phases of the menstrual cycle, emphasizing the need for standardized measurement to menstrual cycle phase (Figure 3). Although treatment decisions regarding these markers may still require repeated samples above the recommended level, standardizing the timing of measurements may improve the interpretability of results and consequently reduce the overall number of tests and the unnecessary retesting of individuals. Moreover, an increase in LDL-C concentration by 10 points over 6 months in blood tests performed to monitor lipoprotein cholesterol changes in response to dietary intervention may lead a physician to conclude that the prescribed diet is not effective in preventing LDL-C deterioration. Alternatively, a physician may conclude that the patient is not compliant with the prescribed diet, leading to a pharmaceutical prescription, when a 10-point change in LDL-C is just as likely due to the first measurement being made during the female patient's luteal menstrual phase and the second measurement during the follicular phase. The opposite scenario could lead to the undertreatment of a woman with active deterioration in cholesterol metabolism and heightened risk of progressing cardiovascular disease. Such “chance” delays in the identification of pathology could contribute to heart disease being the leading cause of death in women and a top 4 cause of preventable death in young women aged 20–44 years (87).
Figure 3.
Percent classification of cardiovascular risk according to total cholesterol (TC) and low density lipoprotein cholesterol (LDL-C) (A) and high sensitivity C-reactive protein (hsCRP) (B) in premenopausal women throughout the menstrual cycle. FSH, follicle-stimulating hormone; LH, luteinizing hormone. Data are adapted from Mumford et al. (47) in A and reprinted with modification from Gaskins et al. (70) with permission by Oxford University Press in B.
It must be acknowledged that layering additional specifications upon the collection of biospecimens for clinical care may introduce additional burden to patients and/or medical staff; however, timing of biospecimen collection or clinical assessment with menstrual cycle phase is not foreign to clinical practice. Indeed, blood specimens for measurement of gonadotropins and anti-mullerian hormone are often timed to occur between the second and fourth day of the menstrual cycle when conducting a clinical work-up for infertility assessment in women. Similarly, assessment of progesterone is often targeted for day 21 of the menstrual cycle to capture the mid luteal phase, post ovulation. Also, Papanicolaou smears are often conducted mid cycle, when a woman has passed menses to enable accurate diagnostic evaluation of the cervical specimen collected. Likewise, patients must have fasting or morning blood and/or urine collected for other diagnosis and/or monitoring needs required by physicians, further proving the feasibility of incorporating a sense of timing into specimen collection. At the same time, such testing often occurs outside the physician's office at a laboratory location convenient to the patient, decreasing both patient and clinic burden. Moreover, increased consideration for the effect of menstrual cycle on biomarker variability may ultimately help reduce the rate of unnecessary retesting, thereby helping alleviate health-care costs and burdens on patients, medical staff, and physicians in ordering and interpreting multiple tests.
Based on these findings, implementation of uniform timing of biomarker measurement in reproductive-aged women would improve interpretation in clinical settings as well as future studies. These findings highlight that the standard of care based on men may not necessarily be appropriate for women, and that women need to be studied directly. Thus, consideration of menstrual cycle phase in the development of clinical guidelines for reproductive-aged women could improve the current standard of care.
CONCLUSION
Overall, evidence indicates that certain markers of oxidative stress, inflammation, lipoprotein cholesterol, glucose metabolism, and uric acid vary across the menstrual cycle in healthy, regularly cycling, premenopausal women. This inherent cyclical variation is an important source of biological variability with implications for research and clinical practice, which is in addition to the other less avoidable sources of biomarker variability attributable to factors further from the researcher's or clinician's control (e.g., laboratory inter- and intraassay repeatability). Although the discussion here has focused on the field of cardiovascular health and disease, these findings are also especially applicable to the study of reproductive cancers and other outcomes affecting premenopausal women. Random measurement of such biomarkers in research studies of cycling, premenopausal women has demonstrated greater measurement error, compared with men or postmenopausal women, potentially leading to decreased power to detect meaningful biological differences across sexes or between pre- and postmenopausal women. In the assessment of cardiovascular disease, for which there is a lower rate of incident disease in women, such variability may have significant implications for study design, power, and analysis. Moreover, random measurement of clinically used biomarkers in premenopausal women may lead to misclassification of disease risk. Considering menstrual cycle phase in the development of clinical guidelines for reproductive-aged women and in the design of research studies will be an important step in the progress of improving our understanding and management of clinical prevention and care of cardiometabolic disease morbidity and mortality in women.
ACKNOWLEDGMENTS
Author affiliations: Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland (Enrique F. Schisterman, Sunni L. Mumford, Lindsey A. Sjaarda).
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health (contract HHSN275200403394C).
Conflict of interest: none declared.
REFERENCES
- 1.National Heart, Lung, and Blood Institute. Morbidity & Mortality: 2009 Chart Book on Cardiovascular, Lung, and Blood Diseases. Bethesda, MD: National Heart, Lung, and Blood Institute, National Institutes of Health; 2009. [Google Scholar]
- 2.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 3.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122(25):2748–2764. doi: 10.1161/CIR.0b013e3182051bab. [DOI] [PubMed] [Google Scholar]
- 4.D'Agostino RB, Grundy S, Sullivan LM, et al. Validation of the Framingham coronary heart disease prediction scores—results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 5.Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 6.Assmann G, Cullen P, Schulte H. Simple scorg scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation. 2002;105(3):310–315. doi: 10.1161/hc0302.102575. Erratum Circulation. 2002;105(7):900. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Buring JE, Rifai N, et al. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611–619. doi: 10.1001/jama.297.6.611. Erratum in JAMA. 2007;297(13):1433. [DOI] [PubMed] [Google Scholar]
- 8.Roeters van Lennep JE, Westerveld HT, Erkelens DW, et al. Risk factors for coronary heart disease: implications of gender. Cardiovasc Res. 2002;53(3):538–549. doi: 10.1016/s0008-6363(01)00388-1. [DOI] [PubMed] [Google Scholar]
- 9.National Cholesterol Education Program. Bethesda, MD: National Heart, Lung, and Blood Institute, National Institutes of Health; 2002. Estimate of 10-year risk for coronary heart disease Framingham point scores. http://www.nhlbi.nih.gov/guidelines/cholesterol/risk_tbl.htm. Accessed July 16, 2013. [Google Scholar]
- 10.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 11.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 12.Cheng W, Lau OD, Abumrad NA. Two antiatherogenic effects of progesterone on human macrophages; inhibition of cholesteryl ester synthesis and block of its enhancement by glucocorticoids. J Clin Endocrinol Metab. 1999;84(1):265–271. doi: 10.1210/jcem.84.1.5379. [DOI] [PubMed] [Google Scholar]
- 13.Miyagawa K, Rosch J, Stanczyk F, et al. Medroxyprogesterone interferes with ovarian steroid protection against coronary vasospasm. Nat Med. 1997;3(3):324–327. doi: 10.1038/nm0397-324. [DOI] [PubMed] [Google Scholar]
- 14.Chu MC, Rath KM, Huie J, et al. Elevated basal FSH in normal cycling women is associated with unfavourable lipid levels and increased cardiovascular risk. Hum Reprod. 2003;18(8):1570–1573. doi: 10.1093/humrep/deg330. [DOI] [PubMed] [Google Scholar]
- 15.Park C, Overton C. Premature menopause linked to CVD and osteoporosis. Practitioner. 2010;254(1727):21–22. 25–26. [PubMed] [Google Scholar]
- 16.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women–principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 17.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Hamelin BA, Methot J, Arsenault M, et al. Influence of the menstrual cycle on the timing of acute coronary events in premenopausal women. Am J Med. 2003;114(7):599–602. doi: 10.1016/s0002-9343(03)00051-2. [DOI] [PubMed] [Google Scholar]
- 19.Mukamal KJ, Muller JE, Maclure M, et al. Variation in the risk of onset of acute myocardial infarction during the menstrual cycle. Am J Cardiol. 2002;90(1):49–51. doi: 10.1016/s0002-9149(02)02386-x. [DOI] [PubMed] [Google Scholar]
- 20.Dubey RK, Oparil S, Imthurn B, et al. Sex hormones and hypertension. Cardiovasc Res. 2002;53(3):688–708. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 21.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286(2):R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 22.Vitale C, Mendelsohn ME, Rosano GM. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol. 2009;6(8):532–542. doi: 10.1038/nrcardio.2009.105. [DOI] [PubMed] [Google Scholar]
- 23.Strauss J, Barbieri R. Yen & Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 6th. Philadelphia, PA: Saunders Elsevier; 2009. [Google Scholar]
- 24.Wactawski-Wende J, Schisterman EF, Hovey KM, et al. BioCycle Study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23(2):171–184. doi: 10.1111/j.1365-3016.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howards PP, Schisterman EF, Wactawski-Wende J, et al. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol. 2009;169(1):105–112. doi: 10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knopp RH, Paramsothy P, Retzlaff BM, et al. Sex differences in lipoprotein metabolism and dietary response: basis in hormonal differences and implications for cardiovascular disease. Curr Cardiol Rep. 2006;8(6):452–459. doi: 10.1007/s11886-006-0104-0. [DOI] [PubMed] [Google Scholar]
- 27.Knopp RH, Zhu X. Multiple beneficial effects of estrogen on lipoprotein metabolism. J Clin Endocrinol Metab. 1997;82(12):3952–3954. doi: 10.1210/jcem.82.12.4472. [DOI] [PubMed] [Google Scholar]
- 28.Campos H, Walsh BW, Judge H, et al. Effect of estrogen on very low density lipoprotein and low density lipoprotein subclass metabolism in postmenopausal women. J Clin Endocrinol Metab. 1997;82(12):3955–3963. doi: 10.1210/jcem.82.12.4437. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava RA, Baumann D, Schonfeld G. In vivo regulation of low-density lipoprotein receptors by estrogen differs at the post-transcriptional level in rat and mouse. Eur J Biochem. 1993;216(2):527–538. doi: 10.1111/j.1432-1033.1993.tb18171.x. [DOI] [PubMed] [Google Scholar]
- 30.Zannis VI, Chroni A, Krieger M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med (Berl) 2006;84(4):276–294. doi: 10.1007/s00109-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 31.Acton S, Rigotti A, Landschulz KT, et al. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271(5248):518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 32.Barnett JB, Woods MN, Lamon-Fava S, et al. Plasma lipid and lipoprotein levels during the follicular and luteal phases of the menstrual cycle. J Clin Endocrinol Metab. 2004;89(2):776–782. doi: 10.1210/jc.2003-030506. [DOI] [PubMed] [Google Scholar]
- 33.Larsen LF, Andersen HR, Hansen AB, et al. Variation in risk indicators of cardiovascular disease during the menstrual cycle: an investigation of within-subject variations in glutathione peroxidase, haemostatic variables, lipids and lipoproteins in healthy young women. Scand J Clin Lab Invest. 1996;56(3):241–249. doi: 10.3109/00365519609088613. [DOI] [PubMed] [Google Scholar]
- 34.Tonolo G, Ciccarese M, Brizzi P, et al. Cyclical variation of plasma lipids, apolipoproteins, and lipoprotein(a) during menstrual cycle of normal women. Am J Physiol. 1995:E1101–E1105. doi: 10.1152/ajpendo.1995.269.6.E1101. (6 Pt 1) [DOI] [PubMed] [Google Scholar]
- 35.Wall PML, Choudhury N, Gerbrandy EA, et al. Increase of high-density-lipoprotein cholesterol at ovulation in healthy women. Atherosclerosis. 1994;105(2):171–178. doi: 10.1016/0021-9150(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 36.Muesing RA, Forman MR, Graubard BI, et al. Cyclic changes in lipoprotein and apolipoprotein levels during the menstrual cycle in healthy premenopausal women on a controlled diet. J Clin Endocrinol Metab. 1996;81(10):3599–3603. doi: 10.1210/jcem.81.10.8855808. [DOI] [PubMed] [Google Scholar]
- 37.Schijf CP, van der Mooren MJ, Doesburg WH, et al. Differences in serum lipids, lipoproteins, sex hormone binding globulin and testosterone between the follicular and the luteal phase of the menstrual cycle. Acta Endocrinol (Copenh) 1993;129(2):130–133. doi: 10.1530/acta.0.1290130. [DOI] [PubMed] [Google Scholar]
- 38.Mattsson LA, Silfverstolpe G, Samsioe G. Lipid composition of serum lipoproteins in relation to gonadal hormones during the normal menstrual cycle. Eur J Obstet Gynecol Reprod Biol. 1984;17(5):327–335. doi: 10.1016/0028-2243(84)90111-4. [DOI] [PubMed] [Google Scholar]
- 39.Ahumada Hemer H, Valles de Bourges V, Juarez Ayala J, et al. Variations in serum lipids and lipoproteins throughout the menstrual cycle. Fertil Steril. 1985;44(1):80–84. [PubMed] [Google Scholar]
- 40.Kim HJ, Kalkhoff RK. Changes in lipoprotein composition during the menstrual cycle. Metabolism. 1979;28(6):663–668. doi: 10.1016/0026-0495(79)90020-9. [DOI] [PubMed] [Google Scholar]
- 41.Haines CJ, Cheung LP, Lam CWK. Changes in atherogenic lipids and lipoproteins during natural and hyperstimulated cycles in healthy women. Fertil Steril. 1997;68(2):231–235. doi: 10.1016/s0015-0282(97)81507-5. [DOI] [PubMed] [Google Scholar]
- 42.Lebech AM, Kjaer A. Lipid metabolism and coagulation during the normal menstrual cycle. Horm Metab Res. 1989;21(8):445–448. doi: 10.1055/s-2007-1009258. [DOI] [PubMed] [Google Scholar]
- 43.Azogui G, Ben-Shlomo I, Zohar S, et al. High density lipoprotein concentration is increased during the ovulatory phase of the menstrual cycle in healthy young women. Gynecol Endocrinol. 1992;6(4):253–257. doi: 10.3109/09513599209024987. [DOI] [PubMed] [Google Scholar]
- 44.Elhadd TA, Neary R, Abdu TA, et al. Influence of the hormonal changes during the normal menstrual cycle in healthy young women on soluble adhesion molecules, plasma homocysteine, free radical markers and lipoprotein fractions. Int Angiol. 2003;22(3):222–228. [PubMed] [Google Scholar]
- 45.Woods M, Schaefer EJ, Morrill A, et al. Effect of menstrual cycle phase on plasma lipids. J Clin Endocrinol Metab. 1987;65(2):321–323. doi: 10.1210/jcem-65-2-321. [DOI] [PubMed] [Google Scholar]
- 46.Mumford SL, Dasharathy S, Pollack AZ, et al. Variations in lipid levels according to menstrual cycle phase: clinical implications. Clin Lipidol. 2011;6(2):225–234. doi: 10.2217/clp.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mumford SL, Schisterman EF, Siega-Riz AM, et al. A longitudinal study of serum lipoproteins in relation to endogenous reproductive hormones during the menstrual cycle: findings from the BioCycle Study. J Clin Endocrinol Metab. 2010;95(9):E80–E85. doi: 10.1210/jc.2010-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 49.Roberts LJ, Morrow JD. Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28(4):505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 50.Lawson JA, Rokach J, FitzGerald GA. Isoprostanes: formation, analysis and use as indices of lipid peroxidation in vivo. J Biol Chem. 1999;274(35):24441–24444. doi: 10.1074/jbc.274.35.24441. [DOI] [PubMed] [Google Scholar]
- 51.Payne RA. Cardiovascular risk. Br J Clin Pharmacol. 2012;74(3):396–410. doi: 10.1111/j.1365-2125.2012.04219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsimikas S. Oxidative biomarkers in the diagnosis and prognosis of cardiovascular disease. Am J Cardiol. 2006;98(11A):9P–17P. doi: 10.1016/j.amjcard.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 53.Tsimikas S, Brilakis ES, Miller ER, et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353(1):46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 54.Nhan S, Anderson KE, Nagamani M, et al. Effect of a soymilk supplement containing isoflavones on urinary F2 isoprostane levels in premenopausal women. Nutr Cancer. 2005;53(1):73–81. doi: 10.1207/s15327914nc5301_9. [DOI] [PubMed] [Google Scholar]
- 55.Schisterman EF, Gaskins AJ, Mumford SL, et al. Influence of endogenous reproductive hormones on F2-isoprostane levels in premenopausal women: the BioCycle Study. Am J Epidemiol. 2010;172(4):430–439. doi: 10.1093/aje/kwq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gross M, Steffes M, Jacobs DR, Jr, et al. Plasma F2-isoprostanes and coronary artery calcification: the CARDIA Study. Clin Chem. 2005;51(1):125–131. doi: 10.1373/clinchem.2004.037630. [DOI] [PubMed] [Google Scholar]
- 57.Ide T, Tsutsui H, Ohashi N, et al. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol. 2002;22(3):438–442. doi: 10.1161/hq0302.104515. [DOI] [PubMed] [Google Scholar]
- 58.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11(4):411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 59.Chakrabarti S, Lekontseva O, Davidge ST. Estrogen is a modulator of vascular inflammation. IUBMB Life. 2008;60(6):376–382. doi: 10.1002/iub.48. [DOI] [PubMed] [Google Scholar]
- 60.Gray GA, Sharif I, Webb DJ, et al. Oestrogen and the cardiovascular system: the good, the bad and the puzzling. Trends Pharmacol Sci. 2001;22(3):152–156. doi: 10.1016/s0165-6147(00)01640-0. [DOI] [PubMed] [Google Scholar]
- 61.Rifai N, Ridker PM. High-sensitivity C-reactive protein: a novel and promising marker of coronary heart disease. Clin Chem. 2001;47(3):403–411. [PubMed] [Google Scholar]
- 62.Boekholdt SM, Hack CE, Sandhu MS, et al. C-reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women: the EPIC-Norfolk prospective population study 1993–2003. Atherosclerosis. 2006;187(2):415–422. doi: 10.1016/j.atherosclerosis.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 63.Pradhan AD, Manson JE, Rossouw JE, et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women's Health Initiative observational study. JAMA. 2002;288(8):980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- 64.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 65.Wunder DM, Yared M, Bersinger NA, et al. Serum leptin and C-reactive protein levels in the physiological spontaneous menstrual cycle in reproductive age women. Eur J Endocrinol. 2006;155(1):137–142. doi: 10.1530/eje.1.02178. [DOI] [PubMed] [Google Scholar]
- 66.Capobianco G, de Muro P, Cherchi GM, et al. Plasma levels of C-reactive protein, leptin and glycosaminoglycans during spontaneous menstrual cycle: differences between ovulatory and anovulatory cycles. Arch Gynecol Obstet. 2010;282(2):207–213. doi: 10.1007/s00404-010-1432-2. [DOI] [PubMed] [Google Scholar]
- 67.Jilma B, Dirnberger E, Loscher I, et al. Menstrual cycle-associated changes in blood levels of interleukin-6, α1 acid glycoprotein, and C-reactive protein. J Lab Clin Med. 1997;130(1):69–75. doi: 10.1016/s0022-2143(97)90060-3. [DOI] [PubMed] [Google Scholar]
- 68.Blum CA, Muller B, Huber P, et al. Low-grade inflammation and estimates of insulin resistance during the menstrual cycle in lean and overweight women. J Clin Endocrinol Metab. 2005;90(6):3230–3235. doi: 10.1210/jc.2005-0231. [DOI] [PubMed] [Google Scholar]
- 69.Wander K, Brindle E, O'Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138–146. doi: 10.1002/ajpa.20785. [DOI] [PubMed] [Google Scholar]
- 70.Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423–431. doi: 10.1093/aje/kwr343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Devries MC, Hamadeh MJ, Phillips SM, et al. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1120–R1128. doi: 10.1152/ajpregu.00700.2005. [DOI] [PubMed] [Google Scholar]
- 72.Anderson LA, McTernan PG, Barnett AH, et al. The effects of androgens and estrogens on preadipocyte proliferation in human adipose tissue: influence of gender and site. J Clin Endocrinol Metab. 2001;86(10):5045–5051. doi: 10.1210/jcem.86.10.7955. [DOI] [PubMed] [Google Scholar]
- 73.Valdes CT, Elkind-Hirsch KE. Intravenous glucose tolerance test-derived insulin sensitivity changes during the menstrual cycle. J Clin Endocrinol Metab. 1991;72(3):642–646. doi: 10.1210/jcem-72-3-642. [DOI] [PubMed] [Google Scholar]
- 74.Pulido JME, Salazar MA. Changes in insulin sensitivity, secretion and glucose effectiveness during menstrual cycle. Arch Med Res. 1999;30(1):19–22. doi: 10.1016/s0188-0128(98)00008-6. [DOI] [PubMed] [Google Scholar]
- 75.Yki-Jarvinen H. Insulin sensitivity during the menstrual cycle. J Clin Endocrinol Metab. 1984;59(2):350–353. doi: 10.1210/jcem-59-2-350. [DOI] [PubMed] [Google Scholar]
- 76.Bingley CA, Gitau R, Lovegrove JA. Impact of menstrual cycle phase on insulin sensitivity measures and fasting lipids. Horm Metab Res. 2008;40(12):901–906. doi: 10.1055/s-0028-1082081. [DOI] [PubMed] [Google Scholar]
- 77.Yeung EH, Zhang C, Mumford SL, et al. Longitudinal study of insulin resistance and sex hormones over the menstrual cycle: the BioCycle Study. J Clin Endocrinol Metab. 2010;95(12):5435–5442. doi: 10.1210/jc.2010-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality: the NHANES I Epidemiologic Follow-up Study, 1971–1992. JAMA. 2000;283(18):2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 79.Culleton BF, Larson MG, Kannel WB, et al. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131(1):7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 80.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Puig JG, Anton FM, Alonso-Vega GG. Renal handling of uric acid: hypouricemia and tubular urate secretion. Arch Intern Med. 1986;146(9):1865. [PubMed] [Google Scholar]
- 82.Yahyaoui R, Esteva I, Haro-Mora JJ, et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J Clin Endocrinol Metab. 2008;93(6):2230–2233. doi: 10.1210/jc.2007-2467. [DOI] [PubMed] [Google Scholar]
- 83.Mumford SL, Dasharathy SS, Pollack AZ, et al. A longitudinal study of serum uric acid in relation to endogenous reproductive hormones during the menstrual cycle: findings from the BioCycle Study. Hum Reprod. 2013;28(7):1853–1862. doi: 10.1093/humrep/det085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pucher GW, Griffith FR, Jr, Brownell KA, et al. Studies in human physiology. VI. Variations in blood chemistry over long periods of time, including those characteristic of menstruation. J Nutr. 1934;7(2):169–193. [Google Scholar]
- 85.Mira M, Stewart PM, Gebski V, et al. Changes in sodium and uric acid concentrations in plasma during the menstrual cycle. Clin Chem. 1984;30(3):380–381. [PubMed] [Google Scholar]
- 86.Fang J, Shaw KM, Keenan NL. Prevalence of coronary heart disease—United States, 2006–2010. MMWR Morb Mortal Wkly Rep. 2011;60(40):1377–1411. [PubMed] [Google Scholar]
- 87.CDC Office of Women's Health. Atlanta, GA: CDC/ATSDR Office of Women's Health, Centers for Disease Control and Prevention; 2009. Leading causes of death in females, United States, 2009. http://www.cdc.gov/women/lcod/2009/index.htm. Accessed March 3, 2013. [Google Scholar]